Abstract
Exposure to severe stress has been linked to negative postpartum outcomes among new mothers including mood disorders and harsh parenting. Non-human animal studies show that stress exposure disrupts the normative adaptation of the maternal brain, thus identifying a neurobiological mechanism by which stress can lead to negative maternal outcomes. However, little is known about the impact of stress exposure on the maternal brain response to infant cues in human mothers. We examined the association of stress exposure with brain response to infant cries and maternal behaviors, in a socioeconomically diverse (low- and middle-income) sample of first-time mothers (N=53). Exposure to stress across socioeconomic, environmental, and psychosocial domains was associated with reduced brain response to infant cry sounds in several regions, including the right insula/inferior frontal gyrus and superior temporal gyrus. Reduced activation in these regions was further associated with lower maternal sensitivity observed during a mother-infant interaction. The findings demonstrate that higher levels of stress exposure may be associated with reduced brain response to an infant’s cry in regions that are important for emotional and social information processing, and that reduced brain responses may further be associated with increased difficulties in developing positive mother-infant relationships.
Keywords: Neuroimaging, Stress, Maternal brain, Infant cry, Maternal sensitivity
1. Introduction
The postpartum period is an important time for new mothers and their infants. This developmental window is when the infant’s brain develops most rapidly following birth (Gilmore et al., 2018), and the quality of mother-infant interactions during the first year of life plays a critical role in supporting that brain development (Perry et al., 2018; Soe et al., 2016). The arrival of a baby also precipitates a significant change in a new mother’s life. While this change brings excitement and joy, it also increases psychological and physical demands with regards to taking care of a baby who is entirely dependent on its caregiver for survival. Thus, if new mothers experience severe stress, beyond the typical demands associated with parenting, they are more vulnerable to negative postpartum outcomes including depression and anxiety. This in turn can negatively influence mother-infant relationships (Brummelte and Galea, 2010; Kim, 2016; Kim and Bianco, 2014). Non-human animal studies suggest that high levels of stress exposure can disrupt normative maternal brain adaptation, which further leads to impairments in maternal behaviors (Hillerer et al., 2012; Pawluski et al., 2016; Slattery and Hillerer, 2016). However, there is still a limited understanding of the underlying neural mechanisms by which stress exposure may influence human mothers’ adaptations to parenthood.
During the postpartum period, human mothers exhibit heightened neural and behavioral sensitivity to infant cues compared to non-parents (Seifritz et al., 2003; Stallings et al., 2001). Infant cues (e.g. cry sounds or images of infant faces) have been associated with activations in a network of brain regions including the mesolimbic-cortical dopaminergic pathway (Laurent and Ablow, 2011; Rigo et al., 2019; Strathearn et al., 2008). This neural pathway incorporates brain regions involved in reward processing including the hypothalamus, ventral tegmental area, substantia nigra, striatum, and medial prefrontal cortex. This pathway is well connected with the salience network including the amygdala, anterior insula, and anterior cingulate cortex (Cole et al., 2013). Non-human animal studies demonstrate that activation in these reward and salience network regions critically supports maternal caregiving behaviors (Lonstein et al., 2015). In human mothers, activation of these networks in response to infant cues has been associated with sensitive maternal behaviors (Atzil et al., 2011; Paul et al., 2019). Infant cues are also associated with activations in brain regions involved in emotional information processing and planning for action including the superior temporal gyrus (STG), the supplementary motor area, as well as brain regions involved in emotion regulation including the medial and lateral prefrontal cortex (PFC) (Kim et al., 2016b; Witteman et al., 2019). These regions are considered to support human mothers’ ability to process infants’ emotional and social cues and to appropriately respond to those cues while regulating their own emotional responses (Rutherford et al., 2015). Activations in the STG and PFC have been positively associated with more sensitive maternal behaviors observed during mother-infant interactions (Bornstein et al., 2017; Feldman, 2017).
Existing evidence from non-human animal studies demonstrates that stress negatively influences maternal brain and behavioral adaptation to parenthood. Exposure to severe stress such as social isolation, overcrowded living environment, or frequent housing mobility during pregnancy or the postpartum period leads to reduced oxytocin-receptor expression in the mesocorticolimbic dopamine pathway (Haim et al., 2014) and impaired structure and activation of medial PFC in dams (Leuner et al., 2014; Sabihi et al., 2014). These changes in the brain due to stress exposure further lead to impaired maternal behaviors (Herzog et al., 2009; Hillerer et al., 2011; Smith et al., 2004) and depressive-like or anxious behaviors in dams (Slattery and Hillerer, 2016).
In human mothers, it is well-established that exposure to severe stressors such as poverty, major adverse life events, and trauma are significant risk factors for suboptimal parenting across different ages of children (Barnett, 2008; Crnic and Low, 2002; Raver and Leadbeater, 1999). During the first year postpartum, a higher number of stressors including domestic and non-domestic violence, negative life events, and homelessness was associated with more severe parenting stress (Nair et al., 2003) and a more negative parenting attitude (Kettinger et al., 2000). Among over 100 predominantly low-income mothers, more stressors during the first four months postpartum including the need for federal or government assistance, negative life events, crowded living conditions, and low perceived resources was also associated with higher levels of parenting stress (Candelaria et al., 2006). Stressful conditions can also increase emotional distress in mothers which can in turn negatively influence their ability to sensitively respond to their infants (Liu and Tronick, 2013; Salm Ward et al., 2017; Soumyadeep et al., 2017).
Few studies address the role of stress exposure in the human mother’s brain, therefore there is limited understanding of how stress exposure is associated with maternal brain responses to infants. In one study, researchers examined low-income status as a stressful environment for new mothers. Low-income mothers reported higher levels of perceived stress (e.g. feeling overwhelmed) (Kim et al., 2016a). Low-income and high perceived stress were further associated with dampened medial and lateral PFC and STG responses to infant cry sounds among these same mothers during the first postpartum year (Kim et al., 2016a). Researchers found similar patterns of brain activation in a study on trauma from interpersonal violence. The mothers who were exposed to interpersonal violence exhibited reduced brain activation in the superior frontal gyrus while seeing their own 1–2 year-old children during a mother-child separation session (Schechter et al., 2012). In another study, mothers exposed to traumatic experiences such as war also exhibited reduced brain responses in the sensorimotor and anterior insula regions while observing others experiencing pain (Levy et al., 2019). Although not specific to child cues, these reduced brain responses were then associated with less sensitive behaviors in the mothers’ interactions with their own children at ages 11–13 (Levy et al., 2019). Together, these findings suggest that mothers’ stress exposure is associated with reduced brain responses to their children in brain regions that are involved in emotional and social information processing and emotion regulation, among other functions. However, these previous studies limit the study of stress exposure to a specific stressor such as poverty or trauma. The associations among stress exposure across domains, mothers’ brain responses to child cues, and parenting behaviors are not yet well understood. It would be important to build knowledge of these associations, particularly for the first postpartum year, when mothers are establishing long-term emotional relationships with their children.
Thus, in the current study, we examined the associations between exposure to stress exposure and human maternal brain activation in response to infant cues, specifically infant cry sounds, during the first year postpartum. We included a sample of first-time mother participants with socioeconomically diverse (low to middle income) backgrounds, and stress exposure was assessed across socioeconomic, environmental, and psychosocial domains. First, we hypothesized that stress exposure would be associated with higher maternal distress such as depressive mood or anxiety symptoms. Second, based on the studies reviewed in the previous paragraph, we hypothesized that higher stress exposure would be associated with reduced brain responses to infant cry sounds compared to white noise sounds, particularly in brain regions involved in emotion information processing and emotion regulation including the medial and lateral PFC, anterior insula, and STG. Last, we examined direct associations between brain activation and parenting behaviors. We hypothesized that reduced brain response to infant cry sounds was associated with lower maternal sensitivity during mothers’ interactions with their own infants.
2. Materials and methods
2.1. Participants
Participants were recruited via flyers and brochures in postpartum clinics in the Denver metro area, as well as through federal and state programs that serve low-income new families (i.e. Women, Infant, and Children (WIC) clinics and Colorado’s Prenatal Plus Programs). Eligibility criteria included first-time mothers with no major birth or pregnancy complications, full-term births, ages 18–40, and English speaking. Exclusion criteria included an IQ below 70 (using WASI-II), a history of a psychiatric diagnosis other than depression or anxiety (based on selfreport), a family income-to-needs ratio (INR; see the measure section for details) above 8, and the mother’s infant having more than a one-night stay in the neonatal intensive-care unit (NICU). We included participants with a history of depression and anxiety for a more representative community sample, as they are the two most common diagnoses during the postpartum period. Because the overarching goal of the research project was to examine the role of stress exposure in low and middle-income women, we oversampled low-income women, and very high-income women were excluded.
Among 77 participants who participated in home visits, 61 of them met MRI specific eligibility criteria (i.e. no magnetic metal in the body and not being claustrophobic) and consented to participate in the neuroimaging portion of the study. The remaining 16 participants completed only the home visit portion of the project. Of the 61 participants in the neuroimaging portion of the study, 53 participants were included in the current analysis. Six participants were removed due to missing data for one of the stress exposure index measures (see measures section), one participant was removed due to excessive motion (see preprocessing section), and one participant was removed due to a technical error (i.e. use of incorrect stimuli). Of the 53 participants included in the analysis, 26 participants overlap with participants published in Kim et al. (2016) using the same fMRI task. However, the current research question of stress exposure has not been previously examined in this sample. Please see supplementary materials for overlaps with other manuscripts using different fMRI tasks from the same research project.
