Abstract
Background.
HLA incompatible renal transplantation still remains one of best therapeutic options for a subgroup of patients who are highly sensitized and difficult to match but not much is known about its long-term graft and patient survival.
Methods.
One hundred thirty-four HLA incompatible renal transplantation patients from 2003 to 2018 with a median follow of 6.93 y were analyzed retrospectively to estimate patient and graft survivals. Outcomes were compared with groups defined by baseline crossmatch status and the type and timings of rejection episodes.
Results.
The overall patient survival was 95%, 90%, and 81%; and graft survival was 95%, 85%, and 70% at 1, 5, and 10 y, respectively. This was similar to the first-time deceased donor transplant cohort. The graft survival for pretreatment cytotoxic-dependent crossmatch (CDC) positive crossmatch group was significantly low at 83%, 64%, and 40% at 1, 5, and 10 y, respectively, compared with other groups (Bead/CDC, P = 0.007; CDC/Flow, P = 0.001; and microbead assay/flow cytometry crossmatch, P = 0.837), although those with a low CDC titer (<1 in 2) have comparable outcomes to the CDC negative group. Female patients in general fared worse in both patient and graft survival outcomes in each of the 3 groups based on pretreatment crossmatch, although this did not reach statistical significance. Antibody-mediated rejection was the most frequent type of rejection with significant decline in graft survival by 10 y when compared with no rejection (P < 0.001). Rejection that occurred or continued to occur after the first 2 wk of transplantation caused a significant reduction in graft survivals (P < 0.001), whereas good outcomes were seen in those with a single early rejection episode.
Conclusions.
One-, 5-, and 10-y HLA incompatible graft and patient survival is comparable to deceased donor transplantation and can be further improved by excluding high-CDC titer cases. Antibody-positive female patients show worse long-term survival. Resolution of early rejection is associated with good long-term graft survival.
INTRODUCTION
HLA-specific antibodies have been considered a significant barrier to successful kidney transplantation since this was first recognized in 1969.1 In the mid-1980s, pretransplant antibody removal, or desensitization, was pioneered to overcome this barrier,2 but these have tended to be high-risk transplants with inferior outcomes. Moreover, in recent years, there has been a downward trend of HLA antibody incompatible (HLAi) renal transplantation worldwide. In the United Kingdom, although approximately 40% of patients on the transplant waiting list are sensitized with HLA-specific antibodies, only 1.5% of the kidney transplantations performed last year were HLAi renal transplantations.3 The reason for the global decrease in HLAi kidney transplantation is the concomitant increase in the paired kidney exchange (PKE) programs and the changes in the local allocation policies.4,5 Nevertheless, there is an ongoing requirement for HLAi transplantation in patients wherein finding a compatible donor through PKE is difficult. For these highly sensitized patients, desensitization could be considered via a combined approach wherein the recipients are offered a lower immunologic risk donor through the PKE or directly from their potential donors after risk stratification.6 Moreover, with the increase in fast track and extended criteria donors, it is likely that more patients will be sensitized in the coming years because of the reduced longevity of these kidneys and lower levels of HLA matching,7 thus potentially increasing the need for HLAi transplantation. Therefore, it becomes important to determine the benefits or detriment of such transplantations so that we can use this appropriately to optimize patient care.
Studies have reported an increase in patient survival with HLAi renal transplantation in comparison to patients remaining on the waiting list or on dialysis.8,9 However, a recent study from the United Kingdom did not show any survival benefit in patients undergoing HLAi in comparison to those who remain on dialysis.10 Many studies have also shown reasonable success in short- and medium-term outcomes in graft survival in HLAi renal transplantation.11,12 Advances in antibodies detection and screening, HLA typing, and desensitization of patients have aided in curtailing the risk associated with HLAi renal transplants.13 Despite this, graft rejection remains a major obstacle in achieving more successful long-term outcomes in HLAi renal transplantations, which is further compounded by the lack of effective treatment for antibody-mediated rejection (AMR).14 There is, however, a lack of published on long-term (10 or more y) outcome data for HLAi kidney transplantation. We now have completed sufficient cases to be able to show 10-y patient and graft survival data and identify key factors that determine long-term outcomes.
MATERIALS AND METHODS
Patients
A retrospective study was conducted at the University Hospitals Coventry and Warwickshire (UHCW) NHS Trust, which is a UK tertiary international referral center for HLAi transplants. The study was approved by the Coventry University Ethical committee and the UHCW Research and Development. Patients who underwent HLAi renal transplantation between 2003 and 2018 were included. Table 1 lists the key characteristics of the study population. These patients were referred from various centers in the United Kingdom and Ireland and were transferred back once stable posttransplant. All relevant clinical data of patients were collated from UHCW and referral centers, whereas all immunologic and histocompatibility-related data were collated from NHS Blood and Transplant Centre, Birmingham. We also compared the overall patient and graft survival data of the HLAi study cohort with (i) first-time deceased donor renal transplant, UK cohort, (ii) first-time deceased donor transplant, (iii) UHCW (our center) cohort, and (iv) the standard live donor transplant of UHCW cohort.
TABLE 1.
