Abstract
Context:
Verrucous lesions pose a diagnostic challenge to the clinicians as well as pathologists. There are few discrete histological features which if looked for carefully can help differentiate them.
Aim:
The aim of this study is to bring into light the histological features of several verrucous lesions occuring on skin and mucosa lined by squamous epithelium.
Settings and Design:
This is a 6-month prospective and retrospective study done on cutaneous and squamous mucosal biopsies with an exophytic pattern of growth. Clinical details along with the diagnosis were retrieved from the case files and correlated with the histological diagnosis.
Subjects and Methods:
Only hematoxylin and eosin-stained sections were studied.
Results:
Of the 35 cases, 10 (28.5%) were female and 25 (71.4%) were male. The size of the lesions ranged from 0.5 cm to 6.5 cm. The site of lesions included anogenital (8 cases, 22.8%), cutaneous (24 cases, 68.5%), and oral mucosal (3 cases, 8.5%) areas. Warts were found to be the most common lesions (14 cases, 40%), of which cutaneous warts comprised 9 cases (64%) and genital warts comprised 5 cases (36%). It was observed that benign warts were clinically confused with other rare cutaneous lesions such as polyps and cysts. Malignant counterpart of a wart or condyloma called as warty carcinoma was not known to many and was mistaken for a conventional squamous cell carcinoma.
Conclusion:
Histology is of utmost importance in differentiating the several verrucous lesions because sometimes clinical appearance may mimic one another.
Keywords: Carcinoma, histology, verrucous, warty
INTRODUCTION
Verrucous lesions are defined as “pertaining to or marked by wart like growth pattern.” In a simplified language, any exophytic/raised growth on the surface of the skin or any organ can be called as verrucous. Contrary to the common belief, all verrucous lesions are not human papillomavirus (HPV) associated. A few of them look like warts to the naked eye but are altogether different in terms of behavior and prognosis. Histopathology plays a crucial role in differentiating them. With this study, we aim to highlight certain histological features as observed on light microscopy, which can make the task of classifying common exophytic/verrucous lesions easy for the pathologists and also differentiating them from their look alike. We also aim to make the clinicians well versed with entities like these so that while taking a biopsy, they are well aware of the differential diagnoses thereby obtaining adequate material for histological examination.
SUBJECTS AND METHODS
This is a combined 6-month retrospective and prospective study conducted on all excisional and incisional biopsies having an exophytic growth pattern. The study included only lesions having a cutaneous or mucosal origin lined by squamous epithelium like oral mucosa and anogenital and cervical mucosa. Lesions occurring in visceral organs, respiratory mucosa, bony tissue, and soft tissue were excluded from the study. A total of 35 such cases were received by us. The clinical and gross findings like age, sex, site of lesion, size of lesion, and the clinical impression were noted. Histopathological features studied in detail were type of papillae, pattern of growth (exophytic, endophytic or both), presence of fibrovascular cores (FV), koilocytic atypia, inclusion bodies (intranuclear [IN] and intracytoplasmic [IC]), keratohyaline granules, status of base and margin, and type of stroma. The final histological diagnosis was compared and correlated with the clinical diagnosis.
For histology, only hematoxylin and eosin-stained sections were studied under a light microscope. No special stains or ancillary techniques were used.
RESULTS
Of the total 35 cases, 10 (28.5%) were female and 25 (71.4%) were male. The age group ranged from 10 years to 73 years. The size of the lesions ranged from 0.5 cm to 6.5 cm. The site of lesions included both anogenital (8 cases, 22.8%) and nongenital (27 cases, 77.1%) areas. Of the nongenital areas, three were located in the oral mucosa, the rest 24 were cutaneous in origin. Warts were found to be the most common lesions (14 cases, 40%), of which cutaneous warts comprised 9 cases (64%) including deep palmoplantar warts and genital warts also called as condyloma accuminatum (CA) comprised 5 cases (36%). It was observed that of the nine cutaneous warts diagnosed on histology, only four correlated with the clinical impression. The rest of all were confused with malignancies (squamous cell carcinoma [SCC]) and other benign lesions such as cysts and polyps. On the other hand, rare lesions such as keratoacanthoma (KA), seborrheic keratosis (SK), inverted follicular keratosis (IFK), Verrucous epidermal nevus (VEN), hypertrophic lichen planus (HLP), verrucous carcinoma (VC), and fibroepithelial polyps (FEP) were confused with more commonly encountered lesions like warts and malignancies, especially SCC owing to their cutaneous location. Second, it has also been observed that the clinicians are not very familiar with entities like warty carcinomas (WCs) and variants like warty basaloid (WB) type and confuse them with proliferative SCCs or condylomas. This in turn leads to limited sampling and missing of either an exophytic component which shows koilocytosis or an endophytic component which shows basaloid and invasive features. Hence, it is advised that all exophytic tumors should have two separate biopsies, (a) from the exophytic part and (b) from the endophytic part including the base. It is because of similar reasons only that rare cases like carcinoma cuniculatum (CC), cutaneous WC, and VC having better prognosis are misdiagnosed as SCC on biopsies. Table 1 enumerates the clinical and histological features of all the 35 cases with a special mention on the key points which can help in differentiating them from one another. Tables 2 and 3 show comparative histological features between the common mimics of verrucae vulgaris (VV).
Table 1.
