Abstract
The Ulva prolifera green tides in the Yellow Sea, China, which have been occurring since 2007, are a serious environmental problem attracting worldwide attention. Despite extensive research, the outbreak mechanisms have not been fully understood. Comprehensive analysis of anthropogenic and natural biotic and abiotic factors reveals that human activities, regional physicochemical conditions and algal physiological characteristics as well as ocean warming and biological interactions (with microorganism or other macroalgae) are closely related to the occurrence of green tides. Dynamics of these factors and their interactions could explain why green tides suddenly occurred in 2007 and decreased abruptly in 2017. Moreover, the consequence of green tides is serious. The decay of macroalgal biomass could result in hypoxia and acidification, possibly induce red tide and even have a long-lasting impact on coastal carbon cycles and the ecosystem. Accordingly, corresponding countermeasures have been proposed in our study for future reference in ecosystem management strategies and sustainable development policy.
Keywords: green tides, Ulva prolifera, outbreak mechanisms, eco-environmental effects, Yellow Sea
INTRODUCTION
Massive green tides, which are mainly caused by Ulva prolifera (U. Prolifera) have successively occurred for 12 years (2007–18) in the Yellow Sea off the coasts of Jiangsu and Shandong Province, China [1,2]. In particular, during 2008, massive U. prolifera fronds floated into the coasts of Qingdao, posing a great challenge to the local government owing to the upcoming Olympic Sailing Regatta to be held in Qingdao. The direct economic losses caused by that macroalgal bloom were as high as 1.3 billion RMB [3]. Every spring (from mid-April to early May) since 2007, macroalgal blooms have initially occurred along the Jiangsu coast with small-scale floating algae, then migrated northward along the coast of the southern Yellow Sea driven by monsoons and ocean currents, accumulating in the near-shore waters of the Shandong Peninsula in June and July, and then declined gradually [4]. With the annual coverage area of over 20 000 km2, U. prolifera bloom in the Yellow Sea is the largest green-tide event in the world so far (Fig. 1) [5].
Figure 1.

Variation of the distribution and coverage areas of the U. prolifera green tides from 2007 to 2018 (http://www.soa.gov.cn/zwgk/hygb/zghyzhgb/).
Research and monitoring over 10 years have shown that the outbreak mechanisms of U. prolifera green tides can be attributed to multiple factors. Remote sensing data have demonstrated that U. prolifera green tides originally occurred along the Jiangsu coast [6–13] and gene sequencing data have confirmed that the U. prolifera growing in Porphyra mariculture areas along the Jiangsu coast is the same species as that found in the Yellow Sea [14]. It also reveals that the unique mud flats for cultivation of Porphyra are favourable habitats for U. prolifera and that more than 20 000 ha of mariculture rafts are good substrata for the propagation of U. prolifera [2,5,15]. Moreover, the Porphyra mariculture area is characterized by high nutrient levels (i.e. NO3–N >10 μM, PO4–P > 0.3 μM and SiO3–Si >15 μM), which could almost meet the nutrient demands of U. prolifera for its rapid growth [16]. In addition, a large number of micropropagules of U. prolifera that germinate on the mariculture rafts in seawater or sediments after the Porphyra harvest grow into new fronds as the temperature increases, thus serving as the original species of green tides occurring in the following spring [17,18].
As indicated by morphology and genomics, U. prolifera is considered to be the dominant green-tide-forming seaweed in the Yellow Sea [19–21]. Other species of the Ulva genus, such as U. intestinalis, U. linza, U. compressa and U. clathrate, have also been observed in Porphyra mariculture areas along the Jiangsu coast [22]. However, U. prolifera can outcompete others owing to its higher nutrient-uptake rate, faster growth and stronger reproduction capacity [23]. The proportion of U. prolifera among the raft-attached macroalgae usually increases with Porphyra growth. When the attached macroalgae are discarded into seawater after the Porphyra harvest, the proportion of U. prolifera in floating algae can increase from 63 to 91% within 24 hours under optimal environmental conditions [2,15]. Moreover, besides its own physiological advantages of U. prolifera, appropriate physicochemical conditions (e.g. favourable temperature, salinity and light intensity), southeastward monsoons, etc., can all contribute greatly to the occurrence of green tides [5].
As a matter of fact, the outbreak mechanisms of green tides in the Yellow Sea are far more complicated than had been thought before. In the past decade, various attempts have been made to prevent the annual occurrence of large-scale green tides but were unsuccessful. However, in 2017, U. prolifera green tides decreased abruptly in the Yellow Sea, which aroused further curiosity and attention. Why did U. prolifera green tides first occur in 2007 and decrease remarkably in 2017? What have not been identified so far that are closely related to the green-tide outbreak? We are gradually unravelling the reasons behind this phenomenon.
Massive macroalgal blooms can have a negative impact on the coastal environment; for example, they can seriously upset the balance of coastal ecosystems, affect air–sea exchanges and even cause the death of cultured organisms, such as sea cucumber and shellfish, in the Yellow Sea [24]. During the late development stage of green tides, the decomposition of massively accumulated fronds can degrade seawater quality and cause foul odours (Fig. 2), both of which can seriously interfere with local tourism and coastal mariculture in the Shandong Peninsula. In addition, the massive macroalgal bloom can have a long-term impact on marine biogeochemical cycles.
Figure 2.

Environmental impacts of the U. prolifera green tides as seen from the situation in the Qingdao coasts in July, 2016. (a) Coastlines covered with U. prolifera fronds accumulated by natural tidal movements; (b) U. prolifera fronds bleached under sunlight; (c) decay of U. prolifera fronds.
This review is aimed at a systematic analysis of multiple factors in facilitating the outbreaks of U. prolifera green tides as well as the impact on the environment of the Yellow Sea. Based on the recognition of the outbreak mechanisms of green tides and their following consequences, we proposed corresponding countermeasures to prevent the frequent occurrence of green tides in the future.
THE BLOOMING PROCESS OF ULVA PROLIFERA GREEN TIDES IN THE YELLOW SEA
The U. prolifera green tide is a trans-regional disaster in the Yellow Sea, China. These macroalgal blooms usually originate in the coastal areas of the southern Yellow Sea during mid-April to early May, and start as small patches of free-floating green algae. Subsequently, the free-floating algal patches move northward and northeastward, driven by seasonal monsoons and ocean currents. During the migration period, U. prolifera grows fast and accumulates gradually. In late May, large floating U. prolifera aggregates into long large floating mats. Nearly all the floating macroalgae are observed in linear bands ranging from hundreds of metres to tens of kilometres in the open sea area. In mid-June, floating U. prolifera accumulates with a huge biomass (∼1.5 × 106 tons) and covers a large area in the coastal sea of the Shandong Peninsula. Thereafter, great social and economic losses emerge due to the large algal biomass piling up on beaches and coastal waters. At the end of June, U. prolifera continues to grow, causing the biomass to reach as high as ∼3.5 × 106 tons. In mid-July, U. prolifera reaches its maximum biomass of ∼1.2 × 107 tons. After that, U. prolifera green tides begin to decline. In early August, the floating U. prolifera can only be sporadically observed in the coastal areas [15,25].
REGIONAL FACTORS FOR MACROALGAL BLOOMS
Every spring from 2007, U. prolifera green tides have developed from small-scale floating patches into massive macroalgal blooms, which is closely related to various factors including seawater temperature, salinity, light intensity, wind direction and ocean currents in the Yellow Sea.
Favourable temperature, salinity and light intensity
Seawater temperature has been identified as a key factor in influencing the growth of U. prolifera [2,15]. Ulva prolifera generally grows well when temperature ranges between 10 and 20°C; its biomass can increase by as much as 23% per day when the temperature exceeds 15°C, while it cannot grow when the temperature is below 10°C [26,27]. The daily average temperature ranges from 6 to 21°C along the Jiangsu coast during spring (from March to May), which is ideal for the growth of U. prolifera [15]. Vegetative propagation is one of the main reproduction modes for U. prolifera, which may contribute to the rapid growth of the fronds. The algal fragment of U. prolifera grows by 28% per day at 20°C and only by 3% per day at 10°C in laboratory incubations [28]. Moreover, the suitable temperature range for the release of spores and gametes of U. prolifera is 20–30°C [29] and the temperature for the germination of these micropropagules is 15–25°C [18,22,30]. This, to some extent, also accounts for the occurrence of annual large-scale macroalgal blooms in late April or early May. Additionally, O2 and CO2 are produced separately in photosynthesis and respiration and fill the fronds of U. prolifera, causing them to float in the surface water [5]. Ulva prolifera can have normal photosynthesis only when the temperature exceeds 10°C; the water temperature during May to June in the Yellow Sea ranges from 15 to 25°C, which is quite suitable for the floating and accumulation of U. prolifera. As mentioned in the introduction, Porphyra mariculture rafts are good substrata for the early propagation of U. prolifera. Considering the obvious impact of temperature on the growth of U. prolifera, earlier harvest of Porphyra in the southern Jiangsu Province, especially before the temperature reaches over 10°C in April, may reduce the biomass of U. prolifera fall off the rafts and thus lower the outbreak scale of green tides [31].
