Abstract
The strong spatial confinement of a nanocavity plasmonic field has made it possible to visualize the inner structure of a single molecule and even to distinguish its vibrational modes in real space. With such ever-improved spatial resolution, it is anticipated that full vibrational imaging of a molecule could be achieved to reveal molecular structural details. Here we demonstrate full Raman images of individual vibrational modes at the ångström level for a single Mg-porphine molecule, revealing distinct characteristics of each vibrational mode in real space. Furthermore, by exploiting the underlying interference effect and Raman fingerprint database, we propose a new methodology for structural determination, which we have called ‘scanning Raman picoscopy’, to show how such ultrahigh-resolution spectromicroscopic vibrational images can be used to visually assemble the chemical structure of a single molecule through a simple Lego-like building process.
Keywords: scanning Raman picoscopy, tip-enhanced Raman spectroscopy, structure determination, vibrational mode imaging, interference effect
INTRODUCTION
The determination of the chemical structure of a molecule is a premier step in chemistry. In the past century, different spectroscopic tools, such as nuclear magnetic resonance [1] and electronic and vibrational spectroscopies [2–4], have been routinely employed for structure characterization. The combination of rich spectroscopic data and chemical intuition helps to identify the basic chemical groups or specific chemical bonds in a molecule. However, the lack of spatial information has made it very difficult to firmly determine the placement and connectivity of the chemical groups from the spectroscopic data alone. Scanning tunneling microscopy (STM) [5–8] and atomic force microscopy (AFM) [9,10] have the remarkable ability to visualize the molecular skeleton, but usually lack sufficient chemical information for precise chemical structure determination. Such deficiencies can in principle be overcome by a combination of scanning probe microscopy and Raman spectroscopy, as demonstrated by the tip-enhanced Raman spectroscopy (TERS) [11–25]. By taking advantage of the strong spatial confinement of the nanocavity plasmon [26–28], sub-nanometer resolution Raman images of a single molecule have been obtained, even resolving vibrational modes [22,25,29–31], which shows a great potential for structural determination. In this study, we present a new methodology for structural determination, which we have called ‘scanning Raman picoscopy’ (SRP), that can be utilized for visually constructing the chemical structure of a single molecule. It is achieved by taking advantage of three key elements. First, the full mapping of individual vibrational modes with ångström-level resolution allows the placements of atoms or chemical bonds to be visually determined. Second, the position-dependent interference effect for local symmetric and anti-symmetric vibrations enables the connectivity of the chemical groups involved to be identified. The third element is the combination of spectromicroscopic images and Raman fingerprints for different chemical groups that conclusively ensures the definite arrangement of constituent components of a single molecule. We demonstrate that the construction of a single Mg-porphine model molecule requires only a few vibrational images through a simple Lego-like building process. The protocol established in this proof-of-principle demonstration is expected to stimulate active research in the field as it develops into a mature and universal technology. To highlight the delicate structure-resolving power of this Raman-based scanning technique, a terminology of scanning Raman picoscopy is adopted for such atomistic near-field tip-enhanced Raman spectromicroscopy.
