Abstract
Purpose
We aimed to investigate the prognostic value of serum β2-microglobulin for patients with Burkitt lymphoma (BL) and to propose a risk-stratifying classification system.
Materials and Methods
A prospective registry-based cohort study of BL patients treated with dose-intensive or effective dose-adjusted chemotherapies (n=81) was conducted. Survival outcomes were compared based on previously reported risk groups and/or serum β2-microglobulin levels. A risk-stratifying classification system incorporating serum β2-microglobulin levels was proposed and validated in an independent validation cohort (n=60).
Results
The median age was 47 years, and 57 patients (70.4%) were male. Patients with high serum β2-microglobulin levels (> 2 mg/L) had significantly worse progression-free survival (PFS) and overall survival (OS) (p < 0.01 for both). Serum β2-microglobulin levels further stratified patients in the low-risk and high-risk groups in terms of PFS (p=0.010 and p=0.044, respectively) and OS (p=0.014 and p=0.026, respectively). Multivariate analyses revealed that a high serum β2-microglobulin level (> 2 mg/L) was independently associated with a shorter PFS (hazards ratio [HR], 3.56; p=0.047) and OS (HR, 4.66; p=0.043). The new classification system incorporating the serum β2-microglobulin level allowed the stratification of patients into three distinct risk subgroups with 5-year OS rates of 100%, 89.5%, and 62.5%. In an independent cohort of BL, the system was validated by stratifying patients with different survival outcomes.
Conclusion
Serum β2-microglobulin level is an independent prognostic factor for BL patients. The proposed β2-microglobulin–based classification system could stratify patients with distinct survival outcomes, which may help define appropriate treatment approaches for individual patients.
Keywords: Burkitt lymphoma, β2-microglobulin, Prognosis, Risk stratification
Introduction
Burkitt lymphoma (BL) is a rare type of non-Hodgkin lymphoma of B cell origin that features highly aggressive biological and clinical behavior [1,2]. The molecular hallmark of BL is a MYC translocation, which results in an extremely high rate of cellular proliferation. BL is highly sensitive to chemotherapy and can potentially be cured with high-intensity chemotherapy regimens [1,2].
Despite the highly aggressive biological behavior of BL, the patterns of disease involvement and clinical course show substantial heterogeneity [1–4]. Population-based studies have identified several risk factors for a poor outcome among patients with BL, such as older age, advanced stage, African ancestry, poor performance status (PS), and high levels of lactate dehydrogenase (LDH) [5–9]. A recent multicenter study reported age, Eastern Cooperative Oncology Group (ECOG) PS, LDH, and central nervous system involvement as independent prognostic factors for the survival outcomes of patients with BL [6]. Based on the known prognostic factors, risk-adapted treatment approaches with dose adjustment have been proposed to effectively treat high-risk patients with dose-intensified regimens while minimizing treatment-related toxicities in low-risk patients by reducing the chemotherapeutic dose intensity [10,11]. In this strategy, the stage of the disease, serum LDH levels, ECOG PS, and the presence of bulky disease are taken into consideration to classify patients into low- and high-risk groups. However, the absence of a validated prognostic scoring system for patients with BL warrants further investigation into additional prognostic factors and the development of risk-stratifying models for these patients.
β2-microglobulin is a small protein that is an essential part of major histocompatibility complex class I molecules, and its serum levels can be elevated under various pathologic conditions. Elevated serum β2-microglobulin levels have been shown to be associated with poor clinical outcomes in various types of lymphoma [12–14], suggesting potential biological roles in the development and progression of lymphomatous disease. In particular, the β2-microglobulin level was shown to be a strong prognostic factor in high-grade non-Hodgkin lymphoma [15]. However, the prognostic value of β2-microglobulin has not been specifically investigated in patients with BL.
In this study, using two independent BL cohorts, we aimed to investigate the prognostic value of β2-microglobulin and to propose a risk-stratifying classification based on serum β2-microglobulin levels.
Materials and Methods
1. Study patients and diagnosis of BL
The study patients were identified from a prospective registry of lymphoma patients at Asan Medical Center (Seoul, Korea) (Asan Lymphoma Registry). Between May 2004 and March 2020, 103 patients were diagnosed as having BL based on both histologic and immunophenotypic findings. After excluding patients with no baseline serum β2-microglobulin value (n=10) and those who did not receive dose-intensive or effective dose-adjusted chemotherapies (n=12), 81 patients were included as the main study population (training cohort). Data such as patient characteristics and survival outcomes were obtained from the prospectively collected database and electronic medical record system of the institution.
The validation cohort was constructed based on 60 BL patients who were treated with intensive chemotherapy regimens and had a baseline serum β2-microglobulin value recorded at Samsung Medical Center between February 2000 and December 2019.
