Abstract
Hyaluronic acid (HA) is a glycosaminoglycan, a natural component of the extracellular matrix. The identical structure of the molecule in all living organisms is its main advantage, as it translates into the minimal probability of immunogenicity. Therefore, it is the closest to the ideal preparation used as a filler, due to its biocompatibility and stability at the site of implantation. This paper includes the discussion of the potential mechanisms of adverse immune reactions to HA along with the mechanisms of reaction following vaccinations against SARS-CoV-2. Based on the literature, we tried to systematize adverse immune reactions with systemic manifestations to HA. The occurrence of unpredictable reactions to hyaluronic acid indicates that they may not be treated as neutral or non-allergenic. The modifications of the chemical structure of HA, additives and individual tendencies in a patient may be the cause of unpredictable reactions, leading to serious health consequences. Preparations of unknown origin, poorly purified, or including bacterial DNA are particularly dangerous. Therefore, long-lasting follow-up of the patient and the selection of a preparation approved by the FDA or EMA are of high importance. Patients are often unaware of the consequences of cheaper procedures performed by persons without suitable knowledge with the use of unregistered products, so the public should be educated and legal regulations should be introduced.
Keywords: hyaluronic acid, fillers, delayed inflammatory reactions, autoimmune/autoinflammatory syndrome induced by adjuvants, SARS-CoV-2
Introduction
Hyaluronic acid (HA) is a glycosaminoglycan, a natural component of the extracellular matrix. It is produced and released to the surrounding extracellular space by dermal fibroblasts, synoviocytes, endothelial cells, smooth muscle cells, adventitial cells and oocytes.1,2 The identical structure of the molecule in all living organisms is its main advantage, which is associated with the minimal risk of immunogenicity. Biocompatibility and stability at the site of implantation make it an almost ideal choice in the whole family of fillers. It has a significant advantage of being able to stimulate neocollagenogenesis as a result of the mechanical expansion of tissues after an injection, and the subsequent activation of dermal fibroblasts.2–4 Hyaluronic acid is highly hydrophilic with the exceptional properties of binding water molecules (over 1000 times its own weight), forming extended conformations whose volumes are enormous compared to the weight, and forming gel even at very low concentrations. It leads to the rapid hydration of tissues and increased skin turgor.3,5,6 Moreover, skin moisturizing and the antioxidant potential of HA promote skin cell regeneration and stimulate the production of collagen.5
A continuous increase in the popularity of esthetic procedures with the use of such substances as HA has been observed for many years. According to the International Society of Aesthetic Plastic Surgery (ISAPS) over 4.3 million esthetic procedures were performed with the use of HA in 2019, which constituted a 15.7% increase compared to 2018.7 The American Society for Dermatologic Surgery (ASDS) reported that dermatologic surgeons performed 2.7 million dermal filler injections in 2019.8 Performing such procedures is becoming a very profitable form of a gainful activity. Therefore, an increasing number of people offer this kind of services, commonly without adequate training or qualifications due to the lack of legal regulations in many countries. Moreover, competitive preparations are available on the market. They may be cheaper and poorer quality, not approved by the FDA or EMA, which is a risk factor for developing new types of adverse reactions. According to research conducted in Belgium, the majority of 14 tested suspicious illegal samples included considerably less product than specified on the packaging.9 The grey area of illicit esthetic procedures is present in many countries. Moreover, the procedures are not registered and taxes due are not paid.
Therefore, there are many reports in the literature concerning adverse events which often contribute to considerable diagnostic and therapeutic problems and unpredictable consequences in patients.7,8 It is particularly important as regards the hypersensitivity to hyaluronic acid. The pathogenesis of some reactions has not been fully elucidated, so the terminology is not uniform in the literature and numerous consensuses concerning the management of complications have not included such reactions yet.10,11
This paper includes data from the overview of the literature. Evaluated articles were identified by searching PubMed using the following phrases: Hyaluronic acid, fillers and adverse effect. The search had been conducted till the 30th of March 2021. One hundred and five articles were found, and 42 of them were analyzed.
Factors Influencing the Immunogenicity of HA Products
Hyaluronic acid is not organ- or species-specific, so it might be assumed that it would not cause allergic reactions.12 However, it needs to be remembered that the injected product also includes additives and HA is received via bacterial biosynthesis.
Individual tendencies were also demonstrated that contributed to the risk of late-onset, immune-mediated, adverse reactions related to dermal fillers in patients bearing HLA-B*08 and DR1*03 haplotypes. Such a combination of HLA subtypes was related to an almost fourfold increase in the probability of developing adverse reactions (OR 3.79).13
Size of the Molecule
Hyaluronic acid exists in the form of multiparticulates which are simply designed, but versatile biological molecules. The size of HA influences opposing actions: it may have pro- or anti-inflammatory properties, promote or inhibit cell migration, activate or stop cell division and differentiation.14–16 Regrettably, no uniform consensus has been reached on the division of HA in terms of the molecular size.14,16,17
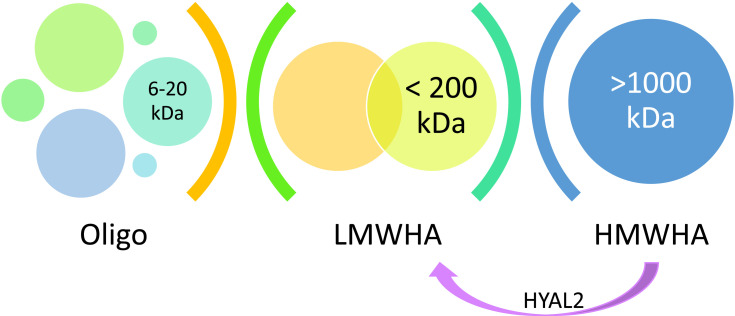
When administering an HMW-HA product, it is worth remembering that natural hyaluronidases trigger its degradation and contribute to LMW-HA formation. HYAL2 (anchored on the cell membrane) cleaves high-molecular-weight HA (>1 MDa) into 20 kDa fragments. Furthermore, if HA hypersensitivity starts, an inflammation promotes its further degradation16 (Figure 1).
Figure 1.
The immune activity of LMW-HA and HMW-HA.16
Some discrepancy may be noted in the definition of molecule size in case of HA products, eg, as regards a group of Juvederm products (Allergan) molecules >500 kDa are considered as LMW-HA, while >5000 kDa – HMW-HA. It influences the improvement of the safety profile of products.18
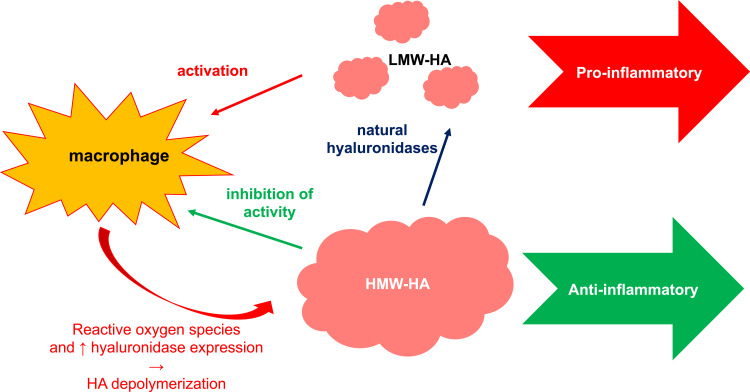
In some situations low-molecular-weight (LMW) HA may induce hypersensitivity14 (Figure 2). It is considered as a proinflammatory molecule. It is abundant at sites of active tissue catabolism, eg, after an injury, initiating an inflammation via the influence on Toll-like receptors (TLR2, TLR4).14–16,19 In this way, LMW-HA promotes the activation and maturation of dendritic cells (DC), stimulates the production of proinflammatory cytokines such as IL-1β, IL-6, IL-12, TNF-α, and TGF-β by various types of cells, manages the expression of chemokines and migration of cells.14,17,20 LMW-HA may act as danger-associated molecular patterns (DAMPs) initiating the mechanisms of innate immunity, similarly to bacterial proteins or heat-shock proteins.14,21 CD44 acts as a receptor for LMW-HA as a form of pattern recognition. It is present on the surface of all human cells and may interact with other ligands, such as osteopontin, collagen and matrix metalloproteinases (MMP).14,16,17
Figure 2.