The impact of salinity on U. prolifera cannot be ignored either. Suitable salinity for the growth of U. prolifera has been reported to range between 8 and 32 [32]. The photosynthetic responses of U. prolifera to short-term salinity fluctuations indicate that U. prolifera is quite sensitive to salinity changes. The photosynthetic rate is significantly higher with high salinity (26–32), optimum at 32 and low salinity (<8) has adverse effects on the photosynthesis. In coastal waters of the Yellow Sea, the salinity usually varies between 28 and 31 during the spring and summer periods (from April to June), which aligns within the suitable salinity ranges for the growth of U. prolifera [22]. Besides its effects on growth, salinity also influences the reproduction of U. prolifera; for example, lower salinity (i.e. 20) promotes its sporangium formation and increases spore germination as well [33]. Furthermore, the growth of U. prolifera was compared under different temperatures (i.e. 15 or 22°C) and salinities (i.e. 10 or 25), the results of which indicate that, with low salinity (i.e. 10), the growth of U. prolifera decreases compared with high salinity (i.e. 25) [34]. All of the above results indicate that U. prolifera can endure drastic variability in salinity and modest salinity variations, to some extent, can benefit its growth and blooming.
Apart from temperature and salinity, light intensity also affects the physiology of U. prolifera, including the growth, photosynthesis, reproduction, nutrient uptake, utilization, etc. [4,35]. Light promotes algae growth, but excessive light inversely inhibits their growth. However, microalgae often have a high tolerance to light conditions [26], owing to their various photosynthetic adaptation strategies, like changing cellular pigment components or dissipating excess energy via photosynthetic quantum control (energy quenching and energy redistribution between PSII/PSI) [21]. For the green-tide-forming species U. prolifera, suitable light intensity to drive its growth has been reported to vary from 18 to 175 μM photons m−2 s−1 [26]. Of course, such light intensity for growth also varies with changes in other environmental factors like nutrients, salinity and temperature [21,35]. Under ammonium-enrichment conditions, its maximum photosynthetic rate can reach 370 μM O2 g FW−1 h−1 at the optimal light level of 120 μM photons m−2 s−1 [35]. The light intensity also affects the reproduction of U. prolifera by regulating the release or germination of spores and gametes. The optimal light level for the release of spores and gametes has been reported to be 240 μM photons m−2 s−1 while, for their germination, it is 120 μM photons m−2 s−1 [36]. For asexual reproduction, however, the highest reproduction rate has been found at a light intensity of 100 μM photons m−2 s−1 [28]. Furthermore, light provides energy to drive nutrient absorption by algae. Ulva prolifera was observed to have optimal ammonium absorption at a light level of 80 μM photons m−2 s−1; as the light level decreases, the nitrogen balance within the fronds is disrupted, leading to non-absorption and even excretion of ammonium [37]. Light intensity in the Yellow Sea varies between 60 and 140 μM photons m−2 s−1 during May to June, which can thus fully satisfy the growth of U. prolifera [9].
Monsoons and ocean currents drive the northward migration of U. prolifera
The southeastward monsoons prevail in the Yellow Sea from April to July, driving surface ocean currents northwards and thus the northward migration of U. prolifera fronds [9,38,39]. Wind direction is one of the most important factors affecting the migration direction of U. prolifera. Remote sensing data show that the distribution of U. prolifera in surface waters appears as a linear band and is consistent with the main path of monsoons [40]. In the Jiangsu coastal area, the U. prolifera fronds that are scraped off the Porphyra mariculture rafts or the fragments mixed up from the bottom by upwelling are scattered throughout the surface water [9,37]. These fronds flow through the sand-ridge channels and out to the open sea with the residual tidal currents. When U. prolifera reaches the open sea, the dynamics of the algal accumulation are driven by a complex ocean dynamic process, including the southeast monsoons, Ekman drift, Langmuir circulation, Subei and Lunan coastal currents [39–41]. In addition to the main northward path, a new southward path along the Subei coastal current was identified, extending from the Jiangsu coast to the East China Sea [12]. The muddy coastal current with U. prolifera fronds reaches the southwestern East China Sea in early May along with severe local eutrophication, leading to the occurrence of green tides in this new area [42,43]. Moreover, in 2009, the wind direction of monsoons was quite different from that in other years, with the southwest monsoon prevailing in the Yellow Sea. Driven by the southwest monsoons, only a very few floating patches of U. prolifera could reach the coastline of the Shandong Peninsula [44]. As U. prolifera did not pile up at the coastline as usual, instead most was floating in the ocean, so the U. prolifera green tides in the Yellow Sea in 2009 had the largest distribution area during the past decade.
ANTHROPOGENIC PERTURBATIONS AND MACROALGAL BLOOMS
Increasing atmospheric CO2 and global warming promote the photosynthetic carbon fixation of U. prolifera
Global warming has become one of the most important issues worldwide and is mainly attributed to increasing population density and intensive anthropogenic activities [45]. Coastal ecosystems are suffering from various negative effects caused by increasing temperature, which can be embodied as harmful red/green tides, anoxia and storm surges. In recent years, global temperature has increased rapidly and the annual global surface temperature anomaly has increased by 0.6°C since 2008, as compared to the average temperature from 1951 to 1980 (Fig. 3). Meanwhile, global CO2 concentration is also increasing. In October 2016, the World Meteorological Organization (WMO) in Geneva claimed that the global average CO2 concentration reached 400 ppm for the first time in 2015 [46]. In 2017, the atmospheric concentration of CO2 reached 406 ppm according to the latest WMO dataset. In fact, both the rising temperature and atmospheric CO2 facilitate the growth and photosynthetic carbon fixation of phytoplankton. Thus, we inferred that rising atmospheric CO2 and continuous global warming primarily caused by anthropogenic activities are likely to be among the important factors that facilitate the early occurrence and continuous expansion of green tides. Ocean acidification has a typical negative impact on the increasing of atmospheric CO2 and global warming. Laboratory studies have indicated that ocean acidification and warming could accelerate the onset and magnitude of gamete settlement and increase the reproduction of Ulva rigida—a causative green-tide species closely related to U. prolifera [47]. Based on the model, it is also predicted that the bloom-forming Ulva would dominate coastal regions as populations of non-blooming species would decrease faster and maintain lower growth rates, even under mild carbon enrichment [48]. Thus, climate changes are considered to be possibly responsible to some extent for more severe green tides, particularly in a scenario in which eutrophication cannot be effectively controlled [47].
Figure 3.
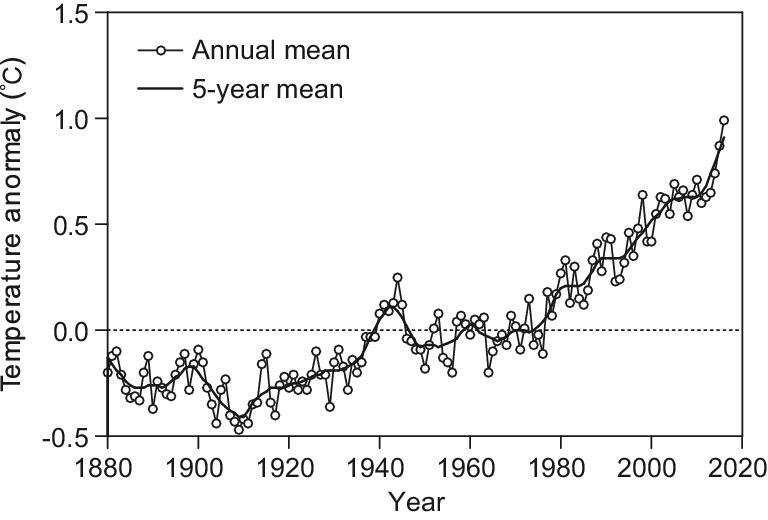
Changes in global surface temperature from 1884 to 2015, as compared to the averaged 1951–80 temperature indicated by the thin dotted line. Source: NASA GISS, https://climate.nasa.gov/vital-signs/global-temperature/.
Increasing nitrogen levels support the growth of U. prolifera
Green macroalgae blooms are occurring more frequently and the magnitudes of the blooms are getting larger worldwide. In recent decades, the Baltic Sea, Knysna Estuary, South Africa, Biscayne Bay, Narragansett Bay, South Korea and Japan coasts all have suffered from massive macroalgal blooms [46,49–55]; Brittany (France) has even been experiencing large-scale macroalgal blooms annually since the 1970s [56], which are closely associated with coastal eutrophication [57].