A total of 53 first-time mothers (age M=26.11 years) were included in the study during 0–10 postpartum months (M=4.46 months). Table 1 depicts information on the demographic and major variables. Overall, participants included 41.5% Caucasian and 43.4% Hispanic backgrounds. The participants were also socioeconomically diverse − 41.5% lived in low income (INR below 2), and 49.1% of the participants reported 13 years or less as their highest educational degree completed.
Table 1.
Characteristics of the participants.
| N (%) | Mean ± SD | Range | |
|---|---|---|---|
| Maternal age (years) at the time of fMRI scans Maternal race/ethnicity | – | 26.11 ± 5.62 | 18–37 |
| Caucasian | 22 (41.5) | – | – |
| Hispanic | 23 (43.4) | – | – |
| African-American | 4 (7.5) | – | – |
| Others | 4 (7.6) | – | – |
| Maternal education (years) | – | 14.15 ± 2.43 | 9–20 |
| Infant sex (female) | 31 (58.5) | – | – |
| Postpartum month at the time of fMRI scans | – | 4.47 ± 2.12 | 0.89–10.66 |
| Maternal sensitivity | – | 5.34 ± 1.24 | 3–7 |
| Depressive symptoms (BDI) | – | 7.45 ± 5.12 | 0–22 |
| Trait anxiety symptoms (STAI-Trait) | – | 36.26 ± 9.81 | 20–60 |
| State anxiety symptoms (STAI-State) | – | 31.57 ± 6.74 | 20–47 |
| History of depression or anxiety diagnosis (Yes) | 21 (39.6) | – | – |
| Anxiety and depression medication use (Yes) | 4 (7.5) | – | – |
| Interval between home and fMRI visits (months) | – | 1.04 ± 1.07 | 0.07–6.25 |
| Breastfeeding | 35 (66.0) | ||
| Right handedness | 48 (90.6) | – | – |
| Relationship status (married/engaged/common law marriage) | 34 (64.2) | ||
| Time away from own infant per week (hours) | – | 13.23 ± 15.31 | 0–50 |
2.2. Procedures
Interested individuals contacted the research lab by phone or email and were invited to participate in the study after completing an eligibility screener. The research study had two visits - a home visit and a neuroimaging visit. During in-home visits, at least two trained researchers visited participants’ homes in the late afternoon. After consenting, researchers conducted interviews with questions regarding income and other demographic information as well as a WASI-II test and questionnaires with participants. Mother-infant interactions were video-recorded for 15 min, and infant cry sounds were recorded during the home visits. Researchers also evaluated housing quality, crowding, and assessed noise levels at home visits. Typically, a few weeks after the home visit, the participant came to a neuroimaging center, the Center for Innovation & Creativity, University of Colorado - Boulder. At the visit, mothers completed a neuroimaging session. Childcare support was provided, and monetary compensation was provided for the participant’s time and participation at the end of each visit. All procedures were approved by the University of Denver IRB board.
2.3. Measures
2.3.1. Stress exposure
Exposure to a total of 9 stressors – 3 stressors in each of the three domains (socioeconomic, environmental, and psychosocial) was assessed (Table 2). The specific stressors were selected to represent the stressful environment that mothers may experience across multiple levels of their ecological system, a theory developed by Bronfenbrenner and Ceci (1994).
Table 2.
Stress exposure variables.
| Mean ± SD | Range | |
|---|---|---|
| Socioeconomic stress | ||
| Income-to-needs ratio | 2.72 ± 1.56 | 0.43–6.24 |
| Financial stress | 2.03 ± 0.86 | 1.00–4.67 |
| Food insecurity | 1.19 ± 2.01 | 0.00–6.00 |
| Environmental stress | ||
| Substandard housing quality | 0.52 ± 0.40 | 0.00–2.38 |
| Noise (Leq, dBA) | 60.20 ± 7.99 | 46.60–106.50 |
| Crowding (ratio of occupants to number of rooms) | 0.57 ± 0.26 | 0.25–1.80 |
| Psychosocial stress | ||
| Marital dissatisfaction (lower score reflects dissatisfaction) | 16.32 ± 4.00 | 5–21 |
| Violence in the community | 0.19 ± 0.44 | 0–2 |
| Troubles with authority | 0.17 ± 0.43 | 0–2 |
| Total score of stress exposure | 1.94 ± 1.80 | 0–8 |
Socioeconomic stressors included low family income-to-needs ratio, high financial stress, and high food insecurity. The low family income-to-needs ratio was assessed based on a detailed interview of family income during the past 12 months. Based on the annually adjusted poverty line from the federal government, low income was defined as a family’s income being lower than twice the federal poverty line (i.e. INR below 2). Financial stress was assessed by the Financial Strain Scale (Vinokur and Caplan, 1987; Vinokur et al., 1996), which reflects subjective socioeconomic stress. The index included three items about how likely it was that the family experienced hardship due to limited income on a 5 Likert scale (1 = not at all to 5 = very much). This index has been used with perinatal samples (Mitchell and Christian, 2017; Wright et al., 2010). Food insecurity was assessed using the U.S. Household Food Security Scale: Six-Item Short Form (Blumberg et al., 1999). The measure included 6 items about how likely it was that the family did not have access to enough food. The response options of the measure were reversed so that a higher score indicated more food insecurity.
The environmental stressors included substandard housing quality, noise, and crowding at the family’s home. Assessment of these three stressors followed the protocol from previous studies of physical environmental stressors in families with children (Dufford and Kim, 2017; Evans and English, 2002; Kim et al., 2013a). Housing quality was assessed by a trained researcher during a home visit, using a standardized observational rating scale (Evans et al., 2000). Subscales included structural quality, maintenance, cleanliness and clutter, safety hazards, and climatic conditions. Scores of each item were first transformed to z-scores, then the mean score of all items was used as a final score. Noise in the home was assessed for at least two hours during the home visit using a decibel meter (Leq, dBA) in the primary social space of the home (typically the living room). Crowding in the house was calculated by dividing the number of rooms by the number of people who resided in the house.
Psychosocial stressors included marital dissatisfaction, violence in the community, and trouble with authorities (i.e. social service agencies, medical/health professionals, teachers, superiors at work). The three stressors were chosen to reflect psychosocial stress in the home, neighborhood, and social systems. Marital quality was assessed using the brief 4-Item Dyadic Adjustment Scale (Sabourin et al., 2005). A lower score indicated more dissatisfaction in marital quality. Violence in the community and trouble with authorities were assessed using the life event interview, CRISYS (Shalowitz et al., 1998; Shalowitz et al., 2006). The scale ‘violence in the community’ included 8 items such as “did you see violence outside your home?” and the scale ‘trouble with authorities’ included 4 items such as “did you have trouble with social service agencies?”
To create an index of stress exposure, each stressor’s score was coded by assigning a 1 if the score was in the top quartile (except marital dissatisfaction which used the bottom quartile, and low income which used INR below 2 as criteria), and 0 otherwise. A summative score of the dichotomous variables of the nine stressors was calculated. The range of the scores in the sample was 0–8 (Supplementary Fig. 1).
2.3.2. Maternal distress
Depressed mood was assessed using the Beck Depression Inventory - I. The version included 21 questions about depressive symptoms in the past week (Beck et al., 1988). Participants rated their symptoms using a 4-point scale (from “absent” to “severe”). The measure has been used with perinatal mothers (Gotlib et al., 1989; O’hara and Swain, 1996). In the current study, 66% of mothers had no depression (scores below 10), 32% had mild depression (scores 10–18), and 2% had moderate depression (scores 19–29). Anxiety symptoms were assessed using another widely-used self-report questionnaire - STAI (The State-Trait Anxiety Inventory) -X (Spielberger et al., 1970). The questionnaire included 20 items for state anxiety (the current feeling) and 20 items for trait anxiety (the general feeling), and participants rated their symptoms based on a 4-point scale (from “not at all” to “very much so”). The assessment has been commonly used to identify anxiety symptoms among pregnant women and new mothers. Scores of 43 and above are considered severe postpartum anxiety symptoms (Feldman et al., 2009). Nine percent of mothers had scores above the cutoff based on state anxiety scores, while 26% of mothers had scores above the cutoff based on trait anxiety scores.
2.3.3. Parenting behaviors
Maternal sensitivity was assessed based on a video-recorded interaction between mother and infant. During the home visit, mothers were asked to interact with their infants naturally for 15 min without toys. Two researchers who were blinded to participants’ demographic backgrounds coded videos in the lab using the Emotional Availability Scales 4th edition (Biringen, 2008; Biringen et al., 2014). The coders were trained and certified by Dr. Biringen who created the EA scales. The scales have four parent-related dimensions: sensitivity, structuring, non-intrusiveness, and non-hostility. We focused on maternal sensitivity because it is a particularly important element of maternal behaviors across the first year of infancy and predicts later secure infant-mother attachment (Feldman et al., 2009; Isabella et al., 1989; Smith and Pederson, 1988). The sensitivity scale was based on appropriate sensitivity of maternal behaviors in affect, clarity, timing, flexibility, acceptance, amount, and conflict. A possible range of the direct sensitivity score was 1–7. High scores reflect optimally sensitive maternal behaviors, mid scores reflect inconsistently sensitive behaviors, and low scores reflect problematic interactions. Two coders coded 24% of the videos together and the average measures intraclass correlation (ICC) was 0.91.