Division of HA according to molecule size.18
After the regression of an inflammation and eliminating the remnants of injured tissues by macrophages, LMW-HA molecules are removed via CD44-dependent endocytosis. Conversely, chronic inflammations are associated with an increased amount of LMW-HA, so they may be considered as a natural biosensor of tissue integrity status.14,20,22,23 The role of CD44 receptor of HA was demonstrated in research on the regulation of inflammation under in vivo conditions. Anti-CD44 treatment inhibited the development of such conditions as collagen-induced arthritis or dermal lesions in the murine model of atopic dermatitis.24
High-molecular-weight (HMW) HA is common in intact tissues. It inhibits the production of proinflammatory mediators (IL-1β, IL-8, IL-17, TNF-α, metalloproteinases), reduces TLR expression and regulates angiogenesis.14,19 HMW-HA also influences the function of macrophages responsible for the regulation of local inflammatory response by stimulating their anti-inflammatory activity.15,24,25
Additives to HA Products
The total amount of hyaluronic acid in a person weighing 70 kg is ~15 g, and its average turnover rate is 5 g/day. About 50% of the total amount of hyaluronic acid in the human body is concentrated in the skin. Its half-life is 24–48 hours.22,26 Therefore, unmodified natural HA has the half-life of only about 12 hours before it is rapidly cleaved by hyaluronidases, natural tissue enzymes and reactive oxygen species.27,28 The crosslinking of HA chains was developed to prolong its stability and produce larger, more stable molecules with prolonged residue time in tissues (about several months) and with similar biocompatibility and viscoelastic filling properties.28 Crosslinking involves the combination of a higher proportion of low-molecular-weight HA and a lower proportion of high-molecular-weight HA. Such a modification changes the natural conformation of HA molecules and may influence its immunogenicity.18
Crosslinking mainly involves polymer crosslinking with the formation of covalent bonds, predominantly including (–COOH) and/or hydroxyl (–OH) skeleton. Crosslinking may be facilitated by some compounds, eg, 1,4-butanediol diglycidyl ether (BDDE) (Juvederm, Restylane, Princess), divinyl sulphone (Captique, Hylaform, Prevelle), or diepoxyoctane (Puragen).29 Nevertheless, BDDE epoxide groups are neutralized following a reaction with HA, so only trace amounts of unreacted BDDE may be found in the product (<2 parts per million).26 Crosslinked HA hydrogel is an adaptable material which leads to the formation of a 3D structure with unique properties (rheology, degradation, fitness for purpose). Such features facilitate the easy distribution of the product with the stimulating effect of the production of molecular components of the extracellular matrix.30,31
In order to increase the hydrophilic properties of the product, some manufacturers add other compounds such as, dextran or mannitol. Each of those additives may become a possible antigen stimulating the immune response.
The Technology of HA Production
Currently, HA preparations are produced by bacterial fermentation from specific strains of Streptococci spp. (Streptococcus equi or Streptococcus zooepidemicus). It reduces the risk of immunogenicity compared to previously used animal-origin preparations, but it does not eliminate the contamination with the molecules of proteins, bacterial nucleic acid and stabilizers. They may become antigens and stimulate the immune response of the host as hypersensitivity to HA products. Therefore, the technology of filler production (eg, Restylane) focused on the reduction of product contamination.32
Biofilm Components
According to another hypothesis, immune response to HA is due to an inflammation triggered by the components of bacterial biofilm which are transported into tissues at the moment of injecting the product.33,34 Biofilm is composed of bacteria, their nutrients and the products of metabolism. It mainly encompasses primarily nonpathogenic species that colonize the healthy skin or mucous membranes (eg, Cutibacterium acnes, Streptococcus oralis, Staphylococcus epidermidis), which was confirmed via polymerase chain reaction tests.33–35
It is frequently difficult to confirm the infective agent in cultures due to their distinct slow-growing character and the fact that they are known as variants of small colonies. Moreover, their metabolism may be decelerated in the biofilm, which facilitates the avoidance of antibiotic influence.35,36 Furthermore, the ability to form the extracellular matrix of exopolysaccharides, which also includes HA, is a preventive factor in terms of phagocytosis. Such bacteria may remain dormant for many years and then be activated by external factors and provoke a response.35–37 Macrophages and giant cells are usually present in the vicinity of those microorganisms. They may be rapidly activated and induce inflammatory reactions.38 Some factors, like bacterial infections with bacterial strains similar to biofilm components, may activate the dormant microorganisms via the mechanism of mimicry. The activation may be due to an injury caused by another procedure of skin filling.38
It is difficult to differentiate between an inflammation triggered by bacterial biofilm and delayed-onset hypersensitivity. If a red indurated lesion appears at any moment after the procedure, regardless of the duration, biofilm should instantly be suspected.38 It may be both asymmetrical and symmetrical, and may sometimes affect all locations at which HA was administered during the procedure. A broad-spectrum antibiotic with good penetration into the skin should be used, even if the culture yields a negative result. If an antibiotic-resistant increasingly fibrous nodule occurs, it is most probably a foreign body granuloma.
The Mechanism of Superantigens
HA may also stimulate inflammatory response via the mechanism of superantigens. Such a reaction does not require the primary phase of inflammation.12,39 Superantigens trigger clonal activation to 40% of naïve T cells and, probably, NKT. The activation of those lymphocytes leads to cytokine storm characterized by the release of large amounts of proinflammatory cytokines, such as IL-1β, IL-2, IL-6 and TNF-α.40
Severe pneumonia frequently accompanied by severe respiratory failure is an example of a pathological reaction to bacterial superantigen (Staphylococcal enterotoxin B) which increases LMW-HA production by fibroblasts in the pulmonary tissue. HA stimulates an increased production of IL-8 and IP-10 chemokines which play an important role in the recruitment of inflammatory cells into the lungs.40,41 A similar mechanism is observed in asthma, chronic obstructive pulmonary disease and pneumonia in the course of COVID-19.41 The increased production of LMW-HA leads to the hyperstimulation of CD44 and proinflammatory cytokine and chemokine release.40 Such a mechanism may also be observed in inflammation triggered by the components of biofilm.
