Abstract
The superconductivity of hydrides under high pressure has attracted a great deal of attention since the recent observation of the superconducting transition at 203 K in strongly compressed H2S. It has been realized that the stoichiometry of hydrides might change under high pressure, which is crucial in understanding the superconducting mechanism. In this study, PH3 was studied to understand its superconducting transition and stoichiometry under high pressure using Raman, IR and X-ray diffraction measurements, as well as theoretical calculations. PH3 is stable below 11.7 GPa and then it starts to dehydrogenate through two dimerization processes at room temperature and pressures up to 25 GPa. Two resulting phosphorus hydrides, P2H4 and P4H6, were verified experimentally and can be recovered to ambient pressure. Under further compression above 35 GPa, the P4H6 directly decomposed into elemental phosphorus. Low temperature can greatly hinder polymerization/decomposition under high pressure and retains P4H6 up to at least 205 GPa. The superconductivity transition temperature of P4H6 is predicted to be 67 K at 200 GPa, which agrees with the reported result, suggesting that it might be responsible for superconductivity at higher pressures. Our results clearly show that P2H4 and P4H6 are the only stable P–H compounds between PH3 and elemental phosphorus, which is helpful for shedding light on the superconducting mechanism.
Keywords: high pressure, hydrides, superconductivity, stoichiometric evolution
INTRODUCTION
Since superconducting mercury was first reported [1,2], scientists have continued to search for new high critical temperature (Tc) materials. In 2004, Ashcroft studied hydrogen-dominant hydrides [3], in which condensed H2 may contribute to a high Tc. Motivated by this work, extensive theoretical investigations on this system have been reported, such as SiH4 [4], GeH4 [5], GaH3 [6], SiH4(H2)2 [7], CaH6 [8] and YH6 [9], etc. A few remarkable high-Tc materials have also been observed in subsequent experimental studies. Recently, Drozdov et al. reported the superconductive transition of H2S at 203 K and 155 GPa [10], which broke the highest Tc record [11]. Many theoretical [12,13] and experimental [14] studies have explored its stoichiometry and structure, which play an important role in understanding the underlying mechanism of superconductivity.
Very recently, PH3, a typical hydrogen-rich hydride, has attracted a great deal of research interest because of its superconducting transition discovered by Drozdov and his co-workers [15–20]. Their experimental work revealed that PH3 might be a high-temperature superconducting candidate. From the resistance measurements, a superconducting transition signature was observed at Tc of 30 K. This increased to 103 K with pressures up to 207 GPa. However, structural information was not provided, and the origin of the superconducting transition remains puzzling. Subsequent theoretical studies [16–19] showed that the P–H compound should also be a complex system, and all the predicted structures were metastable with respect to the elemental phase.
Flores-Livas et al. [16] studied the phase diagram of phosphorus hydrides with different stoichiometries and found that they tended to decompose into phosphorus and hydrogen at high pressure. Liu et al. [17] predicted a PH3 phase with a monoclinic structure (C2/m) and a Tc of 83 K at 200 GPa, which is closer to the observed superconducting transition temperature. Shamp et al. [18] predicted that PH3 is thermodynamically unstable during decomposition into the elemental phases, as well as PH2 and H2. Two PH2 phases with C2/m and I4/mmm symmetry were computed as metastable at 200 GPa. The corresponding superconducting critical temperatures were 76 and 70 K, respectively. Bi et al. [19] found that a dynamically stable PH2 phase was the best according to the observed superconducting transition at 80 GPa. The PH3 phase to PH2 phase reaction was exothermic at that pressure, which proves the spontaneity of the reaction.
Until now, the PH3 phase under compression has remained unknown and no relevant experimental studies have been reported. The high-pressure stoichiometry and structural behavior of PH3 are critical to understanding the superconducting transition in the P–H system, which needs to be experimentally determined. For this purpose, we studied the structural behavior of PH3 under high pressure. We identified the pressure-induced step-by-step polymerization of PH3 and a route to elemental phosphorus that unveiled the unknown transition process and provides experimental evidence for understanding the underlying mechanism of the superconductivity of P–H compounds.
