Abstract
Traumatic brain injury (TBI) in children younger than 4 years old results in cognitive and psychosocial deficits in adolescence and adulthood. At 4 weeks following closed head injury on postnatal day 11, male and female rats exhibited impairment in novel object recognition memory (NOR) along with an increase in open arm time in the elevated plus maze (EPM), suggestive of risk-taking behaviors. This was accompanied by an increase in intrinsic excitability and frequency of spontaneous excitatory post-synaptic currents (EPSCs), and a decrease in the frequency of spontaneous inhibitory post-synaptic currents in layer 2/3 neurons within the medial prefrontal cortex (PFC), a region that is implicated in both object recognition and risk-taking behaviors. Treatment with progesterone for the first week after brain injury improved NOR memory at the 4-week time point in both sham and brain-injured rats and additionally attenuated the injury-induced increase in the excitability of neurons and the frequency of spontaneous EPSCs. The effect of progesterone on cellular excitability changes after injury may be related to its ability to decrease the mRNA expression of the β3 subunit of the voltage-gated sodium channel and increase the expression of the neuronal excitatory amino acid transporter 3 in the medial PFC in sham- and brain-injured animals and also increase glutamic acid decarboxylase mRNA expression in sham- but not brain-injured animals. Progesterone treatment did not affect injury-induced changes in the EPM test. These results demonstrate that administration of progesterone immediately after TBI in 11-day-old rats reduces cognitive deficits in adolescence, which may be mediated by progesterone-mediated regulation of excitatory signaling mechanisms within the medial PFC.
Keywords: Pediatric TBI, Adolescence, Cognition, Risk-taking behavior, E/I balance, Neuronal excitability, Object Recognition, Prefrontal Cortex
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in the pediatric population, with the highest incidence of hospital room visits occurring in children younger than 4 years old (Araki et al 2017, Coronado et al 2011, Emami et al 2017, Faul & Coronado 2015). Survivors of childhood TBI often deal with long-term cognitive and psychosocial deficits. Clinical data demonstrate that brain injury sustained during the pre-school years (between 1 and 5 years old) results in long-term impairments in intellectual and academic performance, executive functioning, and psychosocial problems (Catroppa et al 2008, Ewing-Cobbs et al 2006, Treble-Barna et al 2017). Evidence suggests that these neurobehavioral deficits can persist and even worsen over time (Catroppa et al 2008, Catroppa et al 2012, Treble-Barna et al 2017), particularly when brain trauma is sustained at a younger age (Keenan et al 2018, Treble-Barna et al 2017). Collectively, these data demonstrate that survivors of pediatric TBI are afflicted with a “chronic” disease state in cognitive and emotional function. Although mortality within the first few days has decreased over the past two decades, due to improvements in post-traumatic neurocritical supportive care, there has been a lack of pathology-based therapies to limit these deficits in survivors of childhood TBI (Huh & Raghupathi 2019, Kochanek et al 2019, Thurman 2016).
We and others have demonstrated both acute and long-term spatial learning and memory deficits – examples of hippocampal-directed behaviors – following contusive or diffuse pediatric TBI in rodents of varying ages (Adelson et al 2000, Huh & Raghupathi 2007, Prins & Hovda 1998, Raghupathi & Huh 2007). Recent studies have also documented impairments in non-hippocampal-dependent behaviors. Thus, contusive TBI in 5–7 day-old rabbits led to deficits in novel object recognition (NOR) memory (Zhang et al 2015), whereas contusive TBI in 7-day-old rats resulted in increased anxiety in the elevated plus maze (EPM) test (Baykara et al 2013). One mechanism underlying these latter behaviors may relate to changes in the activity of surviving cortical neurons following TBI. Neocortical lesions in 3-week old rats resulted in an increase in the frequency of spontaneous excitatory post synaptic currents (EPSCs) and a decrease in the frequency of spontaneous inhibitory post synaptic currents (IPSCs) at 2–6 weeks post injury (Li & Prince 2002). Contusive TBI in 17-day-old rats resulted in decreased local field potential amplitude and stimulus evoked neuronal activity in the cortex contralateral to the injury site (Li et al 2014), and increased spontaneous synaptic bursting activity in the peri-injury zone 2 weeks after injury (Nichols et al 2015).
Progesterone has demonstrated efficacy as a neuroprotectant in adult rodent TBI models (Allitt et al 2017, Gilmer et al 2008, Goss et al 2003, Li et al 2012, O’Connor et al 2007, Robertson et al 2006, Roof et al 1994, Roof et al 1992, Si et al 2013, Stein 2008, Wright et al 2001, Yao et al 2005, Zhang et al 2017), although two large, phase 3 clinical trials of progesterone treatment of adult TBI were terminated early for futility (Stein 2015). However, the benefits of progesterone have been documented in animal models of pediatric TBI. Administering progesterone immediately following contusive head trauma in 28-day-old male and female rats attenuated motor function and spatial learning deficits and reduced lesion size (Geddes et al 2016, Geddes et al 2014). Progesterone administration immediately following contusive head trauma in 7-day-old rats reversed TBI-induced spatial learning deficits, reduced apoptosis, and increased neuronal density within the prefrontal cortex, amygdala, and hippocampus (Baykara et al 2013, Uysal et al 2013). Interestingly, progesterone improved mitochondrial respiratory control ratio following contusive trauma in 17 to 21-day old male but not female rats but reduced cortical tissue loss only in female rats (Robertson & Saraswati 2015). In addition to its neuroprotective properties, progesterone can regulate excitatory and inhibitory neurotransmission (Robertson et al 2015, Wei & Xiao 2013). Thus, we hypothesized that treatment with progesterone in the first week after pediatric TBI would reduce deficits in cognitive function and psychological behaviors and the associated alterations in the balance of cortical excitatory and inhibitory signaling following brain injury.
Methods
Traumatic Brain Injury
Surgical procedures were done in accordance with the rules and regulations of the Institutional Animal Care and Use Committee at Drexel University and the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The injury model used in this study was originally characterized by Raghupathi and Huh (2007) and subsequently utilized in multiple studies (Hanlon et al 2017, Hanlon et al 2019). Timed-pregnant dams were ordered from Charles River Laboratories (Wilmington MA) and housed in the vivarium under 12h light:12h dark conditions with access to food and water ad libitum. On postnatal day 11 (the neurological equivalent of a child below the age of 4, Porterfield 1994, Rice & Barone 2000, Yager & Thornhill 1997), male and female Sprague-Dawley rat pups were anesthetized using isoflurane (Patterson Veterinary, Greeley CO, 5% induction, 2–3% maintenance), and a 2 cm incision was made to expose the skull. The anesthesia was stopped, and animals were placed in a plastic rodent restrainer (Braintree Scientific, Braintree MA) and transferred to the stage of an electronic controlled cortical impact device (eCCI, Custom Design and Fabrication, Richmond VA). The impactor tip (5 mm diameter) was positioned over the left parietal cortex midway, between the bregma and lambda sutures, and was driven into the intact skull at a velocity of 5 m/s (3 mm distance from zero point, 100 ms dwell time). After receiving the impact, animals were placed on their backs and the time to right themselves (righting reflex) and time to return to normal breathing (apnea) were recorded. Animals were then re-anaesthetized, and the scalp was sutured closed. Sham-injured animals were surgically prepared but did not receive the impact. Animals were allowed to recover in a separate cage before being placed back with the dam. Surgical procedures and recovery were performed on heating pads at 37°C to maintain the body temperature, as previously described (Raghupathi & Huh 2007).
Experimental Design
Animals in each litter were randomly assigned to receive a closed head injury (N=69) or were treated as sham-injured controls (N=52). Progesterone (Sigma, Cat# 1000917813) was dissolved in sesame oil (Cat# 156621, MP Biomedicals, Solon, OH) the day before surgery. Sham- and brain-injured animals from each litter were randomly assigned to receive either progesterone (16 mg/kg, N=57) or sesame oil (N=64). Progesterone or sesame oil was intraperitoneally injected immediately following the impact or the equivalent time after removal from anesthesia for the sham-injured animals. Subsequent injections via the subcutaneous route were given at 6 hours and every 24 hours for 6 additional days for a total of 8 injections/animal. The dose of progesterone used in the current studies was reported to be optimal for reversing cognitive deficits following TBI in both adolescent (Geddes et al 2014) and adult (Goss et al 2003) rats, relative to lower (4 mg/kg) or higher (32 mg/kg) doses. Progesterone was administered during the first week following injury, the standard treatment paradigm that has been utilized in both adult (Goss et al 2003, Roof et al 1994, Roof et al 1992) and pediatric (Geddes et al 2016, Geddes et al 2014) preclinical TBI studies. Animals were tested in the 4th week after injury in novel object recognition (cognitive function, 25–26 days post-injury = postnatal days 36–37), novelty-induced locomotor activity (27 days post-injury = postnatal day 38) and the elevated plus maze (anxiety-like behavior, 28 days post-injury = postnatal day 39). Behavioral testing was performed in adolescent-aged rats (36–39-days-old) because cognitive and behavioral symptoms become more apparent as brain-injured infants and toddlers age into adolescence (Choe et al 2016, Ryan et al 2015). Following behavioral assessments, subgroups of animals were randomly assigned to generate tissue for histological analysis, quantitative real-time polymerase chain reaction (qRT-PCR), or whole cell patch clamp electrophysiology at 4 weeks after injury (29 days post-injury = postnatal day 40, Table 1).
Table 1. Primers used for analysis of mRNA expression in the medial prefrontal cortex of sham- and brain-injured rats.
All sequences are listed as 5’−3’. vGlut1, vesicular glutamate transporter 1; vGlut2, vesicular glutamate transporter 2; 67kDa glutamic acid decarboxylase, GAD67; GABAA- α1, α1 subunit of the gamma amino butyric acid receptor – A isoform; GluR1, R1 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GluN1, N1 subunit of the N-methyl-D-aspartate receptor; EAAT3, neuronal excitatory amino acid transporter 3; NaVβ3, β3 subunit of the voltage-gated sodium channel.
| Gene | Accession number | Concentration (nM) | Forward | Reverse |
|---|---|---|---|---|
| Cyclophilin-A | NM_017101.1 | 200 | GTGTTCTTCGACATCACGGCT | CTGTCTTTGGAACTTTGTCTGCA |
| vGlut1 | NM_053859.2 | 50 | TGGTGGAGGGGGTCACATAC | CCAGCCGACTCCGTTCTAAG |
| vGlut2 | NM_053427.1 | 50 | CGTGAAGAATGGCAGTATGTCTTC | TGAGGCAAATAGTGCATAAAATATGACT |
| GAD67 | NM_017007.1 | 50 | CAGCTCCCTGTGGCTGAATC | GAAGATGCCATCAGCTCGGT |
| GABAA-α1 | NM_183326.2 | 50 | CCCACACCCCATCAATAGGT | GAGGCAGTAAAGCAGACAGG |
| GluR1 | NM_031608.1 | 200 | GAAATGTGCAGTTCAACGAGAAAG | TTTCGGATTCCATCATGTTTCAT |
| GluN1 | NM_001270602.1 | 100 | CACCAGACTAAAGATAGTGACAATCCA | CCTCTTTGCATGTCCCATCA |
| EAAT3 | NM_013032.3 | 200 | AATGGGGGCTTCTCGGTAGA | CAGTCCCAGGCATCTAAGGC |
| NaVβ3 | NM_139097.3 | 200 | CACCCAGTCACTGCAAATGTATG | CGTTGAGTCAATGGGATTGGA |
Novel Object Recognition
Animals were assessed for cognitive deficits using the NOR memory test performed under normal light conditions. On the day prior to testing, rats were placed in a 61 cm × 41 cm plastic box and habituated for 10 minutes. On the testing day, two identical objects (glass bottles) were placed in opposite corners of the box and the rats were given 5 minutes to explore the objects. After one hour, one of the objects was replaced with a new object of similar dimensions (metal cylinder) and rats were returned to the box and allowed to explore the objects for 5 minutes. Both of the trials were video-recorded, and the number of seconds spent sniffing each of the objects was timed. Rats that showed a preference for one of the objects during the first trial were excluded from further analysis, as were rats that failed to explore both of the objects for a minimum of 10 seconds during either trial. The discrimination index was calculated as:
Locomotor Activity
At the conclusion of NOR testing, rats were assessed for novelty-induced locomotor activity in a 30-minute open field test which was performed in a dark, sound-attenuated chamber. Rats were placed in the center of a 22.1”x22.1”x15.83” SmartFrame Open Field System (Kinder Scientific, Poway CA). The distance traveled, as recorded by the number of beam breaks, was measured by MotorMonitor software (Kinder Scientific). Total distance traveled was averaged in 5-minute bins for each group.