The diagnosis of BL was confirmed by experienced pathologists from each institution (C.S.P., J.H., and Y.H.K) based on morphological characteristics and immunohistochemical features, including CD10, CD20, Bcl-6, Bcl-2, and Ki-67 expression. Chromosomal translocations such as t(8;14), t(8;22), and t(2;8), and c-Myc overexpression, were assessed based on karyotyping and fluorescence in situ hybridization.
2. Chemotherapy regimens
The dose-intensive or dose-adjusted chemotherapy regimens used were as follows: (1) brief-duration high-intensity chemotherapy regimen consisting of one cycle of cyclophosphamide and prednisone followed by cycles containing ifosfamide; high-dose methotrexate, vincristine, dexamethasone, and either doxorubicin or etoposide/cytarabine; or intrathecal triple therapy (B-NHL) [16]; (2) hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone with rituximab (R-HyperCVAD) [17] or without rituximab (HyperCVAD); (3) dose-adjusted infusional etoposide, doxorubicin, and vincristine with prednisone, cyclophosphamide, and rituximab (DA-EPOCH-R) [18]; (4) cyclophosphamide, vincristine, prednisone, doxorubicin and high-dose methotrexate (COPADM); and (5) the LMB protocol where COPADM is delivered in the induction phase, and the consolidation phase is maintained with high-dose methotrexate and cytarabine [19].
3. Serum β2-microglobulin levels
Serum β2-microglobulin values were obtained as part of regular clinical practice for lymphoma staging work-up. Serum β2-microglobulin was measured using a radioimmunoassay kit (Immunotech, Inc., Prague, Czech Republic). The kit manufacturer defined the upper normal limit of serum β2-microglobulin as 2.5 mg/L.
4. Classification of risk groups
The low-risk group was defined as patients having stage I/II disease, normal LDH levels, ECOG PS of ≤ 1, and non-bulky disease (tumor mass with a diameter of < 7 cm) [11], whereas the high-risk group was defined as patients who did not meet the low-risk group criteria [10,11].
5. Statistical analysis
Statistical analyses were performed using R software ver. 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). Progression-free survival (PFS) was defined as the time interval from the time of initial diagnosis (index date) to the date of disease progression [20] or death. Overall survival (OS) was defined as the time interval between the index date and the date of death from any cause. The Kaplan-Meier method was used to estimate survival outcomes, and the log-rank test was used to compare these survival outcomes among the subgroups. The maximal chi-square method was used to determine the optimal cut-off value of serum β2-microglobulin that best segregated the PFS outcomes. Univariate and multivariate analyses of PFS and OS were performed using Cox proportional hazards models. The examined variables included serum β2-microglobulin levels, age, sex, and previously known prognostic factors for BL, such as age, sex, stage, serum LDH levels, presence of bulky disease, and ECOG PS. In multivariate analyses, variables with a potential relationship (p < 0.05) in the univariate analyses were included. A p-value of < 0.05 was considered statistically significant.
Results
1. Patient characteristics
The baseline characteristics of 81 study patients with BL in the training cohort are summarized in Table 1. Their median age was 47 years (range, 16 to 82 years), and 57 patients (70.4%) were male. There were nine (11.1%), 56 (61.5%), 14 (17.3%), and two (2.5%) patients with an ECOG PS of 0, 1, 2, and 3 or 4, respectively. In addition, two patients (2.5%) were infected with human immunodeficiency virus. Stage IV disease (n=62, 76.5%) was the most prevalent, followed by stage I (n=10, 12.3%), stage II (n=8, 9.9%), and stage III (n=1, 1.2%). The majority of patients (n=65, 80.2%) were classified as the high-risk group, and 16 (19.8%) patients were classified as the low-risk group. Median serum LDH and β2-microglobulin levels were 395 IU/L (range, 139 to 18,822 IU/L) and 2.3 mg/dL (range, 0.76 to 22.2 mg/dL), respectively. All of the study patients received dose-intensive or effective dose-adjusted chemotherapies; B-NHL was the most frequently used regimen (n=37, 45.7%), followed by R-HyperCVAD (n=28, 34.6%) and DA-EPOCH-R (n=16, 19.8%).
Table 1.
Clinical characteristics of the patients
| Variable | Training cohort (n=81) | Validation cohort (n=60) |
|---|---|---|
| Age (yr) | 47 (16–82) | 52.5 (18–84) |
| Age > 65 yr | 13 (16.0) | 10 (16.7) |
| Male sex | 57 (70.4) | 42 (70) |
| ECOG PS | ||
| 0 | 9 (11.1) | 10 (16.7) |
| 1 | 56 (61.5) | 31 (51.7) |
| 2 | 14 (17.3) | 19 (31.6) |
| 3/4 | 2 (2.5) | 0 |
| HIV infection | 2 (2.5) | 2 (3.6)a) |
| Stage | ||
| I | 10 (12.3) | 6 (10.0) |
| II | 8 (9.9) | 9 (15.0) |
| III | 1 (1.2) | 5 (8.3) |
| IV | 62 (76.5) | 40 (66.7) |
| Bulky disease | 7 (8.6) | 1 (1.7) |
| Risk group | ||
| Low | 16 (19.8) | 9 (15.0) |
| High | 65 (80.2) | 51 (85.0) |
| LDH > UNL | 55 (67.9) | 45 (75.0) |
| β2-microglobulin (mg/dL) | 2.3 (0.76–22.2) | 2.7 (1.11–22.5) |
Values are presented as median (range) or number (%). ECOG, Eastern Cooperative Oncology Group; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; PS, performance status; UNL, upper normal limit.
