Abstract
Cholesterol 7 alpha-hydroxylase (CYP7A1, EC1.14) is the first and rate-limiting enzyme in the classic bile acid synthesis pathway. Much progress has been made in understanding the transcriptional regulation of CYP7A1 gene expression and the underlying molecular mechanisms of bile acid feedback regulation of CYP7A1 and bile acid synthesis in the last three decades. Discovery of bile acid-activated receptors and their roles in the regulation of lipid, glucose and energy metabolism have been translated to the development of bile acid-based drug therapies for the treatment of liver-related metabolic diseases such as alcoholic and non-alcoholic fatty liver diseases, liver cirrhosis, diabetes, obesity and hepatocellular carcinoma. This review will provide an update on the advances in our understanding of the molecular biology and mechanistic insights of the regulation of CYP7A1 in bile acid synthesis in the last 40 years.
Keywords: Cholesterol 7 alpha-hydroxylase (CYP7A1), Bile acid metabolism, Farnesoid X receptor (FXR), Takeda G protein-coupled receptor 5 (TGR5), Bile acid receptors, Liver metabolism
1. Introduction
Cholesterol 7 alpha-hydroxylase (CYP7A1, EC1.14) is the first and rate-limiting enzyme in the bile acid synthesis pathway. This enzyme is exclusively expressed in the endoplasmic reticulum of hepatocytes and is regulated by the end products, bile acids, returning to the liver via enterohepatic circulation. In 1977, Myant and Mitropoulos1 published the first exclusive review on CYP7A1. Since then, tremendous progress has been made regarding the cloning and characterization of the CYP7A1 gene, the molecular mechanisms of bile acid feedback regulation of CYP7A1 gene expression, the role of bile acid-activated receptor signaling in the regulation of metabolism, and bile acid based-drug therapies for cholestasis and metabolic diseases. This review will provide an in-depth update on CYP7A1 and other sterol hydroxylases in bile acid synthesis pathways.
In early 1990, a breakthrough was achieved in the purification and cloning of the gene coding Cyp7a1.2–4 Availability of specific antibodies and cDNAs helped elucidate the molecular regulatory mechanisms of Cyp7a1 gene transcription. Ten years later, a nuclear receptor, farnesoid X receptor (FXR),5–8 and a membrane G protein-coupled receptor (GPBAR-1, also known as Takeda G protein-coupled receptor 5, TGR5) were identified as bile acid-activated receptors, and the roles of FXR and TGR5 in the regulation of bile acid synthesis, transport and metabolic homeostasis were unveiled.9–20 Several genetically modified mouse models were generated for the study of bile acid synthesis and the regulation of lipid, glucose and energy metabolism.21–26 Distinct species differences in bile acid synthesis and composition between mice and humans have been recognized.20,27 Rodents (rats and mice) synthesize more cholesterol, and they clear and catabolize cholesterol to bile acids more efficiently than humans.28 Mice have higher serum high density lipoprotein (HDL)-cholesterol and very little low density lipoprotein (LDL)-cholesterol compared to humans. Mice also produce 6-hydroxylated muricholic acids as the major primary bile acid in the liver and they produce a larger, more hydrophilic bile acid pool compared to humans.29 In contrast, the human bile acid pool and composition are highly hydrophobic. Different bile acid species have different efficacies for biliary cholesterol secretion, intestinal fat absorption and feedback regulation of bile acid synthesis and signaling. Thus, the species differences in bile acid composition and regulation of metabolism and homeostasis have been a challenge to the translation of results from animal studies to human physiology and pathophysiology.30 Nevertheless, research in bile acid synthesis and signaling using novel mouse models has been crucial for the development of bile acid-based drugs to treat cholestatic liver diseases and metabolic liver diseases such as diabetes, obesity and alcoholic and non-alcoholic fatty liver diseases.17,19,20,31,32 This review will cover the latest advances regarding CYP7A1 and other sterol hydroxylases in bile acid synthesis in mice and humans, and the roles of these enzymes in human health and disease.
2. CYP7A1 and sterol hydroxylases in bile acid synthesis
There are 17 enzymes involved in the synthesis of bile acids from cholesterol which occurs in two main pathways: the classic (or neutral) pathway, which is developed after weaning and becomes the major pathway for bile acid synthesis in humans, and the alternative (or acidic) pathway, which may be the major bile acid synthesis pathway in the neonate.33 The classic pathway is more important in humans and the alternative pathway is more active in mice and rabbits compared to humans.34–36 Bile acid synthesis pathways have been reviewed previously.16,19,20,37,38 Several cytochrome P450 (CYP) hydroxylase enzymes are involved in bile acid synthesis; here, only the key regulatory enzymes in the classic and alternative pathways are shown in Fig. 1.
Fig. 1. Bile acid and oxysterol synthesis in the liver and other tissues.
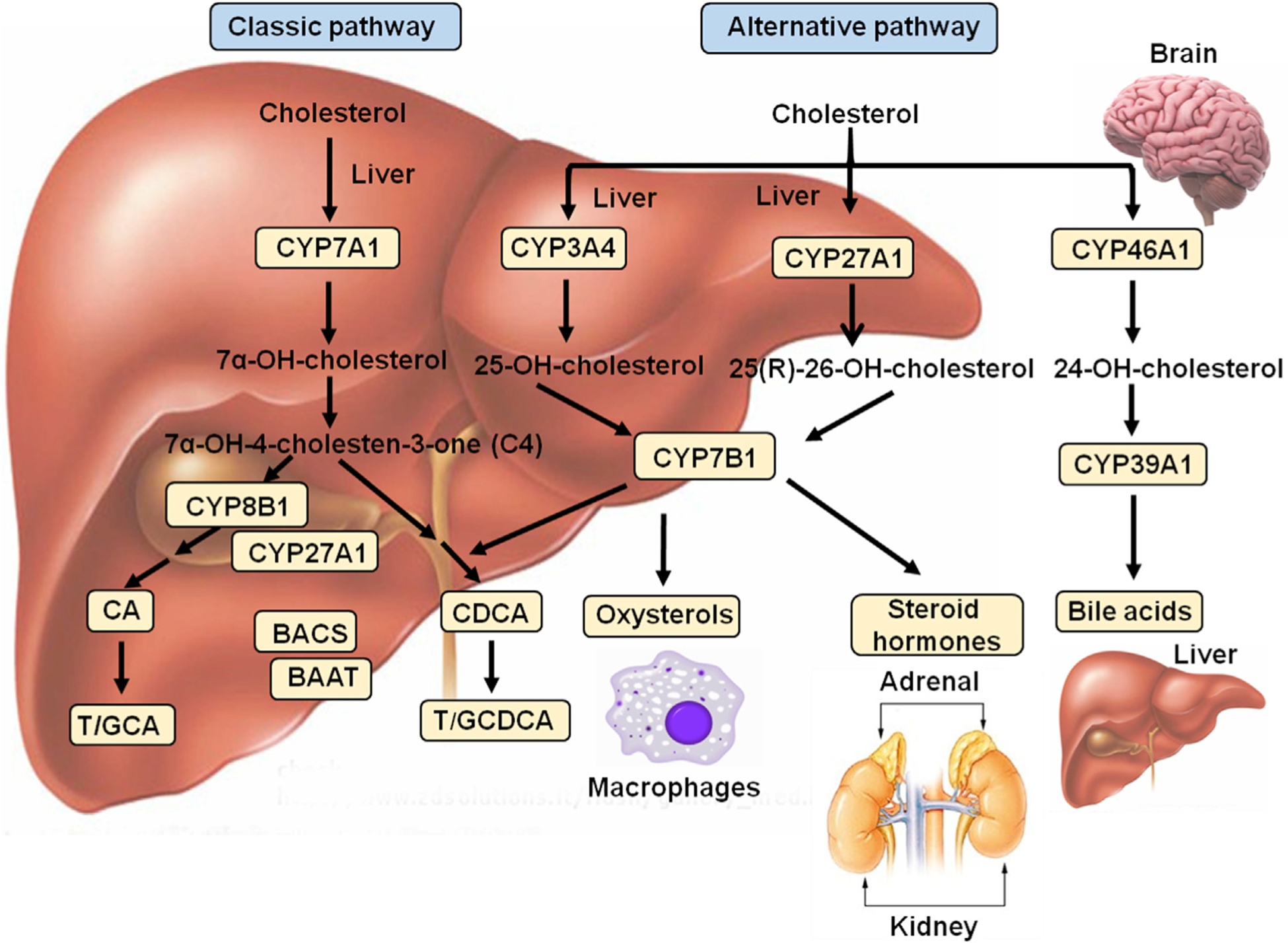
The liver is the only organ that expresses all the enzymes required for bile acid synthesis in the classic bile acid synthesis pathway. The alternative pathways exist in macrophages, adrenal and brain. The classic pathway of bile acid synthesis from cholesterol is catalyzed by cholesterol 7 alpha-hydroxylase (CYP7A1). 7α-hydroxycholesterol is then converted to 7α-hydroxy-4-cholesten-3-one (C4), which is the common precursor for cholic acid (CA) and chenodeoxycholic acid (CDCA) synthesis. C4 is 12α-hydroxylated by sterol 12α-hydroxylase (CYP8B1), producing CA. Without CYP8B1, C4 is converted to CDCA. Mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes steroid side-chain oxidation to form 3α, 7α, 12α-trihydroxycholestanic acid (THCA) and 3α, 7α-dihydroxycholestanic acid (DHCA). THCA and DHCA are activated by bile acid-CoA synthase (BACS, also known as long-chain acyl-CoA synthase, SLC27A5) for peroxisomal β-oxidation reactions to cleave a propionyl-CoA to form cholyl-CoA and chenodeoxycholyl-CoA, which are conjugated to the amino acids glycine (G) or taurine (T) by bile acid-CoA: amino acid N-acyltransferase (BAAT) to form T/GCA and T/GCDCA, respectively. The alternative bile acid synthesis pathway is initiated by CYP27A1, which converts cholesterol to 25(R)-26-hydroxycholesterol (27-hydroxycholesterol) in the liver. Oxysterol 7α-hydroxylase (CYP7B1) catalyzes 7α-hydroxylation of 27-hydroxycholesterol. In the liver, CYP3A4 catalyzes 25-hydroxylation of cholesterol to 25-hydroxycholesterol, followed by hydroxylation by CYP7B1. CYP27A1 and CYP7B1 are also expressed in macrophages for oxysterol metabolism and in adrenal glands for steroid hormone synthesis. In the brain, cholesterol 24-hydroxylase (CYP46A1) hydroxylates cholesterol to 24-hydroxycholesterol, an abundant sterol in the brain, which can be transported to hepatocytes and 7α-hydroxylated by 24-hydroxysterol 7α-hydroxylase (CYP39A1) for bile acid synthesis.
The classic pathway is initiated by CYP7A1, a member of the CYP family 7, subfamily A1. Expression of CYP7A1, which is the only rate-limiting enzyme in the classic pathway, is limited to the liver and it determines the rate of bile acid synthesis. CYPs are mixed function oxidases (monooxygenases) that hydroxylate drugs, fatty acids, steroids, carcinogens and xenobiotics by adding one atom of O2 to the substrate while the second oxygen atom is reduced to water. Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome P450 reductase mediates the transfer of two electrons from NADPH to the substrate-bound CYPs. CYP7A1 is located in the cholesterol-poor endoplasmic reticulum (microsome) and is substrate-specific; that is, it only metabolizes cholesterol to 7α-hydroxycholesterol. Hydroxysteroid dehydrogenase 3B7 converts 7α-hydroxycholesterol to 7α-hydroxy-4-cholesten-3-one (C4), which is a surrogate serum marker for bile acid synthesis. C4 is the common precursor for synthesis of the two major primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA).39,40 Sterol 12α-hydroxylase (CYP8B1) is required for the synthesis of CA, which is a tri-hydroxy-bile acid. Without 12α-hydroxylation of C4, CDCA, a di-hydroxy-bile acid, is produced. Mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes steroid side-chain oxidation to form 3α, 7α, 12α-trihydroxycholestanic acid (THCA) and 3α, 7α-dihydroxycholestanic acid (DHCA). THCA and DHCA are activated by bile acid-CoA synthase (BACS, also known as long-chain acyl-CoA synthase, SLC27A5) and are transported into peroxisomes where peroxisomal β-oxidation reactions cleave a propionyl-CoA to form cholyl-CoA and chenodeoxycholyl-CoA, respectively. These bile acyl-CoAs are subsequently conjugated to the amino acids glycine (G) or taurine (T) by bile acid-CoA: amino acid N-acyltransferase (BAAT) to form T/GCA and T/GCDCA.19 In mice, most bile acids (~95%) are conjugated to taurine,41 whereas in humans bile acids are conjugated to glycine more than taurine in a ratio of about 3:1.42 Conjugation of bile acids increases bile acid solubility under physiological pH to form sodium salts, and bile acids are stored in the gallbladder until food intake stimulates the release of bile.
The alternative bile acid synthesis pathway is initiated by CYP27A1 to form 25(R)-26-hydroxycholesterol, which is 7α-hydroxylated to 3β, 7α-dihydroxy-5-cholestanoic acid by CYP7B1 for synthesis of CDCA and CA.43 CYP7B1 is widely expressed in most tissues, and oxysterols produced in extrahepatic tissues can be converted to bile acids in the liver. In steroidogenic tissues, such as the adrenal glands, CYP7B1 plays a key role in steroid sex hormone synthesis.44 Both CYP27A1 and CYP7B1 are highly expressed in macrophages, where CYP27A1 and CYP7B1 are involved in the metabolism of cholesterol to oxidized sterols (oxysterols). Oxysterols, such as 25-hydroxycholesterol and 27-hydroxycholesterol, are highly abundant in serum and liver. Microsomal cholesterol 25-hydroxylase, a non-CYP enzyme, has been implicated in the hydroxylation of cholesterol to 25-hydroxycholestrol.45 The activity of this enzyme is very low in human and mouse liver and in other tissues. More recent studies demonstrate that human CYP3A4 (mouse Cyp3a11) catalyzes the hydroxylation of cholesterol to 25-hydroxycholesterol in liver.46 CYP7B1 can also hydroxylate cholesterol to 25-hydroxycholesterol in the liver.22 CYP3A4 is the predominant CYP expressed in the liver and intestine and metabolizes more than 70% of drugs and xenobiotics.47 In the brain, CYP46A1 hydroxylates cholesterol to 24-hydroxycholesterol and is the major route for cholesterol catabolism in the brain.48 24-hydroxycholesterol is excreted through blood-brain barrier to the liver, where 24-hydroxysterol 7α-hydroxylase (CYP39A1) hydroxylates it to 7α−24-dihydroxycholesterol for bile acid synthesis.22
CA and CDCA are the two major primary bile acids synthesized in human liver, whereas CA and α-muricholic acid (α-MCA) and β-MCA are the major primary bile acids in rodents. CDCA is 6β-hydroxylated to α-MCA (3α, 6β, 7α) and β-MCA (3α, 6β, 7β) (Top, Fig. 2). Cyp2c70 is a muricholic acid 6β-hydroxylase responsible for the conversion of CDCA to α-MCA and ursodeoxycholic acid (UDCA) to β-MCA.49 Two recent studies reported that Cyp2c70 sequentially converts CDCA to α-MCA and then to β-MCA.50,51 CDCA is 6α-hydroxylated to hyocholic acid (HCA) by Cyp4a12 (3α, 6α, 7α) in humans and pigs. HCA and MCA are 6α/β-stereoisomers and are highly soluble.
Fig. 2. Primary and secondary bile acid synthesis in humans and mice.