Elevated Plus Maze
Animals were assessed for anxiety-like behavior using the EPM. Rats were placed in the center of an EPM and allowed to freely explore the maze for 5 minutes. EPM testing was performed under dim red light and recorded with an overhead camera. Data were collected using EthoVision XT12 software (Noldus Information Technology Inc., Leesburg, VA). The primary outcome measures were entries into and time spent in the open arms of the maze. Animals were scored as being in an open arm only when both forepaws passed into the open arm.
Whole cell patch-clamp recording
Rats were anesthetized with an intraperitoneal injection of Euthasol (100 mg/kg) and transcardially perfused with 60 mL of carboxygenated (5% CO2, 95% H20) slicing artificial cerebrospinal fluid (ACSF) consisting of the following (in mM): 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2, pH of 7.4. Brains were then rapidly removed and glued to the slicing stage of a vibrating microtome (Leica Microsystems, Buffalo Grove, IL), and 300 μM coronal slices containing the medial PFC were obtained. Slices were incubated at 37°C for 1 hour in oxygenated artificial cerebrospinal fluid (ACSF) containing the following (in mM): 126 NaCl, 10 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, and 2 CaCl2, pH 7.4. Brain slices were then kept at room temperature until use.
Brain slices were individually transferred to a recording chamber and continually superfused with oxygenated ACSF maintained at 34°C. Using an Olympus BX51WI microscope and Samsung SCB-2001 camera, individual pyramidal neurons were identified with infrared differential interference contrast imaging. Recordings from pyramidal neurons were made using borosilicate glass patch pipettes (resistance 5–8 MΩ), which were filled with an intracellular solution containing the following (in mM): 128 K gluconate, 10 HEPES, 0.05 CaCl2, 0.3 GTP, 5 ATP, 1 glucose, 4 NaCl, pH 7.4 as previously reported (Prouty et al 2017, Smith et al 2015). Whole-cell recordings were obtained using an axon MultiClamp 700A amplifier, DigiData 1332A digitizer, and ClampEx 9.2 software (Molecular Devices, San Jose, CA). A series of depolarizing current steps (−100 to 220 pA) were applied to the cell to evaluate membrane properties. Only neurons with an action potential (AP) overshoot greater than 0 mV were included in further analysis. Neurons were then held in voltage clamp mode at −70 mV to record spontaneous excitatory post synaptic currents (EPSCs) for 5 minutes, and at 0 mV to record spontaneous inhibitory post synaptic currents (IPSCs). Previous studies have isolated excitatory and inhibitory currents by recording postsynaptic currents at −70 mV and 0 mV, respectively (Cantu et al 2015, Prouty et al 2017). The driving force for cations is much greater than that for chloride ions at −70 mV (which is the reversal potential for currents mediated by both ionotropic glutamate receptors). In contrast, the driving force for chloride ions is much greater than that for cations at 0 mV (which is the reversal potential for gamma amino butyric acid isoform A - GABAA - receptor-mediated currents). In prior studies, EPSCs and IPSCs were recorded in the presence of GABAA and glutamate receptor antagonists, respectively (Prouty et al 2017, Smith et al 2015), precluding the need for pharmacologic confirmation of similar currents in the present study. Recorded data were analyzed using ClampFit 10.5 (Molecular Devices, San Jose, CA). Input resistance, resting membrane potential, rheobase and AP threshold were assessed in current clamp traces. In voltage clamp traces, spontaneous EPCSs and IPSCs were analyzed using an automated template-matching protocol. Mean spontaneous current frequency was calculated for each cell across the full duration of the recording.
Quantitative real-time polymerase chain reaction
The medial prefrontal cortex was micro-dissected from coronal sections taken between 2 and 3 mm anterior to bregma and stored in RNA-Later (Sigma-Aldrich, St. Louis, Montana) until processed. As described previously (Pandey et al 2019), the mRNA was purified using a Qiagen RNeasy kit (Qiagen, Valencia, CA), and cDNA was synthesized using RNA-to-cDNA Master Mix (Life Technologies, Grand Island, NY). The concentration of total RNA was quantified by measuring the absorbance at 260 nm. A SYBR Green PCR core reagents kit (Life Technologies, Grand Island, NY) was used for qRT-PCR. PCR analyses were performed in MicroAmp Optic 96-well reaction plates (Life Technologies, Grand Island, NY) and run on a StepOnePlus Real-Time PCR System (Applied Biosystems). The PCR cycle was carried out at 50°C for 2 min, 95°C for 10 min, and 40 cycles of 15 seconds at 95°C followed by 1 min at 60°C. The levels of target gene expression were quantified relative to the expression of cyclophilin-A using the relative quantification method (ΔΔCT). Expression of mRNA in sham-injured animals that received sesame oil was used as the basis for comparison of mRNA expression in all other groups. Primers were designed using the NCBI primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) and purchased from ThermoFisher Scientific. The primers were validated by testing primer dilutions against a series of cDNA dilutions and determining the efficiency of the standard curve [10^(−1/slope)], as well as running a melting curve analysis to confirm primer specificity. The sequences and concentrations of the primers are listed in Table 1.
Histological Analysis
Rats were transcardially perfused at 4 weeks following injury using 0.9% saline containing heparin (1000 units/L, Sagent Pharmaceuticals, Schaumburg, IL) followed by 10% formalin (Fisher Scientific, Pittsburgh, PA). Brains were post-fixed in formalin for 24 hours before being transferred to a cryoprotective solution containing 30% sucrose. Brains were frozen between −40 and −50°C and 40 μM coronal sections were obtained between 3 mm and 2 mm anterior to bregma using a freezing sliding microtome. Sections were incubated in anti-NeuN (1:1000, Sigma-Aldrich, St. Louis, MO) at 4°C for 16–24 hours. Sections were then incubated in a biotinylated donkey anti-mouse secondary antibody (1:500, Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. Sections were then mounted on gelatin-coated glass slides and cover-slipped using Permount mounting medium. Additional separate sets of adjacent sections were mounted on gelatin-coated slides and stained for Nissl (2% Cresyl violet) as previously described (Huh et al 2008).
Statistical Analysis
All data are presented as mean ± SEM. All statistical tests were performed using Statistica 7 (StatSoft, Tulsa, Oklahoma). All analyses were evaluated using a three-way analysis of variance (ANOVA) for sex, injury status, and treatment. When necessary, post-hoc analyses were conducted using the Neuman-Keuls test. Discrimination ratio and GAD67 mRNA expression in brain-injured animals were correlated using linear regression. In all cases, a p-value of less than 0.05 was considered as significant.
Results
Acute response to injury
Brain injury in 11-day-old rats resulted in a skull fracture and hematoma (data not shown). Brain-injured animals exhibited an increase in the time to right themselves immediately following the impact (Table 2; Injury; F(1,113)=102.9, p<0.001), which was similar in both male and female rats (Sex; F(1,113)=1.04, p=0.3). Brain injury resulted in a brief period of apnea which was similar in both male and female rats (Sex; F(1,65)=0.52, p=0.47). Body weights of animals on the day of injury was similar between sham and injured rats (Injury; F(1,113)=1.1, p=0.29, Table 3). Randomization to treatment was confirmed based on a lack of differences in either righting reflex or apnea times between vehicle and progesterone-treated groups (Table 2). Progesterone treatment did not affect weight gain relative to animals given vehicle during the week that it was administered or at 4 weeks post injury (Table 3).
Table 2. Acute neurologic status of rats in the study.
Eleven-day-old male and female rat pups were randomly assigned to either sham- or brain-injured groups. Sham- and brain-injured rats were randomly assigned to receive daily injections of sesame oil (vehicle) or progesterone (PROG). Subsets of the animals tested in the behavioral assays were randomly assigned to be euthanized for mRNA measurements, histology and whole-cell patch clamp electrophysiology. Latency to regain righting reflex and times of apnea were recorded as described in the Methods.
| Outcome | Group | N (male, female) | Righting reflex (s) |
Apnea (s) |
|---|---|---|---|---|
| Behavior | Sham + vehicle | 29 (13, 16) | 70 ± 8 | NA |
| Sham + PROG | 23 (10, 13) | 63 ± 8 | NA | |
| Injured + vehicle | 35 (17, 18) | 185 ± 14* | 16 ± 2 | |
| Injured + PROG | 34 (16, 18) | 205 ± 15* | 21 ± 3 | |
| qRT-PCR | Sham + vehicle | 9 (3,6) | 73 ± 7 | NA |
| Sham + PROG | 5 (2, 3) | 46 ± 12 | NA | |
| Injured + vehicle | 12 (5, 7) | 144 ± 15* | 14 ± 1 | |
| Injured + PROG | 12 (5, 7) | 182 ± 26* | 16 ± 3 | |
| Histology | Sham + vehicle | 8 (4, 4) | 95 ± 19 | NA |
| Sham + PROG | 6 (2, 4) | 73 ± 19 | NA | |
| Injured + vehicle | 6 (3, 3) | 224 ± 50* | 17 ± 2 | |
| Injured + PROG | 10 (5, 5) | 237 ± 35* | 29 ± 4 | |
| Whole cell patch clamp | Sham + vehicle | 12 (6, 6) | 51 ± 12 | NA |
| Sham + PROG | 12 (6, 6) | 66 ± 11 | NA | |
| Injured + vehicle | 17 (9, 8) | 201 ± 21* | 18 ± 3 | |
| Injured + PROG | 12 (6, 6) | 202 ± 12* | 18 ± 2 |
p<0.05 compared to sham-injured rats.
Table 3. Animal weights during the course of the study.
