Abstract
We present a case of non-arteritic anterior ischaemic optic neuropathy (NAION) with no ocular or systemic risk factors in a patient who recovered from a recent SARS-CoV-2 pneumonia. NAION is the most common acute optic neuropathy among individuals over 50 years of age. It results from a transient hypoperfusion of the optic nerve head circulation, especially in patients with low vascular compliance due to ocular or systemic risk factors. We attribute the ophthalmological condition to a SARS-CoV-2 virus-associated endotheliopathy that can be prevented with timely protection of endothelial function with vitamins D and K2.
Keywords: COVID-19, eye, capillary, cranial nerves
Background
Late complications, mostly cerebral and cardiac, after COVID-19 pneumonia are reported in increasing numbers. These have been linked with hypercoagulability and associated lupus anticoagulant antibodies. However, endothelial dysfunction has also been implied. Non-arteritic anterior ischaemic optic neuropathy (NAION) has not been reported as a sequela of COVID-19 so far.
Case presentation
A 64-year-old Caucasian man presented at the emergency eye clinic with a 4-day history of right eye visual field reduction. Four weeks earlier, he had been discharged after 5-day hospitalisation for COVID-19 pneumonia. In summary, the patient was admitted to hospital because of dyspnoea 1 week after first symptoms of COVID-19. CT of the thorax showed typical ground-glass opacities with a score 15 of 25, compatible with COVID-19 pneumonia. PCR was positive for SARS-CoV-2.
For 4 days he was treated with high-flow nasal cannula oxygen without the need for mechanical ventilation or intubation. He was further treated with hydroxychloroquine (2×400 mg a day for 6 days) and acetaminophen (4×1 g a day for 5 days). Oxygen saturation was always above 90%. Blood pressure during hospitalisation ranged from 123/64 mm Hg to 136/82 mm Hg. No other therapy was given. The patient used no medication, is a non-smoker, had no previous ophthalmological disease, no diabetes, no obesity, no hypercholesterolaemia nor other systemic diseases. No ocular or systemic risk factors for NAION were present.
Investigations
Ophthalmological findings showed a relative afferent pupillary defect in the right eye, relative desaturation of colour perception and unilateral sectorial papillary oedema (figure 1). Visual acuity was preserved. No crowded disc was observed in the other eye. Automatic perimetry demonstrated an inferior altitudinal defect (figure 2). Fluorescein angiography excluded cilioretinal artery occlusion or embolic choroidal hypoperfusion (figure 3). Laboratory investigations are shown in table 1.
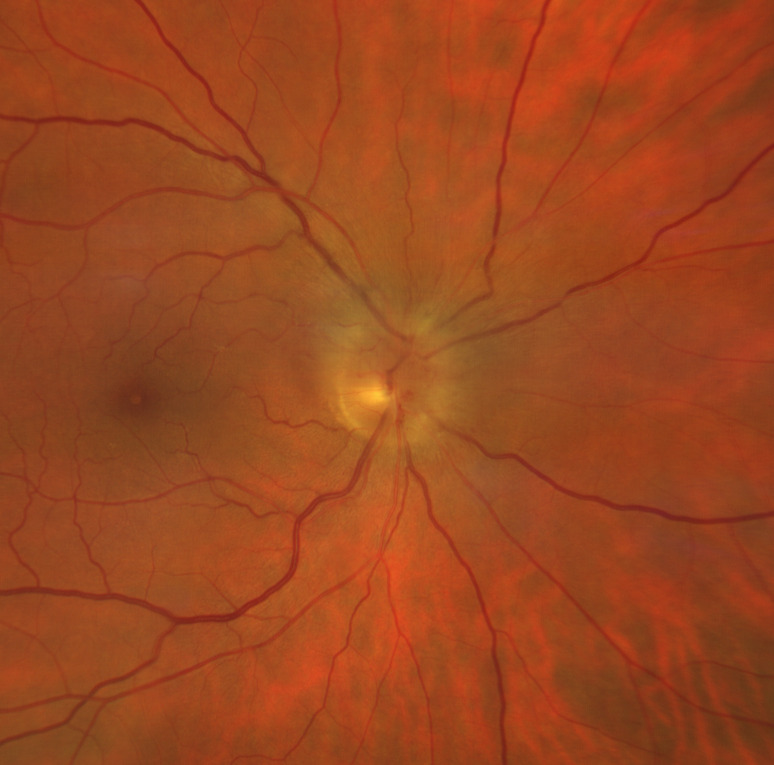
Figure 1.
