Abstract
A 23-year-old young woman with a known history of valvular heart disease of rheumatic origin, post balloon mitral valvotomy 5 years ago, presented with fever, palpitations and breathlessness. ECG showed atrial fibrillation with fast ventricular rate. A 2D transthoracic echocardiography showed severe restenosis of mitral valve and moderate left ventricular dysfunction. She was admitted for evaluation of fever and control of the ventricular rate. She sustained cardiac arrest due to unknown cause and was resuscitated. When the patient sustained another cardiac arrest, torsades de pointes was detected on the monitor. Blood parameters showed hypomagnesaemia, hypocalcaemia and hypokalaemia, causing functional hypoparathyroidism which was treated with intravenous magnesium, oral calcium and vitamin D supplements. Timely detection of a tachyarrhythmia due to a ventricular origin was life saving, which is rarely seen in patients with rheumatic heart disease and mitral stenosis.
Keywords: arrhythmias, valvar diseases, cardiovascular system, endocrinology
Background
Rheumatic heart disease (RHD) with mitral valve disease commonly cause atrial arrhythmias and most commonly atrial fibrillation (AF). Arrhythmias arising from the ventricles are uncommon in RHD. Ventricular arrhythmias arise when there is a cardiac enlargement or congestive heart failure. RHD predisposing to the development of torsades de pointes (TdP) is extremely rare. TdP is an atypical and distinctive form of polymorphic ventricular tachycardia (VT) in which the polarity of the QRS complex twists at points which occurs due to a prolonged QT interval. In this patient, dyselectrolaemia especially hypomagnesaemia, hypokalaemia and hypocalcaemia were noted. Magnesium (Mg2+) is an essential, fourth most abundant cation and ranking second in the intracellular environment, plays a crucial role in membrane integrity, neuromuscular excitability, muscle contraction and hormone secretion.1 Magnesium deficiency leads to QT interval prolongation due to Na+–K+ pump inhibition and change in membrane potential which predisposes to life-threatening polymorphic VT or TdP.2 Hypomagnesaemia if untreated, can lead to tetany, recurrent seizures, status epilepticus and life-threatening arrhythmias. Both hypomagnesaemia and less commonly hypermagnesaemia leads to abnormal functioning of parathyroid glands. This is often called functional hypoparathyroidism because it resolves when magnesium level is restored.3
Case presentation
A 23-year-old young woman with a known history of severe mitral stenosis of rheumatic origin, status post balloon mitral valvotomy (BMV) in 2015 presented with fever, palpitations and breathlessness for a duration of 1 week. There was no history of cough, abdominal pain, burning micturation, loose stools, rashes or any recent travel history. In the emergency room (ER), ECG showed atrial flutter with fibrillation with fast ventricular rate (figure 1). She was haemodynamically stable. AF rate was controlled with an intravenous diltiazem in the ER and the patient was later shifted to isolation ward after nasopharyngeal swab for COVID-19 test was taken. Relevant laboratory investigations at the time of admission has been summarised in table 1. Based on the blood investigations with raised liver enzymes and deranged renal functions, there was a suspicion of an underlying leptospirosis or hepatitis infection for which relevant investigations were sent. Reports are mentioned in table 1. Hepatitis-A IgM titres and bilirubin levels were elevated. Card test for leptospirosis was negative. She was managed with intravenous antibiotics, heart rate-controlling medications and diuretics. Her 2D transthoracic echocardiography showed severe mitral stenosis, moderate pulmonary artery hypertension and moderate left ventricular (LV) dysfunction, left ventricular ejection fraction (LVEF; 35%), but no evidence of infective endocarditis (figure 2). On the fifth day post admission, she was found unresponsive in the ward. Cardiopulmonary resuscitation (CPR) was initiated. She was revived, intubated and connected to mechanical ventilator. Inotropes were started for hypotension.
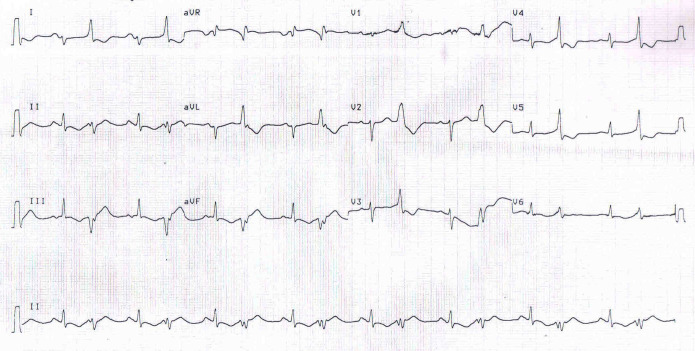
Figure 1.