RESULTS AND DISCUSSION
All STM imaging and Raman spectral measurements were performed on a custom-built optical-STM system operating under ultrahigh vacuum (∼5.0 × 10−11 torr) and in liquid-helium cryogenic conditions (∼7 K) (see Supplementary material S1 in the online supplementary data for more details). The SRP imaging was carried out through a synchronization function between the STM controller and CCD camera, acquiring a Raman spectrum at each pixel during scanning. As shown in the experimental setup in Fig. 1a, a single Mg-porphine model molecule adsorbed on the Ag(100) surface is excited by a confined plasmonic field generated at the apex of a Ag tip with atomic sharpness. The STM topography in Fig. 1a indicates that the molecular size is about 1 nm. Figure 1b shows typical Raman spectra for three representative positions labeled in Fig. 1a (blue, red and green dots represent center, lobe and gap positions, respectively). Although the lateral distances among these three positions are only 3–5 Å, distinct intensity differences for different spectral peaks can already be observed. By scanning the tip over the target molecule, a series of SRP mapping images for all labeled Raman peaks are obtained (Fig. 1c), which represent the nicest vibrational images that have been experimentally observed. Each vibration shows its own characteristic image with rich details, highlighting the extraordinary power of the SRP technique. It can be estimated from the line profile of the vibrational image at 3072 cm−1 in Fig. 1d that the spatial resolution (defined by the full width at the half-maximum) can reach 1.5(1) Å (Fig. 1e). Such a high resolution enables vibrational imaging at the single-chemical-bond level. It is known that each vibrational mode is closely related to the collective motion of different atoms, which provides the information about the placement of specific atoms and their connectivity. An overall analysis of different vibrational images thus offers sufficient information for visually constructing the chemical structure of the target molecule.
Figure 1.
Ångström-resolved Raman images of distinct vibrational modes for a single molecule by scanning Raman picoscopy. (a) Schematic diagram of SRP technique. The nanocavity defined by the silver tip and substrate generates a strong and highly confined plasmonic field, which is used for the excitation and emission enhancement of the Raman signals from a single molecule. The STM topograph of a single target molecule adsorbed on Ag(100) is shown at the bottom (−0.02 V, 2 pA, 2.5 nm × 2.5 nm). (b) Typical Raman spectra acquired at representative positions labeled in (a): lobe (red), gap (green) and center (blue). The spectrum on the bare Ag surface is also shown in black, confirming the clean tip condition free of contamination. Spectral acquisition condition: −0.02 V, 8 nA, 30 s. (c) SRP spatial mapping images (−0.02 V, 8 nA, 2.5 nm × 2.5 nm, 25 × 25 pixels, 2 s per pixel) corresponding to the peaks labeled in (b), revealing different spatial distribution patterns for different Raman modes. (d) SRP image at 3072 cm−1 used for the estimation of spatial resolution. (e) Line profile of Raman signal intensities corresponding to the dashed line in (d), exhibiting a lateral spatial resolution down to 1.5(1) Å.
The high spatial resolution of SRP images for a specific vibrational mode Qk results from the confinement of the plasmonic field at the nanoscale [22,25,30–32]. The Raman intensity for this mode is related to the field-related vibronic transition moment between the vibronic ground (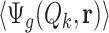 ) and vibronic excited states (
) and vibronic excited states ( ) (see Supplementary material S2 in the online supplementary data for details), i.e.
) (see Supplementary material S2 in the online supplementary data for details), i.e.
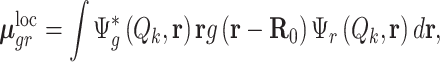 |
(1) |
in which g(r − R0) is the confined field distribution function centered at the tip position R0. In the representation of atomic orbital basis, the wavefunctions of the ground and excited state can be described by
 |
and
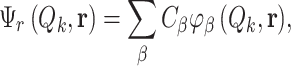 |
respectively. Thus,
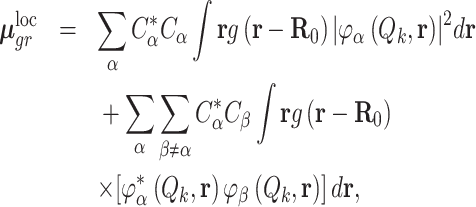 |
(2) |
where ϕα(β) is the atomic orbital of the atom α(β). For a realistic field distribution at the ångström level, two possible situations could occur. When the tip is on the top of an atom α, the Raman signal from this atom becomes dominated, as represented by the first term of Equation (2). When the tip is located at the middle of two atoms, an interference effect is expected to take place due to the cross-term 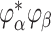 . A positive sign of this term from a symmetric vibrational motion gives a constructive signal, whereas a negative sign from an anti-symmetric vibrational motion results in a destructive signal (see Supplementary material S2 for details). The conceptual demonstration of the interference effect above through Equations (1) and (2) can also be applied to the case where two chemical bonds or multi-centers are covered by the plasmonic field. In other words, in-phase (out-of-phase) local vibrations carry the same (opposite) sign in polarization. The high spatial resolution of out-of-phase anti-symmetric vibrational modes (e.g. the 3072 cm−1 mode in Fig. 1d) arises from the null integral under the sampling window of the local field due to the sign change.