Data of 56 patients were available.
Patients in the validation cohort had comparable baseline characteristics. The chemotherapy regimens used for the patients in the validation cohort were as follows: R-HyperCVAD (n=29, 48.3%), LMB protocol (n=25, 41.7%), COPADM (n=4, 6.7%), and HyperCVAD (n=2, 3.3%).
2. Clinical characteristics and survival outcomes according to the serum β2-microglobulin levels
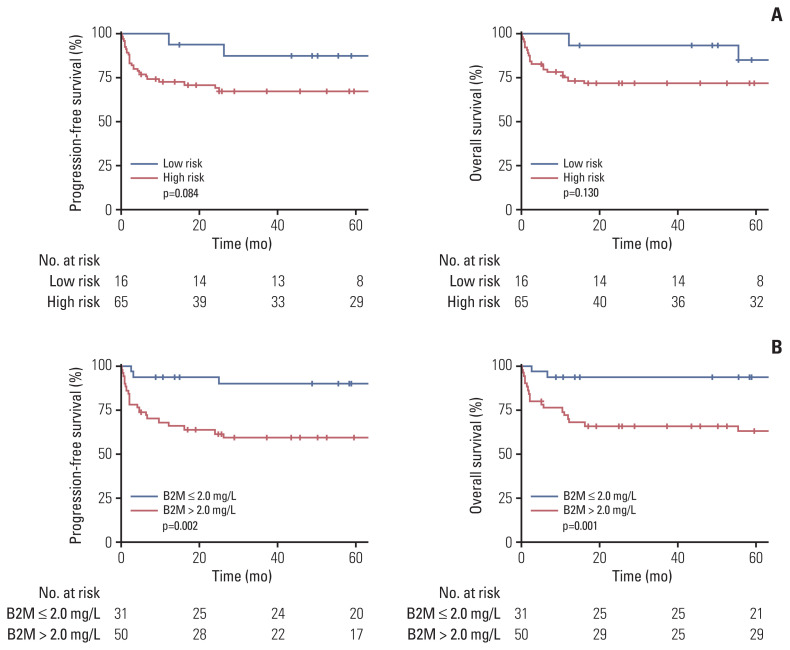
We first compared the clinical outcomes of the study patients according to risk groups. There was no significant difference in the treatment regimens between the low-risk and high-risk groups (p=0.239) (S1 Table). In comparison with the low-risk group, the high-risk group showed a trend toward a shorter PFS and OS (p=0.084 and p=0.130, respectively) (Fig. 1A).
Fig. 1.
Survival outcomes according to risk groups and serum β2-microglobulin (B2M) levels: progression-free survival and overall survival according to risk groups (A) and serum B2M levels (B).
We examined the clinical characteristics and outcomes of the subgroups of BL patients according to their serum β2-microglobulin levels (Table 2). In this regard, we adopted a hot-spot cut-off value of 2 mg/L that best segregated the PFS outcomes. Patients with higher levels of serum β2-microglobulin (the high-B2MG group) were significantly older than those with lower levels (the low-B2MG group) (median age, 53 vs. 38.5 years; p=0.014). The proportion of patients with bulky disease was comparable between the two groups. However, the high-B2MG group had significantly more patients with elevated serum LDH (82.0% vs. 45.2%, p=0.001) and stage III/IV disease (92.0% vs. 51.6%, p < 0.001) (Table 2).
Table 2.
Clinical characteristics according to serum B2MG levels
| Variable | Low-B2MG group (n=31) | High-B2MG group (n=50) | p-value |
|---|---|---|---|
| Age (yr) | 38.5 (28.5–47.5) | 53.0 (30.0–64.0) | 0.014 |
| Male sex | 20 (64.5) | 37 (74.0) | 0.510 |
| Bulky disease | 2 (6.5) | 5 (10.0) | 0.844 |
| Risk group | 0.002 | ||
| Low | 12 (38.7) | 4 (8.0) | |
| High | 19 (61.3) | 46 (92.0) | |
| LDH > UNL | 14 (45.2) | 41 (82.0) | 0.001 |
| ECOG PS ≥ 2 | 1 (3.2) | 15 (30.0) | 0.008 |
| Stage III/IV | 16 (51.6) | 46 (92.0) | < 0.001 |
Values are presented as median (interquartile range) or number (%). B2MG, β2-microglobulin; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PS, performance status; UNL, upper normal limit.
