Supplemental Digital Content is available in the text.
Abstract
Background.
Graft versus host disease (GVHD) is an uncommon but highly morbid complication of intestinal transplantation (ITx). In this study, we reviewed our 17-y experience with GVHD focusing on factors predicting GVHD occurrence and survival.
Methods.
Retrospective review of 271 patients who received 1 or more ITx since program inception in 2003 with survival analysis using Cox proportional hazard modeling.
Results.
Of 271 patients, 28 developed GHVD 34 (18–66) d after ITx presenting with rash or rash with fever in 26, rectosigmoid disease in 1, and hemolysis in 1; other sites, mainly rectosigmoid colon, were involved in 13. Initial skin biopsy demonstrated classic findings in 6, compatible findings in 14, and no abnormalities in 2. Additional sites of GVHD later emerged in 14. Of the 28 patients, 16 died largely from sepsis, the only independent hazard for death (hazard ratio [HR], 37.4181; P = 0.0008). Significant (P < 0.0500) independent hazards for occurrence of GVHD in adults were pre-ITx functional intestinal failure (IF) (HR, 15.2448) and non-IF diagnosis (HR, 20.9952) and early post-ITx sirolimus therapy (HR, 0.0956); independent hazards in children were non-IF diagnosis (HR, 4.3990), retransplantation (HR, 4.6401), donor:recipient age ratio (HR, 7.3190), and graft colon omission (HR, 0.1886). Variant transplant operation was not an independent GVHD hazard.
Conclusions.
Initial diagnosis of GVHD after ITx remains largely clinical, supported but not often confirmed by skin biopsy. Although GVHD risk is mainly recipient-driven, changes in donor selection and immunosuppression practice may reduce incidence and improve survival.
INTRODUCTION
Graft versus host disease (GVHD) is an uncommon complication of solid organ transplantation (SOT) with an incidence ranging between <1% and 10%1-4 that contrasts with the 40%–50% incidence after hematopoietic stem cell transplantation (HSCT).5-7 Incidence of GVHD after intestinal transplantation (ITx) lies at the upper end of the SOT range, attributed to a large number of alloreactive T cells within an intestinal graft. Consequently, interest in GVHD after ITx remains high, especially in the context of mortality that ranges between 40% and 77%.2,8-12
Recent descriptions of GVHD after ITx by several centers have demonstrated a typical presentation with rash mainly appearing 2 wk to 2 mo postoperatively but occasionally delayed for no apparent reason.2,3,8-13 Diagnosis is largely clinical, as routine rash histopathology commonly fails unambiguously to distinguish GVHD from drug reaction or viral infection.8,14,15 Techniques to confirm suspicion of donor T cells in a tissue are not routinely available, and magnitude of peripheral blood T-cell chimerism in ITx recipients with and without clinical evidence of GVHD overlaps.10,16 Response to intensified immunosuppressive drug therapy is often poor, implying that donor alloreactive cells are relatively resistant to standard immunosuppression compared with recipient alloreactive cells. Both inefficacy and toxicity of established treatments contribute to the relatively high mortality.
Recently, we have demonstrated that donor-derived clones in native colon mucosa of selected ITx recipients with typical features of GVHD have a CD69+ resident memory T-cell phenotype.17 Complimentary to this work, we herein report our clinical GVHD experience after ITx with a heightened focus on risks for occurrence compared with ITx recipients not developing GVHD.
MATERIALS AND METHODS
Charts of all patients who underwent ITx at this center from inception of the program in 2003 until June 30, 2019, were reviewed.
Indications for ITx and Variant Operations
Isolated ITx (IITx) was performed for irreversible intestinal failure (IF) with early but progressing intestinal failure–associated liver disease (IFALD), progressive central vein loss, and nonmetastasizing neoplasms compromising the midgut.18 En bloc liver-intestine-pancreas transplant was performed for advanced IFALD with portal hypertension primarily in pediatric patients,19 whereas noncomposite liver and intestinal transplantation (LITx) was performed for the same indication in adults.20 Multivisceral transplant (MVTx) including stomach, small intestine, liver, and pancreas plus splenectomy was performed for advanced IFALD with both unsalvageable foregut and midgut and for extensive portal–mesenteric vein thrombosis.20 Modified multivisceral transplant (MMVTx) that excludes a liver graft was performed for similar indications as IITx but with unsalvageable foregut.
Clinical Transplant Practice
Practices maintained throughout the study period are summarized in Table 1, whereas changes over time potentially relevant to GVHD and other immunological events are summarized in Table 2.18,21,22 Rabbit antithymocyte globulin (r-ATG) was administered to donors during organ procurement except when not logistically feasible or precluded by hemodynamic instability. Early post-ITx exposure to either sirolimus (SIR) or mycophenolate mofetil was defined as a minimum of 14 d of treatment either immediately before onset of GVHD or, for those not developing GVHD, within the first month after ITx including postoperative d 30.
TABLE 1.
ITx management practices throughout the study period 2003–2020
| Induction immunosuppression | ||
| Methylprednisolone, 50 mg/kg (maximum 1600 mg) during first post-ITx week tapered daily | ||
| Then, prednisolone/prednisone 1 mg/kg (maximum 20 mg) daily tapered to 0.1 mg/kg (maximum 5 mg) daily by 6 mo after ITx | ||
| Maintenance immunosuppression | ||
| Prednisone/prednisolone 0.1 mg/kg (maximum 5 mg) daily indefinitely | ||
| Tacrolimus | ||
| Target trough level 25 ng/mL during first post-ITx month | ||
| Target trough level 7–12 ng/mL by eighth post-ITx month, low-end if secondary immunosuppression and high-end if no secondary immunosuppression | ||
| Secondary immunosuppression options | ||
| Sirolimus | ||
| Target trough level 3–5 ng/mL | ||
| Preferred for superior bioavailability | ||
| Discontinued if frequent infections | ||
| Replaced with MMF if renal, pulmonary, or mucosal toxicity | ||
| MMF | ||
| Target trough level 2–4 μg/mL | ||
| Preferred if established renal insufficiency | ||
| Discontinued if frequent infections | ||
| Replaced or discontinued if hypersensitivity including mucosal ulceration | ||
| Nutrition | ||
| Elemental—semielemental diet, starting 1 wk post-ITx and continued 6–12 mo post-ITx | ||
| Dietary fat restriction for 1 mo post-ITx | ||
| Oral intake starting 2–4 wk post-ITx | ||
| Liquid diet by tube if feeding disorder | ||
| Taper parenteral nutrition to maintain age- and size-appropriate body weight | ||
| Graft stoma construction | ||
| Ileostomy | ||
| Preferred | ||
| Closure 3–5 mo post-ITx if no interval rejection | ||
| End colostomy | ||
| If pre-ITx colectomy | ||
| Endorectal pull-through individualized |
ITx, intestinal transplant; MMF, mycophenolate mofetil.
TABLE 2.
Modifications in ITx practices during the study period 2003–2020
| Year | |
|---|---|
| 2008 | |
| Pre-ITx recipient screening for preformed anti-HLA antibodies by single-antigen assay. | |
| Virtual cross-matching for identification of preformed, donor-specific anti-HLA antibodies. | |
| Substitution of induction immune suppression using basiliximab (postoperative d 0 and 4, 10–20 mg/dose), formerly for all ITx recipients, with rabbit antithymocyte globulin for sensitized ITx recipients defined by panel reactive anti-HLA antibody level >20% or positive crossmatch (postoperative d 0–4, 1.5 mg/kg/d). | |
| 2009 | |
| Standardized inclusion of graft ileocecal valve and graft ascending/transverse colon. | |
| Loop in preference to Santulli ileostomy. | |
| 2012 | |
| High-dose intravenous immunoglobulin, rituximab (anti-CD20 monoclonal antibody), and plasmapheresis immediately before and after ITx in HLA antibody-sensitized recipients. | |
| Monitoring for donor-specific anti-HLA antibodies arising de novo after ITx and treatment with intravenous immunoglobulin when present. |
ITx, intestinal transplant.
Graft Monitoring
Protocol surveillance endoscopy and biopsy of the graft via ileostomy or colostomy commenced 1 wk after transplant reduced gradually over time to once yearly panendoscopy after stoma closure. Native colon was inconsistently assessed during protocol surveillance but routinely after suspicion of GVHD elsewhere. Biopsy findings were classified as graft rejection, infection, posttransplant lymphoproliferative disorder, GVHD, and miscellaneous pathologies based on standard criteria.23,24 Humoral rejection was not routinely evaluated.
