Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, methylprednisolone, pneumonia
Abstract
OBJECTIVES:
To determine methylprednisolone’s dose, duration, and administration from onset of symptoms and association with 60 days in hospital survival of coronavirus disease 2019 pneumonia.
DESIGN:
Cohort study.
SETTING:
Thirteen hospitals in New Jersey, United States during March to June 2020.
PATIENTS:
Seven-hundred fifty-nine hospitalized coronavirus disease 2019 patients.
INTERVENTIONS:
We performed a propensity matched cohort study between patients who received methylprednisolone and no methylprednisolone. Patients in the methylprednisolone group were further differentiated into dose (high dose and low dose), duration, and administration from onset of symptoms.
MEASUREMENTS AND MAIN RESULTS:
In the propensity matched sample, 99 out of 380 (26%) in no methylprednisolone, 69 out of 215 (31.9%) in low-dose methylprednisolone, and 74 out of 164 (55.2%) high-dose methylprednisolone expired. Overall median survival for no methylprednisolone (25.0 d), low-dose methylprednisolone (39.0 d), high-dose methylprednisolone (20.0 d), less than or equal to 7 days duration (19.0 d), 7–14 days duration (30.0 d), greater than 14 days duration (44.0 d), onset of symptoms less than or equal to 7 days (20.0 d), and onset of symptoms 7–14 days (27.0 d) were statistically significant (log-rank p ≤ 0.001). Multivariate Cox regression showed nursing home residents, coronary artery disease, and invasive mechanical ventilation were independently associated with mortality. Methylprednisolone was associated with reduced mortality compared with no methylprednisolone (hazard ratio, 0.40; 95% CI, 0.27–0.59; p < 0.001) but no added benefit with high dose. Low-dose methylprednisolone for 7–14 days was associated with reduced mortality compared with less than or equal to 7 days (hazard ratio, 0.45; 95% CI, 0.22–0.91; p = 0.0273), and no additional benefit if greater than 14 days (hazard ratio, 1.27; 95% CI, 0.60–2.69; p = 0.5434). Combination therapy with tocilizumab was associated with reduced mortality over monotherapy (p < 0.0116).
CONCLUSIONS:
Low-dose methylprednisolone was associated with reduced mortality if given greater than 7 days from onset of symptoms, and no additional benefit greater than 14 days. High dose was associated with higher mortality.
As of June 2, 2021, the coronavirus disease 2019 (COVID-19) pandemic has had 170,812,850 confirmed cases, and 3,557,586 deaths (1). Mortality was associated with a “cytokine storm” which can rapidly progress to acute respiratory distress syndrome (ARDS). This period is associated with elevated levels of interleukin (IL)-6, IL-2, IL-7, granulocyte colony-stimulating factor, interferon-γ-inducible protein, monocyte chemoattractant protein-1, macrophage inflammatory protein-1 alpha, and tumor necrosis factor-α (2–4).
Corticosteroids were considered for their anti-inflammatory and anti-fibrotic properties and met the time of need. There were initial reservations due to extrapolated data regarding delayed viral clearance in severe acute respiratory syndrome and Middle Eastern respiratory syndrome, and increased mortality in influenza (5–11). Therefore, corticosteroids were given as rescue for patients who were more hypoxic.
Since then, several studies have investigated the use of different formulation of corticosteroids in COVID-19. The Recovery study showed dexamethasone 6 mg daily improved 28-day mortality with hospitalized COVID-19 patients requiring oxygen supplementation (12). A multicenter observational study in Italy suggested that methylprednisolone (MP) infusion improved 28-day mortality (13). Another study suggested that use of dexamethasone, MP, prednisone, or hydrocortisone within 48 hours of admission and C-reactive protein (CRP) greater than or equal to 20 mg/dL was associated with significant reduction of 28-day mortality or mechanical ventilation (MV) (odds ratio, 0.23; 95% CI, 0.08–0.7) (14). A single-center randomized control trial of MP in Brazil, Methylprednisolone as Adjunct Therapy for Patients Hospitalized with Coronavirus Disease 2019, which used 0.5 mg/kg twice a day for 5 days, did not show reduce mortality in overall population, but it did suggest benefit in patients that are greater than 60 years old with high CRP (15).
Tocilizumab (TOC), a monoclonal antibody and IL-6 antagonist maybe synergistic with dexamethasone and considered adjunct for patients who require at least high-flow (HF) nasal cannula (16–19).
In our institution, we used MP during this initial pandemic surge. There was significant heterogeneity in how it was used and we sought to evaluate the relationship of dose, duration, and timing of administration from onset of symptoms (OOS) with survival.
MATERIALS AND METHODS
Setting and Subjects
We performed a retrospective cohort study on patients admitted to one of 13 Hackensack Meridian Health (HMH) hospitals. Data were obtained from electronic health records (EHRs) in patients with COVID-19. HMH Institutional Review Board approved this study on May 20, 2020, under study number Pro2020-0485. Data were obtained from a prospective observational database registered on ClinicalTrials.gov NCT04347993.