Types of Hyaluronic Acid Hypersensitivity
The risk of delayed reactions after HA injection was determined at 0.7% before 1999 when the technologies of filler production were not so precise. After the introduction of highly purified products, the occurrence of such adverse events decreased to 0.02%.3,42,43 However, the introduction of HA fillers combining high and low HA chains contributed to a higher percentage of AE.44
The first data concerning such reactions were presented in a report concerning the use of NASHA. It was an erythematous-edematous reaction with the infiltration and edema of the surrounding area lasting for up to 15 days. The reaction was observed in 1 out of 1400 patients.3 Other authors reported inflammatory nodules which persisted for longer periods and occurred in 0.8% of the patients.45 They emphasized the etiology associated with protein contamination due to the process of bacterial fermentation. According to the literature, the frequency of adverse reactions was 0.15–0.42%.3,6,43
Numerous attempts at the classification of adverse reactions of HA are available with the criterion of time being applied.46
Bitterman-Deutsch et al classified the causes of adverse reactions and complications occurring after procedures performed with the use of preparations based on hyaluronic acid. They included
the quality of the product (not registered by the FDA, EMA),
inappropriate technique of the procedure,
individual immune sensitivity of the patient.12
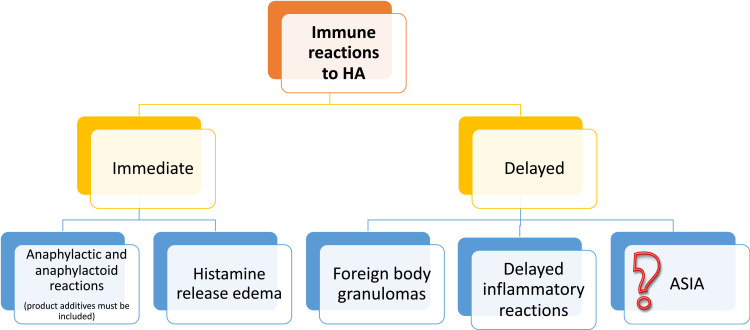
Groups of experts attempted at defining reactions to hyaluronic acid according to the time of appearance post-procedure: “early” (<14 days), “late” (>14 days to 1 year) or “delayed” (>1 year).47–49 Other authors classified the reactions as early (up to a week), intermediate (duration: from a week to a month) and late (over a month).50 Currently, late and delayed reactions are considered as one entity named delayed inflammatory reactions (DIR), as their cause is usually not well defined and the treatment is independent from the etiology.42 The classification of those reactions may be proposed basing on the literature (Figure 3).
Figure 3.
Types of hypersensitivity to HA.
Immediate Reactions
Histamine Release Edema
Transient edema occurring at the injection site immediately after the procedure may be due to the mechanism of histamine release in patients predisposed to type 1 allergic reactions, particularly in individuals with a history of dermographism.51 It manifests only a few minutes after the administration as a result of a mechanical injury to mastocytes and after they release proinflammatory mediators leading to tissue edema and the formation of a wheal. A course of antihistamine treatment is usually sufficient in case of reactions with the participation of mastocytes.51
The larger the skin injury due to an esthetic procedure, the larger the edema which may even develop to 10–50%.52 The frequency of edema occurring after Restylane injections was estimated at 87% based on patient diaries in a randomized double-blind multicenter study.52,53
The areas of the face which seem particularly prone to the development of edema are lips, periorbital and buccal area.52 In order to reduce the risk it is recommended to avoid administering large amounts of fillers, infiltration anesthesia, aggressive massage and preparations with strongly hygroscopic additives (mannitol, dextran).52
Edema at the injection site lasting from several minutes to 2–3 days may be caused by the hygroscopic properties of HA. Such a reaction is usually observed in the perilabial and periorbital area.49,54 It should not be mistaken for edema triggered by the mechanism of immediate allergic reaction (angioedema) which is very rare.49
Type I Hypersensitivity Reactions (Angioedema)
A case of hypersensitivity to angioedema was described after the administration of Restylane (NASHA) into the upper lip. However, the patient was also administered 2% lignocaine which may also trigger type I hypersensitivity reactions. The systemic administration of corticosteroids made the edema regress within 4 days.32
A rapidly developing reaction may be due to the hypersensitivity to residual contamination with the proteins of HA-synthesizing bacteria. An interaction between injected HA and residual mastocytes in tissues is another mechanism clarifying the phenomenon of an immediate reaction. The CD44 receptor on the surface of mastocytes is a receptor for HA and such an interaction may be significant in their migration.32,55
Treatment involves the immediate administration of antihistamine drugs, systemic GCS, or adrenaline.46
Early Reactions
The first reports were published by Turkmani et al and described women aged 22–65 who had undergone procedures with HA manufactured by various companies.39 Lesions presented as erythema and painful edema of the face at the sites of injected fillers. In all cases the reactions started 3–5 days after a flu-like disease (fever, headache, sore throat, cough and fatigue). Moreover, all patients had already undergone HA administration (2 to 6 times) over the period of 4 years prior to developing symptoms at various locations on the face.39
The clinical presentation of the described reactions (erythematous-edematous or urticaria-like exanthem accompanied by systemic manifestations) resemble type III reaction – a pseudo serum sickness reaction. Regrettably, reports to confirm this hypothesis are unavailable in the literature. A case report included a description of a patient with lesions resembling exanthem in the course of Sweet syndrome as a sign of pathergy which developed after 24–48 hours at the site of HA administration.56
Some authors suggested that the mechanism of reaction was due to type IV hypersensitivity. Previous HA injections had provoked the formation of memory lymphocytes, and the subsequent administration of the preparation rapidly triggered the response of CD4+ cells and macrophages.39
The patients were treated with oral prednisolone at a dose of 20–30 mg or methylprednisolone at a dose of 16–24 mg daily for 5 days. The dose was then reduced for another 5 days. After 2 weeks, a complete resolution of symptoms was reported in 10 patients treated with oral steroids. Minor edema persisted in the remaining four patients. It was treated with hyaluronidase for a month after the development of symptoms.39
Delayed Reactions
Delayed Inflammatory Reactions (DIR)
According to the literature, numerous delayed complications may occur after HA injections. However, each author classified them basing on the clinical experience. No uniform terminology or classification has been developed to describe such adverse reactions. The term Persistent Intermittent Delayed Swelling (PIDS) was defined by Brazilian dermatologists in 2017.57 Another term to describe this pathology was introduced by Beleznay et al in 2015: Delayed-Onset Nodules15,58 and by Snozzi et al: Late Inflammatory Response Syndrome (LIRS).58 Another term was proposed in 2020: Delayed Inflammatory Reactions (DIR).48
Chung et al emphasized that DIR included four types of reactions: 1) DTH reactions (correctly called: delayed type IV hypersensitivity); 2) foreign body granulomatous reactions; 3) biofilm; and 4) atypical infections. A DTH reaction is a delayed cellular immune inflammation, which developed in response to an allergen.59
Epidemiology