RESULTS AND DISCUSSION
Stoichiometric evolutions of PH3 at room temperature
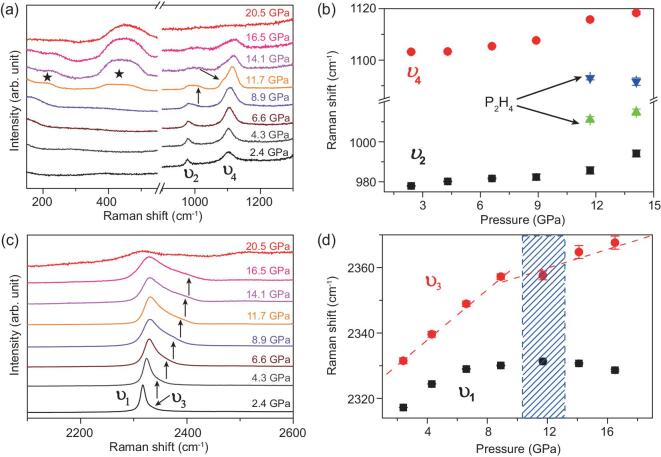
After the PH3 gas was loaded into the sample chamber of the diamond anvil cell (DAC) and returned to room temperature, a colorless and transparent sample (Supplementary Fig. 1, available as Supplementary Data at NSR online) was observed. The characteristic Raman peaks (Supplementary Fig. 1, available as Supplementary Data at NSR online) at 978 (υ2, symmetric bending mode), 1104 (υ4, asymmetric bending mode), 2317 (υ1, stretching mode) and 2331 (υ3, stretching mode; shoulder) cm−1 agreed well with previous reports [21], indicating the existence of PH3 in the chamber.
The X-rays can damage the sample (Supplementary Fig. 2, available as Supplementary Data at NSR online), so Raman and infrared absorption spectroscopy (IR) were mainly used for our in situ studies of PH3 at high pressure. Figure 1a and c shows the Raman spectra of the sample during compression. Under high pressure, these characteristic modes blue shifted and broadened (Fig. 1b and d) and eventually vanished at 20.5 GPa. Several new peaks (marked by black asterisks and arrows inFig. 1a) were observed at around 11.7 GPa, which suggested a phase transition. For the P–H stretching modes, we also noticed a dramatic expansion of the characteristic bonds. Figure 1d shows the peak positions of the υ1 and υ3 modes as a function of pressure. The peak shift of υ1 dramatically decreased and started to red shift at 11.7 GPa. We attributed these changes to a transition in the sample near 11.7 GPa.
Figure 1.
(a, c) Raman spectra of PH3 at various pressures at room temperature. The peak positions of υ2, υ4 (b) and υ1, υ3 (d) as functions of pressure.
These new peaks in the Raman spectra (Fig. 1a) were consistent with previous studies about P2H4 at ambient pressure. The two new peaks at low frequencies correspond to the PH2 rocking mode and P–P stretching mode in the P2H4 molecule, which were observed at around 217 and 436 cm−1, respectively [22,23]. The emergency P–P bond at 11.7 GPa proved the dimerization of PH3 molecules. The other new peaks at 1007 and 1093 cm−1 were from the PH2 scissoring modes in the P2H4 molecule, which also agrees with previous reports. These factors suggest that the pressure-induced transition is due to the dimerization of PH3 at high pressure.