Animal weights were recorded daily immediately prior to injury/surgery (day 0) and every day prior to subcutaneous administration of progesterone. Animals were also weighed at the end of behavioral assessments (day 28). Values represent means and standard errors of the mean.
| Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 28 |
| Sham + sesame oil | 22.8±0.7 | 24.3±0.9 | 26.6±1.1 | 27.8±1.0 | 29.8±1.0 | 31.5±1.0 | 33.6±1.0 | 194.6±15.8 |
| Sham + Progesterone | 22.3±0.7 | 24.7±0.7 | 26.5±0.8 | 28.4±0.9 | 30.3±0.9 | 32.1±0.8 | 34.0±0.9 | 188.5±19.2 |
| Injured + sesame oil | 23.9±0.5 | 25.4±0.6 | 27.4±0.7 | 29.3±0.7 | 31.4±0.8 | 33.2±0.7 | 35.6±0.7 | 202.6±12.6 |
| Injured + Progesterone | 22.8±0.8 | 24.4±0.8 | 26.1±0.8 | 28.2±0.9 | 30.2±0.9 | 32±0.8 | 34.6±0.9 | 199.7±9.4 |
Novel Object Recognition Memory and Locomotor Activity
Brain injury significantly impaired recognition memory in brain-injured rats compared to sham-injured rats (Injury: F(1,85)=11.4, p=0.001, Figure 1A). Progesterone significantly improved recognition memory regardless of injury status (Treatment: F(1,85)=5.9, p=0.017, Figure 1A). There was no effect of sex on the discrimination index in the NOR test (F(1,85)=0.5, p=0.5). Brain injury did not affect novelty-induced locomotor activity, as there were no group differences in the total distance traveled during the 30-minute duration in an open field test (F(1,42)=0.23, p=0.6, Figure 1B). The total times spent sniffing the objects during both the familiarization trial (F(1,85)=5.98, p=0.02, Table 4) and the discrimination trial (F(1,85)=5.84, p=0.02, Table 4) were slightly but significantly decreased in brain-injured rats compared to sham-injured rats. However, the percent of time spent with each object did not differ between sham- and brain-injured animals during the familiarization trial (F(1,85)=0.17, p=0.68, Table 4) indicating that the animals did not exhibit a preference for one of the objects during the familiarization trial. In contrast, brain-injured animals exhibited a decreased preference for the novel object (F(1,85)=11.4, p=0.001, Table 4) and progesterone-injected animals significantly preferred the novel object (F(1,85)=5.9, p=0.017, Table 4).
Figure 1. Progesterone (Prog) improves novel object recognition.
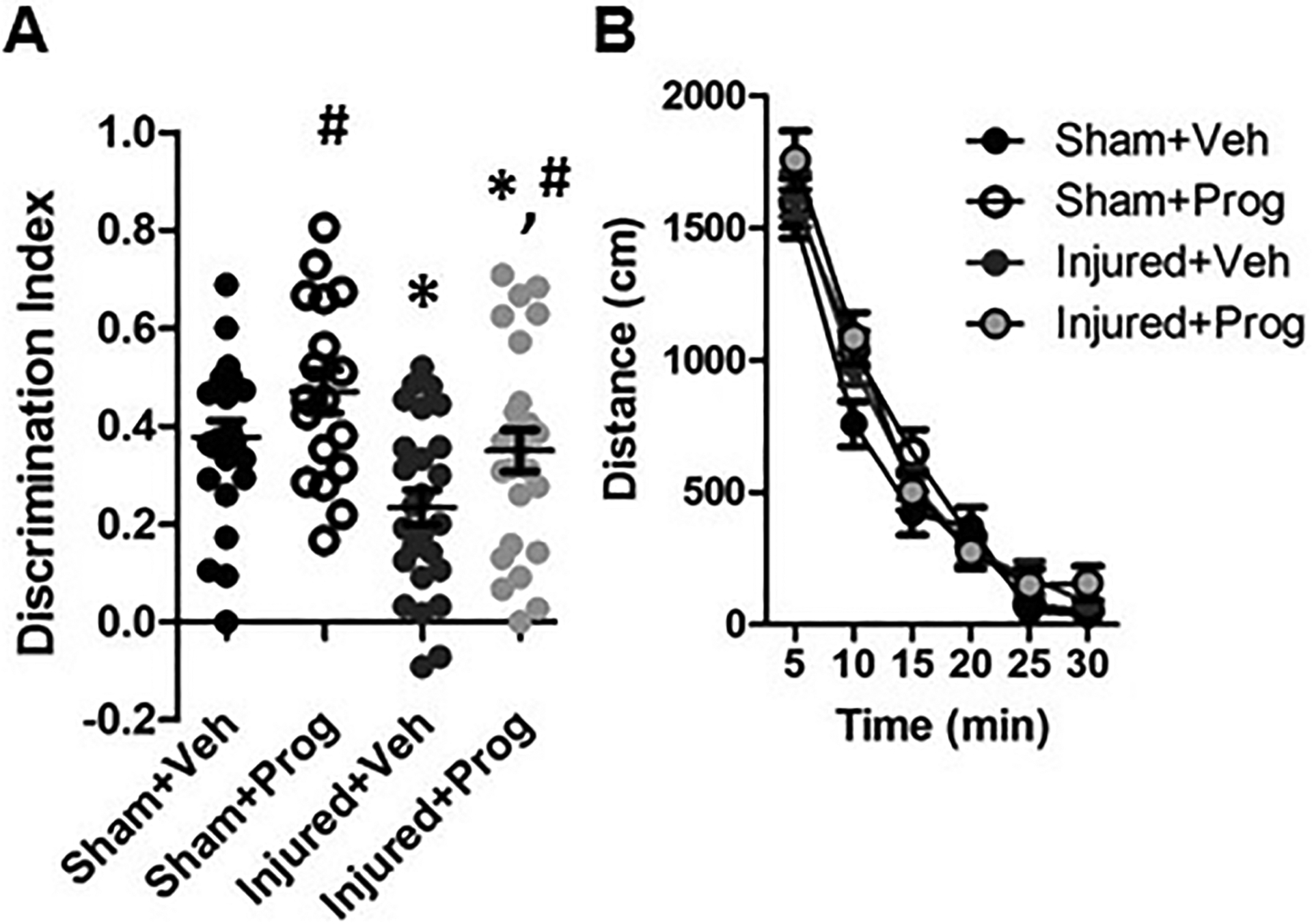
Panel A illustrates the average discrimination index during the novel object discrimination trial. Panel B depicts the average distance traveled during a 30-minute open-field test. Data are presented as means and error bars represent standard error of the mean. *, p<0.05 compared to sham-injured animals; #, p<0.05 compared to animals that received sesame oil (vehicle, Veh).
Table 4.
Times spent by sham- and brain-injured rats with objects during familiarization and discrimination trials in the novel object recognition test.
| Familiarization trial | Discrimination trial | ||||||
|---|---|---|---|---|---|---|---|
| Status | Treatment | Total time (s) |
Object 1 (%) |
Object 2 (%) |
Total time (s) |
Novel object (%) |
Familiar object (%) |
| Sham | Sesame oil | 46.8±3.4 | 51.5±2.0 | 48.5±2.0 | 34.8±3.5 | 68.9±1.7 | 31.1±1.7 |
| Sham | Progesterone | 44.9±2.7 | 48.8±2.0 | 51.2±2.0 | 38.9±2.4 | 73.5±2.2# | 26.5±2.2# |
| Injured | Sesame oil | 39.4±2.1* | 50.5±1.7 | 49.5±1.7 | 29.1±2.0* | 61.7±1.8 | 38.3±1.8 |
| Injured | Progesterone | 37.1±2.4* | 50.4±1.7 | 49.6±1.7 | 30.5±2.1* | 67.5±2.1# | 32.5±2.1# |
p<0.05 compared to sham-injured animals;
p<0.05 compared to rats injected with sesame oil.
Anxiety-like behavior
Brain injury resulted in an increase in exploration of the open arms in the EPM. Figure 2A represents a typical heatmap demonstrating that brain-injured rats spent a greater amount of time in the open arms compared with sham-injured rats. Quantification of the number of entries into the open arm indicated that brain injury significantly increased open arm entries relative to sham-injured rats (Injury: F(1,68)=4.5, p=0.037, Figure 2B). Brain-injured rats also spent significantly more time in the open arm compared to sham-injured rats (Injury: F(1,68)=4.2, p=0.04, Figure 2C). Progesterone treatment did not affect either open arm entries (Figure 2B, Treatment: F(1,68)=0.2, p=0.7) or open arm time (Figure 2C, Treatment: F(1,68)=0.3, p=0.6). Sex did not affect either open arm entries (F(1,68)=0.8, p=0.4) or open arm time (F(1,68)=1.5, p=0.2). Although brain injury did not affect total distance traveled during the 5-minute EPM trial (F(1,68)=0.4, p=0.5, data not shown), there was a significant effect of sex (F(1,68)=6.1, p=0.016), with female rats moving more than male rats (data not shown).
Figure 2. Pediatric TBI increases exploratory behavior in the EPM.
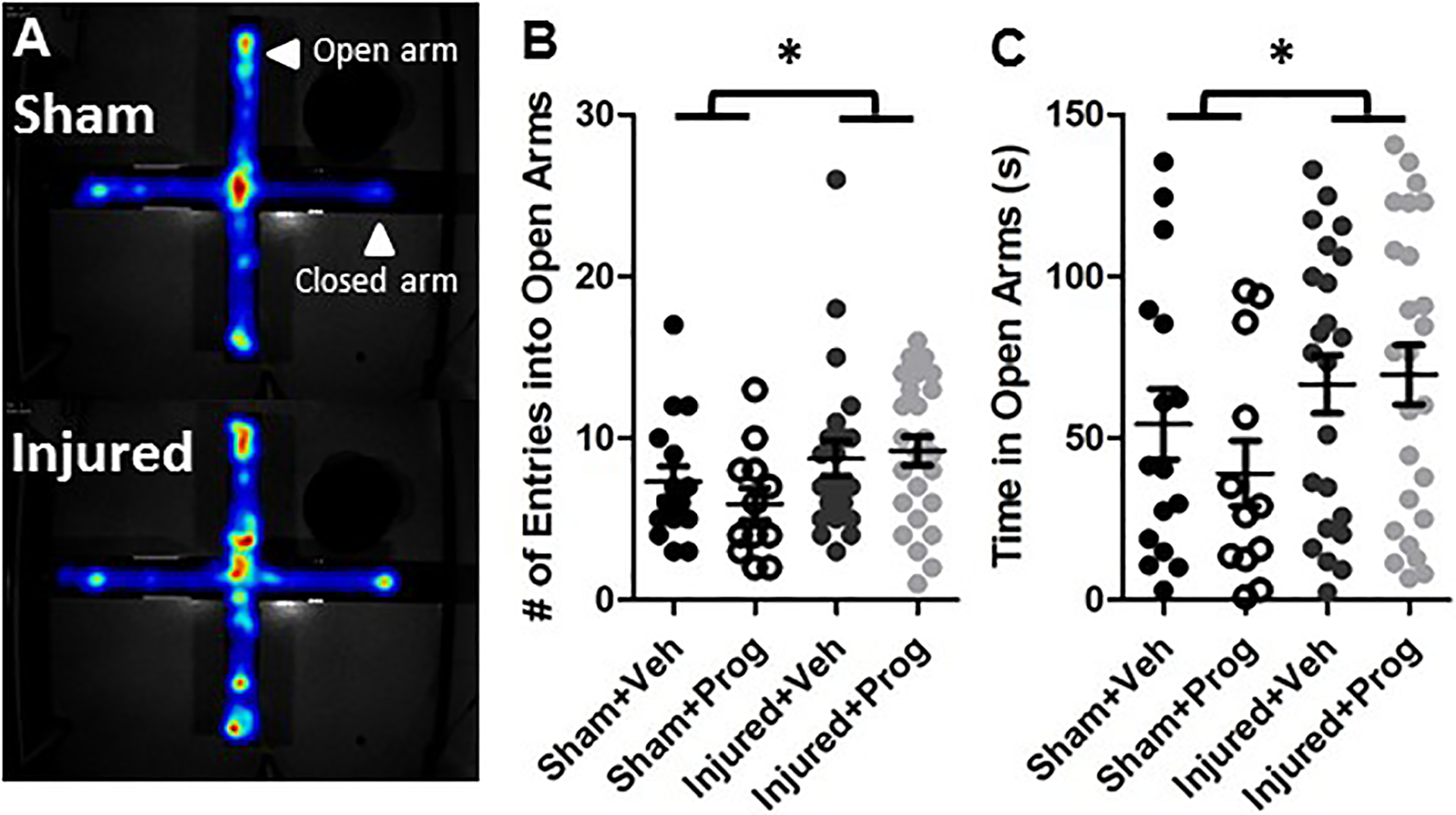
Panel A illustrates a representative heat map from a sham- and a brain-injured rat, depicting the amount of time the animals spent within four arms of the EPM. Graphs depict the average number of entries (B) and the time spent in the open arms (C). Data are presented as means and error bars represent standard error of the mean. Veh, sesame oil; Prog, progesterone. *, p<0.05 compared to sham-injured animals.