Basing on different sources it may be stated that the frequency of such a reaction is variable. A paper authored by researchers from Israel has recently been published. Basing on a questionnaire they assessed the number of adverse events in the form of DIR. The questionnaire was completed by 334 physicians performing HA injections. It revealed that almost half of them had not diagnosed DIR, while 11.4% of them responded that they had observed such a reaction over 5 times.48 Reactions triggered by products manufactured by Allergan were very well documented during registration trials conducted to assess the safety. A similar reaction was reported in about 1% of 103 patients monitored for 24 months after the administration of Juvederm Voluma®.60 A similar morphology of the reaction was observed in 0.5% of the patients during a 68-month retrospective review of 4702 procedures performed with the use of Juvederm Voluma® in 2342 patients.15 A higher percentage was observed with the use of Juvederm Volbella® product which was administered in the areas of the tear trough and lips. Recurrent reactions (3.17 episodes on average) lasting up to 11 months occurred in 4.25% (n=17) after the average of 8 weeks.42 The most recent analysis of the 2-year follow-up of over a thousand patients treated with Vycross fillers revealed the occurrence of delayed nodules in 1%.57 Chung et al was very critical as regards the frequency of reported reactions. The occurrence of delayed inflammatory reactions calculated on the basis of prospective research was 1.1% annually, while in retrospective research it was below 1% in the period from 1 to 5.5 years. Not all reported cases were actually DIRs, because no precise definition was developed.59
Pathomechanism
Delayed inflammatory reactions (DIR) secondary to tissue filler administration develop after at least 2–4 weeks or later following an HA injection.42 The clinical manifestations occur in the form of recurrent episodes of localized solid edema with erythema and tenderness, or in the form of subcutaneous nodules at the site of HA injection.42,48 The nodules may be warm to touch and the surrounding skin may be purple or brownish. The occurrence of the reaction in the majority of patients at all sites at the same time, also in cases of previous HA administration, regardless of the type of a filler or the number of injections is a significant element of the clinical picture of the reaction.15,39 The lesions were more common in persons who had previously been injected with a larger volume of HA.43 Additionally, the accompanying edema is the most visible after awakening and slightly improves throughout the day.42,44,57 Some patients (~40%) developed accompanying systemic flu-like manifestations.15
It is possible that those reactions may be related to contamination with DNA, proteins, bacterial endotoxins, even at much lower concentrations than HA.15 However, LMW-HA may also act as an adjuvant in a direct manner or via related infectious molecules (biofilms) in genetically predisposed individuals.15,44 However, the development of inflammatory nodules in areas located at some distance from the site of injection, resistance of the lesion to long-lasting antibiotic treatment and the exclusion of an infectious agent (cultures and PCR test) raise doubts about the role of biofilm. Moreover, the effectiveness of treatment with hyaluronidase and the dependence on the volume of HA administered suggest the mechanism of delayed hypersensitivity.42,44
The initiation of the reaction occurring as a result of an infection or injury leads to the increase in serum interferon, which may exacerbate previously present inflammations.15,57,61 Furthermore, LMW-HA stimulates CD44 or TLR4 receptors on the surface of macrophages and dendritic cells. It activates them and delivers co-stimulatory signals to T cells.15,19,24 DIR-related inflammatory nodular lesions develop in the period between 3 and 5 months after the injection of HMW-HA filler (with anti-inflammatory properties), which is then disintegrated and converted into LMW-HA with proinflammatory properties.15
The onset of a reaction is most commonly triggered by another infectious process (sinusitis, urinary tract infection, respiratory infection, dental infection), facial injury and dental procedures.57 The response was also provoked by a vaccination and recurred with menstrual bleeding.15,57 Each episode was probably due to an infectious triggering factor.
Some authors also described a genetic predisposition underlying the reaction in individuals with the following subtypes: HLA B * 08 or DRB1 * 03.4 (a fourfold increase in the risk).13,62
Diagnostics
DIR-related lesions are of inflammatory nodular character. They should be differentiated with nodules caused by biofilm, abscesses (softening, fluctuance), granulomatous reactions (hard, inflammatory nodules).58
Chung et al proposed the performance of a skin test with an HA product prior to the planned procedure, although the time necessary to interpret test results would even be 3–4 weeks.59 They recommended such a test particularly in persons in whom adverse events had been previously noted. If the test renders a positive result, the patient should not be treated with the same HA filler again. However, it may not eliminate all reactions, as they are commonly observed as a result of a trigger factor, eg, a concomitant infection, which may occur at any moment.59
Diagnostic work-up should mainly involve the exclusion of an underlying infection. Therefore, the inflammatory nodule requires an incision and drainage. Its content should be tested for an infection with aerobic and anaerobic bacteria, bacilli and fungi prior to the implementation of any treatment.42,43,63 The use of PCR method is very useful in the diagnostics of bacilli, as they are particularly difficult to culture.43
The histopathological examination reveals the features of granulomatous dermatitis (epithelioid histiocytic granulomas, numerous multinucleated foreign body–type giant cells surrounding amorphous material). Literature revealed the negative results of cultures tested for fungal, bacterial and mycobacterial infections and a negative polymerase chain reaction (PCR) result.43,44,64
Ultrasonography is a good noninvasive diagnostic method. It visualizes the presence of HA associated with the diffuse increase in thickness and the increased echogenicity of the surrounding subcutaneous tissue, similarly to the diffuse inflammation of the subcutaneous tissue corresponding with clinical edema. It also facilitates the assessment of filler density.43,57
In case of adverse reactions, the patient has to undergo laboratory testing including the following parameters: complete blood count, C-reactive protein and erythrocyte sedimentation rate (ESR).39,42 CRP is elevated in over 50% of the cases.15 In case of early and delayed reactions, it is recommended to perform ultrasonography, cultures (aspirates), and biopsy (the tissue also has to be sent to be cultured), preferably prior to introducing any treatment.46
Patch tests with HA-Vycross family were developed in order to confirm DIR. They include HA-Voluma, HA-Volift, 0.25% BDDE in petroleum and 15% lidocaine in petroleum (Chemotechnique MB Diagnostics AB, Vellinge, Sweden). It is also possible to administer an intradermal injection of HA-Voluma and HA-Volift, and assess the result after 20 minutes and after 96 hours and 2 months in case of delayed reactions.65
Treatment
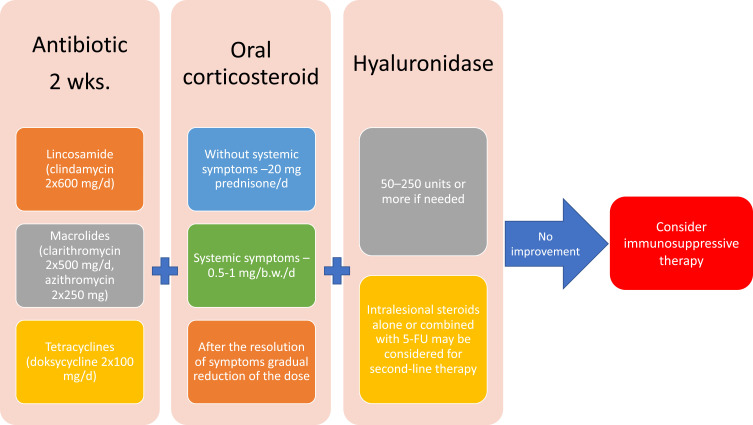
Currently, no uniform consensus on the management in case of HA hypersensitivity is available. The treatment of the complications differs depending on the experience of the physician and developed management procedures.10,42,46,50,58 It is advised to treat the complications with antibiotics, non-steroidal anti-inflammatory drugs, and systemic corticosteroids. Antihistamines are not effective in those reactions because of a different pathomechanism.49,50 The removal of the allergen which stimulates the development of hypersensitivity is the most favorable option to undertake. It may be achieved via the use of hyaluronidase.39,49 Figure 4 shows the present authors’ own algorithm of procedure in case of delayed reactions.
Figure 4.
Management of delayed hypersensitivity due to HA.