Evaluation and Treatment of GVHD
Appearance of a symmetrical, erythematous maculopapular rash most typically on the hands and feet, hairline, chest, and abdomen corresponding to acute disease8,25 prompted a skin biopsy evaluated by a dermatopathologist. Skin biopsies were classified as (1) GVHD-classic, (2) GVHD-consistent, or (3) normal based on pathology reports; GVHD grade in classic disease was recorded when specified. Features required for diagnosis of classic GVHD included vacuolar interface degeneration, full-thickness keratinocyte necrosis, or satellite cell necrosis,14,26-28 whereas less specific but GVHD-compatible features included spongiosis, eosinophilia, less extensive keratinocyte necrosis, and perivascular and perieccrine inflammation.29 Skin biopsies, endoscopy, and other investigations were repeated as necessary to evaluate disease progression and flare. GVHD therapy was not protocolized and varied with individual practitioners and as new therapies became available.
Peripheral blood chimerism was determined if GVHD was suspected clinically and typically rechecked with persistent or worsening disease. Methodology was based on polymorphisms in short tandem repeats with lower limit of detection at 1%. For most of the study, assays detected total peripheral blood chimerism and since 2017, CD3 (T cell)-specific chimerism. Intermediate-resolution HLA typing was obtained by rSSO-XR assay (One Lambda, West Hills, CA) using genomic DNA. A degree of HLA matching was determined using serologic equivalents and HLA class I cross-reactive groups (Tables S1 and S2, SDC, http://links.lww.com/TXD/A344).30,31
Analysis
To facilitate most analyses, pre-ITx diagnoses were classified into 3 groups: (1) anatomic IF, that is, short bowel syndrome; (2) functional IF subdivided into intestinal pseudoobstruction (“dysmotility”) including total to near-total aganglionosis and intractable diarrhea associated with congenital mucosal disease; and (3) non-IF, consisting of intraabdominal and pelvic tumors, most commonly desmoids associated with familial adenomatous polyposis, and portal–mesenteric thrombosis generally due either to a primary hypercoagulable disorder or secondary to advanced liver disease including complications of previous isolated liver transplantation. Chemotherapy for tumors before ITx could not be determined in most cases. Underlying genetic disorders were either confirmed by diagnostic testing or presumed on the basis of typical clinical features. Patients aged ≥18 were classified as adult and those <18 y as pediatric.
Comparisons of continuous variables were made using the Wilcoxon and Mann-Whitney tests, and proportions were compared using the Fisher and Fisher-Freeman-Halton tests; central tendencies were expressed as medians with interquartile range (first quartile–third quartile). Cox proportional hazards regression was used in outcome modeling as indicated; June 30, 2020, was the censor date. Significance was taken as P < 0.0500. Statistical calculations were performed using MedCalc Statistical Software (Ostend, Belgium). This retrospective review was conducted under the authorization of the Institutional Review Board of Georgetown University (ID #2004-008).
RESULTS
Clinical Presentation of GVHD
Twenty-eight patients (14 adults, 16 male individuals), median age at ITx = 16.0 (1.7–41.8) y, were diagnosed with GVHD from among the 271 patients receiving 278 ITx through June 30, 2019, giving a GVHD incidence of 10%. Underlying diseases leading to ITx were anatomic IF in 6, functional IF in 11 (dysmotility in 6 and mucosal disease in 5), and non-IF in 11 (abdominal tumor in 6, primary or secondary portal–mesenteric thrombosis in 5). Incidence of GVHD did not differ between secretory diarrhea and dysmotility within the functional IF group (P = 0.1484) or between tumor and thrombosis diagnoses within the non-IF group (P = 1.0000). Known or presumed genetic diagnoses in 13 of the 28 patients included familial adenomatous polyposis in 4, microvillus inclusion disease in 2, and 1 each of multiple intestinal atresia with severe combined immunodeficiency syndrome, Kabuki syndrome, megacystis microcolon intestinal hypoperistalsis syndrome, total intestinal aganglionosis, trichohepatoenteric syndrome, tufting enteropathy, and prothrombin gene mutation. Types of ITx performed included IITx in 10, LITx in 5, MVTx in 11, and MMVTx in 2.
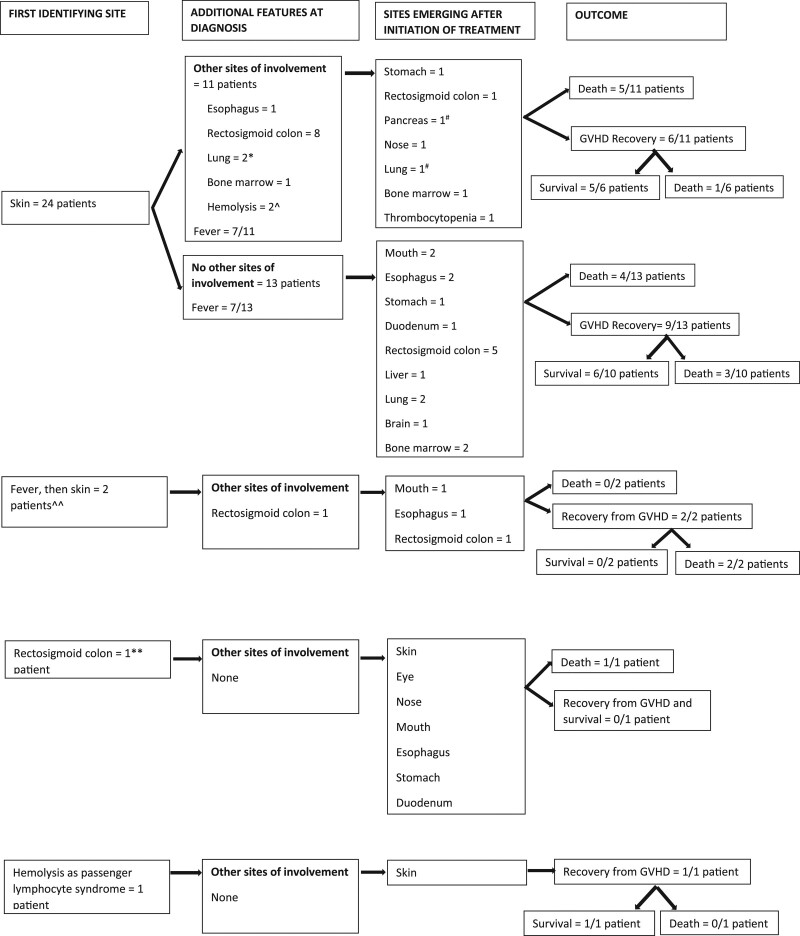
First symptoms and signs of GVHD appeared 34 (18–66) d after ITx in 2 clusters, an early period with a median interval after ITx of 30 (17–55) d in 25 patients and a late period in the remaining 3, median interval after ITx being 353 (309–381) d (early versus late, P = 0.0053). Irrespective of time of onset, all cases had an acute phenotype. Figure 1 summarizes evolving patterns and distributions of disease activity. The first indication of GVHD was a rash in 24 of the 28 patients. Fever (median 38.8 [38.1–40.1] °C) appeared within 24 h of rash in 14 of the 24 patients and preceded the rash by 96 h in 2 additional patients. As indicated in Figure 1, of the 26 patients whose presentation of GVHD was as a rash or fever soon followed by rash, contemporaneous evaluation revealed active disease at other sites in 13, the most common being native rectosigmoid colon. Of these 26 patients, 22 had a skin biopsy available for review that was obtained 6 (2–14) d after onset of rash or fever. Disease was classified as GVHD-classic in 6 patients (grade II in 4, grade I in 1, and unspecified in 1), GVHD-compatible in 14 patients, and normal in 2. In patients with GVHD-compatible biopsies, the most common alternative histopathological diagnosis was drug reaction that was discarded or rejected because of worsening of rash despite medication change or detection of GVHD elsewhere.
FIGURE 1.
Sequence of organ involvement in GVHD after intestinal transplantation in 28 patients. *Clinical—not proven by biopsy in 1. ^Direct antiglobulin negative but remission under steroids and rabbit antithymocyte globulin in 1. #Clinical—not proven by biopsy. ^^Delayed until 96 h after fever in both patients. **Incidental finding. GVHD, graft vs host disease.