A comparison of the survival outcomes of the entire cohort revealed the significantly shorter PFS and OS of the high-B2MG group (p=0.002 and p=0.001, respectively) compared with the low-B2MG group (Fig. 1B). When the study patients were further classified based on their different β2-microglobulin levels (i.e., β2-microglobulin ≤ 2.0 mg/L; 2.0 mg/L < β2-microglobulin ≤ 2.5 mg/L; and β2-microglobulin > 2.5 mg/L), patients with a β2-microglobulin level of > 2.0 mg/L but ≤ 2.5 mg/L had a PFS and OS that were comparable to those with a β2-microglobulin level of > 2.5 mg/L, but inferior to those with a β2-microglobulin level of < 2.0 mg/L (S2 Fig.).
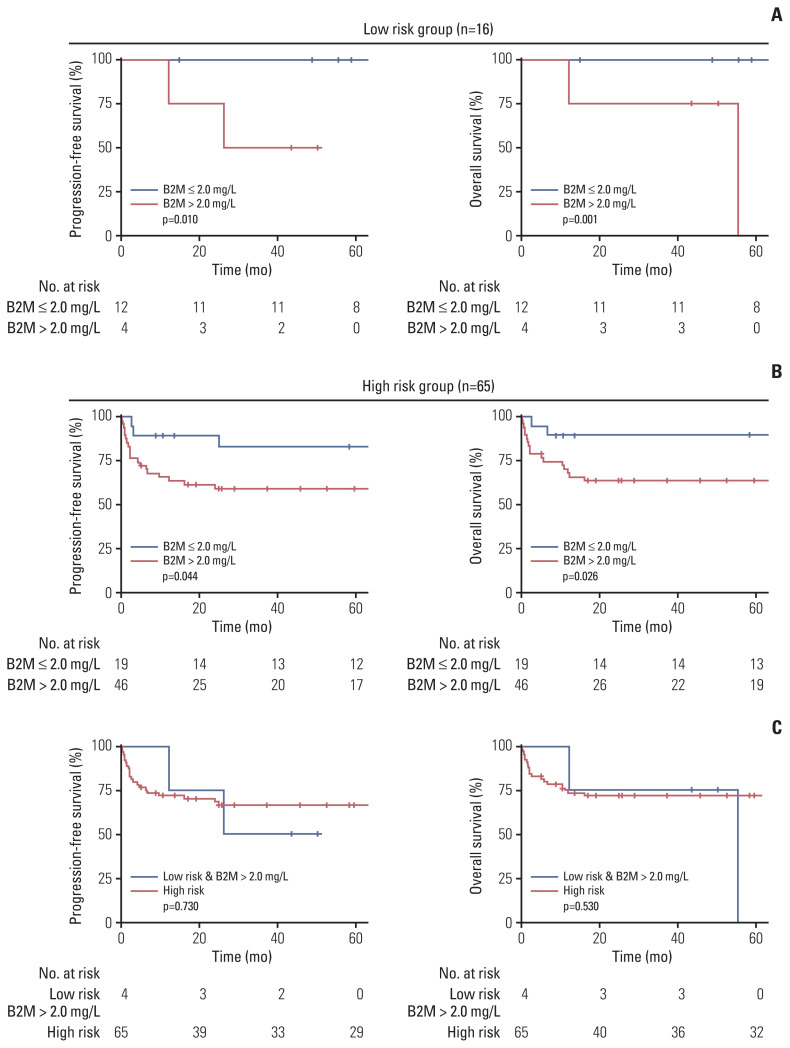
3. Survival outcomes of patients in the low- and high-risk groups according to their serum β2-microglobulin levels
In the low-risk group, 12 patients (75.0%) had low serum β2-microglobulin levels (≤ 2 mg/L), whereas four patients (25.0%) had high serum β2-microglobulin levels (> 2 mg/L). In the high-risk group, 46 patients (70.8%) had low serum β2-microglobulin levels, whereas 19 patients (29.2%) had high serum β2-microglobulin levels. In the low-risk group, patients with β2-microglobulin levels of > 2 mg/L had significantly shorter PFS and OS (Fig. 2A) (p=0.010 and p=0.001, respectively). Notably, neither disease progression nor death events were observed in the low-risk group among patients with low serum β2-microglobulin levels (≤ 2 mg/L). Similarly, in the high-risk group, high serum β2-microglobulin levels (> 2 mg/L) were associated with a significantly shorter PFS and OS (Fig. 2B) (p=0.044 and p=0.026, respectively). When we compared survival outcomes between low-risk patients with elevated serum β2-microglobulin and high-risk patients, there was no significant difference in PFS and OS (p=0.730 and p=0.530, respectively) (Fig. 2C). PFS and OS were also comparable among patients who received different chemotherapy regimens (p=0.760 and p=0.940, respectively).