Histologic analysis
Compared to sham-injury (Figures 3A, 3B), brain injury did not result in any overt lesion or hemorrhage within the prelimbic region of the prefrontal cortex (Figures 3C, 3D) at 4 weeks after injury. Furthermore, brain injury did not result in an overt loss of cells within this region (Figure 3G) compared to sham injury (Figure 3E). Treatment with progesterone had no effect on cellular staining and morphology in either sham (Figure 3F) or brain-injured animals (Figure 3H). The extent of NeuN immunoreactivity was similar in brain-injured (Figure 3K) and sham-injured animals (Figure 3I) and was not affected by treatment with progesterone (Figures 3J, 3L). Assessment of the Nissl-stained sections directly below the impact site of both vehicle- and progesterone-treated brain-injured rats revealed atrophied cortex, hippocampus, and white matter tracts as previously reported (Raghupathi & Huh 2007), indicative of the moderate nature of the injury severity (data not shown).
Figure 3. Pediatric TBI does not result in an overt lesion or loss of NeuN immunoreactivity within the mPFC.
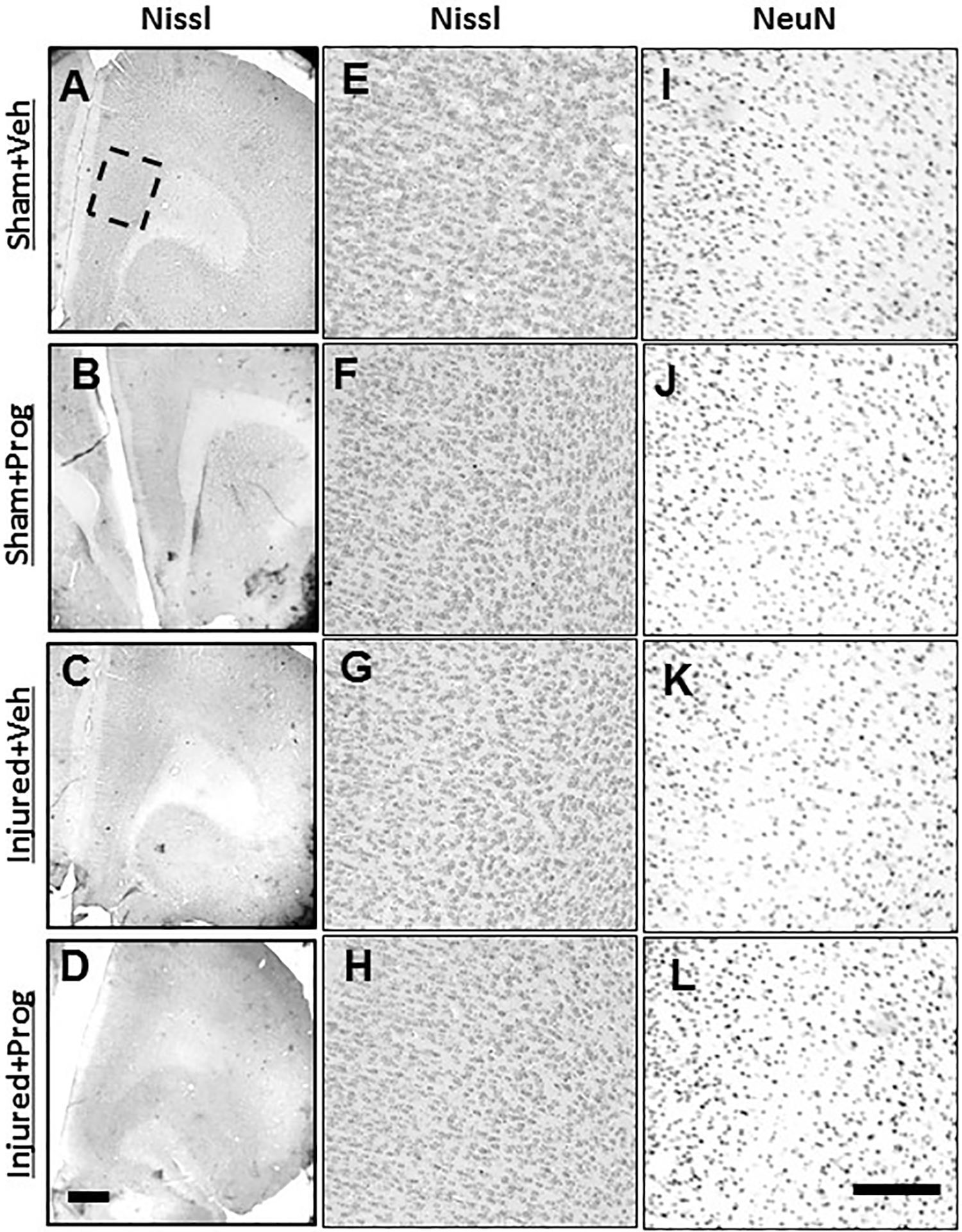
Representative images illustrating Nissl staining in coronal sections containing the mPFC (A-H) and NeuN immunoreactivity (I-L) from sham- (A,B,E,F,I,J) and brain-injured rats (C,D,G,H,K,L) that received sesame oil (Veh; A,C,E,G,I,K) or progesterone (Prog; B,D,F,H,J,L), at 4 weeks after sham- or brain-injury. Boxed area in panel A represents the prelimbic region of medial PFC depicted at higher magnification in panels E-L. Scale bar in panel D = 200 μm for panels A-D and in panel L = 50 μm for panels E-L.
Whole-cell patch clamp electrophysiology
Intrinsic excitability of layer 2/3 neurons:
Brain injury did not affect membrane voltage (F(1,117)=1.35, p=0.25, Figure 4A), AP threshold (F(1,115)=0.75, p=0.39, Figure 4B) or rheobase (F(1,114)=0.47, p=0.49, Figure 4C). However, progesterone treatment significantly increased the AP threshold (F(1,115)=5.4, p=0.02, Figure 4B) and the rheobase (F(1,114)=6.95, p=0.01, Figure 4C) in both sham and brain-injured animals. Brain injury resulted in a significant increase in input resistance (F(1,116)=4.4, p=0.037, Figure 4D) which was not affected by progesterone treatment. Neurons in brain-injured animals responded with significantly greater action potentials in response to varying the levels of current injection relative to neurons from sham-injured animals (F(1,59)=5.3, p=0.024, Figure 4E). The sex of the animal did not affect most of the intrinsic cellular properties that were measured, with the exception of membrane voltage, which was lower in male rats irrespective of injury or treatment with progesterone (F(1,117)=8.6, p=0.004).
Figure 4: Pediatric TBI increases intrinsic excitability in Layer 2/3 pyramidal neurons in the medial PFC.
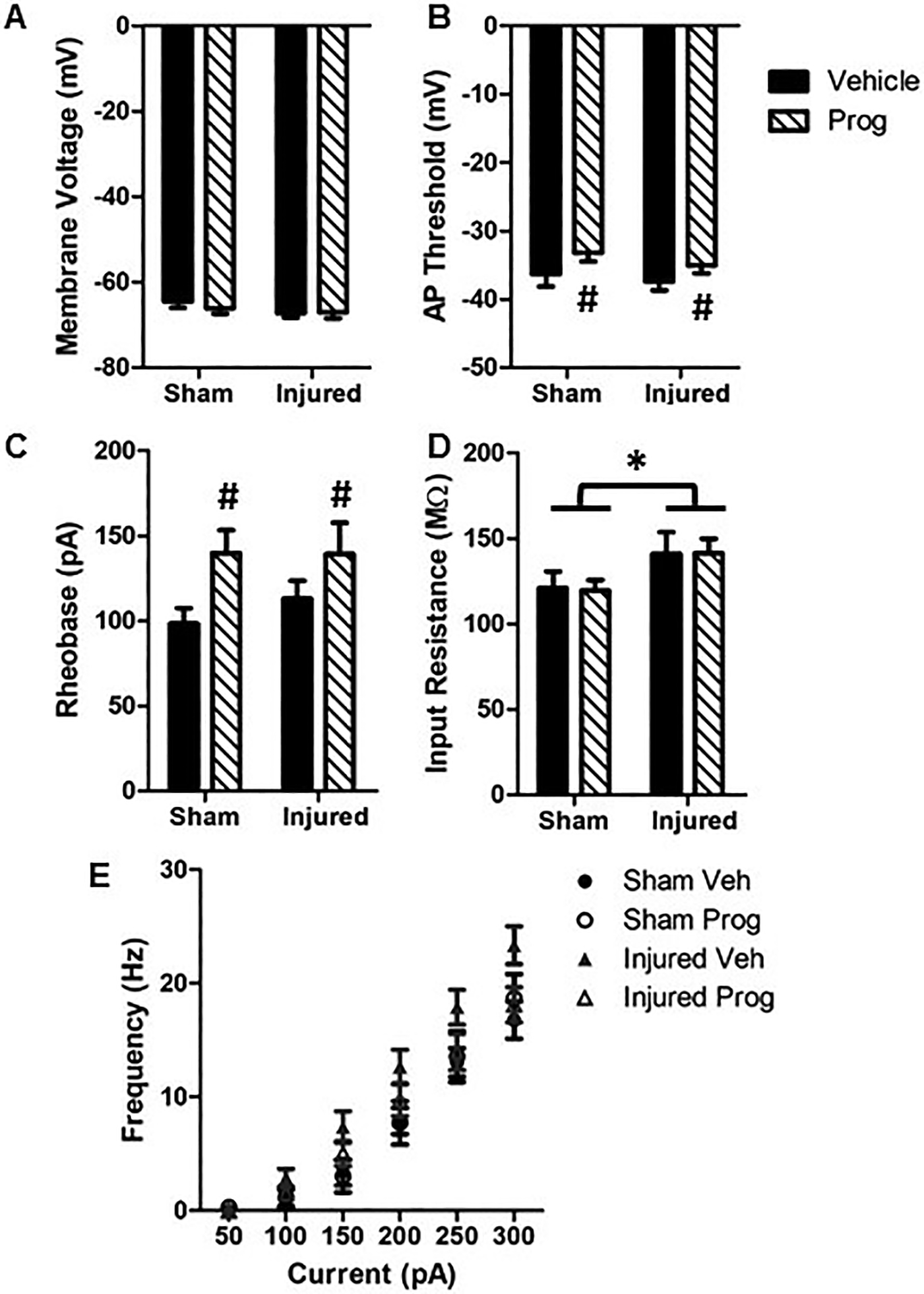
A: Membrane voltage; B: Action potential threshold; C: Rheobase; D: Input resistance; E: Neuron firing rate in response to varying levels of current injection. Bar graphs represent mean and error bars represent standard error of the mean from 26 cells for sham + sesame oil (Veh), 34 cells for sham + progesterone (Prog), 37 cells for injured + vehicle and 28 cells for injured + progesterone animals. *, p<0.05 compared to sham-injured animals; #, p<0.05 compared to sham- and brain-injured animals injected with sesame oil.
