Abstract
Soil organic carbon (SOC) plays critical roles in stabilizing atmospheric CO2 concentration, but the mechanistic controls on the amount and distribution of SOC on global scales are not well understood. In turn, this has hampered the ability to model global C budgets and to find measures to mitigate climate change. Here, based on the data from a large field survey campaign with 2600 plots across China's forest ecosystems and a global collection of published data from forested land, we find that a low litter carbon-to-nitrogen ratio (C/N) and high wetness index (P/PET, precipitation-to-potential-evapotranspiration ratio) are the two factors that promote SOC accumulation, with only minor contributions of litter quantity and soil texture. The field survey data demonstrated that high plant diversity decreased litter C/N and thus indirectly promoted SOC accumulation by increasing the litter quality. We conclude that any changes in plant-community composition, plant-species richness and environmental factors that can reduce the litter C/N ratio, or climatic changes that increase wetness index, may promote SOC accumulation. The study provides a guideline for modeling the carbon cycle of various ecosystem scales and formulates the principle for land-based actions for mitigating the rising atmospheric CO2 concentration.
Keywords: litter carbon-to-nitrogen, wetness index, annual litterfall, soil texture, soil organic carbon
INTRODUCTION
Promoting soil organic carbon (SOC) accumulation is considered a large-scale land-based mitigation option after the Paris Agreement on climate [1,2]. General understanding on the drivers of SOC accumulation is the prerequisite for programming the land-based mitigation actions [3,4] and modeling carbon cycling in support of C mitigation [5]. For this purpose, numerous studies have approached the mechanisms that control SOC dynamics. The studies on scales that are smaller than plots usually tried to address the mechanism of SOC accumulation through exploring how SOC formation is delicately regulated by biotic inputs (litter quality [6,7] and quantity [8–10], root exudate [11] and root contributions [12,13], etc.), SOC composition [14] and the competition between plants and decomposers [15]. At the same time, the studies on scales that are larger than plots tried to find the relationships between SOC and more extensive factors that relate to climate and vegetation types [16–20], edaphic characters [21] and management options [4] for a better quantification of global C pools. With these studies, many processes relating to soil carbon sequestration [22–24] and hypotheses addressing the mechanisms that control the processes [25,26] have been proposed. However, no commonly accepted mechanism underlying SOC accumulation has emerged yet [27]. The increasing understanding of C processes but lack of agreement on the general mechanisms that control SOC dynamics highlight the need for more studies that are based on measurements over various soil types and spatial–temporal scales [3].
On account of this, a nationwide field survey across China's forest ecosystems that aimed to accurately address the C stocks and their related drivers was conducted during 2011–15 [28,29]. We re-compiled a large dataset from the database of the survey campaign to test the effect of litter quality and quantity, climatic condition, soil texture and plant diversity on SOC stocks because numerous studies showed that these variables could dominate SOC-accumulation processes. Here, litter quality and quantity are indicated by the litter C/N ratio and annual litterfall, respectively, representing the primary input that activates soil organic carbon processes. Soil texture is surrogated by the percentage of both clay and silt, or the respective percentages of either clay or silt, representing the impacts of SOC vessels on SOC stock. Plant diversity is shown by the Shannon index, representing the effects of community complexity on SOC accumulation. We did not consider either the mean annual temperature (MAT) or the mean annual precipitation (MAP) because they correlate tightly (Supplementary Fig. 1). Instead, we considered the wetness index P/PET (precipitation-to-potential-evapotranspiration ratio)—a variable that is integrated from MAT and MAP—as the surrogate of climatic conditions that regulates the environment of soil organic carbon processes. The dataset is characterized by these features: (i) data acquisition (sampling and measurement) was conducted using an identical methodology, (ii) all plots are the same size (1000 m2 in area), (iii) all the data are the averages of sufficiently representative samples from a plot and thus realize the transformation in a fine scale (smaller than plot scale) to plot scale (or ecosystem scale), (iv) different scales (e.g. regional scales, biome scales and national scales) can be obtained through combining the corresponding plots in those scales. Apart from the dataset of the China nationwide field survey campaign, we searched the globally published case studies that were based on forest ecosystems for the paired data of SOC vs P/PET, or soil texture, or litter C/N, or annual litterfall or plant-species richness to construct a meta-dataset. The meta-dataset came from 122 case studies and contained 1900 plots distributed in 33 countries.
With the two datasets, we attempt to address how these abiotic and biotic factors affect SOC accumulation and to examine the two known hypotheses: (i) does SOC result from a combination of abiotic and biotic factors [25] and (ii) how about the difference in the contributions to SOC accumulation between soil texture and plant stoichiometry [6].
RESULTS
Based on the consideration of all possible dominant factors (including litter C/N ratio, annual litterfall, P/PET, Shannon index and soil texture) that constrain SOC accumulation, two structural equation models (SEMs) were constructed and are shown in Figs 1 and 2. Before constructing the SEMs, we standardized the original data in accordance with the same province because only by province are the boundary conditions clear. Figure 1 shows that SOC concentration across all soil layers (0–100 cm) is negatively affected by litter C/N ratio and positively affected by wetness index. Apart from the direct effect on SOC accumulation, P/PET also impacts SOC dynamics indirectly, by affecting litter C/N ratio and Shannon index. Partial correlation analysis (r∂) confirms that the contribution of litter C/N ratio to mean SOC concentration over the entire soil profile is the largest (r∂ = −0.35, P < 0.001), followed by wetness index (r∂ = 0.20, P < 0.001). The values of these correlations differ between soil layers. From top to deep soil, the negative dependence of SOC concentration on litter C/N ratio increases, while the positive dependence on P/PET decreases. By contrast, the Shannon index has only a small positive relationship with SOC concentration in the top 20-cm layer. The amount of litterfall does not have any significant statistical effect on SOC across all the plots. The results through the Box and Whiskers Chart (Supplementary Figs 2 and 3) further confirm the results of Fig. 1. The same results were obtained using SOC stocks (Mg C ha−1) instead of SOC concentrations (g C kg−1) (see Supplementary Fig. 4).