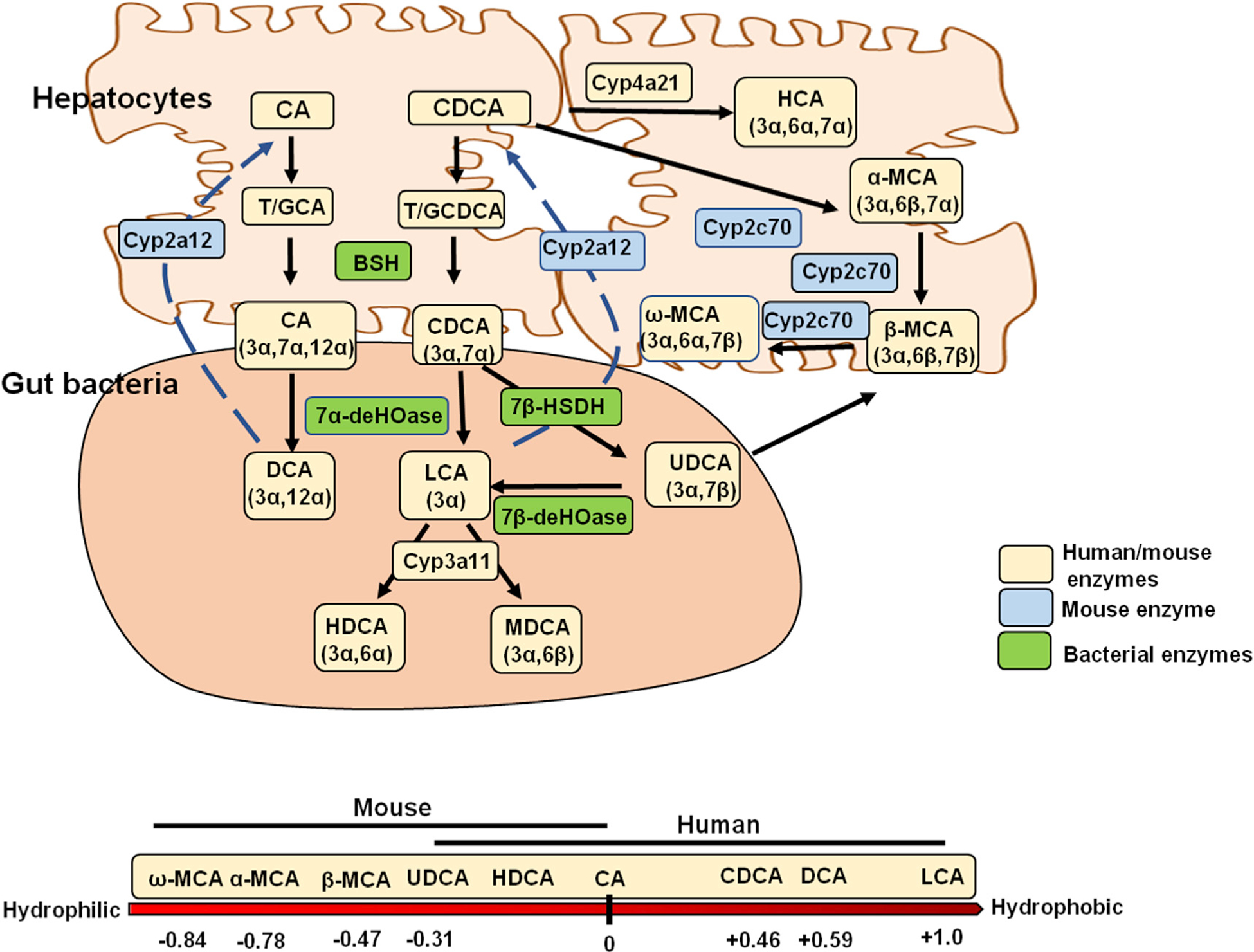
Top: The primary bile acids CA and CDCA are conjugated to taurine (T) or glycine (G) in the liver. In mice, CDCA may be further metabolized to α- and β-muricholic acid (α- and β-MCA) by Cyp2c70. CDCA can be converted to hyocholic acid by Cyp4a12 in humans and pigs. In the intestine, T/GCA and T/GCDCA are deconjugated by bacterial bile salt hydrolase (BSH) and dehydroxylated by bacterial 7α-dehydroxylase, forming deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. In mice, DCA and LCA recycled to the liver can be converted back to CA and CDCA by Cyp2a12. A small amount of CDCA is converted to UDCA in humans, and in mice UDCA can be converted to β-MCA by Cyp2c70. Bottom: Bile acid composition and hydrophobicity in humans and mice. Abbreviations: CA, cholic acid; CDCA, chenodeoxycholic acid; UDCA, ursodeoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid.
3. Bile acid biotransformation by the gut microbiota
Conjugated bile acids are deconjugated by bacterial bile salt hydrolase (BSH) mainly in the colon. Then, bacterial 7α-dehydroxylase converts CA and CDCA to the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. BSH activity is restricted to the genera Clostridium, Enterococcus, Bifidobacterium, Bacteroides, and Lactobacillus, while 7α-dehydroxylase activity mainly originates from Clostridium XIVa clusters.52,53 LCA is sulfate-conjugated and excreted into feces and urine, while some DCA is passively reabsorbed in the colon. In mice, gut bacteria convert β-MCA to ω-MCA (3α, 6α, 7β), a highly soluble bile acid, for fecal and urine excretion. CDCA (3α, 7α) is epimerized by 7β-hydroxysteroid dehydrogenase to UDCA (3α, 7β), a secondary bile acid synthesized in humans. Conversion of the 7α -HO group in CDCA to a 7β -HO group in UDCA changes a hydrophobic bile acid to a hydrophilic bile acid. In general, β-epimers and increasing the number of hydroxy groups increases bile acid solubility and decreases the hydrophobicity of bile acids (Bottom, Fig. 2). Bile acid hydrophobicity increases in the order of ω-MCA<α-MCA<β-MCA < UDCA < CA < CDCA < DCA < LCA.54
In rodents, UDCA recirculated to the liver can be converted to β-MCA by Cyp2c70, and DCA and LCA can be 7α-hydroxylated back to CA and CDCA, respectively, by Cyp2a12 (Fig. 2). Also, in rodents, LCA can be converted to hyodeoxycholic acid (HDCA) and murideoxycholic acid (MDCA) by intestinal 6α- and 6β-hydroxylase (Cyp3a11), respectively.55 In human liver microsomes, LCA is hydroxylated to HDCA by CYP3A4.56 Therefore, DCA and LCA levels are very low in rodents compared to humans. In mice deficient of Cyp2c70, MCAs are not synthesized and CDCA is increased, while in Cyp2a12 deficient mice DCA and LCA accumulate. In mice deficient of both Cyp2c70 and Cyp2a12, DCA, CDCA and LCA are all increased, and bile acid hydrophobicity is increased, similar to humans.51
4. Bile acid homeostasis
Bile acids are detergent molecules that are toxic to cells if accumulated in large amounts. Thus, bile acid synthesis and metabolism must be tightly regulated to maintain homeostasis and prevent cellular toxicity. It should be noted that bile acid composition in serum, liver, gallbladder bile, intestine and feces are different. Serum bile acids contain both conjugated and free bile acids, while bile acids in the liver and gallbladder bile are predominantly conjugated bile acids. In the ileum, most bile acids are conjugated whereas the colon contains more deconjugated secondary bile acids. The total bile acid pool consists of bile acids in liver, gallbladder bile and intestine. Serum bile acid content is typically very low (1–2%) and may not be included in measuring the total bile acid pool. With respect to the bile acid pool in mice, about 80% of bile acids are in the intestine, 15% are in the gallbladder, and the remaining bile acids (5%) are in the liver. In humans, the gallbladder and bile duct have the highest bile acid levels and decrease from the upper intestine to the colon and feces.57
4.1. Enterohepatic circulation of bile acids
Immediately after synthesis, bile acids are conjugated to glycine and taurine to increase their solubility and bile salt export pump (BSEP) excretes conjugated bile acids from hepatocytes into the biliary system. Here cholangiocytes, stimulated by release of the hormone secretin from duodenal S cells, modifying bile content via the secretion of bicarbonate, water and ions (Fig. 3).58 A small portion of bile acids are passively reabsorbed by cholangiocytes and return to hepatocytes, referred to as the cholehepatic shunt. Bile acids form mixed micelles with phosphatidylcholine and cholesterol and are stored in the gallbladder. After meal intake, bile acids are released from the gallbladder into the upper intestine where a small amount of bile acids are passively reabsorbed. In the terminal ileum, most bile acids (95%) are reabsorbed into enterocytes via apical sodium-dependent bile acid transporter (ASBT). Bile acids bind to ileum bile acid binding protein (IBABP) and are transported to the sinusoidal membrane, where the organic solute transporter α and β (OSTα and OSTβ) heterodimer effluxes bile acids to portal blood circulation. Bile acids returning to the liver are taken up by hepatic sinusoidal sodium taurocholate co-transport peptide (NTCP) to inhibit bile acid synthesis. The enterohepatic circulation of bile acids is highly efficient and maintains a consistent total bile acid pool size and composition in the gastrointestinal system.
Fig. 3. Bile acid feedback regulation of bile acid synthesis via enterohepatic circulation of bile acids.
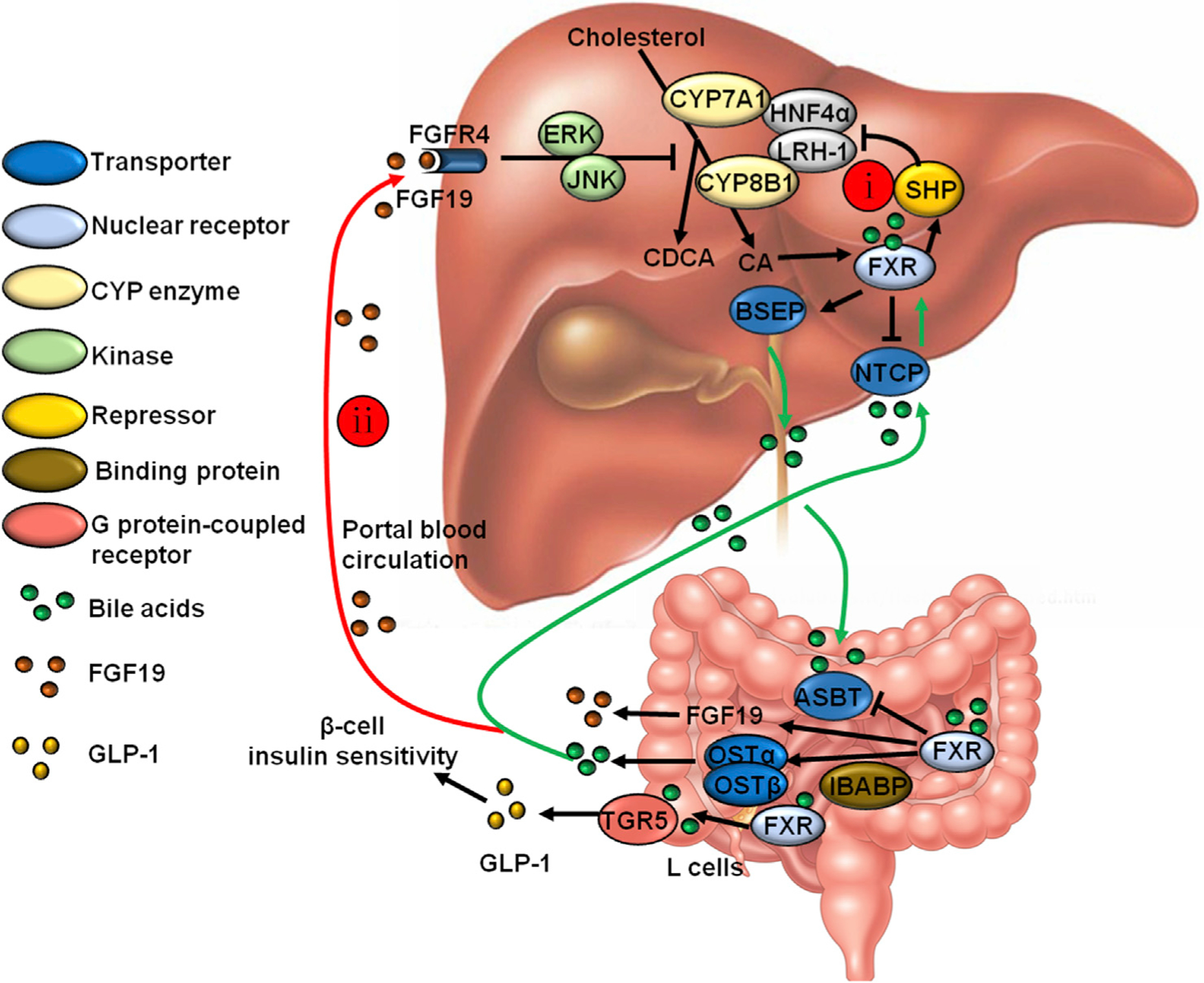
(i) Bile acids activate the nuclear receptor FXR in hepatocytes to inhibit bile acid synthesis and promote excretion of bile acids into bile. FXR induces the negative co-repressor small heterodimer partner (SHP) to inhibit hepatocyte nuclear factor 4 alpha (HNF4α)/LXR-mediated activation of CYP7A1. FXR also activates bile salt export pump (BSEP) to efflux bile acids into gallbladder bile, and FXR inhibits sodium taurocholate co-transport peptide (NTCP), preventing the accumulation of bile acids in the liver. (ii) In the intestine, bile acids activate FXR and induce the release of fibroblast growth factor 19 (FGF19, Fgf15 in mice). FGF19 released into portal circulation inhibits bile acid synthesis via extracellular signal-regulated kinase (ERK)/c-Jun N-terminal kinase (JNK)-mediated blockade of CYP7A1. Also, in the intestine, bile acids activate the G protein-coupled receptor TGR5, stimulating release of glucagon-like peptide 1 (GLP-1) and improving insulin tolerance. Abbreviations: FXR, farnesoid X receptor; CYP7A1, cholesterol 7 alpha-hydroxylase; LXR, liver X receptor; TGR5, Takeda G protein-coupled receptor 5; LRH-1, liver-related homologue-1; CDCA, chenodeoxycholic acid; CA, cholic acid; ASBT, apical sodium-dependent bile acid transporter; OST, organic solute transporter; IBABP, ileum bile acid binding protein.
4.2. FXR
FXR is widely expressed in the gastrointestinal tract and plays a central role in regulation of the enterohepatic circulation of bile acids, though the contribution of FXR to the regulation of bile acid synthesis may be highly tissue-specific.59 FXR was first identified as a nuclear receptor activated by farnesol metabolites.5–8 Taurochenodeoxycholic acid (TCDCA) is the most efficacious endogenous FXR agonist (EC50 = 17 mM), while taurocholic acid (TCA) is a weak FXR agonist (EC50 = ~600 μM).8 Fxr−/− mice have increased serum bile acids, cholesterol, and triglycerides, and increased hepatic cholesterol and a proatherogenic serum lipoprotein profile.25 Bile acid pool and fecal bile acid secretion are reduced in Fxr−/− mice due to reduced biliary bile acid secretion. These mice also have reduced expression of BSEP, IBABP, and multi-drug resistant protein 2 (Mdr2),25 suggesting bile acid transporters and binding proteins are FXR targets and that FXR plays critical roles in regulating bile acid and hepatic lipid homeostasis (Fig. 3). In the liver, FXR induces BSEP to efflux bile acids into bile. FXR inhibits NTCP, whose expression is regulated by the retinoic acid receptor (RAR)/retinoid X receptor (RXR) heterodimer.60 Thus, FXR maintains very low levels of intrahepatic bile acids. In the ileum, FXR inhibits ASBT and induces IBABP,59 which transports bile acids from the apical brush border membrane to the sinusoidal membrane, where FXR induces OSTα/OSTβ to efflux bile acids into portal blood. Therefore, FXR plays a critical role in the control of the enterohepatic circulation of bile acids to maintain a constant circulating bile acid pool (Fig. 3).
In the ileum, bile acid concentrations are high and activate FXR, which induces the expression and release of the enteroendocrine hormone fibroblast growth factor 19 (FGF19; Fgf15 in mice). FGF19 is secreted into portal circulation and activates hepatic membrane fibroblast growth factor receptor 4 (FGFR4)/β-Klotho complex/extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) signaling to inhibit bile acid synthesis.61 This intestinal FXR/FGF19-to-liver FGFR4 signaling pathway may be the primary physiological mechanism for bile acid feedback regulation of bile acid synthesis. When bile acids accumulate in hepatocytes, such as during cholestatic liver injury, bile acids may activate FXR to induce the negative nuclear receptor small heterodimer partner (SHP), which inhibits trans-activation of the CYP7A1 and CYP8B1 genes by interfering with the transcriptional activators hepatocyte nuclear factor 4α (HNF4α) and liver-related homologue-1 (LRH-1) (Fig. 3). Thus, FXR plays a critical role in the regulation of bile acid synthesis, transport and enterohepatic circulation of bile acids to inhibit bile synthesis and prevent cholestatic injury. The small amount of bile acids excreted into feces (5%, ~0.5 g/day in humans) are replenished by de novo synthesis in hepatocytes.