Our inclusion criteria were patients greater than or equal to 18 years old, admitted for greater than or equal to 2 days, hospitalized from the study period of March 1, 2020, to June 15, 2020, and diagnosed with severe COVID-19 pneumonia, which includes a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) and saturation of peripheral oxygen less than 94% on room air at sea level, a respiratory rate greater than 30 breaths/min, Pao2/Fio2 (P/F) less than 300 mm Hg, or lung infiltrates greater than 50%. We excluded patients who were less than 18 years old or pregnant. Waiver of consent and HIPAA authorization was granted due to noninterventional study that gathered data from secondary research purposes.
Demographics, clinical characteristics, laboratory values, treatments, and outcomes were manually abstracted by Internal Medicine Residents. Assignments of patients occurred in real time and not randomized. Data were entered using Research Electronic Data Capture hosted by HMH. Data abstraction occurred daily from June 1, 2020, to December 1, 2020. Quality control was performed by physicians (R.C.G., R.S., T.N., Y.O., J.J.).
Age, gender, and sex were self-reported. Weight and height were measured. Comorbidities were defined prior to COVID-19 and included cardiovascular disease, lung disease, diabetes mellitus, neurologic disease, cancer, and renal disease. Levels of oxygen support include no oxygen supplementation, nasal cannula, high flow nasal cannula, nonrebreather (NRB), and MV. ICU level of care included mechanical ventilatory support, hospitalized within a dedicated ICU stay, and patients assigned to critical care team regardless of geographic placement due to overflow during pandemic crisis.
SARS-CoV-2 was detected in nasal swabs by reverse transcription PCR. Routine blood tests included complete blood count, coagulation profile, complete metabolic profile, inflammatory markers (IL-6, CRP, d-dimer, and ferritin), and arterial blood gas (ABG).
Intervention
Although dexamethasone, prednisone, and hydrocortisone were available, MP was the most commonly used corticosteroid and given to patients as rescue when rapidly decompensating. The dose and duration of the MP were at the discretion of the ordering healthcare provider. The default weight base regimen was actual body weight. Other providers did not use MP despite worsening respiratory status due to concerns of delayed viral clearance.
Outcomes
The primary outcome was 60 days in hospital survival between no MP (NMP) and MP group, which is differentiated by dose, duration, and initiation from OOS. The secondary outcome is to compare survival with monotherapy versus combination therapy with TOC.
Data and Statistical Analysis
A one-to-one propensity score (PS) matched design of those treated with MP versus NMP was pursued. Patients in the two cohorts were matched based on the older age (age ≥ 60 yr vs age < 60 yr), obesity (body mass index [BMI] ≥ 30.0 kg/m2 vs BMI < 30.0 kg/m2), sex (male/female), diabetes (yes/no), hypertension (yes/no), cancer (yes/no), respiratory rate (respiratory rate > 22 vs < 22), renal failure (yes/no), low oxygen (oxygenation < 94% vs oxygenation ≥ 94%), CRP (CRP > 20 mg/dL vs CRP ≤ 20 mg/dL), and quick Sequential Organ Failure Assessment (score: 0, 1, 2, 3). The matched variables were determined due to low number of missing data, have prior studies suggesting they effect survival, and assess severity of critically ill patients (1–15, 20). A nearest-neighbor method (greedy match) was employed using a caliper of 0.20 to obtain the matched sample. We performed a post-match assessment of how distribution of PSs (or logit of PSs) and the adjusted variables are balanced between the NMP and MP using standardized difference and variance ratio and graphical displays produced by the ASSESS statement of PROC PS matched sample, no () in SAS 9.4 (SAS Institute, Cary, NC).
Determination of the optimal cutoff value of dose of MP was conducted using the Youden index method based on logistic regression of the binary outcome, in hospital survival, and total dose/absolute weight/day.
Categorical variables were presented as the frequency and percentage. Continuous variables were presented as the median and interquartile range. Shapiro-Wilk test was used to assess normality of continuous variables. Estimates of time to event such as inhospital survival, start of MV, and discharge were obtained using Kaplan-Meier method which reported median (95% CI), 60-day and 30-day survival rates (95% CI), and the intervals were calculated using the arcsine square root transformation method. Comparison of the dependent (PS matched sample) was performed using stratified log-rank test based on quintiles of the PSs as the strata. To examine association of risk factor of interest, MP treatment, Cox proportional hazard (PH) regression analysis with robust covariance (21) (sandwich estimator) to account paired observations was used conducted and hazard ratios (HRs), 95% CIs, and p values were reported in all univariable and multivariable analysis from PROC PHREG. The PH assumption, critical in Cox regression, was evaluated using a Kolmogorov-type supremum test (22) in ASSESS statement of PROC PHREG. If the PH assumption was violated, then a continuous variable which also violated the PH and its interaction with time were included in the model to adjust for the significant interaction with time to the risk of inhospital mortality (23).