Figure 1.

Structural equation models (SEMs) showing the strong negative relationship between the litter C/N ratio and the SOC concentration (g C kg−1) in the whole soil profile or in the top vs deep soil, and the contributions of the wetness index (P/PET), plant-species diversity (Shannon index) and annual litterfall (t ha−1). Soil layers (a) 0–100 cm, (b) 0–20 cm and (c) 20–100 cm. The blue and red arrows indicate positive and negative relationships, respectively. The width of the arrows indicates the strength of the relationships. Numbers adjacent to arrows are standardized path coefficients and are indicative of the effect size of the relationship. *P < 0.05; **P < 0.01; ***P < 0.001. Dashed arrows indicate non-significant relationships (P > 0.05). The final models fit the data well, as suggested by the χ2 and P-values. (Note P > 0.05 indicates that the final model fits the data well; see the ‘Methods’ section.)
Figure 2.

Structural equation models (SEMs) showing the connections between the litter carbon-to-nitrogen ratio (litter C/N ratio), wetness index (P/PET-precipitation/potential evapotranspiration), tree-species diversity (Shannon index), soil texture (claysilt, cumulative percentages (%) of particles <50 μm in diameter) and annual litterfall (litterfall, t ha−1) with soil organic carbon concentration (SOC, g C kg−1) in soil layers (a) 0–100 cm, (b) 0–20 cm and (c) 20–100 cm. The blue and red arrows indicate positive and negative relationships, respectively. The width of the arrows indicates the strength of the relationships. Numbers adjacent to arrows are standardized path coefficients and are indicative of the effect size of the relationship. *P < 0.05; **P < 0.01; ***P < 0.001. Dashed arrows indicate non-significant relationships (P > 0.05). The final models fit the data well, as suggested by the χ2 and P-values (χ2 = 2.48, P = 0.65, df = 4 and n = 278 in (a); χ2 = 1.99, P = 0.74, df = 4 and n = 278 in (b) and χ2 = 2.21, P = 0.70, df = 4 and n = 278 in (c)).
The SEM in Fig. 2 shows that SOC concentration is not significantly related to soil texture, represented by the percentage of both clay and silt altogether, neither significantly related to the soil texture represented by the respective percentages of either clay or silt (not displayed here). Other relationships shown in Fig. 2 are similar to those of Fig. 1.
Both Figs 1 and 2, as well as Supplementary Fig. 3, show that the litter C/N ratio is negatively correlated with the Shannon index, suggesting that an increase in plant-species richness in China's forest ecosystems reduces the litter C/N ratio significantly (P < 0.001) and thus indirectly promotes SOC accumulation.
The meta-data across the global forest ecosystems also show that SOC concentration is significantly affected by the litter C/N ratio and wetness index, but not by annual litterfall and soil texture (Fig. 3 and Supplementary Fig. 5).
Figure 3.
Boxplot showing the relationships between SOC concentrations (g C kg−1) and (a) P/PET, (b) litter C/N ratio, (c) litterfall (t ha−1 yr−1) and (d) claysilt (%), using data collected from the literature.
The two datasets were merged to inspect whether they are comparable and reach the same results. The SOC concentrations in the meta-dataset were standardized in accordance with the same country and then amalgamated with the provincial-standardized SOC concentration in the dataset of the China's nationwide field survey campaign. National scale, as the largest normalization unit except for continent scale, maintains all the variations resulting from various factors, which facilitates the dataset comparison and analysis. Figure 4, which was based on the amalgamation of the two datasets, also confirms our results—that is, SOC concentrations are significantly related to the P/PET and litter C/N ratio but show no significant relationship between annual litterfall and soil texture (the percentages of clay and silt particles). The two datasets are highly comparable.
Figure 4.
Boxplot showing the relationships between SOC concentrations (g C kg−1) and (a) P/PET, (b) litter C/N ratio, (c) claysilt (%) and (d) annual litterfall (t ha−1 yr−1), using data from both the survey of China's forest ecosystems and the published literature.
DISCUSSION
The paper displayed the respective contributions of the litter C/N ratio, P/PET, plant diversity, litterfall and soil texture to SOC concentrations. Litter with a low C/N ratio and moist conditions with high P/PET favor SOC accumulation. Across China's forest ecosystems, the forest communities with rich plant species usually produce litter of low C/N ratio, which can promote SOC accumulation. Notably, no significant effects of annual litterfall on SOC concentration were found, even though higher litterfall implies higher SOC [30] in the formulations of soil carbon models based on first-order decomposition kinetics. The lack of litterfall effect thus questions common model assumptions and suggests that there are non-linear effects induced by litter quality that might not be captured by current models.
Empirical, independent evidence supports or can be explained by our findings. For example, foresters often transplant the leguminous plants with a low C/N ratio into forests to increase soil organic matter [31]. In monsoon evergreen broadleaved forests, SOC has accumulated rapidly in the past decades following an increase in species number, despite stable litterfall rates [32–34]. Therefore, increased litter amount alone does not necessarily lead to more SOC accumulation [11,35]. FACE experiments showed that elevated atmospheric CO2 concentration increased shoot and root biomass [36], but did not promote SOC accumulation [35] due to increased C/N ratios [35,37].
The underlying mechanisms that dominate the strong and significant correlations of SOC accumulation with biotic and abiotic factors are based on the following knowledge. High P/PET and low litter C/N ratio can increase litter turnover [21,38,39], carbon-use efficiency of decomposer microorganisms [40] and SOC transportability to deep soils.