5. Regulation of CYP7A1 gene transcription
5.1. CYP7A1 gene
CYP7A1, the critical point of regulation in bile acid synthesis, is tightly regulated by bile acids returning to the liver via the enterohepatic circulation.1 The underlying molecular mechanism of bile acid-based regulation of CYP7A1 has been difficult to elucidate. Most early studies of bile acid synthesis and CYP7A1 were carried out using rat, rabbit and hamster models. Purification of CYP7A1 from rat liver and reconstitution with NADPH-cytochrome reductase confirmed that CYP7A1 is a cytochrome P450 enzyme expressed in liver microsomes and utilizes cholesterol as a substrate.3 It was also shown that CYP7A1 specific activity is 2-fold higher in female rats compared to male rats, and its activity is regulated by drugs and hormones: phenobarbital, 3-methylcholanthrene, pregnenolone 16α-carbonitrile and thyroid hormone T3 induce CYP7A1 activity, and dexamethasone inhibits its activity.3,62–64
The CYP7A1 gene encodes multiple mRNA species, which are translated to a protein of 503 amino acid residues with a heme binding sequence typically present in CYP enzymes.2,4 In rats, cholesterol or bile acid-binding resin (sequestrant) feeding induced CYP7A1 mRNA, protein and activity in parallel, suggesting bile acids and cholesterol regulate CYP7A1 mainly by transcriptional mechanisms. The full length 3.6 kb mRNA species contains a coding region of 1509 bp and a 2.5 kb 3′-untranslated region (3′-UTR) with multiple polyadenylation signals and AUUU sequence motifs, indicating short half-life mRNAs and post-translational modification may be also involved in the regulation of CYP7A1 expression. The human CYP7A1 gene spans ~11 kb of the genome.65 The human and rat CYP7A1 amino acid coding sequences are highly homologous (90%), but the human CYP7A1 gene promoter sequence diverges substantially from rat and mouse Cyp7a1 genes,66 and the human CYP7A1 gene responds to few hormones. Thyroid hormone T3 induces rat Cyp7a1,62 but represses human CYP7A1 gene expression and several thyroid hormone response elements have been identified in the human CYP7A1 and rat Cyp7a1 gene promoter.65,67 Glucocorticoid and T3 synergistically induce Cyp7a1 mRNA in rats,68 while glucocorticoid, glucagon and 3′,5′-cyclic adenosine monophosphate (cAMP) all inhibit human CYP7A1 gene transcription.69
5.2. Mechanisms of bile acid regulation of CYP7A1 gene transcription
The underlying molecular mechanism of bile acid feedback regulation of bile acid synthesis appears to be very complex and not completely understood.38,70,71 Analysis of the rat Cyp7a1 gene promoter by DNase I footprinting identified several nuclear hormone response elements (AGGTCA motifs) and two putative bile acid responsive elements (BAREs), BARE-I (direct repeat with 4 nucleotide spacing, DR4, nt−74 to −55) and BARE-II (DR1, nt−149 to −128) (Fig. 4).72–74 Transcription factors bind to the BARE-I and BARE-II to stimulate RNA polymerase and basal transcription of the CYP7A1 gene (Fig. 4).
Fig. 4. Mechanism of regulation of the CYP7A1 gene.
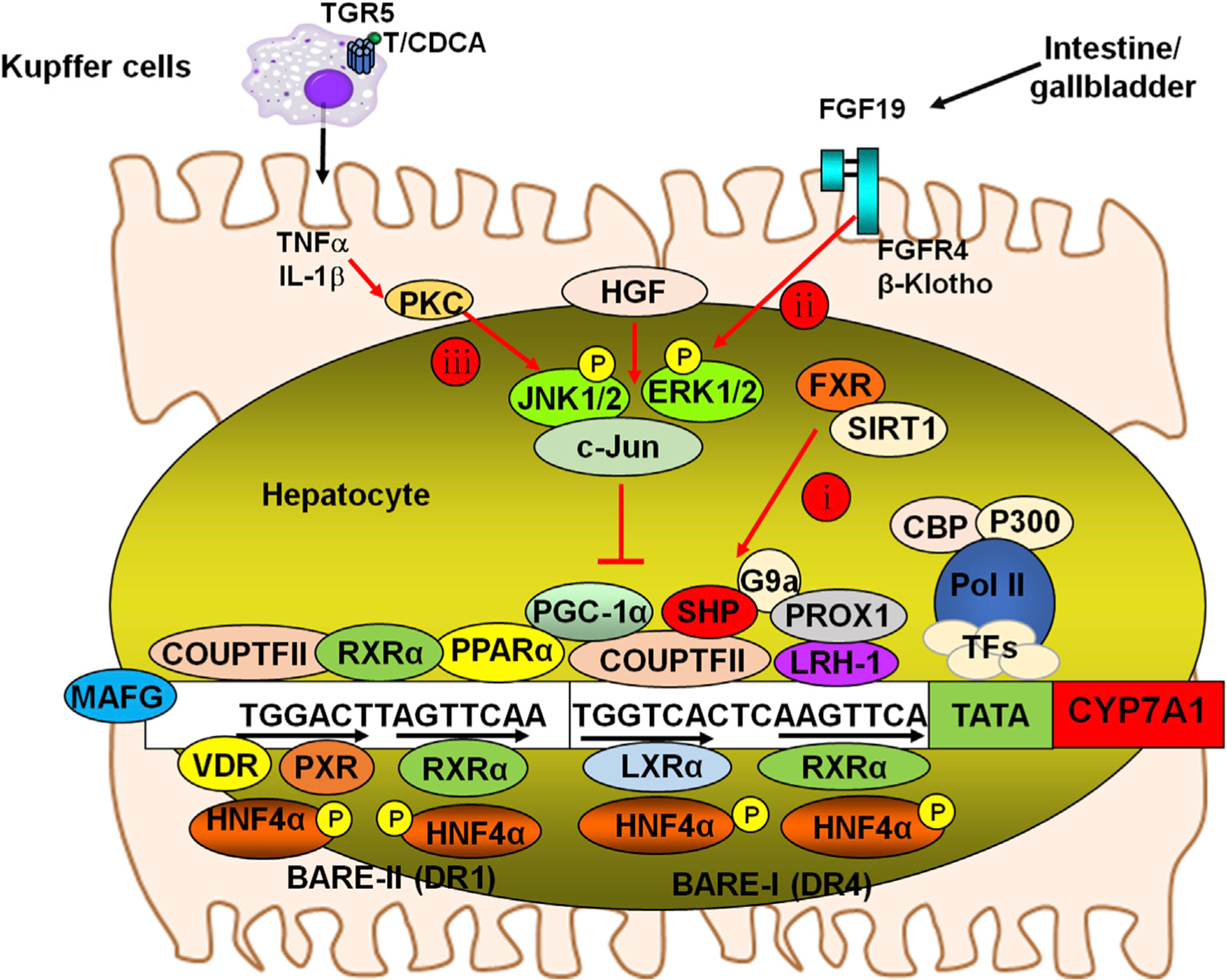
The CYP7A1 gene proximal promoter contains BARE, which binds the nuclear receptors shown. Three mechanisms for bile acid regulation of CYP7A1 gene transcription are illustrated here. The CYP7A1 promoter contains two bile acid responsive elements, BARE-I and BARE-II, which contains a direct repeat with 4 bases spacing (DR4) and 1 base spacing (DR1) motif, respectively. Nucleotide sequences shown are rat Cyp7a1 gene. Mechanisms 1 and 2 are FXR-dependent and mechanism 3 depicts an FXR-independent cell signaling pathway. Mechanism i: Bile acid activation of FXR induces the repressor SHP to inhibit HNF4α and LRH-1-mediated transcriptional activation of CYP7A1. The SHP-mediated mechanisms may also inhibit PXR/RXRα (BARE-II), COUP-TFII/RXRα (BARE-I and BARE-II), LXR/RXRα (BARE-I) and PPARa/RXRα (BARE-II) binding to the CYP7A1 promoter to suppress CYP7A1 gene transcription. FXR also induces MAFG to inhibit CYP7A1. PXR, VDR and PPARa inhibit CYP7A1 gene transcription via competition for HNF4α binding to the BARE-II on the CYP7A1 gene promoter. Mechanism ii: Bile acid activation of intestinal FXR induces FGF19, which is transported from the intestine to the liver and activates the FGFR4/β-Klotho complex to phosphorylate and activate JNK1/2 and ERK1/2 signaling pathways. Phosphorylation of HNF4α inhibits its dimerization and binding to the CYP7A1 gene, resulting in reduced CYP7A1 gene transcription. Phosphorylation of PGC-1α, a co-activator of PPARα, and other nuclear receptors also inhibit trans-activation of the CYP7A1 gene. Mechanism iii: Taurodeoxycholic acid (TDCA)/CDCA activates macrophages and induces the release of inflammatory cytokines including TNFα and IL-1β, which cross the sinusoidal membrane to activate protein kinase C (PKC) and JNK signaling pathways to inhibit CYP7A1 gene transcription. Epigenetic regulation: Histone acetylases (HATs), such as CBP/P300 and histone deacetylases (HDACs) such as sirtuin 1 (SIRT1), regulate FXR, SHP and CYP7A1/CYP8B1 by epigenetic mechanisms. The methytransferase G9a is recruited by SHP and Prox-1 to inhibit CYP7A1 by histone methylation. Abbreviations: CYP7A1, cholesterol 7 alpha-hydroxylase; BARE, bile acid response elements; HNF4α, hepatocyte nuclear factor 4 alpha; FXR, farnesoid X receptor; FGFR4, fibroblast growth factor receptor 4; CDCA, chenodeoxycholic acid; JNK, c-Jun N-terminal kinase; SHP, small heterodimer partner; LRH-1, liver-related homologue-1; PXR, pregnane X receptor; VDR, vitamin D receptor; PPAR, peroxisome proliferator-activated receptor.
5.2.1. Hepatic FXR-dependent mechanisms
It was first proposed that FXR induced the nuclear receptor SHP to inhibit transactivation of the CYP7A1 gene by HNF4α and LRH-1 (Mechanism i, Fig. 4).75,76 However, in Shp knockout mice bile acid-feeding still inhibits Cyp7a1 gene transcription, suggesting that redundant pathways may exist for bile acids to inhibit transcription of the Cyp7a1 gene.77 A recent study showed that FXR induced V-maf avian musculoaponeurotic fibrosarcoma oncogene homologue G (MAFG), which was a negative regulator of bile acid synthesis (Fig. 4).78 Overexpression of MAFG in mouse liver repressed Cyp7a1, Cyp8b1, Cyp7b1 and Cyp27a1 gene transcription. Interestingly, MAFG reduced CA but increased muricholic acids. In Mafg knockdown mice, Cyp7a1 and Cyp8b1 expression were increased with increased CA levels. These results suggest that MAFG may inhibit Cyp8b1 gene transcription more than Cyp7a1 in an FXR-dependent manner. The cause of the differential regulation of Cyp8b1 by MAFG compared to Cyp7a1, Cyp7b1 and Cyp27a1, and the mechanism of MAFG oncogene in the inhibition of these genes, are not clear.
5.2.2. Intestinal FXR/FGF15 to liver FGFR4 pathway
In 1995, Pandak et al.79 first reported that intraduodenal, but not intravenous, infusion of TCA inhibited Cyp7a1 mRNA expression in biliary fistula rats. These authors suggested that a putative intestinal factor released or reabsorbed in the presence of bile acids in the ileum might be involved in the regulation of bile acid synthesis. Years later, a study showed that Cyp7a1 mRNA and bile acid pool size were induced in Fgfr4−/− mice.80 This study also suggested that JNK mediated Fgfr4 repression of the Cyp7a1 gene. Later, Inagaki et al.61 identified that the intestinal FXR-induced endocrine hormone FGF15/FGF19 activated hepatic FGFR4/β-Klotho signaling to inhibit CYP7A1 via JNK (Mechanism ii, Fig. 4). In mice, Fgf15 is produced in the intestine but not liver.81 In human primary hepatocytes, FGF19 is expressed and is induced by bile acids to activate ERK1/2 signaling to inhibit CYP7A1 expression.82 In obstructive cholestatic patients, FGF19 is highly induced in the liver to inhibit CYP7A1 expression and bile acid synthesis.83
Another study reports that intestinal FGF15 was down-regulated by Kruppel-like factor 15 (KLF15) in the ileum and the KLF15-FGF15 signaling axis regulated the circadian expression of Cyp7a1 and bile acid synthesis.84 Klf15 is expressed in mouse liver but did not regulate Cyp7a1 mRNA expression. Overexpression of Kfl15 reduced Fgf15 expression in mouse ileum, which was reversed by Klf15 knockdown. In Klf15−/− mice, Fgf15 was strongly induced in the ileum. However, KLF15-FGF15 regulation of bile acid synthesis is independent of bile acid/FXR/FGF15 signaling. Another study reports that Diet1 is co-expressed with Fgf15 in mouse enterocytes.85 Diet1 encodes a 236 kD protein consisting of LDL receptor meprin-A5-protein phosphatase mu domains. Diet1 deficiency in mice increased the bile acid pool size and impaired bile acid feedback regulation of Cyp7a1, while transgenic overexpression of Diet1 restored Cyp7a1 regulation in these mice. Interestingly, mutations in the Diet1 gene increased FGF19 secretion in bile acid diarrhea (BAD) patients.86 How Diet1 regulates Fgf15 and the relevance of the Diet1/Fgf15 axis in the regulation of bile acid synthesis is not clear.
5.3. Regulation of CYP7A1 by cell signaling
Studies from Roger Davis’ laboratory showed that CDCA activates macrophages to secret cytokines, tumor necrosis factor (TNF) α and interleukin (IL)-1β, which may cross the hepatic sinusoidal membrane to activate protein kinase C and JNK signaling to inhibit CYP7A1 expression independent of FXR (Mechanism iii, Fig. 4).28,87,88 In primary human hepatocytes, CDCA and IL-1β markedly induced c-Jun to suppress CYP7A1 and CYP8B1 expression.89,90 Furthermore, CA feeding induced TNFα and IL-1β, and induction of cytokines was blunted in Jnk−/− mice, suggesting that bile acids may activate the bile acid receptor TGR5 in macrophages and Kupffer cells to stimulate pro-inflammatory cytokine production via the JNK pathway to inhibit CYP7A1 and CYP8B1 gene transcription.88 The cell signaling mechanism initiated by Kupffer cells may be rapidly activated to reduce bile acid synthesis as an adaptive response to cholestatic liver injury. Concurrently, infiltration and accumulation of neutrophils in the liver mediates cholestatic liver injury in mice,91 but evidence for this in humans is still controversial.92
5.4. TGR5 signaling in bile acid synthesis
TGR5 is widely expressed in the epithelial cells of intestine and gallbladder, liver sinusoid endothelial cells, and Kupffer cells, but is not expressed in hepatocytes.9,93 The secondary bile acids DCA and LCA bind to TGR5, increasing intracellular cAMP and activating protein kinase A and cAMP response element binding protein (CREB) signaling pathways to regulate gene transcription.94 Tgr5−/− mice have a reduced bile acid pool size and are protected from lithogenic diet-induced cholesterol gallstone formation.95,96 TGR5 is highly expressed in the gallbladder, and activation of TGR5 causes smooth muscle relaxation and stimulates gallbladder filling.97 Activation of TGR5 stimulates brown adipose tissue energy metabolism by inducing deiodinase type 2 (DIO2), which converts thyroid hormone T4 to trihydroxythyronine T3. White adipose tissue browning and mitochondrial energy metabolism are also stimulated by TGR5.98 Tgr5−/− mice have reduced Cyp7b1 expression, increased TCA and reduced tauromuricholic acid (TMCA) in bile, and are resistant to fasting-induced hepatic steatosis compared to wild type mice.99 Fasted Tgr5−/− mice have increased activation of hepatic growth hormone-signal transducer and activator of transcription 5 (Stat 5) signaling, which may regulate Cyp7b1 gene transcription. FXR and TGR5 are co-expressed in enteroendocrine L cells and activation of FXR induces Tgr5 gene transcription,100 proglucagon synthesis, and glucagon-like peptide-1 (GLP-1) secretion,101 while activation of TGR5 stimulates glucose-induced GLP-1 secretion from L cells and improves insulin sensitivity (Fig. 3).100
5.5. Regulation of CYP7A1 gene transcription by transcription factors
The Cyp7a1 gene promoter regions BARE-I and BARE-II contain several hormone receptor binding sequence motifs that have been shown to bind liver-enriched transcriptional factors and nuclear receptors (Fig. 4).
5.5.1. Liver-enriched transcription factors
In the rat Cyp7a1 gene proximal promoter, HNF4α, HNF1 and CAAT/enhancer-binding protein bind BARE-I (C/EBP).102,103 Nuclear receptors are ligand-activated transcription factors that bind to DNA sequences to activate RNA polymerase II and general transcription factors. A heterodimer comprised of orphan nuclear receptors chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and RXRα binds to both BARE-I and BARE-II (Fig. 4).72,104 HNF4α homodimers and COUP-TFII/RXRα may interact to regulate CYP7A1 gene transcription.74 At physiological concentrations (10 μM), CDCA and CA activate FXR and repress CYP7A1 promoter activity via BARE-II, but FXR/RXRα do not bind to this element, suggesting an indirect mechanism for FXR to mediate bile acid repression.105 Later studies reported that FXR induced SHP to inhibit CYP7A1 gene transcription activated by HNF4α and LRH-1 (Fig. 4).75,76 The human CYP7A1 promoter sequence diverges from the rat Cyp7a1 gene promoter sequence but the BARE sequences are preserved among different species.65 HNF4α is a liver-enriched transcription factor that plays a critical role in the basal transcription of Cyp7a1 and many other liver-specific genes.