In the multivariable analysis, all covariates (p < 0.10) were included in an initial full model fit and backward elimination selection procedure was performed until significant variables (at 5% levels) were retained. To this final model, an interaction term of the non-PH (NPH) covariate and time to in hospital mortality and the NPH covariate were added to adjust for the NPH property of the MP or MP dose. Since MP administration (MP vs NMP) and MP dose level (NMP, LD, HD) could not be included in the same model, two final models were fit to include each these variables separately.
In a third model, this study examined the association between risk of in hospital mortality and a combination of MP dose (NMP, LD, HD) and MP dose durations (≤ 7 d, 7–14 d, ≥ 14 d), defined as MP dose (NMP; LD MP, ≤ 7 d; LD MP, 7–14 d; LD MP, ≥ 14 d; HD MP, ≤ 7 d; HD MP, 7–14 d; HD MP, ≥ 14 d), both a univariable and multivariable analysis that used the adjustment for NPH property using Fio2 as described above.
Univariable and multivariable Cox regression model were used to determine synergy with TOC. Model 4 consisted of MP (NMP, MP), TOC and no TOC (NTOC). Four levels (NMP + NTOC; NMP + TOC; MP + NTOC; MP + TOC) were considered. Model 5 consisted of MP dose (NMP, LD MP, HD MP) and TOC (NTOC, TOC) so that six levels (NMP + NTOC; NMP + TOC; LD MP + NTOC; LD MP + TOC; HD MP + NTOC; HD MP + TOC) were considered.
RESULTS
Population
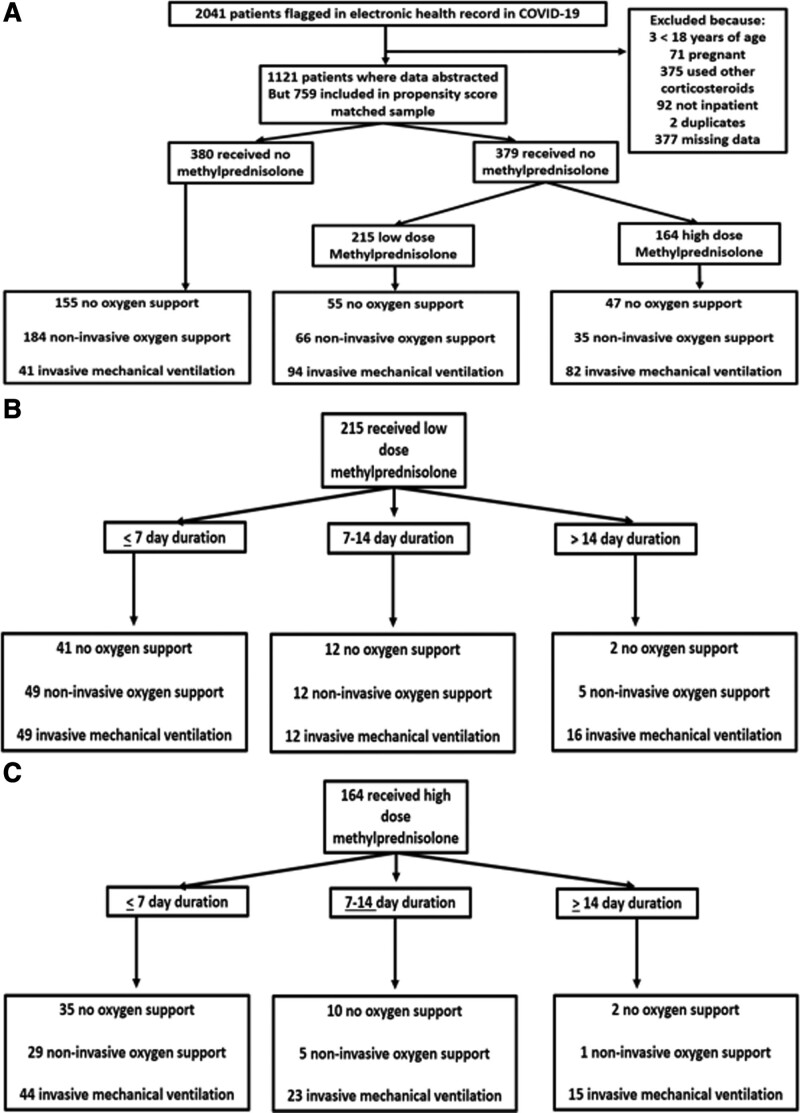
Between March 1, 2020, and June 15, 2020, 2,041 patients were flagged in the EHR with a diagnosis of COVID-19 and pneumonia. Five-hundred forty-three patients were removed because they were less than 18 years old, pregnant, received other formulations of corticosteroids, or were not inpatient status. One-thousand one-hundred twenty-one patients had their data abstracted (Fig. 1).
Figure 1.
Flow charts. A, Overall cohort flow chart. B, Flow chart of low dose methylprednisolone, duration and oxygen support. C, Flow chart of high dose methylprednisolone, duration and oxygen support.. COVID-19 = coronavirus disease 2019.
The distribution of the baseline characteristics is shown in (Table S1, http://links.lww.com/CCX/A721). In the unmatched population, 645 patients did not receive MP and 476 patients received MP.