Clinicopathological features of all the cases
| Age/sex | Site | Size (greatest dimension in cm) | Pattern (exo/endo) with/without FV cores and type of keratosis | Koilocytotic atypia/inclusions | Margins and base | Clinical impression | Histology diagnosis | Key points |
|---|---|---|---|---|---|---|---|---|
| 30/male | Finger | 0.8 | Exo + endo with cores and church spire like hyperkeratosis | +/+ (keratohyaline granules) | Inward sloping/cupping of rete ridges ending at same level | Papilloma | VV | Parakeratosis with keratohyalin granules is the key in differentiation from AC which also occurs on dorsum of hand |
| 62/male | Preauricular | 1.2 | Endo without cores Ortho + parakeratosis |
+/− | Inward sloping/cupping of rete ridges ending at same level | Sebaceous cyst | VV | Parakeratosis with koilocytotic atypia and noninfiltrating margins is the key in differentiation from KA which also occurs on face |
| 28/male | Finger | 0.5 | Exo without cores Ortho + parakeratosis |
+/− | Inward sloping/cupping of rete ridges ending at same level | Wart | VV | Koilocytosis |
| 20/male | Finger | 0.8 | Exo without cores Ortho + parakeratosis |
+/− | Inward sloping/cupping of rete ridges ending at same level | Wart | VV | Koilocytosis |
| 20/male | Chin | 0.6 | Exo with cores focally Hyperkeratosis |
−/+ (keratohyaline granules) | Papillary projections ending at same level as that of the epidermis | ?Boil | VV | Sometimes parakeratosis and koilocytosis may not be apparent. Size of lesion and keratohyaline granules help in differentiating it from others |
| 45/male | Eyelid | 0.5 | Exo with cores Hyperkeratosis |
−/+ (keratohyaline granules) | Finger-like papillary projections ending at same level as that of the epidermis | Squamous papilloma/polyp | VF/squamous papilloma of eyelid | Keratohyaline granules |
| 46/male | Eyelid | 1.0 | Exo without cores mild Hyperkeratosis |
−/− | Small finger-like undulating epidermal projections ending at the same level | Polyp | FEP | Atrophic/normal epidermis Fat extending upto superficial dermis |
| 57/male | Thigh | 2.0 | Exo without cores | −/− | Undulating surface | Papilloma | FEP | Size is larger compared to VV |
| 55/female | Chest wall | 2.5 | Exo without cores | −/− | Undulating surface | Skin tag | FEP | Same as above |
| 53/male | Anal verge | 1.4 | Exo with cores Ortho + parakeratosis |
+/− | Noninfiltrating acanthotic epithelial margins | Anal polyp | CA | Koilocytosis |
| 54/female | Cervix | 1.8 | Exo + endo with occassional cores Parakeratos |
+/− | Noninfiltrating acanthotic epithelial margins | SCC | CA | Koilocytosis Noninfiltrating margins Low grade dysplasia |
| 43/male | Anal area | 2.8 | Same as above | +/− | Same as above | Sentinel pile | CA | Same as above |
| 55/male | Perianal | 2.6 | Same as above | +/− | Noninfiltrating | Anal tag | CA | Same as above |
| 65/female | Cervix | 0.5 | Same as above | +/− | Noninfiltrating | Cervical polyp | CA | Same as above |
| 65/female | Cervix | 3.0 | Exo + endo with cores Ortho + parakeratosis |
+/− | Infiltrating Inflammatory stroma |
SCC | Warty Ca or condylomatous carcinoma | Koilocytotic atypia in exophytic component Keratin pearls Well differentiated |
| 44/female | Cervix | 4.7 | Exo + endo with cores Ortho + parakeratosi |
+/− | Infiltrating Desmoplastic stroma |
SCC | Warty Ca | Same as above |
| 70/male | Leg (anteriorly) | 1.2 | Exo + endo with cores Ortho + parakeratosis |
+/− | Infiltrating with interconnected rete ridges | SCC | Cutaneous warty Ca | Same as above |
| 66/female | Anal region | 0.7 | Exo + endo without cores Parakeratosis |
+/− | Infiltrating with basaloid cells showing peripheral palisading | SCC | Warty basaloid Ca | Both warty and basaloid components should be>10% |
| 69/male | Buccal mucosa | 5.6 | Exo + endo with cores Ortho + parakeratosis |
−/− | Infiltrating jagged margins with neutrophilic abscesses | SCC | CC | Deep sinuses/crypts filled with keratin Well differentiated Keratin pearls No koilocytotic atypia |
| 70/male | Back | 3.2 | Exo + endo with thin cores Ortho + parakeratosis |
−/− | Broad pushing noninfiltrating margins not ending at the same level | SCC | VC | Papillae up and down (not ending at the same level) No koilocytotic atypia No dysplasia Pushing irregular margins Larger size than VV |
| 73/male | Chest wall | 2.0 | Exo + endo with thin cores Ortho + parakeratosis |
−/− | Same as above | SCC | VC | Same as above |
| 62/male | Buccal mucosa | 1.3 | Exo + endo with thin cores Orthokeratosis |
−/− | Same as above | SCC | VC | Same as above |
| 52/male | Back | 1.1 | Exo<endo with occassional cores Orthokeratosis |
−/− | Acanthotic epidermis with noninfiltrating margins, irregular base | Papilloma | SK | Presence of ‘horn cysts’in the epidermis Basaloid cell proliferation No mitosis/atypia Rete ridges may/may not end at the same level |