Patient characteristics
| Patient characteristics | HLAi cohort |
|---|---|
| Total number of patients | 134 |
| Gender, n (%) | |
| Female | 82 (61.2) |
| Male | 52 (38.8) |
| Mean age at time of transplantation, mean ± SD | 42.93 ± 11.52 |
| Median follow-up time in y, median ± SD | 6.93 ± 3.33 |
| Donor-specific antibodies, n (%) | |
| Class I HLA | 52 (38.8) |
| Class II HLA | 41 (30.6) |
| Class I + II HLA | 41 (30.6) |
| Crossmatch, n (%) | |
| Bead positive | 47 (35.1) |
| FC positive | 64 (47.8) |
| CDC positive | 23 (17.2) |
| Transplantation type, n (%) | |
| Living donor transplantation | 118 (88.1) |
| Deceased donor transplantation | 16 (12.0) |
| Comorbidities | |
| Yes, n (%) | 61 (45.5) |
| Hypertension, n | 8 |
| Hypotension, n | 12 |
| Obesity, n | 4 |
| Diabetes, n | 3 |
| Others, n | 34 |
| No, n (%) | 73 (55.5) |
| Number of previous transplants, n (%) | |
| 0 | 51 (38.1) |
| 1 | 61 (45.5) |
| 2 | 15 (11.2) |
| 3 | 7 (5.2) |
| aTreatment approach, n | |
| OKT3 | 8 |
| ATG | 35 |
| IVIG | 6 (including 2 preop) |
| Rituximab | 2 (preop) |
| Campath | 3 |
aPatients with >1 treatment for rejection.
ATG, antithymocyte globulin; Bead, microbead assay; CDC, cytotoxic-dependent crossmatch; FC, flow cytometry crossmatch; HLAi, HLA antibody incompatible; OKT3, muromomab-CD3.
Patients sensitized to HLA antigens were selected for antibody incompatible transplantation if they had reactivity with donor HLA antigens measured by cytotoxic-dependent crossmatch (CDC), flow cytometry crossmatches (FC), or microbead assay (Bead). Patients who had ABO incompatibility or had combined HLAi and ABOi transplants were excluded.
Pretransplant patients were typically treated with 5 alternate day sessions of double filtration plasmapheresis (DFPP) with the aim of achieving negative flow crossmatch at the time of surgery. In some cases, depending on the starting levels of DSA, fewer or more sessions of DFPP were administered and the transplant was performed in the presence of FC-positive crossmatch.12,15
Immunosuppression Protocols
Immunosuppression consisted of mycophenolate mofetil, tacrolimus, and prednisolone as previously described12 and methylprednisolone 500 mg was given as a single intravenous dose intraoperatively. Two doses of basiliximab 20 mg were given, at days 0 and 4. Posttransplant serum samples for antibody analysis were taken daily for the first 2 wk and then 3 times a week for the next 2 wk.
Treatment of Rejection
Rejection was diagnosed by renal biopsy or clinically if there was rapid onset of oliguria with a rise in both serum creatinine and in DSA levels. During the initial few years of our AiT program, rejection was treated with high dose of methylprednisolone for 3 d and with OKT3 (muromomab-CD3) if the rejection was steroid resistant. After 2007, antithymocyte globulin (ATG, Genzyme) replaced OKT3.
HLA Antibody Testing
HLA antibodies were characterized and monitored by microbead assay as previously described.15,16 Lymphocyte crossmatching was performed at baseline (pretreatment) and using a serum sample taken within 24 h pretransplant according to our previous description.16
Data Analysis
Patient Survival and Graft Survival
Overall, patient survival and death-censored graft survival were analyzed. Patient survival was calculated from the date of renal transplantation to date of death or last follow-up. Death-censored graft survival was calculated from the date of transplantation to the date of graft failure or last follow-up. Graft failure was defined as a return to requiring renal replacement therapy as indicated in the clinical records of the patients.
Crossmatch Status
Patients were divided into 3 groups: CDC, FC, and Bead based on the crossmatch assay on the pretreatment/pretransplant serum samples. If the patients underwent DFPP before transplant, pretreatment samples before the start of the first DFPP session were used. If not, the pretransplantation serum sample was used. One-, 5-, and 10-y graft and patient survival were analyzed, and outcomes were compared between the groups. A further subgroup analysis based on the gender was also performed in each of the groups. In the CDC group, an analysis was performed based on CDC titer level.
HLA Antibodies
The study subjects were categorized based on their donor-specific antibody (DSA) status (class I, class II, class I + II antibody groups). One-, 5-, and 10-y graft survival outcomes between the groups were analyzed.
Rejection
Patients were classified as those who had rejection and those who did not based on renal biopsy as described previously.16 Each case was confirmed by independent review, and all cases reported as “suspicious rejection” were included as rejection, and comparative 1-, 5-, and 10-y graft survival were analyzed. Patients were further categorized based on biopsy-proven definitive evidence of the type of rejection—AMR, T-cell–mediated rejection (TCMR), or mixed rejection,17 and the data were reanalyzed retaining only definitive diagnosis of rejection with “suspicious rejections” included as no rejections.
Further analyses were performed by grouping patients based on the number of rejection episodes, that is, no rejection, 1 episode of rejection, and 2 or more episodes of rejection. The 1-, 5-, and 10-y graft survivals were compared between the 3 groups.
The patients who had rejection were regrouped based on the timing of rejection as rejection occurring within the first 2 wk after transplantation and after the first 2 wk of transplantation. The 1-, 5-, and 10-y graft survivals were compared between the 2 groups.
Statistical Analysis
Statistical analysis of comparison between groups was performed with nonparametric testing for interdependent groups using IBM SPSS Version 25.0 (SPSS Institute, Chicago, IL). Kaplan-Meier death-censored survival analysis was used to calculate all survival estimates. Chi-square (Log Rank [Mantel-Cox]) was used to determine statistical significance in the survival analysis between groups. A probability of values (P value) <0.05 was considered statistically significant.
RESULTS
A total of 165 consecutive antibody incompatible transplants performed at our unit were reviewed and a final of 134 was analyzed in this study. Thirty-one patients were excluded for the following reasons; 1 patient did not provide consent, 23 patients had blood group (ABO) incompatibility, or both HLA and ABO incompatibility, and 7 did not proceed to transplant.