A PS matched sample was constructed out of 759 patients (380 in NMP and 379 in MP). They were balanced for variables used in the matching. MP group had a higher percentage of patients from nursing homes and on MV.
There was a constantly evolving universal protocol that initially advocated against the use of MP due to concern for viral shedding. As new literature supported the use of corticosteroids, MP was used. Level of oxygen support was the main trigger. In all 13 hospitals, the peak of the surge was April 11–17, 2020 (Figs. S1 and S2, http://links.lww.com/CCX/A721).
Primary Analysis
In the PS matched sample, 99 out of 380 NMP and 143 out of 379 MP patients expired, although the difference in inhospital overall survival between the MP (median, 25.0 d; 95% CI, 22.0–32.0 d) and NMP (median, 19.0 d; 95% CI, 14.0–25 d) was statistically significant (stratified log-rank p < 0.0001) (Tables 1 and 2). The cause of death in both cohorts was 100% ARDS.
TABLE 1.
Summary of the Propensity Score Matching for Methylprednisolone and No Methylprednisolone Treated Coronavirus Disease 2019 Patients
| Propensity Score Information | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observations | Treated (Methylprednisolone) | Control (None) | Treated—Control | ||||||||
| n | Mean | sd | Minimum | Maximum | n | Mean | sd | Minimum | Maximum | Mean Difference | |
| All | 392 | 0.4155 | 0.1045 | 0.1878 | 0.7103 | 616 | 0.3720 | 0.0961 | 0.1686 | 0.6977 | 0.0435 |
| Region | 392 | 0.4155 | 0.1045 | 0.1878 | 0.7103 | 615 | 0.3723 | 0.0958 | 0.1956 | 0.6977 | 0.0431 |
| Matched | 384 | 0.4109 | 0.1004 | 0.1878 | 0.7103 | 384 | 0.4074 | 0.0966 | 0.1956 | 0.6977 | 0.0035 |
TABLE 2.
Standardized Mean Differences (Methylprednisolone–No Methylprednisolone)
| Variable | Observations | Mean Difference | sd | Standardized Difference | Percent Reduction | Variance Ratio |
|---|---|---|---|---|---|---|
| Logit propensity score | All | 0.18717 | 0.432253 | 0.43300 | 1.1296 | |
| Region | 0.18546 | 0.42905 | 0.91 | 1.1395 | ||
| Matched | 0.01401 | 0.03242 | 92.51 | 1.0738 | ||
| Obesity status (body mass index ≥ 30.0 kg/m2) | All | –0.12778 | 0.490334 | –0.26060 | 1.0820 | |
| Region | –0.12719 | –0.25940 | 0.46 | 1.0812 | ||
| Matched | –0.00781 | –0.01593 | 93.89 | 1.0016 | ||
| Older age (> 60 yr) | All | –0.03502 | 0.483048 | –0.07249 | 0.9623 | |
| Region | –0.03565 | –0.07381 | 0.00 | 0.9618 | ||
| Matched | 0.02604 | 0.05391 | 25.63 | 1.0353 | ||
| Sex | All | –0.00812 | 0.480332 | –0.01690 | 0.9903 | |
| Region | –0.00871 | –0.01813 | 0.00 | 0.9896 | ||
| Matched | –0.02083 | –0.04337 | 0.00 | 0.9765 | ||
| Diabetes | All | –0.04801 | 0.477468 | –0.10054 | 1.0636 | |
| Region | –0.04747 | –0.09942 | 1.12 | 1.0628 | ||
| Matched | 0.00781 | 0.01636 | 83.73 | 0.9914 | ||
| Hypertension | All | –0.05334 | 0.495965 | –0.10755 | 0.9754 | |
| Region | –0.05248 | –0.10581 | 1.62 | 0.9756 | ||
| Matched | 0.01042 | 0.02100 | 80.47 | 1.0069 | ||
| Cancer | All | –0.02157 | 0.308368 | –0.06994 | 1.1960 | |
| Region | –0.02141 | –0.06944 | 0.72 | 1.1942 | ||
| Matched | 0.00000 | 0.00000 | 100.00 | 1.0000 | ||
| Respiratory rate > 22 breaths/min | All | –0.06401 | 0.396891 | –0.16127 | 1.2802 | |
| Region | –0.06374 | –0.16059 | 0.42 | 1.2785 | ||
| Matched | –0.00781 | –0.01968 | 87.79 | 1.0263 | ||
| Renal failure | All | –0.01252 | 0.259765 | –0.04821 | 1.1722 | |
| Region | –0.01241 | –0.04779 | 0.86 | 1.1704 | ||
| Matched | –0.00781 | –0.03008 | 37.62 | 1.0978 | ||
| Oxygen < 94% | All | –0.10506 | 0.496524 | –0.21158 | 0.9776 | |
| Region | –0.10428 | –0.21003 | 0.73 | 0.9775 | ||
| Matched | –0.01042 | –0.02098 | 90.08 | 0.9943 | ||
| C-reactive protein > 20 mg/L | All | –0.06192 | 0.389826 | –0.15884 | 1.2908 | |
| Region | –0.06166 | –0.15818 | 0.41 | 1.2891 | ||
| Matched | –0.01823 | –0.04676 | 70.56 | 1.0699 |
sd of all observations used to compute standardized differences.