2.4. fMRI infant cry paradigm
The infant cry task has been used in several previous studies with postpartum women, and brain activation has been associated with a range of maternal factors including mood and parenting behavior styles (Guo et al., 2018; Ho and Swain, 2017; Kim et al., 2016a, 2011, 2010a; Laurent and Ablow, 2011; Musser et al., 2012). The task included the mother’s own infant’s cry sounds which were recorded during a home visit. The cry was a natural cry during a diaper change, when a baby was seeking attention, or when feeding time was approaching. The control infant cry was collected using a similar home visit protocol. Samples of the infant cries from mothers who did not participate in the current study were rated by seven adults on a 1–10 scale for emotional intensity. One with an average level of emotional intensity was selected for the control baby-cry stimuli. Any non-cry noise and background sounds for both own and control infant cry sounds were removed from the recording using sound editing software (Cool Edit Pro Version 2.0, Syntrillium Software, Phoenix, AZ). The own infant cry and control infant cry sounds were matched for volume (total root-mean-square sound intensity of − 8 dB), but no further changes in the acoustic properties were made in order to maintain the natural properties of the cry sounds. White noises were synthesized by generating a spectral average of the cry, which was then matched to the gross temporal envelope of the own infant and control infant cry sounds. The control infant cry was identical for all participants.
The infant cry task was organized into two functional runs, in which blocks of cry stimuli and control sounds lasted 20 s. Each stimulus block was separated by an average 10-s rest period (ranged 8–12 s) at which time only background scanner noise could be heard. Each run contained blocks of four sound stimuli—(A) own infant cry, (B) control infant cry, (C) own infant cry matched white noise, and (D) control infant cry matched white noise. The order of the blocks was randomized. Each block was presented a total of 10 times and a white crosshair was displayed in the center of a black screen during the task. The total duration of the task was 21 min. During the task, mothers were asked to pay attention to each sound and experience feelings and thoughts as they naturally occurred. This was to ensure the task would capture the natural thoughts and feelings in response to sounds while minimizing any unrelated cognitive processing of the stimuli by answering questions. After completing the scan session, mothers participated in an out-of-scanner behavioral task. In this task, mothers listened to the sounds used in the scan task again and rated their emotional response to each sound.
2.5. fMRI parameters
The study experienced a scanner update in the middle; thus, two different scanners were used in the study − 3.0 T Siemens Trio scanner (N=31) and 3.0 T Siemens Prisma scanner (N=22). Both scanners used a standard 32-channel head coil. Functional data was acquired using matching parameters (540 T2* -weighted echo-planar-imaging (EPI) volumes; TR= 2300 ms; TE=27 ms; flip angle=73°; field of view=192 mm2; matrix size, 64 × 64; 36 axial slices; voxels=3 mm3). High resolution anatomical T1-weighted images using the 3D magnetization-prepared rapid gradient-echo (MPRAGE) protocol were also acquired. For the Trio scanner, the parameters were 192 sagittal slices, TR = 2530 ms, TE = 1.64 ms, flip angle = 7°, FOV = 256 mm2 and voxels = 1 mm3. For the Prisma scanner, the parameters were 224 sagittal slices, TR = 2400 ms, TE = 2.07 ms, flip angle = 8°, FOV = 256 mm2 and voxels = 0.8 mm3.
2.6. fMRI Processing
Analysis of Functional Neuroimages software (AFNI version 18.3.12) (Cox, 1996) was used for preprocessing and statistical analysis. The first two TRs (in addition to 4 dummy TRs) of each run were discarded to account for magnetic equilibrium. After slice timing correction, images within each run were realigned to the last image to correct for movement. If the motion was greater than 0.5 mm in any direction between TRs or more than 10% of voxels were outliers, individual TRs were censored. One participant who had more than 20% of the total images removed was excluded from the analysis. Among data included in the analysis, the range of number of TRs censored was 0–97 (M =14.15; 2.6% of the total volumes), and the range of mean frame-wise displacement was 0.04–0.20 mm (M = 0.09 mm). An average number of TRs that were censored ranged from 3.26 to 4.24 across four conditions. After motion correction, realigned functional images were co-registered to anatomical images and functional images were anatomically normalized to Talairach space. Images were spatially smoothed with a 6-mm root-mean-square deviation Gaussian blur, and signals were scaled to percent change relative to the mean in each voxel.
At the individual participant level, general linear models were used to estimate the shape of the hemodynamic response to each stimulus (own infant cry, control infant cry, own infant cry matched noise, control infant cry matched noise). The design matrix included four conditions (boxcar function convolved with hemodynamic response function), third-order polynomials, and 6 motion parameters. Beta images, represented estimated activation in each condition for each participant, were then used in group-level analyses.
2.7. Analysis
2.7.1. Correlations and behavioral ratings
First, the bivariate Pearson correlation analyses or Chi-squared test (if variables were categorical variables) were conducted including all main and demographic variables. Second, two separate repeated-measures ANCOVAs were conducted to test the associations among emotional (pleasantness, distressing) rating of the sounds and stress exposure. Stress exposure was a between-subjects variable, and sound (cry, noise) and condition (own, control) were within-subject factors, and maternal age and postpartum months were covariates. In addition to postpartum months, which may influence mothers’ emotional ratings, stress exposure was associated with maternal age (see Results), thus was included as a covariate. One of the participants was missing the out-of-scanner rating data, and was thus excluded from the analyses.
2.7.2. fMRI analysis
At the group-level of fMRI analyses, AFNI’s 3dLME was utilized to create a whole-brain linear mixed-effects model with stress exposure as a between-subjects factor, and sound (cry, noise) and condition (own, control) as within-subject factors. The statistical approach is similar to other approaches i.e. using first-level contrasts of conditions then conducting second-level analysis (Madhyastha, et al. 2018; Telzer, et al., 2018). Covariates including maternal age, postpartum months, and scanner type were included in the group AFNI 3dLME models. The covariates were also included in follow-up analyses which were used to decompose effects. While stress exposure was not associated with postpartum months or scanner type (see Results), the two variables may independently influence maternal brain responses to infant cry sounds, thus we included them in the analysis as covariates. An EPI based mask (90% overlap across participants) was applied to exclude data outside of the brain from the analysis. Results were corrected for multiple comparisons within the whole brain using the cluster extent threshold of k ≥ 29 with a height threshold of p < 0.001, equivalent to a whole-brain corrected false positive probability of p < 0.05, as calculated by 3dClustSim using the spatial autocorrelation function (ACF) option. To rule out the potential role of variables that are associated with stress exposure on brain activation, additional separate whole-brain models were tested by including variables that were associated with stress exposure or potential confounders (i.e. maternal education, a history of mood disorders, current intake of psychotropic medication, maternal anxious or depressed symptoms). The activation from each cluster was extracted and entered into a SPSS dataset for further analyses. Using the extracted clusters in SPSS, Pearson correlation analyses were conducted to test the associations among brain activations and emotional ratings to infant cry sounds.
2.7.3. Associations between brain responses and parenting behaviors
A regression analysis was used to examine associations between brain activation of the extracted clusters in SPSS and maternal sensitivity, including covariates to account for maternal distress levels (BDI and trait-STAI) in the model. Because trait-STAI scores, not state-STAI scores, were associated with stress exposure, we chose to include trait anxiety scores. We checked for extreme values in all variables, but no outliers were detected. A mediation analysis among stress exposure, brain activation, and maternal sensitivity was not performed due to the cross-sectional nature of the data.
3. Results
3.1. Characteristics of the sample
Among maternal mood variables, stress exposure was positively associated with higher trait anxiety, r(51) = 0.30, p = 0.03. We examined the associations between stress exposure and maternal behaviors, however, the correlations were not significant. Among demographic variables, stress exposure was associated with younger mother’s age, r(51) = −0.43, p = 0.001, and fewer years of maternal education, r(51) = −0.48, p < 0.001. Thus, to avoid multicollinearity due to the high correlations between maternal age and education years [r(51)=0.73, p < 0.001], we included maternal age as a covariate in the main whole-brain analysis and included maternal education years as a covariate in an additional whole-brain analysis. All other relationships between stress exposure and demographic variables included in Table 1 were not significant (Supplementary Table 1).
3.2. Emotional ratings of sounds
A repeated-measure ANCOVA analysis revealed that three-way interaction was not significant for either rating of how pleasant or distressing stimuli were to mothers. The two-way interaction between stress exposure X sound (cry vs noise) was significant for the pleasantness rating, F(1,48) = 7.83, p = 0.007. Higher stress exposure was associated with a higher pleasantness rating of infant cry sounds, r(50)=0.36, p = 0.009 (Supplementary Fig. 2). Stress exposure was not associated with the rating of white noise sounds, r(50)=−0.15, p > 0.01. The main effects of postpartum months were significant for both pleasantness ratings, F(1,48) = 4.07, p = 0.049, and distressing ratings, F(1,48) = 6.80, p = 0.01. Later postpartum months were associated with greater pleasantness ratings [r(50)=0.26, p=0.06] and lower distressing ratings [r(50)=−0.34, p=0.014] across all sounds. All other effects and interactions were not significant, which suggests that the emotional response ratings of own and control infant cries were not significantly different among mothers.