Fundus examination of right eye shows a pale optic nerve disc with sectorial swelling, dilated capillaries in the disc surface and some intrapapillary haemorrhages. The macula appears within normal limits with no signs of cilioretinal artery occlusion.
Figure 2.
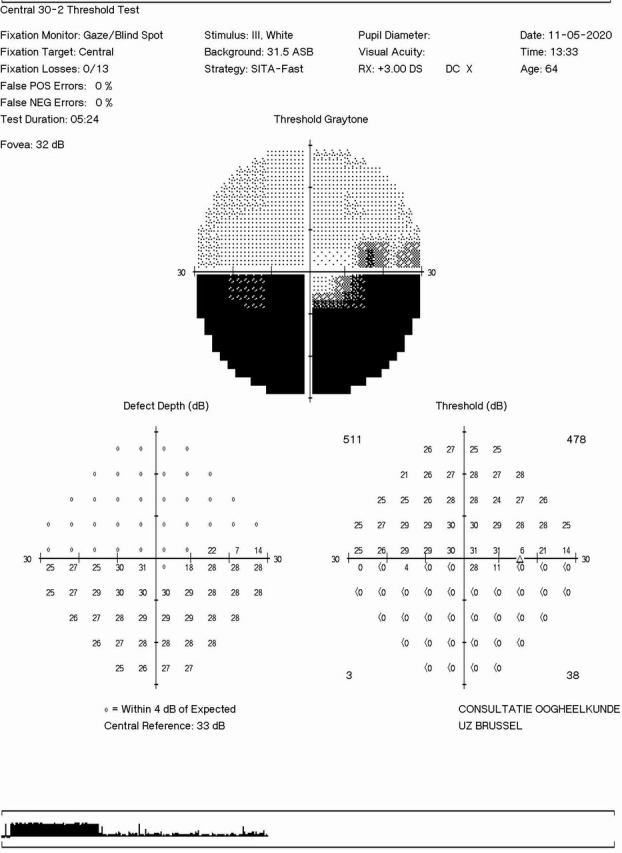
Inferior altitudinal defect in right visual field at the presentation in the eye clinic.
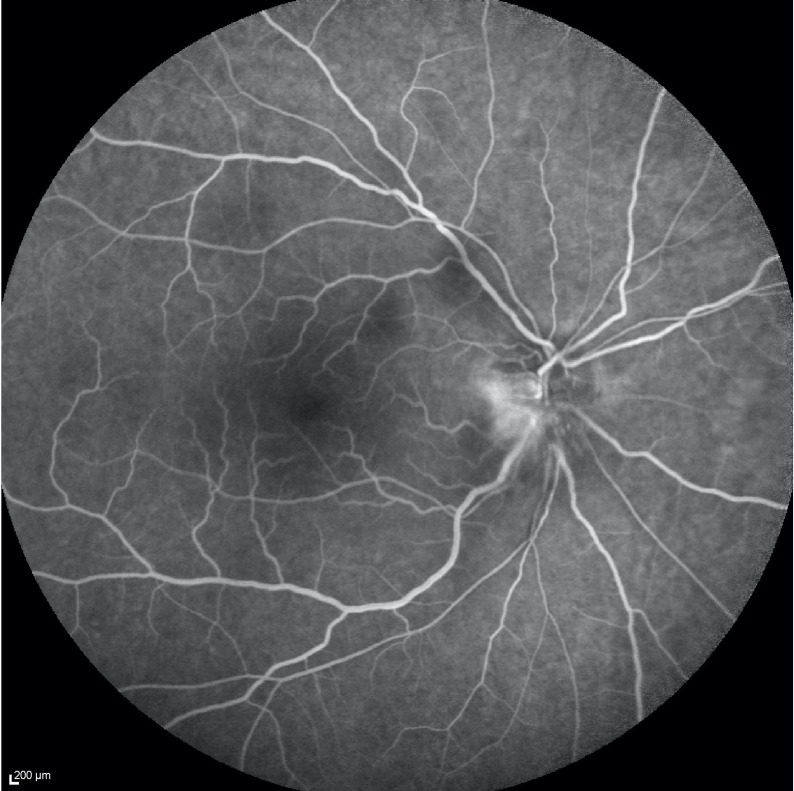
Figure 3.