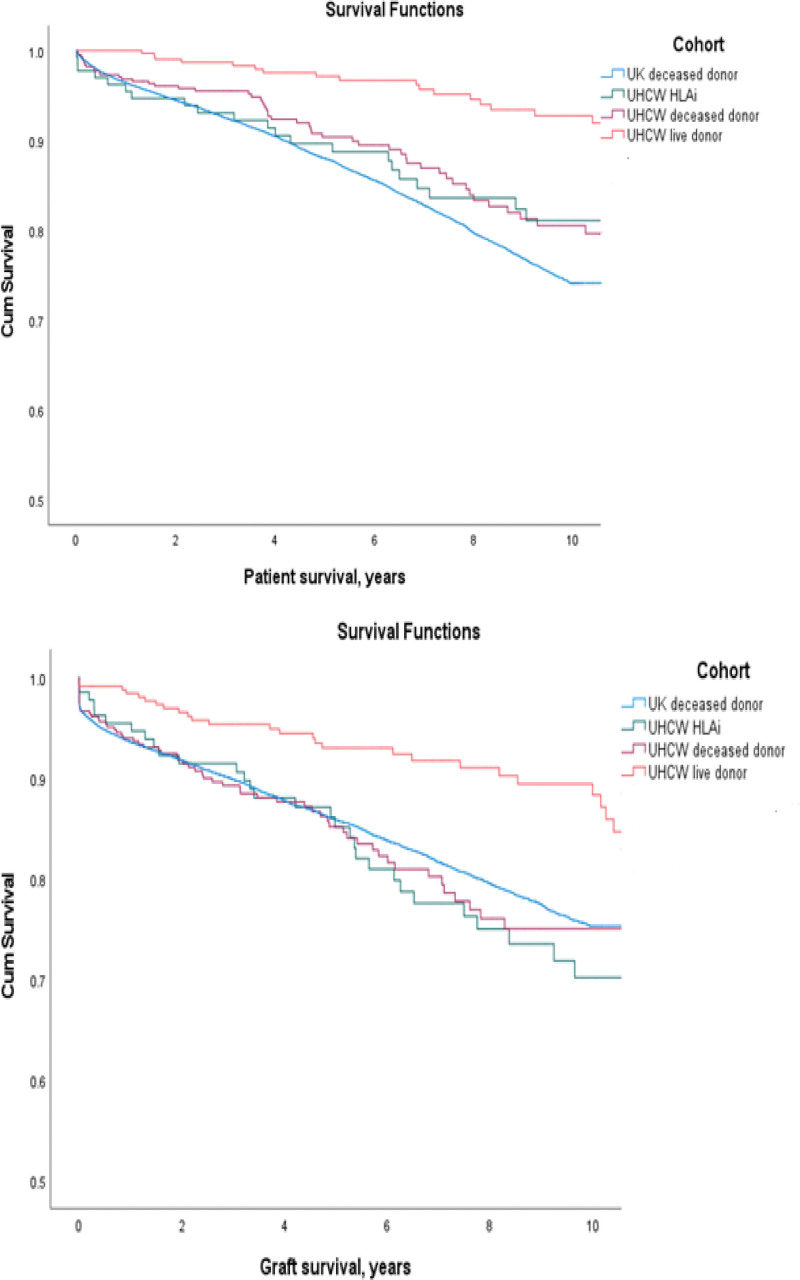
The median follow-up for the HLAi study cohort was 6.93 ± 3.33 y. The patient and graft survival estimates of our HLAi study cohort were compared with (i) first-time deceased donor renal transplant, UK cohort, (ii) first-time deceased donor transplant, UHCW cohort, and (iii) the standard live donor transplant of UHCW cohort from the same time period of January 1, 2003 to December 31, 2018, as shown in Table 2. There was a significant difference in patient survival between the UHCW live donor cohort versus (i) UHCW HLAi cohort (P = 0.007), (ii) UK deceased donor cohort (P < 0.001), and (iii) UHCW deceased donor cohort (P < 0.001), as shown in Figure 1A. Similarly, there was a significant difference in graft survival between the UHCW live donor versus (i) UHCW HLAi (P = 0.003), (ii) UK deceased donor (P = 0.001), and (iii) UHCW deceased donor (P = 0.001), as shown in Figure 1B. However, there were no significant differences between other pairs in both patient and graft survival.
TABLE 2.
One-, 5-, and 10-y patient and graft survival estimates of the different comparative cohorts
| Transplant cohorts from January 2003 to December 2018 | HLAi cohort (study)—UHCW | First-time deceased donor cohort—UK | First-time deceased donor cohort—UHCW | Live-donor cohort—UHCW |
|---|---|---|---|---|
| Total number | 134 | 22 277 | 441 | 325 |
| 1-y patient survival, % | 95.4 | 99.2 | 96.8 | 100 |
| 5-y patient survival, % | 88.7 | 97.6 | 90.4 | 97.1 |
| 10-y patient survival, % | 81.1 | 74.1 | 80.5 | 92.7 |
| 1-y graft survival, % | 95.4 | 93.6 | 93.9 | 98.4 |
| 5-y graft survival, % | 85.1 | 85.8 | 85.2 | 93.0 |
| 10-y graft survival, % | 70.2 | 75.3 | 75 | 89.4 |
HLAi, HLA antibody incompatible; UHCW, University Hospitals Coventry and Warwickshire.
FIGURE 1.