Synaptic inputs to layer 2/3 neurons:
Figures 5A and 5B illustrate representative traces of spontaneous excitatory post-synaptic currents (EPSCs) and spontaneous inhibitory post-synaptic currents (IPSCs), respectively. Although brain injury did not affect the frequency of EPSCs (F(1,94)=3.6, p=0.06), there was an effect of treatment wherein progesterone decreased the average EPSC frequency (F(1,94)=13.9, p=0.0003, Figure 5C). Based on the significant interaction between injury status and treatment (F(1,94)=13.4, p=0.0004), post-hoc analyses revealed that neurons from brain-injured animals administered with sesame oil exhibited significantly higher EPSC frequencies than those from sham-injured animals (Figure 5C, p=0.0003). Importantly, treatment of brain-injured rats with progesterone attenuated this increase in frequency (Figure 5C, p=0.0004). Brain injury significantly decreased the amplitude of EPSCs (F(1,94)=10.8, p=0.001, Figure 5D), which was not affected by progesterone (Injury X Treatment, F(1,94)=3.4, p=0.07). Brain injury also resulted in a significant decrease in the frequency of IPSCs (F(1,73)=8.5, p=0.005, Figure 5E), which was unaffected by progesterone treatment (Injury X Treatment; F(1,73)=0.1, p=0.75). The amplitude of IPSCs was reduced in neurons from brain-injured animals (F(1,72)=4.1, p=0.047, Figure 5F), which was similarly not affected by progesterone treatment (F(1,72)=0.23, p=0.63). Whereas sex was not a determinant for most parameters measured, the amplitude of the IPSCs was higher in female rats (irrespective of injury and treatment status) compared to their male counterparts (F(1,72)=4.6, p=0.036).
Figure 5: Pediatric TBI increases excitatory drive in Layer 2/3 pyramidal neurons in the medial PFC.
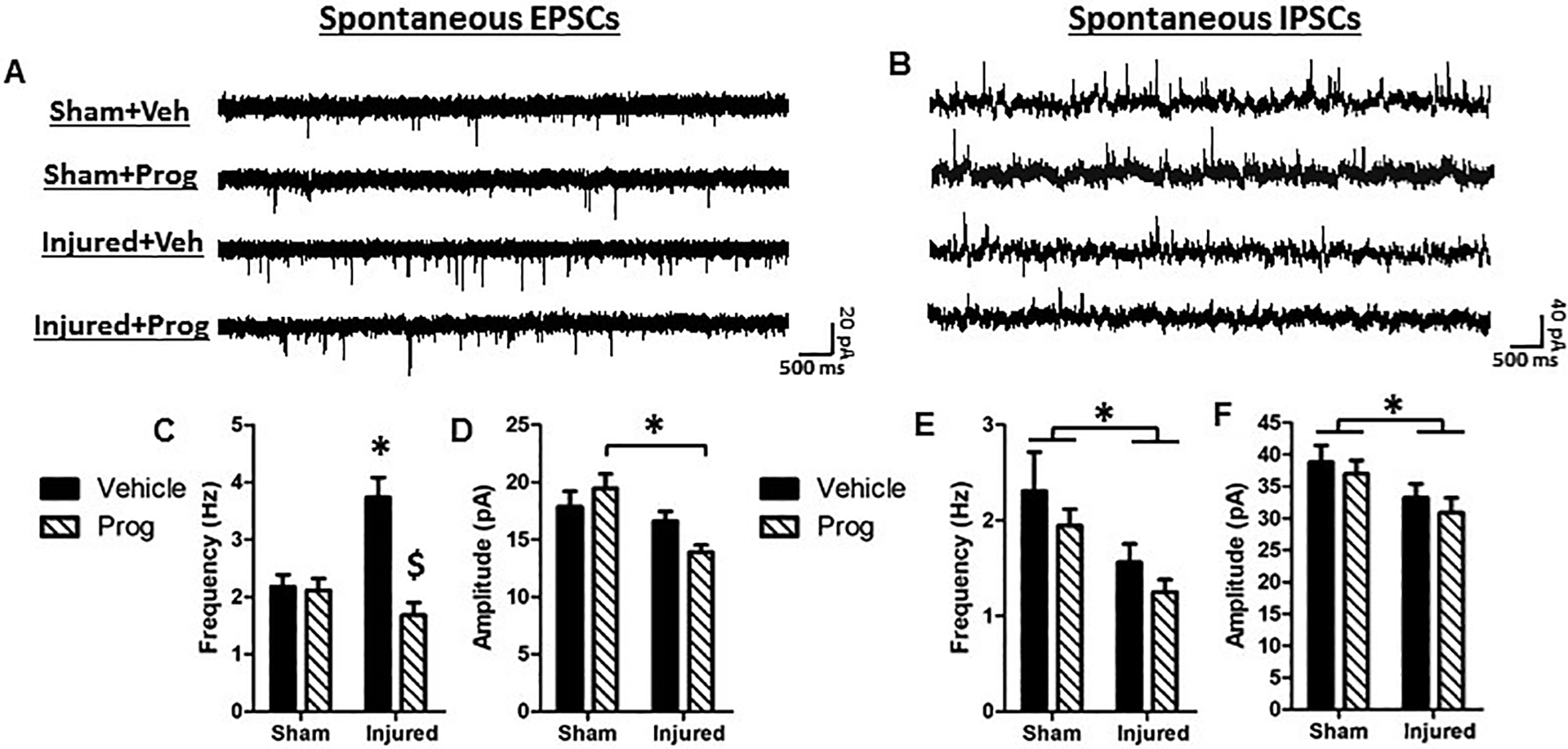
Representative traces of spontaneous excitatory post-synaptic currents (EPSCs, A) and spontaneous inhibitory post-synaptic currents (IPSCs, B). C: EPSC frequency; D: EPSC amplitude; E: IPSC frequency; F: IPSC amplitude. Bar graphs represent mean and error bars represent standard error of the mean. *, p<0.05 compared to sham-injured animals; $, p<0.05 compared to brain-injured animals injected with sesame oil.
Intrinsic excitability of layer 5 neurons:
There was no effect of brain injury on membrane voltage (F(1,100)=0.8, p=0.37, Figure 6A), AP threshold (F(1,100)=0.1, p=0.79, Figure 6B), rheobase (F(1,99)=0.3, p=0.56, Figure 6C) or firing frequency (F(1,56)=1.3, p=0.26, Figure 6E). Brain injury resulted in an increase in the input resistance of layer 5 neurons (F(1,99)=4.3, p=0.04, Figure 6D), which was unaffected by progesterone treatment (F(1,99)=0.1, p=0.73). Progesterone treatment did however lead to significantly fewer action potentials in response to current injection (F(1,56)=6.8, p=0.01, Figure 6E).
Figure 6: Pediatric TBI does not affect the intrinsic excitability of Layer 5 pyramidal neurons in the medial PFC.
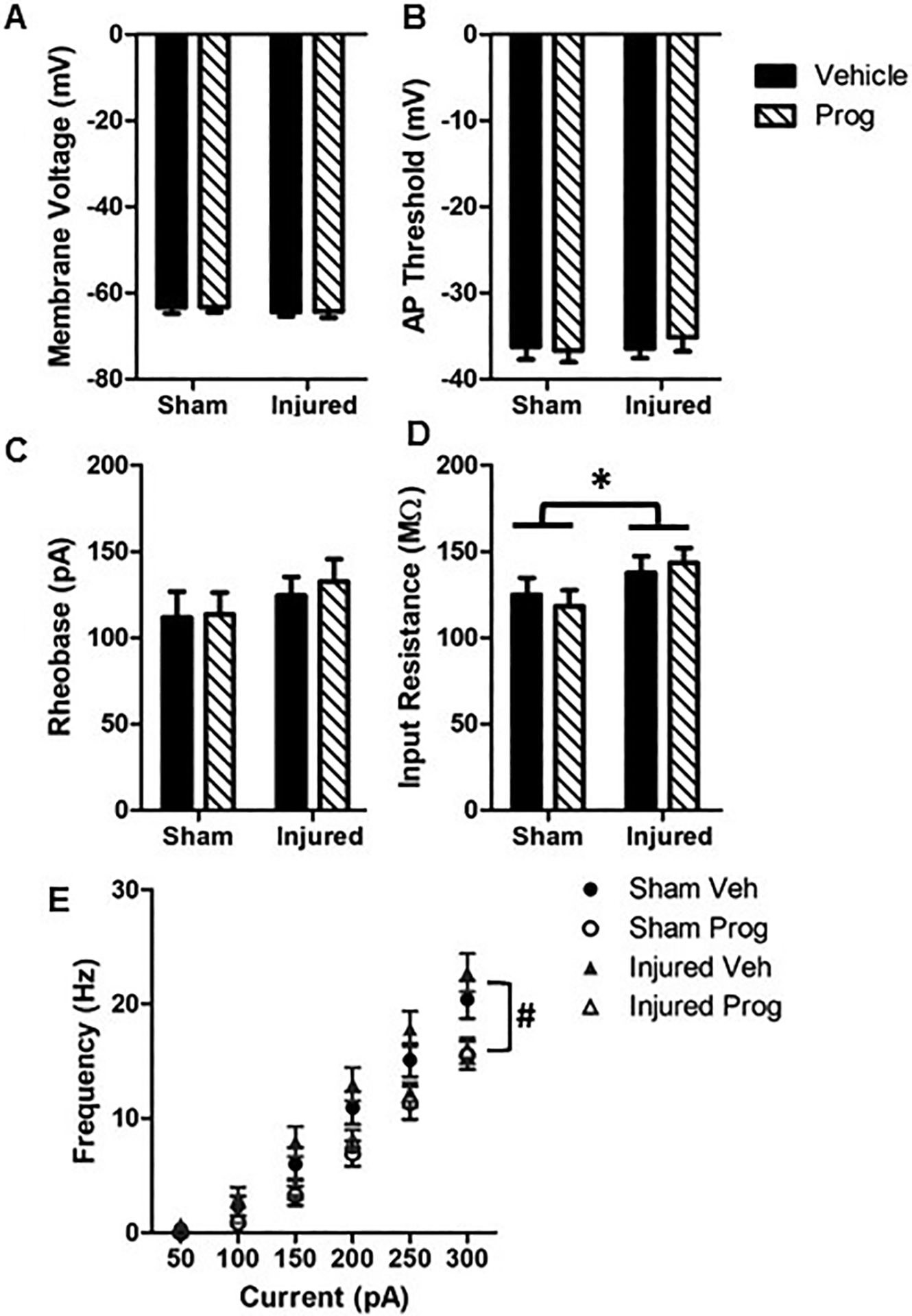
A: Membrane voltage; B: Action potential threshold; C: Rheobase; D: Input resistance; E: Neuron firing rate in response to varying levels of current injection. Bar graphs represent mean and error bars represent standard error of the mean from 24 cells for sham + sesame oil (Veh), 22 cells for sham + progesterone (Prog), 39 cells for injured + vehicle and 23 cells for injured + progesterone animals. *, p<0.05 compared to sham-injured animals; #, p<0.05 compared to sham- and brain-injured animals injected with sesame oil.