The human CYP7A1 transcription factor (CTF), mouse LRH-1, and α-fetal protein transcription factor (FTF) belong to the NR5A2 family of nuclear receptors and control the basal level expression of the CYP7A1 gene.106 Several mechanisms have been implicated in the modulation of HNF4α/LRH-1 binding to the human and rat BAREs to inhibit CYP7A1 promoter activity (Fig. 4). As described previously, FXR induces SHP to inhibit transactivation of the CYP7A1 gene by HNF4α and LRH-1 (Mechanism i, Fig. 4). FGF19/FGFR4 activates JNK1/2 and ERK1/2 to phosphorylate HNF4α and reduce HNF4α homodimer binding to the BARE (Mechanism ii, Fig. 4),82,107 while bile acids and cytokines activate ERK1/2 and JNK1/2 pathways to inhibit human CYP7A1 gene transcription (Mechanism iii, Fig. 4).90
In addition, hepatocyte growth factor (HGF) has been shown to inhibit human CYP7A1 via c-Jun, JNK1/2 and ERK1/2 signaling (Fig. 4).108 HGF is known to activate the tyrosine protein kinase c-Met to phosphorylate PI3K, Ras and Src kinases mediating cell proliferation and regeneration. Knockdown of c-Met by siRNA increased CYP7A1 mRNA levels and blocked HGF inhibition of CYP7A1 mRNA in human hepatocytes. Further, HGF recruited c-Jun and SHP but blocked the co-activators peroxisome proliferator activated receptor γ-coactivator 1 (PGC-1α) and CREB-binding protein (CBP) from binding to CYP7A1 chromatin, thus repressing CYP7A1 gene expression (Fig. 4).
Lastly, prospero-related homeodomain protein 1 (Prox1) has been shown to interact with FTF and repress Cyp7a1 expression (Fig. 4) by interacting with HNF4α and PGC-1α to inhibit CYP7A1 expression in human hepatocytes.109 siRNA knockdown of Prox1 significantly increased CYP7A1 mRNA expression in human hepatocytes.
5.5.2. Peroxisome proliferator-activated receptor alpha (PPARα)
PPARα is highly expressed in the liver. Activation of PPARa by fibrates stimulates lipolysis of triglycerides in adipose tissue as well as efflux of free fatty acids to the liver and muscle for fatty acid oxidation and energy metabolism. However, activation of the PPARα/RXRα heterodimer by fibrates enhances its binding to DR1 in the BARE-II, thus preventing HNF4α binding to the BARE and ultimately inhibiting human CYP7A1 gene transcription (Fig. 4).110
5.5.3. Pregnane X receptor (PXR)
PXR is a bile acid, steroid and drug-activated nuclear receptor that binds to the BARE-II in the human CYP7A1 gene promoter (Fig. 4) and is involved in coordinate regulation of drug metabolism and bile acid homeostasis.111 PXR is activated by LCA and induces CYP3A4 in liver and intestine, leading to induction of phase I drug oxidation CYPs, phase II drug conjugation enzymes and phase III drug transporters to detoxify and secrete drugs and LCA. Rifampicin is a specific human PXR agonist that inhibits CYP7A1 gene expression via blockade of PGC-1α/HNF4α interaction in human hepatocytes (Fig. 4),112 but has no effect in mice, while pregnane activates mouse PXR, but not human PXR.113 In Cyp3a11 deficient mice, Cyp7a1 is activated and bile acid synthesis is increased, which increases de novo cholesterol synthesis.114
5.5.4. Liver X receptor (LXR)
Humans are resistant to diet-induced hypercholesterolemia due to the presence of the ATP-binding cassette subfamily G member 5/8 (ABCG5/8) heterodimer, which effluxes most dietary cholesterol and plant sterols absorbed in the intestine.115 Cholesterol homeostasis is maintained by the conversion of cholesterol to bile acids, which facilitates biliary excretion of cholesterol. Cholesterol feeding induces Cyp7a1 via activating LXRα in mice, but not in humans (Fig. 4).116 In LXRα deficient mice, dietary cholesterol accumulates in liver and fails to upregulate Cyp7a1. LXRα is an oxysterol-activated receptor expressed in hepatocytes and macrophages that is involved in lipogenesis.117 LXRα binds to the DR4 motif in the rat BARE-I to induce Cyp7a1 gene transcription, but the human CYP7A1 promoter lacks the DR4 motif, and thus, does not bind LXRα.118 Cholesterol is a substrate of CYP7A1, but cholesterol feeding inhibits CYP7A1 and bile acid synthesis in human hepatocytes.119 It is possible that activation of FXR signaling may override the stimulation by LXRα to inhibit CYP7A1.120
5.5.5. Vitamin D receptor (VDR)
VDR is activated by 1α,25-dihydroxyvitamin D3, the active form of vitamin D3 produced in the kidney. VDR is closely related to PXR and plays a critical role not only in calcium and phosphate homeostasis and bone formation, but also in immunomodulation, cell growth and differentiation.121 Deficiency of VDR or mutations of the VDR gene cause low serum calcium, type II rickets and bone malformation. VDR is also a bile acid-activated nuclear receptor in the intestine and that is efficaciously activated by LCA.122 VDR is abundantly expressed in the kidney, intestine and bone but is expressed in very low levels in other tissues. Activation of VDR induces CYP3A4 and sulfotransferases in intestine and liver to sulfonate and detoxify drugs for biliary and renal excretion. VDR is expressed in mouse stellate cells but not hepatocytes. Activation of VDR inhibits stellate cell activation by Tgfβ1/small mothers against decapentaplegic (Smad) signaling and protects against liver fibrosis in mice.123 On the contrary, VDR is expressed in human hepatocytes and activation of VDR by LCA inhibits CYP7A1 expression by interacting with HNF4α and competing with co-activators.124 VDR also has non-genomic actions at the plasma membrane. Activation of VDR by LCA and 1α, 25 (OH)2-D3 induces VDR translocation from the cytosol to the plasma membrane to induce tyrosine phosphorylation of c-Src and the downstream c-Raf/MEK1/2/ERK1/2 pathway, which phosphorylates HNF4α to inhibit Cyp7a1 gene expression.125 This may be a rapid response of bile acids to protect hepatocytes from cholestatic injury.
5.5.6. Regulation of the CYP8B1 gene
CYP8B1 is also inhibited by bile acids.126 CYP8B1 determines the ratio of CA to CDCA in humans and the ratio of CA to MCA in mice, and thus, the hydrophobicity of circulating bile acids. The rat and human CYP8B1 gene promoters contain HNF4α and LRH-1/FTF binding sequences.126–129 Thyroid hormone suppresses Cyp8b1 activity and mRNA expression.130 In contrast to Cyp7a1, cholesterol feeding suppresses Cyp8b1 expression in mice.131 Interestingly, steroid responsive element binding protein (Srebp)-1a and Srebp1-c, but not Srebp-2, stimulates Cyp8b1 mRNA expression and SREBP response element (SRE) and E boxes were identified in the rat Cyp8b1 gene promoter. SREBP activation and procession are inhibited by oxysterols, consistent with cholesterol inhibition of Cyp8b1 via Srebp-1. A recent study reports that the anabolic steroid hormone epistane elevated GCA by upregulation of CYP8B1 in human hepatocytes.132 This recreational steroid activates androgen receptor, LXR α, and PXR, leading to increasing expression of CYP8B1. Interestingly, an in vitro assay showed that CYP8B1 can convert CDCA to CA.133 Similar to Cyp7a1, Cyp8b1 gene transcription is suppressed by the FXR/SHP mechanism.134 Furthermore, the liver FXR/SHP pathway preferentially suppresses Cyp8b1, while the intestine FXR/FGF15 pathway preferentially inhibits Cyp7a1.107
5.5.7. Regulation of the CYP7B1 gene
Bile acids modestly reduce Cyp7b1 expression in mice.135,136 Inhibition of cholesterol synthesis by squalestatin decreased CYP7B1, whereas cholesterol increased CYP7B1 activity and mRNA expression. The human CYP7B1 coding sequence shares about 40% sequence identity to CYP7A1.137 CYP7B1 catalyzes the 7⍺-hydroxylation of cholesterol, 25- and 27-hydroxycholesterol, and dehydroepiandrosterone (DHEA) and CYP7B1 is critical for the metabolism of oxysterols.44,138 SREBP inhibits LXRα-induced CYP7B1 gene transcription.139 Thus, cholesterol activates LXRα to inhibit SREBP and induce CYP7B1 to metabolize oxysterols to bile acids. In Cyp7b1 deficient mice, bile acid pool size and serum cholesterol and triglycerides are maintained, but 25- and 27-hydroxycholesterol are increased compared to wild type mice.26 CYP7B1 expression is upregulated by retinoic acid-related orphan receptor alpha (RORα) and is repressed by LXRα.140 Cyp7b1 expression is sexually diphormic and male-specific, and is regulated by growth hormone/Stat5.99,141 Estrogen regulates CYP7B1 expression via insulin/phospho-inositol-3 kinase signaling.142 Interestingly, a recent study reported that cold exposure in mice induces cholesterol synthesis and Cyp7b1 expression to convert cholesterol to bile acids via reshaping the gut microbiota.143 This study suggests that bile acids are important metabolic effectors in sustained brown adipose tissue activation during thermogenesis.
5.5.8. Regulation of the CYP27A1 gene
CYP27A1 is located in the inner mitochondrial membrane. CYP27A1 catalyzes oxidation of the steroid side chain of cholesterol for bile acid synthesis. Transport of cholesterol into mitochondria is mediated by steroidogenic acute regulatory protein D1 (StarD1) to activate CYP27A1.44,144,145 Bile acids inhibit HNF4α-mediated trans-activation of human CYP27A1 gene transcription.146 Growth hormone, insulin-like growth factor-1, steroidogenic acute regulatory protein and dexamethasone increase CYP27A1, whereas T4 and PMA reduce CYP27A1 gene promoter activity.147 CYP27A1 is induced by dexamethasone and suppressed by cyclosporin A, while CA reduced CYP27A1 mRNA stability.148 Glucocorticoid receptor mediates dexamethasone induction of CYP27A1 gene transcription.142 Interestingly, PXR induces Cyp27a1 and the cholesterol efflux transporters ABCA1 and ABCG1 by activating LXRα in the intestine rather than in liver.149
6. Post-transcriptional regulation of CYP7A1
Several post-transcriptional mechanisms have been implicated in the regulation of Cyp7a1 expression. These include regulation of mRNA stability, regulation by microRNAs (miRNAs), and epigenetic regulation.
6.1. Regulation of mRNA stability
The 3′-UTR of Cyp7a1 mRNA transcripts have multiple AUUUU sequences indicative of short half-life mRNA, which is regulated by bile acids.150 It has been reported that Apob mRNA editing enzyme catalytic polypeptide 1 (Apobec-1)-deficient mice had significantly lower expression of Cyp7a1 mRNA and protein, indicating Apobec-1 may stabilize Cyp7a1 mRNA.151 Apobec-1 is an AU-rich RNA binding protein and a cytidine deaminase that converts a cytosine in the glutamine (CAA) codon to uracil, creating a stop codon (UAA) in the Apob100 gene, thus synthesizing a truncated Apob48 in human and mouse intestine and in mouse liver. It was demonstrated that Apobec-1 binds to AU-rich regions of the 3′-UTR of Cyp7a1 mRNA to stabilize the transcript. Thus, bile acids and lipids coordinately regulate bile acid synthesis and Apob expression.151 On the other hand, a recent study reports that activation of FXR induces the RNA binding protein ZFP36L1 which binds to the 3′-UTR region to destabilize Cyp7a1 mRNA.152 ZFP36L1-deficient mice have altered bile acid synthesis, reduced adiposity and are protected from diet-induced obesity and hepatic steatosis. This study uncovered SHP-independent FXR signaling for rapid inhibition of Cyp7a1 expression and bile acid synthesis by post-transcriptional mechanisms.
6.2. Regulation by miRNA
MiRNAs are small non-coding RNAs (about 19e25 nucleotides) that bind to complementary sequences in the 3′-UTR of mRNA to inhibit mRNA transcription. The liver-specific miRNA miR-122a is the predominant miRNA expressed in hepatocytes and has been shown to regulate cholesterol synthesis and lipid metabolism.153,154 MiR-122 expression level is decreased in hepatocellular carcinoma and non-alcoholic steatohepatitis (NASH).155,156 Screening of a human miRNA microarray identified 5 differentially expressed miRNAs, including miR-122a, in human hepatocytes treated with CDCA, the FXR agonist GW4064 or FGF19. Cyp7a1 expression is down-regulated by miR-122a mimic and induced by miR-122a antagomir. A miR-122a binding sequence has been identified in the 3′-UTR of human CYP7A1 mRNA near the Apobec-1 binding site.157 MiR-122a also regulates many liver-specific transcription factors, including HNF1, HNF3, HNF4 and C/EBP. MiR-422a binding sites were also identified in the 3′-UTR of human CYP7A1 and CYP8B1 mRNAs. MiR-33a, which is located in the intron on the SREBP-2 gene, has been shown to regulate CYP7A1 and plays a key role in regulation of cholesterol homeostasis.158–160 Overexpression of miR-33a in mouse liver increased hepatic cholesterol and reduced the bile acid pool and serum cholesterol.158
6.3. Regulation by epigenetic mechanisms
It was first reported that bile acid synthesis is regulated by epigenetic mechanisms through SHP-mediated chromatin remodeling.161 Acetylation of FXR by histone acetylase p300 is important for ligand-activated FXR to induce SHP.162 SHP is rapidly degraded through a ubiquitin-proteasomal degradation pathway, and FGF19 increased SHP stability by inhibiting ERK-dependent ubiquitin-proteasomal degradation.163 Interestingly, SHP stability was increased in leptin-deficient mice and diet-induced obese mice. FXR is also regulated by sirtuin 1 (SIRT1), a histone acetylase that mediates nutrient and hormone regulation of hepatic metabolism.164 Acetylation of FXR increases its stability but inhibits its interaction with RXRα, as well as its DNA binding and transcriptional activity. In metabolic disease, FXR interaction with SIRT1 and p300 was altered and FXR acetylation was increased. Chromatin immunoprecipitation assay showed that bile acids induced recruitment of the mSin3A and Swi/Snf complex containing Brm ATPase to interact with SHP and repress CYP7A1 promoter activity. Bile acids or FGF19 induced protein kinase C zeta to phosphorylate and translocate SHP to the nuclei to regulate its target genes in liver metabolism.165 Phosphorylation of Thr-55 is critical for SHP function and interaction with chromatin modifiers at bile acid responsive genes. Mutation of Thr-55 attenuates SHP-mediated epigenomic and metabolic effects. Another interesting study showed that FXR is acetylated in diet-induced obese mice.166 Acetylation of FXR blocked interaction with small ubiquitin like modifier (SUMO) ligase, resulting in reduced SUMOylation and activation of inflammatory gene expression. Thus, dysregulation of acetylation and SUMOylation of FXR reduced the anti-inflammatory effect of FXR signaling in metabolism and obesity.167
Glucose stimulates CYP7A1 transcription in human hepatocytes.168 Activation of AMP-activated protein kinase (AMPK) reduces CYP7A1 expression by inhibiting HNF4α binding to CYP7A1 chromatin. Glucose is metabolized to acetyl-CoA, which stimulates histone acetylation and decreases H3K9 di- and tri-methylation of CYP7A1 chromatin. This study suggested that glucose regulates CYP7A1 and bile acid synthesis by epigenetic mechanisms in diabetes. In streptozocin-induced diabetic mice and genetically obese mice, hyperglycemia increased histone acetylation status of the Cyp7a1 gene promoter, leading to elevated basal levels of Cyp7a1 expression and enlarged bile acid pool size with altered bile acid composition.169 In these mice, CA levels are increased while MCAs are decreased, total bile acid pool size is increased, and Cyp7a1 chromatin is hyperacetylated. The fasting to refeeding response of Cyp7a1 is impaired and may exacerbate metabolic disorders in diabetic mice.
G9a, a methyltransferase, inhibits Cyp8b1 gene transcription via epigenetic modification of its chromatin. G9a is co-localized with SHP and inhibits CYP7A1 by inducing H3K9 methylation.170 SHP is associated with lysine-9-methylated histone 3. The naturally occurring SHP mutant R213C interacts less avidly with lysine-9-methylated histone and has lower transcriptional repressor activity.171 SHP repression involves a multistep mechanism, including histone deacetylation followed by H3K9 methylation by G9a to stabilize the association of SHP with chromatin. Another study reports that Prox-1 inhibits Cyp7a1 by interacting with the lysinespecific demethylase 1 (LSD1)/nucleosome remodeling and histone deacetylase (NuRD) repressive complex.172 Prox-1 was shown to recruit LSD1 and histone deacetylase 2 (HDAC2) to the Cyp7a1 promoter to increase H3K4 demethylation, while bile acid treatment increased Prox-1 and LSD1/NuRD occupation at the Cyp7a1 promoter to stimulate deacetylation.