Survival estimates of patients transplanted between January 1, 2003 and December 31, 2018, in the different cohorts—(1) HLA incompatible transplants, UHCW (our center) study cohort, (2) first-time deceased donor renal transplant, UK cohort, (3) first-time deceased donor transplant, UHCW cohort, and (4) the standard live donor transplant of UHCW cohort. Associated P values were all different between the live UHCW donor and all other donor types as given below; however, there were no significant differences between other pairs. A, Patient survival: statistically significant difference in patient survival between the UHCW live donor cohort vs (i) UHCW HLAi cohort (P = 0.007), (ii) UK deceased donor cohort (P < 0.001), and (iii) UHCW deceased donor cohort (P < 0.001). B, Graft survival: statistically significant difference in graft survival between the UHCW live donor vs (i) UHCW HLAi (P = 0.003), (ii) UK deceased donor (P = 0.001), and (iii) UHCW deceased donor (P = 0.001). HLAi, HLA antibody incompatible; UHCW, University Hospitals Coventry and Warwickshire.
Antibody Variables Relating to Outcome
Graft Survival
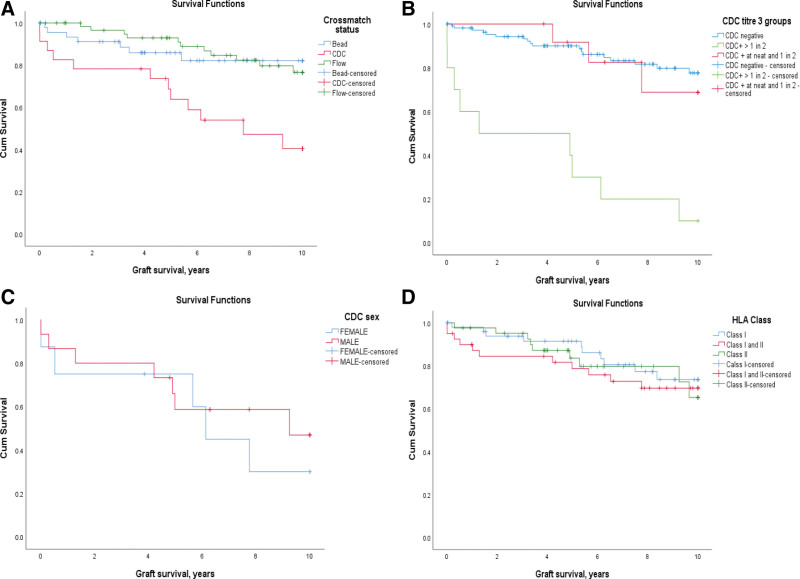
Graft survival was analyzed in term of baseline (immediately pretreatment) DSA levels characterized by Bead positive only, FC positive (Bead+, CDC–), and CDC positive (FC+, Bead+). The graft survival for CDC positive group was 83%, 64%, and 40% at 1, 5, and 10 y, respectively, which is significantly lower than the other 2 groups (Bead versus CDC, P = 0.007, CDC versus Flow, P = 0.001, and Bead versus Flow, P = 0.837) (Figure 2A). The graft survival for FC-positive patient group was 100%, 93%, and 77%, and Bead positive patient group was 95%, 86%, and 82% at 1, 5, and 10 y, respectively.
FIGURE 2.
Graft survival based on (A) pretreatment crossmatch status donor-specific antibody levels according to Bead, Flow, or CDC positivity. Significantly reduced graph survival when the baseline crossmatch was CDC positive (Bead vs CDC, P = 0.007; CDC vs Flow, P = 0.001; and Bead vs Flow, P = 0.837). B, CDC positivity and gender effect: Within the CDC+ group, overall outcome is poorer for the female recipients, although not statistically significant (P = 0.572). C, CDC titers: In those with a CDC+ titer of 1 in 2 or below, graph survival matches the CDC negative group. The group with a CDC+ titer of >1 in 2 have significantly worse outcome compared with the low titer group P < 0.001. D, Antibody specificity (HLA class type): no difference in graft survival based on class of donor-specific antibodies. Bead, microbead assay; CDC, cytotoxic-dependent crossmatch; Flow, flow cytometry crossmatch.
The graft survival for the 9 female CDC positive patients is 75%, 75%, and 30%, which is worse, although not significantly different than the 14 male CDC positive patients with survival of 87%, 59%, and 47% at 1, 5, and 10 y, respectively (P = 0.572) (Figure 2B). A similar trend was noticed in FC-positive and Bead-positive groups in which graft survival in females was lower than males, but this did not reach statistical significance.
CDC titer was also seen to influence graft survival (Figure 2C). As a group, those with a cytotoxic titer >1 in 2 did significantly worse than those with a titer at 1 in 2 or below. The 1-, 5-, and 10-y graft survival for those with CDC+ titer of 1 in 2 or below was 100%, 92%, and 70%, which is similar to the CDC negative group and not statistically different. However, the group with a CDC+ titer of >1 in 2 have significantly worse outcomes with 1-, 5-, and 10-y survival of 60%, 30%, and 10% (P < 0.001).
Graft survival was also analyzed with respect to DSA specificity. The transplants were divided on the basis of antibody specificity for donor HLA class I, class II, or both. Although there appeared to be poor early graft survival in the group with both class I and II DSAs, overall, there is no significant difference between these 3 groups (Figure 2D).