| 45/female | Nose | 0.9 | Exo<endo with occassional cores Orthokeratosis |
−/− | Cup shaped lesion with pseudo-infiltrating margins in a noninfllammatory stroma | SCC | KA | Loose keratin unlike compact type in VV Cup is not fully filled with keratin (no plugging of mouth) Mild atypia, no mitosis |
| 67/female | Eyelid | 0.6 | Exo<endo with occassional cores Orthokeratosis |
−/− | Regular, noninfiltrating | Squamous papilloma | IFK | Squamous eddies Plugging of hair follicle No atypia/mitosis |
| 59/male | Postauricular | 4.5 | Exo without cores Orthokeratosis alternating with parakeratosis |
−/− | Rete ridges are regularly elongated and end at same level | SCC | ILVEN ILVEN |
Alternating foci of orthokeratosis witj hypergranulosis and parakeratosis with hypogranulosis Rete rigdes elongated |
| 16/male | Umblicus | 2.5 | Exo without cores Orthokeratosis |
−/− | Finger like papillary projections ending at same level as normal epidermis | Granuloma | VEN | Exophytic finger like projections with only orthokeratosis. And thinned epidermis No koilocytosis or keratohyaline granules |
| 55/male | Leg (anteriorly) | 2.0 | Exo + endo without cores Orthokeratosis |
−/− | Irregular pseudoinfiltrating | SCC | HLP | Band of lymphocytes at the dermoepidermal junction causing basal layer vacuolation No atypia/mitosis |
| 37/male | Lip | 0.5 | Exo without cores Orthokeratosis |
−/− | Regular border with thickened epithelial extensions ending at the same level in an inflammatory stroma | VC or SCC | VH | Predominantly exophytic pattern only Well-differentiated squamous epithelium with keratin pearls |
| 55/female | Finger | 2.5 | Exo with broad cores showing lobular capillary proliferation Orthokeratosis |
−/− | Irregular, noninfiltrating ending at different levels | Malignant skin adnexal tumor | Verr He | Like a pyogenic granuloma with verrucous surface |
| 14/male | Foot | 0.8 | Exo without cores Ortho + parakeratosis |
−/variably sized eosinophilic intracytoplasmic inclusions | Regular noninfiltrating margins | Corn | Myrmecia | Variable sized and shaped intranuclear and intracytoplasmic inclusions are characteristic |
| 50/female | Right toe | 0.5 | Endo without cores Ortho + parakeratosis |
Same as above | Same as above | Wart | Myrmecia | Same as above |
| 70/male | Left toe | 1.0 | Exo + endo without cores Ortho + parakeratosis |
Same as above | Same as above | Corn | Myrmecia | Same as above |
| 12/male | Right arm | 0.6 | Exo without cores | −/large keratinocytes showing cytopathic effect | Same as above | EDV | EDV | Cytopathic effect in epidermal keratinocytes is characteristic |
| 10/male | Face | 0.5 | Exo + endo without cores | −/large keratinocytes and same sized eosinophilic intracytoplasmic inclusions | Same as above | MC | EDV+MC | MC inclusions are of uniform size and shape compared to Myrmecia |
VV: Verrucae vulgaris, FV: Fibrovascular, PEP: Fibroepithelial polyps, CA: Condyloma accuminatum, SCC: Squamous cell carcinoma, VC: Verrucous carcinoma, SK: Seborrheic keratosis, KA: Keratoacanthoma, IFK: Inverted follicular keratosis, VEN: Verrucous epidermal nevus, ILVEN: Inflammatory linear VEN, HLP: Hypertrophic lichen planus, VH: Verrucous hyperplasia, EDV: Epidermodysplasia verruciformis, MC: Molluscum contagiosum, VF: Verrucae filiformis
Table 2.
Differentiating features between VV, KA, SK and IFK on histology
| VV | KA | SK | IFK |
|---|---|---|---|
| 1) The exophytic papillae may lie above the level of normal epidermis | 1) The exophytic papillae lie below the level of normal epidermis | 1) Papillae are predominantly above the surface of normal epidermis (exo >> endo) | 1) Predominantly endophytic ingrowing but may have papillae (endo >> exo) |
| 2) Compact (tight) hyperkeratosis filling the entire cup and plugging the mouth | 2) Acantholytic (loose) type of hyperkeratosis, partially filling the cup and open mouth | 2) Hperkeratosis is seen but is less compared to VV, KA | 2) Hyperkeratosis is less. Keratin-filled crypts containing hair shaft seen |
 |
 |
||
| 3) The rete ridges end at the same level | 3) May not end at same level and show pseudo-infilrating margins mimicking a carcinoma | 3) The rete ridges end at the same level | 3) The rete ridges end at the same level |
| 4) Koilocytosis is present | 4) Koilocytosis is absent | 4) Koilocytosis is absent | 4) Koilocytosis is absent |
| 5) Cytological and nuclear atypia is absent | 5) Cytological and nuclear atypia may be seen | 5) Cytological and nuclear atypia is absent | 5) Cytological and nuclear atypia is absent |
| 6) Keratin pearls not seen in the acanthotic epidermis | 6) Keratin pearls may be seen in the acanthotic epidermis | 6) Keratin pearls/horn cysts are always seen in the acanthotic epidermis | 7) “Squamous eddies” seen |
VV: Verrucae vulgaris, KA: Keratoacanthoma, SK: Seborrheic keratosis, IFK: Inverted follicular keratosi
Table 3.