Fig. 2.
Survival outcomes of patients with different serum β2-microglobulin (B2M) levels in different risk groups. Progression-free survival and overall survival according to the serum B2M levels in the low-risk group (A) and high-risk group (B). (C) Survival outcomes were compared between low-risk group patients with serum B2M levels of > 2.0 mg/L and high-risk group patients.
4. Multivariate analyses for PFS and OS
We performed Cox regression analyses for PFS and OS (Table 3). Multivariate analyses revealed that a serum β2-microglobulin level of > 2 mg/L was independently associated with a shorter PFS (hazard ratio [HR], 3.56; 95% confidence interval [CI], 1.02 to 12.45; p=0.047) and OS (HR, 4.66; 95% CI, 1.04 to 20.85; p=0.043). Older age (> 65 years) was marginally associated with a shorter PFS (HR, 2.43; 95% CI, 0.98 to 6.01; p=0.055), whereas poor PS (ECOG PS ≥ 2) was an independent negative prognostic factor for PFS (HR, 4.35; 95% CI, 1.89 to 10.02; p < 0.001). Both older age (> 65 years) and poor PS (ECOG PS ≥ 2) were independently associated with a shorter OS (HR, 3.02; 95% CI, 1.17 to 7.79; p=0.021 and HR, 4.50; 95% CI, 1.84 to 10.99; p < 0.001, respectively).
Table 3.
Factors associated with progression-free survival and overall survival
| Variable | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| B2MG > 2 mg/dL | 5.65 (1.69–18.90) | 0.005 | 3.56 (1.02–12.45) | 0.047 | 7.66 (1.79–32.82) | 0.006 | 4.66 (1.04–20.85) | 0.043 |
|
| ||||||||
| Age > 65 yr | 2.68 (1.11–6.45) | < 0.001 | 2.43 (0.98–6.01) | 0.055 | 3.33 (1.34–8.26) | 0.010 | 3.02 (1.17–7.79) | 0.021 |
|
| ||||||||
| Male sex | 0.91 (0.39–2.12) | 0.833 | - | - | 0.77 (0.32–1.83) | 0.548 | - | - |
|
| ||||||||
| Stage III/IV | 4.50 (10.06–19.11) | 0.042 | - | - | 3.80 (0.89–16.27) | 0.072 | - | - |
|
| ||||||||
| LDH > UNL | 2.33 (0.87–6.20) | 0.092 | - | - | 2.64 (0.89–7.81) | 0.080 | - | - |
|
| ||||||||
| Bulky disease | 0.95 (0.22–4.03) | 0.095 | - | - | 1.10 (0.26–4.72) | 0.896 | - | - |
|
| ||||||||
| ECOG PS ≥ 2 | 5.64 (2.54–12.53) | < 0.001 | 4.35 (1.89–10.02) | < 0.001 | 5.90 (2.52–13.82) | < 0.001 | 4.50 (1.84–10.99) | < 0.001 |
B2MG, β2-microglobulin; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazards ratio; LDH, lactate dehydrogenase; PS, performance status; UNL, upper normal limit.
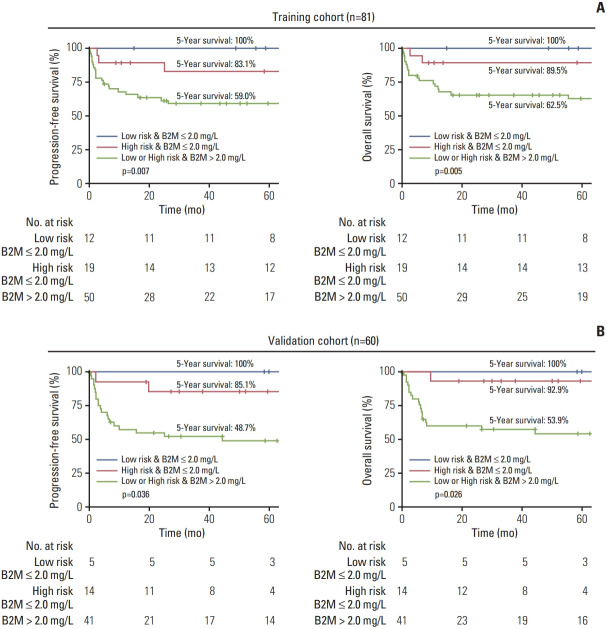
5. Proposal of a novel risk stratification classification incorporating serum β2-microglobulin
Considering the ability of serum β2-microglobulin levels to stratify BL patients with distinct clinical outcomes, we proposed a new risk stratification classification system with distinct survival outcomes (Fig. 3A): (1) a low-risk group with serum β2-microglobulin levels of ≤ 2 mg/L with 5-year PFS and OS rates of 100% (95% CI, 100 to 100) and 100% (95% CI, 100 to 100), respectively (minimal risk subgroup); (2) a high-risk group with serum β2-microglobulin levels of ≤ 2 mg/L with 5-year PFS and OS rates of 83.1% (95% CI, 67.2 to 100) and 89.5% (95% CI, 76.7 to 100), respectively (the intermediate risk subgroup), and (3) any-risk group with serum β2-microglobulin levels of > 2 mg/L with 5-year PFS and OS rates of 59.0% (95% CI, 46.6 to 74.7) and 62.5% (95% CI, 50 to 78.2), respectively (the highest risk subgroup). Patients in the three new risk subgroups showed statistically different PFS and OS (p < 0.01 for both).