7. Circadian regulation of CYP7A1 and bile acid synthesis
Circadian rhythms coordinate transcription and epigenetic regulation of many metabolic pathways linked to energy metabolism and play a critical role in liver function and disease.173–176 Circadian rhythms are generated by the central biological clock located in the suprachiasmatic nucleus (SCN) in the hypothalamus, which synchronizes physiological and cellular responses with environment cues such as light, and non-photic cues including nutrient availability, behavioral activity, drugs and alcohol.177 At the molecular level, rhythms are generated by transcription and translation of core clock genes both in the SCN and in the periphery. The core clock protein products Clock and brain and muscle ARNT-like 1 (Bmal1) heterodimerize and induce two negative clock genes, Cryptochrome 1 and 2 (Cry1/2) and Period 1 and 2 (Per1/2), whose proteins inhibit Clock and Bmal1 gene transcription. As Per and Cry proteins accumulate, they are phosphorylated by casein kinase, tagged for degradation, and Clock and Bmal1 gene transcription is disinhibited (Fig. 5). Clock mutant mice are obese and hyperphagic, and have increased hepatic cholesterol when fed a high cholesterol and CA diet, suggesting Clock and circadian rhythms play an important role in liver and lipid metabolism.178
Fig. 5. Circadian regulation of CYP7A1.
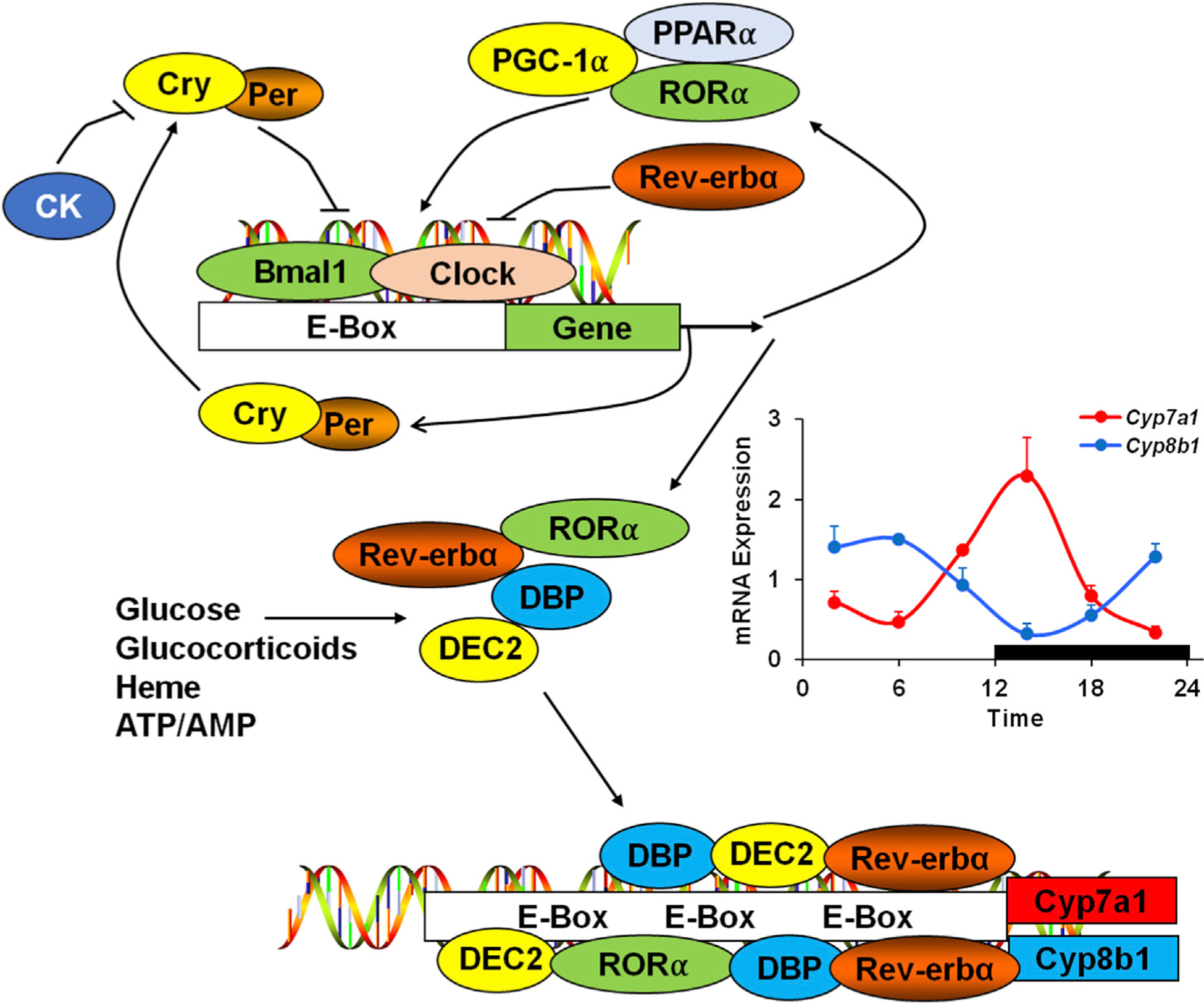
Top: The molecular circadian clock exists in nearly all cells and consists of a cycling transcriptional-translational feedback loop. The core clock proteins Clock and Bmal1 activate the transcription of the negative clock regulators Per and Cry. Per and Cry heterodimers inhibit the Clock/Bmal1 forward limb of the clock, thus regulating Per/Cry synthesis. Per/Cry accumulation leads to phosphorylation by casein kinase, which tags the proteins for degradation and releases the inhibition of Clock/Bmal1. Middle: Clock/Bmal1 also regulates the transcription of clock-controlled genes involved in glucose and energy metabolism. Several of these genes play roles in regulating CYP7A1 and CYP8B1, which exhibit inverse rhythms of gene expression. Bottom: Several clock/controlled transcription factors regulate CYP7A1 and CYP8B1 gene transcription, including D-site binding protein (DBP), DEC2, and Reverb-α and RORα, which also negatively and positively regulate the core clock mechanism above, respectively. Clock proteins bind to the E-box motifs (CANNTG) on the gene promoters. Abbreviations: CYP7A1, cholesterol 7 alpha-hydroxylase; CYP8B1, sterol 12 alpha-hydroxylase; Bmal1, brain and muscle ARNT-like 1; Per, Period; Cry, Cryptochrome; RORα, retinoic acid-related orphan receptor alpha.
The expression of liver Cyp7a1 mRNA, protein and activity levels in rats and mice, which are nocturnal, exhibit a distinct diurnal rhythm peaking at mid-night and declining to a nadir at midday.3,177,179 Serum bile acids exhibit two peaks in mice, at the beginning and end of the dark phase. Primary bile acids were increased during the dark phase while secondary bile acids were increased during the light phase.180 Unconjugated bile acids peaked during the day and were lowest at night. Serum Fgf15 peaked at approximately 4 h after the peak in Cyp7a1, consistent with Fgf15 inhibition of bile acid synthesis.
The postprandial rise in bile acid synthesis and Cyp7a1 expression corresponds to the time of feeding.19 Fasting and refeeding differentially affects clock gene expression, dependent upon the tissue and its metabolic activity.181,182 Feeding rapidly induced Cyp7a1 expression and altered its diurnal expression pattern, which declined during the post absorptive and fasting states.179 Circadian disruption affects liver and bile aid metabolism. Even short-term sleep disruption (6 h/day for 1 week) markedly altered the diurnal rhythm and amplitude of Cyp7a1 mRNA expression in mice. The increase in Clock and Bmal1 expression parallels the increase in Cyp7a1 and HNF4α mRNA expression levels, which peaks 2 h into the dark phase in mice.179 The circadian transcription of Cyp7a1 mRNA also parallels the circadian expression of D-site binding protein (DBP), a liver-enriched b-ZIP protein, suggesting DBP regulates rhythmic expression of Cyp7a1.183 Dbp is a clock-controlled gene that peaks at night and is undetectable in the morning in rat liver.184,185 Dbp mRNA expression peaks 4 h before Cyp7a1 mRNA peaks and multiple functional DBP binding sites were identified in the rat Cyp7a1 gene promoter.186 The nuclear receptors Rev-erBα, Rorα, Pparα, and Pgc1α are clock-controlled genes that regulate Clock and Bmal1 expression by binding to the E-box motifs (CANNTG) on the clock gene promoter (Fig. 5).187 Rev-erbα is a transcriptional repressor and a heme sensor that coordinately regulates the cellular clock, glucose homeostasis and energy metabolism.188 Rev-erbα expression peaks earlier than Dbp and decreases to basal levels during the dark phase when Cyp7a1 peaks. Dbp and Rev-erbα may be the major positive and negative regulators, respectively, of the Cyp7a1 diurnal rhythm in mice.169,179
Clock/Bmal1-controlled Rev-erbα plays a key role in the regulation of rhythmicity of bile acid synthesis and is the first direct metabolic output of the circadian clock.173 In mice, Rev-erbα regulates the circadian expression of Srebp, which regulates its target LXRα to induce Cyp7a1 expression.189 Upon feeding, Rev-erbα inhibits the cyclic expression of insulin sensitive gene 2, which sequesters Srebp in the Golgi membrane and blocks its translocation to the endoplasmic reticulum. Interestingly, Rev-erbα deficient mice have increased lipogenesis through induction of Srebp1c and Srebp2. Shp and E4bp4, negative regulators of Cyp7a1, are increased in Rev-erbα−/− mice, indicating they are direct targets of Rev-erbα.190 Another clock-controlled gene, Dec2, is a basic-helix-loop-helix transcriptional repressor that binds to the E-box and suppresses Cyp7a1 gene expression induced by Dbp in rat liver.191 In free-fed mice the circadian rhythm of Cyp8b1 is opposite to that of Cyp7a1 (Fig. 5). RORa induces Cyp8b1 expression while Rev-erbα suppresses Cyp8b1 expression and Dec2 suppresses Cyp8b1 expression induced by Dbp.191,192 Another study reports that restricted feeding shifted the peripheral clock and increased bile acid synthesis, activating PXR and constitutive active receptor (CAR) to increase serum aspartate amino transferase and cholestatic injury.193 Per1/Per2 double knockout mice have disrupted rhythms in Cyp7a1, Ntcp and Dbp which altered bile acid homeostasis and caused hepatic cholestasis.193 Another interesting study reports that in Klf15−/− mice, the oscillation of Cyp7a1 and Cyp7b1 mRNA and protein was attenuated.84 KLF15 plays a role in nutrient flux and utilization during fasting and feeding cycles. Oscillation of KLF15 regulates the circadian expression of Fgf15, which inhibits Cyp7a1 expression in mouse liver. Klf15 mRNA expression shows a strong circadian rhythm that coincides with the peak in Cyp7a1 mRNA. Klf15 may not directly regulate circadian rhythms in bile acid synthesis but could play a more important role in nutrient supply during starvation.
There are a few studies of circadian rhythms of bile acid synthesis in human patients. One study reports bile acid synthesis (assayed by serum C4 levels) in humans has a rapid diurnal variation over 24 h, with peaks at 1 p.m. and 9 p.m., coinciding with food intake and declining at night.194 These variations are not synchronized with the cholesterol synthesis marker lathosterol, which peaks at night. Other studies indicate conjugated and unconjugated bile acids have asynchronous rhythms, or that unconjugated bile acids peak late at night. The diurnal change in serum C4 is not synchronized with serum lipids or the postprandial rise of serum bile acids. Limited studies in human patients showed serum FGF19 levels vary greatly among individuals and have a pronounced diurnal variation that precedes the decline of bile acid synthesis during fasting conditions.195 Bile acid synthesis varies greatly in normal individuals and is typically higher in men than women.196 Serum bile acid levels are positively correlated to serum triglycerides. Fasting induces FGF21 in serum, which varied 250-fold among individuals, did not display any diurnal variation, and was unrelated to serum bile acids.197 Postprandial transintestinal bile acid flux increases circulating FGF19 levels and suppresses bile acid synthesis, supporting the role of FGF19 in the regulation of bile acid synthesis in humans.198
8. CYP7A1 in human diseases and therapies
Inborne errors of bile acid synthesis have been identified in 13 of the 17 enzymes involved in bile acid synthesis (reviewed in Ref 19 and 199)199. Most inborne errors of bile acid synthesis are identified in neonates and newborns. Deficiency of bile acid synthesis genes causes malabsorption of fats, steroids and nutrients, and accumulation of toxic steroid intermediates, which cause liver injury and cholestatic liver diseases.
8.1. Mutations and polymorphisms of CYP7A1 and other sterol hydroxylase genes
8.1.1. CYP7A1 mutations
Only one family of patients with a CYP7A1 gene mutation has been identified. These patients have dyslipidemia, premature atherosclerosis and gallstone disease, consistent with the role of bile acids in maintaining cholesterol and lipid metabolism.200 The double mutation in the CYP7A1 coding sequence results in a frame shift mutation that causes early termination and translation of a truncated peptide. These patients have reduced CA and DCA, which are likely synthesized via the alternative bile acid synthesis pathway. The first single nucleotide polymorphism (SNP) (−203A < C) identified in the human CYP7A1 gene promoter is linked to increased LDL-cholesterol.201 Several SNPs in the CYP7A1 promoter and coding sequences have been shown to affect bile acid synthesis,202 LDL-cholesterol lowering response to statins,203,204 cholesterol and serum lipids,205–208 coronary artery disease,209 and gallstone disease.210–212 Interestingly, the −203A < C polymorphism has been shown to alter the diurnal rhythm of CYP7A1 activity, monitored by modeling the dynamics of serum C4 in patients.213
As a proof of concept study, Cyp7a1 transgenic mice (Cyp7a1-Tg) were created by cloning a rat Cyp7a1 coding sequence over-expressing Cyp7a1 to study bile acid and lipid metabolism. These mice had increased insulin sensitivity and glucose tolerance and were protected from western high fat diet-induced obesity and hepatic steatosis.214 In Cyp7a1-Tg mice, Cyp7a1 mRNA levels were increased 10-fold, but Cyp7a1 protein expression, enzyme activity and bile acid pool sized only increased ~2–3 fold. In bile, TCA was absent and TCDCA became the predominant bile acid. It is likely that increased TCDCA activated the FXR/SHP pathway to inhibit Cyp8b1 expression. Microarray gene profiling showed that the top up-regulated genes and pathways were de novo cholesterol synthesis and transport, Ldl receptors and Srebp.215 These mice had increased biliary secretion of bile acids and cholesterol to maintain cholesterol homeostasis. Interestingly, in Cyp7a1-Tg mice acetyl-CoA was preferentially used for cholesterol synthesis rather than fatty acid synthesis, explaining observed reduced lipogenesis and increased cholesterol synthesis. This mouse model demonstrates that increasing Cyp7a1 and the bile acid pool, and decreasing Cyp8b1, has metabolic benefits against fatty liver diseases, diabetes and obesity. The original Cyp7a1 knockout mice (Cyp7a1−/−) in a mixed genetic background had a malnutrition phenotype and exhibited reduced survival, as 80% of pups died and surviving mice required vitamin supplementation.21 Surprising, Cyp7a1−/− mice bred into a pure C57BL/6J background survived well and were phenotypically normal.216 Cyp8b1, Cyp7b1 and Cyp27a1 were all upregulated, indicating the alternative bile acid synthesis pathway was upregulated to produce a smaller (60%) but more hydrophobic bile acid pool compared to wild type mice. It is interesting that Cyp8b1 expression was upregulated in Cyp7a1−/− mice to produce substantial amount of TCA (32% of the bile acid pool), indicating the alternative pathway also can produce CA. It has been reported that human CYP8B1 can convert CDCA to CA.133 It is also surprising that Cyp7a1−/− mice are insulin sensitive and are protected from high fat/high cholesterol diet-induced metabolic disorder. All these studies suggest that both the classic and alternative bile acid synthesis pathways are important in maintaining bile acid homeostasis and whole-body metabolic homeostasis. Cyp7a1 expression and bile acid pool and composition can be targeted for therapeutic treatment of metabolic disorders.