With the increase in population and rapid economic development, nutrient loadings have been influenced by anthropogenic perturbations in the coastal waters of the Yellow Sea [16,58]. At present, eutrophication along the Jiangsu coast is becoming more and more serious. The area-weighted nutrient pollution (AWCPI-NP) index of this area for 2007–12 increased by 45% compared to that of 2001–06 (P < 0.05) [59]. The Sheyang and Guan Rivers are the two largest rivers flowing through Jiangsu Province. The annual N and P loadings of these two rivers into coastal waters between 2007 and 2012 were approximately three to five times higher than those between 2004 to 2006 [16]. Moreover, the Jiangsu coastal area is the largest shrimp and crab aquaculture zone in China, with thousands of aquaculture ponds along the coast. Over 50 000 tons of fertilizers such as fermented chicken manure, which is rich in organic N and P, are poured into the ponds yearly [60–62]. Discharge of nutrient-rich wastewater from these ponds supplies abundant nutrients to near-shore waters. As shown in Fig. 4, the N and P loadings markedly increased from 2003 to 2016 due to both river inputs and aquaculture wastewater discharge along the Jiangsu coast, which contributed greatly to the eutrophication of this coastal area. Besides the Yellow Sea, the Gouqi Island (in the East China Sea) underwent a U. prolifera green tide for the first time in 2012, indicating a close relationship between U. prolifera green tides and severe local eutrophication [43].
Figure 4.
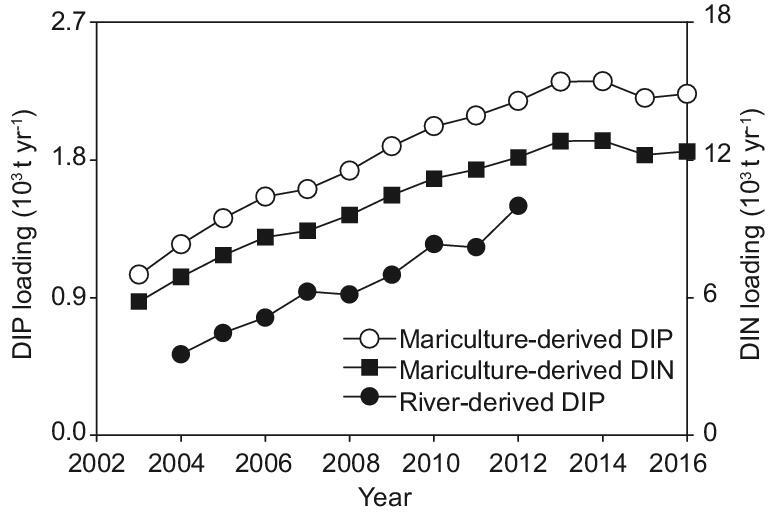
Nutrient loadings from river inputs and aquaculture along the Jiangsu coast from 2003 to 2016. Nutrient loadings from river inputs refer to the sum from the Guanhe and Sheyang Rivers; nutrient loadings from aquaculture are calculated based on yearly aquaculture total production (wet weight) (data between 2003 and 2012 obtained from [71], data between 2013 and 2016 obtained from the Jiangsu Fishery Statistical Yearbook). DIN, dissolved inorganic nitrogen; DIP, dissolved inorganic phosphorus.
Nitrogen and phosphorus are two essential nutrients for the growth of U. prolifera [63,64]. The maximum growth rate of U. prolifera can reach 56% day−1 with sufficient N and P supplies [4]. Dissolved inorganic nitrogen (DIN) is particularly critical for the growth of U. prolifera. Based on a mesocosm experiment, U. prolifera fronds can grow vigorously by consuming DIN quickly, even though the P concentration is fairly low in seawater [64]. Lianyungang, Yancheng and Nantong are three coastal cities situated along the Jiangsu coast where DIN concentrations vary from 10 to 80 μM. The DIN values are only 0.80–5.8 and 0.70–16 μM in the Yellow Sea and in Qingdao coastal waters, respectively [5]. Based on nine surveys conducted between March and September 2012, DIN concentration in the surface water gradually decreased with the green-tide bloom, whereas the lowest DIN level was still over 7.0 μM in the coastal waters of Jiangsu Province [58]. Moreover, the average DIN concentration in the surface water has increased by two to three times along the Jiangsu coast since 1996 to the most recent 3 years (Fig. 5a), indicating that the level of N is sufficient for macroalgal blooms [2]. The PO4–P concentration showed a decreasing trend during the study period (Fig. 5b) and the unbalanced changes in N and P caused a significant increase in the N/P ratio during 2005–14 (Fig. 5d). Ulva prolifera might adapt well to high N/P conditions and grow vigorously under P-limited conditions [16,64]. There were obvious changes in the silicate concentration and Si/N and Si/P ratios from 1996 to 2017 (Fig. 5c, e, f), but Si is generally thought to be a non-limiting factor for the growth of U. prolifera [65].
Figure 5.
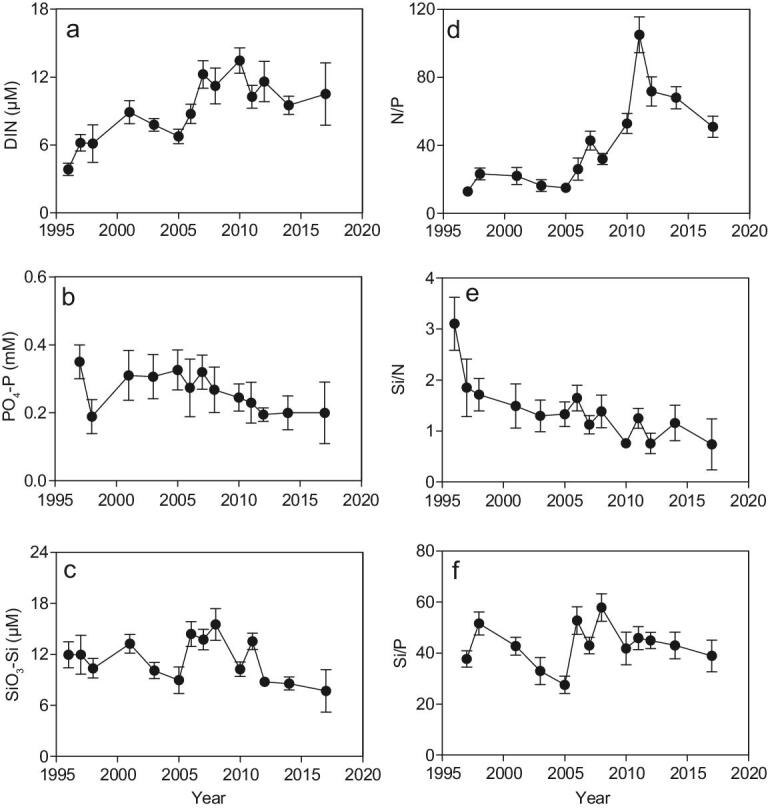
Variations in DIN (a), PO4–P (b) and SiO3–Si (c) concentrations and N/P (= DIN/PO4–P) (d), Si/N (= SiO3–Si/DIN) (e) and Si/P (= SiO3–Si/PO4–P) (f) ratios in the surface waters (0–0.5 m) of the Jiangsu coast. The data were collected from March to May (data between 1996 and 2014 were obtained from [71]; there are no data during 2015–16; data of 2017 were obtained from field surveys).
Porphyra mariculture rafts provide substrata for the macroalgal blooms
Besides eutrophication, the rapid expansion of Porphyra mariculture is another important anthropogenic factor that contributes to the development of green tides [4]. Jiangsu Province has a long history of Porphyra mariculture, with the annual fresh Porphyra production reaching approximately 12 600 tons, and it is the second largest Porphyra yezoensis mariculture zone in East Asia, closely following Japan [60,66,67]. The annual Porphyra mariculture starts in August and ends in the following April, during which massive ropes, bamboo poles and nylon nets are installed in the mariculture regions [59]. At present, the coverage area for Porphyra mariculture is approximately 4.1 × 104 ha with about 4500 m of ropes per ha being used for the cultivation, which is thought to provide substrata for U. prolifera [16]. When Porphyra is harvested from mid-April to mid-May, U. prolifera fronds that are attached to the rafts fall into seawater, together with the U. prolifera fragments removed from the ropes by local farmers, both of which provide the most direct and initial biomass supply for the green tides [68]. Annually, approximately 6500 tons of U. prolifera fall off the Porphyra rafts, 62% of which float in the surface water, spread out with wind and ocean currents, and finally bloom into massive green tides [15]. The area used for Porphyra mariculture in 2016 was about eight times larger than that in 1995, which clearly increased since the first macroalgal bloom in 2007 (Fig. 6). The sharp increase of Porphyra mariculture area in 2007 is possibly one of the most important causes leading to the outbreak of the world's largest U. prolifera green tide in 2008. Before 2006, most Porphyra mariculture activities were in the intertidal zone near the coastline of Jiangsu Province. The U. prolifera that falls from the Porphyra rafts could hardly reach the offshore areas due to the reverse movement of tidal currents. However, in the following few years after 2006, the Porphyra mariculture expanded very rapidly to the out area of the sand ridges along the Jiangsu coast. These sand ridges are about 200 km long and 100 km wide. The unique radial geomorphology there can frequently induce the formation of upwelling in the sand-ridge channels. Meanwhile, under the driving force of southeast wind, the large amounts of U. prolifera fragments falling from Porphyra mariculture rafts floated on the surface water and moved northwest. It eventually induced the outbreak of large-scale green tides in the Yellow Sea near the Shandong Peninsula in 2008 [4,5,8,9].