Patient Survival
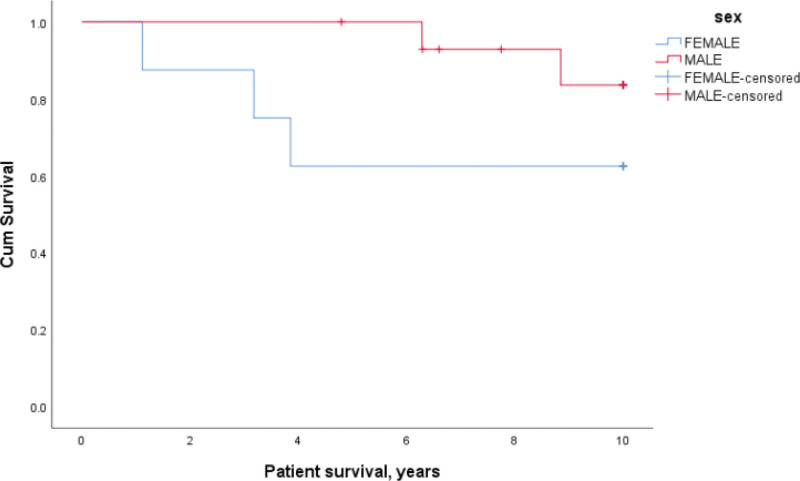
There was no difference in patient survival between the 3 groups based on DSA levels characterized by Bead positive (FC–, CDC–), FC positive (Bead+, CDC–), and CDC positive (FC+, Bead+). Patient survival for CDC positive females was lower but not statistically significant at 88%, 63%, and 63% when compared with males at 100%, 100%, and 83% at 1, 5, and 10 y, respectively (P = 0.176) (Figure 3). There was a similar trend in the FC-positive and Bead-positive groups with lower survival in the females but not reaching statistical significance. There was also no difference in patient survival based on CDC titer or antibody specificity to HLA class (I, II, or I+II).
FIGURE 3.
Effect of cytotoxic-dependent crossmatch positivity and gender on patient survival. Patient survival for cytotoxic-dependent crossmatch positive females was lower at 88%, 63%, and 63% at 1, 5, and 10 y, respectively when compared with males, P = 0.176.
Rejection on Graft Survival Outcomes
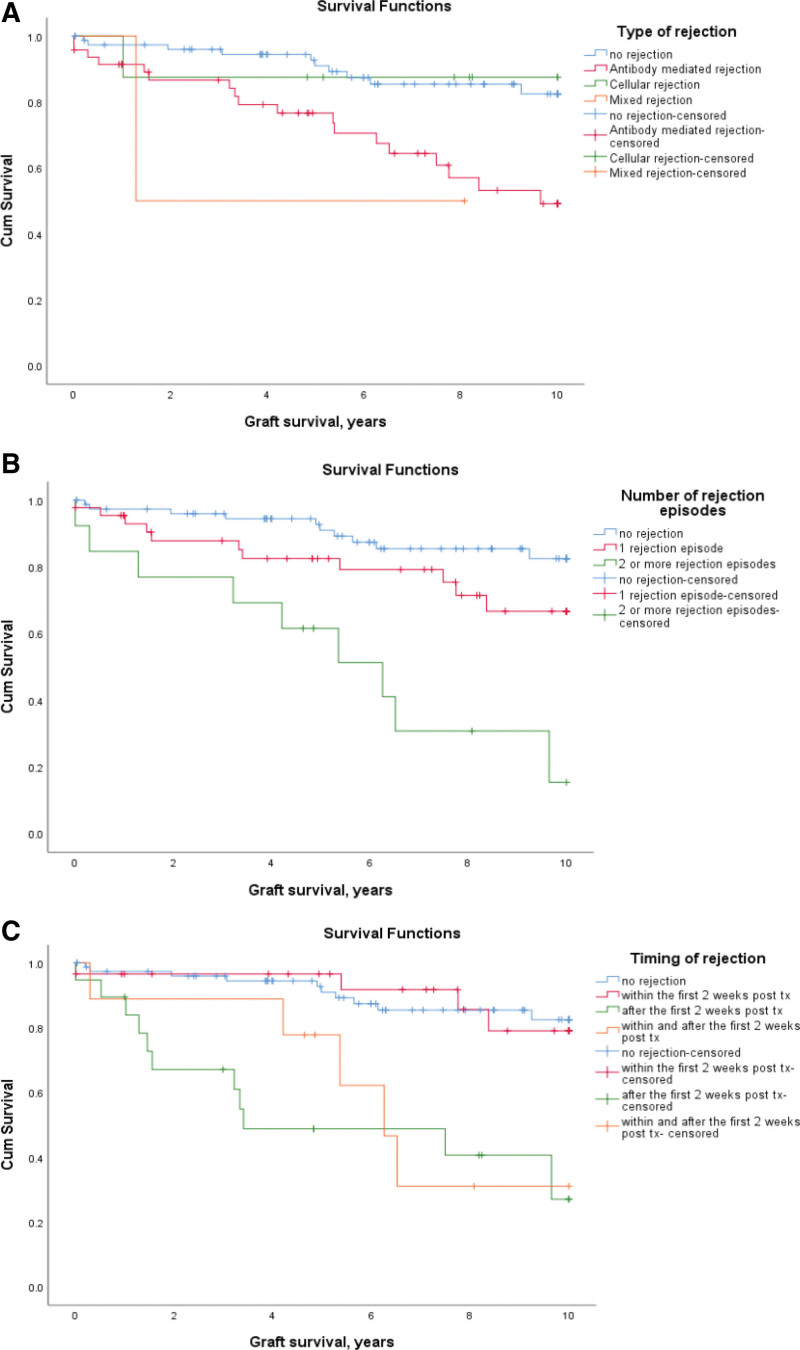
The 1-, 5-, and 10-y graft survival for patients who had rejection was 93%, 77%, and 54% when compared with those who did not have rejection which was 97%, 91%, and 82%. This was statistically significant, P = 0.002.
Of 80 cases that were crossmatch positive (Flow or CDC) preplasmapheresis (DFPP), 48 cases were flow crossmatch positive at pretransplant analyses. There was no increased rejection based on flow crossmatch positivity in this group (P = 0.26, Fisher Exact 2-tailed test). However, higher relative median frequency on flow cytometry was associated with increased rates of rejection (Mann-Whitney analysis P = 0.024). A higher median RMF would relate to higher total DSA MFI values.