8.1.2. CYP7B1 mutations
In contrast to the mild metabolic phenotype in mice and humans with CYP7A1 gene mutations, mutations in the CYP7B1 gene cause severe neonatal cholestasis, cirrhosis and liver failure in neonates.217 In these patients, serum levels of 27-hydroxycholesterol are markedly elevated, leading to accumulation of hepatoxic unsaturated monohydroxy-cholenoic acids. These findings indicate that the alternative bile acid synthesis pathway is quantitatively important in the neonate. A functional SNP in the CYP7B1 gene promoter has been reported.218 The allele frequency differs in Asians and Caucasians and has been linked to prostate cancer. CYP7B1 is an oxysterol 7α-hydroxylase involved in steroid sex hormone synthesisand is regulated by estrogen and androgens in the prostate in humans.219–222 Many CYP7B1 mutations have been identified in hereditary paraplegias and neurodegenerative disorders.223–228
8.1.3. CYP8B1 polymorphism
CYP8B1 gene mutations have not been identified in humans. Only one SNP in the CYP8B1 gene has been reported and is associated with gallstone disease in the Han Chinese population.229 Cyp8b1 expression is increased in obese and diabetic mice and increased serum 12α-hydroxylated bile acids are associated with obesity and insulin resistance in humans.230–232 Cyp8b1−/− mice have increased TMCAs, which antagonize FXR and reduce Fgf15 to stimulate bile acid synthesis, increase bile acid pool and improve glucose homeostasis by stimulating GLP-1 secretion, and prevent western diet-induced obesity and hepatic steatosis.233–237 Overexpression of Cyp8b1 exacerbated dyslipidemia and diet-induced obesity and diabetes via stimulation the ceramide/mechanistic target of rapamycin complex 1 (mTORC1)/SREBP1 pathway.230 In hibition of CYP8B1 and reduction of CA protect against NAFLD, diabetes, and obesity, while activation of CYP8B1 causes cholestasis.132 Several inhibitors of Cyp8b1 have been identified.132
8.1.4. CYP27A1 mutations
There are over 200 reported cases of human CYP27A1 gene mutations, which have been linked to cerebrotendinous xanthomatosis (CTX), a rare autosomal recessive disorder of bile acid synthesis.238–240 CTX patients have abnormally high levels of cholestanol in the blood and accumulate cholestanol and cholesterol in the brain and tendons, forming tendon xanthomas. However, Cyp27a1 deficiency in mice does not accumulate cholestanol and does not induce CTX-like symptoms because Cyp27a1−/− mice and CTX patients accumulate different bile acid intermediates.241 In mice, accumulation of 5β-cholestane-3α,7α,12α -triol activates mouse PXR to induce Cyp3a11, which metabolizes triol and reduces accumulation of this toxic metabolite,242 while human PXR is not activated by triol. CDCA treatment inhibits CYP7A1 to reduce cholestanol and bile acid metabolites and improve clinical and neurophysiological outcomes of CTX.243 It has been reported that 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator that inhibits cardiovascular effects of estrogen,244 but activates estrogen receptor-dependent growth and LXR-dependent metastasis of breast cancer in mice.245 CYP27A1 expression and 27-hydroxycholesterol are associated with breast cancer pathogenesis in human patients.246
8.2. Bile acid-based drug therapies
Most studies on bile aid synthesis were performed using animal models. Despite marked differences in bile acid composition and pool size between humans and mice, results obtained from mouse studies have been translated to therapies for metabolic diseases (reviewed in Refs. 17, 19 and 20). For decades, bile acids have been used as supplements to increase the bile acid pool in patients with inborne errors of bile acid synthesis. Bile acid sequestrants have long been used to reduce the bile acid pool and stimulate bile acid synthesis. More recently, bile acid derivatives are used to activate FXR signaling and reduce the bile acid pool in cholestasis patients. Drugs that regulate bile acid synthesis, bile acid pool size and bile acid composition are in various phases of clinical trials for treating liver-related diseases, such as cholestasis and NASH. NASH is a progressive form of non-alcoholic fatty liver disease (NAFLD), which is a significant complication of obesity and type 2 diabetes mellitus (T2DM), and an independent risk factor for cardiovascular disease. NAFLD has become the most common chronic liver disease, affecting about 30% of the US population. About 30% of NAFLD patients progress to NASH cirrhosis, while about 0.5% of patients with NAFLD progress to hepatocellular carcinoma.247,248 NAFLD patients have increased CA, CDCA and bile acid synthesis, and increased ratio of primary bile acids to secondary bile acids.249 NASH patients also have increased circulating conjugated primary bile acids, an increased ratio of conjugated CA to CDCA, and decreased secondary bile acids.250 Currently, no Food and Drug Administration-approved drug therapy is available for NASH fibrosis or NAFLD.
8.2.1. Increasing the bile acid pool
CA (Cholbam®) and CDCA (Chenodiol®) have been used to treat inborne errors of bile acid synthesis by increasing the bile acid pool in these patients.251 CDCA and UDCA (Ursodiol®, Actigall®) are used to dissolve cholesterol gallstones. UDCA is highly soluble and is used to reduce bile acid toxicity in patients with primary biliary cholangitis (PBC). PPARα agonists (fibrates) and selective modulators have been used in combination with UDCA to treat cholestasis in primary biliary cirrhosis patients.20 Fibrates may induce cholesterol gallstones as a side effect in humans, likely due to repression of CYP7A1 and bile acid synthesis.
8.2.2. Reducing the bile acid pool size
During cholestasis, the bile acid pool is increased and bile acids accumulate in the liver, causing liver inflammation and injury. Reducing CYP7A1 and bile acid synthesis by bile acid sequestrants and FXR agonists reduces the bile acid pool during cholestasis.
Bile acid sequestrants bind bile acids, reduce the bile acid pool to induce CYP7A1, and stimulate bile acid synthesis and reduce serum LDL cholesterol. Cholestyramine is an established drug used for gallstone dissolution, hypercholesterolemia, and BAD.252 Second generation sequestrants, such as Colesevelam and Colestimide, stimulate GLP-1 secretion and thermogenesis in brown adipose tissue to improve insulin sensitivity and glycemic control in NASH patients.253–255
The FXR agonist obeticholic acid (OCA, OCALIVA®) is a CDCA derivative that activates FXR/FGF19 to inhibit CYP7A1 and bile acid synthesis. OCA can be used to treat BAD and is approved to treat PBC and is in phase 3 clinical trials for NASH,256–260 though side effects of OCA treatment may include pruritus, increased LDL-cholesterol and decreased HDL, and gallstone disease.259,261 The non-steroidal FXR agonist Cilifexor improves cholestasis and liver injury in primary sclerosing cholangitis patients and Tropifexor is in clinical trials for PBC and NASH.262,263 The FXR antagonists GUDCA and Gly-MCA reduce weight and improve diabetes,264,265 while the non-tumorigenic FGF19 analogue NGM282 reduces CYP7A1 expression and improves inflammation and is in clinical trials for NASH fibrosis.266 However, non-steroidal FXR agonists also have the same side effects as bile acid-based FXR agonists.
9. Conclusions and future perspective
CYP7A1 has attracted increasing attention in the last two decades as an important enzyme in hepatic metabolism and as a regulator of whole body metabolic homeostasis.38 CYP7A1 and bile acid synthesis are increasingly used as biomarkers for monitoring liver metabolism and disease progression. The molecular mechanisms that regulate CYP7A1 expression in health and disease have been slowly elucidated but require further study. Targeting the bile acid receptor FXR may have therapeutic benefit for treating liver diseases and injury,20 however, unwanted side effects of bile acid-based drugs have been recognized. The proof-of-concept studies that manipulated bile acid synthesis and bile acid composition in mouse liver by transgenic overexpression of Cyp7a1 demonstrated Cyp7a1 may protect against diet-induced obesity and diabetes.214,215 The genomic editing of the CYP7A1 gene, miRNA silencing or epigenetic regulation of CYP7A1 translation and mRNA stability may alter CYP7A1 expression to control the rate of bile acid synthesis, pool size and composition and could lead to effective therapies for treating liver-related diseases.
Acknowledgements
This research is supported by grants DK58379 and DK44442 from the USA National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Myant NB, Mitropoulos KA. Cholesterol 7α-hydroxylase. J. Lipid Res 1977;18: 135–153. [PubMed] [Google Scholar]
- 2.Jelinek DF, Andersson S, Slaughter CA, Russell DW. Cloning and regulation of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J. Biol. Chem 1990;265:8190–8197. [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JY, Miller WF, Lin GM. Regulation of cholesterol 7α-hydroxylase in the liver. Purification of cholesterol 7α-hydroxylase and the immunochemical evidence for the induction of cholesterol 7α-hydroxylase by cholestyramine and circadian rhythm. J. Biol. Chem 1990;265:3889–3897. [PubMed] [Google Scholar]
- 4.Li YC, Wang DP, Chiang JY. Regulation of cholesterol 7α-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7α-hydroxylase mRNA. J. Biol. Chem 1990;265:12012–12019. [PubMed] [Google Scholar]
- 5.Forman BM, Goode E, Chen J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. [DOI] [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. [DOI] [PubMed] [Google Scholar]
- 7.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 1999;3:543–553. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem 2003;278:9435–9440. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun 2002;298: 714–719. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest 2004;113: 1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5–connecting nutrition and metabolism. Thyroid. 2008;18: 167–174. [DOI] [PubMed] [Google Scholar]
- 13.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabol. 2009;10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev 2009;89:147–191. [DOI] [PubMed] [Google Scholar]
- 15.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol 2011;54:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. 2018;18:71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and non-alcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2016;65:350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keitel V, Häussinger D. Role of TGR5 (GPBAR1) in liver disease. Semin. Liver Dis 2018;38:333–339. [DOI] [PubMed] [Google Scholar]
- 19.Chiang JYL, Ferrell JM. Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr 2019;39:175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol 2020;318: G554–G573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishibashi S, Schwartz M, Frykman PK, Hertz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. I. Postnatal lethality reversed by bile acid and vitamin supplementation. J. Biol. Chem 1996;271:18017–18023. [DOI] [PubMed] [Google Scholar]
- 22.Li-Hawkins J, Lund EG, Bronson AD, Russell DW. Expression cloning of an oxysterol 7α -hydroxylase selective for 24- hydroxycholesterol. J. Biol. Chem 2000;275:16543–16549. [DOI] [PubMed] [Google Scholar]
- 23.Repa JJ, Lund EG, Horton JD, et al. Disruption of th sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglycridemia: reversal by cholic acid feeding. J. Biol. Chem 2000;275:39685–39692. [DOI] [PubMed] [Google Scholar]
- 24.Li-Hawkins J, Gåfvels M, Olin M, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Invest 2002;110:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. [DOI] [PubMed] [Google Scholar]
- 26.Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol. Chem 2000;275:16536–16542. [DOI] [PubMed] [Google Scholar]
- 27.Guo GL, Chiang JYL. Is CYP2C70 the key to new mouse models to understand bile acids in humans? J. Lipid Res 2020;61:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RA, Miyake JH, Hui TY, Spann NJ. Regulation of cholesterol 7α-hydroxylase. Barely missing a SHP. J. Lipid Res 2002;43:533–543. [PubMed] [Google Scholar]
- 29.Thakare R, Alamoudi JA, Gautam N, Rodrigues AD, Alnouti Y. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol 2018;38:1323–1335. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Dawson PA. Animal models to study bile acid metabolism. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis 2019;1865:895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang JYL. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017;1:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrell JM, Chiang JYL. Understanding bile acid signaling in diabetes: from pathophysiology to therapeutic targets. Diabetes Metab. J 2019;43:257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massimi M, Lear SR, Huling SL, Jones AL, Erickson SK. Cholesterol 7alpha-hydroxylase (CYP7A): patterns of messenger RNA expression during rat liver development. Hepatology. 1998;28:1064–1072. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KE, Kok E, Javitt NB. Bile acid synthesis in man: metabolism of 7 -hydroxycholesterol-14 C and 26-hydroxycholesterol- 3 H. J. Clin. Invest 1972;51:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda A, Salen G, Shefer S, et al. Regulation of 25- and 27-hydroxylation side chain cleavage pathways for cholic acid biosynthesis in humans, rabbits, and mice. Assay of enzyme activities by high-resolution gas chromatography;-mass spectrometry. J. Lipid Res 2000;41:442–451. [PubMed] [Google Scholar]
- 36.Björkhem I, Araya Z, Rudling M, Angelin B, Einarsson C, Wikvall K. Differences in the regulation of the classical and the alternative pathway for bile acid synthesis in human liver. No coordinate regulation of CYP7A1 and CYP27A1. J. Biol. Chem 2002;277:26804–26807. [DOI] [PubMed] [Google Scholar]
- 37.Russell DW. The Enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem 2003;72:137–174. [DOI] [PubMed] [Google Scholar]
- 38.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res 2009;50(Suppl):S120–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda A, Yoshida T, Xu G, et al. Significance of plasma 7α-hydroxy-4-cholesten-3-one and 27-hydroxycholesterol concentrations as markers for hepatic bile acid synthesis in cholesterol-fed rabbits. Metabolism. 2004;53: 42–48. [DOI] [PubMed] [Google Scholar]
- 40.Honda A, Yamashita K, Numazawa M, et al. Highly sensitive quantification of 7α-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J. Lipid Res 2007;48:458–464. [DOI] [PubMed] [Google Scholar]
- 41.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjövall J Fifty years with bile acids and steroids in health and disease. Lipids. 2004;39:703–722. [DOI] [PubMed] [Google Scholar]
- 43.Pandak WM, Bohdan P, Franklund C, et al. Expression of sterol 12α-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. [DOI] [PubMed] [Google Scholar]
- 44.Kakiyama G, Marques D, Takei H, et al. Mitochondrial oxysterol biosynthetic pathway gives evidence for CYP7B1 as controller of regulatory oxysterols. J. Steroid Biochem. Mol. Biol 2019;189:36–47. [DOI] [PubMed] [Google Scholar]
- 45.Lund EG, Kerr TA, Sakai J, Li WP, Russell DW. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem 1998;273:34316–34327. [DOI] [PubMed] [Google Scholar]
- 46.Honda A, Miyazaki T, Ikegami T, et al. Cholesterol 25-hydroxylation activity of CYP3A. J. Lipid Res 2011;52:1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guengerich FP, Cheng Q. Orphans in the human cytochrome P450 super-family: approaches to discovering functions and relevance in pharmacology. Pharmacol. Rev 2011;63:684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. U. S. A 1999;96:7238–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi S, Fukami T, Masuo Y, et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res 2016;57:2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Boer JF, Verkade E, Mulder NL, et al. A human-like bile acid pool induced by deletion of Cyp2c70 modulates effects of farnesoid X receptor activation in mice. J. Lipid Res 2019;61:291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honda A, Miyazaki T, Iwamoto J, et al. Regulations of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res 2019;61: 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol 2014;30:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikegami T, Honda A. Reciprocal interactions between bile acids and gut microbiota in human liver diseases. Hepatol. Res 2018;48:15–27. [DOI] [PubMed] [Google Scholar]
- 54.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res 1989;30:719–730. [PubMed] [Google Scholar]
- 55.Hrycay E, Forrest D, Liu L, et al. Hepatic bile acid metabolism and expression of cytochrome P450 and related enzymes are altered in Bsep (−/−) mice. Mol. Cell. Biochem 2014;389:119–132. [DOI] [PubMed] [Google Scholar]
- 56.Xie W, Radominska-Pandya A, Shi Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. U. S. A 2001;98:3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voronova V, Sokolov V, Al-Khaifi A, et al. A physiology-based model of bile acid distribution and metabolism under healthy and pathologic conditions in human beings. Cell Mol Gastroenterol Hepatol. 2020;10(1):149–170. S2352–345X(20)30026–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyer JL, Graf J, Meier PJ. Hepatic transport systems regulating pHi, cell volume, and bile secretion. Annu. Rev. Physiol 1992;54:415–438. [DOI] [PubMed] [Google Scholar]
- 59.Kim I, Ahn SH, Inagaki T, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res 2007;48: 2664–2672. [DOI] [PubMed] [Google Scholar]