Fluorescein angiography of the right eye shows optic nerve fluorescein leak from superficial dilated capillaries during the retinal venous phase and the classical hyperfluorescence of superficial and deep portions of the optic disc in the late phase. No signs of cilioretinal artery occlusion were found.
Table 1.
Laboratory findings during hospital admission and discharge for COVID-19 pneumonia, at eye clinic presentation and at follow-up
| Laboratory findings | Hospital admission for COVID-19 pneumonia | Hospital discharge for COVID-19 pneumonia | Eye clinic presentation | Follow-up | Normal range |
| PCR detection of COVID-19 (SARS-CoV-2) | Positive | ||||
| Clinical chemistry blood | |||||
| Glucose (mg/dL) | 120 ! | 70–100 | |||
| pH | 7.487 ! | 7.35–7.45 | |||
| PaCO2 (mm Hg) | 30.6 ! | 35–45 | |||
| PaO2 (mm Hg) | 87.7 ! | 90–100 | |||
| Bicarbonate (mmol/L) | 23.1 | 22–26 | |||
| Total CO2 (mmol/L) | 24.1 | 22–27 | |||
| Base excess (mmol/L) | 0.7 | −2 +2 | |||
| Oxygen content (%) | 96.8 | >97% | |||
| Lactate (mmol/L) | 0.8 | 0.7–2.1 | |||
| Urea (mg/dL) | 32 | 28 | 19–43 | ||
| Creatinine (IDMS-norm) (mg/dL) | 0.92 | 0.89 | 0.87 | 0.95 | 0.67–1.17 |
| eGFR (MDRD-IDMS) (mL/min/1.73 m²) | >60 | >60 | >60 | >60 | >60 |
| eGFR (CKD-EPI-IDMS) (mL/min/1.73 m²) | 88 | 90 | 91 | 84 | >60 |
| Ionogram | |||||
| Sodium (mmol/L) | 136 ! | 141 | 137–145 | ||
| Potassium (mmol/L) | 3.7 | 3.7 | 3.4–5.0 | ||
| Chloride (mmol/L) | 101 | 100 | 98–107 | ||
| Calcium (mmol/L) | 2.18 | 2.08 ! | 2.10–2.55 | ||
| Bicarbonate (mmol/L) | 25 | 32 | 22–30 | ||
| Anion gap (mmol/L) | 14 | 13 | |||
| CRP (mg/L) | 170.2 ! | 145.5 ! | 12.7 ! | 0.8 | <5 |
| Creatine kinase (U/L) | 75 | 86 | 107 | 55–170 | |
| Lactate dehydrogenase (U/L) | 888 ! | 654 ! | 481 | 313–618 | |
| Aspartate aminotransferase (U/L) | 57 | 37 | 17–59 | ||
| Alanine aminotransferase (U/L) | 55 | 55 | 21–72 | ||
| Alkali aminotransferase (U/L) | 55 | 55 | 29–33 | ||
| Gamma-glutamyl transferase (U/L) | 45 | 9–48 | |||
| Cholesterol (mg/dL) | 165 | <190 | |||
| HDL-cholesterol (mg/dL) | 50 | >40 | |||
| Non-HDL cholesterol (mg/dL) | 114 | <130 | |||
| LDL cholesterol (mg/dL) | 108 | <100 | |||
| Triglyceride (mg/dL) | 118 | <150 | |||
| Bilirubin total (mg/dL) | 0.67 | 0.2–1.3 | |||
| Haematological profile | |||||
| Platelet (x109/L) | 215 | 305 | 217 | 219 | 158–450 |
| Red blood cell (x1012/L) | 5.0 | 4.4 | 4.4 | 5.0 | 4.2–5.7 |
| Haemoglobin (g/L) | 144 | 130 | 150 | 151 | 130–165 |
| Haematocrit (%) | 43.9 | 39.4 ! | 45.2 | 45.3 | 39.6–49.2 |
| MCV (fL) | 88.5 | 89.8 | 91.3 | 83.0–98.0 | |
| MCH (pg) | 29.0 | 29.6 | 30.3 | 27.0–33.0 | |
| MCHC (g/dL) | 32.8 | 32.9 | 33.2 | 33.2 | 32.0–35.0 |
| White cell count (×10³/mm³) | 5.4 | 6.3 | 5.5 | 5.8 | 3.6–9.6 |
| Formula | |||||
| Neutrophile (%) | 68.7 | 66.8 | 60.0 | 59.8 | 41.0–74.0 |
| Basophile (%) | 0.1 | 0.2 | 0.3 | 0.2 | 0.0–2.0 |
| Eosinophile (%) | 1.5 | 3.0 | 3.1 | 2.6 | 0.0–6.0 |
| Lymphocyte (%) | 16.4 ! | 17.2 ! | 26.3 | 26.5 | 19.0–44.0 |
| Monocyte (%) | 13.3 ! | 12.8 | 10.3 | 10.9 | 3.0–13.0 |
| Erythrocyte sedimentation velocity (mm/hour) | 10 | 4 | 0–10 | ||