Fig. 3.
Survival outcomes in subgroups determined by a novel serum β2-microglobulin (B2M)–based risk stratification system. Progression-free survival and overall survival of low-risk group patients with serum B2M levels of ≤ 2 mg/L, high-risk group patients with serum B2M levels of ≤ 2 mg/L, and any-risk group patients with serum B2M levels of > 2 mg/L in the training cohort (A) and validation cohort (B).
To validate the generalizability of our classification system, we used it for patients in the validation cohort (Fig. 3B). Similarly, the system was able to stratify patients with different PFS and OS (p=0.036 and p=0.026, respectively). Patients in the minimal, intermediate, and highest risk subgroups showed 5-year PFS rates of 100% (95% CI, 100 to 100), 85.1% (95% CI, 68.3 to 100), and 48.7% (95% CI, 35.1 to 67.5), respectively. The 5-year OS rates for the minimal, intermediate, and highest risk subgroups were 100% (95% CI, 100 to 100), 92.9% (95% CI, 80.3 to 100), and 53.9% (95% CI, 40.2 to 72.3), respectively.
Discussion
In the current study, we investigated the clinical value of serum β2-microglobulin for the risk stratification of BL patients. To the best of our knowledge, this study is the first to highlight the level of serum β2-microglobulin as a robust prognosticator for patients with BL. Our β2-microglobulin-based stratification of risk subgroups raises an important question regarding the application of dose adjustment of chemotherapy for patients with BL. The clinical value of this study is considerably improved by the validation of our risk-stratifying system in an independent cohort.
The prognostic value of serum β2-microglobulin has been widely investigated in various types of lymphoproliferative disorders such as extranodal natural killer/T cell lymphoma [13], non-gastric mucosa-associated lymphoid tissue lymphoma [14], follicular lymphoma [21], diffuse large B cell lymphoma [22], Hodgkin lymphoma [23], and multiple myeloma [24]. Owing to its robustness as a prognosticator, serum β2-microglobulin has been incorporated into the prognostic staging system for multiple myeloma and follicular lymphoma [21,24]. In agreement with these findings, serum β2-microglobulin was found to be an independent prognosticator in our BL cohort.
The exact mechanism linking β2-microglobulin to the poor clinical outcomes of patients with various lymphoproliferative diseases remains poorly understood. In our analyses, high serum β2-microglobulin levels were associated with a more advanced disease status and reflected a higher tumor burden represented by elevated serum LDH. This is consistent with the findings of previous studies showing an association between elevated serum β2-microglobulin levels and a higher tumor burden [13,25]. Notably, the cut-off value of β2-microglobulin was 2.0 mg/L (identified as the best prognostic segregator in the training cohort), which was lower than the upper normal limit of serum β2-microglobulin (2.5 mg/L). This suggests that only a slight increase in the β2-microglobulin value may reflect the aggressive biological and clinical features of BL. Indeed, patients with a β2-microglobulin level of > 2.0 mg/L but ≤ 2.5 mg/L showed survival outcomes comparable to those with a β2-microglobulin level of > 2.5 mg/L, but inferior to those with a β2-microglobulin level of < 2.0 mg/L. These results suggest that adopting a β2-microglobulin cut-off of 2.0 mg/L would be more reasonable in terms of stratifying BL patients with distinct survival outcomes. Unlike LDH, for which the upper normal limit varies widely due to different assay methods yielding different results, the quantification of β2-microglobulin levels is generally considered standardized. Indeed, the International Staging System for the staging of multiple myeloma adopts β2-microglobulin levels as a continuous variable [24] with cut-off values of 3.5 mg/L and 5.5 mg/L, rather than the upper normal limit (2.5 mg/L). Nevertheless, our cut-off value of 2 mg/L will need to be further validated in cohorts from other institutions employing different β2-microglobulin assay methods.