It is recommended to introduce systemic antibiotic treatment with intralesional hyaluronidase injections in order to remove the allergen.15,39,57,61,62 Some authors advocated the use of antibiotic treatment beforehand to proceed with HYAL injections.10,39 As regards nodules provoked by biofilm, it is important to remove the filler, so hyaluronidase should be used 24–48 hours after introducing antibiotics. It may be highly effective in breaking down the matrix, thus increasing antibiotic efficiency.58
As regards antibiotics, it is recommended to use oral ciprofloxacin (500–750 mg twice daily) with tetracycline or macrolide for 3–6 weeks.42,48 Other authors recommended treatment with broad-spectrum antibiotics (doxacillin or rifampicin for at least 3 weeks) combined with multiple hyaluronidase injections (30–100 units intralesionally).49,63 However, the use of ciprofloxacin is seen as contributing to severe adverse events (an increased risk of tendinitis and tendon rupture at all ages).66
Various authors proposed the intralesional injections of GCS (triamcinolone acetonide) or oral prednisone.15,57,62 Prednisone was most commonly recommended as oral treatment. The dose was 40 mg/day for 3 days. Subsequently, it was down-titrated. Artizi et al demonstrated that corticosteroids administered orally, intramuscularly and intravenously were less effective than if they were administered intralesionally.43 Alternative methods involved the intralesional administration of 5-FU, radiofrequency, laser therapy or filler evacuation.44,54
The cooperation between Artzi et al and clinicians from 10 countries who administered HA resulted in the development of a therapeutic algorithm of DIR in 2020.42 They proposed that first-line treatment should include 3–6 weeks of fluoroquinolones (ciprofloxacin 2×500 mg) with tetracycline (minocycline 1×100 mg) or macrolides (azithromycin 2×250 mg for 6 days or clarithromycin 2×500 mg). If an improvement was not achieved within 2–3 weeks, they proposed the use of hyaluronidase (30–100 units per nodule). The authors recommended that the treatment should start with 5 units of hyaluronidase. The dose should be doubled in case of more resistant fillers (eg, Vycross family manufactured by Allergan). The recommended dose of hyaluronidase was 10–20 U for a single injection into a site of <2.5 mm, or 2–4 points of injection, 10–20 U each for site sizes of 2.5 mm–1 cm. If necessary, injections might be repeated. Lesions might also be managed by the intralesional administration of triamcinolone acetonide (10 mg/mL) or a 1:1:1 mixture of 5-FU:GCs: normal saline or 1% lignocaine. With resistant processes, it was recommended to administer oral GCs at a dose of 0.5–0.75 mg/b.w./day for 7–21 days with gradual reduction. If such management did not lead to an improvement, it was necessary to perform biopsy and collect swabs for culture tests. Subsequent procedures included immunosuppression, laser therapy or the surgical resection of material from the nodule.42
However, this first and very useful algorithm did not explain how to manage DIRs which are associated with systemic, usually flu-like symptoms. From our viewpoint, therapy should involve one-stage administration of antibiotic, systemic GCs and hyaluronidase. Hyaluronidase supports the removal of the antigen which stimulates the reaction whose small amounts may even trigger a response, which is typical of hypersensitivity. The administration of GCs facilitates the suppression of systemic symptoms and prevents the formation of immune memory cells. Delaying GC administration may contribute to the exacerbation of systemic manifestations
It needs to be emphasized that the use of noninvasive ultrasonography allows for the precise determination of lesion location for the effective administration of hyaluronidase. It accelerates the removal of the product which triggered the inflammatory reaction and reduces the amount of hyaluronidase used, which may also cause allergic reactions.
Foreign Body Reaction
All injected implants induce the inflow of neutrophils and mononuclear cells with phagocytosis by concentrated macrophages and fibroblast activation, followed by collagen deposition. It is not only a physiological reaction but also a beneficial phenomenon, as it maintains the injected product in a proper location. Such a reaction occurs due to the fact that the immune system is unable to perform the enzymatic degradation or phagocytosis of a foreign body. In case of HA, the process of molecule crosslinking increases its size which makes phagocytosis impossible and stimulates chronic cellular response.1,33,49 Foreign body granulomas are not a typical allergic reaction, but they are caused by a sudden stimulation of macrophage memory.62 The reasons for the development of severe inflammatory granulomatous processes have not been precisely elucidated.49
It is not fully known why such a reaction only occurs in some patients (from 0.01% to 1.0%) and usually develops after 6–24 months at all injection sites at the same time.49,65
The incidence of foreign body granuloma following HA filler injection ranges from 0.02% to 0.4%.1,67,68 Clinical lesions are characterized by solid erythematous papules or nodules, which developed via fibrosis. The foreign body is typically surrounded by multinuclear giant cells with lymphohistiocytic infiltration.1,67,68 A rare form presenting as cellulitis was also described.33 An infectious etiology should be ruled out in case of fluctuance.3
The administration of hyaluronidase significantly improves the clinical status, which was demonstrated in clinical research.69 Moreover, intralesional GC injections may be used. 5-Fluorouracil or laser therapy may be implemented in case of resistance.1,49,65 Despite the risk of skin atrophy, the initial dose of triamcinolone should be high (40 mg/mL), but it is important that it should be administered into the tissue of the nodule and not beneath. If necessary, the injections should be repeated every 3–4 weeks. Seemingly, starting the treatment of granulomas with low doses of triamcinolone (5 and 10 mg/mL) leads to their resistance and is associated with a risk of relapse.65 Surgical resection is the treatment of choice in case of the unsuccessful treatment of foreign body granuloma with other methods.49,65
Autoimmune/Autoinflammatory Syndrome Induced by Adjuvants (ASIA) (Shoenfeld’s Syndrome)
Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) is a spectrum of immune disorders triggered by chronic exposure to adjuvants, ie, substances strengthening antigen-specific immune response.70 Adjuvant substances include aluminium hydroxide in vaccines (eg, HBV, influenza), foreign bodies (eg, silicone, hyaluronic acid, methacrylate, polylactic acid, paraffin, metal implants), microorganisms (EBV) or toxic substances (eg, mercury, crude oil), which are omnipresent. However, the occurrence of ASIA is due to genetic predispositions (HLA-DRB1 polymorphism).70,71 Regrettably, the actual adjuvant substance may remain unidentified in some patients.
The pathogenesis is associated with the activation of the innate immune pattern recognition receptor by the adjuvant. As an adjuvant, HA strengthens antigen-specific immune response, induces the release of inflammatory cytokines and interacts with Toll-like receptors and inflammasome. The reaction leads to the stimulation of innate and adaptive immune response (polyclonal B cell activation, influence on cellular immunity) and may trigger the symptoms of autoimmunization or an autoimmune disease.70
Reaction to silicone is the best elucidated ASIA reaction. Silicone had been considered as a neutral substance for a long time. Regrettably, numerous cases of autoimmune reactions were noted due to the presence of silicone implants. They mostly presented as undifferentiated disorders of the connective tissue, but also as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), vasculitis and systemic sclerosis. Increased levels of profibrotic cytokines and anti-silicone and anti-collagen antibodies were identified.70,72
The group of symptoms is varied in individual patients and the symptoms are not highly specific. Therefore, it was assumed that the diagnosis of ASIA might be made if the patient met at least two major or one major and two minor criteria (Table 1).