Differentiating features between CC, SCC, VC and VH on histology
| CC | SCC, conventional type | VC | VH |
| 1) Exophytic papillary component is always present | 1) Exophytic papillary component is absent | 1) Exophytic papillae are present but with thin/without FV cores | 1) Exophytic pattern only |
| 2) Tumor is always well differentiated | 2) Tumor may be well, moderately or poorly differentiated | 2) Always well differentiated | 2) Papillae are absent, well differentiated squamous cells |
| 3) Infiltrating jagged margins with a inflammatory and mild desmoplastic stroma | 3) Infiltrating margins with extensive desmoplasia in stroma | 3) Pushing broad margins | 3) Rete ridges lie at the same level as the normal epithelium, inflammatory stroma |
| 4) Crypts or sinuses filled with keratinous debris are present | 4) Keratin filled crypts are not seen Variable keratinization | 4) Hyperkeratosis with parakeratosis is seen | 4) Orthokeratosis only |
| 5) Mild cellular atypia may be seen | 5) Atypia is always present in variable degrees and proportion | 5) Atypia is never present | 5) No cellular atypia |
| 6) Koilocytotic atypia is absent | 6) Koilocytotic atypia is absent | 6) Koilocytotic atypia is absent | 6) Koilocytotic atypia is absen |
| 7) Locally aggressive May infiltrate bone | 7) Locally aggressive and may show distant metastasis | 7) Not aggressive No propensity to metastasize | 7) May progress to VC |
| 8) Good prognosis | 8) Bad prognosis | 8) Very good prognosis | 8) Considered as a precursor to VC |
SCC: Squamous cell carcinoma, VC: Verrucous carcinoma, VH: Verrucous hyperplasia, CC: Carcinoma cuniculatum, FV: Fibrovascular
DISCUSSION
The verruciform group of neoplasms is usually site specific like CA and its malignant counterparts called as pure WC [Figure 1] and WB carcinomas [Figure 2] which occur in anogenital areas.[1,2] Warty component comprises exophytic papillae with FV cores lined by polygonal cells with well-defined cellular borders, eosinophilic cytoplasm, and prominent koilocytotic atypia (wrinkled hyperchromatic nuclei with perinuclear halo and frequent multinucleation). The base is infiltrating and stroma shows desmoplasia. In addition to this, if there is a monotonous population of poorly differentiated cells with scant basophilic cytoplasm, hyperchromatic nuclei, inconspicuous nucleoli, and frequent mitotic figures accounting for at least 10% of the tumor, it is called as WB.[3] Identification of basaloid features is important because it has a worse prognosis than the pure form and requires an aggressive therapeutic approach.[3] Chaux et al.[3] have described three microscopic patterns of WB carcinomas and arranged pure basaloid carcinoma, SCC/WB carcinoma, and warty carcinoma in an increasing order of prognosis and decreasing order of incidence of metastasis.[3,4] On the other hand, cutaneous origin of WC is very rare. We came across one case of pure cutaneous WC on the anterior aspect of leg in a 70-year-old male having no previous history of any anogenital malignancy [Figure 3]. Similar to our case, Aroni et al.[5] also have reported a case of cutaneous WC on the thigh. CA is a type of verrucae which is sexually transmitted and is commonly caused by HPV-6, 10, and 11.[6,7] It shows an arborescent papillary pattern with prominent central FV cores, surface koilocytosis, sharply defined base without invasion, and orderly maturation of epithelial cells [Figure 4]. Other less common and rare types of verrucous lesions comprise deep plamoplantar warts (Myrmecia), epidermodysplasia verruciformis (EDV), and molluscum contagiosum (MC) which are differentiated on the basis of characteristic IN and IC inclusions. Deep palmoplantar warts are caused by HPV and show large eosinophilic keratohyaline granules (IN and IC inclusions) of variable shapes and sizes in the keratinocytes [Figure 5]. MC on the other hand shows uniform appearing eosinophilic IC inclusions in the keratinocytes different from the granules seen in myrmecia [Figure 6].[8] EDV is a rare autosomal recessive genetic disorder manifesting by increased susceptibility to cutaneous HPV 5 and 8[9] having oncogenic potential. Clinically, it can present as wart-like papules, SK, and pityriasis like plaques.[10] Microscopic examination shows orthokeratosis, acanthosis, koilocytes, and occasional large keratinocytes with enlarged nuclei in the granular layer [Figure 6]. Our study included two cases of EDV in HIV-positive siblings. One of them had a co-existing MC as well suggesting an HIV-induced immunosuppression, a likely cause of defect in acquired cell-mediated immunity.
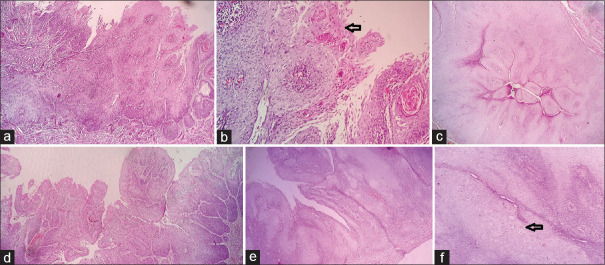
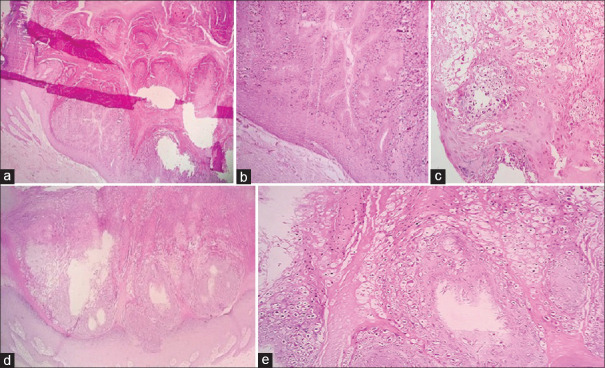
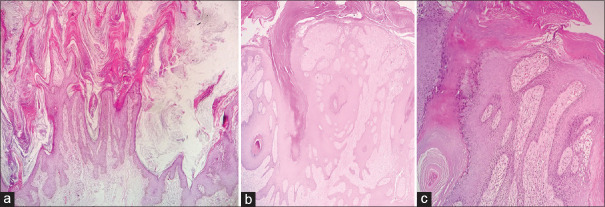
Figure 1.