Absolute lymphopenia was common at diagnosis in the 25 patients with early onset GVHD, typically more severe after r-ATG compared with basiliximab induction (median 600 [400–760] per μL versus 900 [670–1300] per μL, P = 0.0466). In contrast, simultaneous median absolute neutrophil count was 7600 (2730–11 700) per μL and did not vary with induction method (P = 0.4663). Total peripheral blood chimerism determined 4 (0–10) d after first symptoms or signs in 19 of the 28 patients demonstrated a median donor percentage of 0% (0–2.8), the 2 highest values equaling 25% and 50%. Peripheral blood CD3 cell chimerism at GVHD onset in 3 of the most recently transplanted patients with multisite disease equaled 6.0% (4.5–13.8), which equivocally exceeded simultaneous total peripheral blood chimerism of 0.0% (0.0–1.3) in these 3 (P = 0.0625).
Treatment and Late Disease Activity
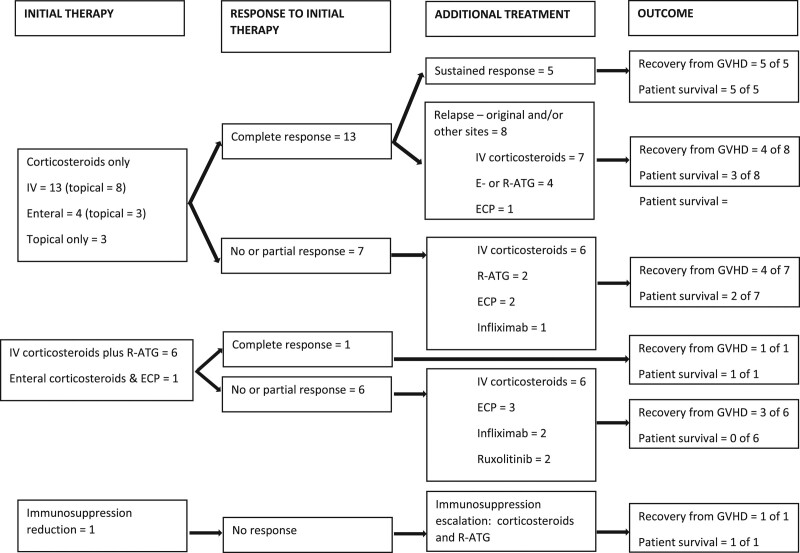
Treatment of GVHD was as outlined in Figure 2. Initial therapy included corticosteroids, mostly intravenous, except for 1 patient whose immunosuppression was curtailed in an unsuccessful attempt to stimulate rejection of donor alloreactive cells. Additional agents, mostly r-ATG, supplemented corticosteroids at diagnosis mainly based on severity of clinical presentation and in 1 patient, 50% donor total peripheral blood chimerism referred to above. Mycophenolate mofetil or SIR was maintained in 21 patients, in 19 for 98 (38–174) d after GVHD diagnosis, similarly distributed between patients receiving corticosteroids alone or in combination with other therapies.
FIGURE 2.
Sequential treatment of graft vs host disease after intestinal transplantation in 28 patients. E-ATG, antithymocyte globulin (equine); ECP, extracorporeal photopheresis. GVHD, graft vs host disease; IV, intravenous; r-ATG, antithymocyte globulin (rabbit).
Responses to treatment included sustained complete GVHD remission, a transient complete response followed by relapse at the initial or new site(s), or partial or no response. Partial and nonresponders received additional therapy that included more corticosteroids plus other treatments shown in Figure 2. Cutaneous disease commonly recurred or worsened as initially normal or GVHD-compatible skin biopsies progressed to GVHD grade I in 1 patient, grade II in 6 patients, grade III in 2 patients, and grade IV in 1 patient. Furthermore, 14 patients demonstrated new sites of disease at a median of 73 (36–190) d after GVHD onset including skin disease in 2 patients initially without this finding. GVHD remained purely cutaneous in 5 of the 28; features of this mild phenotype group are summarized in Table 3.
TABLE 3.
Patients with cutaneous GVHD alone
| Patienta | Skin biopsy | Fever at presentation | Initial chimerismb (%) | Treatment | GVHD recovery |
|---|---|---|---|---|---|
| 1 | Initial: classicc grade II | Yes | 0 | • Topical tacrolimus and steroids• Methylprednisolone IVd | Yes |
| 2 | Initial: compatibleeLater: classic grade II | No | 2 | • Topical steroids• ECP | Yes |
| 3 | Initial: compatible | Yes | 0 | • Methylprednisolone IV | Yes |
| 4 | Initial: compatible | Yes | 0 | • Topical tacrolimus• Methylprednisolone IV and rabbit antithymocyte globulin | Yes |
| 5 | No biopsy | No | 0 | • Topical steroids | Yes |
aIn chronological order of transplant date.
bTotal peripheral blood.
cClassic GVHD histopathology defined as vacuolar interface degeneration, full-thickness keratinocyte necrosis, or satellite cell necrosis.
dMethylprednisolone started before chimerism testing.
eGVHD-compatible features included spongiosis, eosinophilia, less extensive keratinocyte necrosis, and perivascular and perieccrine inflammation.
ECP, extracorporeal photopheresis; GVHD, graft vs host disease; IV, intravenous.
Outcome of GVHD
Of the 28 patients with GVHD, 12 (43%) were alive on June 30, 2020, 3.0 (2.1–4.8) y after ITx. Of the 16 patients who died, GVHD was ongoing in 10 at death 10.4 (7.6–11.6) mo after disease onset, while the remaining 6 patients died after GVHD remission 27.9 (8.6–35.0) mo after disease onset (P = 0.0824). Of those dying with active GVHD, sepsis was the primary cause of death or important contributor to death in all 9 patients in whom details about death were known. The most common fatal pathogen was aspergillus (5 patients); others included cytomegalovirus, toxoplasma, and Enterobacter cloacae. The 6 deaths among the 18 patients who had recovered from GVHD resulted from infections that included adenovirus, Candida glabrata, and aspergillus. In total, 8 patients experienced aspergillus infection, of whom 3 had received intermittent prophylaxis with micafungin (death in 2). All 7 patients who developed an Epstein-Barr virus-driven posttransplant lymphoproliferative disorder recovered with treatment. Grade I (mild) graft rejection occurred in 1 patient 17 d before recognition of GVHD that resolved despite relapsing skin and lung involvement. Grade III (severe) rejection developed 15.0 (4.5–23.2) mo after onset of GVHD in 6 patients of whom 5 had experienced GVHD recovery. The sole patient with ongoing GVHD had persistent ulcerative rectosigmoid colon disease when rejection was identified in graft ileum and colon 3 wk before death.
Characteristics of the 12 patients surviving and 16 patients dying after GVHD are summarized in Table 4. As shown in Table 4, nonsurvivors demonstrated greater peripheral blood chimerism, clinical indications of more severe and refractory disease including emergence of new sites of involvement, and more numerous and severe complications. Reflecting employment for worsening and refractory disease, no individual therapy was associated with favorable outcome. Univariable Cox modeling (Table 5) also indicated an increased hazard for death with an underlying genetic disease, equivocal death hazard from splenectomy-inclusive ITx, and reduced death hazard with IITx. Nevertheless, the multivariable Cox model also shown in Table 5 demonstrated that only sepsis was independently associated with fatal GVHD outcome. There was no association between survival and initial skin biopsy findings (GVHD-classic versus compatible, P = 0.9351, and normal versus GVHD-compatible, P = 0.2679).
TABLE 4.