Table 1.
| Major Criteria | Minor Criteria |
|---|---|
| → Myalgia, myositis or muscle weakness Arthralgia and/or arthritis → Chronic fatigue, sleep disturbances → Neurological manifestations (particularly associated with demyelination) → Cognitive impairment, memory loss → Fever, xerostomia → The removal of the inducing factor leads to an improvement → Histopathological confirmation of affected organs |
→ The presence of autoantibodies or antibodies directed at the specific adjuvant or RF, ANCA, ANA, anti-Sm, anti-RNP, anti-SSA/Ro, anti-SSB/antithyroid → Other clinical manifestations (eg, related to irritable bowel disease) → Specific HLA (ie, HLA DRB1, HLA DQB1) → The development of autoimmune diseases (SLE, MS, Sjögren’s syndrome, autoimmune thyroiditis, autoimmune hepatitis, etc) |
The risk of developing ASIA is higher in individuals with a history of post-vaccination reactions, individual tendency towards autoimmunization (concomitant diseases), a history of severe allergic reactions and a family history of autoimmune diseases.71 The possibility of developing ASIA should also be considered in patients with arthralgia, myalgia, chronic fatigue, sleep disturbances, neurological/cognitive disorders and unexplained respiratory and cutaneous disorders.71
Adverse Reactions to Hyaluronic Fillers and the SARS-CoV-2 Epidemic
The SARS-CoV-2 epidemic affected numerous medical aspects. Some reports were published on the issue of inflammatory reactions at the sites of previous filler administration after a vaccination and after a COVID-19 infection. The occurrence of delayed reactions, both at injection sites and distant from vaccine administration sites, was described by Blumenthal et al. The histopathological skin examination of the site of a delayed reaction revealed superficial perivascular and perifollicular lymphocytic infiltration with the presence of eosinophils and diffuse mastocytes.73
The first description was prepared by Munavalli et al and referred to cases of delayed inflammatory reactions (DIR) to HA dermal fillers in women.62 One of those cases occurred after a COVID-19 infection (cheeks, lips and tear trough – Restylane Lift, RestylaneL), one case was noted after the administration of the first dose of a generally available mRNA-1273 vaccine (Moderna, Cambridge MA) (Juvederm® VolumaTM in the tear trough and 1 cm3 of Juvederm® Ultra). Another case was noted after the second dose of BNT162b2 vaccine (Pfizer, New York, NY) (no data concerning filler type), and the fourth case was observed after mRNA-1273 clinical Phase III trial after administering placebo (normal saline).62
Binding and blocking the receptors of angiotensin-converting enzyme 2 (ACE2) by the S (spike) protein of SARS-CoV-2 in order to access the cell is a possible mechanism of DIR development in case of HA fillers associated with COVID-19. The interaction between spike proteins and dermal ACE2 receptors promotes the proinflammatory activation of Th1, which is beneficial for a reaction which is mediated by CD8+ T lymphocytes.62 CD8+ T lymphocytes are an important line of protection against infection. Moreover, they are already present in the infiltration which develops quite quickly around the deposits of HA injected into the skin and subcutaneous tissue.62
In human skin, ACE2 is expressed in the keratinocytes, fibroblasts, dermal vascular endothelium and adipocytes of the subcutaneous tissue where HA filler is deposited.62,74 ACE2 is membrane-bound and soluble. It catalyzes the conversion of proinflammatory angiotensin II into angiotensin metabolites with anti-inflammatory potential.74 The spike proteins of SARS-CoV-2 are irreversibly bound to membrane-bound ACE2 and the accumulation of angiotensin II triggers a proinflammatory reaction (increased TNF-α, IL-6 and IL-8), increases the activity of CD44 glycoprotein which has affinity for LWM-HA with proinflammatory properties.74,75
The patients required the administration of high doses of GCs (even up to 60 mg of prednisone) and hyaluronidase administration. Interestingly, one patient refused to take GCs in order not to weaken the effect of vaccination. Therefore, she received an angiotensin convertase inhibitor (lisinopril 5 mg) in order to reduce proinflammatory angiotensin II. The reactions persisted from several days to weeks.59 The subsequent report published in 2021 by Munavalli et al presented four cases of DIR (Juvederm products) induced by Moderna vaccines.62 The treatment included ACEII inhibitors (lisinopril 5–10 mg), without the necessity of administering potentially immunosuppressive doses of oral corticosteroids.74
Interestingly, the reactions observed so far have only occurred in case of mRNA vaccines including pegylated polyethylene glycol (PEG) (Pfizer BioNTech, Moderna). Such compounds are widely used in medical products (they sustain the content of drugs in systemic fluids by inhibiting their metabolism or protecting the drug from immune degradation), cosmetics and household products (eg, creams and lotions, shampoos, hair dyes, and oral hygiene products) and HA fillers (Neauvia, MatexLab SA, Lugano, CH).76,77 Positive results of patch tests with propylene glycol at the concentrations of 5%, 10% and 20% were demonstrated in data obtained at Mayo Clinic. It was claimed that it might lead to allergic reactions and irritation.78 The compound is present in COVID-19 vaccines, because the introduction of a pegylated nanoparticle surrounding mRNA impairs its enzymatic degradation, increases its water-solubility and, thereby, the bioavailability of lipid nanoparticles.75,79 Despite the fact that PEGs are considered to be compounds with the low potential of triggering allergic reactions, the most commonly described hypersensitivity reactions were IgE-dependent. Moreover, some authors reported cases of anaphylactic shock and complement activation-related pseudoallergy.76,79 Propylene glycol may also induce immediate reactions after an injection. According to the literature, one patient required mechanical ventilation due to respiratory failure which occurred as a result of the self-injection of e-cigarette liquid including propylene glycol.80 However, delayed reactions were also described.81
The Italian Society of Aesthetic Medicine recommended making patients aware of the possibility of developing a reaction and developing a management regimen:
informing the patients before the procedure of HA filler administration about the possible reactions and noting it in the informed consent for the procedure;
avoiding the performance of the procedure directly before a vaccination;
refraining from the performance of the procedure between the administration of the first and second vaccine dose, and for a month after a vaccination;
a history of HA procedures may not be considered as a contraindication against COVID-19 vaccines or any vaccines!82
Conclusions
The occurrence of unpredictable reactions to hyaluronic acid indicates that they may not be treated as neutral or non-allergenic. The modifications of the chemical structure of HA, additives and individual tendencies in a patient may be the cause of unpredictable reactions, leading to serious health consequences. Therefore, original products approved by the FDA or EMA should be used to minimize the risk and the procedures should only be performed by appropriately trained physicians. Patients are often unaware of the consequences of cheaper procedures performed by persons without suitable knowledge with the use of unregistered products, so the public should be educated and legal regulations should be introduced.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Abduljabbar M, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Dermatol Surg. 2016;20(2):100–106. doi: 10.1016/j.jdds.2016.01.001 [DOI] [Google Scholar]
- 2.Alcântara CEP, Noronha MS, Cunha JF, et al. Granulomatous reaction to hyaluronic acid filler material in oral and perioral region: a case report and review of literature. J Cosmet Dermatol. 2018;17(4):578‐583. doi: 10.1111/jocd.12374 [DOI] [PubMed] [Google Scholar]
- 3.Friedman PM, Mafong EA, Kauvar AN, et al. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28(6):491–494. doi: 10.1046/j.1524-4725.2002.01251.x [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163. doi: 10.1001/archderm.143.2.155 [DOI] [PubMed] [Google Scholar]
- 5.Bukhari SNA, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120(120):1682–1695. doi: 10.1016/j.ijbiomac.2018.09.188 [DOI] [PubMed] [Google Scholar]
- 6.Matarasso SL, Herwick R. Hypersensitivity reaction to nonanimal stabilized hyaluronic acid. J Am Acad Dermatol. 2006;55(1):128‐131. doi: 10.1016/j.jaad.2006.02.039 [DOI] [PubMed] [Google Scholar]
- 7.International Society of Aesthetic Plastic Surgery. 2019. Available from: https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf. Accessed July1, 2021.
- 8.The American Society for Dermatologic Surgery. ASDS survey. Available from: https://www.asds.net/_Media.aspx?id=9449. Accessed May17, 2018.