Low-power view of a Warty carcinoma of the cervix showing an exophytic papillary growth with fibrovascular cores (a-e×100 H and E). High-power view highlighting the fibrovascular cores and koilocytes (f, H and E×400)
Figure 2.
Low-power view of warty basaloid carcinoma (a and c, H and E×100). High-power view showing koilocytic atypia (black arrow) and basaloid cells (yellow arrow) (b,H and E×400)
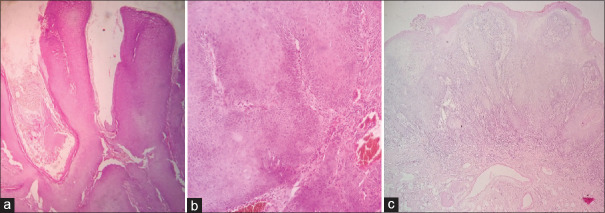
Figure 3.
Low-power view of (a) exo and (b) endophytic components of Warty cutaneous carcinoma (H and E×100). High-power view of koilocytosis (c, H and E×400)
Figure 4.
Exophytic papillary noninfitrating pattern of condyloma accuminatum (a, H and E × 100) low-power view of well differentiated squamous cells within an acanthotic mucosa (b, H and E × 100). High-power view of koilocytic atypia (c, H and E × 400)
Figure 5.
Low-power view of a deep palmoplantar wart (myrmecia) (a and d H and E×100). High-power view showing the characteristic intranuclear and intracytoplasmic inclusions (c and e H and E×400)
Figure 6.
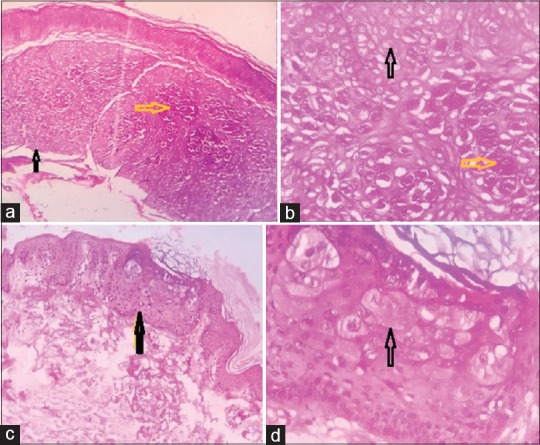
Low-power view showing combined epidermodysplasia verruciformis + molluscum contagiosum (a) and epidermodysplasia verruciformis only (c) (H and E × 100). High-power view of molluscum contagiosum inclusions (yellow arrow) and epidermodysplasia verruciformis keratinocytes (black arrow) (b and d, H and E ×400)
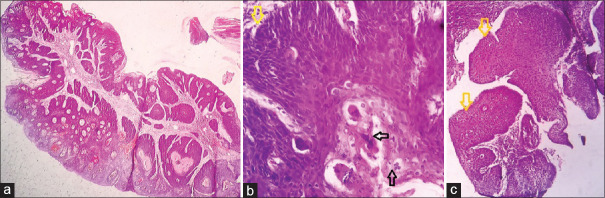
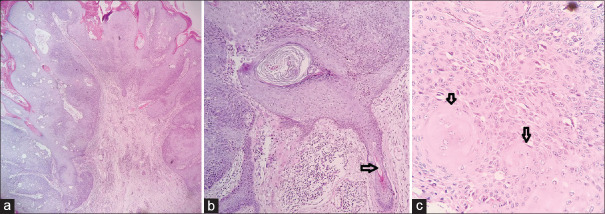
CC (CA) is a distinct and rare variant of oral SCC which is easily mistaken for VC and SCC of conventional type.[11] We encountered a similar case in which biopsy from buccal mucosa growth was reported as SCC, but the resection specimen showed a tumor with both exophytic and endophytic infiltrating components [Figure 7a]. Microscopic examination revealed a papillomatous growth lined by well-differentiated squamous cells sending complex branching crypts/sinuses filled with keratinous debris and burrowing into the stroma with jagged margins, surrounded by an inflammatory infiltrate. Koilocytic atypia was absent [Figure 7b–d].[12,13,14] Alcohol and tobacco have been implicated as the likely etiological factors in the development of CC in the upper aerodigestive tract, whereas HPV is seen in CC at cutaneous sites. It is rarely associated with lymph node matastasis and distant disease.[15] VC is a rare, low-grade variant of well-differentiated SCC commonly seen in the oral mucosa and infrequently on the skin where it is mistaken for the more frequent VV.[16] VC shows an exophytic papillary growth with hyperkeratosis and endophytic papillae ending at different levels with broad pushing margins [Figure 8a and b]. Its precursor called as verrucous hyperplasia (VH) has overlapping clinicopathologic features. Differentiation of the two is based on the location of hyperplastic epithelium in relation to the adjacent normal epithelium. In VH, the rete ridges are broad and lie at the same level as that of the adjacent epithelium [Figure 8c] unlike VC which has an uneven pushing margin.[17,18] Both of them exhibit no dysplasia or atypia throughout, and a local excision with wide margins is an ideal treatment for both.[19] VC does not metastasize locally or distally unlike SCC which has infiltrating margins and shows metastasis.