Factors associated with survival and death after GVHD in 28 patients following intestinal transplantation
| Explanatory variable | P a | ||
|---|---|---|---|
| Pretransplant recipient factors | |||
| Primary disease type | Short bowel syndrome—survived | 50.0%, n = 3/6 | 0.8948 |
| Functional intestinal failure—survivedb | 36.4%, n = 4/11 | ||
| Nonintestinal failurec—survived | 45.5%, n = 5/11 | ||
| Gender | Male—survived | 43.8%, n = 7/16 | 1.0000 |
| Female—survived | 41.7%, n = 5/12 | ||
| Genetic diagnosis presentd | Survived | 25.0%, n = 3/12 | 0.0671 |
| Died | 62.5%, n = 10/16 | ||
| Perioperative factors | |||
| Age group at transplant | Adult—survived | 42.9%, n = 6/14 | 1.0000 |
| Pediatric—survived | 42.9%, n = 6/14 | ||
| Age at transplant, median (interquartile range) | Survived, n = 12 | 17.0 (5.4–32.8) y | 0.9630 |
| Died, n = 16 | 20.0 (2.2–36.7) y | ||
| Donor age at transplant, median (interquartile range) | Survived, n = 12 | 11.5 (1.6–17.5) y | 0.7983 |
| Died, n = 16 | 8.0 (2.6–16.5) y | ||
| Donor to recipient age ratio, median (interquartile range) | Survived, n = 12 | 0.45 (0.20–0.90) | 0.3156 |
| Died, n = 16 | 0.70 (0.35–1.1) | ||
| Repeat intestinal transplant | Survived | 25.0%, n = 3/12 | 1.0000 |
| Died | 18.8%, n = 3/16 | ||
| Transplant type | Small intestine—survived | 70.0%, n = 7/10 | 0.0631 |
| Liver and small intestine—survived | 40.0%, n = 2/5 | ||
| Multivisceral and modified multiviscerale—survived | 23.1%, n = 3/13 | ||
| Liver graft included | Survived | 41.7%, n = 5/12 | 0.2495 |
| Died | 68.8%, n = 11/16 | ||
| Splenectomy with transplantf | Survived | 25.0%, n = 3/12 | 0.0671 |
| Died | 62.5%, n = 10/16 | ||
| Colon graft included | Survived | 100%, n = 12/12 | 0.2381 |
| Died | 81.2%, n = 13/16 | ||
| Number of DR locus mismatches, median (interquartile range) | Survived, n = 12 | 2.0 (2.0–2.0) | 0.3250 |
| Died, n = 13 | 2.0 (1.8–2.0) | ||
| Immunological factors | |||
| Immunosuppression induction with rabbit antithymocyte globuling | Survived | 33.3%, n = 4/12 | 0.6908 |
| Died | 25.0%, n = 4/16 | ||
| Early secondary immunosuppression after transplanth | Received sirolimus and survived | 33.3%, n = 1/3 | 0.8261 |
| Received mycophenolate mofetil and survived | 25.0%, n = 1/4 | ||
| Received neither and survived | 47.6%, n = 10/21 | ||
| Graft rejection before onset GVHD | Survived | 8.3%, n = 1/12 | 0.4286 |
| Died | 0%, n = 0/16 | ||
| Clinical features of GVHD | |||
| Time of onset after transplant, median (interquartile range) | Survived, n = 12 | 32 (12–42) d | 0.2094 |
| Died, n = 16 | 48 (22–72) d | ||
| Fever present at onset | Survived | 41.7%, n = 5/12 | 0.2495 |
| Died | 68.8%, n = 11/16 | ||
| Classic skin biopsy at onseti | Survived | 22.2%, n = 2/9 | 0.5372 |
| Died | 30.8%, n = 4/13 | ||
| Highest biopsy grade of skin disease | Grade I—survived | 0%, n = 0/1 | 0.9999 |
| Grade II—survived | 30.8%, n = 4/13 | ||
| Grade III—survived | 0%, n = 0/2 | ||
| Absolute lymphocyte count at presentation, median (interquartile range) | Survived, n = 12 | 850 (650–1200)/μL | 0.3888 |
| Died, n = 16 | 750 (350–1200)/μL | ||
| Absolute neutrophil count at presentation, median (interquartile range) | Survived, n = 12 | 7850 (2900–10 850)/μL | 0.7103 |
| Died, n = 16 | 4650 (2700–10 100)/μL | ||
| Total donor peripheral blood chimerism, median (interquartile range) | Survived, n = 11 | 0.0 (0.0–0.0)% | 0.0050 |
| Died, n = 14 | 2.5 (0.0–5.3)% | ||
| Number of disease sites at presentation, median (interquartile range) | Survived, n = 12 | 1.0 (1.0–2.0) | 0.8540 |
| Died, n = 16 | 1.0 (1.0–2.0) | ||
| More than 1 disease site at presentation | Survived | 33.3%, n = 4/12 | 1.0000 |
| Died | 37.5%, n= 6/16 | ||
| Number of GVHD sites emerging after treatment, median (interquartile range) | Survived, n = 12 | 0 (0.0–0.0) | 0.0014 |
| Died, n = 16 | 1.5 (0.5–3.0) | ||
| Any GVHD site emerging after treatment | Survived | 16.7%, n = 2/12 | 0.0063 |
| Died | 75.0%, n = 12/16 | ||
| Time of late site emergence after GVHD onset, median (interquartile range) | Survived, n = 2 | 153 d | 0.2733 |
| Died, n = 12 | 58 (35–174) d | ||
| Total number of involved sites, median (interquartile range) | Survived, n = 12 | 2.0 (1.0–2.0) | 0.0017 |
| Died, n = 16 | 3.0 (2.0–4.5) | ||
| Treatment of GVHD | |||
| Initial steroid response | Survived | 100%, n = 11/11 | 1.0000 |
| Died | 93.3%, n = 14/15 | ||
| Steroid resistancej | Survived | 50.0%, n = 6/12 | 0.0031 |
| Died | 100%, n = 15/15 | ||
| Anti-interleukin 2 receptor antibody | Survived | 0%, n = 0/12 | 1.0000 |
| Died | 6.2%, n = 1/16 | ||
| Antitumor necrosis factor antibody | Survived | 0%, n = 0/12 | 0.2381 |
| Died | 18.8%, n = 3/16 | ||
| Antithymocyte globulink | Survived | 33.3%, n = 4/12 | 0.2761 |
| Died | 56.2%, n = 9/16 | ||
| Sirolimus or mycophenolate mofetill | Survived | 83.3%, n = 10/12 | 0.6618 |
| Died | 68.8%, n = 11/16 | ||
| Extracorporeal photopheresis | Survived | 0%, n = 0/12 | 0.0103 |
| Died | 43.8%, n = 7/16 | ||
| Ruxolitinib | Survived | 0%, n = 0/12 | 0.4921 |
| Died | 12.5%, n = 2/16 | ||
| Elapsed time from onset GVHD to end of studym | Survived, n = 12 | 3.0 (2.1–4.8) y | 0.0853 |
| Died, n = 16 | 6.1 (3.0–8.6) y | ||
| Complications of GVHD and treatment | |||
| Insulin therapy during treatment | Survived | 33.3%, n = 4/12 | 0.1283 |
| Died | 66.7%, n = 10/15 | ||
| Renal replacement therapy | Survived | 0%, n = 0/12 | 0.0031 |
| Died | 53.3%, n = 8/15 | ||
| Aspergillus infection | Survived | 8.3%, n = 1/12 | 0.0433 |
| Died | 46.7%, n = 7/15 | ||
| Cytomegalovirus infection | Survived | 8.3%, n = 1/12 | 0.6051 |
| Died | 20.0%, n = 3/15 | ||
| Epstein-Barr virus infection | Survived | 25.0%, n = 3/12 | 0.6828 |
| Died | 40.0%, n = 6/15 | ||
| Pseudomonas infection | Survived | 0%, n = 0/12 | 0.1060 |
| Died | 26.7%, n = 4/15 | ||
| Sepsis of all causes | Survived | 8.3%, n = 1/12 | <0.0001 |
| Died | 93.3%, n = 14/15 | ||
| New tumor developing during treatment | Survived | 16.7%, n = 2/12 | 0.2232 |
| Died | 43.8%, n = 7/16 | ||
| Eventual remission of GVHD | Survived | 100%, n = 12/12 | 0.0009 |
| Died | 37.5%, n = 6/16 | ||
| Graft rejection after GVHD | Survived | 8.3%, n = 1/12 | 0.1965 |
| Died | 31.2%, n = 5/16 |
aFisher exact test, Fisher-Freeman-Halton test, or Mann-Whitney test as appropriate.
bFunctional intestinal failure equivalent to congenital mucosal disease and dysmotility.
cNonintestinal failure equivalent to abdominal tumors and portomesenteric thrombosis, primary and secondary.
dProven by testing or assumed because of known inheritance patterns.
eMultivisceral and modified multivisceral transplants in combination equivalent to splenectomy.
fSplenectomy with transplant in comparison with spleen-preserving transplants: small intestine and liver-small intestine.
gRabbit antithymocyte globulin in place of basiliximab.
hSirolimus or mycophenolate mofetil given for at least 2 wk before onset of GVHD or for at least 2 wk after transplant including postoperative d 30 when GVHD absent.
iClassic histopathology defined as vacuolar interface degeneration, full-thickness keratinocyte necrosis, or satellite cell necrosis.
jLack of initial response or recurrence of disease after initial response.
kRabbit antithymocyte globulin in 10 and equine antithymocyte globulin in 3.
lSirolimus in 11, mycophenolate mofetil in 8, combination of sirolimus and mycophenolate mofetil in 2.
mEnd of study: June 30, 2020. P values in bold font denote significance at < 0.0500.
GVHD, graft vs host disease.
TABLE 5.