. A positive sign of this term from a symmetric vibrational motion gives a constructive signal, whereas a negative sign from an anti-symmetric vibrational motion results in a destructive signal (see Supplementary material S2 for details). The conceptual demonstration of the interference effect above through Equations (1) and (2) can also be applied to the case where two chemical bonds or multi-centers are covered by the plasmonic field. In other words, in-phase (out-of-phase) local vibrations carry the same (opposite) sign in polarization. The high spatial resolution of out-of-phase anti-symmetric vibrational modes (e.g. the 3072 cm−1 mode in Fig. 1d) arises from the null integral under the sampling window of the local field due to the sign change.
The basic information desirable for constructing a molecular structure is the type of atomic elements of the target molecule, which are known to consist of C, N, H and Mg elements for Mg-porphine. The large collection of Raman spectra for different molecules in literature and database provides tentative assumptions about the plausible functional groups involved. We start the assembling from certain well-defined Raman spectral features associated with the highly localized carbon–hydrogen (C−H) stretching vibrations in the range of 2800–3400 cm−1 typically reported in the literature [33]. Upon slightly changing the tip position by about 0.2 nm from the blue dot position to the more symmetric green dot position (Fig. 2a), the Raman spectrum evolves from a two-peak feature into a single-peak feature at 3092 cm−1 with the complete disappearance of the 3072 cm−1 peak, as shown in Fig. 2b. Such evolution suggests the existence of two types of C–H vibrations with different characters in the small vicinity. In fact, these two vibrations can be identified as the sp2 C−H stretching modes since the observed C−H stretching frequencies fall into the sp2 C−H stretching region [33,34]. The SRP images for these two modes show distinct patterns in spatial distribution, as illustrated in Fig. 2c and d. The one at 3072 cm−1 is composed of ‘eight bright dots’ while the other at 3092 cm−1 consists of ‘four lobes’, which can be well explained by the presence of the interference effect proposed above (see Supplementary material S2 online for more details). Specifically, the well-resolved eight small dots mark the positions of eight C−H bonds, stemming from the out-of-phase destructive interference associated with an anti-symmetric vibration shown on the right of Fig. 2c (see Supplementary Video in the online supplementary data for details). As a result, the intensities in-between the neighboring dots become the weakest. On the other hand, each lobe in the image for the 3092 cm−1 mode is generated from the in-phase constructive interference between two neighboring C−H bonds associated with a symmetric vibration (right of Fig. 2d), which leads to the brightest spot in the lobe close to the center of two connected C−H bonds. The first Lego piece of the molecule can thus be determined to be a H−C = C−H group, and the positions of the four pieces are illustrated in Fig. 2e.
Figure 2.

Interference effect between two neighboring sp2 C−H stretching vibrations. (a) STM topograph of the target molecule (−0.02 V, 2 pA) with the dashed line marking the outline of the molecule. (b) Typical single-pixel Raman spectra extracted from SRP images in high-wavenumber region acquired on slightly different lobe positions of the molecule. Blue (green) spectrum corresponds to the blue (green) dot position marked in (a). The characteristic spectral regions of different C−H stretching vibrations are marked on the top. (c) and (d) SRP mapping images for the two high-wavenumber Raman peaks at 3072 cm−1 and 3092 cm−1, showing destructive and constructive interference features, respectively. The dashed boxes on the right show schematic atomic vibrations, with the red arrow pairs illustrating the anti-symmetric and symmetric vibrations. (e) Partially determined molecular structure with four H−C = C−H at the lobe positions of the molecule.