- 9.Vanhee C, Desmedt B, Baudewyns S, et al. Characterization of suspected dermal fillers containing hyaluronic acid. Anal Methods. 2017;9(28):4175. doi: 10.1039/C7AY01130J [DOI] [Google Scholar]
- 10.Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol. 2017;31(7):1088–1095. doi: 10.1111/jdv.14295 [DOI] [PubMed] [Google Scholar]
- 11.Philipp-Dormston WG, Goodman GJ, De Boulle K, et al. Global approaches to the prevention and management of delayed-onset adverse reactions with hyaluronic acid-based fillers. Plast Reconstr Surg Glob Open. 2020;8(4):e2730. doi: 10.1097/GOX.0000000000002730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Rep. 2015;7(1):5851. doi: 10.4081/dr.2015.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decates TS, Velthuis PJ, Schelke LW, et al. Increased risk of late-onset, immune-mediated, adverse reactions related to dermal fillers in patients bearing HLA-B*08 and DRB1*03 haplotypes. Dermatol Ther. 2021;34(1):e14644. doi: 10.1111/dth.14644 [DOI] [PubMed] [Google Scholar]
- 14.Ruppert SM, Hawn TR, Arrigoni A, et al. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol Res. 2014;58(2–3):186–192. doi: 10.1007/s12026-014-8495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929–939. doi: 10.1097/DSS.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 16.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88. [PubMed] [Google Scholar]
- 18.Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125–134. doi: 10.1111/jocd.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mummert ME. Immunologic roles of hyaluronan. Immuno Res. 2005;31(3):189–205. doi: 10.1385/IR:31:3:189 [DOI] [PubMed] [Google Scholar]
- 20.Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286(15):2883–2908. doi: 10.1111/febs.14777 [DOI] [PubMed] [Google Scholar]
- 21.Rowley JE, Amargant F, Zhou LT, et al. Low molecular weight hyaluronan induces an inflammatory response in ovarian stromal cells and impairs gamete development in vitro. Int J Mol Sci. 2020;21(3):1036. doi: 10.3390/ijms21031036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83(7):317–325. doi: 10.1078/0171-9335-00392 [DOI] [PubMed] [Google Scholar]
- 23.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Lee YS, Hahn JH, et al. Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta, Rac1, ROS, and MAPK to exert anti-allergic effect. Mol Immunol. 2008;45(9):2537–2547. doi: 10.1016/j.molimm.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 25.Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1(7):481–493. doi: 10.1021/acsbiomaterials.5b00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39(12):1758–1766. doi: 10.1111/dsu.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antônio CR, Antônio JR, Coura MGG, et al. Microcânulas em dermatologia: especificações. Surg Cosmet Dermatol. 2015;07:241–244. [Google Scholar]
- 28.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19(3):141–150. doi: 10.1111/j.1529-8019.2006.00068.x [DOI] [PubMed] [Google Scholar]
- 29.Allemann IB, Baumann L. Hyaluronic acid gel (Juvéderm™) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3:629–634. doi: 10.2147/CIA.S3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zerbinati N, D’Este E, Farina A, et al. Morphological evidences following pegylated filler treatment in human skin. J Biol Regul Homeost Agents. 2017;31(2Suppl. 2):79–85. [PubMed] [Google Scholar]
- 31.Monticelli D, Martina V, Mocchi R, et al. Chemical characterization of hydrogels crosslinked with polyethylene glycol for soft tissue augmentation. Open Access Maced J Med Sci. 2019;7(7):1077–1081. doi: 10.3889/oamjms.2019.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonhardt JM, Lawrence N, Narins RS. Angioedema acute hypersensitivity reaction to injectable hyaluronic acid. Dermatol Surg. 2005;31(5):577‐579. doi: 10.1097/00042728-200505000-00017 [DOI] [PubMed] [Google Scholar]
- 33.Mamelak AJ, Katz TM, Goldberg LH, Graves JJ, Kaye VN, Friedman PM. Foreign body reaction to hyaluronic acid filler injection: in search of an etiology. Dermatol Surg. 2009;35(Supplement 2):1701e3. doi: 10.1111/j.1524-4725.2009.01350.x [DOI] [PubMed] [Google Scholar]
- 34.Shin YS, Kwon WJ, Cho EB, et al. A case of cellulitis-like foreign body reaction after hyaluronic acid dermal filler injection. Dermatol Sin. 2018;36(1):46–49. doi: 10.1016/j.dsi.2017.06.004 [DOI] [Google Scholar]
- 35.Beer K, Kaufman-Janette J, Bank D, et al. Safe and effective chin augmentation with the hyaluronic acid injectable filler, VYC-20L. Dermatol Surg. 2021;47(1):80–85. doi: 10.1097/DSS.0000000000002795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constantine RS, Constantine FC, Rohrich RJ. The everchanging role of biofilms in plastic surgery. Plast Reconstr Surg. 2014;133(6):865e–872e. doi: 10.1097/PRS.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 37.Sadashivaih A, Mysore V. Biofilms: their role in dermal fllers. J Cutan Aesthet Surg. 2010;3(1):20–22. doi: 10.4103/0974-2077.63257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;12(6):295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turkmani MG, De Boulle K, Philipp-Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin Cosmet Investig Dermatol. 2019;29(12):277–283. doi: 10.2147/CCID.S198081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchakina ON, Castillejo CM, Bridges CC, McKallip RJ. The role of hyaluronic acid in SEB-induced acute lung inflammation. Clin Immunol. 2013;146(1):56–69. doi: 10.1016/j.clim.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 41.Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF. Interplay of extracellular matrix and leukocytes in lung inflammation. Cell Immunol. 2017;312:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Artzi O, Cohen JL, Dover JS, et al. Delayed Inflammatory Reactions to hyaluronic acid fillers: a literature review and proposed treatment algorithm. Clin Cosmet Investig Dermatol. 2020;13:371–378. doi: 10.2147/CCID.S247171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Artzi O, Loizides C, Verner I, Landau M. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg. 2016;42(1):31–37. doi: 10.1097/DSS.0000000000000562 [DOI] [PubMed] [Google Scholar]
- 44.Sadeghpour M, Quatrano NA, Bonati LM, et al. Delayed-onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45(8):1085–1094. doi: 10.1097/DSS.0000000000001814 [DOI] [PubMed] [Google Scholar]
- 45.Lowe NJ, Maxwell CA, Lowe P, Duick MG, Shah K. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001;45(6):930–933. doi: 10.1067/mjd.2001.117381 [DOI] [PubMed] [Google Scholar]
- 46.Trindade de Almeida A, Banegas R, Boggio R, et al. Diagnosis and treatment of hyaluronic acid adverse events: latin American ex- pert panel consensus recommendations. Surg Cosmet Dermatol. 2017;9(3):204–213. [Google Scholar]
- 47.Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open. 2019;7(6):e2172. doi: 10.1097/GOX.0000000000002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shalmon D, Cohen JL, Landau M, Verner I, Sprecher E, Artzi O. Management patterns of delayed inflammatory reactions to hyaluronic acid dermal fillers: an online survey in Israel. Clin Cosmet Investig Dermatol. 2020;13:345‐349. doi: 10.2147/CCID.S247315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510. doi: 10.1007/s00266-017-1063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikkilineni R, Wipf A, Farah R, Sadick N. New classification schemata of hypersensitivity adverse effects after hyaluronic acid injections: pathophysiology, treatment algorithm, and prevention. Dermatol Surg. 2020;46(11):1404–1409. doi: 10.1097/DSS.0000000000002385 [DOI] [PubMed] [Google Scholar]