Figure 6.
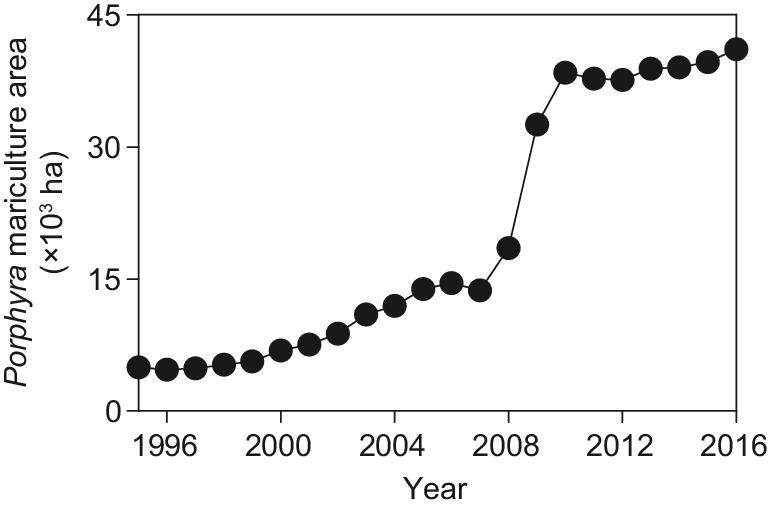
Annual variations in the Porphyra mariculture area in Jiangsu Province between 1995 and 2015 (data obtained from the Chinese Fishery Statistical Yearbook).
BIOLOGICAL CHARACTERIETICS OF ULVA PROLIFERA CONFER ITS DOMINANCE IN THE YELLOW SEA
Based on its morphology and genomic characteristics, U. prolifera is considered as the dominant green-tide-forming seaweed in the Yellow Sea. It accounts for over 90% of green-tide macroalgae, owing to its high nutrient-uptake rate, high photosynthetic capacity and multiple reproduction modes. These biological characteristics endow U. prolifera with a strong ability to tolerate changes in seawater temperature, light intensity and nutrient levels during floating. It also owns high growth and reproduction capacities. All these factors might lead to the massive green tides.
High nutrient-uptake rate
Ulva prolifera assimilates inorganic N and P very quickly and has high uptake rates under nutrient-rich conditions [69]. The nitrate- and ammonium-uptake rates of U. prolifera (Vmax-NO3-N = 124 and Vmax-NH4-N = 284 μM g−1 DM h−1, DM refers to the algal dry weight) are higher than those of other Ulva species (e.g. Vmax-NO3-N = 109 and Vmax-NH4-N = 250 μM g−1 DM h−1 for U. linza) in Jiangsu coastal waters. Accordingly, the growth rate of U. prolifera is higher than that of U. linza (SU. prolifera = 13% d−1 vs SU. linza = 10% d−1) [23]. Moreover, eukaryotic microalgae normally cannot survive when co-cultivated with U. prolifera, as most of the nutrients in seawater are absorbed and utilized by U. prolifera [70]. The maximum DIN uptake rate of U. prolifera can be as high as 110 μM g−1 DM h−1 [71], which makes U. prolifera outcompete microalgae by reducing N to levels below which microcells cannot grow [70]. Hence, green and red tides have seldom occurred simultaneously in Chinese coastal waters.
In addition to inorganic nutrients, some low-molecular-weight organic nutrients like urea, glycine and adenosine triphosphate can be absorbed by U. prolifera [71]. The uptake of both inorganic and organic nutrients by macroalgae can be described as a saturating function of substrate concentrations using the Michaelis–Menten equation (Fig. 7). During the early blooms of green tides, U. prolifera is most prone to taking up inorganic nutrients and subsequently large amounts of DIN and dissolved inorganic phosphorus (DIP) are utilized in the development of green tides [71]. Hence concentrations of dissolved inorganic nutrients are relatively low during the mid-late development periods of green tides, while the uptake ability of U. prolifera to organic nutrients satisfies their nutrient demand and subsequent blooms. Temporal variations of dissolved nutrients were investigated in the southern Yellow Sea during the green-tide-blooming period, and it was found that the DIN decreased rapidly and varied from 21 to 8.0 μM during late April and early May (i.e. early blooms). The DIN decreased to below 5 μM in June and July; simultaneously, the concentrations of dissolved organic nitrogen (DON) began decreasing very fast and varied from 28 to 16.5 μM. Even in late July and August (i.e. late blooms), the DON always maintained ∼15 μM in seawater and the DON provided sufficient nutritional conditions for the growth of U. prolifera [72]. Similar results were also observed in another field investigation [58], the concentrations of DON and dissolved organic phosphorus (DOP) decreasing from 31 to 15 μM and from 0.40 to 0.10 μM during the macroalgal blooms. These results indicate that, besides inorganic forms, organic nutrients indeed play an important role in the growth of U. prolifera [4,15], especially during the mid-late blooms of green tides.
Figure 7.
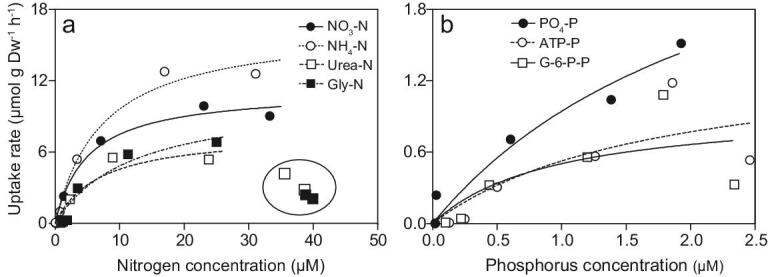
The concentration-dependent uptake rates of (a) dissolved nitrogen and (b) phosphorus of U. prolifera during an incubation experiment (modified from [70]). The fitting curves excluded the circled data points.
High photosynthetic capacity
Most marine algae primarily perform C3 photosynthesis. Ulva prolifera fronds also have the key enzymes for C4 photosynthesis, so it may perform both C3 and C4 photosynthesis [73,74]. Ulva prolifera also has the lhc SR gene and non-photochemical quenching mechanism, both of which give U. prolifera a high photosynthetic carbon-fixation capacity and environmental adaptability to changing temperatures and light levels [75,76]. Moreover, U. prolifera has high photosynthetic plasticity, which may ensure its ability to obtain sufficient energy to increase its biomass and to adapt to a long-distance migration [21]. For example, U. prolifera can be periodically transferred from the surface to the bottom of the water column due to vertical mixing when floating from the southern to northern Yellow Sea. When exposed to the surface water, U. prolifera fronds can dissipate excess energy via photosynthetic quantum control (energy quenching and energy redistribution between PSII/PSI) to avoid light-caused damages to the photosynthetic system; when mixed into deeper waters, the fronds can promote light utilization by increasing their cellular chlorophyll a (Chl a), chlorophyll b (Chl b) and Chl b/Chl a ratio [21]. The higher photosynthetic capacity of U. prolifera contributes to its free-floating proliferation and prevalence among floating green algae species in the Yellow Sea [77].
Multiple reproduction modes
The life history of U. prolifera is very complicated. Ulva prolifera has multiple reproduction modes, including sexual, asexual and vegetative propagation [78], among which asexual and vegetative propagation primarily contribute to its rapid expansion within 1 or 2 months. The fronds of U. prolifera can grow by (i) increasing algal tubular diameter, (ii) forming new branches, (iii) releasing spores and (iv) expanding fronds [28]. Ulva prolifera can release 1.2 × 107 spores or 2.3 × 107 gametes per cm2 frond, and 92–97% of them can bind to new micropropagules to germinate [79]. These unique and multiple reproductive methods allow the opportunistic macroalgae U. prolifera to maintain colonization and excessive growth, resulting in the formation of massive green tides.
ROLES OF MICROBIAL ORGANISMS DURING MACROALGAL BLOOMS
Algae are closely associated with bacteria in natural seawater. It is extremely difficult to obtain one sterile alga without using antibiotics or a streaking-based algal separation method with multiple plates. The relationship between algae and bacteria is sophisticated, involving symbiosis, competition, lethality, gene transfer, etc. Ulva prolifera, being no exception, is closely associated with microbial organisms. Ulva prolifera contains endophytes within and around its fronds. All these microbial organisms may play a vital role in regulating the formation and recession of macroalgal blooms. For example, the morphogenesis of U. linza, which is similar to that of U. prolifera, depends on the regulation of the key microbial group Cytophaga-Flexibacter-Bacteroides that ensures the normal growth of its fronds [80]. Moreover, the fronds of U. prolifera are commonly surrounded by abundant heterotrophic diazotrophs during the blooms [24]. Our previous studies indicate that dissolved organic N could support the growth of U. prolifera during the mid or late development stages of green tides [71]. Given that the inorganic N concentration is low in the late stage of green tides, a large number of diazotrophs attached on the fronds may satisfy the organic N demand of U. prolifera through N fixation.