ECG strip showing atrial fibrillation.
Table 1.
Relevant laboratory investigations on the day of admission
| Investigations | Observed value | Reference range | Units |
| Total leucocyte count | 11 100 | 400–11 000 | cells/mm3 |
| Differential count | Neutrophils: 75, Lymphocytes: 24, Eosinophils: 1 |
Neutrophils: 40–75 Lymphocytes: 20–40 Eosinophils: 1–6 |
% |
| Platelet count | 127 000 | 150 000–400 000 | cells/mm3 |
| Potassium | 5.93 | 3.5–5.1 | mmol/L |
| Sodium | 129 | 136–145 | mmol/L |
| Total bilirubin, direct bilirubin, indirect bilirubin | 7.96, 4.69, 3.27 | Upto 1.2,<0.2, 0–0.75 |
mg/dL |
| Serum glutamic oxaloacetic transaminase | 4963.6 | 0–32 | U/L |
| Serum glutamic pyruvic transaminase | 1884.6 | 0–33 | U/L |
| Alkaline phosphatase | 102 | 60–170 | U/L |
| Urea | 91.3 | 16.6–48.5 | mg/dL |
| Creatinine | 1.91 | 0.7–1.4 | mg/dL |
| Thyroid Stimulating Hormone (TSH) | 1.92 | 0.27–4.2 | uIU/mL |
| Leptospirosis card test, dengue card test, malaria parasite test (fluroscent) | Negative | ||
| COVID-19 swab test | Negative | ||
| Leptospirosis IgM | Equivocal | ||
| HAV IgM | 1.58 | (>1.2 = reactive) |
Abnormal values have been marked as bold.
HAV, hepatitis A virus.
Figure 2.
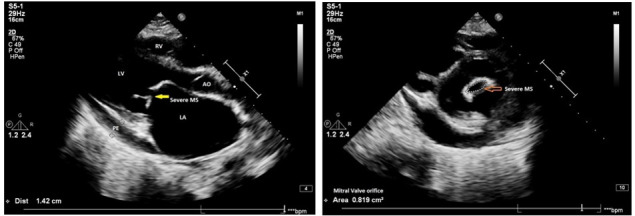
A 2D transthoracic echocardiogram—RHD, severe mitral restenosis, MVOA: 0.8 cm2, MVG: 18/11 mm Hg, trivial MR, non-coapting tricuspid valve, severe TR, peak gradient: 53 mm Hg, moderate PAH, grade 1 PR, grossly dilated LA and RA, dilated RV, reduced right ventricular function, moderate left ventricular dysfunction, LVEF: 35%, global hypokinesia LV, moderate PE seen. LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MVG, mitral valve gradient; MVOA, mitral valve orifice area; PAH, pulmonary artery hypertension; PE, pericardial effusion; PR, pulmonary regurgitation; RA, right atrium; RHD, rheumatic heart disease; RV, right ventricle; TR, tricuspid regurgitation.
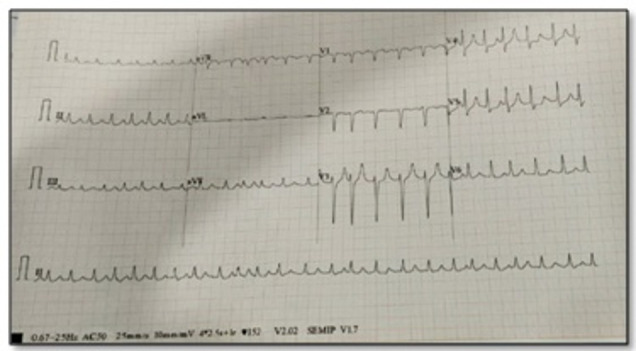
She continued to desaturate inspite of ventilatory support. Her chest X-ray showed left lower lobe collapse (figure 3). Video bronchoscopy showed a thick blood clot in the left main bronchus which was removed. On ninth day post admission, she had another cardiac arrest, CPR was initiated and she was revived. Just prior to cardiac arrest, her cardiac rhythm revealed runs of polymorphic VT (figure 4) leading to ventricular fibrillation (VF) and cardiac arrest. Following cardioversion, ECG revealed prolonged QTc (figure 5) which is a common forerunner of polymorphic VT. Table 2 shows laboratory investigations on ninth day post arrest.