| Haematocrit (%) | 46.6 | 39.6–49.2 | |||
| Coagulation profile | |||||
| PT (%) | 80 | 104 | >70 | ||
| PT (INR) | 1.2 | 1.0 | 0.8–1.3 | ||
| APTT (s) | 29.8 | 22.2–34.4 | |||
| Von Willebrand factor antigen (%) | 104 | >52 | |||
| D-dimer (ng/mL) | 832 ! | 300 | <215 | <500 | |
| Lupus anticoagulant | Negative | ||||
| Cardiolipine antibody IgG (U/mL) | <2.6 | <20 | |||
| Cardiolipine antibody IgM (U/mL) | 4.2 | <20 | |||
| Radioimmunology | |||||
| Hydroxy vitamin D (µg/L) | 11.4 ! | 22.3 | 20–50 | ||
| Ferritin (µg/L) | 411 ! | 113 | 30–400 | ||
| Serology | |||||
| Mycoplasma pneumonia IgM–IgG | Negative | ||||
| Humoral immunology | |||||
| Antinuclear antibody | Negative | ||||
| Antineutrophilic cytoplasm antibody | Negative | ||||
APTT, activated partial thromboplastin time; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IDMS, isotope-dilution mass spectrometry; INR, international normalised ratio; LDL, low-density lipoprotein; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; MDRD, Modification of Diet in Renal Disease; n/a, not available; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; PT, prothrombin time.
Differential diagnosis
Embolism of short posterior ciliary arteries of optic nerve head is a rare cause of NAION.1 Embolic occlusion of these arteries is seen on fluorescein fundus angiography as hypoperfusion choroidal area. Fluorescein fundus angiography did not show signs of short posterior ciliary artery embolism (figure 3).
A hypercoagulable state due to antiphospholipid antibodies2 was ruled out. Also, anticardiolipin and lupus anticoagulant were negative, D-dimer had normalised and Von Willebrand factor antigen was normal (table 1).
Arteritic anterior ischaemic optic neuropathy (AION) is a mandatory differential diagnosis to rule out in patient presenting with an ischaemic optic neuropathy.1 The primary cause of AION is giant cell arteritis (GCA).1
The differential diagnosis between NAION and GCA of posterior ciliary arteries is based on systemic and clinical features.
The patient had no clinical signs of GCA, that is, no jaw claudication, no headache, no scalp tenderness or other features.
Erythrocyte sedimentation rate increased in GCA was completely normal the day of the patient’s presentation at the eye clinic (10 mm/hour) and in 2-month follow-up time (4 mm/hour). At presentation, C reactive protein (CRP) was within normal range (12.7 mg/L) and significantly decreased compared with hospitalisation value (170.2 mg/L). At 2-month follow-up, CRP was completely normal (0.8 mg/L). Thus, the systemic and clinical features do not support GCA.
Treatment
Acute management of AION requires systemic corticosteroid therapy in arteritic AION but not in NAION.1 1No systemic corticosteroid therapy was started in our patient.
Vitamin D deficiency was treated with oral supplementation. At cardiological screening, 8 weeks after hospital discharge, a moderate systemic hypertension (150/90) was measured. Valsartan was started with intake in the morning to avoid nocturnal hypotension (80 mg per day) together with aspirin (80 mg per day) as secondary cardiovascular protection.