Univariable and multivariable Cox proportional hazards modeling of factors associated with death following GVHD after intestinal transplantation
| Explanatory variable | Univariable hazard ratio (95% confidence interval) | P | Multivariable hazard ratio (95% confidence interval) | P |
|---|---|---|---|---|
| Pretransplant recipient factors | ||||
| Genetic diagnosis presenta | 2.8821 (1.0324–8.0462) | 0.0433 | ||
| Perioperative factors | ||||
| Transplant type | ||||
| Isolated intestinal transplant relative to splenectomy-associated transplantb | 0.2303 (0.0628–0.8442) | 0.0267 | ||
| Splenectomy-associated transplantb | 2.7377 (0.9916–7.5584) | 0.0519 | ||
| Clinical features of GVHD | ||||
| Donor total lymphocyte chimerism | 1.0434 (1.0030–1.0854) | 0.0349 | ||
| Number of GVHD sites emerging after treatment | 1.3646 (1.0971–1.6972) | 0.0052 | ||
| No GVHD site emerging after treatment | 0.1319 (0.0357–0.4870) | 0.0024 | ||
| Total number of involved sites | 1.4275 (1.1248–1.8116) | 0.0034 | ||
| Complications of GVHD | ||||
| Renal replacement therapy during treatment | 8.9834 (2.7885–28.9413) | 0.0002 | ||
| Aspergillus infection during and after treatment | 4.2105 (1.3687–12.9525) | 0.0122 | ||
| Sepsis of all types during treatment | 41.5925 (5.1243–337.5959) | 0.0005 | 37.4181 (4.5517, 307.6056) | 0.0008 |
| Nonremission of GVHD | 15.9832 (3.4046–75.0349) | 0.0004 |
aProven by testing or assumed because of known inheritance patterns.
bSplenectomy-associated transplant equivalent to multivisceral and modified multivisceral transplant in combination. Modified multivisceral transplant excludes simultaneous en bloc liver transplant. P values in bold font denote significance at <0.0500.
GVHD, graft vs host disease.
Risk Factors for Occurrence of GVHD
A comparison of the 28 patients with GVHD with 206 ITx recipients without GVHD who survived at least 1 y after ITx is summarized in Table 6. As shown in Table 6, patients developing GVHD more frequently had functional IF, non-IF, and genetic diagnoses; 1 or more previous transplants; more mismatching at the HLA-DR locus; and received colon- and splenectomy-inclusive ITx. Conversely, those developing GVHD less commonly received early secondary immunosuppression with SIR or experienced graft rejection. Contrasting with HLA-DR, those developing and not developing GVHD had similar numbers of mismatches at other HLA loci, including A, B, C, DQ, DRB, Bw4, Bw6, and A and B cross-reactive groups; they also did not differ in frequency of donor HLA homozygosity. Of the 6 patients developing GVHD with a history of previous transplant, 3 received a MVTx, 2 received a LITx, and 1 received an IITx; all 6 received a colon graft. The 22 patients developing GVHD after primary ITx did not differ from retransplanted patients in distribution of variant ITx operations (P = 0.3404) or graft colon inclusion (P = 1.0).
TABLE 6.
Factors associated with occurrence of GVHD in 28 of 234 patients after intestinal transplantation
| GVHD present | GVHD absenta | P b | ||
|---|---|---|---|---|
| Demographic variables | ||||
| Percentage male | 57.1%, n = 16/28 | 57.8%, n = 119/206 | 1.0000 | |
| Percent adult | 50.0%, n = 14/28 | 49.0%, n = 101/206 | 1.0000 | |
| Primary diseases typec | Short bowel syndrome | 21.4%, n = 6/28 | 77.2%, n = 159/206 | <0.0001 |
| Dysmotility | 21.4%, n = 6/28 | 15.0%, n = 31/206 | ||
| Congenital mucosal disease | 17.9%, n = 5/28 | 4.4%, n = 9/206 | ||
| Abdominal tumor | 21.4%, n = 6/28 | 1.9%, n = 4/206 | ||
| Portomesenteric thrombosis, primary and secondary | 17.9%, n = 5/28 | 1.5%, n = 3/206 | ||
| Genetic diagnosis presentd | 46.4%, n = 13/28 | 20.9%, n = 43/206 | 0.0077 | |
| NOD mutation present | 15%, n = 3/20 | 23.1%, n = 42/182 | 0.5743 | |
| Perioperative factors | ||||
| Hospitalized at transplant | 11.5%, n = 3/26 | 14.4%, n = 24/167 | 1.0000 | |
| Age at transplant (y), median (interquartile range) | 18.2 (2.8–33.5) y, n = 28 | 15.0 (1.7–42.9) y, n = 206 | 0.6989 | |
| Donor to recipient age ratio, median (interquartile range) | 0.53 (0.29–1.0), n = 28 | 0.40 (0.24–0.65), n = 206 | 0.0534 | |
| Donor to recipient weight ratio, median (interquartile range) | 0.87 (0.61 to 1.05) | 0.76 (0.63–0.91) | 0.2154 | |
| Transplant type | Small intestine | 35.7%, n = 10/28 | 59.7%, n = 123/206 | <0.0001 |
| Liver - intestine | 17.9%, n = 5/28 | 28.6%, n = 59/206 | ||
| Multivisceral and modified multiviscerale | 46.4%, n = 13/28 | 11.7%, n = 24/206 | ||
| Liver graft included | 57.1%, n = 16/28 | 38.3%, n = 79/206 | 0.0665 | |
| Colon graft included | 89.3%, n = 25/28 | 55.8%, n = 115/206 | 0.0004 | |
| Splenectomy with transplantf | 46.4%, n = 13/28 | 11.7%, n = 24/206 | <0.0001 | |
| Previous transplant—any type | 21.4%, n = 6/28 | 8.7%, n = 18/206 | 0.0490 | |
| Total class I mismatches, median (interquartile range) | 5.0 (5.0–5.2), n = 25 | 5.0 (5.0–6.0), n = 170 | 0.4405 | |
| Number of DR locus mismatches, median (interquartile range) | 2.0 (2.0–2.0), n = 25 | 2.0 (1.0–2.0), n = 193 | 0.0268 | |
| Total class II mismatches, median (interquartile range) | 4.0 (4.0–5.0), n = 23 | 4.0 (3.0–5.0), n = 178 | 0.1382 | |
| Immunological factors | ||||
| Panel reactive antibodies at transplant, median (interquartile range) | 0.0 (0.0–20.8) %, n = 27 | 0.0 (0.0–19.8) %, n = 201 | 0.4960 | |
| Pretreatment of donor with rabbit antithymocyte globulin | 75.0%, n = 18/24 | 71.4%, n = 125/175 | 0.8126 | |
| Induction immunosuppression | ||||
| Basiliximab | 71.4%, n = 20/28 | 69.4%, n = 143/206 | 0.8001 | |
| Rabbit antithymocyte globulin | 28.6%, n = 8/28 | 30.6%, n = 63/206 | ||
| Early secondary immunosuppression after transplantg | 0.0058 | |||
| Sirolimus | 10.7%, n = 3/28 | 39.5%, n = 62/157 | ||
| Mycophenolate mofetil | 14.3%, n = 4/28 | 12.7%, n = 20/157 | ||
| None | 75.0%, n = 21/28 | 47.8%, n = 75/157 | ||
| Absolute neutrophil count at 37 d after transplant, median (interquartile range) | 5450/μL (2700–10 850/μL), n = 28 | 5000/μL (3200–7300/μL), n = 130 | 0.6260 | |
| Absolute lymphocyte count at 37 d after transplant, median (interquartile range) | 800/μL (550–1200/μL), n = 28 | 1100/μL (400–2300/μL), n = 130 | 0.1962 | |
| Rejection within 90 d after intestinal transplant (if no GVHD) or before GVHD | 3.6%, n = 1/28 | 18.9%, n = 39/206 | 0.0572 | |
| Overall incidence of graft rejection | 25.0%, n = 7/28 | 51.0%, n = 105/206 | 0.0144 | |
| Transplant outcomes | ||||
| 5-y graft survivalh | 36.4%, n= 4/11 | 71.1%, n = 121/157 | 0.0067 | |
| 5-y patient survivalh | 36.4%, n = 4/11 | 81.8%, n = 130/159 | 0.0019 |
aOne-year patient survival required for inclusion in the GVHD absent cohort.
bFisher exact test, Fisher-Freeman-Halton test, or Mann-Whitney test as appropriate.
cCategories consolidated into 3 composite groups for analysis including short bowel syndrome (anatomic intestinal failure), functional intestinal failure equivalent to congenital mucosal disease and dysmotility, and nonintestinal failure equivalent to abdominal tumors and portomesenteric thrombosis, primary and secondary.
dProven by testing or assumed because of known inheritance patterns.
eMultivisceral and modified multivisceral transplants in combination equivalent to splenectomy. Modified multivisceral transplant excludes simultaneous en bloc liver transplant.
fSplenectomy with transplant in comparison with spleen-preserving transplants: small intestine and liver-small intestine.
gSirolimus or mycophenolate mofetil given for at least 2 wk before onset of GVHD or for at least 2 wk after transplant including postoperative d 30 when GVHD absent. Ten patients receiving both drugs in this timeframe excluded from analysis.
hPatients transplanted before June 30, 2015. P values in bold font denote significance at <0.0500.