Litters with rich N and a low C/N ratio not only speed up decomposition [38], but also increase the decomposer carbon-use efficiency [6,40], resulting in a higher microbial biomass [6] and a higher proportion of DOC [41] and fine residues, which can be transported and incorporated effectively into the deeper soil matrix [6,7] and ultimately stabilized [6,42,43]. Experiments demonstrated that litters of low lignin concentration promote SOC accumulation due to the high decomposition rate (high litter turnover) [41,44], similar to that of the high P/PET and low C/N ratio. However, litter lignin concentration is independent of litter C/N ratio (Supplementary Fig. 6) and the litter of low lignin does not mean a low C/N ratio and will not necessarily increase carbon-use efficiency of decomposer microorganisms. Through an incubation experiment with 13C-labelled lignin, it was concluded that lignin is rather unreactive in soils either due to the lack of easily available organic matter for co-metabolism or due to enhanced adsorption properties [45], which differs from the effect of low C/N ratio on SOC accumulation.
Here, below-ground litter is taken as the same input as above-ground litter (see the ‘Methods’ section). However, root litter may make other contributions to SOC accumulation [13]. It was estimated that the mean residence time of root-derived SOC is 2.4 times that of shoot-derived SOC due to physico-chemical protection, micrometer-scale physical protection, chemical interactions with metal ions and higher chemical recalcitrance of root tissues [12]. Another root-related C input is root exudate. Root exudates increase organic carbon input and bring bioactive substances to soils [46]. The former plays the same roles as litter does due to similar C/N ratios between live and dead roots [47]. The latter will alter the microbe communities and environments in soils [11,48], and thus plays different roles in SOC accumulation, deserving further studies.
High P/PET provides suitable climatic conditions for litter decomposition [21,39] and keeps soil in a moister state to accelerate the transport of SOC to deep soil layers. Moreover, high P/PET could maintain anaerobic conditions if soils are not well drained, which facilitates SOC preservation [49]. Ultraviolet radiation can also affect SOC accumulation through increasing the litter-decomposition rate in arid grasslands [50], but this effect is probably very limited in forests of wet regions, especially for soils that are covered by forest canopies.
High plant-species richness can increase the biomass [51] and activity [52] of decomposer microorganisms and thus produce more necromass that is easily stabilized in soils. This potential mechanism could explain the finding that a high Shannon index corresponds to a high SOC concentration in top soils (Fig. 1). More importantly, our field survey dataset shows that plant-species richness causes a decrease in the litter C/N ratio and thus could boost SOC accumulation, which is consistent with the previous results [53]. It should be noted that our finding relies on the average of a large area. Therefore, the phenomenon may not be observed in some specific conditions. For example, the plant communities that are composed of species with low C/N ratios will unlikely decrease the litter C/N ratio in a short time, even if plant-species richness is increasing. Plant-species composition in plantations is artificially controlled and thus the litter C/N ratios are also artificially dominated.
The result of decoupling between SOC and annual litterfall is contrary to many studies [54] but is supported by the report that the spatial distribution of SOC differs substantially from that of above- and below-ground biomass [4]. Some soil carbon models that predict an increasing SOC with higher litterfall might miss some key processes that constrain SOC accumulation.
As the vessel of SOC, the mineral soil matrix is expected to contribute substantially to SOC stabilization and preservation through the effects of soil physical structures and soil chemistry [55,56]. Biogeochemical models rely almost exclusively on clay content to modify the rates of SOC turnover and CO2 emission to the atmosphere [55]. However, we did not find a significant effect of soil texture on SOC accumulation across China's forest ecosystems, consistently with the conclusions of other reports [57]. This evidence suggests that a different parameter rather than clay or clay + silt could have more predictive power by capturing the role of soil physicochemical properties in SOC cycling [55]. The study did not investigate the effects of chemical processes because it is difficult to ascertain which chemical component drives the stabilization and preservation of soil organic matter; all the components (pH, ion, organic matter, etc.) in the soil chemical process are in dynamic equilibrium [56]. Previous studies showed that enhanced N deposition acidified tropical forest soils [58], promoting SOC accumulation through two different processes. One is through increasing cation exchange capacity [58] and the other is through decreasing the activity of decomposer microorganisms [59–61]. N addition was also observed to significantly promote SOC accumulation through decreasing the C/N ratio in leaves and roots [62,63]. Interestingly, if nitrogen fertilization increases only the net primary productivity [64] or biomass [62], SOC storage changes uncertainly, either increasing or decreasing or remaining unchanged [62,64], probably depending on whether the C/N ratios are changed or not.
This contribution elucidated the general drivers of SOC accumulation. Although almost all the drivers had been separately identified in previous studies, here we clarify, for the first time, how a range of possible drivers (climate, quality and quantity of litter and root exudate, plant-species richness and soil texture) affect SOC accumulation using a unique dataset. With this dataset, we demonstrated that changes in environmental factors and plant-community composition that reduce the litter C/N ratio or increase the wetness index and plant-species richness are expected to promote SOC accumulation. In contrast, soil texture and litterfall flux have little predictive power for SOC. This finding challenges the C cycling models that predict the dependence of SOC accumulation on litter production and soil texture, and suggests some guiding principles for land-based actions of mitigating rising atmospheric CO2 concentrations.