Outcome and follow-up
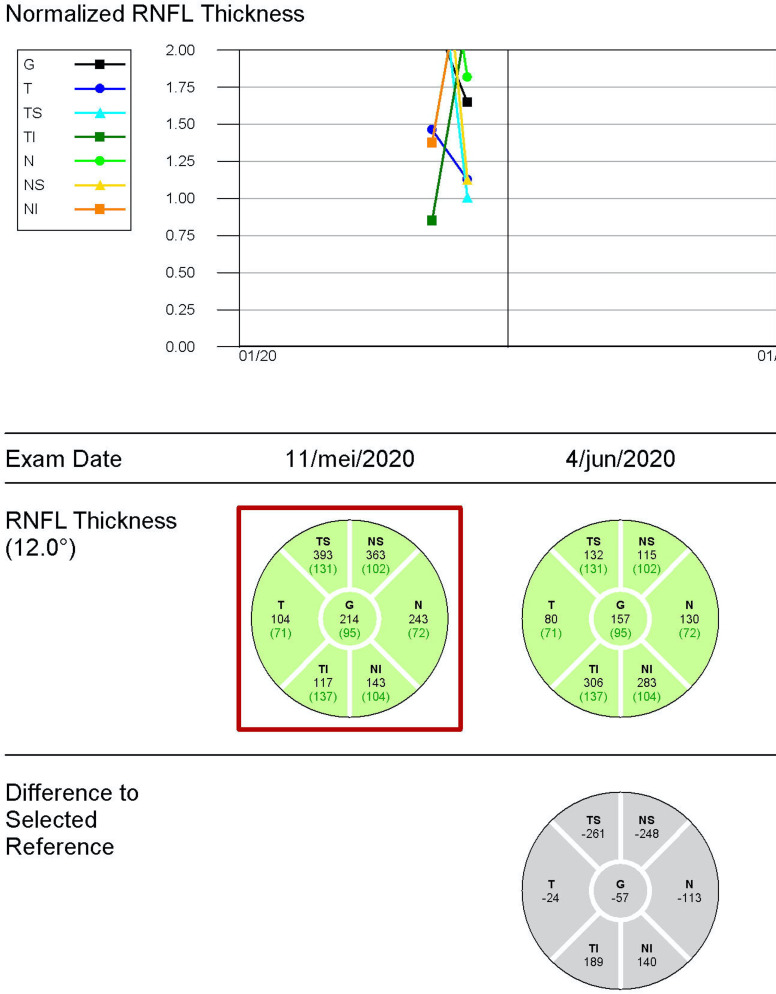
During 2-month follow-up, the visual acuity remained 20/20 (0.0 logMAR) in both eyes. Optic disc oedema decreased (optical coherence tomography/OCT) (figure 4) and optic disc pallor developed (figure 5). The automatic visual field at 1-month follow-up showed a generalised reduction of light perception threshold in the residual upper hemifield (figure 6). Visual fields remained stable at 2-month follow-up with moderate improvement of mean deviation value (figure 6). Macular ganglion cell layer analysis with OCT showed a progressive decrease in thickness in the right eye at 2-month follow-up (figure 4). Vitamin D was within normal range (22.3 µg/L) at 2-month follow-up.
Figure 4.
Pseudo-thickening of the nerve fibre layer of the right eye due to papillary oedema. The abnormal thickening reduced in 2-month follow-up examination. RNFL, retinal nerve fibre layer.