- 60.Denson LA, Auld KL, Schiek DS, McClure MH, Mangelsdorf DJ, Karpen SJ. Interleukin-1beta suppresses retinoid transactivation of two hepatic transporter genes involved in bile formation. J. Biol. Chem 2000;275:8835–8843. [DOI] [PubMed] [Google Scholar]
- 61.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabol. 2005;2:217–225. [DOI] [PubMed] [Google Scholar]
- 62.Ness GC, Pendleton LC, Li YC, Chiang JY. Effect of thyroid hormone on hepatic cholesterol 7α-hydroxylase, LDL receptor, HMG-CoA reductase, farnesyl pyrophosphate synthetase and apolipoprotein A-I mRNA levels in hypophysectomized rats. Biochem. Biophys. Res. Commun 1990;172:1150–1156. [DOI] [PubMed] [Google Scholar]
- 63.Hylemon PB, Gurley EC, Stravitz RT, et al. Hormonal regulation of cholesterol 7α-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J. Biol. Chem 1992;267:16866–16871. [PubMed] [Google Scholar]
- 64.Pandak WM, Vlahcevic ZR, Chiang JY, Heuman DM, Hylemon PB. Bile acid synthesis. VI. Regulation of cholesterol 7α-hydroxylase by taurocholate and mevalonate. J. Lipid Res 1992;33:659–668. [PubMed] [Google Scholar]
- 65.Wang DP, Chiang JY. Structure and nucleotide sequences of the human cholesterol 7 alpha-hydroxylase gene (CYP7). Genomics. 1994;20:320–323. [DOI] [PubMed] [Google Scholar]
- 66.Wang DP, Stroup D, Marrapodi M, Crestani M, Galli G, Chiang JY. Transcriptional regulation of the human cholesterol 7α-hydroxylase gene (CYP7A) in HepG2 cells. J. Lipid Res 1996;37:1831–1841. [PubMed] [Google Scholar]
- 67.Drover VA, Wong NC, Agellon LB. A distinct thyroid hormone response element mediates repression of the human cholesterol 7α-hydroxylase (CYP7A1) gene promoter. Mol. Endocrinol 2002;16:14–23. [DOI] [PubMed] [Google Scholar]
- 68.Hylemon P, Gurley E, Stravitz R, et al. Hormonal regulation of cholesterol 7α-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J. Biol. Chem 1992;267:16866–16871. [PubMed] [Google Scholar]
- 69.Song KH, Chiang JY. Glucagon and cAMP inhibit cholesterol 7α-hydroxylase (CYP7a1) gene expression in human hepatocytes: discordant regulation of bile acid synthesis and gluconeogenesis. Hepatology. 2006;43:117–125. [DOI] [PubMed] [Google Scholar]
- 70.Russell DW. Nuclear orphan receptors control cholesterol catabolism. Cell. 1999;97:539–542. [DOI] [PubMed] [Google Scholar]
- 71.Ge MX, Shao RG, He HW. Advances in understanding the regulatory mechanism of cholesterol 7α-hydroxylase. Biochem. Pharmacol 2019;164:152–164. [DOI] [PubMed] [Google Scholar]
- 72.Stroup D, Crestani M, Chiang JY. Identification of a bile acid response element in the cholesterol 7 alpha-hydroxylase gene CYP7A. Am. J. Physiol 1997;273: G508–G517. [DOI] [PubMed] [Google Scholar]
- 73.Stroup D, Chiang J. Hepatocyte nuclear factor 4 binds the bile acid response element II of the rat cholesterol 7 alpha-hydroxylase gene. Faseb. J. 1998: A1390. [Google Scholar]
- 74.Stroup D, Chiang JY. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7α-hydroxylase gene (CYP7A1). J. Lipid Res 2000;41:1–11. [PubMed] [Google Scholar]
- 75.Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000;6:517–526. [DOI] [PubMed] [Google Scholar]
- 76.Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000;6:507–515. [DOI] [PubMed] [Google Scholar]
- 77.Wang L, Lee YK, Bundman D, et al. Redundant pathways for negative feedback regulation of bile Acid production. Dev. Cell 2002;2:721–731. [DOI] [PubMed] [Google Scholar]
- 78.de Aguiar Vallim TQ, Tarling EJ, Ahn H, et al. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metabol. 2015;21: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pandak WM, Heuman DM, Hylemon PB, Chiang JY, Vlahcevic ZR. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7α-hydroxylase in rats with biliary fistulas. Gastroenterology. 1995;108:533–544. [DOI] [PubMed] [Google Scholar]
- 80.Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J. Biol. Chem 2005;280:17707–17714. [DOI] [PubMed] [Google Scholar]
- 81.Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7α-hydroxylase gene expression. Hepatology. 2009;49:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. [DOI] [PubMed] [Google Scholar]
- 84.Han S, Zhang R, Jain R, et al. Circadian control of bile acid synthesis by a KLF15-Fgf15 axis. Nat. Commun 2015;6:7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vergnes L, Lee JM, Chin RG, Auwerx J, Reue K. Diet1 functions in the FGF15/19 enterohepatic signaling axis to modulate bile acid and lipid levels. Cell Metabol. 2013;17:916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JM, Ong JR, Vergnes L, et al. Diet1, bile acid diarrhea, and FGF15/19: mouse model and human genetic variants. J. Lipid Res 2018;59:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyake JH, Wang SL, Davis RA. Bile acid induction of cytokine expression by macrophages correlates with repression of hepatic cholesterol 7α-hydroxylase. J. Biol. Chem 2000;275:21805–21808. [DOI] [PubMed] [Google Scholar]
- 88.Lou G, Ma X, Fu X, et al. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PloS One. 2014;9,e93567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jahan A, Chiang JY. Cytokine regulation of human sterol 12α-hydroxylase (CYP8B1) gene. Am. J. Physiol. Gastrointest. Liver Physiol 2005;288: G685–G695. [DOI] [PubMed] [Google Scholar]
- 90.Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7α-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 2006;43:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Feng D, Cai Y, et al. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology. 2018;68:1604–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45: 695–704. [DOI] [PubMed] [Google Scholar]
- 94.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig. Liver Dis 2014;46:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J. Endocrinol 2006;191:197–205. [DOI] [PubMed] [Google Scholar]
- 96.Vassileva G, Golovko A, Markowitz L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J 2006;398: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol 2011;25:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439: 484–489. [DOI] [PubMed] [Google Scholar]
- 99.Donepudi AC, Boehme S, Li F, Chiang JY. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology. 2017;65:813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pathak P, Liu H, Boehme S, et al. Farnesoid X receptor induces takeda G-protein receptor 5 crosstalk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem 2017;292:11055–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun 2015;6: 7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chiang JY, Stroup D. Identification and characterization of a putative bile acid responsive element in cholesterol 7α-hydroxylase gene promoter. J. Biol. Chem 1994;269:17502–17507. [PubMed] [Google Scholar]
- 103.Crestani M, Stroup D, Chiang JY. Hormonal regulation of the cholesterol 7 alpha-hydroxylase gene (CYP7). J. Lipid Res 1995;36:2419–2432. [PubMed] [Google Scholar]
- 104.Stroup D, Crestani M, Chiang JY. Orphan receptors chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and retinoid X receptor (RXR) activate and bind the rat cholesterol 7α-hydroxylase gene (CYP7A). J. Biol. Chem 1997;272:9833–9839. [DOI] [PubMed] [Google Scholar]
- 105.Chiang JY, Kimmel R, Weinberger C, Stroup D. FXR responds to bile acids and represses cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem 2000;275:10918–10924. [DOI] [PubMed] [Google Scholar]
- 106.Nitta M, Ku S, Brown C, Okamoto AY, Shan B. CPF: an orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7α-hydroxylase gene. Proc. Natl. Acad. Sci. U. S. A 1999;96:6660–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song KH, Ellis E, Strom S, Chiang JY. Hepatocyte growth factor signaling pathway inhibits cholesterol 7α-hydroxylase and bile acid synthesis in human hepatocytes. Hepatology. 2007;46:1993–2002. [DOI] [PubMed] [Google Scholar]
- 109.Song KH, Li T, Chiang JY. A prospero-related homeodomain protein is a novel co-regulator of hepatocyte nuclear factor 4α that regulates the cholesterol 7α-hydroxylase gene. J. Biol. Chem 2006;281:10081–10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marrapodi M, Chiang JY. Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J. Lipid Res 2000;41:514–520. [PubMed] [Google Scholar]
- 111.Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab. Dispos 2001;29:1467–1472. [PubMed] [Google Scholar]
- 112.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor (PXR) inhibition of human cholesterol 7α-hydroxylase gene (CYP7A1) transcription. Am. J. Physiol. Gastrointest. Liver Physiol 2005;288:G74–G84. [DOI] [PubMed] [Google Scholar]
- 113.Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. [DOI] [PubMed] [Google Scholar]
- 114.Hashimoto M, Kobayashi K, Watanabe M, et al. Knockout of mouse Cyp3a gene enhances synthesis of cholesterol and bile acid in the liver. J. Lipid Res 2013;54:2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu L, Hammer RE, Li-Hawkins J, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. U. S. A 2002;99:16237–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peet DJ, Turley SD, Ma W, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. [DOI] [PubMed] [Google Scholar]
- 117.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXRa. Nature. 1996;383: 728–731. [DOI] [PubMed] [Google Scholar]
- 118.Chiang JY, Kimmel R, Stroup D. Regulation of cholesterol 7α-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRa). Gene. 2001;262: 257–265. [DOI] [PubMed] [Google Scholar]
- 119.Chen W, Owsley E, Yang Y, Stroup D, Chiang JY. Nuclear receptor-mediated repression of human cholesterol 7α-hydroxylase gene transcription by bile acids. J. Lipid Res 2001;42:1402–1412. [PubMed] [Google Scholar]
- 120.Shang Q, Pan L, Saumoy M, et al. An overlapping binding site in the CYP7A1 promoter allows activation of FXR to override the stimulation by LXRα. Am. J. Physiol. Gastrointest. Liver Physiol 2007;293:G817–G823. [DOI] [PubMed] [Google Scholar]
- 121.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. [DOI] [PubMed] [Google Scholar]
- 122.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. [DOI] [PubMed] [Google Scholar]
- 123.Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han S, Chiang JY. Mechanism of vitamin D receptor inhibition of cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. Drug Metab. Dispos 2009;37:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol. Endocrinol 2010;24: 1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): roles of hepatocyte nuclear factor 4α (HNF4α) in mediating bile acid repression. J. Biol. Chem 2001;276:41690–41699. [DOI] [PubMed] [Google Scholar]
- 127.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr. Rev 2002;23:443–463. [DOI] [PubMed] [Google Scholar]
- 128.Yang Y, Zhang M, Eggertsen G, Chiang JY. On the mechanism of bile acid inhibition of rat sterol 12α- hydroxylase gene (CYP8B1) transcription: roles of alpha-fetoprotein transcription factor and hepatocyte nuclear factor 4α. Biochim. Biophys. Acta 2002;1583:63–73. [DOI] [PubMed] [Google Scholar]
- 129.del Castillo-Olivares A, Gil G. Alpha 1-fetoprotein transcription factor is required for the expression of sterol 12α-hydroxylase, the specific enzyme for cholic acid synthesis. Poential role in the bile acid-mediated regulation of gene transcription. J. Biol. Chem 2000;275:17793–17799. [DOI] [PubMed] [Google Scholar]
- 130.Andersson U, Yang YZ, Björkhem I, Einarsson C, Eggertsen G, Gåfvels M. Thyroid hormone suppresses hepatic sterol 12α-hydroxylase (CYP8B1) activity and messenger ribonucleic acid in rat liver: failure to define known thyroid hormone response elements in the gene. Biochim. Biophys. Acta 1999;1438:167–174. [DOI] [PubMed] [Google Scholar]
- 131.Yang Y, Eggertsen G, Gåfvels M, et al. Mechanisms of cholesterol and sterol regulatory element binding protein regulation of the sterol 12α-hydroxylase gene (CYP8B1). Biochem. Biophys. Res. Commun 2004;320:1204–1210. [DOI] [PubMed] [Google Scholar]
- 132.Petrov PD, Fernández-Murga L, Conde I, et al. Epistane, an anabolic steroid used for recreational purposes, causes cholestasis with elevated levels of cholic acid conjugates, by upregulating bile acid synthesis (CYP8B1) and cross-talking with nuclear receptors in human hepatocytes. Arch. Toxicol 2020;94:589–607. [DOI] [PubMed] [Google Scholar]
- 133.Fan L, Joseph JF, Durairaj P, Parr MK, Bureik M. Conversion of chenodeoxycholic acid to cholic acid by human CYP8B1. Biol. Chem 2019;400:625–628. [DOI] [PubMed] [Google Scholar]
- 134.Miao J, Choi SE, Seok SM, et al. Ligand-dependent regulation of the activity of the orphan nuclear receptor, small heterodimer partner (SHP), in the repression of bile acid biosynthetic CYP7A1 and CYP8B1 genes. Mol. Endocrinol 2011;25:1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schwarz M, Lund EG, Lathe R, Björkhem I, Russell DW. Identification and characterization of a mouse oxysterol 7α-hydroxylase cDNA. J. Biol. Chem 1997;272:23995–24001. [DOI] [PubMed] [Google Scholar]
- 136.Pandak WM, Hylemon PB, Ren S, et al. Regulation of oxysterol 7α-hydroxylase (CYP7B1) in primary cultures of rat hepatocytes. Hepatology. 2002;35: 1400–1408. [DOI] [PubMed] [Google Scholar]
- 137.Wu Z, Martin KO, Javitt NB, Chiang JY. Structure and functions of human oxysterol 7α-hydroxylase cDNAs and gene CYP7B1. J. Lipid Res 1999;40: 2195–2203. [PubMed] [Google Scholar]
- 138.Norlin M, Chiang J. Transcriptional regulation of human oxysterol 7α-hydroxylase (CYP7B1) by sterol response element binding protein (SREBP) and alpha-fetoprotein transcription factor (FTF). Faseb. J 2003;316(1):158–164. [DOI] [PubMed] [Google Scholar]
- 139.Norlin M, Chiang JY. Transcriptional regulation of human oxysterol 7α-hydroxylase by sterol response element binding protein. Biochem. Biophys. Res. Commun 2004;316:158–164. [DOI] [PubMed] [Google Scholar]
- 140.Wada T, Kang HS, Angers M, et al. Identification of oxysterol 7α-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORα) (NR1F1) target gene and a functional cross-talk between RORα and liver X receptor (NR1H3). Mol. Pharmacol 2008;73:891–899. [DOI] [PubMed] [Google Scholar]
- 141.Holloway MG, Laz EV, Waxman DJ. Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4α. Mol. Endocrinol 2006;20:647–660. [DOI] [PubMed] [Google Scholar]
- 142.Tang W, Norlin M, Wikvall K. Glucocorticoid receptor-mediated upregulation of human CYP27A1, a potential anti-atherogenic enzyme. Biochim. Biophys. Acta 2008;1781:718–723. [DOI] [PubMed] [Google Scholar]
- 143.Worthmann A, John C, Ruhlemann MC, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med 2017;23:839–849. [DOI] [PubMed] [Google Scholar]
- 144.Pandak WM, Ren S, Marques D, et al. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J. Biol. Chem 2002;277:48158–48164. [DOI] [PubMed] [Google Scholar]
- 145.Hall EA, Ren S, Hylemon PB, et al. Detection of the steroidogenic acute regulatory protein, StAR, in human liver cells. Biochim. Biophys. Acta 2005;1733: 111–119. [DOI] [PubMed] [Google Scholar]
- 146.Chen W, Chiang JY. Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4a (HNF4a). Gene. 2003;313:71–82. [DOI] [PubMed] [Google Scholar]
- 147.Araya Z, Tang W, Wikvall K. Hormonal regulation of the human sterol 27-hydroxylase gene CYP27A1. Biochem. J 2003;372:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Segev H, Honigman A, Rosen H, Leitersdorf E. Transcriptional regulation of the human sterol 27-hydroxylase gene (CYP27) and promoter mapping. Atherosclerosis. 2001;156:339–347. [DOI] [PubMed] [Google Scholar]
- 149.Li T, Chen W, Chiang JY. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J. Lipid Res 2007;48:373–384. [DOI] [PubMed] [Google Scholar]
- 150.Agellon LB, Cheema SK. The 3’-untranslated region of the mouse cholesterol 7α-hydroxylase mRNA contains elements responsive to post-transcriptional regulation by bile acids. Biochem. J 1997;328:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie Y, Blanc V, Kerr TA, et al. Decreased expression of cholesterol 7α-hydroxylase and altered bile acid metabolism in apobec-1−/− mice lead to increased gallstone susceptibility. J. Biol. Chem 2009;284:16860–16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tarling EJ, Clifford BL, Cheng J, et al. RNA-binding protein ZFP36L1 maintains posttranscriptional regulation of bile acid metabolism. J. Clin. Invest 2017;127:3741–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chang J, Nicolas E, Marks D, et al. MiR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. [DOI] [PubMed] [Google Scholar]
- 154.Esau C, Davis S, Murray SF, et al. MiR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metabol. 2006;3:87–98. [DOI] [PubMed] [Google Scholar]