METHODS
Study sites
For the field survey campaign during 2011–15 that was first reported by Tang et al. [29], we divided the country into three types of grid sizes—100, 400 and 900 km2—on the basis of vegetation distribution using a 1:1 000 000 vegetation map [65]. A grid size of 100 km2 was designed for tropical and subtropical regions with rich species diversity, and 400 and 900 km2 were used for temperate and alpine vegetation regions where species diversity is relatively poor. In total, 35 800 grids were documented across China, and then they were overlaid on vegetation and administrative maps to obtain the number of grids for forests and for each of the 31 provinces; 3–5% of the grids in each province and a total of ∼380 forest grids across China's forest ecosystems were randomly chosen. About seven locations in each grid were selected based on forest types and the information on historical inventory data and there were 2600 locations (380 grids × 7 locations = 2660) in total. The locations in each grid were determined in advance before the field survey to minimize the subjective preference of investigators. For each location, three plots were selected: one was for the most dominant forest type and the other two were for the main forest types according to the proportions of area. The plot for the most dominant forest type in each location was taken as the permanent plot [29]. In brief, an inventory system composed of 7800 plots (1000 m2 in area for each, a few plantation plots of 600 m2 in area) across China's forest ecosystems was set up in 2011, in which 2600 plots (which amounted to one-third of 7800 plots; 1000 m2 in area for each) were selected in advance as permanent plots that are precisely and spatially positioned (Supplementary Fig. 7). This paper refers to the 2600 permanent plots.
Field surveying and sampling
In the field surveys, the species of all individuals with DBH (diameter at breast height = 1.3 m) ≥5 cm in the entire plot and all understory plants, including the woody individuals with DBH <5 cm, shrubs, seedlings and herbaceous plants in three randomly selected quadrats (2 × 2 m2) of each plot were recorded. The samples of different organs (leaf, branch, stem and root) from the main species in the canopy layer were taken to measure the carbon and nitrogen concentrations.
Each plot was divided into 10 subplots (10 × 10 m2) and one quadrat (1 × 1 m2) was randomly selected in each subplot. In total, 10 quadrats in each plot were selected and spatially fixed to collect litter. Before determining the needed minimum quadrats of each plot, we performed many experiments and analysed the existing data of CERN (Chinese Ecosystem Research Network). Our experiments and analysis showed that the composition and proportion of species in the litters gathered from 10 quadrats were not significantly (P < 0.01) different from these gathered from 20 quadrats. Therefore, we took 10 quadrats as a uniform number applied to all plots across China's forest ecosystem. All the litter (leaves, twigs and woody debris) collected in the 10 quadrats was fully mixed and some samples were brought to the laboratory for analysis.
Soil samples were taken using a soil auger to measure SOC concentration, soil bulk density and soil texture. The soil profile of 1 m in depth was divided into five layers, namely 0–10, 10–20, 20–30, 30–50 and 50–100 cm. Nine sampling points were positioned equidistantly along the two diagonal lines. In the vicinity of each sampling point, three parallel samples were taken as a composite sample for each soil layer. In total, there were 45 composite samples (9 sampling points × 5 soil layers) in a plot.
Field sampling was performed in the summer (July–September) of 2011–15.
Chemical analysis
To ensure the accuracy of measurement, two special laboratories were set up to be responsible for all the chemical analysis of the project. One was in South China Botanical Garden, CAS (Chinese Academy of Sciences); the other was in the Institute of Botany, CAS. Two parallel chemical analysis methods were used to measure the concentrations of SOC, carbon and nitrogen in live organs (leaf, branch, stem and root) and litter.
Carbon concentrations of live organs, litters and soils were measured using a CN analyser (PE-2400 II, Perkin Elmer, Waltham, MA, USA). For some measurement results beyond normal ranges, we used the Walkley-Black wet digestion method [66] to confirm or to adjust the measurements by the CN analyser.
Nitrogen concentrations of live organs, litters and soils were measured using a CN analyser (PE-2400 II, Perkin Elmer, Waltham, MA, USA). Similarly, for some measurement results beyond normal ranges, we used the Semimicro-Kjeldahl method to confirm or to adjust the measurements by the CN analyser.
After removal of the organic matter and carbonates, the diameters of various particles in each soil layer (0–10, 10–20, 20–30, 30–50 and 50–100 cm) were separately determined using a Laser Scattering Particle Size Distribution Analyzer (LA-960, Haan, Germany). The particles in the three ranges of diameter <1.981, 1.981–51.471 and 51.471–5000 μm are usually called clay, silt and sand, respectively. The cumulative percentages of particles for clay, silt and both (i.e. the diameters of particles <1.981, 1.981–51.471 and <51.471 μm) were taken to characterize soil texture. In this study, we analysed the soil texture of 315 permanent plots that belonged to 315 different grids.
Calculation of the litter C/N ratio
The above-ground litter C/N ratios were directly measured and calculated as the ratio of carbon concentration (g C kg−1) to nitrogen concentration (g N kg−1) of the same litter sample. The below-ground litter (root litter) C/N ratios were substituted for the C/N ratios of live roots because they have non-significant difference [47]. The C/N ratios of all litter (both above-ground and below-ground) were calculated as:
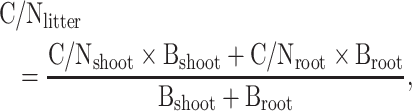 |
(1) |
where 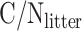 is the C/N ratio of all litter (both above-ground and below-ground) and
is the C/N ratio of all litter (both above-ground and below-ground) and 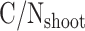 and
and 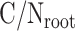 are the C/N ratios of shoot litter and live root, respectively.
are the C/N ratios of shoot litter and live root, respectively.  and
and  are the biomasses of shoot and root, respectively.
are the biomasses of shoot and root, respectively.