[20] CC is more aggressive than VC locally and may invade underlying bone. It can be said that VC > CC > SCC in terms of prognosis and behavior. VV or common wart by standard definition is an HPV-associated infective lesion of the skin exhibiting a well-circumscribed exophytic growth pattern with marked hyperkeratosis.[21] However, we observed endophytic (with cupping margins) and combined exo + endophytic patterns of VV as well [Figures 9–14] which mimic acrokeratosis verruciformis (AKV), KA, IFK, SK, FEPs, verrucous hemangioma (VH), and verrucous epidermal nevi (VEN). Identification of prominent granular layer with keratohyaline granules or/with koilocytes and compact hyper and parakeratosis with dilated blood vessels in the papillary dermis is the characteristic finding of VV. IFK is a predominantly endophytic ingrowing tumor-like lesion representing proliferation of the external sheath of hair follicles. It shows a lobular configuration of squamous cells with peripheral lining by basaloid cells. “Squamous eddies” is characteristic of IFK [Figure 11].[22] KA is a benign keratinocytic neoplasm arising from hair follicle usually seen in sun-exposed areas. It frequently shows a dome-shaped nodule with a central crater of keratin, cupping of margins, and acanthosis of the epidermis forming papillary outgrowths sometimes.[23] Epidermal nevi (EN) are hamartomatous proliferation of the epithelium including the keratinocytes, sebocytes, pilosebaceous units, eccrine glands, or apocrine glands.[24] We observed two cases of EN in a child and an adult. On histology, VEN shows acanthosis, papillomatosis, and thickening of the epidermis with elongation of rete ridges and perivascular infiltration of lymphocytes in the upper dermis [Figure 13a]. A variant called as inflammatory linear verrucous epidermal nevus shows alternation of orthokeratosis with hypergranulosis and parakeratosis lacking a granular layer with mild spongiosis [Figure 13b]. HLP is a warty growth usually seen on the pretibial and perimalleolar regions. On light microscopy, it shows marked hyperkeratosis, uneven acanthosis, elongated rete ridges ending at different levels, and moderate dermal infiltrate of lymphocytes and histiocytes causing basal layer vacuolation and making the border unclear. In long-standing cases, it may transform to SCC [Figure 14b–d].[25]
Figure 7.
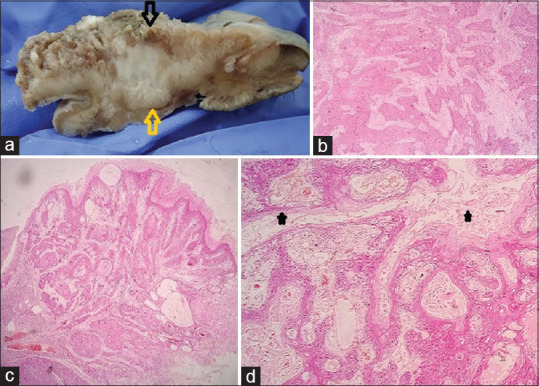
Gross picture of CC (a). Yellow and black arrows showing the gross endophytic and exophytic components and their corresponding light microscopic images respectively in (b) and (c) (Hand E X100). High power view showing keratin filled sinuses (black asterisk) (d H&E X400)
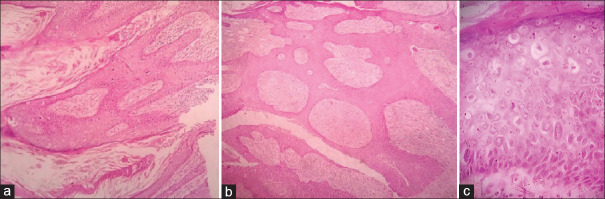
Figure 8.
Low-power view of a verrucous carcinoma showing (a) exophytic papillae and (b) endophytic pushing margins. Verrucous hyperplasia showing an exophytic pattern composed of well-differentiated squamous cells in an inflammatory stroma (c) (H and E×100)
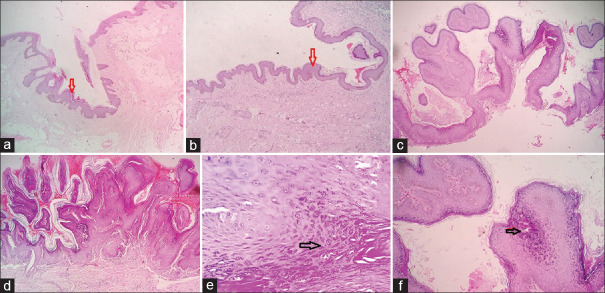
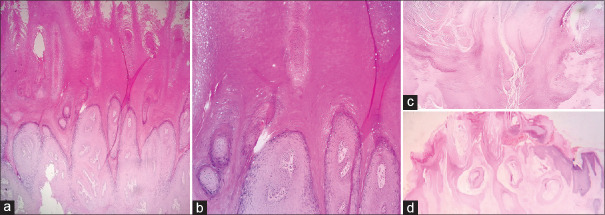
Figure 9.
Low-power view of fibroepithelial polyps (a and b) showing undulating papillomatous atrophic epidermal surface with focal hyperkeratosis (H and E ×100). Verrucae vulgaris for comparison showing an acanthotic papillary epidermis with fibrovascular cores (c and d, H and E ×100). High-power view highlighting the keratohyaline granules in verrucae vulgaris (e and f, H and E × 400)
Figure 14.