3.3. fMRI analysis of the associations between stress exposure and brain activation
The three-way interaction of stress exposure X sound (cry, white noise) X condition (own infant, control infant) was not significant. However, the two-way interaction of stress exposure X sound (cry vs noise) revealed five significant clusters (Table 3). The first cluster includes both the anterior insula and inferior frontal gyrus (IFG) (Fig. 1a, b). The results also include a cluster in the right superior temporal gyrus (STG) (Fig. 1c, d). In all clusters, stress exposure was negatively associated with cry vs. white noise activation. Therefore, higher stress exposure was associated with reduced brain activation in response to infant cry sounds compared to white noise across own and control infant conditions.
Table 3.
Brain areas showing stress exposure X sound (cry, white noise) interactions.
| Regions | BA | Side | x | y | z | Cluster size | F |
|---|---|---|---|---|---|---|---|
| Insula | 47 | R | 29 | 26 | 2 | 86 | 20.62 |
| Cerebellum | – | L | −22 | −64 | −22 | 52 | 21.33 |
| Medial frontal gyrus | 8 | R | 5 | 38 | 38 | 41 | 15.38 |
| Supramarginal gyrus | 40 | R | 56 | −49 | 32 | 40 | 16.58 |
| Superior temporal gyrus | 41 | R | 44 | −31 | 5 | 39 | 17.60 |
p < 0.05, corrected; BA = Brodmann area, R = right, L = left; the Talairach coordinates, and F- statistics represent the voxel with maximum signal intensity (i.e. peak value) for each cluster.
Fig. 1.
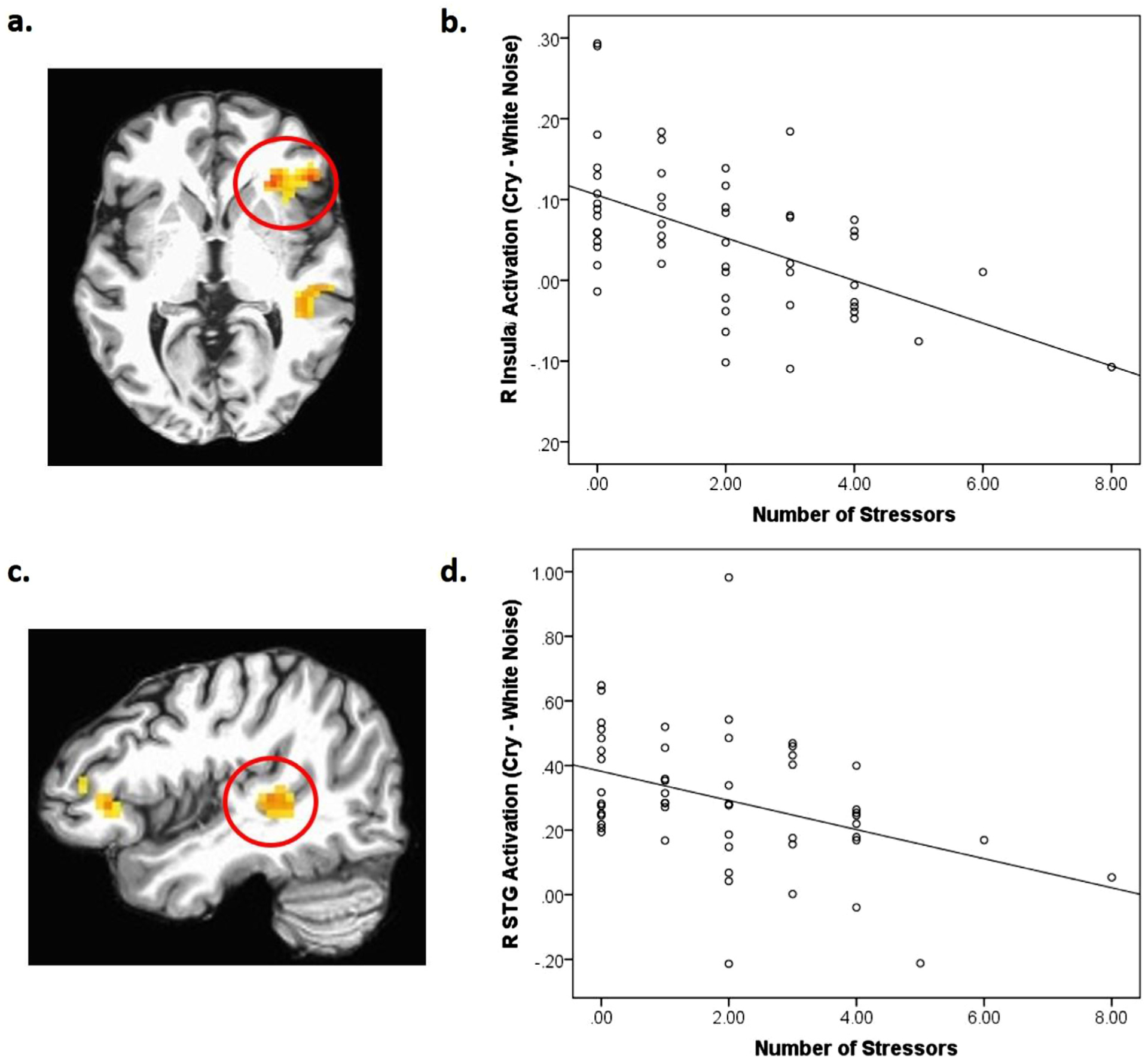
(a) The right (R) insula cluster (BA47; x, y, z = 29, 26, 2; k=86) which also included a part of the inferior frontal gyrus (IFG) in a red circle showing stress exposure X sound interaction, p < 0.05, corrected; (b) scatterplot describing the negative associations between stress exposure and brain responses to infant cry sounds (both own and control infant cry sounds) in the region; (c) The right (R) superior temporal gyrus (STG) (BA41; x, y, z = 44, −31, 5l; k=39) in a red circle showing stress exposure X sound interaction, p < 0.05, corrected; (d) a scatterplot describing the negative associations between stress exposure and brain responses to infant cry sounds (both own and control infant cry sounds) in the region.
We decomposed interactions to examine whether the findings were driven by brain activation in response to infant cry or white noise. First, brain activation data from each condition (own infant cry, control infant cry, own infant cry matched white noise and control infant cry matched white noise) was extracted using the masks of the functional clusters. Then, the averaged percent signal change values across the two cry conditions and across the two white noise conditions were included in the following correlation analyses with a stress exposure variable. Stress exposure was negatively associated with brain response to infant cry sounds in the right insula, r(51) = −0.41, p = 0.002, the left cerebellum, r(51) = −0.27, p = 0.048, and left superior temporal gyrus, r(51) = −0.38, p = 0.006. Stress exposure was positively associated with brain response to white noise in the right supramarginal gyrus, r(51) = 0.30, p = 0.03. In the medial frontal gyrus, although the contrast of cry vs. white noise was negatively associated with stress exposure, brain responses to infant cry or white noise alone were not significantly associated with stress exposure.
In the whole-brain analysis, there was no significant main effect of stress exposure or the postpartum months and scanner type. The main effect of maternal age was detected in one brain region in the left supramarginal gyrus (BA 40; x, y, z = −55, −46, 29, k=30). Older maternal age was associated with greater brain responses across all conditions in this region. From the same whole-brain analysis, the two-way interactions between sound (cry, white noise) and condition (own, control) and the main effect of sound (cry, white noise) revealed significant results and were reported in the Supplementary Material (Supplementary Tables 2 and 3).
We conducted further whole-brain analyses while controlling for additional variables (maternal education years, history of mood disorders, current intake of psychotropic medication, maternal anxiety or depressive symptoms). In the separate whole-brain models, the two-way interactions of stress exposure and sound (cry vs noise) were identified in all five clusters from the main analysis in Table 3, p < 0.05, corrected.
Last, to examine the associations of brain activations with the emotional rating of the cry sounds, we used the extracted data from the functional clusters and conducted correlation analyses. Higher right insula activation in response to cry sounds was associated with lower pleasantness ratings, r(50) = −0.36, p = 0.01, and higher distressing ratings, r(50) = 0.40, p = 0.003. Higher right medial frontal gyrus response to cry sounds was associated with lower pleasantness ratings, r(50) = −0.32, p = 0.02, and higher left cerebellum response to cry sounds was associated with higher distressing ratings, r(50) = 0.35, p = 0.01.
3.4. Associations between brain responses to infant cry and parenting behaviors
We tested associations between maternal sensitivity and maternal brain activation in the three functional clusters where brain response to cry was associated with stress exposure while controlling for depressive mood and anxiety symptoms. Maternal sensitivity was positively associated with brain response to cry in the right insula, B = 0.33, p = 0.02 (Fig. 2a), and superior temporal gyrus, B = 0.29, p = 0.04 (Fig. 2b). Maternal sensitivity was associated with brain response to infant cry sounds in the left cerebellum at a trend level only, B = 0.24, p = 0.095. The main effects of maternal moods were not significant in the analyses.
Fig. 2.