GVHD, graft vs host disease; NOD, nucleotide-binding oligomerization domain.
Results of univariable and multivariable Cox modeling of factors associated with development of GVHD are summarized in Table 7; explanatory variables associated with an increased hazard of GVHD mostly paralleled greater cumulative incidences shown in Table 6. As shown in Table 7, multivariable modeling emphasized the overriding importance of original disease (non-IF, Wald’s chi-square 18.5428; functional IF, Wald’s chi-square 4.9578) and greater donor to recipient age ratio (DRAR; Wald’s chi-square 5.7501) to increased GVHD risk and of early exposure to SIR (Wald’s chi-square 5.2650) to reduced GVHD risk. Type of ITx operation was not associated with GVHD risk independent of original disease in multivariable modeling; illustrative of this finding, splenectomy-inclusive ITx was performed in 7.9% (13/165) of patients with anatomic IF, 19.6% (10/51) of patients with functional IF, and 77.8% (14/18) of patients without IF (P < 0.0001).
TABLE 7.
Univariable and multivariable Cox proportional hazard regression modeling of risks for occurrence of graft vs host disease after intestinal transplantation in patients of all ages
| Explanatory variable | Univariable hazard ratio (95% confidence interval) | P | Multivariable hazard ratio (95% confidence interval) | P |
|---|---|---|---|---|
| Primary disease | ||||
| Nonintestinal failurea relative to anatomic intestinal failure | 22.0157 (8.1254–59.6515) | <0.0001 | 11.3181 (3.7509–34.1520) | <0.0001 |
| Functional intestinal failureb relative to anatomic intestinal failure | 7.1053 (2.6243–19.2376) | 0.0001 | 3.9210 (1.1777–13.0542) | 0.0260 |
| Genetic diagnosisc | 3.3190 (1.5773–6.9837) | 0.0016 | ||
| Retransplant | 3.1715 (1.2798–7.8598) | 0.0127 | ||
| Donor to recipient age ratio | 2.4316 (1.1490–5.1457) | 0.0202 | 4.2798 (1.3041–14.0451) | 0.0165 |
| Number of DR locus mismatches | 2.9843 (1.0538–8.4519) | 0.0395 | ||
| Transplant type | ||||
| Splenectomy-associated transplantd | 5.1395 (2.4448–10.8043) | <0.0001 | ||
| Colon graft omission | 0.1272 (0.0379–0.4265) | 0.0008 | ||
| Early secondary immunosuppression with sirolimuse | 0.1775 (0.0529–0.5963) | 0.0052 | 0.1750 (0.0395–0.7756) | 0.0218 |
aNonintestinal failure consists of tumor and portomesenteric thrombosis.
bFunctional intestinal failure consists of congenital mucosal disease and dysmotility.
cProven by testing or assumed because of known inheritance patterns.
dSplenectomy-associated transplant equivalent to multivisceral and modified multivisceral transplant in combination. Modified multivisceral transplant excludes simultaneous en bloc liver transplant.
eMinimum of 14 d of treatment either immediately before onset of graft vs host disease or within first month after transplant including postoperative d 30 in those not developing graft vs host disease. Ten patients receiving both drugs in this timeframe excluded from analysis.
P values in bold font denote significance at <0.0500.
Comparison of multivariable Cox modeling of the whole GVHD group (Table 7) with adult (Table 8) and pediatric (Table 9) subgroups indicated age-associated divergence in independent GVHD hazards. Thus, the whole group association of functional IF with increased GVHD risk and of early SIR therapy with reduced GVHD risk originated from strength of these associations in the adult subgroup, whereas whole group association of DRAR with GVHD originated from this association in infants and children. Only pediatric patients incurred independently increased risks for GVHD from inclusion of a colon graft and retransplantation. A high DRAR was the most important covariate associated with GVHD in pediatric patients (Wald’s chi-square 14.4284); median DRAR in those with GVHD was 1.0 (0.43–1.2) compared with 0.33 (0.21–0.63) in those without(P = 0.0065.) Receiver operating characteristic curve analysis indicated that a DRAR equal to 0.9 was 64.3% sensitive and 90.5% specific for GVHD (P = 0.021). In contrast, the interquartile range of DRAR in the entire adult population (0.30–0.68) was comparatively narrow (pediatric to adult variance ratio = 2.0376, P < 0.001), and the median DRAR of 0.46 did not differ between adult patients who did and did not develop GVHD (P = 0.6101).
TABLE 8.
Univariable and multivariable Cox proportional hazard regression modeling of hazards for occurrence of graft vs host disease after intestinal transplantation in adultsa
| Explanatory variable | Univariable hazard ratio (95% confidence interval) | P | Multivariable hazard ratio (95% confidence interval) | P |
|---|---|---|---|---|
| Primary disease | ||||
| Nonintestinal failureb relative to anatomic intestinal failure | 26.2683 (5.5751–123.7689) | <0.0001 | 20.9952 (4.4248–99.6202) | 0.0001 |
| Functional intestinal failurec relative to anatomic intestinal failure | 8.5846 (1.5648–47.0964) | 0.0133 | 15.2448 (2.7266–85.2365 | 0.0019 |
| Genetic diagnosisd | 2.8615 (0.9909–8.2637) | 0.0520 | ||
| Transplant type | ||||
| Splenectomy-associated transplante | 4.5962 (1.6088–13.1309) | 0.0044 | ||
| Liver graft inclusion | 3.9134 (1.3079–11.7097) | 0.0147 | ||
| Early secondary immunosuppression with sirolimusf | 0.1074 (0.0137–0.8406) | 0.0335 | 0.0956 (0.0116–0.7900) | 0.0293 |
aAdults equivalent to age 18 y and above at transplant.
bNonintestinal failure consists of tumor and portomesenteric thrombosis.
cFunctional intestinal failure consists of congenital mucosal disease and dysmotility.
dProven by testing or assumed because of known inheritance patterns.
eSplenectomy-associated transplant equivalent to multivisceral and modified multivisceral transplant in combination. Modified multivisceral transplant excludes simultaneous en bloc liver transplant.
fMinimum of 14 d of treatment either immediately before onset of graft vs host disease or within first month after transplant including postoperative d 30 in those not developing graft vs host disease. Eight patients receiving both drugs in this timeframe excluded from analysis.
P values in bold font denote significance at <0.0500.
TABLE 9.
Univariable and multivariable Cox proportional hazard regression modeling of risks for occurrence of graft vs host disease after intestinal transplantation in pediatric patientsa
| Explanatory variable | Univariable hazard ratio (95% confidence interval) | P | Multivariable hazard ratio (95% confidence interval) | P |
|---|---|---|---|---|
| Primary disease | ||||
| Nonintestinal failureb relative to anatomic intestinal failure | 22.7173 (4.9699–103.8405) | 0.0001 | 4.3990 (1.1000–17.5924) | 0.0362 |
| Functional intestinal failurec relative to anatomic intestinal failure | 6.4578 (1.8868–22.1022) | 0.0030 | ||
| Genetic diagnosisd | 3.7073 (1.2951–10.6124) | 0.0146 | ||
| Retransplant | 5.0418 (1.6860–15.0770) | 0.0038 | 4.6401 (1.3974–15.4082) | 0.0122 |
| Donor to recipient age ratio | 3.5809 (1.6615–7.7179) | 0.0011 | 7.3190 (2.6206–20.4410) | 0.0001 |
| Transplant type | ||||
| Splenectomy-associated transplante | 5.3718 (1.8604–15.5105) | 0.0019 | ||
| Colon graft omission | 0.2610 (0.0712–0.9570) | 0.0427 | 0.1886 (0.0416–0.8555) | 0.0306 |
aPediatric patients correspond to age <18 y at transplant.
bNonintestinal failure equivalent to tumor and portomesenteric thrombosis.
cFunctional intestinal failure equivalent to congenital mucosal disease and dysmotility.
dProven by testing or assumed because of known inheritance patterns.
eSplenectomy-associated transplant equivalent to multivisceral and modified multivisceral transplant in combination. Modified multivisceral transplant excludes simultaneous en bloc liver transplant.