- 51.Wei JF, Wei XL, Mo YZ, He SH. Induction of mast cell accumulation, histamine release and skin edema by N49 phospholipase A2. BMC Immunol. 2009;28(10):21. doi: 10.1186/1471-2172-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King M. Management of edema. J Clin Aesth Dermatol. 2017;10:e1–e4. [PMC free article] [PubMed] [Google Scholar]
- 53.Narins RS, Brandt F, Leyden J, et al. A randomized, double- blind, multicentre comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29(6):588–595. doi: 10.1046/j.1524-4725.2003.29150.x [DOI] [PubMed] [Google Scholar]
- 54.Alam M, Gladstone H, Kramer EM, et al.; American Society For Dermatologic Surgery. ASDS guidelines of care: injectable fillers. Dermatol Surg. 2008;34(Suppl 1):S115–1148. doi: 10.1111/j.1524-4725.2008.34253.x [DOI] [PubMed] [Google Scholar]
- 55.Wagner RD, Fakhro A, Cox JA, Izaddoost SA. Etiology, prevention, and management of infectious complications of dermal fillers. Semin Plast Surg. 2016;30(02):83–86. doi: 10.1055/s-0036-1580734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brys AK, Cox SE. Early-onset sweet-like dermatitis after facial hyaluronic acid filler injection. Dermatol Surg. 2020;46(12):1759–1761. doi: 10.1097/DSS.0000000000002215 [DOI] [PubMed] [Google Scholar]
- 57.Aquino Cavallieri F, Klotz de Almeida Balassiano L, Totti de Bastos J, et al. Persistent, intermitent delayed swelling PIDS: late adverse reaction to hyaluronic acid fillers. Surg Cosmet Dermatol. 2017;9(3):218–222. [Google Scholar]
- 58.Snozzi P, van Loghem JAJ. Complication management following rejuvenation procedures with hyaluronic acid fillers-an algorithm-based approach. Plast Reconstr Surg Glob Open. 2018;6(12):e2061. doi: 10.1097/GOX.0000000000002061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung KL, Convery C, Ejikeme I, Ghanem AM. A systematic review of the literature of delayed inflammatory reactions after hyaluronic acid filler injection to estimate the incidence of delayed type hypersensitivity reaction. Aesthet Surg J. 2020;40(5):NP286–NP300. doi: 10.1093/asj/sjz222 [DOI] [PubMed] [Google Scholar]
- 60.Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81–89. doi: 10.2147/CCID.S40581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alijotas-Reig J, Fernandez-Figueras MT, Puig L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43(2):241–258. doi: 10.1016/j.semarthrit.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 62.Munavalli GG, Guthridge R, Knutsen-Larson S, Brodsky A, Matthew E, Landau M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2021;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiang YZ, Pierone G, Al-Niaimi F. Dermal fillers: pathophysiology, prevention and treatment of complications. J Eur Acad Dermatol Venereol. 2017;31(3):405–413. doi: 10.1111/jdv.13977 [DOI] [PubMed] [Google Scholar]
- 64.Pérez-Pérez L, García-Gavín J, Wortsman X, Santos-Briz Á. Delayed adverse subcutaneous reaction to a new family of hyaluronic acid dermal fillers with clinical, ultrasound, and histologic correlation. Dermatol Surg. 2017;43(4):605–608. doi: 10.1097/DSS.0000000000000945 [DOI] [PubMed] [Google Scholar]
- 65.Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006;118(Suppl):92–107. doi: 10.1097/01.prs.0000234672.69287.77 [DOI] [PubMed] [Google Scholar]
- 66.Cipro (ciprophloxacin chloride). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019537s074,020780s032lbl.pdf. Accessed July1, 2021.
- 67.Alsaad SM, Fabi SG, Goldman MP. Granulomatous reaction to hyaluronic acid: a case series and review of the literature. Dermatol Surg. 2012;38(2 Part 1):271e6. doi: 10.1111/j.1524-4725.2011.02214.x [DOI] [PubMed] [Google Scholar]
- 68.Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42(2):232–239. doi: 10.5999/aps.2015.42.2.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rzany B, Becker-Wegerich P, Bachmann F, Erdmann R, Wollina U. Hyaluronidase in the correction of hyaluronic acid-based fillers: a review and a recommendation for use. J Cosmet Dermatol. 2009;8(4):317–323. doi: 10.1111/j.1473-2165.2009.00462.x [DOI] [PubMed] [Google Scholar]
- 70.Watad A, Bragazzi NL, McGonagle D, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: insights from an analysis of 500 cases. Clin Immunol. 2019;203:1–8. doi: 10.1016/j.clim.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 71.Kaczor T. Recognizing Autoimmune/Autoinflammatory Syndrome Induced by Adjuvants (ASIA). Useful knowledge for identifying patients with underlying immune dysregulation. Nat Med J. 2017;9. [Google Scholar]
- 72.Perricone C, Colafrancesco S, Mazor RD, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1–16. doi: 10.1016/j.jaut.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 73.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munavalli GG, Knutsen-Larson S, Lupo MP, Geronemus RG. Oral angiotensin converting enzyme inhibitors for the treatment of delayed inflammatory reaction of dermal hyaluronic acid fillers following COVID-19 vaccination - a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63–68. doi: 10.1016/j.jdcr.2021.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scholzen TE, Ständer S, Riemann H, Brzoska T, Luger TA. Modulation of cutaneous inflammation by angiotensin-converting enzyme. J Immunol. 2003;170(7):3866–3873. doi: 10.4049/jimmunol.170.7.3866 [DOI] [PubMed] [Google Scholar]
- 76.Turner PJ, Ansotegui IJ, Campbell DE, et al.; Anaphylaxis Committee. COVID-19 vaccine-associated anaphylaxis: a statement of the world allergy organization anaphylaxis committee. World Allergy Organ J. 2021;14(2):100517. doi: 10.1016/j.waojou.2021.100517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zerbinati N, Esposito C, Cipolla G, et al. Chemical and mechanical characterization of hyaluronic acid hydrogel cross-linked with polyethylene glycol and its use in dermatology. Dermatol Ther. 2020;33(4):e13747. doi: 10.1111/dth.13747 [DOI] [PubMed] [Google Scholar]
- 78.Lalla SC, Nguyen H, Chaudhry H, et al. Patch testing to propylene glycol: the Mayo Clinic experience. Dermatitis. 2018;29(4):200–205. doi: 10.1097/DER.0000000000000393 [DOI] [PubMed] [Google Scholar]
- 79.Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9(3):221. doi: 10.3390/vaccines9030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belkoniene M, Socquet J, Njemba-Freiburghaus D, Pellaton C. Near fatal intoxication by nicotine and propylene glycol injection: a case report of an e-liquid poisoning. BMC Pharmacol Toxicol. 2019;20(1):28. doi: 10.1186/s40360-019-0296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caballero ML, Quirce S. Delayed hypersensitivity reactions caused by drug excipients: a literature review. J Investig Allergol Clin Immunol. 2020;30(6):400–408. doi: 10.18176/jiaci.0562 [DOI] [PubMed] [Google Scholar]
- 82.Collegio Italiana. Collegio Italiano delle Società Scientifiche di Medicina Estetica. Available from: https://www.societamedicinaestetica.it/post/vaccino-covid-19-moderna-filler-nessuna-controindicazione-se-si-adottano-pratiche-prudenziali. Accessed July1, 2021.