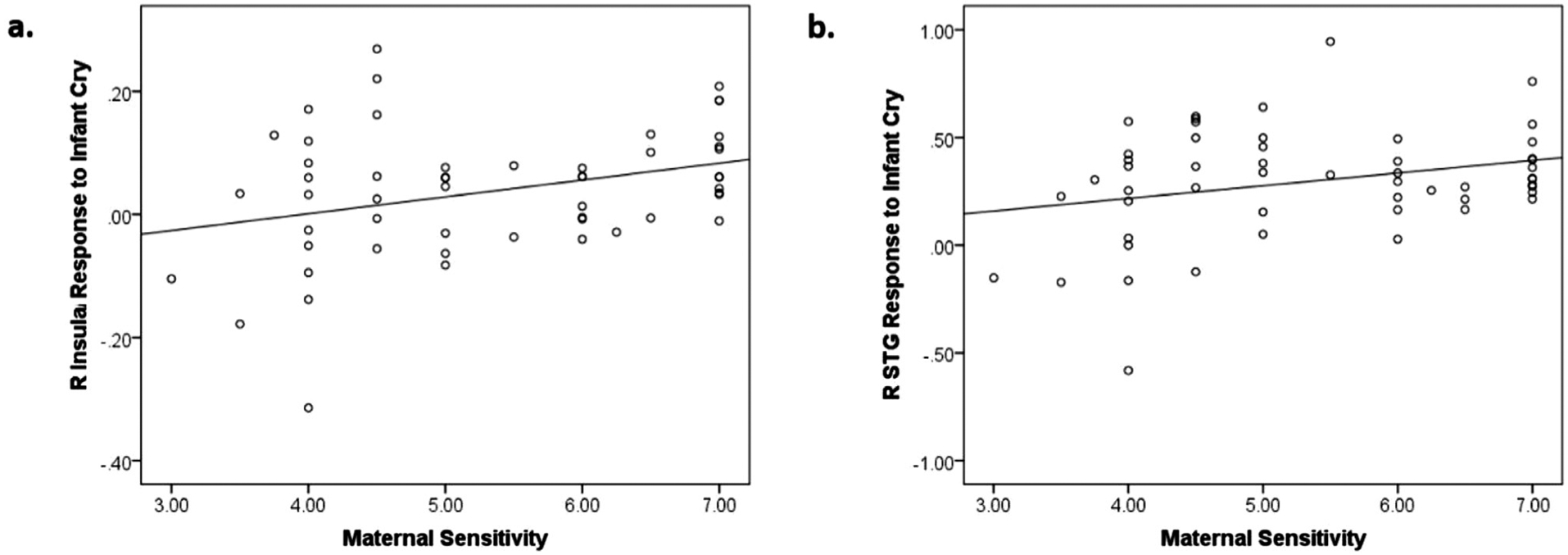
(a) A scatterplot describing the positive associations between maternal sensitivity and brain responses to infant cry sounds (both own and control infant cry sounds) in the right (R) insula (BA47; x, y, z = 29, 26, 2; k=86; Table 3) and maternal sensitivity; (b) a scatterplot describing the positive associations between maternal sensitivity and brain responses to infant cry sounds (both own and control infant cry sounds) in the right (R) superior temporal gyrus (STG) (BA41; x, y, z = 44, −31, 5l; k=39; Table 3).
4. Discussion
In the current study, we provide evidence for the role of stress exposure in brain responses to infant cry sounds and parenting behaviors among new mothers. We measured the stress mothers experience across multiple domains of their postpartum environment. We found that stress exposure was associated with elevated anxiety symptoms among new mothers. Stress exposure was also associated with reduced activation in brain regions that are involved in emotion information processing and emotion regulation including the right insula, medial frontal gyrus, and STG, in response to infant cry sounds compared to white noise. The dampened activation in response to infant cry sounds in the insula and STG were further associated with lower maternal sensitivity observed during mothers’ interactions with their own infants. Existing literature suggests that mothers experiencing severe stress are at a greater risk for postpartum mood disorders and harsh parenting. The findings of the current study provide evidence that reduced response to infant cry sounds in certain brain regions may be involved in the link between stress exposure and difficulties in adjustment to motherhood.
We examined stress exposure across multiple domains among low- and middle-income mothers during the first postpartum year. While low- income new mothers are, on average, at greater risk for stress exposure, they have been under-represented in research. Therefore, it was important for the current study to oversample this group. Factors were selected based on existing evidence that higher exposure can present a significant amount of stress. Each factor was coded as a stressor if the scores were in the top-quartile or otherwise met the threshold for a high level of exposure. In the current study, the mean number of stressors the mothers experienced was slightly below 2 (M = 1.94). In a possible range of 0–9, this mean number may seem low. However, it should be noted that each of these stressors presents significant stress to individuals. Previous research that included large sample sizes and a similar number of stressors also found the mean number of stressors to be between 1 and 2 (Appleyard et al., 2005; Evans and Kim, 2007; Evans et al., 2007; Liu and Tronick, 2013). Because our sample size is relatively small, it is important for our findings to be replicated by future studies with a larger sample. More participants with a higher number of stressors may reveal a stronger association of stress exposure with maternal mood symptoms and parenting behaviors than the associations found in the current study.
As expected, when we examined the associations between stress exposure and maternal distress, we found that stress exposure was associated with negative mood, specifically trait anxiety symptoms. Higher stress exposure was associated with higher anxiety symptoms reported by mothers. A possible reason as to why only trait anxiety symptoms, not state anxiety or depressive mood symptoms, was associated with stress exposure is the difference in the range of scores on each measure. In the current study, high trait anxiety symptoms had the greatest amount of variability, with 26% of participants scoring above the cutoff. Only 2% and 9% of participants scored above the cutoff for moderate depression and state high anxiety symptoms, respectively. Compared to other studies, a lower percentage of participants had depressive mood and state anxiety scores that rose above cutoffs (Gotlib et al., 1989; Grant et al., 2008; O’hara and Swain, 1996). A possible reason is that we included a wide range of postpartum months (1–10 months) and depressive mood and anxiety symptoms tend to decrease toward the later postpartum period (Dennis et al., 2013; Kim et al., 2013b; O’hara and Swain, 1996; Stuart et al., 1998). Another possibility is that the recruitment design and demands of the current study may have attracted mothers with fewer postpartum difficulties. The current research protocol involved both home visits and neuroimaging scans during the very busy postpartum period and mothers contacted the research team about their interests in participating. These aspects of the research project were likely to appeal to participants who are relatively well-functioning. For this reason, it is possible that the mothers experiencing high levels of stress exposure in the current study may be more resilient compared to mothers who did not participate in the study.
When we examined the brain response to infant cry, stress exposure was significantly associated with reduced activation in several brain regions that are involved in parenting. The identified cluster with insula at its peak coordinates also included a part of the IFG. The right insula, IFG, and medial frontal gyrus are involved in detecting the salience of stimuli and evaluating the emotional values of infant cues (Moses-Kolko et al., 2014; Parsons et al., 2013; Zaki et al., 2012). Non-human animal studies suggest that disrupted function (e.g. lesion, oxytocin receptor antagonist) of the medial frontal gyrus can lead to abnormal parenting behaviors and higher anxiety in mothers, and this region is also particularly vulnerable to stress exposure (Febo et al., 2010; Leuner et al., 2014; Sabihi et al., 2014). Right STG activation has been shown to be involved in processing the emotional content of an auditory stimulus such as infant cry sounds (Bornstein et al., 2017; Montoya et al., 2012; Sander et al., 2007). The insula, IFG, medial frontal gyrus, and STG are also structurally and functionally interconnected (Ghaziri et al., 2017), which highlights their role in processing infant cues. Activations in the cerebellum (involved in emotional information processing; (Adamaszek et al., 2017)) may also play an important role in mothers’ sensitive and appropriate responses to infant cues. Therefore, it is concerning that stress exposure is associated with reduced activation in these brain regions.
Furthermore, we found that reduced brain activation in the right insula and STG in response to cry sounds were associated with lower maternal sensitivity observed during mother-infant interactions. Reduced brain response to infant stimuli in these regions has previously been associated with suboptimal parenting behaviors (Atzil et al., 2011; Hipwell et al., 2015; Kim et al., 2011; Musser et al., 2012). This may further support the importance of the role of insula and STG regions in detecting and evaluating an infant’s distress cues, given their role in understanding one’s own and other’s emotional states (Singer et al., 2009; Uddin, 2015). This is consistent with findings from previous studies. For example, low-income mothers’ reduced STG activations in response to infant’s cry were associated with less positive perceptions of parenting (Kim et al., 2016a), and reduced anterior insular activation in response to others’ pain was associated with less sensitive maternal behaviors among trauma-exposed mothers (Levy et al., 2019). Although maternal sensitivity was associated with brain activation in the regions that were also associated with stress exposure, maternal sensitivity was not directly associated with stress exposure in this sample. One possibility is that maternal brain response to infant cues may be more proximal and sensitive measures to explain the variance in maternal sensitivity, compared to environmental measures such as stress exposure, in this relatively small sample of mothers.