Calculating the SOC concentration and soil texture of considered soil layers based on the measured values
Based on the measured SOC concentration and soil textures of soil layers 0–10, 10–20, 20–30, 30–50 and 50–100 cm, the values of SOC and soil texture in 0–100, 0–20 and 20–100 cm in this paper were calculated according to Equations (2)–(4):
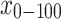 |
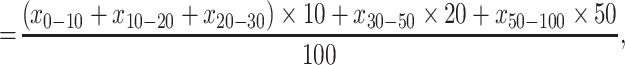 |
(2) |
 |
(3) |
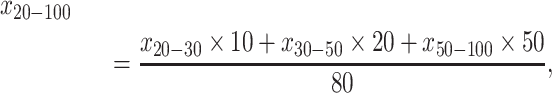 |
(4) |
where, in Equations (2)–(4), x0–100, x0–20 and x20–100 are the SOC concentration (g kg−1) or soil texture (%) (percentage of both clay and silt or the respective percentages of either clay or silt) in soil layers 0–100, 0–20 and 20–100 cm, respectively. x0–10, x10–20, x20–30, x30–50 and x50–100 are the measured SOC concentration (g kg−1) or soil texture (%) (percentage of both clay and silt or the respective percentages of either clay or silt) of soil layers 0–10, 10–20, 20–30, 30–50 and 50–100 cm, respectively.
Calculating the SOC stock of considered soil layers based on the measured values
Based on the measured SOC concentration and soil sulk density of layers 0–10, 10–20, 20–30, 30–50 and 50–100 cm, the values of SOC density in 0–100, 0–20 and 20–100 cm in this paper were calculated according to Equations (5)–(7):
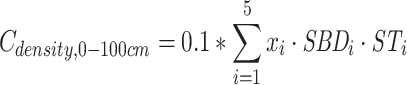 |
(5) |
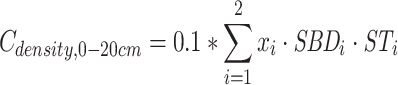 |
(6) |
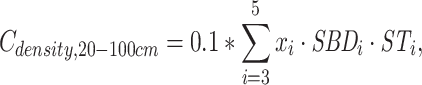 |
(7) |
where 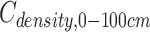 ,
, 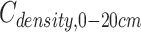 and
and 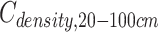 are the SOC density (Mg C ha−1) of soil layer 0–100, 0–20 and 20–100 cm, respectively. i = soil layer 1 (0–10 cm), 2 (10–20 cm), …, 5 (50–100 cm). xi, SBDi and STi are the SOC concentrations (g kg−1), soil bulk density (g cm−3) and the thickness (cm) of soil layer i, respectively.
are the SOC density (Mg C ha−1) of soil layer 0–100, 0–20 and 20–100 cm, respectively. i = soil layer 1 (0–10 cm), 2 (10–20 cm), …, 5 (50–100 cm). xi, SBDi and STi are the SOC concentrations (g kg−1), soil bulk density (g cm−3) and the thickness (cm) of soil layer i, respectively.
Shannon index of plant diversity
Based on the recorded species, the Shannon index was calculated as:
 |
(8) |
where H = Shannon index, n = number of species, pi = proportion of individuals of the ith species out of all individuals in the plot [67].
Annual litterfall
Chen et al. [28] provided the annual litterfall of China's natural forest plots, which was estimated using the function established by Wang et al. [68]:
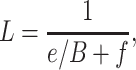 |
(9) |
in which L is the annual litterfall in a plot (t ha−1 year−1), e and f are constants for a specific forest type and B is the total biomass (including roots) in a plot (t ha−1) that was estimated by Tang et al. [29] based on the nationwide field campaign from 2011 to 2015. In this paper, we additionally estimated the annual litterfall of artificially originated forests (including plantation) using the same approach. The calculated annual litterfall refers to the total litter of an ecosystem including both the above-ground litter and the below-ground dead roots, but no root exudates.
To test the accuracy of Equation (9), we compared the calculated and the observed annual litterfall from 30 permanent plots of CERN (see Supplementary Table 1 for the original data) and found that the two groups of values are highly comparable (Observed values = 0.95 × Calculated values + 0.78, R2 = 0.96, P < 0.0001, n = 30).
Climate data
Temperature and precipitation were obtained from Tang et al. [29], which were interpolated from the observations at 1098 stations across China (Chinese National Meteorological Information Center, http://data.cma.cn) using ANUSPLIN software [69,70].
Mean annual potential evapotranspiration (PET) and wetness index (P/PET)
Yearly PET is estimated using Hamon's method [71,72]:
 |
(10) |
where D is the time from sunrise to sunset in multiples of 12 h, varies with date, latitude, slope and aspect of a watershed (if the influences of slope and aspect are not considered, the average daily D of an entire year is 1); Vd is the saturated vapor density (g m−3) at the annual mean temperature (T, °C), 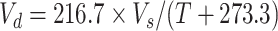 ; Vs is the saturated vapor pressure (mb),
; Vs is the saturated vapor pressure (mb),  ; K is the correction coefficient to adjust PET calculated using Hamon's method to realistic values, which ranges from 1.2 to 1.4. Thus, for consistency, we used K = 1.3 to calculate the PETs of all plots. After the yearly PET is estimated, the annual P/PET can be easily calculated. The same methodology was used by Zhou et al. [73].
; K is the correction coefficient to adjust PET calculated using Hamon's method to realistic values, which ranges from 1.2 to 1.4. Thus, for consistency, we used K = 1.3 to calculate the PETs of all plots. After the yearly PET is estimated, the annual P/PET can be easily calculated. The same methodology was used by Zhou et al. [73].
Summary for the dataset of China's nationwide field survey campaign
Based on the above method, we obtained the dataset of China's nationwide field survey campaign for this paper. The overview of the dataset is shown in Supplementary Figs 8 and 9.