Next, we move on to analyze the second spectral region in 1300–1700 cm−1 correlated with C = C stretching vibrations. The strong position-dependent spectral features shown in Fig. 3a ensure a large contrast for the SRP images. Indeed, four vibrational images at 1359 cm−1, 1377 cm−1, 1463 cm−1 and 1499 cm−1 in Fig. 3b all clearly exhibit a ‘four-lobe’ structure and each lobe has a size of about 3 Å. Considering the ultrahigh spatial resolution of 1.5 Å achieved (Fig. 1e) and the presence of sp2 carbon atoms involved in C−H stretching vibrations (Fig. 2e), the absence of detailed structures within the lobe itself suggests similar Raman polarizabilities over the lobe. In other words, the electronic density over the lobe is likely to be polarized together, thus suggesting a conjugated ring structure. The SRP images in Fig. 3b for the four vibrational modes also reveal the phase relations between the vibrations of these four conjugated rings. The sharper contrast of four lobes for modes 1359 cm−1 and 1463 cm−1, together with negligible intensities between the lobes and at the molecular center, suggest a destructive interference associated with the anti-symmetric vibrational motions between neighboring rings. By contrast, the other two images at 1377 cm−1 and 1499 cm−1 exhibit a smaller contrast with considerable intensities between the neighboring lobes, resulting from a constructive interference associated with the symmetric vibrational motions. Moreover, the measurable intensities at the molecular center for these two symmetric modes clearly imply the presence of an atom in the center that is chemically connected to the rings (Fig. 3a). This is based on the fact that the molecular cavity size is over 4 Å, much larger than the 1.5 Å spatial resolution; consequently, the interference effect would be too small to generate the intensity in the center. With the help of the Raman frequency analysis shown in Fig. 3a, one can thus conclude that the most likely structure of the ring is a five-membered pyrrole with a N atom capable of bonding to a metal. Thus, we can further build up the molecular structure by placing four pyrrole rings in the lobe positions, as shown in Fig. 3b.
Figure 3.

Vibrational analysis for pyrrole-ring vibrations. (a) Typical single-pixel Raman spectra in the intermediate wavenumber region acquired on the representative positions labeled in the inset STM topograph (red for lobe, green for gap and blue for center). Characteristic fingerprint regions for ring vibrations of pyrrole and pyridine are marked on the top [33]. (b) SRP mapping images for the four Raman peaks at 1359 cm−1, 1377 cm−1, 1463 cm−1 and 1499 cm−1 marked in (a). The schematic vibrations and phase relations between pyrrole rings are shown on the right of each SRP image based on the partially determined molecular structure. The pink (blue) pentagon represents the dominant ‘compression’ (‘stretching’) motion of the pyrrole ring. The vibration and phase relation for the 1475 cm−1 mode are analyzed in Supplementary material S3 in the online supplementary data.
The next Lego piece is the one that connects the four pyrrole rings. At the vicinity of the connecting positions, the image for the vibrational mode at 841 cm−1 shown in Fig. 4a provides the direct evidence, where four nearly isolated spots can be observed. It is known from the Raman frequency analysis that the vibrations in this frequency region are associated with C−H out-of-plane bending motions, although the C−H bending motion is not as local as the C−H stretching vibration. The possibility of a conjugated N atom acting as the bridging unit, as usually seen in porphyrazine [35], can be excluded chemically since no N−H bonds are expected for a bridging N atom, not to mention the appearance of related out-of-plane vibrations. Further support for the assignment of the bridging unit to a C−H bond is the SRP image at 925 cm−1 for another type of C−H out-of-plane bending vibration (Supplementary material S3 in the online supplementary data). The brightest spot center corresponds to the bridging C−H while the elongated feature on the two sides is likely to arise from the in-phase out-of-plane bending vibrations of the two neighboring C−H on the pyrrole rings. Such a feature is not possible if the bridging unit is a N atom. The determination of the bridging ‘Lego’ allows us to further build up a nearly complete molecular structure, which is illustrated in Fig. 4c, showing a nice porphine structure.