Importantly, our study demonstrated the ability of serum β2-microglobulin levels to further stratify the clinical outcomes of patients in addition to known risk stratification criteria that were originally developed for selecting different chemotherapeutic dose intensities [11,26]. The risk-stratifying criteria were proposed based on the International Prognostic Index and the presence of bulky disease [26]. Subsequently, a recent phase 2 study of risk-adapted DA-EPOCH-R adopted this classification system for patient stratification and allocation to different chemotherapeutic intensities [11]. Although each factor comprising this risk-stratifying criterion was shown to be prognostic in patients with BL [5–9], to our knowledge, the validity of the previously suggested system has not been thoroughly confirmed as a robust prognostic index for BL patients. Since the chemotherapy regimens were not significantly different between the different risk groups based on the previously suggested criteria, no definite difference in our study in the survival outcomes of patient subgroups based on these criteria indicates that they have room for improvement. Our results suggest that additional incorporation of the β2-microglobulin level may be one of the feasible options to improve the risk stratification of BL patients.
Our results showed that patients in the low-risk group with elevated serum β2-microglobulin levels exhibited clinical outcomes comparable to those of patients in the high-risk group, suggesting that extreme caution should be taken when treating these patients. Therefore, additional studies are required to confirm whether serum β2-microglobulin levels are elevated in low-risk group patients with poor clinical outcomes in other BL cohorts. This is important given that a fair proportion (25%) of patients in our study in the low-risk group had elevated serum β2-microglobulin levels. On the other hand, serum β2-microglobulin was able to significantly stratify patients in the high-risk group in terms of PFS and OS. This suggests that high-risk group patients with low serum β2-microglobulin levels may be treated with less intense chemotherapeutic regimens, which may lead to reduced acute treatment-related morbidities such as severe myelosuppression or long-term sequelae such as cognitive dysfunction, secondary malignancy, and disabling neuropathy [27,28].
Considering the strong prognostic impact of serum β2-microglobulin levels, we proposed a new classification system that could stratify patients into three distinct risk subgroups with 5-year OS rates of 100%, 89.5%, and 62.5%. Importantly, the risk-stratifying ability of our system was well validated in an independent cohort, with 5-year OS rates of 100%, 92.9%, and 53.9% for the risk subgroups. Our newly proposed risk-stratifying system should be further investigated in the context of risk-adapted therapy with dose adjustment. The optimization of therapeutic strategies, including the chemotherapeutic regimen and dose intensity for the re-classified patients (i.e., the low-risk group with elevated serum β2-microglobulin levels and the high-risk group with low serum β2-microglobulin levels) is a topic of particular interest. From a practical point of view, the level of serum β2-microglobulin is easily determined with excellent reproducibility, and our results suggest that serum β2-microglobulin level measurements in daily practice may guide treatment decisions and the prediction of clinical outcomes. However, the retrospective nature of our study and the relatively small number of patients, may limit the interpretation and generalizability of our data.
In conclusion, the serum β2-microglobulin level is an independent prognostic factor, which allows for further risk stratification of BL patients. This risk-stratifying classification system incorporating the serum β2-microglobulin level may be useful in stratifying BL patients with distinct survival outcomes. This classification system warrants further investigation in future studies dealing with the issue of applying risk-adapted treatment approaches with dose adjustment.
Footnotes
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board (IRB) (No.2020-1229). Informed consent from the patients was waived.
Author Contributions
Conceived and designed the analysis: Kim HD, Suh C.
Collected the data: Kim HD, Cho H, Kim S, Lee K, Kang EH, Park JS, Park CS, Huh J, Ryu JS, Lee SW, Yoon DH, Kim SJ, Ko YH, Kim WS, Suh C.
Contributed data or analysis tools: Kim HD, Cho H, Suh C.
Performed the analysis: Kim HD, Suh C.