Type of Rejection
Fifty-seven patients had any type of rejection diagnosed by biopsy, including “suspicious” (Table 3). Of these, 47 had AMR with 1-, 5-, and 10-y graft survival of 91%, 77%, and 49%, which is significantly lower than those with no rejection, P < 0.001. The graft survival of patients with TCMR was 87% at 1, 5, and 10 y with no significant difference in overall graft survival when compared with patients who had no rejection, P = 0.97(Table 3 and Figure 4A). Although the estimated graft survival of patients with mixed rejection is comparable to AMR, as the numbers were low this did not reach statistical significance. The analysis was repeated including only definitive diagnosis of biopsy-proven rejection, and the results were similarly statistically significant, although 10-y graft survival of AMR was lower at 39% (data not shown).
TABLE 3.
Graft survival outcomes based on rejection
| Patients, n | Cumulative proportion surviving at, % | ||||
|---|---|---|---|---|---|
| Total | Not within total that had graft failure | 1 y | 5 y | 10 y | |
| Total number | 134 | 30 | 95.4 | 85.1 | 70.2 |
| No rejection | 77 | 17 | 97 | 91 | 82 |
| Antibody-mediated rejection | 47 | 14 | 91 | 77 | 49 |
| Cellular rejection (TCMR) | 8 | 1 | 87 | 87 | 87 |
| Mixed rejection | 2 | 1 | 100 | 50 | 50 |
| Within the first 2 wk | 29 | 2 | 97 | 97 | 79 |
| After the first 2 wk | 19 | 9 | 84 | 49 | 27 |
| Within and after the first 2 wk | 9 | 5 | 89 | 78 | 31 |
| 1 rejection episode | 44 | 7 | 95 | 82 | 67 |
| 2 or more rejection episodes | 13 | 9 | 85 | 61 | 15 |
TCMR, T-cell–mediated rejection.
FIGURE 4.
Graft survival based on (A) rejection types: the graft survival in antibody-mediated rejection was 91%, 77%, and 49% when compared with rejection-free patients with survival of 97%, 91%, and 82% at 1, 5, and 10 y (P < 0.001). B, Number of rejection episodes: If 2 or more rejection episodes the graft survival rate were significantly lower, P < 0.001 if no rejection and P = 0.003 if 1 episode of rejection. C, Timing of rejection: if rejection occurred or continued to occur after 2 wk, the graft survival was significantly lower, P < 0.001. tx, transplant.
Number of Rejection Episodes
As the number of rejection episodes increases, the graft survival decreases. When there was a single rejection episode, the graft survival rates are 95%, 82%, and 67% and with 2 or more rejection episodes it was 85%, 61%, and 15% at 1, 5, and 10 y, respectively. This is significantly lower compared with patients who had a single episode (P = 0.003) and those who had no rejection (P < 0.001) (Figure 4B and Table 3). The results are similar when the data were reanalyzed including only definitive diagnosis of rejection as rejection episodes.
Timing of Rejection
Rejection occurring only within the first 2 wk of transplantation did not cause any significant decline in the long-term graft survival when compared with rejection-free patients with rates of 97%, 91%, and 82% at 1, 5, and 10 y, P = 0.95. In contrast, graft survival was significantly lower in those with rejection occurring for the first time after 2 wk (P < 0.001) or continuing to occur after the first 2 wk of transplantation (P < 0.001) compared with rejection-free patients. The 1-, 5-, and 10-y graft survival for patients who had rejection after the first 2 wk of transplantation was 84%, 49%, and 27%, and for those who had rejection within and after 2 wk this was 89%, 78%, and 31%. There was a significant difference in the graft survival estimates for patients who had rejection in <2 wk posttransplant when compared with those who had rejection after 2 wk (P < 0.001) and for those who had rejection episodes within and after the first 2 wk of transplantation (P = 0.003) (Figure 4C and Table 3). The results were also similar when the data were reanalyzed including only definitive diagnosis of rejection as rejection episodes.
DISCUSSION
There has been a lack of published, long-term outcome data for HLAi renal transplantation and we have addressed this. The patient and graft survival estimates of our HLAi cohort for 1, 5, and 10 y is 95%, 90%, and 81% and 95%, 85%, and 70%, respectively. This is similar to the outcomes seen for first time, deceased brain dead donor transplants in the United Kingdom18 and at our center for the same time period. However, there was a significant difference in overall patient and graft survival between the HLAi study cohort and the standard live donor transplant cohort at our center. Given the higher risk nature of our cases, more than half of whom had repeat transplants, this report provides reassurance that HLAi transplantation can, overall, offer good long-term outcomes for highly sensitized patients with a low prospect of a compatible donor.
We have looked in detail at 2 factors that significantly affect outcome: measurements of baseline sensitization and the nature of the rejection responses. Graft survival for the group with a positive baseline CDC crossmatch is significantly lower when compared with the FC- and Bead-positive groups. This association between cytotoxic reactivity against donor HLA and poor outcome is now well described.11,12,18-21 However, in our experience, it is only the higher levels of cytotoxic reactivity, that is CDC titer >1 in 2 that determine reduced graft survival. The graft survival of the CDC low titer group was similar to the CDC negative group. This observation therefore should widen access to transplantation to almost half of cases referred with a positive CDC crossmatch who might otherwise be declined.