Synaptic inputs to layer 5 neurons:
Figures 7A and 7B illustrate representative traces of spontaneous EPSCs and IPSCs, respectively. There was no effect of brain injury on the EPSC frequency (F(1,91)=0.03, p=0.86, Figure 7C) or amplitude (F(1,92)=2.1, p=0.15, Figure 7D). Brain injury resulted in a significant decrease in the frequency of spontaneous IPSCs compared to sham-injured rats (F(1,78)=6.0, p=0.017), which was not affected by progesterone treatment (F(1,78)=0.12, p=0.73, Figure 7E). The amplitude of the IPSCs was not significantly affected either by brain injury (F(1,79)=0.51, p=0.48) or by progesterone treatment (F(1,79)=0.09, p=0.75, Figure 7F).
Figure 7. Pediatric TBI decreases inhibitory drive in layer 5 pyramidal neurons in the medial PFC.
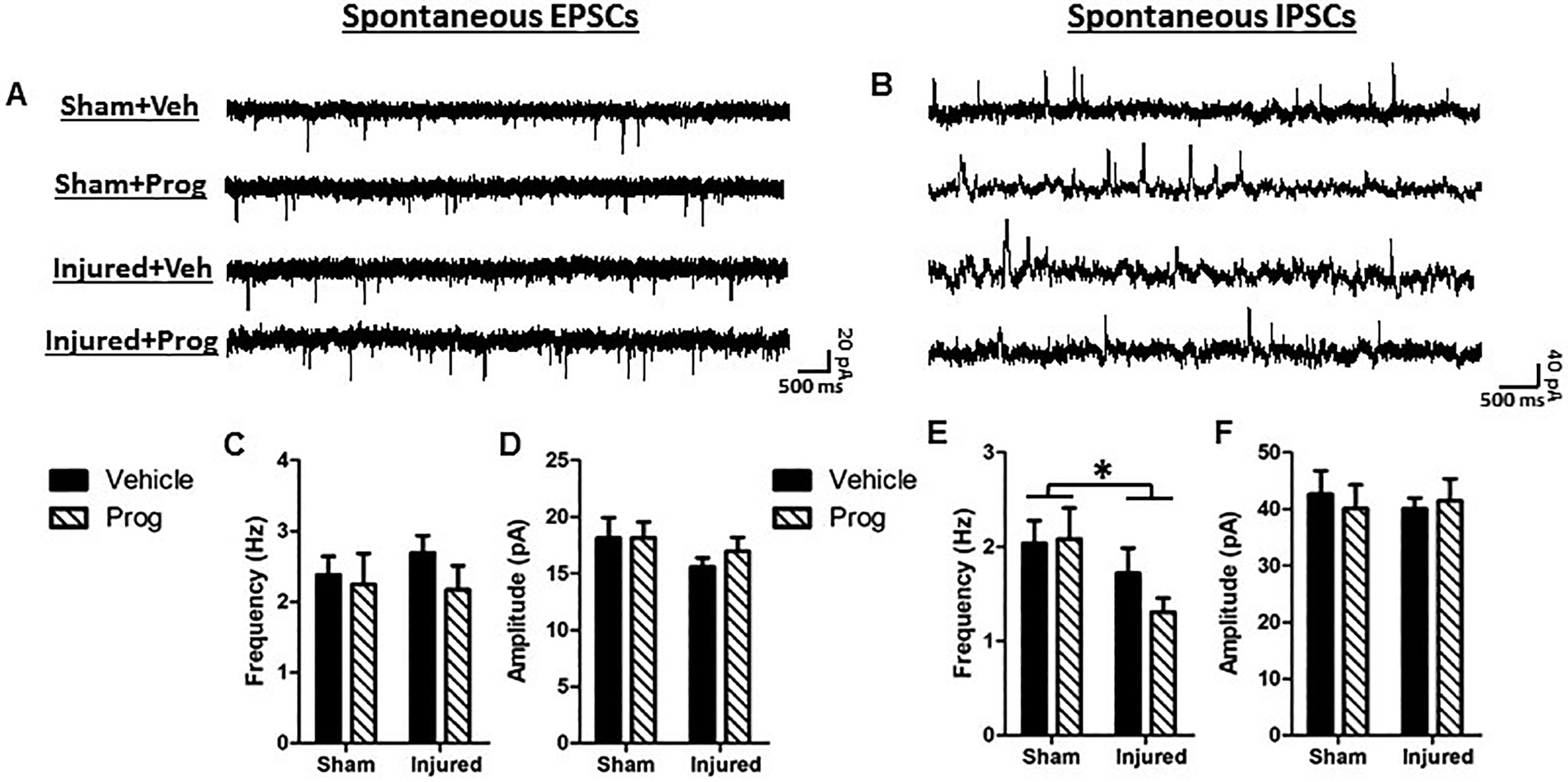
Representative traces of spontaneous excitatory post-synaptic currents (EPSCs, A) and spontaneous inhibitory post-synaptic currents (IPSCs, B). C: EPSC frequency; D: EPSC amplitude; E: IPSC frequency; F: IPSC amplitude. Bar graphs represent mean and error bars represent standard error of the mean. *, p<0.05 compared to sham-injured animals.
Expression of mRNA for GAD67, EAAT-3, and NaVβ3
Neither brain injury (F(1,20)=2.6, p=0.12, Figure 8A) nor progesterone administration (F(1,20)=1.5, p=0.23, Figure 8A) affected the expression of mRNA for GAD67 in the medial PFC. However, there was a significant interaction effect between injury status and treatment (F(1,20)=5.9, p=0.02), and post-hoc tests revealed that GAD67 mRNA was significantly elevated in sham-injured rats given progesterone compared with their sesame oil-administered counterparts (p=0.037, Figure 8A). In contrast, progesterone treatment did not affect GAD67 mRNA expression in brain-injured rats (p=0.91). In both sham- and brain-injured rats, progesterone treatment resulted in a significant increase in mRNA for the neuronal excitatory amino acid transporter (EAAT3, F(1,16)=4.4, p=0.05, Figure 8B), as well as a significant decrease in mRNA for the voltage-gated sodium channel β3 subunit (NaVβ3, F(1,16)=5.8, p=0.03, Figure 8C), compared with animals given sesame oil. A significant regression co-efficient was observed between the discrimination index in the NOR test and GAD67 mRNA expression in the medial PFC (F(1,21)=5.1, R2 = 0.195, p=0.03, Figure 8D). There was no effect of brain injury or progesterone treatment on mRNA for the ionotropic glutamate receptor subunits GluR1 or GluN1, the α1 subunit of the GABAA receptor, or the vesicular glutamate transporters vGlut1 and vGlut2 (data not shown). No significant correlation was observed between EAAT3 or NaVβ3 mRNA and discrimination index (data not shown).
Figure 8. Progesterone regulates the expression of glutamatergic and GABAergic markers in the medial PFC.
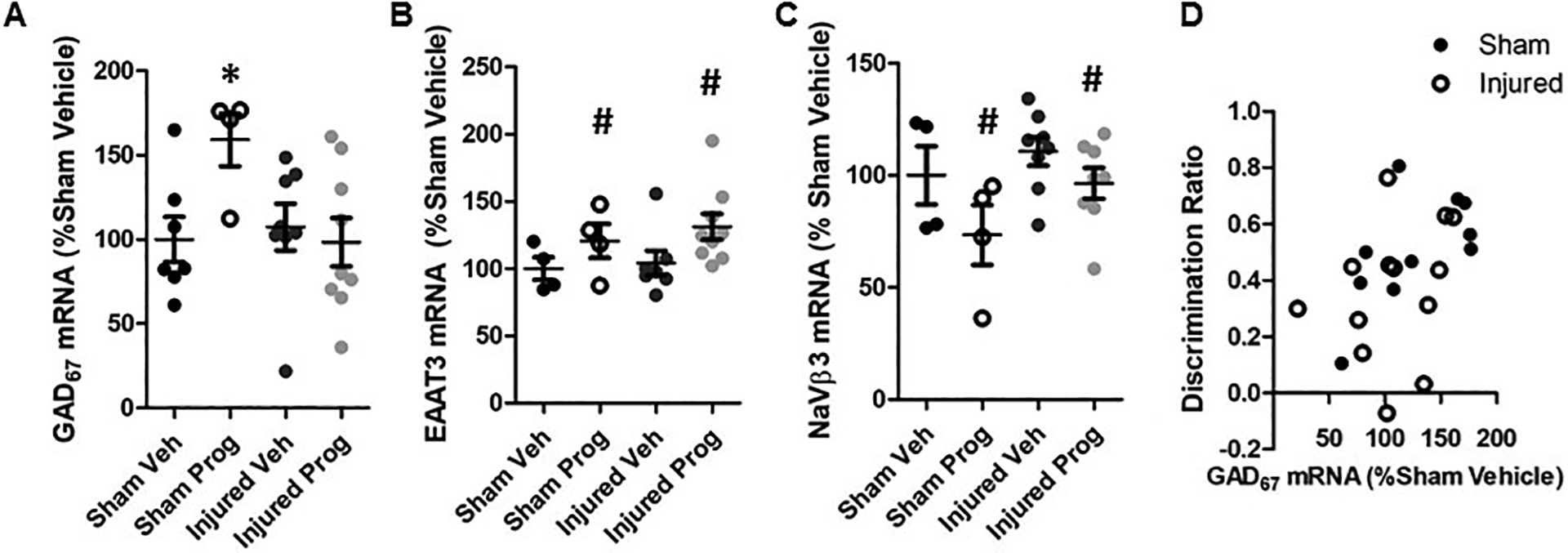
Gene expression of GAD67 (A), excitatory amino acid transporter 3 (EAAT3, B), and the β subunit of the voltage-gated sodium channel (NaVβ3, C) within the medial PFC. Data are presented relative to values for sham-injured animals injected with sesame oil (Veh). Scatter plots represent each individual data point and error bars represent standard errors of the mean. D: Correlation between discrimination index in the novel object recognition test and GAD67 mRNA expression for sham- and brain-injured animals that were injected with either sesame oil (Veh) or progesterone (Prog). *, p<0.05 compared to sham-injured animals injected with sesame oil. #, p<0.05 compared to sham- and brain-injured animals injected with sesame oil.