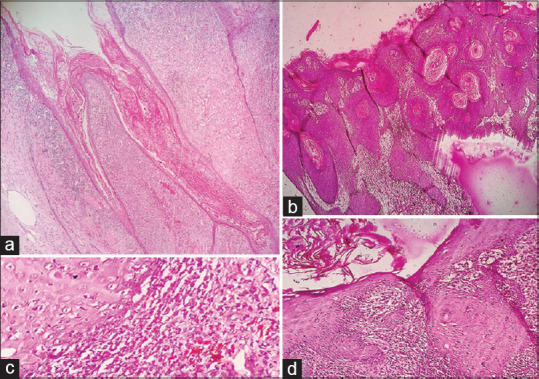
Low-power view of verrucous Hemangioma (VeH) and a hypertrophic lichen planus respectively (a and b, H and E×100X). Band of lymphoctes at the dermoepidermal junction in hypertrophic lichen planus (d, H and E×100) causing basal layer vacuolation (c, H and E×400)
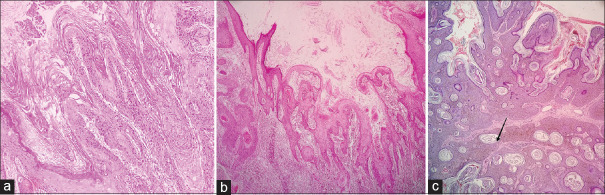
Figure 11.
Low-power view of inverted follicular keratosis (a, H and E × 100). High-power view showing entrapped hair follicle (black arrow in b) and squamous eddies (black arrow in c) (H and E × 400)
Figure 13.
Low-power view showing exophytic finger-like papillary projections with tiers of keratin in VEN (a, H and E×100). Another case of VEN with psoriasiform pattern of the epidermis showing alternating bands of ortho and parakeratosis consistent with inflammatory linear verrucous epidermal nevus (b, H and E×100) (c, H and E×400)
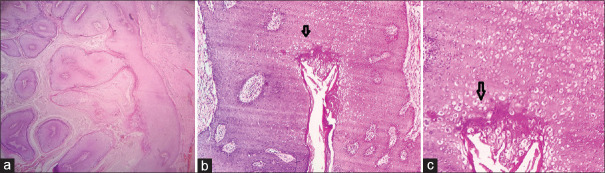
Figure 10.
Respective low (a, H and E×100) and high (b, H and E×400) power views of a verrucae vulgaris showing "church spire" like keratosis mimicking an AKV. The presence of parakeratosis or keratohyaline granules helps in differentiation. Low-power view of a characteristic predominantly endophytic (c) and exophytic pattern (d) of verrucae vulgaris (H and E×100)
Figure 12.
Low-power view of a keratoacanthoma showing papillary tips lined by “loose” keratin (a) and endophytic cupping margins with pseudoinfiltrating base (b) (H and E×100). Low-power view of SK (black arrows showing horn cysts in an acanthotic epidermis with basaloid cells) (c H and E×100)
CONCLUSION
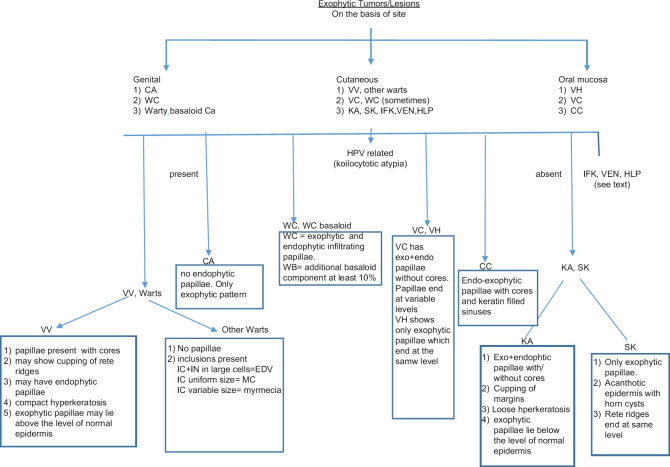
It can be said that a verruciform group of lesions has a broad spectrum of histomorphology and behavior. Hence, a careful histological examination is essential to reach a correct diagnosis. Figure 15 shows an algorithmic approach to the differentiation of verrucous lesions on histology, as described above. Certain features to be kept in mind while reporting are:-
Figure 15.
Algorithmic approach to the differentiation of verrucous lesions on histology. Abbreviations: CA: Condyloma Accuminatum, WC: Warty Carcinoma, WC basaloid: Wart Carcinoma with Basaloid features, VV: Verrucae Vulgaris, VC: Verrucous carcinoma, KA: Keratoacanthoma, SK: Seborrheic Keratosis, VH: Verrucous Hyperplasia, CC: Carcinoma Cunniculatum, HPV: Humanpapilloma Virus, IN: Intranuclear, IC: Intracytoplasmic, FV: Fibrovascular cores, IFK: Inverted follicular keratosis, HLP: Hypertrophic lichen planus, VEN: Verrucpus epidermal nevus
WC are not confined to genital areas, cutaneous origin are rare but if present, it offers a better prognosis than SCC
Never miss a basaloid component
Type and pattern of hyperkeratosis is important (ortho/para, compact/loose,)
Koilocytotic atypia is the key to identify HPV association, if not seen, then parakeratosis with dark basophilic and large keratohyaline granules are also pathognomic of HPV cytopathic effect
Orientation of all verrucous lesions with respect to normal epithelium/epidermis is a very helpful in making a correct diagnosis. Hence, a part of normal epithelium should always be included in the section along with the lesion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are thankful to the technical staff of histopathology department especially Mr. Kamal, Miss priyanka for their cooperation, our Laboratory Manager Dr. Ritu Bisht for her immense support and guidance and the Chairman of hospital for allowing us to conduct this study.