We did not find any statistical differences in death-censored graft survival when comparing cases with either HLA class I, class II, or both class I and II DSAs. It has been shown that patients with complement fixing HLA class I DSAs had inferior graft survival compared with HLA class II DSAs.22 On looking at our data further, we noted an unexpected distribution (P < 0.001) of DSA types between the crossmatch cases, with a clear bias towards combined class I and II DSAs in the patients with a positive CDC crossmatch (data not shown). There were 12 graft losses in the CDC+ group and the earliest 5 (4 within 1 y) were in patients with both class I and II DSAs. It might be that the reaction complexity of the sera enhances complement activation on donor cells, and this might not be so apparent using single antigen capture assays to characterize complement activation. The CDC test has the advantage that it is directly donor-specific, however, it is not a standardized test. We do not use the more sensitive antihuman globulin enhanced method and therefore our baseline measurements of overall reactivity would represent higher levels compared with results using the enhancement approach.
We also saw a trend in which female recipients with DSAs had poorer graft and patient survival compared with male recipients. Although consistent with our previous observations that pregnancy stimulated DSAs rebound more aggressively in this cohort,23 this does not reach statistical significance. Studies in pregnancy have shown an anti-inflammatory state in pregnancy with an increase in the T helper 2 cytokines and immune modulatory proteins with a shift postpartum.24,25 It could be speculated that the proinflammatory state postpartum in general and increased sensitivity to pregnancy induced antigens specifically, could result in decreased graft and patient survival. The relationship between pregnancy and subsequent HLAi transplantation needs further study because the consequences seem severe.
AMR was the only rejection type that significantly reduced graft survival in comparison to TCMR or no rejection in our study. We see graft survival estimates of 49% at 10 y if there was an occurrence of definitive or suspicious AMR, compared with 82.4% if there was no rejection. Conversely, TCMR in HLAi transplantation, though there were relatively low numbers in our study, seems to be more amenable to successful treatment and does not necessarily precipitate graft loss. Our analysis extends the time frame and is consistent with findings from similar studies showing significantly lower 5-y graft survival in patients who had AMR.26,27
Finally, we have made the observation that the timing of an early rejection episode, either side of 2 wk posttransplant, is significant in terms of short and long-term graft survival. No significant difference was observed in graft survival probability between patients who only had rejection within the first 2 wk following transplantation and those who had no rejection at all. However, there was a significant impact on graft survival if rejection occurred after the first 2 wk of transplantation or within and after the first 2 wk of transplantation. In addition, experiencing >1 rejection episode caused a significant reduction in graft survival rates in our study. This latter finding has been shown before in standard transplants.28 In our cohort, the majority of transplants with >1 rejection episode experienced an event both within and after 2 wk from transplantation. Several factors could contribute to these observations in HLAi transplantations. Intensive monitoring of patients during the first few wk following transplantation, could detect rejection early, resulting in prompt and efficient management.29 Whereas, rejection occurring later on is often diagnosed when patients present with overt symptoms or upon routine checkup. Interestingly, we observed that of the patients who experienced 2 or more rejection episodes, the majority were those with rejections occurring within and after the first 2 wk of transplantation and was therefore associated with nonresolution of the first episode. AMR is considered to be a disease process with a continuum of severity, beginning at any time after transplantation and developing at varying levels of intensity, progressively leading to the development of chronic allograft damage, dysfunction, and loss.30 However, we found significantly better outcomes in those with the earlier, compared with those with later rejection episodes. Thus, the earlier rejections seem to be more effectively treated, for operational reasons, as above, or perhaps because of fundamental immunologic reasons, thereby limiting AMR progression.
STRENGTHS AND LIMITATIONS
This is a study from a tertiary international referral center for HLAi renal transplantation in the United Kingdom. As our unit has considerable experience in such transplantations, these results may not necessarily be extrapolated to other practices. Also, the number of cases is limited given the constraints of a single center. As it is a retrospective study, the protocols were not standardized, which could have resulted in a bias in the outcomes.
This is the first long-term study of a relatively large cohort of HLAi transplants that shows equivalent graft and patient survival to deceased donor transplantation. We have identified 2 key areas that allow very good outcomes in what is considered high-risk transplantation. Exclusion of the highest immunologic risk cases, defined as those with a CDC titer of >1 in 2, gives us an estimated 10-y graft survival of almost 75%. Similarly, those with AMR or TCMR diagnosed and treated within the first 2 wk posttransplantation can be expected to have a good chance of long-term transplant survival.
CONCLUSIONS
Long-term graft and patient survival for HLAi renal transplantation is similar to deceased donor transplantation. There are certain subgroups, in particular those with a crossmatch CDC titer of >1 in 2, antibody-positive female patients, and unresolving or recurrence of AMR, which are associated with a poor prognosis. Given that the need for HLAi transplantation exists and is likely to increase in the future, despite PKE programs, further large studies looking at risk stratification, early diagnosis, and management of rejection are needed.
ACKNOWLEDGMENTS
We thank Dr D. Mitchell, University of Warwick, for contributing to design and acquisition of materials for the work; Dr David Lowe, NHSBT, Birmingham, for contributing to design, acquisition of materials, and analysis; Dr Colm Magee, Renal Unit, Beaumont Hospital, Republic of Ireland, for contributing to referral, assessment, and management of patients; Dr H Kanji, Renal Unit, UHCW, for contributing to data collection and management of patients; Dr S. Talwar, Renal Unit, UHCW, for contributing to data collection; Mr Matthew Robb, Department of Statistics, NHSBT, Bristol, United Kingdom; past and current staff of Hemodialysis, Renal Unit, UHCW, for helping with management of patients; past and current Transplant coordinators, Renal Unit, UHCW, for helping with assessment and management of patients; past and current Transplant nurses, Renal Unit, UHCW, for helping with management of patients; renal research nurses, UHCW for helping with sample collection; Renal Pharmacists, Renal Unit, UHCW, for helping with management of patients; R&D department, Renal Unit, UHCW, for helping with getting ethical approval for the study; and all UK and international referral units for contributing to referral, assessment, and management of patients.