Figure 4.
Completing full molecular structure by assembling bridging units and central metal atom. (a) and (b) SRP mapping images for the Raman peaks at 841 cm−1 (a) and 925 cm−1 (b), respectively. (c) Partially determined molecular structure including the bridging units. (d) and (e) SRP mapping images for the two low-wavenumber Raman peaks at 211 cm−1 (d) and 362 cm−1 (e), respectively. (f) Fully determined molecular structure of the Mg-porphine molecule. (g) Merged SRP image by overlaying four different image patterns shown on the right for the vibration modes at 211 cm−1, 841 cm−1, 1463 cm−1 and 3072 cm−1. (h) Artistic view of the Mg-porphine molecule showing how four colored ‘Legos’ in (g) are assembled into a complete molecular structure, with pyrrole rings in red, pyrrole C−H bonds in yellow, bridging C−H bonds in green and central Mg atom in blue.
The last step is to determine the position of the metal atom in the center, which can be easily realized by analyzing the images of low-frequency vibrations at 211 cm−1 and 361 cm−1, respectively, as shown in Fig. 4d and e. The large centralized spot indicates that a metal atom is chemically connected with the surrounding groups, which again confirms the assignment of pyrrole groups. The relatively large spot size suggests that the motion of the metal atom can cause wider electron density changes, beyond the porphine core area. The center atom can be assigned to Mg, since these two vibrational frequencies agree well with the Mg−N bond vibrations (Supplementary material S3) reported for Mg-porphine [36–38]. It should be noted that the observed SRP images in Fig. 1c all exhibit an approximately four-fold symmetry, thus ruling out the possibility of a metal-free porphine.
With the last piece in place, the chemical structure of the target Mg-porphine molecule is fully determined in real space in Fig. 4f. Moreover, the colored merged Raman image in Fig. 4g, generated by overlaying the representative individual vibrational images showing on the right side, clearly demonstrates that the spatial arrangement of individual chemical groups nicely coincides with the artistic view of the Mg-porphine molecule in Fig. 4h. The computed SRP images for the representative Raman modes that have been employed to construct the molecular structure agree very well with their experimental counterparts (see Supplementary material S4 in the online supplementary data for details), further confirming the experimental observation of full vibrational images and justifying the validity of the methodology proposed here.
CONCLUSIONS
We have presented a new structural determination methodology (SRP) for visually assembling the chemical structure of a single molecule. It is achieved by combining Raman spectral fingerprints for individual chemical groups with ångström-resolved Raman images and the interference effects involved. The rich spectral data and detailed spatial images of various vibrational modes themselves are already sufficient to provide a panoramic and global view of the molecular structure, which is more comprehensive than the verbal descriptions could give here. The Lego-like building process employed here can be easily generalized with the aid of imaging recognition and machine learning techniques, or by further combination with non-contact AFM and inelastic tunneling probe techniques. The SRP protocol established in this proof-of-principle demonstration can be widely applied for identifying the chemical structure of different materials at the level of chemical bonds.
Supplementary Material
Acknowledgements
We thank Prof. B. Wang for helpful discussions.
FUNDING
This work was supported by the National Key R&D Program of China (2016YFA0200600 and 2017YFA0303500), the National Natural Science Foundation of China, the Chinese Academy of Sciences, and Anhui Initiative in Quantum Information Technologies.
AUTHOR CONTRIBUTIONS
Z.C.D. and J.G.H. conceived and supervised the project. B.Y., A.G., Y.F.Z. and R.P.W. performed experiments and analyzed data. Yao Z. and Y.L. derived the theory and performed theoretical simulations. Yao Z., B.Y., A.G., Yang Z., J.L.Y., Y.L., Z.C.D. and J.G.H. contributed to the data interpretation. Yao Z., B.Y., Y.L., Z.C.D. and J.G.H. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Conflict of interest statement . None declared.