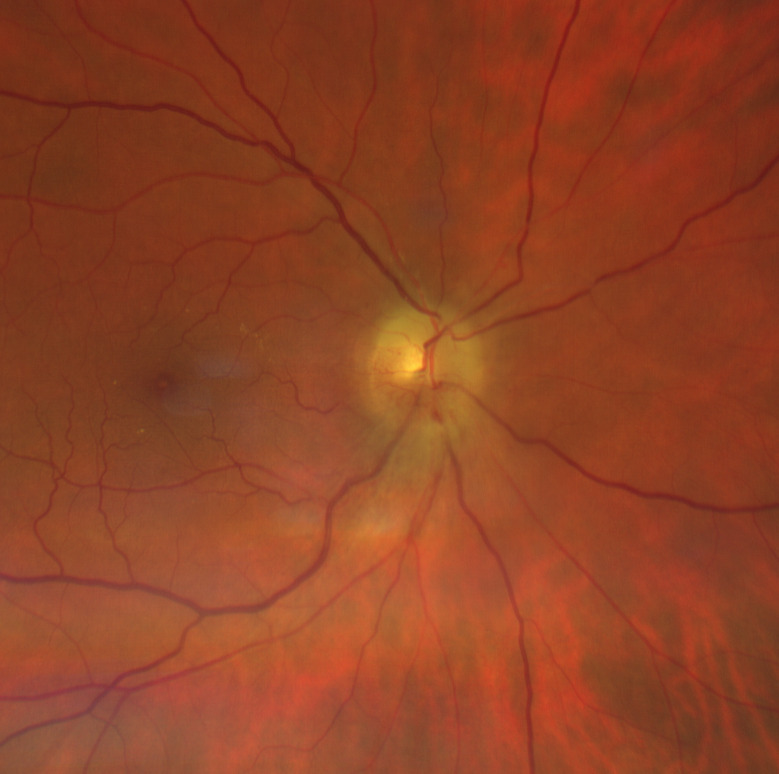
Figure 5.
At 2-month follow-up, superior swelling was reduced but inferior part was also involved. This aspect is highly suggestive of papillary oedema in progressive non-arteritic anterior ischaemic optic neuropathy. The macula appears within normal limits with no signs of cilioretinal artery occlusion.
Figure 6.
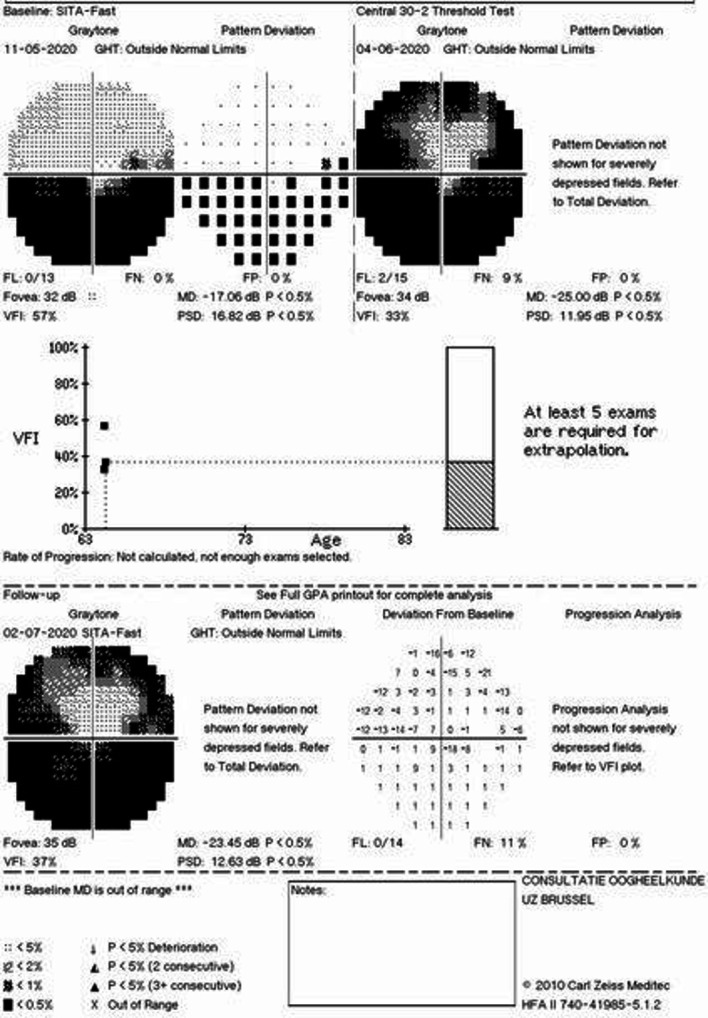
Generalised reduction of light perception threshold in 1-month follow-up with preserved central and paracentral superior visual field area. Moderate improvement in mean deviation parameter in 2-month follow-up examination. MD: mean deviation, PSD: pattern standard deviation, dB: decibel
Discussion
We attribute the ophthalmological condition in our case to SARS-CoV-2 virus-associated endotheliopathy.