To verify the dimerization, we also studied the decompressed sample. A liquid sample was obtained after quenching to ambient conditions, as shown in the microphotograph of the decompressed sample (inset optical images in Fig. 2a). It is well known that P2H4 is a liquid at ambient pressure [22,24], which confirms that pressure drives the dimerization of PH3 to form P2H4 via this reaction:
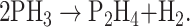 |
(1) |
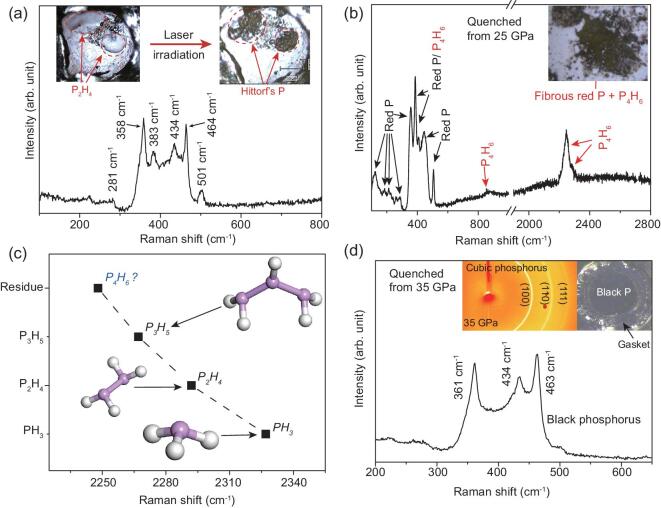
We further employed Raman to measure the recovered liquid sample. However, after laser irradiation, the liquid sample decomposed and generated Hittorf's phosphorus [25,26] (Fig. 2a) according to the photodecomposition properties of P2H4 [24]. This offers more evidence of our findings.
Figure 2.
(a) The Raman spectrum of the Hittorf's phosphorus transformed from the liquid sample after laser irradiation. The inset images show the photo-induced transition of the liquid residue before and after laser irradiation. (b) The Raman spectrum of the sample decompressed from 25 GPa. The inset picture shows the optical micrograph of the decompressed sample. (c) The frequency trend of the P–H stretching in PnHn+2 (n = 1, 2, 3 and 4). (d) The Raman spectrum of the sample quenched from 35 GPa. The inset shows the XRD pattern of the sample at 35 GPa and the optical micrograph of the decompressed sample.
We also employed IR to trace the in situ information of the new product at high pressure. Supplementary Fig. 3a, available as Supplementary Data at NSR online, shows the IR peak near 1095 cm−1 broadened and shifted slightly to a lower frequency with increasing pressure, but an obvious new shoulder was observed at around 1058 cm−1 after decompressing the sample to 11.8 GPa (Supplementary Fig. 3c and d, available as Supplementary Data at NSR online). This new shoulder matched the P2H4 scissors mode well, which was observed at around 1052 cm−1 in a solid state at ambient pressure [27]. This characteristic mode confirms the existence of P2H4. In addition to the P–H stretching modes in the IR spectra (Supplementary Fig. 3b, available as Supplementary Data at NSR online), a new shoulder at around 2329 cm−1 was observed, and it became stronger and stronger with increasing pressure. After it had quenched to 11.8 GPa, the new shoulder peak was more obvious compared to the IR spectrum measured at 12 GPa during compression. This proves dimerization.
As the pressure increased, the P2H4 showed piezochromism. It became yellow, then red and darkened, and eventually became opaque at pressures higher than 25 GPa, consistently with the observations of Drozdov et al. at low temperatures (180 K). As the sample became totally opaque, the vibrational signal vanished and hindered the in situ high-pressure vibrational spectra measurement. Therefore, we had to quench the sample to ambient conditions from different pressures (25 and 35 GPa) and employed Raman spectroscopy to investigate the different quenched residues. Interestingly, once the sample became completely opaque above 25 GPa, it maintained its opaque solid state even when decompressed to room pressure. This irreversible process suggests that a new transition occurred at higher pressures.
Figure 2b shows that the Raman spectrum of the residue quenched from 25 GPa after the opaque transition. A weak peak near 873 cm−1 belonging to PH2 twisting and a strong peak at 2248.5 cm−1 belonging to P–H stretching exist in the spectrum. This new P–H stretching peak is located at a much lower wave number than in PH3, P2H4 (∼2292 cm−1) and P3H5 (∼2267 cm−1) [24], suggesting that the residue contained a new kind of phosphorus hydride. Figure 2c shows the P–H stretching mode of PnHn+2 shifts to lower frequency as n becomes larger. Following this trend, we deduced that the new phosphorus hydride was P4H6, which suggests that the P2H4 molecules continued to dimerize and form P4H6 at higher pressure.