We should note that stress exposure was not associated with different brain activations to own infant cry vs. other infant cry sounds. These findings are similar to previous findings from Kim et al. (2016a) studying the role of low income-to-needs ratio in maternal brain response to infant cry. In that study, low income-to-needs ratio was associated with reduced brain activation in response to infant cry (across own and control infant cry sounds) vs. white noise in the medial and lateral PFC and STG (Kim et al., 2016a). A recent meta-analysis suggests that brain response to own infant images is associated with many of the mesocorticolimbic pathway regions including the amygdala, nucleus accumbens, striatum, and thalamus (Rigo et al., 2019). However, mothers exhibit a heightened neural and physiological reactivity to unfamiliar infant cry sounds compared to non-parents (Boukydis and Burgess, 1982; Seifritz et al., 2003; Stallings et al., 2001). Common brain responses across own and control infant cry sounds are reported in the brain regions of the PFC and STG in the current study (Table S2) as well as other studies (Bornstein et al., 2017; Witteman et al., 2019) with human mothers. Thus, it is possible that new mothers have heightened emotional information processing and reactivity across the identities of infants due to greater emotional urgency compared to infant visual cues. Furthermore, the findings of the current study suggest that brain regions involved in emotional and social information processing are more likely to be associated with stress exposure compared to brain regions involved in maternal motivation, and the impact of stress exposure may not be specific to hearing one’s own baby cries, but more generally across all infant cries.
The emotion rating findings for the infant cry sounds further support the role of heightened maternal reactivity to infant cues, regardless of the identity of the infant. There was no significant difference between emotion ratings of one’s own and control infant cry sounds. However, stress exposure was associated with higher pleasantness ratings across own and control cry sounds. The higher pleasantness rating and lower distressing rating of infant cry sounds were further associated with lower brain response to infant cry sound in the right insula. In the postpartum period, particularly during the early postpartum period, mothers have normative increases in their worries and concerns about their own baby (Kim et al., 2013b; Leckman et al., 1999). Worry thoughts and behaviors are considered important prompts for mothers to invest a high number of hours in caregiving, thus developing close emotional bonds with infants (Leckman and Mayes, 1999). One potential interpretation is that when the mother’s environment is already highly stressful, she may adapt her emotional response to infant cries by perceiving them as more pleasant and less distressing. This may help her to be less emotionally overwhelmed by negative infant cues. However, this adaptation may also be linked to reduced brain and behavioral responses to infants as observed in the current study.
While the current study focused on stress exposure during the postpartum months, it is also important to consider the potential impact of prior stress exposure on maternal brain responses to infants. Mothers who report unresolved attachment-related trauma from their childhood exhibit also exhibit reduced amygdala and behavioral sensitivity to their own infants’ cues, especially the infants’ distress cues (Beebe et al., 2010; Kim et al., 2014). Other studies have also reported potential associations between negative childhood experience and mothers’ dampened neural responses to infant cues (Kim et al., 2010b; Olsavsky et al., 2019). Childhood attachment-related trauma has been associated with disrupted maternal behaviors which include positive or surprised maternal expressions in response to infant distress (Beebe et al., 2010). This may reflect mothers’ difficulties in understanding their own emotional states as well as their infants’ emotional states. This may also reflect mothers’ blunted responses, which may also be related to higher levels of pleasantness ratings of infant cry sounds among stressed mothers in the current study. Unfortunately, some of the stressors such as poverty and family dysfunction can be chronic and intergenerationally transmitted (Iyengar et al., 2014; Tribble and Kim, 2019). Therefore, further investigation of the role of childhood adversities as well as postpartum stress on the maternal brain and behavioral sensitivity will be important for identifying targets for prevention and intervention.
These findings must be considered in light of several limitations. First, we included a relatively wide range of postpartum months - first month to ten months at the time of the fMRI scan. The majority of participants (82%) were scanned within 6 months postpartum. Some of the later fMRI scans were related to difficulties in follow-up between the home visits and fMRI scans. Most participants (81%) participated in the fMRI scans less than 40 days after the home visit, with the mean around three weeks. In the current study, the intervals and postpartum months were not associated with stress exposure or other demographic variables. However, it is important to consider that the data represent a wide range of postpartum months, and there is a wide interval range between home visits and fMRI visits. Therefore, future studies can consider targeting specific postpartum months and reducing the time intervals between the home and fMRI visits.
Second, the current study had a cross-sectional design. Thus, the associations among stress exposure, brain activation, and maternal behaviors are correlational, do not permit a mediation analysis (Maxwell and Cole, 2007), and do not empirically suggest a directionality among the variables. Thus, the suggested directionality is a hypothesis based on the existing literature. Moreover, as discussed earlier, while this study focused on concurrent exposure to stress, some participants may have been experiencing stress exposure for a longer period including their childhood and pregnancy (Olsavsky et al., 2019; Schechter et al., 2012). The current study design does not allow us to separate the unique roles of current vs. previous exposure to stress. Thus, a longitudinal study starting from childhood or pregnancy would be critical to confirm the directionality among the variables, such as whether exposure to stress during different time periods can lead to unique changes in brain response to infant cry, and further lead to altered maternal behaviors. Furthermore, a larger sample of new mothers will help identify the unique effects of different types of stressors. The current study addresses a gap in the literature by providing evidence for the role of higher stress exposure in maternal brain function by assessing stress exposure across multiple domains of a new mother’s life. However, future studies with a larger sample will allow for comparison of different types of stressors, as each stressor may have unique impacts on brain functions (Harnett et al., 2019; McLaughlin et al., 2014).
Third, the current study did not include other biological markers that interact with maternal brain activation and are influenced by chronic stress exposure. In animal studies, stress was associated with higher cortisol and lower oxytocin levels in new mothers which were further associated with differential brain response to own offspring (Hillerer et al., 2012; Zelkowitz et al., 2014). Thus, future studies should consider examining the impact of stress exposure on maternal hormones. Fourth, the current study used an identical and single control cry stimulus to avoid between-subject variability in brain response to control stimuli. Participants did not rate the own vs. control cry sounds differently in terms of their pleasantness or distress levels. However, because own baby cry sounds vary in terms of emotional intensity across participants, future studies can consider using multiple control cry stimuli with different emotional intensities or selecting a control cry stimulus that matches the own cry stimuli in terms of emotional intensity per participant. Fifth, while it is a strength of the current study that all participants are first-time mothers and therefore individual differences in parity are controlled, findings may not be generalized to multiparous women. Previous parenting experience may buffer the impact of environmental stress on maternal brain responses to infant cues as multiparous women show reduced anxiety symptoms (Kim et al., 2013b) and increased brain responses to their infants (Afonso et al., 2008; Akbari et al., 2013). Finally, while the current research included a more socioeconomically diverse sample compared to some previous studies by oversampling low-income mothers and recruiting 43% of participants with a Hispanic background, the sample cannot be generalized to other underrepresented groups such as African Americans, nor can it be generalized to a very high-income group. Future studies that examine the effects of stress exposure in new mothers should include a wide range of racial, ethnic, and socioeconomically diverse samples.
5. Conclusion
We have provided evidence that among first-time new mothers, exposure to stress is associated with dampened brain response to infant cry sounds in brain regions that are important to emotional information processing, including the insula and STG. The dampened brain response was further associated with lower maternal sensitivity, which suggests reduced brain sensitivity to cry sounds may be associated with reduced behavioral sensitivity during interactions with a mother’s own infant. Reduced maternal sensitivity is a risk factor for suboptimal child development (Bornstein, 2002; Feldman, 2007). Thus, for preventions and intervention work, it may be important to target women experiencing high levels of stress exposure and support their transition to parenthood. The findings of the current study may also suggest that stress exposure impacts brain regions that help mothers understand the urgency and meaning of their infants’ distress cues. Therefore, we can consider providing support for mothers who are exposed to high stress to be emotionally attuned to their infants when they are under distress.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of Health [R01HD090068; R21HD078797; R21DA046556]; the Professional Research Opportunity for Faculty (PROF) and Faculty Research Fund (FRF), University of Denver; NARSAD Independent Investigator Grant, and the Victoria S. Levin Award For Early Career Success in Young Children’s Mental Health Research, Society for Research in Child Development (SRCD). AKO was supported during work on this manuscript by the Developmental Psychobiology Research Group Postdoctoral Fellowship T32MH015442 and the National Institutes of Health Loan Repayment Program. For disclosure, AKO is the recipient of additional non-governmental funding sources which did not support this research project (American Academy of Child and Adolescent Psychiatry Pilot Award and Developmental Psychobiology Endowment Fund small grant award), and AKO’s husband works for Thermo Fisher Scientific, a biomedical company unrelated to this line of research. All other authors declare that they have no conflicts of interest in the research. The authors thank the families that participated in the study and the individuals that supported recruitment. The authors also wish to acknowledge Amy Anderson, Lindsay Blanton, Christian Capistrano, Christina Congleton, Tanisha Crosby-Attipoe, Victoria Everts, Rachel Gray, Claire Jeske, Laura Jeske, and Nanxi Xu for research assistance.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2020.117360.