REFERENCES
- 1.Yordanov AD, Slavchev SH, Strashilov SA, Malkodanski IT, Dimitrova BI, Ivanov IN, et al. Warty carcinoma of the uterine cervix with lymph node metastasis: A case report with a literature review. J Clin Exp Oncol. 2018;7:1. [Google Scholar]
- 2.Jang YH, Kim YC, Lee ES. Warty squamous cell carcinoma of the vulva in older women: Association with human papillomavirus. Yonsei Med J. 2005;46:155–8. doi: 10.3349/ymj.2005.46.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaux A, Tamboli P, Ayala A, Soares F, Rodríguez I, Barreto J, et al. Warty-basaloid carcinoma: Clinicopathological features of a distinctive penile neoplasm. Report of 45 cases. Mod Pathol. 2010;23:896–904. doi: 10.1038/modpathol.2010.69. [DOI] [PubMed] [Google Scholar]
- 4.Thapa S, Ghosh A, Shrestha S, Ghartimagar D, Narasimhan R, Talwar OP. Warty carcinoma penis: An uncommon variant. Case Rep Pathol. 2017;2017:2937592. doi: 10.1155/2017/2937592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aroni K, Lazaris AC, Ioakim-Liossi A, Paraskevakou H, Davaris PS. Histological diagnosis of cutaneous “warty” carcinoma on a pre-existing HPV lesion. Acta Derm Venereol. 2000;80:294–6. doi: 10.1080/000155500750012216. [DOI] [PubMed] [Google Scholar]
- 6.Al Aboud AM, Nigam PK. StatPearls. Treasure Island (FL): StatPearls Publishing; 2018. Wart (Plantar, Verruca Vulgaris, Verrucae) [Google Scholar]
- 7.McCutcheon T. Anal condyloma acuminatum. Gastroenterol Nurs. 2009;32:342–9. doi: 10.1097/SGA.0b013e3181b85d4e. [DOI] [PubMed] [Google Scholar]
- 8.Stulberg DL, Hutchinson AG. Molluscum contagiosum and warts. Am Fam Physician. 2003;67:1233–40. [PubMed] [Google Scholar]
- 9.Emsen IM, Kabalar ME. Epidermodysplasia verruciformis: An early and unusual presentation. Can J Plast Surg. 2010;18:21–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Bhat YJ, Ashraf S, Hassan I. Epidermodysplasia verruciformis in two siblings responding to retinoids. Indian J Paediatr Dermatol. 2016;17:322–4. [Google Scholar]
- 11.Datar UV, Kale A, Mane D. Oral carcinoma cuniculatum: A new entity in the clinicopathological spectrum of oral squamous cell carcinoma. J Clin Diagn Res. 2017;11:ZD37–9. doi: 10.7860/JCDR/2017/23437.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thavaraj S, Cobb A, Kalavrezos N, Beale T, Walker DM, Jay A. Carcinoma cuniculatum arising in the tongue. Head Neck Pathol. 2012;6:130–4. doi: 10.1007/s12105-011-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad SG, Kaur K, Gupta S. Carcinoma cuniculatum: A review. Indian J Dent Sci. 2012;4:87–9. [Google Scholar]
- 14.Prasad RS, Moorthy A, Bhadranna A, Pai A. Proliferative endophytic lesion of the maxilla: A diagnostic challenge. J Oral Maxillofac Pathol. 2018;22:S82–S86. doi: 10.4103/jomfp.JOMFP_248_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allon D, Kaplan I, Manor R, Calderon S. Carcinoma cuniculatum of the jaw: A rare variant of oral carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:601–8. doi: 10.1067/moe.2002.126913. [DOI] [PubMed] [Google Scholar]
- 16.Vandeweyer E, Sales F, Deraemaecker R. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:168–70. doi: 10.1054/bjps.2000.3440. [DOI] [PubMed] [Google Scholar]
- 17.Shear M, Pindborg JJ. Verrucous hyperplasia of the oral mucosa. Cancer. 1980;46:1855–62. doi: 10.1002/1097-0142(19801015)46:8<1855::aid-cncr2820460825>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Hazarey VK, Ganvir SM, Bodhade AS. Verrucous hyperplasia: A clinico-pathological study. J Oral Maxillofac Pathol. 2011;15:187–91. doi: 10.4103/0973-029X.84492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grover S, Jha M, Sharma B, Kapoor S, Mittal K, Parakkat NK, et al. Verrucous hyperplasia: Case report and differential diagnosis. Sultan Qaboos Univ Med J. 2017;17:e98–102. doi: 10.18295/squmj.2016.17.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadasivan A, Thankappan K, Rajapurkar M, Shetty S, Sreehari S, Iyer S. Verrucous lesions of the oral cavity treated with surgery: Analysis of clinico-pathologic features and outcome. Contemp Clin Dent. 2012;3:60–3. doi: 10.4103/0976-237X.94548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swetha P, Supriya NA, Kumar GR. Characterization of different verrucous mucosal lesions. Indian J Dent Res. 2013;24:642–4. doi: 10.4103/0970-9290.123421. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal PK, Gupta S, Pandey R, Yadav N. Inverted Follicular Keratosis Scalp. People's Journal of Scientific Research. 2016;9:65–8. [Google Scholar]
- 23.Zargaran M, Baghaei F. A clinical, histopathological and immunohistochemical approach to the bewildering diagnosis of keratoacanthoma. J Dent (Shiraz) 2014;15:91–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Kosann MK. Inflammatory linear verrucous epidermal nevus. Dermatol Online J. 2003;9:15. [PubMed] [Google Scholar]
- 25.Paravina M. Hypertrophic lichen planus – A case report. Serbian J Dermatol Venerol. 2014;6:73–80. [Google Scholar]