Microorganisms have been reported to grow vigorously in seawater at the end of red tides [81]. These microorganisms are generally thought to be a key factor in inducing the recession of red tides and, accordingly, the ‘use of microbial organisms for algal bloom control’ has been proposed [81]. Similarly, massive growth of U. prolifera was observed to be related to or to reciprocally have a significant effect on certain microbial communities, and the abundance of microorganisms increased significantly by the end of U. prolifera green tides [82,83]. So far, it is still unclear whether or not microorganisms contribute significantly to the recession of green tides in the Yellow Sea. At least, it is certain that microbial activities, via decomposition of the massive dead-and-sunk U. prolifera after the green-tide demise, do influence the environment of the Yellow Sea.
ENVIRONMENTAL IMPACTS OF MACROALGAL BLOOMS
Green tides have profound and long-lasting effects on the environment. In the early and middle development stages of U. prolifera green tides, efficient photosynthesis of U. prolifera quickly assimilates inorganic carbon, which supports its growth, thus leading to an increase of pH in seawater. The field investigation carried out in 2014 showed that the pH increased up to 8.6 at the centre of a free-floating Ulva patch (about 100 000 m2) along the Dafeng coast (Jiangsu Province). Massive carbon fixation and abrupt pH increase caused by large green tides may have a significant impact on the phytoplankton population and the community structure and ecosystem functions at the regional scale. At the end of green tides (mid-July to early August), massive U. prolifera fronds that fail to be salvaged will sink down to the bottom or suspend at certain depths where they die and are decomposed gradually by microorganisms [84]. The decomposition of U. prolifera fronds consumes a large amount of oxygen, resulting in low-oxygen or even hypoxic conditions in the coastal waters. Hypoxia can not only cause massive deaths of cultured organisms (e.g. sea cucumbers and abalone) and induce considerable economic losses, but also affect benthic community structure, biomass and inhabitant density [85,86]. Simultaneously, the decay of U. prolifera fronds can release abundant C, N and P, lowering the water quality and affecting the reproduction and respiratory metabolism of heterotrophic microorganisms [53]. Accordingly, large amount of CO2 will dissolve into seawater, decreasing the pH of seawater and accelerating coastal acidification, which are well known to have a profound impact on marine ecosystems [87,88]. As shown in the two surveys conducted in the Yellow Sea in 2008, the dissolved inorganic carbon concentration and sea–air CO2 partial pressure (pCO2) increased markedly while the pH decreased substantially during the late-bloom period (22–25 July 2008) of green tides, indicating that coastal waters become a carbon source after a green tide [89]. Owing to microbial activities, decomposition of U. prolifera releases substantial dissolved organic matter into the surrounding waters, promoting the growth of microbial organisms, which in turn accelerates the decomposition of dead U. prolifera. Accompanied with this process is the release of more CO2 driven by microbial respiration, resulting in worse coastal acidification. On the other hand, the released dissolved organic carbon (DOC) during U. prolifera decomposition is partially transformed into refractory DOC (RDOC) via microbial metabolism according to the microbial carbon pump theory [90–93]. The RDOC is hard for microorganisms to utilize or degrade, and thus accumulates in the water for a long time, which contributes to carbon sequestration in the ocean.
On 23 July 2016, in the late development stage of a green tide, massive U. prolifera fronds floated into the coastal waters of Qingdao, with the largest coverage area in the Golden Beach waters (120°14′38′E, 35°57′25′N), followed by Tuandao (120°18′22′E, 36°03′07′N), Maidao (120°25′47′E, 36°3′29′N) and Shilaoren (120°29′9′E, 36°5′40′N) near-shore waters. We monitored the water physiochemical parameters in the above four waters and found the lowest seawater pH and dissolved oxygen (DO) appeared in the Golden Beach near-shore waters, along with the highest chemical oxygen demand (COD), DOC and bacterial abundance. The DON concentration in this area was similar to that in the Maidao near-shore waters (Table 1). When compared to the seawater quality during the pre-bloom period of green tides (pH: 8.1, DO: 6.3 mg L−1, COD: 2.2 mg L−1), in the Golden Beach, the pH and DO decreased by 7 and 68%, respectively, whereas the COD during the post-bloom period was 22 times higher than that of the pre-bloom period (Table 1). These results indicate that a great mass of U. prolifera in the surface water greatly influences the seawater quality and even causes serious negative environmental effects such as coastal hypoxia and acidification.
Table 1.
The pH, concentrations of dissolved oxygen (DO), chemical oxygen demand (COD), dissolved oxygen carbon (DOC) and dissolved organic nitrogen (DON) and bacterial abundance (mean ± SD from triplicate samples) in the four sampling sites along the Qingdao coastal areas.
| Sampling | pH | DO | COD | DOC | DON | Bacterial abundance |
|---|---|---|---|---|---|---|
| sites | (mg L–1) | (mg L–1) | (mg L–1) | (mg L–1) | (×106 cells mL–1) | |
| Golden Beach | 7.6 | 2.0 | 48.9 | 13.8 | 0.5 | 10.5 |
| ±0.03 | ±0.2 | ±1.5 | ±1.2 | ±0.08 | ±1.6 | |
| Tuandao | 8.2 | 6.8 | 25.6 | 3.6 | 0.2 | 4.8 |
| ±0.02 | ±0.1 | ±1.2 | ±0.6 | ±0.05 | ±0.7 | |
| Maidao | 8.0 | 8.0 | 36.7 | 10.8 | 1.3 | 12.8 |
| ±0.01 | ±0.2 | ±1.9 | ±0.8 | ±0.03 | ±1.3 | |
| Shilaoren | 8.4 | 7.2 | 33.3 | 5.2 | 0.2 | 4.6 |
| ±0.02 | ±0.2 | ±0.5 | ±0.6 | ±0.06 | ±0.3 |
The decomposition of U. prolifera releases high concentrations of transudates that may inhibit the growth of some phytoplankton species; conversely, these transudates containing abundant N and P may enhance the growth of some other opportunistic species. When U. prolifera is co-cultured with microalgae, the microalgae growth, especially for diatoms, is generally inhibited by monopolizing nutrients or toxic exudates from U. prolifera [94]. Nevertheless, substantial amounts of nitrogen nutrients, mainly ammonium, are released after U. prolifera decomposition, which is likely to cause regional eutrophication and promote the growth of red tide species, especially dinoflagellates and raphidophytes [95,96].
WHY DID GREEN TIDES DECREASE IN 2017?
In 2017, the U. prolifera blooming scales shrank sharply compared to those in the previous years, which could be attributable to several aspects. Shrinkage of aquaculture due to worsening water quality and increasing disease may be one of the main reasons [97], since such shrinkage can decrease the nutrient inputs into the Jiangsu coast where U. prolifera green tides originate [14]. Adjustment of the Porphyra harvest mode and time in 2017 (i.e. the harvest time was earlier than before) may also account greatly for the green-tide shrinkage in Qingdao, as it can decrease the amount of U. prolifera released into the water. Moreover, during this period, ‘golden tides’ of Sargassum blooms, another macroalgae, occurred in the Yellow Sea, covering an area of over 47 713 km2 under uncommon currents and wind conditions [98]. The Sargassum thallus has well-developed gas bladders and differentiated parts resembling leaves and stems, giving it a competitive advantage for space and nutrients compared to the Ulva thallus, thus inhibiting the growth of U. prolifera. Moreover, the floating Sargassum golden tide occurred in the Porphyra aquaculture area, resulting in the decrease of attached U. prolifera seaweed and further the shrinkage of the U. prolifera green tide during the year.
Furthermore, U. prolifera is an edible macroalgae, as its protein content reaches as high as 35%. In recent years, U. prolifera has been considered good material for foods and the economic benefits thus incentivize local freshmen to widely collect the attached or floating Ulva seaweed in the Yellow Sea. Such Ulva collection would finally decrease the number of floating blooms in the northern Yellow Sea.
In addition, the abnormal weather conditions caused by the El Niño phenomenon were likely related to the reduction of the U. prolifera green-tide scale in 2017; the El Niño-caused decrease in precipitation and increase in temperature may respectively lower the levels of nutrients that are derived from the land (e.g. in 2017, there was more drought during January to April than ever before in Jiangsu and Shandong Province) or from the stratified bottom layer [99]. Furthermore, El Niño might have altered the marine hydrodynamics, such as weakening the northward currents (e.g. the Subei coastal current) that typically drift the U. prolifera fronds northward [14].
During 2018, the maximum distribution area of green tides was reported to be 34 097 km2 in the southern Yellow Sea (Fig. 1, data of 2018 were from the website of North China Sea Branch of State Oceanic Administration). Though the macroalgal bloom scale exceeded that of 2017, the outbreak scale of green tides in 2018 was much smaller than those of previous years (i.e. 2014–16). The shrinkage of aquaculture along the Jiangsu coast in recent years and the adjustment of Porphyra harvest mode and time were potentially two main reasons for the shrinkage of green tides during 2017 and 2018.
POTENTIAL CONTROL METHODS FOR GREEN TIDES IN THE YELLOW SEA
Reduction of nutrient input from diverse sources
Nutrient inputs from diverse sources are considered to strongly influence the reoccurrence of U. prolifera green tides in the Yellow Sea. For a truly integrated management of the coastal zone, reduction in nutrient input and control of effluents, especially for N-source nutrients from the coastal pond systems, are needed to control eutrophication and prevent green tides in the future.