References
- Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby K, Leggio M, Mariën P, Molinari M, Moulton E, 2017. Consensus paper: cerebellum and emotion. Cerebellum 16, 552–576. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Grella SL, Chatterjee D, Fleming AS, 2008. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Res. 1198, 115–123. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Akbari EM, Shams S, Belay HT, Kaiguo M, Razak Z, Kent CF, Westwood T, Sokolowski MB, Fleming AS, 2013. The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: A microarray study. Behav. Neurosci 127, 913. doi: 10.1037/a0034884. [DOI] [PubMed] [Google Scholar]
- Appleyard K, Egeland B, van Dulmen MH, Alan Sroufe L, 2005. When more is not better: The role of cumulative risk in child behavior outcomes. J. Child Psychol. Psychiatry 46, 235–245. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R, 2011. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology 36, 2603–2615 1111/j.1469–7610.2004.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MA, 2008. Economic disadvantage in complex family systems: Expansion of family stress models. Clin. Child Fam. Psychol. Rev 11, 145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG, 1988. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clin. Psychol. Rev 8, 77–100. [Google Scholar]
- Beebe B, Jaffe J, Markese S, Buck K, Chen H, Cohen P, Bahrick L, Andrews H, Feldstein S, 2010. The origins of 12-month attachment: A microanalysis of 4-month mother-infant interaction. Attach. Hum. Dev 12, 3–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringen Z, 2008. The Emotional Availability (EA) Scales and the Emotional Attachment & Emotional Availability (EA2) Clinical Screener, 4th ed. Boulder, CO. [Google Scholar]
- Biringen Z, Derscheid D, Vliegen N, Closson L, Easterbrooks MA, 2014. Emotional availability (EA): Theoretical background, empirical research using the EA Scales, and clinical applications. Dev. Rev 34, 114–167. doi: 10.1016/j.dr.2014.01.002. [DOI] [Google Scholar]
- Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR, 1999. The effectiveness of a short form of the Household Food Security Scale. Am. J. Publ. Health 89, 1231–1234. doi: 10.2105/AJPH.89.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, 2002. Parenting infants. In: Bornstein MH (Ed.), Handbook of Parenting. Erlbaum, Mahwah, N.J., pp. 3–43. [Google Scholar]
- Bornstein MH, Putnick DL, Rigo P, Esposito G, Swain JE, Suwalsky JTD, Su X, Du X, Zhang K, Cote LR, De Pisapia N, Venuti P, 2017. Neurobiology of culturally common maternal responses to infant cry. In: Proceedings of the National Academy of Sciences, 114, p. E9465 0.1073/pnas.1712022114, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukydis CZ, Burgess RL, 1982. Adult physiological response to infant cries: Effects of temperament of infant, parental status, and gender. Child Dev. 1291–1298. [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ, 1994. Nature-nuture reconceptualized in developmental perspective: A bioecologicalmodel. Psychol. Rev 101, 568. doi: 10.1037/0033-295X.101.4.568. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA, 2010. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 34, 766–776. [DOI] [PubMed] [Google Scholar]
- Candelaria MA, O’Connell MA, Teti DM, 2006. Cumulative psychosocial and medical risk as predictors of early infant development and parenting stress in an African-American preterm sample. J. Appl. Dev. Psychol 27, 588–597. doi: 10.1016/j.appdev.2006.08.006. [DOI] [Google Scholar]
- Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, Beckmann CF, 2013. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb. Cortex 23, 1509–1516. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Crnic K, Low C, 2002. Everyday stresses and parenting. Handbook of Parenting. Volume 5: Practical Issues in Parenting, 242. [Google Scholar]
- Dennis C-L, Coghlan M, Vigod S, 2013. Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the State-Trait Anxiety Inventory? J. Affect. Disord 150, 1217–1220. doi: 10.1016/j.jad.2013.05.049. [DOI] [PubMed] [Google Scholar]
- Dufford AJ, Kim P, 2017. Family income, cumulative risk exposure, and white matter structure in middle childhood. Front. Hum. Neurosci 11. doi: 10.3389/fn-hum.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, English K, 2002. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev 73, 1238–1248 0.1111/1467–8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, 2007. Childhood poverty and health cumulative risk exposure and stress dysregulation. Psychol. Sci 18, 953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D, 2007. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev. Psychol 43, 341 0.1037/0012–1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Evans GW, Wells NM, Chan E, Saltzman H, 2000. Housing and mental health. J. Consult. Clin. Psychol 68, 526–530. doi: 10.1037/0022-006X.68.3.526. [DOI] [PubMed] [Google Scholar]
- Febo M, Felix-Ortiz AC, Johnson TR, 2010. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Res. 1325, 77–88. doi: 10.1016/j.brainres.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, 2007. Parent-infant synchrony and the construction of shared timing; Physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatry 48, 329–354 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, 2017. The neurobiology of human attachments. Trends Cognit. Sci 21, 80–99. doi: 10.1016/j.tics.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E, 2009. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J. Am. Acad. Child Adolesc. Psychiatry 48, 919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Houde J-C, Boucher O, Gilbert G, Descoteaux M, Lippé S, Rainville P, Nguyen DK, 2017. The corticocortical structural connectivity of the human insula. Cereb. Cortex 27, 1216–1228. doi: 10.1093/cercor/bhv308. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W, 2018. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci 19, 123. doi: 10.1093/cercor/bhv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI, 1989. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J. Consult. Clin. Psychol 57, 269. doi: 10.1037/0022-006X.57.2.269. [DOI] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Austin MP, 2008. Maternal anxiety during the transition to parenthood: a prospective study. J. Affect. Disord 108, 101–111. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Guo C, Moses-Kolko E, Phillips M, Swain JE, Hipwell AE, 2018. Severity of anxiety moderates the association between neural circuits and maternal behaviors in the postpartum period. Cognit. Affect. Behav. Neurosci 18, 426–436. doi: 10.3758/s13415-017-0516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Sherer M, Leuner B, 2014. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur. J. Neurosci 40, 3766–3773. doi: 10.1111/ejn.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Wheelock MD, Wood KH, Goodman AM, Mrug S, Elliott MN, Schuster MA, Tortolero S, Knight DC, 2019. Negative life experiences contribute to racial differences in the neural response to threat. NeuroImage 202, 116086. doi: 10.1016/j.neuroimage.2019.116086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, Flugge G, Fuchs E, 2009. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience 159, 982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Neumann ID, Slattery DA, 2012. From stress to postpartum mood and anxiety disorders: how chronic peripartum stress can impair maternal adaptations. Neuroendocrinology 95, 22–38. doi: 10.1159/000330445. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Reber SO, Neumann ID, Slattery DA, 2011. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: implications for postpartum anxiety and mood disorders. Endocrinology 152, 3930–3940. doi: 10.1210/en.2011-1091. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Guo C, Phillips ML, Swain JE, Moses-Kolko EL, 2015. Right frontoinsular cortex and subcortical activity to infant cry is associated with maternal mental state talk. J. Neurosci 35, 12725–12732. doi: 10.1523/JNEUROSCI.1286-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Swain JE, 2017. Depression alters maternal extended amygdala response and functional connectivity during distress signals in attachment relationship. Behav. Brain Res 325, 290–296. doi: 10.1016/j.bbr.2017.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella RA, Belsky J, von Eye A, 1989. Origins of infant-mother attachment: An examination of interactional synchrony during the infant’s first year. Dev. Psychol 25, 12. doi: 10.1037/0012-1649.25.1.12. [DOI] [Google Scholar]
- Iyengar U, Kim S, Martinez S, Fonagy P, Strathearn L, 2014. Unresolved trauma in mothers: intergenerational effects and the role of reorganization. Front. Psychol 5, 966. doi: 10.3389/fpsyg.2014.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettinger LA, Nair P, Schuler ME, 2000. Exposure to environmental risk factors and parenting attitudes among substance-abusing women. Am. J. Drug Alcohol Abuse 26, 1–11. doi: 10.1081/ADA-100100586. [DOI] [PubMed] [Google Scholar]
- Kim P, 2016. Human maternal brain plasticity: adaptation to parenting. New Direct. Child Adolesc. Dev 2016, 47–58. doi: 10.1002/cad.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Bianco H, 2014. How motherhood and poverty change the brain. Zero Three 34, 29–36. [Google Scholar]