The standardized mean differences in the variables range from the variables in the tables have a maximum of 0.0539, which is below upper limit of 0.10 used by (11) and certainly way below the upper limit of 0.25 recommended.
An examination of the PH assumption, MP and Fio2 significantly violated it (both with p < 0.0001) (Tables S3-S10, http://links.lww.com/CCX/A721). The supremum test also indicated that nonproportionality was observed in other variables such as nursing home, lack of taste or smell, WBC less than 11,000 cells/mL, creatinine greater than 1.5 ng/mL, respiratory rate greater than 22 breaths/min, hydroxychloroquine, MP, HD or LD MP, calcium, and initial diastolic blood pressure. The Fio2 was selected to adjust for the significant interaction between MP and time to expiration since it is available in 95% of our patients and it is a denominator of P/F ratio (Figs. S3-S19, http://links.lww.com/CCX/A721). All variables with NPH were adjusted using Fio2, as indicated above. The analysis of the propensity matched scored sample showed that MP was associated with statistically and significantly longer inhospital survival (HR, 0.44; 95% CI, 0.33–0.60; p < 0.0001), adjusted by Fio2 survival time interaction. Then, we further wanted to differentiate MP into dose, duration, and administration from OOS.
The Youden index method yielded a MP dose cutoff of 1.36 mg/kg/d. Low-dose (LD) MP was defined as less than 1.36 mg/kg/d and high-dose (HD) MP was defined as greater than or equal to 1.36 mg/kg/d.
When stratified for dose, 69 out of 215 patients (31.9%) in LD MP and 74 out of 164 patients (55.2%) in HD MP expired (Table S2, http://links.lww.com/CCX/A721). The difference in inhospital survival between NMP (median, 25.0 d; 95% CI, 22.0–32.0 d) and LD MP (median, 39.0 d; 95% CI, 27.0–not available [NA] d) and HD MP (median, 20.0 d; 95% CI, 17.0–24 d) was statistically significant (stratified log-rank p < 0.0001). Compared with NMP, HD MP had survival benefit however this diminished by day 18.
When comparing MP duration, 89 out of 247 patients (35.0%) with MP for less than or equal to 7 days, 26 out of 91 patients (39.5%) with MP for 7–14 days, and 18 out of 41 patients (44%) expired during hospitalization. The difference in inhospital survival between the MP dose less than or equal to 7 days (median, 19.0 d; 95% CI, 17.0–23.0 d), MP dose 7–14 d (median, 30.0 d; 95% CI, 19.0–NA d), and MP dose greater than 14 days (median, 44.0 d; 95% CI, 32.0–60 d) was statistically significant (log-rank p = 0.0011). In patients who received LD MP, 45 out of 139 (32%) with duration less than or equal to 7 days, 16 out of 53 (30.19%) with duration 7–14 days, and eight out of 23 (34.8%) with duration greater than 14 days expired. In patients who received HD MP, 44 out of 108 (40.7%) with duration less than or equal to 7 days, 20 out of 38 (52.6%) with duration 7–14 days, and 10 out of 18 (55.6%) expired.
When stratified for administration of MP from OOS, 86 out of 181 patients (47.3%) who received MP less than 7 days from OOS, nine out of 41 patients (22%) who received MP 7–14 days from OOS, and 48 out of 153 patients (31.4%) who received MP greater than 14 days from OOS expired. The difference in inhospital survival with administration of MP from OOS less than or equal to 7 days (median, 20.0 d; 95% CI, 17.0–26.0 d) and 7–14 days (median, 27.0 d; 95% CI, 23.0–42 d) was statistically significant (log-rank p = 0.0008). The survival in cohort with greater than 14 days from OOS did not drop below 0.5 for the median to be estimable.
Multivariate Cox Regression
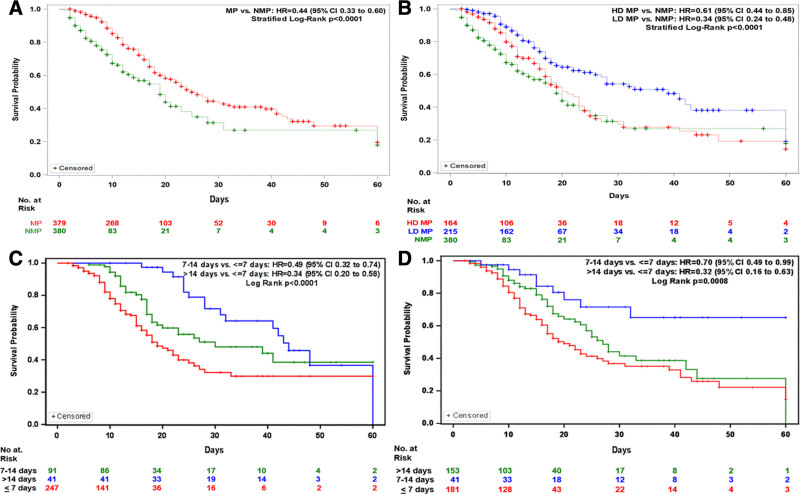
A multivariate time interaction adjusted Cox regression was performed on covariate that had demonstrated significant association with in hospital survival with p value of less than 0.10 (Tables S11-S14 and Figs. S20-S22, http://links.lww.com/CCX/A721). Three models were performed with model 1 containing NMP versus MP; model 2 containing LD MP, HD MP, and NMP; and model 3 containing MP dose and duration (Fig. 2). In all three models, nursing home resident, coronary artery disease, and invasive MV are independently associated with risk of in hospital mortality.