Discussion
The present study demonstrates that a single closed-head injury of moderate severity in the 11-day-old rat resulted in object recognition memory deficits and an increase in exploratory behavior in the elevated plus maze in the 4th week after injury at an age equivalent to adolescence. These behavioral effects occurred in the absence of overt neurodegenerative changes in the medial PFC although atrophy of the cortex and underlying white matter immediately below the site of the impact has been previously reported (Raghupathi & Huh 2007). Closed head injury in the 11-day-old rat resulted in an increase in the frequency of spontaneous EPSCs and a concomitant decrease in spontaneous IPSCs in layer 2/3 pyramidal neurons within the medial PFC, suggesting that the behavioral deficits may be a consequence of functional changes in these neurons. Daily administration of progesterone for the first week following brain injury improved NOR memory, decreased injury-induced neuronal excitability and frequency of EPSCs in medial PFC neurons, decreased gene expression of the β3 subunit of the voltage-gated sodium channel (NaVβ3), and increased gene expression of the excitatory amino acid transporter 3 (EAAT3) in the PFC.
Both closed head injury in neonate rabbits (Zhang et al 2015) and lesions to the medial PFC of adult rats resulted in impairments in recognition memory (Hannesson et al 2004a, Hannesson et al 2004b). Previous studies have linked progesterone to performance in the NOR task, wherein adult female rats in the proestrus phase (when progesterone levels are elevated) exhibit better object recognition compared to their counterparts in other phases of the estrous cycle (Frye et al 2007, Walf et al 2006). Similarly, progesterone administered at the time of training enhanced performance in object recognition tasks in adult ovariectomized rodents (Frye et al 2009, Frye & Walf 2008). Impairment in NOR memory has been linked to activation of excitatory dopaminergic D1 receptors in the PFC (Pezze et al 2015, Watson et al 2012), which in turn enhances EPSC amplitude in layer 2/3 pyramidal neurons (Gonzalez-Islas & Hablitz 2003), suggesting that enhancing excitatory transmission within the PFC observed in the present study may provide a cellular basis for the impairment in short term NOR memory. The beneficial effects of progesterone on NOR memory may therefore be a result of attenuating the hyperexcitability of medial PFC neurons. Treatment with progesterone led to a significant decrease in the level of gene expression of the β3 subunit of the sodium channel, suggesting that progesterone may alter cellular hyperexcitability by regulating the gating and kinetics of voltage-gated sodium channels (Cheng et al 2008, Knock et al 2001, Morales-Lazaro et al 2019, Stell et al 2003, Brackenbury & Isom 2011).
The observation that brain injury in the 11-day-old rat resulted in an increase in the intrinsic excitability of layer 2/3 neurons of the medial PFC, which was accompanied by an increase in spontaneous EPSCs and decrease in spontaneous IPSCs, support previous studies demonstrating changes in excitability and synaptic input to neurons in the upper cortical layers of brain-injured adult animals (Alwis et al 2016, Carron et al 2016, Smith et al 2015). Changes in glutamatergic neurotransmission have also been implicated as a mechanism for hyperexcitability following adult TBI (Guerriero et al 2015). However, progesterone did not affect the expression of the vesicular glutamate transporters or ionotropic glutamate receptor subunits GluR1 and GluN1 in the present study, suggesting that it may not influence the release of glutamate or the activation of glutamate receptors within the PFC. Progesterone has also been reported to upregulate glutamate transporter expression (Liu et al 2012), and to suppress glutamatergic neuronal responses (Smith et al 1987a, Smith et al 1987b). Interestingly, progesterone treatment increased the mRNA expression of the neuronally expressed excitatory amino acid transporter 3 (EAAT3) within the medial PFC in both sham and brain-injured animals, suggesting that progesterone may reduce the frequency of EPSCs in brain-injured rats by increasing the reuptake of extracellular glutamate (Bjorn-Yoshimoto & Underhill 2016, O’Donovan et al 2017).
Following TBI in adult animals, imbalance of the cortical E/I balance leading to a shift towards excitation has been extensively documented (Bonislawski et al 2007, Brizuela et al 2017, Cantu et al 2015, Carron et al 2016, Ding et al 2011, Guerriero et al 2015, Witgen et al 2005, Witkowski et al 2019) and this has been linked to decreases in the number of inhibitory interneurons (Brizuela et al 2017, Cantu et al 2015) and reductions in inhibitory neurotransmission (Bonislawski et al 2007, Cantu et al 2015, Carron et al 2016, Witgen et al 2005). The lack of an effect of progesterone on inhibitory neurotransmission following pediatric brain trauma was surprising considering the preponderance of evidence documenting the potentiation of GABAergic signaling in adult animals by progesterone (Brinton et al 2008, Majewska & Vaupel 1991). This may be because the effects of progesterone were evaluated immediately after drug administration whereas in the present study the effects on inhibitory neurotransmission were evaluated 4 weeks following the conclusion of treatment. Although progesterone treatment did not affect inhibitory neurotransmission, it did lead to an increase in GAD67 mRNA in sham animals given progesterone relative to those given sesame oil, but not in brain-injured rats given progesterone, suggesting that brain injury may impair progesterone-mediated regulation of GAD expression. Closed head injury did not affect the excitability of layer 5 neurons within the medial PFC which is similar to that observed within the peri-injury cortex following contusive TBI in the 17-day-old rat (Nichols et al 2015). The lack of an effect in layer 5 neurons may reflect the difference in vulnerability of these pyramidal tract neurons compared to the intratelencephalic neurons (Smith et al 2015), although the molecular mechanisms underlying the differential vulnerability between layer 2/3 and layer 5 neurons remain to be explored.
The observation of increased open arm time in the EPM following closed head injury in the 11-day-old rat may be related to the increased excitability of neurons within the medial PFC. Chemogenetic activation of excitatory neurons in, or direct electrical stimulation of, the medial PFC of adult rodents increased open arm time in the EPM (Pati et al 2018, Reznikov et al 2018, Salvi et al 2019). Although progesterone reduced the injury-induced excitability of the PFC neurons, it did not alter the behavior in the EPM. In part, these disparate effects may be related to the known effects of progesterone on important developmental processes such as cell differentiation, myelination, and circuit formation that occur during the first few weeks after birth (Gonzalez-Orozco & Camacho-Arroyo 2019). The present observations are also in contrast to reports that progesterone decreased anxiety-like behavior following a contusion injury in 7-day-old rats (Baykara et al 2013) and in adult mice (Frye et al 2004), suggesting that age and injury type may influence the efficacy of progesterone.
In the present study we observed minimal sex differences in the effects of progesterone treatment, which is in line with an absence of reported sex differences in the neuroprotective effects of progesterone on cognitive and motor function following pediatric TBI in previous studies (Geddes et al 2016, Geddes et al 2014, Uysal et al 2013). However, other studies have reported sex differences in the beneficial effects of progesterone on mitochondrial function (Robertson & Saraswati 2015) and microglial activation (Peterson et al 2015) following pediatric brain injury, suggesting that the sex-dependent effects of progesterone may depend on the outcome being evaluated following pediatric brain injury. Taken together, these studies demonstrate that a single, moderate closed head injury in 11-day-old rats results in cognitive deficits which are accompanied by alterations in neuronal functionality within the medial PFC, and suggest that progesterone may reverse post-traumatic cognitive deficits by affecting TBI-induced changes in excitatory signaling mechanisms.
Acknowledgements
These studies were supported, in part, by grants from the National Institutes of Health HD061963 and a grant from the Pennsylvania Department of Health SAP4100077079.