Emerging studies over SARS-CoV-2 virus and COVID-19 disease suggest that endotheliopathy is a central feature of its pathophysiology and leads to severe vascular systemic sequelae by loss of vascular compliance2 including at the level of optic nerve.3
Savastano et al3 have recently reported the impact of SARS-CoV-2 infection on the microvascular network of optic nerve head in patients who recovered from COVID-19, 4 weeks after discharge. It results in an impairment in the blood supply to the peripapillary retinal nerve fibre layer characterised by a reduction of radial peripapillary capillary plexus density that has been previously correlated to visual field loss in NAION.2
Furthermore, a recent study over lung perfusion abnormalities in patients with COVID-19 proposed possible underlying mechanisms, not only microthrombosis but also perfusion defects caused by vasoconstriction that brings to subsequential SARS-CoV-2-induced vasoplegia.4 The transient hypoperfusion of optic nerve head circulation is indeed the most common cause of NAION.1
No treatment is available to restore visual function in NAION.1 Therefore, prevention is paramount. Endothelial function and integrity can be improved by vitamins D and K2.5–9 Moreover, there is support that vitamin D deficiency is particularly common in patients with COVID-19 developing endothelial dysfunction sequels elsewhere.5 6 Vitamin K2 status may also be reduced in patients with COVID-19 and related to poor prognosis.7 8 No test is available to measure vitamin K2 status, but vitamin D level in our patient was markedly reduced (11.4 µg/L).
Based on the growing understanding of SAR-CoV-2 endotheliopathy, we propose considering endothelial reserve in patients with COVID-19 and initiating prophylactic protection to prevent ophthalmic and other systemic sequelae at the earliest moment of diagnosis.
Patient’s perspective.
I was hospitalized with a corona infection from March 30 to April 3. On April 3, my saturation value was 96. I had to stay in quarantine for 14 days at home. On 11 May I got up and immediately noticed that my right eye was malfunctioning. I only had a partial view. I could not make visual observations with the bottom half of my right eye. My left eye was functioning normally. I have not experienced any headaches or pain in my right eye. After the consultation of 4 June 2020, two medicines were prescribed: Valsartan and Aspirin. I have also been following a vitamin D cure since 4 June 2020. The diminished vision in the right eye has stabilized. The left eye is functioning normally. At the consultation on 2 July it was established that vision in the right was slightly improved. However, I was not aware of that improvement.
Learning points.
Endotheliopathy is a central feature of COVID-19 disease.
SARS-CoV-2 virus can impact the vascular compliance of the optic nerve head with a reduction of radial peripapillary capillary plexus density that can result in non-arteritic anterior ischaemic optic neuropathy.
Endothelial protection by vitamins D and K2 should be considered in all patients with COVID-19.
Intervention studies are needed to assess the effect of the administration of vitamins D and K2 on reducing the severity and complications of COVID-19 pneumonia.
Footnotes
Contributors: LM made substantial contribution examining the patient and drafting the article. GF contributed to writing the article. RWK made the conceptual input for the article, scientific interpretation and writing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Hayreh S. Management of ischemic optic neuropathies. Indian J Ophthalmol 2011;59:123–36. 10.4103/0301-4738.77024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 2020;7:e575–82. 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savastano A, Crincoli E, Savastano MC, et al. Peripapillary retinal vascular involvement in early Post-COVID-19 patients. J Clin Med 2020;9:2895. 10.3390/jcm9092895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santamarina MG, Boisier Riscal D, Beddings I, et al. COVID-19: what iodine maps from perfusion CT can reveal-A prospective cohort study. Crit Care 2020;24:619. 10.1186/s13054-020-03333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanham-New SA, Webb AR, Cashman KD, et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr Prev Health 2020;3:106–10. 10.1136/bmjnph-2020-000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D-H, Meza CA, Clarke H, et al. Vitamin D and endothelial function. Nutrients 2020;12:575. 10.3390/nu12020575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dofferhoff ASM, Piscaer I, Schurgers LJ, et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin Infect Dis 2020:ciaa1258. 10.1093/cid/ciaa1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goddek S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int J Infect Dis 2020;99:286–90. 10.1016/j.ijid.2020.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Yin J, Ding H, et al. Vitamin K2 ameliorates damage of blood vessels by glucocorticoid: a potential mechanism for its protective effects in glucocorticoid-induced osteonecrosis of the femoral head in a rat model. Int J Biol Sci 2016;12:776–85. 10.7150/ijbs.15248 [DOI] [PMC free article] [PubMed] [Google Scholar]