Establishment of the meta-dataset
A comprehensive search of relevant peer-reviewed articles and dissertations published from 1993 to 2018 was conducted using the databases of the Web of Science® and ProQuest. We also cross-checked the references of the relevant articles to identify other potential book chapters and peer-reviewed reports using combinations of the keywords: soil carbon (C), SOC, litter carbon-to-nitrogen (C/N) ratio, litterfall, etc. We also collected data for the soil C stock and calculated the soil C concentration by bulk density. The vegetation included natural forests and plantations with different species or different stand ages. Numerical values were extracted from graphically presented data by digitizing the figures using an Engauge Digitizer (Free Software Foundation, Inc., Boston, USA). In total, 122 case studies met our requirements, in which 1900 plots were included, distributed in 33 countries. The meta-dataset contains 1535 plots for SOC vs P/PET, 313 plots for SOC vs soil texture, 90 plots for SOC vs litter C/N and 166 plots for SOC vs annual litterfall. The case studies that reported both SOC and plant-species richness (or all species) were not found. The publications that provided the data are listed in Supplementary Table 1.
Additionally, we also searched globally published studies for the paired data of lignin concentration and C/N ratio. The paired data are shown in Supplementary Fig. 6. The studies that provided the data are listed in Supplementary Table 2.
Data standardization
Before the SEM analysis and the amalgamation of the two datasets (the meta-dataset and the dataset of China's nationwide field survey), all selected variables on the dataset were standardized to delete the background difference in original data among different countries and provinces. We did not consider the forest type as a category to standardize original data because the boundary conditions between any two forest types are not clear. The standardized variable was calculated as:
 |
(11) |
where, Xk,i,j is the normalized value, xk,i,j is the original value;  is the average value of xk,i,j across all j plots in country k or province k;
is the average value of xk,i,j across all j plots in country k or province k; 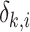 is the standard deviation of xk,i,j in country k or province k; the subscript indicates: k = countries (USA, UK …) or provinces (Guangdong, Beijing …); i = variables (SOC0–100 cm, SOC0–20 cm, SOC20–100 cm, litter C/N ratio, P/PET, Shannon index, annual litterfall, claysilt0–100 cm, claysilt0–20 cm, claysilt20–100 cm); and j = plots.
is the standard deviation of xk,i,j in country k or province k; the subscript indicates: k = countries (USA, UK …) or provinces (Guangdong, Beijing …); i = variables (SOC0–100 cm, SOC0–20 cm, SOC20–100 cm, litter C/N ratio, P/PET, Shannon index, annual litterfall, claysilt0–100 cm, claysilt0–20 cm, claysilt20–100 cm); and j = plots.
Statistical analyses
SEMs were constructed to quantify the multivariate causal network in which SOC, litter C/N ratio, wetness index (P/PET), Shannon index, annual litterfall and soil texture (claysilt) are involved. SEM analyses were performed using AMOS 20 (Amos Development, Spring House, PA, USA). According to the instructions, the hypothesized model is considered to fit the data well if the chi-square test is not significant (P > 0.05) and it will be rejected if the chi-square test is significant (P < 0.05) [74,75]. The Box and Whiskers Chart was used to show the relationships between SOC concentrations and P/PET, litter C/N ratio, litterfall and claysilt based on both the global data and China's forest data. The data of P/PET, C/N ratio, litterfall and claysilt were partitioned into a series of groups and then the Box and Whiskers Chart was performed using Sigmaplot version 11.0 (Systat Software, Inc.). All the linear regressions were performed using Sigmaplot version 11.0 (Systat Software, Inc.).
Supplementary Material
FUNDING
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDA05050000), the National Natural Science Foundation of China (NSFC41430529), the CAS Distinguished Research Fellow Program and the Swedish Research Councils, Formas (2015-468) and VR (2016-04146).
AUTHOR CONTRIBUTIONS
Dr. Yongfei Bai and Shiping Chen provided the published annual litterfall of natural forests. Xin Xiong, Weixin Geng, Jianqi Zhao, Yonghong Deng, Mengdi Zhao, Huiling Zhang, Shun Zou and Jianping Wu took part in the measurements of soil texture and the search for globally published data. G.Z. was responsible for the China's forest field survey campaign in the XDA05050000 project, designed the study and wrote the manuscript. S.X. calculated and analysed the data, provided references, searched the globally published data and drew the figures. P.C. and S.M. made significant contributions to the design of the study and wrote and revised the manuscript. J.F. and G.Y. were responsible for the China's terrestrial ecosystem survey campaign in the XDA05050000 project. All other authors contributed to the field survey, chemical analysis, data check and management.