Figure 2.
Kaplan-Meier (KM) Plots on 30 and 60 d in hospital survival. A, KM Plot shows 30- and 60-d in hospital survival for the no methylprednisolone (NMP) cohort were 31.5% (95% CI, 19.0–45.4%) and 18.0% (95% CI, 4.5–37.7%), respectively. Thirty- and 60-d in hospital survival for the methylprednisolone (MP) cohort were 43.6% (95% CI, 36.5–50.9%) and 19.7% (95% CI, 8.7–33.8%), respectively. B, KM plot shows 30- and 60-d in hospital survival for the low-dose (LD) MP cohort were 54.2% (95% CI, 44.8–63.5%) and 19.1% (95% CI, 1.1–51.4%), respectively. Thirty- and 60-d in hospital survival for the high-dose (HD) MP cohort were 29.6% (95% CI, 19.8–40.4%) and 14.4% (95% CI, 5.0–27.9%), respectively. C, Thirty- and 60-d in hospital survival for MP less than or equal to 7 d duration were 32.2% (95% CI, 22.3–42.9%) and 29.9% (95% CI, 20.0–40.9%), respectively. Thirty- and 60-d in hospital survival for the MP 7–14 d duration were 48.1% (95% CI, 35.0–61.3%) and 38.5% (95% CI, 23.4–55.0%), respectively. Thirty- and 60-d in hospital survival for the MP dose greater than 14 d duration were 71.7% (95% CI, 54.9–85.9%) and 36.6% (95% CI, 16.4–59.7%), respectively. D, Thirty- and 60-d in hospital survival for the MP dose initiation less than or equal to 7 d since onset of symptoms were 36.8% (95% CI, 27.4–46.6%) and 14.8% (95% CI, 3.8–31.1%), respectively. Thirty- and 60-d inhospital survival for the MP dose initiated between 7 and 14 d from onset of symptoms were 41.4% (95% CI, 28.9–54.6%) and 0, respectively. Thirty- and 60-d in hospital survival for the MP dose initiation after 14 d from onset of symptoms were 71.6% (95% CI, 53.2–86.8%) and 65.1% (95% CI, 44.5–83.0%), respectively. HR = hazard ratio.
In model 1, containing MP (NMP vs MP), MP (HR, 0.40; 95% CI, 0.27–0.5; p < 0.0001) was associated with reduced risk of in hospital mortality at 60-day follow-up.
In model 2, containing MP dose levels (NMP, LD MP, HD MP), LD MP (HR, 0.35; 95% CI, 0.22–0.53; p < 0.0001) and HD MP (HR, 0.48; 95% CI, 0.30–0.77; p = 0.0025) were associated with significantly reduced risk of in hospital mortality compared with NMP.
In model 3 with dose and duration, there was no difference in mortality when comparing LD versus HD MP for less than 7 days duration, 7–14 days duration, and greater than 14 days duration. HD MP 7–14 days was associated with low mortality compared with LD MP greater than or equal to 14 days (HR, 2.42; 95% CI, 1.17–5.03; p = 0.0174). HD MP greater than or equal to 14 days was associated with worse survival compared with HD MP less than or equal to 7 days (HR, 0.28; 95% CI, 0.12–0.66; p = 0.0035). HD MP greater than or equal to 14 days was associated with prolonged survival compared with LD MP less than or equal to 7 days (HR, 0.38; 95% CI, 0.17–0.86; p = 0.0192). HD MP less than 7 days was associated with worse survival compared with LD MP 7–14 days (HR, 3.03; 95% CI, 1.42–6.48; p = 0.0042) or LD MP greater than or equal to 14 days (HR, 3.85; 95% CI, 2.09–7.08; p < 0.001). LD MP 7–14 days duration and LD MP greater than or equal to 14 days were associated with longer survival compared with LD MP less than or equal to 7 days, with HR, 0.45 (95% CI, 0.22–0.91; p = 0.0273) and (HR, 0.35; 95% CI, 0.20–0.62; p = 0.0003), respectively. There was no difference between LD MP 7–14 days versus LD MP greater than or equal to 14 days (HR, 1.27; 95% CI, 0.60–2.69; p = 0.5434).