Wrote the paper: Kim HD, Suh C.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Perkins AS, Friedberg JW. Burkitt lymphoma in adults. Hematology Am Soc Hematol Educ Program. 2008:341–8. doi: 10.1182/asheducation-2008.1.341. [DOI] [PubMed] [Google Scholar]
- 2.Kalisz K, Alessandrino F, Beck R, Smith D, Kikano E, Ramaiya NH, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi: 10.1186/s13244-019-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang I, Go H, Jeon YK, Ko YH, Yoon DH, Suh C, et al. Expression of JL1 in Burkitt lymphoma is associated with improved overall survival. Virchows Arch. 2011;459:353–9. doi: 10.1007/s00428-011-1134-6. [DOI] [PubMed] [Google Scholar]
- 4.Jang SJ, Yoon DH, Kim S, Yoon S, Kim DY, Park CS, et al. A unique pattern of extranodal involvement in Korean adults with sporadic Burkitt lymphoma: a single center experience. Ann Hematol. 2012;91:1917–22. doi: 10.1007/s00277-012-1531-1. [DOI] [PubMed] [Google Scholar]
- 5.Wästerlid T, Jonsson B, Hagberg H, Jerkeman M. Population based study of prognostic factors and treatment in adult Burkitt lymphoma: a Swedish Lymphoma Registry study. Leuk Lymphoma. 2011;52:2090–6. doi: 10.3109/10428194.2011.593274. [DOI] [PubMed] [Google Scholar]
- 6.Evens AM, Danilov AV, Jagadeesh D, Sperling AL, Kim SH, Vaca RA, et al. Burkitt lymphoma in the modern era: real world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137:374–386. doi: 10.1182/blood.2020006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakobsen LH, Ellin F, Smeland KB, Wasterlid T, Christensen JH, Jorgensen JM, et al. Minimal relapse risk and early normalization of survival for patients with Burkitt lymphoma treated with intensive immunochemotherapy: an international study of 264 real-world patients. Br J Haematol. 2020;189:661–71. doi: 10.1111/bjh.16425. [DOI] [PubMed] [Google Scholar]
- 8.Castillo JJ, Winer ES, Olszewski AJ. Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:3672–9. doi: 10.1002/cncr.28264. [DOI] [PubMed] [Google Scholar]
- 9.Sweetenham JW, Pearce R, Taghipour G, Blaise D, Gisselbrecht C, Goldstone AH. Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma: outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in first remission or at relapse: results from the European Group for Blood and Marrow Transplantation. J Clin Oncol. 1996;14:2465–72. doi: 10.1200/JCO.1996.14.9.2465. [DOI] [PubMed] [Google Scholar]
- 10.Mead GM, Barrans SL, Qian W, Walewski J, Radford JA, Wolf M, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial) Blood. 2008;112:2248–60. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschewski M, Dunleavy K, Abramson JS, Powell BL, Link BK, Patel P, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Bur-kitt lymphoma. J Clin Oncol. 2020;38:2519–29. doi: 10.1200/JCO.20.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chronowski GM, Wilder RB, Tucker SL, Ha CS, Sarris AH, Hagemeister FB, et al. An elevated serum beta-2-microglobulin level is an adverse prognostic factor for overall survival in patients with early-stage Hodgkin disease. Cancer. 2002;95:2534–8. doi: 10.1002/cncr.10998. [DOI] [PubMed] [Google Scholar]
- 13.Yoo C, Yoon DH, Jo JC, Yoon S, Kim S, Lee BJ, et al. Prognostic impact of beta-2 microglobulin in patients with extranodal natural killer/T cell lymphoma. Ann Hematol. 2014;93:995–1000. doi: 10.1007/s00277-014-2015-2. [DOI] [PubMed] [Google Scholar]
- 14.Yoo C, Yoon DH, Yoon S, Kim S, Huh J, Park CJ, et al. Prognostic impact of beta(2)-microglobulin in patients with non-gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma. 2015;56:688–93. doi: 10.3109/10428194.2014.917640. [DOI] [PubMed] [Google Scholar]
- 15.Johnson PW, Whelan J, Longhurst S, Stepniewska K, Matthews J, Amess J, et al. Beta-2 microglobulin: a prognostic factor in diffuse aggressive non-Hodgkin’s lymphomas. Br J Cancer. 1993;67:792–7. doi: 10.1038/bjc.1993.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, Petroni GR, Schiffer CA, Freter CE, Johnson JL, Barcos M, et al. Brief-duration high-intensity chemotherapy for patients with small noncleaved-cell lymphoma or FAB L3 acute lymphocytic leukemia: results of cancer and leukemia group B study 9251. J Clin Oncol. 2001;19:4014–22. doi: 10.1200/JCO.2001.19.20.4014. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DA, Faderl S, O’Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–80. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 18.Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369:1915–25. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi MK, Jun HJ, Lee SY, Kim KH, Lim DH, Kim K, et al. Treatment outcome of adult patients with Burkitt lymphoma: results using the LMB protocol in Korea. Ann Hematol. 2009;88:1099–106. doi: 10.1007/s00277-009-0729-3. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–62. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Guillermo A, Colomo L, Jimenez M, Bosch F, Villamor N, Arenillas L, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23:2797–804. doi: 10.1200/JCO.2005.07.155. [DOI] [PubMed] [Google Scholar]
- 23.Vassilakopoulos TP, Nadali G, Angelopoulou MK, Siakantaris MP, Dimopoulou MN, Kontopidou FN, et al. The prognostic significance of beta(2)-microglobulin in patients with Hodgkin’s lymphoma. Haematologica. 2002;87:701–8. [PubMed] [Google Scholar]
- 24.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantinides IP, Pathouli C, Karvountzis G, Papadopoulos P, Varvoutsi-Constantinides M, Eliakis P, et al. Serum beta 2 microglobulin in malignant lymphoproliferative disorders. Cancer. 1985;55:2384–9. doi: 10.1002/1097-0142(19850515)55:10<2384::aid-cncr2820551014>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264–74. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 27.Rizzieri DA, Johnson JL, Niedzwiecki D, Lee EJ, Vardiman JW, Powell BL, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer. 2004;100:1438–48. doi: 10.1002/cncr.20143. [DOI] [PubMed] [Google Scholar]
- 28.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–29. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.