REFERENCES
- 1. Rumpel C, Farshad A, Koutika LSet al.. Put more carbon in soils to meet Paris climate pledges. Nature 2018; 564: 32–4. [DOI] [PubMed] [Google Scholar]
- 2. Smith P. Soil carbon sequestration and biochar as negative emission technologies. Glob Change Biol 2016; 22: 1315–24. [DOI] [PubMed] [Google Scholar]
- 3. Harden JW, Hugelius G, Ahlström Aet al.. Networking our science to characterize the state, vulnerabilities, and management opportunities of soil organic matter. Glob Change Biol 2018; 24: e705–18. [DOI] [PubMed] [Google Scholar]
- 4. Scharlemann JPW, Tanner EVJ, Hiederer Ret al.. Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Manag 2014; 5: 81–91. [Google Scholar]
- 5. Luo YQ, Ahlström A, Allison SDet al.. Toward more realistic projections of soil carbon dynamics by Earth system models. Global Biogeochem Cy 2016; 30: 40–56. [Google Scholar]
- 6. Cotrufo MF, Wallenstein MD, Boot CMet al.. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 2013; 19: 988–95. [DOI] [PubMed] [Google Scholar]
- 7. Cotrufo MF, Soong JL, Horton AJet al.. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nature Geosci 2015; 8: 776–9. [Google Scholar]
- 8. Sayer EJ, Heard MS, Grant HKet al.. Soil carbon release enhanced by increased tropical forest litterfall. Nature Clim Change 2011; 1: 304–7. [Google Scholar]
- 9. Leff JW, Wieder WR, Taylor PGet al.. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob Change Biol 2012; 18: 2969–79. [DOI] [PubMed] [Google Scholar]
- 10. Xu S, Liu LL, Sayer EJ. Variability of above-ground litter inputs alters soil physicochemical and biological processes: a meta-analysis of litterfall-manipulation experiments. Biogeosciences, 2013; 10: 7423–33. [Google Scholar]
- 11. Keiluweit M, Bougoure JJ, Nico PSet al.. Mineral protection of soil carbon counteracted by root exudates. Nat Clim Change 2015; 5: 588–95. [Google Scholar]
- 12. Rasse DP, Rumpel C, Dignac MF. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005; 269: 341–56. [Google Scholar]
- 13. Jackson RB, Lajtha K, Crow SEet al.. The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. Annu Rev Ecol Evol Syst 2017; 48: 419–45. [Google Scholar]
- 14. Cusack DF, Halterman SM, Tanner EVJet al.. Decadal-scale litter manipulation alters the biochemical and physical character of tropical forest soil carbon. Soil Biol Biochem 2018; 124: 199–209. [Google Scholar]
- 15. Averill C, Turner BL, Finzi AC. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014; 505: 543–5. [DOI] [PubMed] [Google Scholar]
- 16. De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 2008; 11: 516–31. [DOI] [PubMed] [Google Scholar]
- 17. Batjes NH. Total carbon and nitrogen in the soils of the world. Eur J Soil Science 1996; 47:151–63. [Google Scholar]
- 18. Batjes NH, Sombroek WG. Possibilities for carbon sequestration in tropical and subtropical soils. Global Change Biol 1997; 3: 161–73. [Google Scholar]
- 19. Jobbagy EG, Jackson RB. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 2000; 10: 423–36. [Google Scholar]
- 20. Carvalhais N, Forkel M, Khomik Met al.. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014; 514: 213–7. [DOI] [PubMed] [Google Scholar]
- 21. Doetterl S, Stevens A, Six Jet al.. Soil carbon storage controlled by interactions between geochemistry and climate. Nature Geosci 2015; 8: 780–3. [Google Scholar]
- 22. Drinkwater LE, Wagoner P, Sarrantonio M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998; 396: 262–5. [Google Scholar]
- 23. Schlesinger WH, Lichter J. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature 2001; 411: 466–9. [DOI] [PubMed] [Google Scholar]
- 24. Clemmensen KE, Bahr A, Ovaskainen Oet al.. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013; 339: 1615–8. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt MWI, Torn MS, Abiven Set al.. Persistence of soil organic matter as an ecosystem property. Nature 2011; 478: 49–56. [DOI] [PubMed] [Google Scholar]
- 26. Lehmann J, Kleber M. The contentious nature of soil organic matter. Nature 2015; 528: 60–8. [DOI] [PubMed] [Google Scholar]
- 27. O’Rourke SM, Angers DA, Holden NMet al.. Soil organic carbon across scales. Glob Change Biol 2015; 21: 3561–74. [DOI] [PubMed] [Google Scholar]
- 28. Chen S, Wang WT, Xu WTet al.. Plant diversity enhances productivity and soil carbon storage. Proc Natl Acad Sci USA 2018; 115: 4027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang XL, Zhao X, Bai YFet al.. Carbon pools in China's terrestrial ecosystems: new estimates based on an intensive field survey. Proc Natl Acad Sci USA 2018; 115: 4021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lajtha K, Townsend KL, Kramer MGet al.. Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 2014; 119: 341–60. [Google Scholar]
- 31. Johnson DW, Curtis PS. Effects of forest management on soil C and N storage: meta analysis. Forest Ecol Manag 2001; 140: 227–38. [Google Scholar]
- 32. Tang XL, Wang YP, Zhou GYet al.. Different patterns of ecosystem carbon accumulation between a young and an old-growth subtropical forest in Southern China. Plant Ecol. 2011; 212: 1385–95. [Google Scholar]
- 33. Zhou GY, Peng CH, Li YLet al.. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob Change Biol 2013; 19: 1197–210. [DOI] [PubMed] [Google Scholar]
- 34. Zhou GY, Liu SG, Li ZAet al.. Old-growth forests can accumulate carbon in soils. Science 2006; 314: 1417. [DOI] [PubMed] [Google Scholar]
- 35. Talhelm AF, Pregitzer KS, Zak DR. Species-specific responses to atmospheric carbon dioxide and tropospheric ozone mediate changes in soil carbon. Ecol Lett 2009; 12: 1219–28. [DOI] [PubMed] [Google Scholar]
- 36. King JS, Kubiske ME, Pregitzer KSet al.. Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2. New Phytol 2005; 168: 623–36. [DOI] [PubMed] [Google Scholar]
- 37. van Groenigen KJ, Qi X, Osenberg CWet al.. Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 2014; 344: 508–9. [DOI] [PubMed] [Google Scholar]
- 38. Parton W, Silver WL, Burke ICet al.. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007; 315: 361–4. [DOI] [PubMed] [Google Scholar]