Secondary Analysis
TOC was given to 59 out 65 (77.6%) on MV, eight out of 65 (10.5%) on noninvasive oxygen support, and nine out of 65 (11.8%) on room air. Multivariate Cox regression on MP versus TOC was associated with lower mortality (HR, 0.32; 95% CI, 0.12–0.86; p = 0.0231). Combination of MP and TOC was associated with improved 60-day survival compared with TOC (HR, 0.26; 95% CI, 0.09–0.7; p = 0.0116).
DISCUSSION
This retrospective propensity matched cohort study showed an association with prolonged survival in patients with LD MP if given greater than 7 days from OOS for at least 7 days compared with other cohorts (NMP and HD MP). HD MP was associated with longer survival over NMP but that effect appears to diminish after the 18th day.
These findings may be due to MP used as “rescue,” with higher doses given to patients with more severe disease. Nursing home residents and MV, which were independently associated with higher mortality, were also more prevalent in the MP cohort. Due to shortages, invasive MV were reserved for patients who failed other oxygen support and had advanced disease severity.
One study on pulse MP showed increased survival time over no methylprednisolone (24). However, their dose of 250 mg IV daily would fall under our weight-based definition of LD MP. We had similar results regarding duration, since we found that LD MP for less than 7 days was associated with prolonged survival compared with NMP.
Another explanation is that MP has paradoxical effects of anti-inflammation and prolongation of viral shedding, which maybe dependent on timing, dose, and duration (25–27). Viral shedding reaches its peak during the first week, followed by rapid decline in viral shedding in nonsevere patients during second week. In patients with severe disease, there is a prolonged duration of viral shedding (> 14 d) and protracted inflammatory response (28–31). Viral shedding and inflammation phases are not mutually exclusive but benefit of MP maybe maximized at the time of minimal viral shedding and more inflammation. Administration of MP less than 7 days from OOS was associated with shorter survival likely due to prolongation of viral shedding. Regarding dose, prior studies have failed to show clinically significant correlation with prolonged viral shedding, although the doses in those studies have been less than or equal to 1 mg/kg/body weight (25, 26).
The duration of MP is important since there is a prolonged inflammation in ARDS, regardless of etiology. Down-regulation of cytokines and chemokines in both non-COVID and COVID ARDS have require corticosteroids for duration of greater than 7 days (12, 32, 33). One study in COVID-19 ARDS, a shorter course failed to show mortality benefit in patients less than 60 years old (15). In this study, duration greater than 7 days was associated with longer survival compared with duration less than 7 days, which was also seen in a recent meta-analysis (34).
The current recommendation of TOC as an adjunct to corticosteroids is based on studies with corticosteroids in both control and treatment arms (16–18). One of the strengths of this study is that we were able to compare in hospital survival between TOC versus TOC with MP. Four-hundred mg IV is not 8 mg/kg unless the weight is 50 kg. Only two patients had weight less than or equal to 55 kg and the rest greater than 55 kg. Despite being under dosed, TOC with MP was associated with prolonged survival compared with MP alone.
Organizing pneumonia and its variant, Acute Fibrinous Organizing Pneumonia, are pulmonary histopathologic patterns found in some patients with COVID-19 (35–39). They are steroid-sensitive and their presence can suggest which COVID-19 patients can respond to MP.
Our study has several limitations. First, since it is an observational study, we cannot draw causal inferences based on known and unknown confounders. We tried to limit the known confounders through propensity matching. Second, misclassification of data are possible due to manual extraction of structured and unstructured data for medical health records. We did review the data multiple times by different reviewers. Third, there was a higher prevalence of older patients with multiple comorbidities and during pandemic surge, which could have skewed the mortality rates. Fourth, even after achieving balanced propensity matching, there is a possibility of sampling bias due to obtaining data from convenience sample that could be ascribed to presence of unmeasured confounders. Fifth, we use Fio2 because not all the patients had ABGs, therefore, unable to obtain Pao2 or P/F ratio. Sixth, MP group were more likely to require MV, although MP was still associated with prolonged survival than NMP. At that time, noninvasive ventilation (NIV) was not used due to aerosolization risk. Pre-COVID-19, the American Thoracic Society/European Respiratory Society guidelines have made no recommendation regarding the use of NIV in de novo respiratory failure and respiratory failure from viral pandemics (40). Currently, there is no conclusive evidence to support NIV over invasive MV in COVID-19. It is not necessarily protective of self-inflicted lung injury, which is believed to catalyze the transition from L type to H type ARDS (41, 42).
CONCLUSIONS
This real-world data study showed MP was associated with longer in hospital survival compared with NMP. There was no associated survival benefit with HD compared with LD. There was no associated prolongation of survival if LD MP was less than 7 days or longer than greater than 14 days. Combination therapy with MP and TOC was associated with prolonged survival compared with monotherapy.