REFERENCES
- 1. Günther H. NMR Spectroscopy Basic Principles, Concepts and Applications in Chemistry. New York: Wiley, 2013. [Google Scholar]
- 2. Scott AI. Interpretation of Ultraviolet Spectra of Natural Products. Amsterdam: Elsevier, 2013. [Google Scholar]
- 3. Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Springer, 2006. [Google Scholar]
- 4. Butler HJ, Ashton L, Bird Bet al.. Using Raman spectroscopy to characterize biological materials. Nat Protoc 2016; 11: 664–87. [DOI] [PubMed] [Google Scholar]
- 5. Stipe BC, Rezaei MA, Ho W. Single-molecule vibrational spectroscopy and microscopy. Science 1998; 280: 1732–5. [DOI] [PubMed] [Google Scholar]
- 6. Kim Y, Komeda T, Kawai M. Single-molecule reaction and characterization by vibrational excitation. Phys Rev Lett 2002; 89: 126104. [DOI] [PubMed] [Google Scholar]
- 7. Hou JG, Yang JL, Wang HQet al.. Topology of two-dimensional C60 domains. Nature 2001; 409: 304–5. [DOI] [PubMed] [Google Scholar]
- 8. Chiang CL, Xu C, Han ZMet al.. Real-space imaging of molecular structure and chemical bonding by single-molecule inelastic tunneling probe. Science 2014; 344: 885–8. [DOI] [PubMed] [Google Scholar]
- 9. Giessibl FJ. Atomic resolution of the silicon (111)-(7x7) surface by atomic force microscopy. Science 1995; 267: 68–71. [DOI] [PubMed] [Google Scholar]
- 10. Gross L, Mohn F, Moll Net al.. The chemical structure of a molecule resolved by atomic force microscopy. Science 2009; 325: 1110–4. [DOI] [PubMed] [Google Scholar]
- 11. Stöckle RM, Suh YD, Deckert Vet al.. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem Phys Lett 2000; 318: 131–6. [Google Scholar]
- 12. Anderson MS. Locally enhanced Raman spectroscopy with an atomic force microscope. Appl Phys Lett 2000; 76: 3130–2. [Google Scholar]
- 13. Hayazawa N, Inouye Y, Sekkat Zet al.. Metallized tip amplification of near-field Raman scattering. Opt Commun 2000; 183: 333–6. [Google Scholar]
- 14. Pettinger B, Picardi G, Schuster Ret al.. Surface enhanced Raman spectroscopy: towards single molecular spectroscopy. Electrochemistry 2000; 68: 942–9. [Google Scholar]
- 15. Zhang WH, Yeo BS, Schmid Tet al.. Single molecule tip-enhanced Raman spectroscopy with silver tips. J Phys Chem C 2007; 111: 1733–8. [Google Scholar]
- 16. Neacsu CC, Dreyer J, Behr Net al.. Scanning-probe Raman spectroscopy with single-molecule sensitivity. Phys Rev B 2007; 73: 193406. [Google Scholar]
- 17. Steidtner J, Pettinger B. Tip-enhanced Raman spectroscopy and microscopy on single dye molecules with 15 nm resolution. Phys Rev Lett 2008; 100: 236101. [DOI] [PubMed] [Google Scholar]
- 18. Bailo E, Deckert V. Tip-enhanced Raman spectroscopy of single RNA strands: towards a novel direct-sequencing method. Angew Chem Int Ed 2008; 47: 1658–61. [DOI] [PubMed] [Google Scholar]
- 19. Yano TA, Verma P, Saito Yet al.. Pressure-assisted tip-enhanced Raman imaging at a resolution of a few nanometres. Nat Photon 2009; 3: 473–7. [Google Scholar]
- 20. Liu Z, Ding SY, Chen ZBet al.. Revealing the molecular structure of single-molecule junctions in different conductance states by fishing-mode tip-enhanced Raman spectroscopy. Nat Commun 2011; 2: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sonntag MD, Klingsporn JM, Garibay LKet al.. Single-molecule tip-enhanced Raman spectroscopy. J Phys Chem C 2012; 116: 478–83. [Google Scholar]