- 39. Prescott CE. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010; 101: 133–49. [Google Scholar]
- 40. Manzoni S, Čapek P, Mooshammer Met al.. Optimal metabolic regulation along resource stoichiometry gradients. Ecol Lett 2017; 20: 1182–91. [DOI] [PubMed] [Google Scholar]
- 41. Huang YH, Li YL, Xiao Yet al.. Controls of litter quality on the carbon sink in soils through partitioning the products of decomposing litter in a forest succession series in South China. Forest Ecol Manag 2011; 261: 1170–7. [Google Scholar]
- 42. Sanderman J, Amundson R. A comparative study of dissolved organic carbon transport and stabilization in California forest and grassland soils. Biogeochemistry 2008; 89: 309–27. [Google Scholar]
- 43. Berg B. Litter decomposition and organic matter turnover in northern forest soils. Forest Ecol Manag 2000; 133: 13–22. [Google Scholar]
- 44. Sumiyoshi Y, Crow SE, Litton CMet al.. Belowground impacts of perennial grass cultivation for sustainable biofuel feedstock production in the tropics. GCB Bioenergy 2017; 9: 694–709. [Google Scholar]
- 45. Bahri H, Rasse DP, Rumpel Cet al.. Lignin degradation during a laboratory incubation followed by 13C isotope analysis. Soil Biol Biochem 2008; 40: 1916–22. [Google Scholar]
- 46. Bais HP, Weir TL, Perry LGet al.. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 2006; 57: 233–66. [DOI] [PubMed] [Google Scholar]
- 47. Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer Met al.. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol Monogr 2015; 85: 133–55. [Google Scholar]
- 48. Phillips RP, Finzi AC, Bernhardt ES. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 2011; 14: 187–94. [DOI] [PubMed] [Google Scholar]
- 49. Schuur EAG, Chadwick OA, Matson PA. Carbon cycling and soil carbon storage in Mesic to wet Hawaiian Montane forests. Ecology 2001; 82: 3182–96. [Google Scholar]
- 50. Austin AT, Vivanco L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006; 442: 555–8. [DOI] [PubMed] [Google Scholar]
- 51. Zak DR, Holmes WE, White DCet al.. Soil microbial communities, and ecosystem function: are there any links? Ecology 2003; 84: 2042–50. [Google Scholar]
- 52. Lange M, Eisenhauer N, Sierra CAet al.. Plant diversity increases soil microbial activity and soil carbon storage. Nat Commun 2015; 6: 6707. [DOI] [PubMed] [Google Scholar]
- 53. Huang YY, Ma YL, Zhao Ket al.. Positive effects of tree species diversity on litterfall quantity and quality along a secondary successional chronosequence in a subtropical forest. J Plant Ecol 2017; 10: 28–35. [Google Scholar]
- 54. Luo ZK, Feng WT, Luo YQet al.. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob Change Biol 2017; 23: 4430–9. [DOI] [PubMed] [Google Scholar]
- 55. Rasmussen C, Heckman K, Wieder WRet al.. Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018; 137: 297–306. [Google Scholar]
- 56. McCauley A, Jones C, Jacobsen J. Soil pH and Organic Matter: Nutrient Management Modules 8, #4449-8. Bozeman: Montana State University Extension Service, 2009, 1–12. [Google Scholar]
- 57. Wynn JG, Bird MI, Vellen Let al.. Continental-scale measurement of the soil organic carbon pool with climatic, edaphic, and biotic controls. Global Biogeochem Cy 2006; 20: GB1007, doi: 10.1029/2005GB002576. [DOI] [Google Scholar]
- 58. Lu XK, Mao QG, Gilliam FSet al.. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob Change Biol 2014; 20: 3790–801. [DOI] [PubMed] [Google Scholar]
- 59. Mo J, Zhang W, Zhu Wet al.. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Chang Biol 2008; 14: 403–12. [Google Scholar]
- 60. Hobbie SE. Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 2008; 89: 2633–44. [DOI] [PubMed] [Google Scholar]
- 61. Riggs CE, Hobbie SE. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol Biochem 2016; 99: 54–65. [Google Scholar]
- 62. Fornara DA, Tilman D. Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 2012; 93: 2030–6. [DOI] [PubMed] [Google Scholar]
- 63. Ziter C, MacDougall AS. Nutrients and defoliation increase soil carbon inputs in grassland. Ecology 2013; 94: 106–16. [DOI] [PubMed] [Google Scholar]
- 64. Baer SG, Blair JM. Grassland establishment under varying resource availability: a test of positive and negative feedback. Ecology 2008; 89: 1859–71. [DOI] [PubMed] [Google Scholar]
- 65. Zhang XS. Vegetation Map of the People's Republic of China. Beijing: The Geological Publishing House, 2007. [Google Scholar]
- 66. Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds). Methods of Soil Analysis. Madison: ASA-SSSA, 1982, 539–76. [Google Scholar]
- 67. Spellerberg IF, Fedor PJ. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Global Ecol Biogeogr 2003; 12: 177–9. [Google Scholar]
- 68. Wang B, Huang JY, Yang XSet al.. Estimation of biomass, net primary production and net ecosystem production of China's forests based on the 1999–2003 National Forest Inventory. Scand J For Res 2010; 25: 544–53. [Google Scholar]
- 69. Hutchinson MF. ANUSPLIN Version 4.4 User Guide. http://fennerschoolanueduau/research/publications/software-datasets/anusplin. 2004.
- 70. Wang J, Wang J, Ye Het al.. An interpolated temperature and precipitation dataset at 1-km grid resolution in China (2000–2012). China Scientific Data 2017; 2: 88–95. [Google Scholar]
- 71. Federer CA, Brook LD. A Hydrologic Simulation Model For Eastern Forests . NH, Research Report 19, 84 (Water Resources Research Center). Manchester: University of New Hampshire. 1978. [Google Scholar]
- 72. Vörösmarty CJ, Federer CA, Schloss AL. Potential evaporation functions compared on US watersheds: possible implications for global-scale water balance and terrestrial ecosystem modeling. J Hydrol 1998; 207: 147–69. [Google Scholar]
- 73. Zhou GY, Wei XH, Chen XZet al.. Global pattern for the effect of climate and land cover on water yield. Nat Commun 2015; 6, 5918. [DOI] [PubMed] [Google Scholar]
- 74. Grace JB. Structural Equation Modeling and Natural Systems. Cambridge: Cambridge University Press, 2006. [Google Scholar]
- 75. Hox JJ, Bechger TM. An introduction to structural equation modeling. Fam Sci Rev 2007; 11: 354–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