Setting intercept lines to control the spread of the green tides in the Yellow Sea
Controlling the green tides in the source region would be the most effective method to eliminate the outbreak of green tides. Generally, the initial blooms will take 30–40 days to drift to the northern Yellow Sea. Thus, it would be better to set one intercept line in the origination sea area to stop the northward migration of U. prolifera. Another intercept line could be set in the coastal area of the Rizhao district, which is next to the southern coast line of Qingdao city, so as to clean the scattered floating fronds that may escape the first intercept line. These two intercept lines would play a key role in preventing the spread of the green tides in the Yellow Sea. Besides, the collected Ulva seaweeds can be processed into valuable products.
Compensation mechanism for harvesting U. prolifera
It has been suggested that more hygienic harvesting practices for P. yezoensis, such as land disposal of Ulva species on the bamboos and ropes, should be adopted to prevent the recurrence of green tides in the Yellow Sea [8]. Further, if a high biomass of floating Ulva species was collected before the two intercept lines mentioned above, the negative impact of green tides on the coastal ecology and economy of the Yellow Sea would be greatly reduced. However, due to the low value of Ulva species compared to Porphyra and other aquatic animals, farmers would not want to collect a high biomass of the Ulva species that are attached on Porphyra rafts and floating on the sea surface. Therefore, it would be better for governmental policy makers to set up a compensation mechanism for the harvesting of Ulva species. For example, farmers can collect Ulva species attached or floating biomass and sell them to manufacturers at a certain price to produce food, feed and other products.
In addition, the earlier harvest of Porphyra in the southern Jiangsu Province, especially before the temperature increases to over 10°C in April, might reduce the biomass of U. prolifera dropping off the rafts and thus shrink the outbreak scale of the green tides. This is because temperature has an important impact on the growth of U. Prolifera; for example, U. prolifera can exhibit normal photosynthesis only if the temperature is higher than 10°C [26,27]. A reasonable and scientific compensation mechanism is very important for the farmers to stay positive.
Comprehensive utilization of the huge biomass of green-tide algae
Ulva prolifera is a natural biotic resource. In Zhejiang and Fujian Province, U. prolifera has been treated as a delicious food that can often be found in the market. However, the current utilization of U. prolifera is relatively simple, mainly as fertilizer, feed, food, additives, etc. It is of great significance to further develop and utilize U. prolifera [100]. Turning the massive U. prolifera ‘waste’ into ‘treasure’ not only benefits the local economy, but also helps to reduce the negative impact on the ecological environment.
CONCLUDING REMARKS
The annual outbreak of U. prolifera green tides in the Yellow Sea since 2007 has been attracting worldwide attention. Even though U. prolifera fronds are considered to be a natural asset, which could be used to produce organic fertilizer, polysaccharides, etc., their serious negative effects on local mariculture, tourism and environment have caused heavy economic losses. The monitoring and extensive studies in the past decade have shown that the outbreak mechanisms of U. prolifera green tides are far more complicated than expected. Multiple factors have been proven to induce the outbreak of large-scale green tides in the Yellow Sea, including regional physicochemical conditions (e.g. temperature, salinity, light intensity, monsoons and ocean currents), algal physiological advantages (e.g. high nutrient-uptake rate, high photosynthetic capacity and the coexistence of multiple reproduction modes) and human activities (e.g. nutrient discharge, CO2 emissions), etc. In addition, it has been gradually realized that climate changes, aquaculture modes and biological interactions (e.g. with both microorganism and other macroalgae) also significantly affect the occurrences of U. prolifera green tides in the Yellow Sea. Moreover, the consequences of green tides are serious. In the short to medium term, green tides can degrade water quality, increase the outbreak risk of red tides and cause coastal hypoxia and acidification, while, in the long term, they may have a long-lasting impact on coastal carbon cycles. The identification of the outbreak mechanisms of green tides and the following consequences could provide implications for the corresponding countermeasures to be adopted to avoid future occurrences of green tides in the Yellow Sea, and provide reference for the future in ecosystem management and sustainable development.
FUNDING
This work was supported by the National Key Research and Development Program of China (2016YFA0601402 and 2016YFC1402101–03), the Open Task of Qingdao National Laboratory for Marine Science and Technology (QNLM2016ORP0311), the National Natural Science Foundation of China (41876174, 41606092 and 41606134) and the ‘One Three Five’ research project of Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences. This study is a contribution to the international Integrated Marine Biosphere Research project.
Conflict of interest statement . None declared.
REFERENCES
- 1. Jiang P, Wang JF, Cui YLet al.. Molecular phylogenetic analysis of attached Ulvaceae species and free-floating Enteromorpha from Qingdao coasts in 2007. Chin J Ocean Limnol 2008; 26: 276–9. [Google Scholar]
- 2. Liu XQ, Wang ZL, Zhang XLet al.. A review of the green tides in the Yellow Sea, China. Mar Environ Res 2016; 119: 189–96. [DOI] [PubMed] [Google Scholar]
- 3. Ye N, Zhang X, Mao Yet al.. ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world's largest example. Ecol Res 2011; 26: 477–85. [Google Scholar]
- 4. Zhou MJ, Liu DY, Anderson DMet al.. Introduction to the Special Issue on green tides in the Yellow Sea. Estuar Coast Shelf Sci 2015; 163: 3–8. [Google Scholar]
- 5. Liu D, Keesing JK, He PMet al.. The world's largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuar Coast Shelf Sci 2013; 129: 2–10. [Google Scholar]
- 6. Sun S, Wang F, Li CLet al.. Emerging challenges: massive green algae blooms in the Yellow Sea. Nat Preced 2008; http://hdl.handle.net/10101/npre.2008.2266.1 [Google Scholar]
- 7. Liu D, Keesing JK, Xing Qet al.. World's largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Pollut Bull 2009; 58: 888–95. [DOI] [PubMed] [Google Scholar]
- 8. Liu D, Keesing JK, Dong Zet al.. Recurrence of the world's largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar Pollut Bull 2010; 60: 1423–32. [DOI] [PubMed] [Google Scholar]
- 9. Keesing JK, Liu D, Fearns Pet al.. Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar Pollut Bull 2011; 62: 1169–82. [DOI] [PubMed] [Google Scholar]
- 10. Hu S, Yang H, Zhang JHet al.. Small-scale early aggregation of green tide macroalgae observed on the Subei Bank, Yellow Sea. Mar Pollut Bull 2014; 81: 166–73. [DOI] [PubMed] [Google Scholar]
- 11. Qi L, Hu CM, Xing QGet al.. Long-term trend of Ulva prolifera blooms in the western Yellow Sea. Harmful Algae 2016; 58: 35–44. [DOI] [PubMed] [Google Scholar]
- 12. Xing QG, Hu CM. Mapping macroalgal blooms in the Yellow Sea and East China Sea using HJ-1 and Landsat data: application of a virtual baseline reflectance height technique. Remote Sens Environ 2016; 178: 113–26. [Google Scholar]
- 13. Xiao YF, Zhang J, Cui TW. High-precision extraction of nearshore green tides using satellite remote sensing data of the Yellow Sea, China. Int J Remote Sens 2017; 38: 1626–41. [Google Scholar]
- 14. Zhang QC, Liu Q, Kang ZJet al.. Development of a fluorescence in situ hybridization (FISH) method for rapid detection of Ulva prolifera. Estuar Coast Shelf Sci 2015; 163: 103–11. [Google Scholar]
- 15. Wang ZL, Xiao J, Fan SLet al.. Who made the world's largest green tide in China? An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol Oceanogr 2015; 60: 1105–17. [Google Scholar]
- 16. Li HM, Zhang CS, Han XRet al.. Changes in concentrations of oxygen, dissolved nitrogen, phosphate, and silicate in the southern Yellow Sea, 1980–2012: sources and seaward gradients. Estuar Coast Shelf Sci 2015; 163: 44–55. [Google Scholar]