To confirm the second dimerization, we calculated the Raman modes of P4H6 using the Gaussian 09 program at the B3LYP/6–311(d, p) level [28]. Supplementary Table 1, available as Supplementary Data at NSR online, shows the calculated Raman modes of two typical P4H6 conformers, in which the four phosphorus atoms are linear and U-type (Supplementary Fig. 4a and b, available as Supplementary Data at NSR online). The calculated Raman spectra show that they both have four characteristic bands corresponding to the stretching vibration (350–450 cm−1) of the P–P bond, twisting vibration (700-900 cm−1) of the PH2 group, scissoring vibration (∼1070 cm−1) of the PH2 group and stretching vibration of the P–H bond, respectively. Moreover, the P–H stretching mode can further shift to a lower frequency (2278 cm−1). From Supplementary Table 1, available as Supplementary Data at NSR online, we can see that the P–P stretching bonds and the twisting vibration of the PH2 group from linear P4H6 are closer to our observed peak, suggesting that the linear type P4H6 is the more possible conformer in the residue.
Besides the peaks from P4H6, several other obvious characteristic modes (123.8, 184.8, 218.9, 285, 357.2, 386.5, 407.7, 443.2 and 505.8 cm−1) were observed below 550 cm−1. These peaks are similar to fibrous red phosphorus characteristic modes [25,26], which indicated that parts of P4H6 were thoroughly dissociated when exposed to laser or decompression. At ambient pressure, phosphorus hydrides often undergo disproportionation into phosphorus-rich phosphanes upon exposure to light and heat [24]. However, we did not observe the Raman peaks from other phosphanes from the residue, which proves that P2H4 dimerized directly into P4H6 at high pressure, corresponding to this equation:
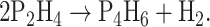 |
(2) |
From the recovered sample, it is confirmed that P2H4 dimerizes at high pressure. However, as both Raman and IR signal disappeared at above 20 GPa, we could not get in situ high-pressure vibrational modes. Therefore, it might be possible that other compounds generated at high pressure, such as P4H6·H2, which may easily decompose back to P4H6 and H2 upon decompression.
Figure 2d shows that the Raman spectra of the residue quenched from 35 GPa. After decompression to 1 atm, typical black phosphorus modes were observed [29,30]. Therefore, P4H6 eventually decomposed into elemental phosphorus at 35 GPa. Hence, the corresponding reaction is as follows:
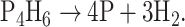 |
(3) |
From the in situ high-pressure XRD (Fig. 2d), the typical diffraction rings of cubic phosphorus further confirmed the thorough decomposition of P4H6 at high pressure.
Stoichiometric evolutions of PH3 at low temperature
The superconductivity of elemental phosphorus has already been studied both experimentally and theoretically [31–33]. The maximum Tc is about 9.5 K at 32 GPa before it decreases with pressure. Near 100 GPa, the Tc is about 4.3 K at 160 GPa, and no superconducting transition was detected in the temperature range from 4 to 40 K. The much lower Tc of phosphorus compared to 100 K indicates that PH3 or other phosphorus hydrides should be responsible for the superconductivity observed at 200 GPa in Drozdov's work. Since Drozdov et al. increased the pressure at low temperature (T <200 K) [15], we speculate that this discrepancy is due to the different experimental protocols used in these two works. Low temperature could hinder the polymerization/decomposition of phosphorus hydrides and secure phosphorus hydrides to much higher pressure.