Figure 3.
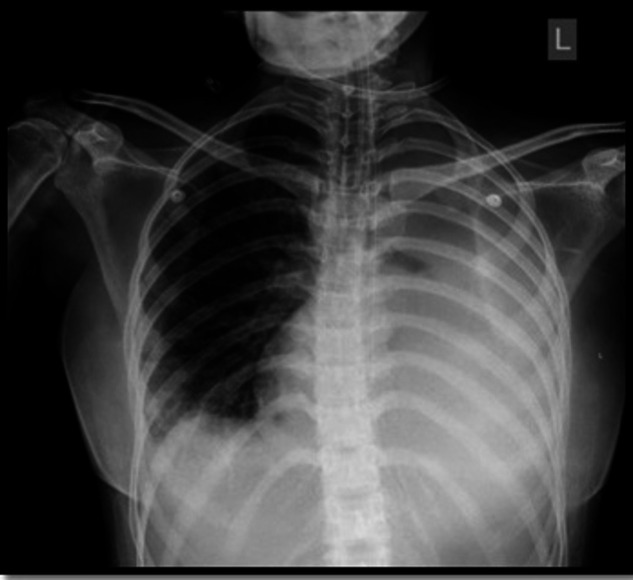
Chest X-ray showing left lower lobe collapse and cardiomegaly.
Figure 4.
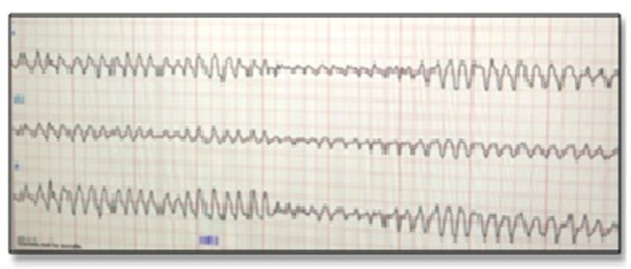
ECG strip showing torsades de pointes.
Figure 5.
ECG following resuscitation which shows QTc prolongation and ventricular bigeminy.
Table 2.
Relevant laboratory investigations on day 9
| Investigations | Observed value | Reference range | Units |
| Sodium | 132 | 136–145 | mmol/L |
| Potassium | 3.37 | 3.5–5.1 | mmol/L |
| Magnesium | 1.6 | 1.6–2.6 | mg/dL |
| Calcium | 8.2 | 8.6–10 | mg/dL |
Treatment
Considering dyselectrolytaemia being the cause of TdP, prompt correction of the same with both intravenous and oral potassium, magnesium, calcium and vitamin D supplements were given. Gastroenterology opinion was sought in view of hepatitis A infection and was started on hepatoprotective medications like vitamin E, S adenyl methionine for viral hepatitis. Intravenous antibiotics were continued. Faeco-oral transmission due to poor sanitation and hand hygiene for the occurrence of hepatitis A could not be ruled out. Her serum electrolytes, liver function test, renal function test showed improvement as shown in table 3. The left lung collapse recovered with bronchoscopic blood clot removal from left main bronchus. On 13th day, she was extubated and was tolerating face mask. Endocrinology opinion was sought in view of hypomagnesaemia and hypocalcaemia. As the parathyroid hormone levels were also less, a diagnosis of functional hypoparathyroidism due to hypomagnesaemia was made. There was no further runs of VT after adequate correction of serum potassium, serum magnesium and serum calcium levels.
Table 3.
Relevant laboratory investigations at the time of discharge
| Investigations | Observed value | Reference range | Units |
| Sodium | 132 | 136–145 | mmol/L |
| Potassium | 4.86 | 3.5–5.1 | mmol/L |
| Magnesium | 2.00 | 1.6–2.6 | mg/dL |
| Calcium | 8.7 | 8.6–10 | mg/dL |
| Total bilirubin, direct bilirubin, indirect bilirubin | 3.15, 2.26, 0.89 | Upto 1.2,<0.2, 0–0.75 |
mg/dL |
| Serum glutamic oxaloacetic transaminase | 82.4 | 0–32 | U/L |
| Serum glutamic pyruvic transaminase | 109.1 | 0–33 | U/L |
| Urea | 4.7 | 16.6–48.5 | mg/dL |
| Creatinine | 0.9 | 0.7–1.4 | mg/dL |
| Serum intact parathyroid hormone | 9.30 | 15–65 | pg/mL |
Outcome and follow-up
After discharge, on her first follow-up after 2 weeks, she was asymptomatic. The blood investigations are as mentioned in table 4. She was continued on oral calcium, magnesium, vitamin D, diuretics, beta blockers and ACE inhibitors. Her LVEF improved from 20% to 50% over 3 months. There were no episodes of palpitation, presyncope or syncope. A repeat BMV was done after 4 months. Her mitral valve area increased from 0.8 cm2 to 1.7 cm2 (figure 6). She tolerated the procedure well and was discharged after 3 days.