- 17. Song W, Li Y, Fang Set al.. Temporal and spatial distributions of green algae micro-propagules in the coastal waters of the Subei Shoal, China. Estuar Coast Shelf Sci 2015; 163: 29–35. [Google Scholar]
- 18. Song W, Peng KQ, Xiao Jet al.. Effects of temperature on the germination of green algae micro-propagules in coastal waters of the Subei Shoal, China. Estuar Coast Shelf Sci 2015; 163: 63–8. [Google Scholar]
- 19. Duan WJ, Guo LX, Sun Det al.. Morphological and molecular characterization of free-floating and attached green macroalgae Ulva spp. in the Yellow Sea of China. J Appl Phycol 2012; 24: 97–108. [Google Scholar]
- 20. Zhao J, Jiang P, Liu ZYet al.. The Yellow Sea green tides were dominated by one species, Ulva (Enteromorpha) prolifera, from 2007 to 2011. Chin Sci Bull 2013; 58: 2298–302. [Google Scholar]
- 21. Zhao XY, Tang XX, Zhang HXet al.. Photosynthetic adaptation strategy of Ulva prolifera floating on the sea surface to environmental changes. Plant Physiol Biochem 2016; 107: 116–25. [DOI] [PubMed] [Google Scholar]
- 22. Fan SL, Fu MZ, Wang ZLet al.. Temporal variation of green macroalgal assemblage on Porphyra aquaculture rafts in the Subei Shoal, China. Estuar Coast Shelf Sci 2015; 163: 23–8. [Google Scholar]
- 23. Luo MB, Liu F, Xu ZLet al.. Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat Bot 2012; 100: 18–24. [Google Scholar]
- 24. Zhang XL, Song YJ, Liu DYet al.. Macroalgal blooms favor heterotrophic diazotrophic bacteria in nitrogen-rich and phosphorus-limited coastal surface waters in the Yellow Sea. Estuar Coast Shelf Sci 2015; 163: 75–81. [Google Scholar]
- 25. Liu XQ, Li Y, Wang ZLet al.. Cruise observation of Ulva prolifera bloom in the southern Yellow Sea, China. Estuar Coast Shelf Sci 2015; 163: 17–22. [Google Scholar]
- 26. Taylor R, Fletcher RL, Raven JA. Preliminary studies on the growth of selected green tide algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Bot Mar 2005; 44: 327–36. [Google Scholar]
- 27. Largo DB, Sembrano J, Hiraoka Met al.. Taxonomic and ecological profile of ‘green tide’ species of Ulva (Ulvales, Chlorophyta) in Central Philippines. Hydrobiologia 2014; 512: 247–53. [Google Scholar]
- 28. Zhang JH, Kim JK, Yarish Cet al.. The expansion of Ulva prolifera O.F. Müller macroalgal blooms in the Yellow Sea, PR China, through asexual reproduction. Mar Pollut Bull 2016; 104: 101–6. [DOI] [PubMed] [Google Scholar]
- 29. Han HB, Wei ZL, Huo YZet al.. Effects of temperature and light intensity on the release and germination of Ulva prolifera spores and gametes. Mar Fish 2015; 37: 517–24. [Google Scholar]
- 30. Huo YZ, Hua L, Wu HLet al.. Abundance and distribution of Ulva microscopic propagules associated with a green tide in the southern coast of the Yellow Sea. Harmful Algae 2014; 39: 357–64. [Google Scholar]
- 31. Keesing JK, Liu DY, Shi YJet al.. Abiotic factors influencing biomass accumulation of green tide causing Ulva spp. on Pyropia culture rafts in the Yellow Sea, China. Mar Pollut Bull 2016; 105: 88–97. [DOI] [PubMed] [Google Scholar]
- 32. Xiao J, Zhang XH, Gao CLet al.. Effect of temperature, salinity and irradiance on growth and photosynthesis of Ulva prolifera. Acta Oceanol Sin 2016; 35: 114–21. [Google Scholar]
- 33. Luo MB, Liu F. Effects of salinity on the growth and reproduction of green tide Ulva prolifera from Yellow Sea. Nat Con Agro-Environ Sci 2011; 148(in Chinese). [Google Scholar]
- 34. Liu YS, Wang D, Xu XTet al.. Combined effects of salinity and temperature on the growth and photophysiological performances in Ulva prolifera. Acta Hydrobiol Sin 2016; 40: 1127–233. [Google Scholar]
- 35. Xu ZG, Wu HY, Zhan DMet al.. Combined effects of light intensity and NH4+-enrichment on growth, pigmentation, and photosynthetic performance of Ulva prolifera (Chlorophyta). Chin J Ocean Limnol 2014; 32: 1016–23. [Google Scholar]
- 36. Wang JW, Yan BL, Lin APet al.. Ecological factor research on the growth and induction of spores release in Enteromorpha prolifera (Chlorophyta). Mar Sci Bull 2007; 26: 60–5. [Google Scholar]
- 37. Sun KM, Li RX, Li Yet al.. Responses of Ulva prolifera to short-term nutrient enrichment under light and dark conditions. Estuar Coast Shelf Sci 2015; 163: 56–62. [Google Scholar]
- 38. Lee JH, Pang IC, Moon IJet al.. On physical factors that controlled the massive green tide occurrence along the southern coast of the Shandong Peninsula in 2008: a numerical study using a particle-tracking experiment. J Geophys Res 2011; 116: C12036. [Google Scholar]
- 39. Qiao FL, Wang GS, Lü XGet al.. Drift characteristics of green macroalgae in the Yellow Sea in 2008 and 2010. Chin Sci Bull 2011; 56: 2236–42. [Google Scholar]
- 40. Cui TW, Zhang J, Sun LEet al.. Satellite monitoring of massive green macroalgae bloom (GMB): imaging ability comparison of multi-source data and drifting velocity estimation. Int J Remote Sens 2012; 33: 5513–27. [Google Scholar]
- 41. Bao M, Guan WB, Yang Yet al.. Drifting trajectories of green algae in the western Yellow Sea during the spring and summer of 2012. Estuar Coast Shelf Sci 2015; 163: 9–16. [Google Scholar]
- 42. Li HM, Tang HJ, Shi XYet al.. Increased nutrient loads from the Changjiang (Yangtze) River have led to increased harmful algal blooms. Harmful Algae 2014; 39: 92–101. [Google Scholar]
- 43. Zhang JH, Liu CC, Yang LLet al.. The source of the Ulva blooms in the East China Sea by the combination of morphological, molecular and numerical analysis. Estuar Coast Shelf Sci 2015; 164: 418–24. [Google Scholar]
- 44. Yi L, Zhang S P, Yin Y Q. Influnce of environmental hydro-meteorological conditions to Ulva prolifera blooms in Yellow Sea, 2009. Periodical Ocean Univ China 2010; 40: 15–23. [Google Scholar]
- 45. Cox PM, Betts RA, Jones CDet al.. Erratum: Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000; 408: 184–7. [DOI] [PubMed] [Google Scholar]
- 46. Blunden J, Arndt DS, Wolken GJ. State of the Climate in 2015. Bull Am Meteorol Soc 2016; 97: S1–275. [Google Scholar]
- 47. Gao G, Clare AS, Rose Cet al.. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar Pollut Bull 2017; 114: 439–47. [DOI] [PubMed] [Google Scholar]
- 48. Allanson BR, Human LRD, Claassens L. Observations on the distribution and abundance of a green tide along an intertidal shore, Knysna Estuary. S Afr J Bot 2016; 107: 49–54. [Google Scholar]
- 49. Xu D, Schaum CE, Lin Fet al.. Acclimation of bloom-forming and perennial seaweeds to elevated pCO2 conserved across levels of environmental complexity. Glob Change Biol 2017; 23: 4828–39. [DOI] [PubMed] [Google Scholar]
- 50. Choi HG, Kim YS, Kim JHet al.. Effects of temperature and salinity on the growth of Gracilariaverrucosa and G. chorda, with the potential for mariculture in Korea. J Appl Phycol 2006; 18: 269–77. [Google Scholar]
- 51. Tsagkamilis P, Danielidis D, Dring MJet al.. Removal of phosphate by the green seaweed Ulva lactuca in a small-scale sewage treatment plant (Ios Island, Aegean Sea, Greece). J Appl Phycol 2010; 22: 331–9. [Google Scholar]
- 52. Collado-Vides L, Avila C, Blair Set al.. A persistent bloom of Anadyomene J.V. Lamouroux (Anadyomenaceae, Chlorophyta) in Biscayne Bay, Florida. Aquat Bot 2013; 111: 95–103. [Google Scholar]
- 53. Ogawaa T, Ohkib K, Kamiya Met al.. High heterozygosity and phenotypic variation of zoids in apomictic Ulva prolifera from brackish environments. Aquat Bot 2015; 120: 185–92. [Google Scholar]
- 54. Conover J, Green LA, Thornber CS. Biomass decay rates and tissue nutrient loss in bloom and non-bloom-forming macroalgal species. Estuar Coast Shelf Sci 2016; 178: 58–64. [Google Scholar]
- 55. Kwon HK, Kang H, Oh YHet al.. Green tide development associated with submarine groundwater discharge in a coastal harbor, Jeju, Korea. Sci Rep 2017; 7: 6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perrot T, Rossi N, Ménesguen Aet al.. Modelling green macroalgal blooms on the coasts of Brittany, France to enhance water quality management. J Mar Syst 2014; 132: 38–53. [Google Scholar]
- 57. Dailer ML, Smith JE, Smith CM. Responses of bloom forming and non-bloom forming macroalgae to nutrient enrichment in Hawai‘i, USA. Harmful Algae 2012; 17: 111–25. [Google Scholar]
- 58. Shi XY, Qi MY, Tang HJet al.. Spatial and temporal nutrient variations in the Yellow Sea and their effects on Ulva prolifera blooms. Estuar Coast Shelf Sci 2015; 163: 36–43. [Google Scholar]