References
- Adelson PD, Dixon CE, Kochanek PM. 2000. Long-term dysfunction following diffuse traumatic brain injury in the immature rat. J Neurotrauma 17: 273–82 [DOI] [PubMed] [Google Scholar]
- Allitt BJ, Johnstone VPA, Richards KL, Yan EB, Rajan R. 2017. Progesterone Sharpens Temporal Response Profiles of Sensory Cortical Neurons in Animals Exposed to Traumatic Brain Injury. Cell Transplant 26: 1202–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwis DS, Yan EB, Johnstone V, Carron S, Hellewell S, et al. 2016. Environmental Enrichment Attenuates Traumatic Brain Injury: Induced Neuronal Hyperexcitability in Supragranular Layers of Sensory Cortex. J Neurotrauma 33: 1084–101 [DOI] [PubMed] [Google Scholar]
- Araki T, Yokota H, Morita A. 2017. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol Med Chir (Tokyo) 57: 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykara B, Aksu I, Buyuk E, Kiray M, Sisman AR, et al. 2013. Progesterone treatment decreases traumatic brain injury induced anxiety and is correlated with increased serum IGF-1 levels; prefrontal cortex, amygdala, hippocampus neuron density; and reduced serum corticosterone levels in immature rats. Biotech Histochem 88: 250–7 [DOI] [PubMed] [Google Scholar]
- Bjorn-Yoshimoto WE, Underhill SM. 2016. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem Int 98: 4–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonislawski DP, Schwarzbach EP, Cohen AS. 2007. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol Dis 25: 163–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. 2011. Na Channel beta Subunits: Overachievers of the Ion Channel Family. Front Pharmacol 2: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, et al. 2008. Progesterone receptors: form and function in brain. Front Neuroendocrinol 29: 313–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela M, Blizzard CA, Chuckowree JA, Pitman KA, Young KM, Dickson T. 2017. Mild Traumatic Brain Injury Leads to Decreased Inhibition and a Differential Response of Calretinin Positive Interneurons in the Injured Cortex. J Neurotrauma 34: 2504–17 [DOI] [PubMed] [Google Scholar]
- Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, et al. 2015. Traumatic Brain Injury Increases Cortical Glutamate Network Activity by Compromising GABAergic Control. Cereb Cortex 25: 2306–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carron SF, Alwis DS, Rajan R. 2016. Traumatic Brain Injury and Neuronal Functionality Changes in Sensory Cortex. Front Syst Neurosci 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. 2008. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI). J Pediatr Psychol 33: 707–18 [DOI] [PubMed] [Google Scholar]
- Catroppa C, Godfrey C, Rosenfeld JV, Hearps SS, Anderson VA. 2012. Functional recovery ten years after pediatric traumatic brain injury: outcomes and predictors. J Neurotrauma 29: 2539–47 [DOI] [PubMed] [Google Scholar]
- Cheng ZX, Lan DM, Wu PY, Zhu YH, Dong Y, et al. 2008. Neurosteroid dehydroepiandrosterone sulphate inhibits persistent sodium currents in rat medial prefrontal cortex via activation of sigma-1 receptors. Exp Neurol 210: 128–36 [DOI] [PubMed] [Google Scholar]
- Choe MC, Valino H, Fischer J, Zeiger M, Breault J, et al. 2016. Targeting the Epidemic: Interventions and Follow-up Are Necessary in the Pediatric Traumatic Brain Injury Clinic. J Child Neurol 31: 109–15 [DOI] [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, et al. 2011. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. MMWR Surveill Summ 60: 1–32 [PubMed] [Google Scholar]
- Ding MC, Wang Q, Lo EH, Stanley GB. 2011. Cortical excitation and inhibition following focal traumatic brain injury. J Neurosci 31: 14085–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami P, Czorlich P, Fritzsche FS, Westphal M, Rueger JM, et al. 2017. Impact of Glasgow Coma Scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: a retrospective, multicenter cohort study. J Neurosurg 126: 760–67 [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Kramer L, Cox CS Jr., Baumgartner J, et al. 2006. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. J Neurosurg 105: 287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Coronado V. 2015. Epidemiology of traumatic brain injury. Handb Clin Neurol 127: 3–13 [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. 2007. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem 88: 208–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC, Walf AA. 2009. Progesterone can enhance consolidation and/or performance in spatial, object and working memory tasks in Long-Evans rats. Anim Behav 78: 279–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. 2008. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem 90: 171–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. 2004. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 alpha-reductase. Brain Res 1004: 116–24 [DOI] [PubMed] [Google Scholar]
- Geddes RI, Peterson BL, Stein DG, Sayeed I. 2016. Progesterone Treatment Shows Benefit in Female Rats in a Pediatric Model of Controlled Cortical Impact Injury. PLoS One 11: e0146419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes RI, Sribnick EA, Sayeed I, Stein DG. 2014. Progesterone treatment shows benefit in a pediatric model of moderate to severe bilateral brain injury. PLoS One 9: e87252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, Scheff SW. 2008. Efficacy of progesterone following a moderate unilateral cortical contusion injury. J Neurotrauma 25: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. 2003. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 23: 867–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Orozco JC, Camacho-Arroyo I. 2019. Progesterone Actions During Central Nervous System Development. Front Neurosci 13: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. 2003. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav 76: 231–42 [DOI] [PubMed] [Google Scholar]
- Guerriero RM, Giza CC, Rotenberg A. 2015. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon LA, Raghupathi R, Huh JW. 2017. Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp Neurol 290: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon LA, Raghupathi R, Huh JW. 2019. Depletion of microglia immediately following traumatic brain injury in the pediatric rat: Implications for cellular and behavioral pathology. Exp Neurol 316: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG. 2004a. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24: 4596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson DK, Vacca G, Howland JG, Phillips AG. 2004b. Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behav Brain Res 153: 273–85 [DOI] [PubMed] [Google Scholar]
- Huh JW, Raghupathi R. 2007. Chronic cognitive deficits and long-term histopathological alterations following contusive brain injury in the immature rat. J Neurotrauma 24: 1460–74 [DOI] [PubMed] [Google Scholar]
- Huh JW, Raghupathi R. 2019. Therapeutic strategies to target acute and long-term sequelae of pediatric traumatic brain injury. Neuropharmacology 145: 153–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. 2008. Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp Neurol 213: 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan HT, Clark AE, Holubkov R, Cox CS, Ewing-Cobbs L. 2018. Psychosocial and Executive Function Recovery Trajectories One Year after Pediatric Traumatic Brain Injury: The Influence of Age and Injury Severity. J Neurotrauma 35: 286–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knock GA, Tribe RM, Hassoni AA, Aaronson PI. 2001. Modulation of potassium current characteristics in human myometrial smooth muscle by 17beta-estradiol and progesterone. Biol Reprod 64: 1526–34 [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, et al. 2019. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines. Pediatr Crit Care Med 20: S1–S82 [DOI] [PubMed] [Google Scholar]
- Li H, Prince DA. 2002. Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol 88: 2–12 [DOI] [PubMed] [Google Scholar]
- Li N, Yang Y, Glover DP, Zhang J, Saraswati M, et al. 2014. Evidence for impaired plasticity after traumatic brain injury in the developing brain. J Neurotrauma 31: 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang B, Kan Z, Zhang B, Yang Z, et al. 2012. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma 29: 343–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Mao D, Liu L, Huang Y, Bo T. 2012. Effects of progesterone on glutamate transporter 2 and gamma-aminobutyric acid transporter 1 expression in the developing rat brain after recurrent seizures. Neural Regen Res 7: 2036–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Vaupel DB. 1991. Steroid control of uterine motility via gamma-aminobutyric acidA receptors in the rabbit: a novel mechanism? J Endocrinol 131: 427–34 [DOI] [PubMed] [Google Scholar]
- Morales-Lazaro SL, Gonzalez-Ramirez R, Rosenbaum T. 2019. Molecular Interplay Between the Sigma-1 Receptor, Steroids, and Ion Channels. Front Pharmacol 10: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Perez R, Wu C, Adelson PD, Anderson T. 2015. Traumatic brain injury induces rapid enhancement of cortical excitability in juvenile rats. CNS Neurosci Ther 21: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Johnson F, Vink R. 2007. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol 205: 145–53 [DOI] [PubMed] [Google Scholar]
- O’Donovan SM, Sullivan CR, McCullumsmith RE. 2017. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Badve PS, Curtis GR, Leibowitz SF, Barson JR. 2019. Neurotensin in the posterior thalamic paraventricular nucleus: inhibitor of pharmacologically relevant ethanol drinking. Addict Biol 24: 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S, Sood A, Mukhopadhyay S, Vaidya VA. 2018. Acute pharmacogenetic activation of medial prefrontal cortex excitatory neurons regulates anxiety-like behaviour. J Biosci 43: 85–95 [PubMed] [Google Scholar]
- Peterson BL, Won S, Geddes RI, Sayeed I, Stein DG. 2015. Sex-related differences in effects of progesterone following neonatal hypoxic brain injury. Behav Brain Res 286: 152–65 [DOI] [PubMed] [Google Scholar]
- Pezze MA, Marshall HJ, Cassaday HJ. 2015. Dopaminergic modulation of appetitive trace conditioning: the role of D1 receptors in medial prefrontal cortex. Psychopharmacology (Berl) 232: 2669–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield SP. 1994. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ Health Perspect 102 Suppl 2: 125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hovda DA. 1998. Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. J Neurotrauma 15: 799–811 [DOI] [PubMed] [Google Scholar]
- Prouty EW, Waterhouse BD, Chandler DJ. 2017. Corticotropin releasing factor dose-dependently modulates excitatory synaptic transmission in the noradrenergic nucleus locus coeruleus. Eur J Neurosci 45: 712–22 [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Huh JW. 2007. Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. J Neurotrauma 24: 1596–608 [DOI] [PubMed] [Google Scholar]
- Reznikov R, Bambico FR, Diwan M, Raymond RJ, Nashed MG, et al. 2018. Prefrontal Cortex Deep Brain Stimulation Improves Fear and Anxiety-Like Behavior and Reduces Basolateral Amygdala Activity in a Preclinical Model of Posttraumatic Stress Disorder. Neuropsychopharmacology 43: 1099–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108 Suppl 3: 511–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Fidan E, Stanley RM, Noje C, Bayir H. 2015. Progesterone for neuroprotection in pediatric traumatic brain injury. Pediatr Crit Care Med 16: 236–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. 2006. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol 197: 235–43 [DOI] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M. 2015. Progesterone protects mitochondrial function in a rat model of pediatric traumatic brain injury. J Bioenerg Biomembr 47: 43–51 [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. 1994. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol 129: 64–9 [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. 1992. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor Neurol Neurosci 4: 425–7 [DOI] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, et al. 2015. The emergence of age-dependent social cognitive deficits after generalized insult to the developing brain: a longitudinal prospective analysis using susceptibility-weighted imaging. Hum Brain Mapp 36: 1677–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi SS, Pati S, Chaudhari PR, Tiwari P, Banerjee T, Vaidya VA. 2019. Acute Chemogenetic Activation of CamKIIalpha-Positive Forebrain Excitatory Neurons Regulates Anxiety-Like Behaviour in Mice. Front Behav Neurosci 13: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si D, Wang H, Wang Q, Zhang C, Sun J, et al. 2013. Progesterone treatment improves cognitive outcome following experimental traumatic brain injury in rats. Neurosci Lett 553: 18–23 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Xiong G, Elkind JA, Putnam B, Cohen AS. 2015. Brain Injury Impairs Working Memory and Prefrontal Circuit Function. Front Neurol 6: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. 1987a. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res 400: 353–9 [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. 1987b. Locally applied progesterone metabolites alter neuronal responsiveness in the cerebellum. Brain Res Bull 18: 739–47 [DOI] [PubMed] [Google Scholar]
- Stein DG. 2008. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev 57: 386–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. 2015. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj 29: 1259–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. 2003. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A 100: 14439–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ. 2016. The Epidemiology of Traumatic Brain Injury in Children and Youths: A Review of Research Since 1990. J Child Neurol 31: 20–7 [DOI] [PubMed] [Google Scholar]
- Treble-Barna A, Zang H, Zhang N, Taylor HG, Yeates KO, Wade S. 2017. Long-Term Neuropsychological Profiles and Their Role as Mediators of Adaptive Functioning after Traumatic Brain Injury in Early Childhood. J Neurotrauma 34: 353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal N, Baykara B, Kiray M, Cetin F, Aksu I, et al. 2013. Combined treatment with progesterone and magnesium sulfate positively affects traumatic brain injury in immature rats. Turk Neurosurg 23: 129–37 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem 86: 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC. 2012. Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 37: 770–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Xiao GM. 2013. The neuroprotective effects of progesterone on traumatic brain injury: current status and future prospects. Acta Pharmacol Sin 34: 1485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, et al. 2005. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience 133: 1–15 [DOI] [PubMed] [Google Scholar]
- Witkowski ED, Gao Y, Gavsyuk AF, Maor I, DeWalt GJ, et al. 2019. Rapid Changes in Synaptic Strength After Mild Traumatic Brain Injury. Front Cell Neurosci 13: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DW, Bauer ME, Hoffman SW, Stein DG. 2001. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma 18: 901–9 [DOI] [PubMed] [Google Scholar]
- Yager JY, Thornhill JA. 1997. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci Biobehav Rev 21: 167–74 [DOI] [PubMed] [Google Scholar]
- Yao XL, Liu J, Lee E, Ling GS, McCabe JT. 2005. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J Neurotrauma 22: 656–68 [DOI] [PubMed] [Google Scholar]
- Zhang M, Wu J, Ding H, Wu W, Xiao G. 2017. Progesterone Provides the Pleiotropic Neuroprotective Effect on Traumatic Brain Injury Through the Nrf2/ARE Signaling Pathway. Neurocrit Care 26: 292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Saraswati M, Koehler RC, Robertson C, Kannan S. 2015. A New Rabbit Model of Pediatric Traumatic Brain Injury. J Neurotrauma 32: 1369–79 [DOI] [PMC free article] [PubMed] [Google Scholar]