Table 4.
Investigations at the time of follow-up
| Investigations | Observed value | Reference range | Units |
| Serum calcium | 9.2 | 8.6–10 | mg/dL |
| Serum phosphorous | 5.7 | 2.5–4.5 | mg/dL |
| Serum magnesium | 2 | 1.7–2.6 | mg/dL |
| Total bilirubin, direct bilirubin, indirect bilirubin | 0.88, 0.28, 0.60 | Upto 1.2,<0.2, 0–0.75 |
mg/dL |
| Serum glutamic oxaloacetic transaminase | 17.2 | 0–32 | U/L |
| Serum glutamic pyruvic transaminase | 8.3 | 0–33 | U/L |
| Alkaline phosphatase | 63 | 60–170 | U/L |
Figure 6.
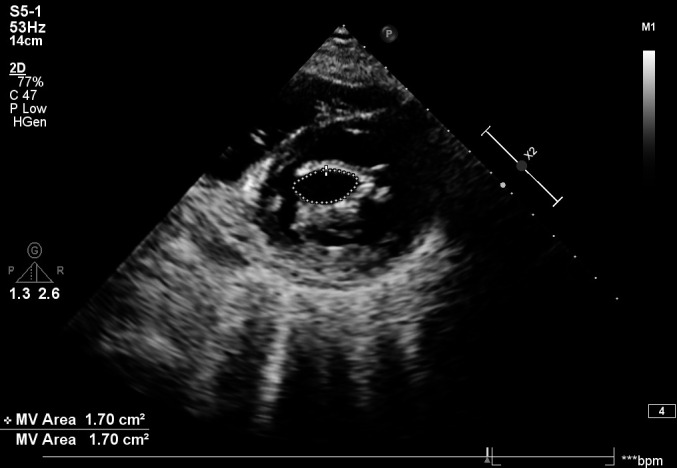
Post BMV 2D transthoracic echocardiogram; MVOA : 1.7 cm2. BMV, balloon mitral valvotomy; bpm, beats per min; MV, mitral valve; MVOA, mitral valve orifice area.
Discussion
TdP is a unique form of polymorphic VT. Harrap’s New Shorter French and English Dictionary, 1967 shows how the word torsades de pointes is described, ‘torsade’ means twisted fringe and ‘pointe’ means point.4 QT prolongation is almost always a forerunner of TdP. QTc >450 ms in males and >460 ms in females is considered prolonged and >500 ms is at higher risk of developing TdP.5 TdP is precipitated by conditions causing prolongation of QT intervals which can be classified as primary (idiopathic) and secondary causes. Primary causes include Jervell-Lange-Nielsen syndrome and Romano-Ward syndrome.5 Secondary or acquired causes are most often drug induced mainly antiarrhythmics, antipsychotics, antiemetics, antifungals, antimicrobials and dyselectrolytaemia due to hypokalaemia, hypomagnesaemia and hypocalcaemia.6 TdP can be self-limiting or can degenerate to VF and cause sudden cardiac arrest.
Our patient presented with palpitation and breathlessness and had a sudden cardiac arrest in the ward which was thought to be due to AF with fast ventricular rate producing haemodynamic instability. In the intensive care unit, while continuously monitoring, her cardiac rhythm revealed TdP due to prolongation of QTc (514 ms) resulting in cardiac arrest. This changed the course of treatment of the patient.
The serum biochemistry revealed hypomagnesaemia (1.6 mg/dL), hypocalcaemia (8.2 mg/dL) and hypokalaemia (3.37 mg/dL) which was eventually corrected with intravenous magnesium, calcium and potassium infusions. Patient had two more cardiac arrest during the course of correction of dyselectrolytaemia from which she was revived.