- 155.Kutay H, Bai S, Datta J, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J. Cell. Biochem 2006;99:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156.Cheung O, Puri P, Eicken C, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song KH, Li T, Owsley E, Chiang JY. A putative role of micro RNA in regulation of cholesterol 7-α-hydroxylase expression in human hepatocytes. J. Lipid Res 2010;51:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rayner KJ, Suarez Y, Dávalos A, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marquart TJ, Allen RM, Ory DS, Baldan A. MiR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. U. S. A 2010;107: 12228–12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kemper JK, Kim H, Miao J, Bhalla S, Bae Y. Role of an mSin3A-Swi/Snf chromatin remodeling complex in the feedback repression of bile acid biosynthesis by SHP. Mol. Cell Biol 2004;24:7707–7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fang S, Tsang S, Jones R, et al. The p300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. J. Biol. Chem 2008;283:35086–35095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miao J, Xiao Z, Kanamaluru D, et al. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 2009;23:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kemper JK, Xiao Z, Ponugoti B, et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metabol. 2009;10:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seok S, Kanamaluru D, Xiao Z, et al. Bile acid signal-induced phosphorylation of small heterodimer partner by protein kinase czeta is critical for epigenomic regulation of liver metabolic genes. J. Biol. Chem 2013;288:23252–23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim DH, Xiao Z, Kwon S, et al. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J. 2015;34:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kemper JK. Regulation of FXR transcriptional activity in health and disease: emerging roles of FXR cofactors and post-translational modifications. Biochim. Biophys. Acta 2011;1812:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li T, Chanda D, Zhang Y, Choi HS, Chiang JY. Glucose stimulates cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. J. Lipid Res 2010;51:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li T, Francl JM, Boehme S, et al. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J. Biol. Chem 2012;287:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fang S, Miao J, Xiang L, Ponugoti B, Treuter E, Kemper JK. Coordinated recruitment of histone methyltransferase G9a and other chromatin modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol. Cell Biol 2007;27:1407–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boulias K, Talianidis I. Functional role of G9a-induced histone methylation in small heterodimer partner-mediated transcriptional repression. Nucleic Acids Res. 2004;32:6096–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ouyang H, Qin Y, Liu Y, Xie Y, Liu J. Prox1 directly interacts with LSD1 and recruits the LSD1/NuRD complex to epigenetically co-repress CYP7A1 transcription. PloS One. 2013;8, e62192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol. Rev 2013;93:107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eckel-Mahan K, Sassone-Corsi P. Epigenetic regulation of the molecular clockwork. Prog Mol Biol Transl Sci. 2013;119:29–50. [DOI] [PubMed] [Google Scholar]
- 176.Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J. Hepatol 2019;71:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ferrell JM, Chiang JY. Circadian rhythms in liver metabolism and disease. Acta Pharm. Sin. B 2015;5:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kudo T, Kawashima M, Tamagawa T, Shibata S. Clock mutation facilitates accumulation of cholesterol in the liver of mice fed a cholesterol and/or cholic acid diet. Am. J. Physiol. Endocrinol. Metab 2008;294:E120–E130. [DOI] [PubMed] [Google Scholar]
- 179.Ferrell JM, Chiang JY. Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell Mol Gastroenterol Hepatol. 2015;1:664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PloS One. 2011;6, e16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. [DOI] [PubMed] [Google Scholar]
- 182.Kawamoto T, Noshiro M, Furukawa M, et al. Effects of fasting and re-feeding on the expression of Dec1, Per1, and other clock-related genes. J. Biochem 2006;140:401–408. [DOI] [PubMed] [Google Scholar]
- 183.Lavery DJ, Schibler U. Circadian transcription of the cholesterol 7α-hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993;7: 1871–1884. [DOI] [PubMed] [Google Scholar]
- 184.Wuarin J, Schibler U. Expression of the liver enriched transcription activator protein DBP follows a stringent circadian rhythm. Cell. 1990;63:1257–1266. [DOI] [PubMed] [Google Scholar]
- 185.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–689. [PMC free article] [PubMed] [Google Scholar]
- 186.Lee YH, Alberta JA, Gonzalez FJ, Waxman DJ. Multiple, functional DBP sites on the promoter of the cholesterol 7α-hydroxylase P450 gene, CYP7. J. Biol. Chem 1994;269:14681–14689. [PubMed] [Google Scholar]
- 187.Preitner N, Damiola F, Lopez-Molina L, et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. [DOI] [PubMed] [Google Scholar]
- 188.Yin L, Wu N, Curtin JC, et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. [DOI] [PubMed] [Google Scholar]
- 189.Le Martelot G, Claudel T, Gatfield D, et al. Rev-erbα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7, e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Duez H, van der Veen JN, Duhem C, et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbα. Gastroenterology. 2008;135:689–698. [DOI] [PubMed] [Google Scholar]
- 191.Noshiro M, Kawamoto T, Furukawa M, et al. Rhythmic expression of DEC1 and DEC2 in peripheral tissues: DEC2 is a potent suppressor for hepatic cytochrome P450s opposing DBP. Gene Cell. 2004;9:317–329. [DOI] [PubMed] [Google Scholar]
- 192.Pathak P, Li T, Chiang JY. Retinoic acid-related orphan receptor alpha regulates diurnal rhythm and fasting induction of sterol 12α-hydroxylase in bile acid synthesis. J. Biol. Chem 2013;288:37154–37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ma K, Xiao R, Tseng HT, Shan L, Fu L, Moore DD. Circadian dysregulation disrupts bile acid homeostasis. PloS One. 2009;4, e6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. [DOI] [PubMed] [Google Scholar]
- 195.Lundåsen TG €alman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med 2006;260:530–536. [DOI] [PubMed] [Google Scholar]
- 196.Gälman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J. Intern. Med 2011;270:580–588. [DOI] [PubMed] [Google Scholar]
- 197.Gälman C, Lundåsen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metabol. 2008;8:169–174. [DOI] [PubMed] [Google Scholar]
- 198.Al-Khaifi A, Straniero S, Voronova V, et al. Asynchronous rhythms of circulating conjugated and unconjugated bile acids in the modulation of human metabolism. J. Intern. Med 2018;284:549–559. [DOI] [PubMed] [Google Scholar]
- 199.Vaz FM, Ferdinandusse S. Bile acid analysis in human disorders of bile acid biosynthesis. Mol. Aspect. Med 2017;56:10–24. [DOI] [PubMed] [Google Scholar]
- 200.Pullinger CR, Eng C, Salen G, et al. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest 2002;110:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nakamoto K, Wang S, Jenison RD, et al. Linkage disequilibrium blocks, haplotype structure, and htSNPs of human CYP7A1 gene. BMC Genet. 2006;7: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lenícek M, Komárek V, Zimolov a M, et al. CYP7A1 promoter polymorphism-203A>C affects bile salt synthesis rate in patients after ileal resection. J. Lipid Res 2008;49:2664–2667. [DOI] [PubMed] [Google Scholar]
- 203.Jiang XY, Zhang Q, Chen P, et al. CYP7A1 polymorphism influences the LDL cholesterol-lowering response to atorvastatin. J. Clin. Pharm. Therapeut 2012;37:719–723. [DOI] [PubMed] [Google Scholar]
- 204.Liu N, Yang G, Liu Y, et al. Effect of cytochrome P450 7A1 (CYP7A1) polymorphism on lipid responses to simvastatin treatment. J. Cardiovasc. Pharmacol 2020;75:168–173. [DOI] [PubMed] [Google Scholar]
- 205.Hofman MK, Weggemans RM, Zock PL, Schouten EG, Katan MB, Princen HM. CYP7A1 A-278C polymorphism affects the response of plasma lipids after dietary cholesterol or cafestol interventions in humans. J. Nutr 2004;134: 2200–2204. [DOI] [PubMed] [Google Scholar]
- 206.Kovár J, Suchánek P, Hub acek JA, Poledne R. The A-204C polymorphism in the cholesterol 7α-hydroxylase (CYP7A1) gene determines the cholesterolemia responsiveness to a high-fat diet. Physiol. Res 2004;53:565–568. [PubMed] [Google Scholar]
- 207.Barcelos AL, Chies R, Almeida SE, et al. Association of CYP7A1 −278A>C polymorphism and the response of plasma triglyceride after dietary intervention in dyslipidemic patients. Braz. J. Med. Biol. Res 2009;42:487–493. [DOI] [PubMed] [Google Scholar]
- 208.Abdullah MM, Eck PK, Couture P, Lamarche B, Jones PJH. The combination of single nucleotide polymorphisms rs6720173 (ABCG5), rs3808607 (CYP7A1), and rs760241 (DHCR7) is associated with differing serum cholesterol responses to dairy consumption. Appl. Physiol. Nutr. Metabol 2018;43: 1090–1093. [DOI] [PubMed] [Google Scholar]
- 209.Iwanicki T, Balcerzyk A, Niemiec P, et al. CYP7A1 gene polymorphism located in the 5’ upstream region modifies the risk of coronary artery disease. Dis. Markers 2015;2015:185969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cai Q, Wang ZQ, Cai Q, Li C, Chen EZ, Jiang ZY. Relationship between CYP7A1–204A>C polymorphism with gallbladder stone disease and serum lipid levels: a meta-analysis. Lipids Health Dis. 2014;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Juzyszyn Z, Kurzawski M, Lener A, Modrzejewski A, Pawlik A, Droździk M. Cholesterol 7a-hydrolase (CYP7A1) c.−278A>C promoter polymorphism in gallstone disease patients. Genet. Test 2008;12:97–100. [DOI] [PubMed] [Google Scholar]
- 212.Sánchez-Cuén J, Aguilar-Medina M, Arámbula-Meraz E, et al. ApoB-100, ApoE and CYP7A1 gene polymorphisms in Mexican patients with cholesterol gallstone disease. World J. Gastroenterol 2010;16:4685–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vlachova M, Blahova T, Lanska V, et al. Diurnal variation in cholesterol 7ahydroxylase activity is determined by the −203A>C polymorphism of the CYP7A1 gene. Croat. Med. J 2016;57:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 2010;52:678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li T, Matozel M, Boehme S, et al. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ferrell JM, Boehme S, Li F, Chiang JY. Cholesterol 7α-hydroxylase-deficient mice are protected from high fat/high cholesterol diet-induced metabolic disorders. J. Lipid Res 2016;57:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Setchell KD, Schwarz M, O’Connell NC, et al. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7α-hydroxylase gene causes severe neonatal liver disease. J. Clin. Invest 1998;102:1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jakobsson J, Karypidis H, Johansson JE, Roh HK, Rane A, Ekström L. A functional C-G polymorphism in the CYP7B1 promoter region and its different distribution in orientals and caucasians. Pharmacogenomics J. 2004;4:245–250. [DOI] [PubMed] [Google Scholar]
- 219.Tang W, Eggertsen G, Chiang JY, Norlin M. Estrogen-mediated regulation of CYP7B1: a possible role for controlling DHEA levels in human tissues. J. Steroid Biochem. Mol. Biol 2006;100:42–51. [DOI] [PubMed] [Google Scholar]
- 220.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5α-androstane-3β,17β-diol, and CYP7B1, regulates prostate growth. Proc. Natl. Acad. Sci. U. S. A 2002;99:13589–13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tang W, Norlin M. Regulation of steroid hydroxylase CYP7B1 by androgens and estrogens in prostate cancer LNCaP cells. Biochem. Biophys. Res. Commun 2006;344:540–546. [DOI] [PubMed] [Google Scholar]
- 222.Olsson M, Gustafsson O, Skogastierna C, et al. Regulation and expression of human CYP7B1 in prostate: overexpression of CYP7B1 during progression of prostatic adenocarcinoma. Prostate. 2007;67:1439–1446. [DOI] [PubMed] [Google Scholar]
- 223.Omoto Y, Lathe R, Warner M, Gustafsson JA. Early onset of puberty and early ovarian failure in CYP7B1 knockout mice. Proc. Natl. Acad. Sci. U. S. A 2005;102:2814–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tsaousidou MK, Ouahchi K, Warner TT, et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet 2008;82:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Biancheri R, Ciccolella M, Rossi A, et al. White matter lesions in spastic paraplegia with mutations in SPG5/CYP7B1. Neuromuscul. Disord 2009;19: 62–65. [DOI] [PubMed] [Google Scholar]
- 226.Criscuolo C, Filla A, Coppola G, et al. Two novel CYP7B1 mutations in Italian families with SPG5: a clinical and genetic study. J. Neurol 2009;256: 1252–1257. [DOI] [PubMed] [Google Scholar]
- 227.Schüle R, Brandt E, Karle KN, et al. Analysis of CYP7B1 in non-consanguineous cases of hereditary spastic paraplegia. Neurogenetics. 2009;10:97–104. [DOI] [PubMed] [Google Scholar]
- 228.Arnoldi A, Crimella C, Tenderini E, et al. Clinical phenotype variability in patients with hereditary spastic paraplegia type 5 associated with CYP7B1 mutations. Clin. Genet 2012;81:150–157. [DOI] [PubMed] [Google Scholar]
- 229.Qin J, Han TQ, Yuan WT, et al. Single nucleotide polymorphism rs3732860 in the 3’-untranslated region of CYP8B1 gene is associated with gallstone disease in Han Chinese. J. Gastroenterol. Hepatol 2013;28:717–722. [DOI] [PubMed] [Google Scholar]
- 230.Pathak P, Chiang JYL. Sterol 12α-hydroxylase aggravates dyslipidemia by activating the ceramide/mTORC1/SREBP1C pathway via FGF21 and FGF15. Gene Expr. 2019;19:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12a-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat. Rev. Endocrinol 2019;15: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64:1168–1179. [DOI] [PubMed] [Google Scholar]
- 234.Bertaggia E, Jensen KK, Castro-Perez J, et al. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am. J. Physiol. Endocrinol. Metab 2017;313:E121–E133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Patankar JV, Wong CK, Morampudi V, et al. Genetic ablation of Cyp8b1 preserves host metabolic function by repressing steatohepatitis and altering gut microbiota composition. Am. J. Physiol. Endocrinol. Metab 2018;314: E418–E432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chevre R, Trigueros-Motos L, Castano D, et al. Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice. Faseb. J 2018;32:3792–3802. [DOI] [PubMed] [Google Scholar]
- 237.Hu X, Bonde Y, Eggertsen G, Rudling M. Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J. Intern. Med 2014;275:27–38. [DOI] [PubMed] [Google Scholar]
- 238.Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem 1991;266:7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 239.Leitersdorf E, Reshef A, Meiner V, et al. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous Xanthomatosis in Jews of Moroccan origin. J. Clin. Invest 1993;91:2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Salen G, Steiner RD. Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX). J. Inherit. Metab. Dis 2017;40:771–781. [DOI] [PubMed] [Google Scholar]
- 241.Honda A, Salen G, Matsuzaki Y, et al. Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27(−/−) mice and CTX. J. Lipid Res 2001;42:291–300. [PubMed] [Google Scholar]
- 242.Dussault I, Yoo HD, Lin M, et al. Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc. Natl. Acad. Sci. U. S. A 2003;100:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Amador MDM, Masingue M, Debs R, et al. Treatment with chenodeoxycholic acid in cerebrotendinous xanthomatosis: clinical, neurophysiological, and quantitative brain structural outcomes. J. Inherit. Metab. Dis 2018;41: 799–807. [DOI] [PubMed] [Google Scholar]
- 244.Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med 2007;13:1185–1192. [DOI] [PubMed] [Google Scholar]
- 245.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342: 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kimbung S, Chang CY, Bendahl PO, et al. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr. Relat. Canc 2017;24: 339–349. [DOI] [PubMed] [Google Scholar]
- 247.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 248.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol 2019;16:411–428. [DOI] [PubMed] [Google Scholar]
- 249.Mouzaki M, Wang AY, Bandsma R, et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PloS One. 2016;11, e0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Puri P, Daita K, Joyce A, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chiang JYL, Ferrell JM. Bile acid biology, pathophysiology, and therapeutics. Clin. Liver Dis 2020;15:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Camilleri M Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015;9:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes. Metabol 2010;12:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am. J. Physiol. Gastrointest. Liver Physiol 2010;298: G419–G424. [DOI] [PubMed] [Google Scholar]
- 255.Potthoff MJ, Potts A, He T, et al. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am. J. Physiol. Gastrointest. Liver Physiol 2013;304:G371–G380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid x receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582 (e1). [DOI] [PubMed] [Google Scholar]
- 257.Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761 (e8). [DOI] [PubMed] [Google Scholar]
- 258.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med 2016;375:631–643. [DOI] [PubMed] [Google Scholar]
- 259.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kowdley KV, Luketic V, Chapman R, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Al-Dury S, Wahlström A, Panzitt K, et al. Obeticholic acid may increase the risk of gallstone formation in susceptible patients. J. Hepatol 2019;67:1890–1902. [DOI] [PubMed] [Google Scholar]
- 262.Trauner M, Gulamhusein A, Hameed B, et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology. 2019;70:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pedrosa M, Seyedkazemi S, Francque S, et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: study design of the TANDEM trial. Contemp. Clin. Trials 2019;88:105889. [DOI] [PubMed] [Google Scholar]
- 264.Mueller M, Thorell A, Claudel T, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol 2015;62:1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med 2018;24:1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2019;71:1198–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]