Footnotes
Published online 19 July, 2021.
N.K., D.B., and N.K. received funds from Chiesi, Sandoz, Astella, VH Bio, One Lambda, Immucor, Biomerieux, and Warwick School of Engineering toward organizing an International Transplantation Conference. N.K. and D.B. have been part of scientific advisory board for HANSA Ltd. The remaining authors have any conflict of interest.
N.K., R.H., and D.B. made substantial contributions to the conception, the design of the work and the acquisition, analysis, and the interpretation of the data. A.A., N.M., D.Z., R.C., S.D., and N.K. made substantial contributions to the acquisition, analysis, and interpretation of the data. K.G., F.T.L., L.C.T., H.K., C.I., and C.C. made substantial contributions to the design of the work. All authors contributed to the revising of the article and approval of the final version. The authors are also in agreement to be accountable for all aspects of the work. A.A. and N.M. have contributed equally to this work.
REFERENCES
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. [DOI] [PubMed] [Google Scholar]
- 2.Taube DH, Welsh KI, Kennedy LA, et al. Successful removal and prevention of resynthesis of anti-HLA antibody. Transplantation. 1984;37:254–255. [DOI] [PubMed] [Google Scholar]
- 3.Organ Donation and Transplantation, NHS Blood and Transport. Kidney activity. 2019. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19191/section-5-kidney-activity.pdf. Accessed November 20, 2020.
- 4.Parsons RF, Locke JE, Redfield RR, III, et al. Kidney transplantation of highly sensitized recipients under the new kidney allocation system: A reflection from five different transplant centers across the United States. Hum Immunol. 2017;78:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manook M, Johnson R, Robb M, et al. Changing patterns of clinical decision making: are falling numbers of antibody incompatible transplants related to the increasing success of the UK Living Kidney Sharing Scheme? A national cohort study. Transpl Int. 2021;34:153–162. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010;10:449–457. [DOI] [PubMed] [Google Scholar]
- 7.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. [DOI] [PubMed] [Google Scholar]
- 8.Marfo K, Lu A, Ling M, et al. Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 2011;6:922–936. [DOI] [PubMed] [Google Scholar]
- 9.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manook M, Koeser L, Ahmed Z, et al. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: a matched cohort analysis. Lancet. 2017;389:727–734. [DOI] [PubMed] [Google Scholar]
- 11.Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–589. [DOI] [PubMed] [Google Scholar]
- 12.Higgins R, Lowe D, Hathaway M, et al. Human leukocyte antigen antibody-incompatible renal transplantation: excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92:900–906. [DOI] [PubMed] [Google Scholar]
- 13.Higgins RM, Bevan DJ, Carey BS, et al. Prevention of hyperacute rejection by removal of antibodies to HLA immediately before renal transplantation. Lancet. 1996;348:1208–1211. [DOI] [PubMed] [Google Scholar]
- 14.Higgins RM, Daga S, Mitchell DA. Antibody-incompatible kidney transplantation in 2015 and beyond. Nephrol Dial Transplant. 2015;30:1972–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins R, Lowe D, Hathaway M, et al. Rises and falls in donor-specific and third-party HLA antibody levels after antibody incompatible transplantation. Transplantation. 2009;87:882–888. [DOI] [PubMed] [Google Scholar]
- 16.Khovanova N, Daga S, Shaikhina T, et al. Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int. 2015;28:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas M, Sis B, Racusen LC, et al. ; Banff meeting report writing committee. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 18.NHS Blood and Transport. Survival rates following transplantation. 2019. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19197/section-11-survival-rates-following-transplantation.pdf. Accessed November 20, 2020.
- 19.Haririan A, Nogueira J, Kukuruga D, et al. Positive cross-match living donor kidney transplantation: longer-term outcomes. Am J Transplant. 2009;9:536–542. [DOI] [PubMed] [Google Scholar]
- 20.Thielke JJ, West-Thielke PM, Herren HL, et al. Living donor kidney transplantation across positive crossmatch: the University of Illinois at Chicago experience. Transplantation. 2009;87:268–273. [DOI] [PubMed] [Google Scholar]
- 21.Riella LV, Safa K, Yagan J, et al. Long-term outcomes of kidney transplantation across a positive complement-dependent cytotoxicity crossmatch. Transplantation. 2014;97:1247–1252. [DOI] [PubMed] [Google Scholar]
- 22.Wahrmann M, Exner M, Schillinger M, et al. Pivotal role of complement-fixing HLA alloantibodies in presensitized kidney allograft recipients. Am J Transplant. 2006;6(5 Pt 1):1033–1041. [DOI] [PubMed] [Google Scholar]
- 23.Higgins R, Lowe D, Daga S, et al. Pregnancy-induced HLA antibodies respond more vigorously after renal transplantation than antibodies induced by prior transplantation. Hum Immunol. 2015;76:546–552. [DOI] [PubMed] [Google Scholar]
- 24.Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31:81–95. [DOI] [PubMed] [Google Scholar]
- 25.Bränn E, Edvinsson Å, Rostedt Punga A, et al. Inflammatory and anti-inflammatory markers in plasma: from late pregnancy to early postpartum. Sci Rep. 2019;9:1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vo AA, Sinha A, Haas M, et al. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization. Transplantation. 2015;99:1423–1430. [DOI] [PubMed] [Google Scholar]
- 27.Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015;26:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokado Y, Takahara S, Hatori M, et al. Acute rejection episodes predict long-term renal transplantation survival. Transplant Proc. 1997;29:1537–1540. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan NS, Zehnder D, Briggs D, et al. Human leukocyte antigen antibody incompatible renal transplantation. Indian J Nephrol. 2012;22:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loupy A, Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379:1150–1160. [DOI] [PubMed] [Google Scholar]