P values in bold font denote significance at <0.0500.
DISCUSSION
Many findings in this review of GVHD after ITx are consistent with those of previous investigations including typical presentation with rash and fever within 2 mo after ITx8,11 and similar incidence in adult and pediatric patients.3,32 However, prevalence of extracutaneous GVHD approximated 80% in this series, contrasting with previous estimates that have ranged between about 30% and 50%.9,10,13,32 High incidence of GVHD involvement at extracutaneous sites, especially colon, facilitated diagnosis in individual patients. Furthermore, multiple site involvement was a hazard for reduced survival, being associated with refractoriness to successive immunosuppressive therapies9,33 that set the stage for fatal opportunistic infection.8-10 Notable among these infections was invasive aspergillosis that has also figured prominently in negative outcomes after HSCT.34,35
This study identified several factors related to the balance between donor and recipient immune function critical to development of GVHD after SOT.3,36,37 Most prominently, GVHD after ITx tended to occur when the original disease was associated with abnormal immune function, either subtle, examples including intestinal pseudoobstruction38 and trichohepatoenteric syndrome,39 or severe, particularly multiple intestinal atresia syndrome.13,40 GVHD was remarkably uncommon with a background of stand-alone short bowel. GVHD hazard was also increased by use of grafts from pediatric donors similar in age to recipients in comparison with younger donors presumably less immunocompetent.37,41 The strong connection of DRAR to GVHD risk implies that age-related physiological disparity in immune function, like absolute recipient immunodeficiency, is highly relevant to GVHD risk. This phenomenon was not observed in adults, presumably due to greater consistency in age difference between donors and recipients in the adult cohort. However, it is notable that transplantation of liver grafts into adults from donors >20 y younger, and presumably more immunocompetent, also increases GVHD risk,42 the mirror image of the relationship prevailing in our pediatric patients.
In some ITx recipients, potential recipient immune deficiencies favoring GVHD appeared to be acquired rather than inborn such as that associated with retransplantation,12 although we have no explanation for restriction of this risk to pediatric patients in this study. Transience of recipient immune incompetence was also implied by occurrence of severe graft rejection in some patients generally after GVHD recovery. Like others,9,13 we found a strong association between recipient splenectomy necessitated by MVTx and MMVTx and GVHD risk, presumably by weakening recipient defense against donor-derived alloreactive T-cell clones. However, because our practice reserves MVTx and MMVTx primarily for non-IF failure and functional IF diagnoses, multivariable regression modeling rejected splenectomy as an independent hazard for GVHD. Because of this bias, an independent effect of splenectomy on GVHD risk may not have been detected. GVHD risk was independently increased by colon graft inclusion that was anticipated by an early experimental ITx model.43 The relatively greater colon lymphocyte mass in infants and children compared with older persons44 may explain confinement of this risk to pediatric patients and emphasizes the need to weigh risks of graft colon inclusion against the clear benefits,45 particularly in younger ITx candidates.
Attempts to reduce GVHD risk by weakening donor-derived immune function with r-ATG during ITx organ procurement is a long-established practice. In this study, r-ATG failed in this objective, possibly due to insufficient time to achieve meaningful donor lymphocyte depletion46 or to intrinsic resistance of key donor memory T cells to r-ATG.47 Failure of induction immunosuppression with r-ATG to reduce GVHD incidence compared with basiliximab may be another expression of that resistance. We are reassessing r-ATG in donor procurement to ascertain if a subset of patients can be identified for whom donor r-ATG might yet prove beneficial. In contrast with r-ATG, SIR given during the first month after ITx reduced GVHD risk in adults. Given the efficacy of SIR in reducing ITx rejection risk,48 its prophylactic value against GVHD provides an additional rationale for early employment after ITx when risks of both acute rejection and GVHD are at their highest. There is evidence that mismatch at the HLA-DR locus is related to graft rejection,49,50 whereas in this study, the HLA-DR locus was associated with GVHD. Although magnitude of GVHD risk from HLA-DR mismatch was small, this commonality further emphasizes the dynamic, 2-way immune balance that prevails after ITx.37
Limitations of this study include primary reliance on routine clinical assessment including standard histopathology to diagnose GVHD without proof of local tissue chimerism or peripheral blood T-cell chimerism data in most cases, although magnitude of T-cell chimerism in those with and without GVHD overlaps.10,37 Conversely, there was no accounting of suspected GVHD cases ultimately rejected on clinical or immunological grounds to compare with affirmative GVHD diagnoses. Furthermore, the uncontrolled nature of treatment undermined identification and optimal employment of potentially useful interventions. Clinical management practices inevitably evolved during the almost 17-y study period, potentially altering GVHD expression.
CONCLUSIONS AND LESSONS LEARNED
GVHD risk after ITx was related more to original disease than to ITx variant operation in patients of all ages. Pretreatment of donors with r-ATG before graft recovery did not reduce GVHD risk. In pediatric but not adult patients, use of grafts from donors significantly younger than intended recipients reduced GVHD risk, whereas including colon with the ITx and repeat transplantation increased GVHD risk. In adult but not pediatric patients, use of SIR soon after ITx reduced GVHD risk, reason for the differential effect based on age being unclear.
Established therapies for GVHD in SOT are based on practices in HSCT. Corticosteroids constitute first-line therapy, and with steroid refractoriness, other immunosuppressives with inconsistent efficacy are used that further predispose to infection.5 Before recent regulatory agency approval of the Janus kinase signal transduction inhibitor ruxolitinib for corticosteroid-refractory GVHD after HSCT,6 2 patients in this study received ruxolitinib off-label in a final but unsuccessful effort to control GVHD unresponsive to numerous therapies including r-ATG.51 In both cases, delayed employment likely contributed to ruxolitinib failure in the setting of advanced disease and patient debility. We now use ruxolitinib as second-line therapy for GVHD once corticosteroid refractoriness has been established on the presumption that earlier use in the GVHD course shall increase probability of extended remission with reduced risk of morbid and fatal complications.
Supplementary Material
Footnotes
Published online 19 July, 2021.
S.S.K., O.T. participated in research design, writing of the article, performance of the research, and data analysis. E.H. participated in research design and performance of the research. A.K., N.A.Y., K.M.K. participated in research design, writing of the article, and data analysis. H.B.P., J.F.G., J.S.H. participated in the writing of the article and data analysis. U.D.E., S.S., R.G., S.S.G., T.M.F., and C.S.M. participated in the writing of the article.
The authors declare no funding or conflicts of interest.