ACKNOWLEDGMENTS
We thank Andrew Ip, MD and Stuart Goldberg, MD for access to Hackensack Meridian Health Real World Database.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Go has done consulting work for Hoffman-La Roche. The article was prepared by Dr. Go. The data were analyzed by Dr. Nyirenda. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.World Health Organization. World Health Organization (WHO) Coronavirus Disease 19 Dashboard. 2021. Available at: https://covid19.who.int/. Accessed June 2, 2021
- 2.Wang D, Hu B, Hu C, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. : Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection Is Suspected: Interim Guidance. 2019. Available at: Who.int/publications/i/item/WHO-MERS-Clinical-15-1-Revision-1. Accessed March 1, 2021
- 6.Bhimraj A, Morgan RL, Schumaker AH, et al. : Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 Apr 27. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers of Disease Control and Prevention. Interim Clinical Guidance for Management of Patients With Confirmed Coronavirus Disease (COVID-19). 2021. Available at: Cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed March 1, 2021
- 8.Arabi YM, Mandourah Y, Al-Hameed F, et al. ; Saudi Critical Care Trial Group. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018; 197:757–767 [DOI] [PubMed] [Google Scholar]
- 9.Lee N, Allen Chan KC, Hui DS, et al. : Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004; 31:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni YN, Chen G, Sun J, et al. : The effect of corticosteroids on mortality of patients with influenza pneumonia: A systematic review and meta-analysis. Crit Care. 2019; 23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGee S, Hirschmann J: Use of corticosteroids in treating infectious diseases. Arch Intern Med. 2008; 168:1034–1046 [DOI] [PubMed] [Google Scholar]
- 12.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 – Preliminary Report. N Engl J Med. 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salton F, Confalonieri P, Meduri GU, et al. : Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020; 7:ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller MJ, Kitsis EA, Arora S, et al. : Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020; 15:489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jernimo CMP, Farias ME, Val FFA, et al. : Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease (Metcovid): A randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Disc. 2021; 72:e373–e381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas IO, Bräu N, Waters M, et al. : Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021; 384:1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021; 397:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021; 384:1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute of Health. COVID-19 Treatment Guidelines. 2021. Available at: https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/. Accessed June 6, 2021
- 20.Leisman DE: Ten pearls and pitfalls of propensity scores in critical care research: A guide for clinicians and researchers. Crit Care Med. 2019; 47:176–185 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC: The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014; 33:1242–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DY, Wei LJ, Ying Z: Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993; 80:557–572 [Google Scholar]
- 23.Allison PD: Survival Analysis Using SAS®: A Practical Guide. Second Edition. Cary, NC, SAS Institute, 2010 [Google Scholar]
- 24.Edalatifard M, Akhtari M, Salehi M, et al. : Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur Respir J. 2020; 56:2002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li TZ, Cao ZH, Chen Y, et al. : Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2021; 93:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Hu Z, Song X: High dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2021; 72:1297–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. : Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun. 2021; 12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou R, Li F, Chen F, et al. : Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis. 2020; 96:288–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.To KKW, Tsang OTY, Leung WS, et al. : Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect Dis. 2020; 20:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Lau EHY, Wu P, et al. : Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020; 26:672–675 [DOI] [PubMed] [Google Scholar]
- 31.Xu K, Chen Y, Yuan J, et al. : Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-10). Clin Infect Dis. 2020; 71:799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meduri GU, Annane D, Chrousos GP, et al. : Activation and regulation of systemic inflammation in ARDS: Rationale for prolonged glucocorticoid therapy. Chest. 2009; 136:1631–1643 [DOI] [PubMed] [Google Scholar]
- 33.Villar J, Ferrando C, Martínez D, et al. ; dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir Med. 2020; 8:267–276 [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri D, Sasaki K, Karkar A, et al. : Corticosteroids in COVID-19 and non-COVID-19 ARDS: A systematic review and meta-analysis. Intensive Care Med. 2021; 47:521–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kory P, Kanne JP: SARS-CoV-2 organising pneumonia: “Has there been a widespread failure to identify and treat this prevalent condition in COVID-19? BMJ Open Resp Res. 2020; 7:e000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hariri LP, North CM, Shih AR, et al. : Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory sydrome and H1N1 influenza: A systematic review. Chest. 2021; 159:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogatchnik BP, Swenson KE, Sharifi H, et al. : Radiology-pathology correlation demonstrating organizing pneumonia in patient who recovered from COVID-19. Am J Respir Crit Care Med. 2020; 202:598–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Travis WD, Costabel U, Hansell DM, et al. ; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013; 188:733–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beasley MB, Franks TJ, Galvin JR, et al. : Acute fibrinous and organizing pneumonia: A histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002; 126:1064–1070 [DOI] [PubMed] [Google Scholar]
- 40.Rochwerg B, Brochard L, Elliott MW, et al. : Official ERS/ATS clinical practice guidelines: Non-invasive mechanical ventilation for acute respiratory failure. Eur Respir J. 2017; 50:1602526. [DOI] [PubMed] [Google Scholar]
- 41.Gorman E, Connolly B, Couper K, et al. : Non-invasive respiratory support strategies in COVID-19. Lancet Respir Med. 2021; 9:553–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grieco DL, Menga LS, Eleuteri D, et al. : Patient self-inflicted lung injury: Implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019; 85:1014–1023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.