Serum Mg2+ level <1.8 mg/dL is referred as hypomagnesaemia. The cause of hypomagnesaemia in this patient could be due to the nutritional deficiency or chronic diuretic usage. Profound hypomagnesaemia result in functional hypoparathyroidism leading to impaired synthesis and secretion of parathormone. This causes hypocalcaemia.7 Polymorphic VT due to QT interval prolongation can be a life-threatening consequence of hypomagnesaemia. LV dysfunction in this patient could be due to tachyarrhythmia-induced cardiomyopathy which recovered promptly after the correction of dyselectrolytaemia.
Patient’s perspective.
I was known to have a heart condition called rheumatic heart disease causing mitral stenosis, and had underwent balloon opening of my heart valve in the past. This time I came to the hospital with fever, palpitations and breathing difficulty. I was admitted in the hospital ward for evaluation and blood tests were done.
On the fifth day, I woke up early in the morning, with a sense of dread and apprehension. I was trying to get out of my bed, but I felt giddy and had a blackout. I could not remember afterwards. Once I recovered my consciousness, I found myself in the bed with the beeping sound and pain over the chest and had a tube in my mouth and connected to a machine. I realised I’m in the intensive care unit (ICU). Many doctors and nurses were monitoring me and reassuring me. Lots of infusions were connected to both of my hands, so I felt I was in critical shape. Nurses were consoling me as I seemed apprehensive.
I continued to be in the ICU for almost 2 weeks with the oxygen tube in my mouth. I remember getting shocked twice during this time and later when I woke up, I felt burning sensation and pain over my chest. I thought during the ICU stay whether at all I could go back to my normal life.
Finally, I was disconnected from oxygen tube and all infusions. I was shifted to room and rehabilitated and was sent home. I don’t have words to express my gratitude for all the sustained efforts put by the doctors and nurses who took care of me and gave me a second life.
Four months later, I was called back for another session of balloon dilatation of heart valve and it went uneventful. Now, I am asymptomatic.
Learning points.
In rheumatic heart disease with mitral valve disease, the most common arrhythmia is of atrial origin and ventricular arrhythmias are rare. Ventricular arrhythmias are life-threatening and needs to be treated immediately, but also a thorough work-up for the cause is important to prevent recurrence.
Magnesium deficiency leads to QT interval prolongation due to Na+–K+ pump inhibition and change in membrane potential predisposes to life-threatening polymorphic ventricular tachycardia or torsades de pointes.
Hypoparathyroidism should be kept in mind in the presence of hypomagnesaemia. As in this case, the aetiology of functional hypoparathyroidism was magnesium deficiency, which was corrected with magnesium supplementation.
Footnotes
Contributors: JJ: resuscitation and critical care management, compiling of information needed for the case report. SR: resuscitation and critical care management, compiling of information needed for the case report. SK: overall management of the patient and supervision of the case report. DJ: management, formatting and supervision of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Rude RK, Rude Robert K. Physiology of magnesium metabolism and the important role of magnesium in potassium deficiency. Am J Cardiol 1989;63:G31eG34. 10.1016/0002-9149(89)90216-6 [DOI] [PubMed] [Google Scholar]
- 2.Cohen L, Laor A, Kitzes R. Prolonged Q-Tc interval and decreased lymphocyte magnesium in congestive heart failure. Magnesium 1984;3:164e168. [PubMed] [Google Scholar]
- 3.Rude RK, Oldham SB, Singer FR. Functional hypoparathyroidism and parathyroid hormone end-organ resistance in human magnesium deficiency. Clin Endocrinol 1976;5:209–24. 10.1111/j.1365-2265.1976.tb01947.x [DOI] [PubMed] [Google Scholar]
- 4.Krikler DM, Curry PV. Torsade de pointes, an atypical ventricular tachycardia. Heart 1976;38:117–20. 10.1136/hrt.38.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang C-E. Congenital and acquired long QT syndrome. current concepts and management. Cardiol Rev 2004;12:222–34. 10.1097/01.crd.0000123842.42287.cf [DOI] [PubMed] [Google Scholar]
- 6.Roden DM. A practical approach to torsade de pointes. Clin Cardiol 1997;20:285–90. 10.1002/clc.4960200318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis [published correction appears in Clin J Am Soc Nephrol. 2015 Oct 7;10(10):1886-7]. Clin J Am Soc Nephrol 2015;10:1257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]