- 22. Zhang R, Zhang Y, Dong ZCet al.. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature 2013; 498: 82–6. [DOI] [PubMed] [Google Scholar]
- 23. Jiang S, Zhang Y, Zhang Ret al.. Distinguishing adjacent molecules on a surface using plasmon-enhanced Raman scattering. Nat Nanotechnol 2015; 10: 865–9. [DOI] [PubMed] [Google Scholar]
- 24. Zhang R, Zhang XB, Wang HFet al.. Distinguishing individual DNA bases in a network by non-resonant tip-enhanced Raman scattering. Angew Chem Int Ed 2017; 56: 5561–4. [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Crampton KT, Tallarida Net al.. Visualizing vibrational normal modes of a single molecule with atomically confined light. Nature 2019; 568: 78–82. [DOI] [PubMed] [Google Scholar]
- 26. Alonso-González P, Albella P, Schnell Met al.. Resolving the electromagnetic mechanism of surface-enhanced light scattering at single hot spots. Nat Commun 2012; 3: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barbry M, Koval P, Marchesin Fet al.. Atomistic near-field nanoplasmonics: reaching atomic-scale resolution in nanooptics. Nano Lett 2015; 15: 3410–9. [DOI] [PubMed] [Google Scholar]
- 28. Benz F, Schmidt MK, Dreismann Aet al.. Single-molecule optomechanics in ‘picocavities’. Science 2016; 354: 726–9. [DOI] [PubMed] [Google Scholar]
- 29. Chiang NH, Chen X, Goubert Get al.. Conformational contrast of surface-mediated molecular switches yields Ångstrom-scale spatial resolution in ultrahigh vacuum tip-enhanced Raman spectroscopy. Nano Lett 2016; 16: 7774–8. [DOI] [PubMed] [Google Scholar]
- 30. Duan S, Tian GJ, Ji YFet al.. Theoretical modeling of plasmon-enhanced Raman images of a single molecule with subnanometer resolution. J Am Chem Soc 2015; 137: 9515–8. [DOI] [PubMed] [Google Scholar]
- 31. Liu PC, Chulhai DV, Jensen L. Single-molecule imaging using atomistic near-field tip-enhanced Raman spectroscopy. ACS Nano 2017; 11: 5094–102. [DOI] [PubMed] [Google Scholar]
- 32. Duan S, Tian GJ, Luo Y. Theory for modeling of high resolution resonant and nonresonant Raman images. J Chem Theory Comput 2016; 12: 4986–95. [DOI] [PubMed] [Google Scholar]
- 33. Socrates G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Chichester: John Wiley & Sons, 2004. [Google Scholar]
- 34. Bellamy LJ. The Infra-Red Spectra of Complex Molecules. New York: Springer, 2013. [Google Scholar]
- 35. Kopranenkov VN, Luk’yanets EA. Porphyrazines: synthesis, properties, application. Russ Chem Bull 1995; 44: 2216–32. [Google Scholar]
- 36. Jarzçcki AA, Kozlowski PM, Pulay Pet al.. Scaled quantum mechanical and experimental vibrational spectra of magnesium and zinc porphyrins. Spectrochim Acta A 1997; 53: 1195–209. [Google Scholar]
- 37. Ogoshi H, Saito Y, Nakamoto K. Infrared spectra and normal coordinate analysis of metalloporphins. J Chem Phys 1972; 57: 4194–202. [Google Scholar]
- 38. Zhang XX, Zhang YX, Jiang JZ. Infrared spectra of metal-free, N′,N-dideuterio, and magnesium porphyrins: density functional calculations. Spectrochim Acta A 2005; 61: 2576–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