- 59. Xing QG, Tosi L, Braga F. Interpreting the progressive eutrophication behind the world's largest macroalgal blooms with water quality and ocean color data. Nat Hazards 2015; 78: 7–21. [Google Scholar]
- 60. Pang SJ, Liu F, Shan TFet al.. Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar Environ Res 2010; 69: 207–15. [DOI] [PubMed] [Google Scholar]
- 61. Liu F, Pang SJ, Zhao XBet al.. Quantitative, molecular and growth analyses of Ulva microscopic propagules in the coastal sediment of Jiangsu province where green tides initially occurred. Mar Environ Res 2012; 74: 56–63. [DOI] [PubMed] [Google Scholar]
- 62. Liu F, Pang SJ, Chopin Tet al.. Understanding the recurrent large-scale green tide in the Yellow Sea: temporal and spatial correlations between multiple geographical, aquacultural and biological factors. Mar Environ Res 2013; 83: 38–47. [DOI] [PubMed] [Google Scholar]
- 63. Li SX, Yu KF, Huo YZet al.. Effects of nitrogen and phosphorus enrichment on growth and photosynthetic assimilation of carbon in a green tide-forming species (Ulva prolifera) in the Yellow Sea. Hydrobiologia 2016; 776: 161–71. [Google Scholar]
- 64. Li RX, Wu XW, Win QSet al.. Growth of Ulva prolifera under different nutrient conditions. Adv Mar Sci 2011; 27: 211–6. [Google Scholar]
- 65. Lobban CS, Wynne MJ. The biology of seaweeds (1st ed.) Oakland: University of California Press, 1981. [Google Scholar]
- 66. Wang XH, Qiao FL, Lu Jet al.. The turbidity maxima of the northern Jiangsu shoal-water in the Yellow Sea, China. Estuar Coast Shelf Sci 2011; 93: 202–11. [Google Scholar]
- 67. Fang S, Wang ZL, Li Yet al.. The dynamics of micro-propagules before the Green tide (Ulva prolifera) outbreak in the Southern Huanghai Sea and Changjiang (Yangtze) River Estuary area. Acta Oceanol Sin 2012; 34: 147–54. [Google Scholar]
- 68. Liu CC, Xu R, He PMet al.. Research on the relations between green tide and Porphyra cultivation in the south Yellow Sea. Mar Sci 2017; 41: 35–43. [Google Scholar]
- 69. Fan X, Xu D, Wang YTet al.. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: implications for the explosion in green tides. J Appl Phycol 2014; 26: 537–44. [Google Scholar]
- 70. Smith DW, Horne AJ. Experimental measurement of resource competition between planktonic microalgae and macroalgae (seaweeds) in mesocosms simulating the San Francisco Bay-Estuary, California, California. Hydrobiologia 1988; 159: 259–68. [Google Scholar]
- 71. Li HM, Zhang YY, Han XRet al.. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Sci Rep 2016; 6: 26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li HM, Zhang YY, Tang HJet al.. Spatiotemporal variations of inorganic nutrients along the Jiangsu coast, China, and the occurrence of macroalgal blooms (green tides) in the southern Yellow Sea. Harmful Algae 2017; 63: 164–72. [DOI] [PubMed] [Google Scholar]
- 73. Roberts K, Granum E, Leegood RCet al.. C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 2007; 145: 230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu J, Fan X, Zhang X. Evidence of coexistence of C3 and C4 photosynthetic pathways in a green-tide-forming alga, Ulva prolifera. PLoS One 2012; 7: 37438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dong MT, Zhang XW Zou J et al. Identification and expression analysis of the gene lhcSR associated with adaptation to light and low temperature stress in the green tide forming alga Ulva prolifera. Mar Biol Res 2012; 8: 746–55. [Google Scholar]
- 76. Zhang XW, Mou SL, Cao SN. Roles of the transthylakoid proton gradient and xanthophyll cycle in the non-photochemical quenching of the green alga Ulva linza. Estuar Coast Shelf Sci 2015; 163: 69–74. [Google Scholar]
- 77. Wang Y, Qu T, Zhao Xet al.. A comparative study of the photosynthetic capacity in two green tide macroalgae using chlorophyll fluorescence. Springerplus 2016; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin A, Shen S, Wang Jet al.. Reproduction diversity of Enteromorpha prolifera. J Integ Plant Biol 2008; 50: 622–9. [DOI] [PubMed] [Google Scholar]
- 79. Zhang JH, Huo YZ, Yu KFet al.. Growth characteristics and reproductive capability of green tide algae in Rudong coast, China. J Appl Phycol 2013; 25: 795–803. [Google Scholar]
- 80. Marshall K, Joint I, Callow MEet al.. Effect of marine bacterial isolates on the growth and morphology of axenic plantlets of the green alga Ulva linza. Microb Ecol 2006; 52: 302–10. [DOI] [PubMed] [Google Scholar]
- 81. Li Y, Zhu H, Lei XQ. The first evidence of deinoxanthin from Deinococcus sp. Y35 with strong algicidal effect on the toxic dinoflagellate Alexandrium tamarense. J Hazard Mater 2015; 290: 87–95. [DOI] [PubMed] [Google Scholar]
- 82. Liu M, Dong Y, Zhao Yet al.. Structures of bacterial communities on the surface of Ulva prolifera and in seawaters in an Ulva blooming region in Jiaozhou Bay, China. World J Microbiol Biotechnol 2011; 27: 1703–12. [Google Scholar]
- 83. Lin GR, Sun FL, Wang CZet al.. Assessment of the effect of Enteromorpha prolifera on bacterial community structures in aquaculture environment. PLoS One 2017; 12: e0179792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hardison AK, Canuel EA, Anderson ICet al.. Fate of macroalgae in benthic systems: carbon and nitrogen cycling within the microbial community. Mar Ecol Prog Ser 2010; 414: 41–55. [Google Scholar]
- 85. Wang HF, Li XZ, Wang JBet al.. The ecological research of the macrobenthic community from sea areas around Qingdao during the upsurge of green seaweed Ulva prolifera in summer of 2008. Mar Sci 2011; 35: 10–8. [Google Scholar]
- 86. Luherne EL, Réveillac E, Ponsero Aet al.. Fish community responses to green tides in shallow estuarine and coastal areas. Estuar Coast Shelf Sci 2016; 175: 79–92. [Google Scholar]
- 87. Witt V, Wild C, Anthony KRNet al.. Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef. Environ Microbiol 2011; 13: 2976–89. [DOI] [PubMed] [Google Scholar]
- 88. Kroeker KJ, Micheli F, Gambi MC. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat Clim Change 2013; 3: 156–9. [Google Scholar]
- 89. Hu Y, Liu CY, Yang GPet al.. The response of the carbonate system to a green algal bloom during the post-bloom period in the southern Yellow Sea. Cont Shelf Res 2015; 94: 1–7. [Google Scholar]
- 90. Jiao NZ, Herndl GJ, Hansell DAet al.. The microbial carbon pump and the oceanic recalcitrant dissolved organic matter pool. Nat Rev Microbiol 2011; 9: 555. [DOI] [PubMed] [Google Scholar]
- 91. Zhang Y, Zhao MX, Cui Qet al.. Processes of coastal ecosystem carbon sequestration and approaches for increasing carbon sink. Sci China Earth Sci 2017; 60: 809–20. [Google Scholar]
- 92. Zhang YY, Zhang JX, Liang YTet al.. Carbon sequestration processes and mechanisms in coastal mariculture environments in China. Sci China Earth Sci. 2017; 60: 2097–107. [Google Scholar]
- 93. Jiao NZ, Cai RH, Zheng Qet al.. Unveiling the enigma of refractory carbon in the ocean. Natl Sci Rev 2018; 5: 459–63. [Google Scholar]
- 94. Nan CR, Zhang HZ, Zhao GQet al.. Allelopathic interactions between the macroalga Ulva pertusa and eight microalgal species. J Sea Res 2004; 52: 259–68. [Google Scholar]
- 95. Wang Y, Yu ZM, Song XXet al.. Effects of macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on growth of four species of bloom-forming dinoflagellates. Aquat Bot 2007; 86: 139–47. [Google Scholar]
- 96. Wang C, Yu RC, Zhou MJ. Effects of the decomposing green macroalga Ulva (Enteromorpha) prolifera on the growth of four red-tide species. Harmful Algae 2012; 16: 12–9. [Google Scholar]
- 97. Chen HG, Zhang ZH, Wang MBet al.. Sales delay, amounts decrease and prices increase: predict and analysis of the crab culture situation of 2016 in Jiangsu Province (in Chinese). China Fisheries 2016; 9: 16–8. [Google Scholar]
- 98. Zhang JH, Shi JT, Gao Set al.. Annual patterns of macroalgal blooms in the Yellow Sea during 2007–2017. PLoS One 2019; 14: e0210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thirumalai K, DiNezio PN, Okumura Yet al.. Extreme temperatures in Southeast Asia caused by El Niño and worsened by global warming. Nat Commun 2017; 8: 15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ebata H, Sato Y, Shimada Set al.. Possibility of land-based cultivation of green alga Ulva prolifera by saline groundwater. Aquacult Sci 2010; 55: 103–8. [Google Scholar]