The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Rai V, Dietz NE, Agrawal DK. Immunological basis for treatment of graft versus host disease after liver transplant. Expert Rev Clin Immunol. 2016;12:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andres AM, Santamaría ML, Ramos E, et al. Graft-vs-host disease after small bowel transplantation in children. J Pediatr Surg. 2010;45:330–336. [DOI] [PubMed] [Google Scholar]
- 3.Mazariegos GV, Abu-Elmagd K, Jaffe R, et al. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004;4:1459–1465. [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Yazawa K, Madono K, et al. Acute graft-versus-host-disease in kidney transplantation: case report and review of literature. Transplant Proc. 2009;41:3949–3952. [DOI] [PubMed] [Google Scholar]
- 5.Zeiser R, von Bubnoff N, Butler J, et al. ; REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800–1810. [DOI] [PubMed] [Google Scholar]
- 6.Przepiorka D, Luo L, Subramaniam S, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. 2020;25:e328–e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SE, Cho BS, Kim JH, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48:587–592. [DOI] [PubMed] [Google Scholar]
- 8.Feito-Rodríguez M, de Lucas-Laguna R, Gómez-Fernández C, et al. Cutaneous graft versus host disease in pediatric multivisceral transplantation. Pediatr Dermatol. 2013;30:335–341. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Selvaggi G, Nishida S, et al. Graft-versus-host disease after intestinal and multivisceral transplantation. Transplantation. 2011;91:219–224. [DOI] [PubMed] [Google Scholar]
- 10.Shin CR, Nathan J, Alonso M, et al. Incidence of acute and chronic graft-versus-host disease and donor T-cell chimerism after small bowel or combined organ transplantation. J Pediatr Surg. 2011;46:1732–1738. [DOI] [PubMed] [Google Scholar]
- 11.Cromvik J, Varkey J, Herlenius G, et al. Graft-versus-host disease after intestinal or multivisceral transplantation: a Scandinavian single-center experience. Transplant Proc. 2016;48:185–190. [DOI] [PubMed] [Google Scholar]
- 12.Kubal CA, Pennington C, Fridell J, et al. Challenges with intestine and multivisceral re-transplantation: importance of timing of re-transplantation and optimal immunosuppression. Ann Transplant. 2018;23:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer RT, Friend B, Talmon GA, et al. Intestinal transplantation in children with multiple intestinal atresias and immunodeficiency. Pediatr Transplant. 2014;18:190–196. [DOI] [PubMed] [Google Scholar]
- 14.Haimes H, Morley KW, Song H, et al. Impact of skin biopsy on the management of acute graft-versus-host disease in a pediatric population. Pediatr Dermatol. 2019;36:455–459. [DOI] [PubMed] [Google Scholar]
- 15.Cruysmans C, Ferneiny MG, Fraitag S, et al. Severe skin complications after small bowel transplantation: graft-versus-host disease, DRESS, virus, or drug toxicity? Transplantation. 2016;100:2222–2225. [DOI] [PubMed] [Google Scholar]
- 16.Zuber J, Rosen S, Shonts B, et al. Macrochimerism in intestinal transplantation: association with lower rejection rates and multivisceral transplants, without GVHD. Am J Transplant. 2015;15:2691–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner J, Svetlicky N, Kang J, et al. CD69+ resident memory T cells are associated with graft-versus-host disease in intestinal transplantation. Am J Transplant. 2021;21:1878–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishbein TM. Intestinal transplantation. N Engl J Med. 2009;361:998–1008. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman SS, Pehlivanova M, Fennelly EM, et al. Predicting liver failure in parenteral nutrition-dependent short bowel syndrome of infancy. J Pediatr. 2010;156:580–585.e1. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto CS, Subramanian S, Fishbein TM. Adult intestinal transplantation. Gastroenterol Clin North Am. 2018;47:341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawksworth JS, Rosen-Bronson S, Island E, et al. Successful isolated intestinal transplantation in sensitized recipients with the use of virtual crossmatching. Am J Transplant. 2012;12 Suppl 4:S33–S42. [DOI] [PubMed] [Google Scholar]
- 22.Elsabbagh AM, Hawksworth J, Khan KM, et al. Long-term survival in visceral transplant recipients in the new era: a single-center experience. Am J Transplant. 2019;19:2077–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remotti H, Subramanian S, Martinez M, et al. Small-bowel allograft biopsies in the management of small-intestinal and multivisceral transplant recipients: histopathologic review and clinical correlations. Arch Pathol Lab Med. 2012;136:761–771. [DOI] [PubMed] [Google Scholar]
- 24.Ganoza A, Mazariegos GV, Khanna A. Current status of graft-versus-host disease after intestinal transplantation. Curr Opin Organ Transplant. 2019;24:199–206. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatol Clin. 2019;37:569–582. [DOI] [PubMed] [Google Scholar]
- 26.Fischer A, Jakubowski AA, Lacouture ME, et al. Histopathologic features of cutaneous acute graft-versus-host disease in T-cell-depleted peripheral blood stem cell transplant recipients. Am J Dermatopathol. 2015;37:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim GY, Schmelkin LA, Davis MD, et al. Clinical and histopathologic manifestations of solid organ transplantation-associated graft-versus-host disease involving the skin: a single-center retrospective study. J Cutan Pathol. 2018;45:817–823. [DOI] [PubMed] [Google Scholar]
- 28.Massi D, Fondi C, Nozzoli C, et al. The impact of histopathologic examination of graft-versus-host disease in the era of reduced-intensity conditioning regimen: a study from the Gruppo Italiano Trapianto di Midollo Osseo. Hum Pathol. 2011;42:254–268. [DOI] [PubMed] [Google Scholar]
- 29.Lehman JS, Gibson LE, el-Azhary RA, et al. Acute cutaneous graft-vs.-host disease compared to drug hypersensitivity reaction with vacuolar interface changes: a blinded study of microscopic and immunohistochemical features. J Cutan Pathol. 2015;42:39–45. [DOI] [PubMed] [Google Scholar]
- 30.Spellman S, Setterholm M, Maiers M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008;14(suppl 9):37–44. [DOI] [PubMed] [Google Scholar]
- 31.Wade JA, Hurley CK, Takemoto SK, et al. HLA mismatching within or outside of cross-reactive groups (CREGs) is associated with similar outcomes after unrelated hematopoietic stem cell transplantation. Blood. 2007;109:4064–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clouse JW, Kubal CA, Fridell JA, et al. Post-intestine transplant graft-vs-host disease associated with inclusion of a liver graft and with a high mortality risk. Clin Transplant. 2019;33:e13409. [DOI] [PubMed] [Google Scholar]
- 33.Qian L, Wu Z, Shen J. Advances in the treatment of acute graft-versus-host disease. J Cell Mol Med. 2013;17:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labbé AC, Su SH, Laverdière M, et al. High incidence of invasive aspergillosis associated with intestinal graft-versus-host disease following nonmyeloablative transplantation. Biol Blood Marrow Transplant. 2007;13:1192–1200. [DOI] [PubMed] [Google Scholar]
- 35.Mikulska M, Raiola AM, Bruno B, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 2009;44:361–370. [DOI] [PubMed] [Google Scholar]
- 36.Davison J, Darbyshire P, Beath SV, et al. Refractory graft versus host disease after pediatric intestinal transplantation-beware of pre-existing immunodeficiency. Transplantation. 2008;86:179. [DOI] [PubMed] [Google Scholar]
- 37.Fu J, Zuber J, Shonts B, et al. Lymphohematopoietic graft-versus-host responses promote mixed chimerism in patients receiving intestinal transplantation. J Clin Invest. 2021;131:e141698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forchielli ML, Young MC, Flores AF, et al. Immune deficiencies in chronic intestinal pseudo-obstruction. Acta Paediatr. 1997;86:1077–1081. [DOI] [PubMed] [Google Scholar]
- 39.Vély F, Barlogis V, Marinier E, et al. Combined immunodeficiency in patients with trichohepatoenteric syndrome. Front Immunol. 2018;9:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jardine S, Dhingani N, Muise AM. TTC7A: steward of intestinal health. Cell Mol Gastroenterol Hepatol. 2019;7:555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elfeki MA, Pungpapong S, Genco PV, et al. Graft-versus-host disease after orthotopic liver transplantation: multivariate analysis of risk factors. Clin Transplant. 2015;29:1063–1066. [DOI] [PubMed] [Google Scholar]
- 43.Pirenne J, Gruessner A, Benedetti E, et al. Addition of the colon to small bowel grafts causes lethal graft-versus-host disease in FK 506-treated pigs. Transplant Proc. 1996;28:886–887. [PubMed] [Google Scholar]
- 44.Albuquerque A. Nodular lymphoid hyperplasia in the gastrointestinal tract in adult patients: a review. World J Gastrointest Endosc. 2014;6:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto CS, Kaufman SS, Fishbein TM. Inclusion of the colon in intestinal transplantation. Curr Opin Organ Transplant. 2011;16:312–315. [DOI] [PubMed] [Google Scholar]
- 46.Shaffer D, Ubhi CS, Simpson MA, et al. Prevention of graft-versus-host disease following small bowel transplantation with polyclonal and monoclonal antilymphocyte serum. The effect of timing and route of administration. Transplantation. 1991;52:948–952. [DOI] [PubMed] [Google Scholar]
- 47.Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10:2132–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauro A, Dazzi A, Ercolani G, et al. Rejection episodes and 3-year graft survival under sirolimus and tacrolimus treatment after adult intestinal transplantation. Transplant Proc. 2007;39:1629–1631. [DOI] [PubMed] [Google Scholar]
- 49.Nitta D, Kinugawa K, Imamura T, et al. Association of the number of HLA-DR mismatches with early post-transplant acute cellular rejection among heart transplantation recipients: a cohort study in Japanese population. Transplant Proc. 2017;49:125–129. [DOI] [PubMed] [Google Scholar]
- 50.Tajik N, Singal D, Pourmand G, et al. Prospective study of microchimerism in renal allograft recipients: association between HLA-DR matching, microchimerism and acute rejection. Clin Transplant. 2001;15:192–198. [DOI] [PubMed] [Google Scholar]
- 51.Ghobrial S, Gonzalez C, Yazigi N, et al. Efficacy and feasibility of ruxolitinib in chronic steroid-refractory GVHD in a pediatric intestine transplant. Pediatr Transplant. 2021;25:e13836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.