- Kim P, Capistrano C, Congleton C, 2016a. Socioeconomic disadvantages and neural sensitivity to infant cry: role of maternal distress. Soc. Cognit. Affect. Neurosci 11, 1597–1607. doi: 10.1093/scan/nsw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL, 2013a. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci 110, 18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE, 2011. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiatry 52, 907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE, 2010a. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosci 124, 695. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE, 2010b. Perceived quality of maternal care i n childhood and structure and function of mothers’ brain. Dev. Sci 13, 662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Mayes L, Feldman R, Leckman JF, Swain JE, 2013b. Early postpartum parental preoccupation and positive parenting thoughts: Relationship with parent-infant interaction. Infant Ment. Health J 34, 104–116. doi: 10.1002/imhj.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE, 2016b. The maternal brain and its plasticity in humans. Horm. Behav 77, 113–123. doi: 10.1016/j.yhbeh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, Strathearn L, 2014. Mothers’ unresolved trauma blunts amygdala response to infant distress. Soc. Neurosci 9, 352–363. doi: 10.1080/17470919.2014.896287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, 2011. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc. Cognit. Affect. Neurosci 7 (2), 125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, 1999. Preoccupations and behaviors associated with romantic and parental love. Perspectives on the origin of obsessive-compulsive disorder. Child Adolesc. Psychiatr. Clin. N. Am 8 (3), 635–665. doi: 10.1016/S1056-4993(18)30172-X. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ, 1999. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatrica Scandinavica. Supplementum 396 (S396), 1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C, Albin-Brooks C, 2014. Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. PLoS One 9 (3). doi: 10.1371/journal.pone.0089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Yirmiya K, Goldstein A, Feldman R, 2019. Chronic trauma impairs the neural basis of empathy in mothers: Relations to parenting and children’s empathic abilities. Dev. Cognit. Neurosci 38, 100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Tronick E, 2013. Re-conceptualising prenatal life stressors in predicting postpartum depression: Cumulative-, specific-, and domain-specific approaches to calculating risk. Paediatr. Perinat. Epidemiol 27 (5), 481–490. doi: 10.1111/pp3.12072. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS, 2015. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm. Behav 73, 156–185. doi: 10.1016/j.yhbeh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA, 2007. Bias in cross-sectional analyses of longitudinal mediation. Psychol. Methods 12, 23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK, 2014. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Christian LM, 2017. Financial strain and birth weight: the mediating role of psychological distress. Arch. Women’s Ment. Health 20, 201–208. doi: 10.1007/s00737-016-0696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl WE, Mayes LC, Potenza MN, 2012. Regional brain responses in nulliparous women to emotional infant stimuli. PLoS One 7. doi: 10.1371/journal.pone.0036270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE, 2014. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J. Neuroendocrinol 26, 665–684. doi: 10.1111/jne.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, Ablow JC, 2012. The neural correlates of maternal sensitivity: An fMRI study. Dev. Cognit. Neurosci 2, 428–436. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha T, Peverill M, Koh N, McCabe C, Flournoy J, Mills K, King K, Pfeifer J, McLaughlin KA, 2018. Current methods and limitations for longitudinal fMRI analysis across development. Dev. Cognit. Neurosci 33, 118–128. doi: 10.1016/j.dcn.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P, Schuler ME, Black MM, Kettinger L, Harrington D, 2003. Cumulative environmental risk in substance abusing women: early intervention, parenting stress, child abuse potential and child development. Child Abuse Neglect 27, 997–1017. doi: 10.1016/S0145-2134(03)00169-8. [DOI] [PubMed] [Google Scholar]
- O’hara MW, Swain AM, 1996. Rates and risk of postpartum depression-a meta-analysis. Int. Rev. Psychiatry 8, 37–54. doi: 10.3109/09540269609037816. [DOI] [Google Scholar]
- Olsavsky AK, Stoddard J, Erhart A, Tribble RC, Kim P, 2019. Neural processing of infant and adult face emotion and maternal exposure to childhood maltreatment. Soc. Cognit. Affect. Neurosci 14, 997–1008. doi: 10.1093/scan/nsz069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Stark EA, Young KS, Stein A, Kringelbach ML, 2013. Understanding the human parental brain: a critical role of the orbitofrontal cortex. Soc. Neurosci 8, 525–543. doi: 10.1080/17470919.2013.842610. [DOI] [PubMed] [Google Scholar]
- Paul S, Austin J, Elliott R, Ellison-Wright I, Wan MW, Drake R, Downey D, Elmadih A, Mukherjee I, Heaney L, Williams S, Abel KM, 2019. Neural pathways of maternal responding: systematic review and meta-analysis. Arch. Women’s Mental Health 22, 179–187. doi: 10.1007/s00737-018-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Lambert KG, Kinsley CH, 2016. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm. Behav 77, 86–97. doi: 10.1016/j.yhbeh.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Perry RE, Finegood ED, Braren SH, Dejoseph ML, Putrino DF, Wilson DA, Sullivan RM, C. C, Blair C, Investigators, F.L.P.K., 2018. Developing a neurobehavioral animal model of poverty: Drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Dev. Psychopathol. 31, 399–418. 10.1017/S095457941800007X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Leadbeater BJ, 1999. Mothering under pressure: Environmental, child, and dyadic correlates of maternal self-efficacy among low-income women. J. Fam. Psychol 13, 523–524. doi: 10.1037/0893-3200.13.4.523. [DOI] [Google Scholar]
- Rigo P, Kim P, Esposito G, Putnick DL, Venuti P, Bornstein MH, 2019. Specific maternal brain responses to their own child’s face: an fMRI meta-analysis. Dev. Rev 51, 58–69. doi: 10.1016/j.dr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, Wallace NS, Laurent HK, Mayes LC, 2015. Emotion regulation in parenthood. Dev. Rev 36, 1–14. doi: 10.1016/j.dr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Durosko NE, Leuner B, 2014. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front. Behav. Neurosci 8, 258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin S, Valois P, Lussier Y, 2005. Development and validation of a brief version of the dyadic adjustment scale with a nonparametric item analysis model. Psychol. Assess 17, 15–27. doi: 10.1037/1040-3590.17.1.15. [DOI] [PubMed] [Google Scholar]
- Salm Ward T, Kanu FA, Robb SW, 2017. Prevalence of stressful life events during pregnancy and its association with postpartum depressive symptoms. Arch. Women’s Ment. Health 20, 161–171. doi: 10.1007/s00737-016-0689-2. [DOI] [PubMed] [Google Scholar]
- Sander K, Frome Y, Scheich H, 2007. FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Hum. Brain Mapp 28, 1007–1022. doi: 10.1002/hbm.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, Wang ZS, Marsh R, Hao XJ, Duan YS, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, Peterson BS, 2012. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc. Cognit. Affect. Neurosci 7, 969–979. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F, 2003. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol. Psychiatry 54, 1367–1375. doi: 10.1016/S0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Shalowitz M, Berry C, Rasinski K, Dannhausen-Brun C, 1998. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv. Res 33, 1381–1402. [PMC free article] [PubMed] [Google Scholar]
- Shalowitz MU, Mijanovich T, Berry CA, Clark-Kauffman E, Quinn KA, Perez EL, 2006. Context matters: a community-based study of maternal mental health, life stressors, social support, and children’s asthma. Pediatrics 117, e940–e948. doi: 10.1542/peds.2005-2446. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K, 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cognit. Sci 13, 334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Hillerer KM, 2016. The maternal brain under stress: Consequences for adaptive peripartum plasticity and its potential functional implications. Front. Neuroendocrinol 41, 114–128. doi: 10.1016/j.yfrne.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW, 2004. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29, 227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Smith PB, Pederson DR, 1988. Maternal sensitivity and patterns of infant-mother attachment. Child Dev. 1097–1101. doi: 10.2307/1130276. [DOI] [PubMed] [Google Scholar]
- Soe NN, Wen DJ, Poh JS, Li Y, Broekman BFP, Chen H, Chong YS, Kwek K, Saw S-M, Gluckman PD, Meaney MJ, Rifkin-Graboi A, Qiu A, 2016. Pre- and post-natal maternal depressive symptoms in relation with infant frontal function, connectivity, and behaviors. PloS One 11, e0152991. doi: 10.1371/journal.pone.0152991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyadeep M, Stefany C, Kristopher F, Purnima M, Jo TM, 2017. Antenatal stressful life events and postpartum depressive symptoms in the united states: the role of women’s socioeconomic status indices at the state level. J. Women’s Health 26, 276–285. doi: 10.1089/jwh.2016.5872. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, 1970. State-Trait Anxiety Scale. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Stallings J, Fleming AS, Corter C, Worthman C, Steiner M, 2001. The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpostpartum women. Parenting 1, 71–100. doi: 10.1080/15295192.2001.9681212. [DOI] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR, 2008. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics 122, 40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S, Couser G, Schilder K, O’hara MW, Gorman L, 1998. Postpartum anxiety and depression: onset and comorbidity in a community sample. J. Nerv. Ment. Dis 186, 420–424. doi: 10.1097/00005053-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Telzer EH, McCormick EM, Peters S, Cosme D, Pfeifer JH, van Duijvenvoorde AC, 2018. Methodological considerations for developmental longitudinal fMRI research. Dev. Cognit. Neurosci 33, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble R, Kim P, 2019. Intergenerational transmission of poverty: how low socioeconomic status impacts the neurobiology of two generations. In: Biobehavioral Markers in Risk and Resilience Research. Springer, pp. 49–67. [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 16, 55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Vinokur A, Caplan RD, 1987. Attitudes and social support: determinants of job-seeking behavior and well-being among the unemployed 1. J. Appl. Soc. Psychol 17, 1007–1024. doi: 10.1111/j.1559-1816.1987.tb02345.x. [DOI] [Google Scholar]
- Vinokur AD, Price RH, Caplan RD, 1996. Hard times and hurtful partners: How financial strain affects depression and relationship satisfaction of unemployed persons and their spouses. J. Person. Soc. Psychol 71, 166. doi: 10.1037/0022-3514.71.1.166. [DOI] [PubMed] [Google Scholar]
- Witteman J, Van IJzendoorn M, Rilling J, Bos P, Schiller N, Bakermans-Kranenburg M, 2019. Towards a neural model of infant cry perception. Neurosci. Biobehav. Rev 99, 23–32. doi: 10.1016/j.neubiorev.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, Lee-Parritz A, Wood RA, Kattan M, Bloomberg GR, 2010. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am. J. Respir. Crit. Care Med 182, 25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN, 2012. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62, 493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P, 2014. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm. Behav 66, 351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