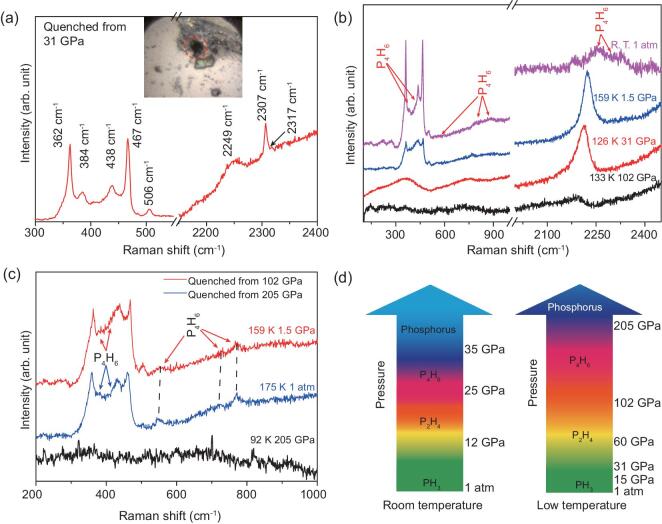
To find out whether low temperature can hinder the reactions and further indentify the superconducting candidate, we studied the high-pressure behavior of PH3 at low temperature (<200 K). First, we compressed PH3 up to 31 GPa, when P2H4 dimerized into P4H6 at room temperature. However, after we decompressed the sample to ambient pressure at low temperature, the sample became transparent again (the inset image in Fig. 3a). As shown in Fig. 3a, the sample decomposed after laser irradiation and the resulting opaque solid (the inset image in Fig. 3a was identified as Hittorf's phosphorus) and two characteristic Raman modes from P2H4 (2307 and 2317 cm−1) were also found. The transparency of the residue and strong peaks from P2H4 suggest P2H4 was dominant in the sample decompressed from 31 GPa at low temperature. However, it can only survive below 25 GPa at room temperature, proving that low temperature can greatly hinder the polymerization of phosphorus hydrides. We further compressed the sample up to 60 GPa, and studied the Raman spectrum of the quenched residue. Similar photodecomposition and typical Hittorf's P Raman modes (Supplementary Fig. 5, available as Supplementary Data at NSR online) were observed, which further suggested that P2H4 could remain up to 60 GPa at low temperature.
Figure 3.
(a) The Raman spectrum of the sample quenched from 31 GPa. The inset image shows the photo-induced transition after laser irradiation. (b) The Raman spectra of the sample collected during decompression from 102 GPa. (c) The Raman spectra of the residue quenched from 205 (blue line) and 102 (red line) GPa, respectively. (d) The phase diagrams of PH3 at room temperature and low temperature.
As the superconductivity was observed at pressures >80 GPa, we compressed PH3 at low temperature up to 102 and 205 GPa, respectively, to investigate the responsible superconducting candidate. As shown in Fig. 3b and c, we did not observe any peaks from the Raman spectra at 102 and 205 GPa, due to its metallic state as identified by Drozdov et al. After decompressing to 31 GPa, a strong peak at around 2212 cm−1 was observed, and it shifted to around 2250 cm−1 after the sample was quenched to ambient conditions. We also observed several other peaks at around 383, 418, 798 and 880 cm−1, which agreed well with our simulated P4H6 (Supplementary Table 1, available as Supplementary Data at NSR online) Raman, confirming the residue recovered from 102 GPa and 133 K was P4H6. As shown in Fig. 3c, the Raman spectrum of the sample decompressed from 205 GPa is almost the same as that from 102 GPa, suggesting P4H6 could be stable up to 205 GPa at low temperature. As P4H6 was observed after decompression from 102 and 205 GPa, we propose that the corresponding superconducting candidate in Drozdov's work could be P4H6. By combining the PH3 structure evolutions at both room and low temperatures, we could obtain the phase diagram of PH3 under high pressure (Fig. 3d). As shown in Fig. 3d, at room temperature, two-step dimerization occurred at around 12 and 25 GPa, and P4H6 finally decomposed into elemental phosphorus at 35 GPa. However, at low temperature, P2H4 could exist up to 60 GPa. P4H6 was maintained from 102 to 205 GPa.
Theoretical calculations
We further performed structural searches on P4H6 at 100, 150 and 200 GPa with maximum simulation cells up to 4 formula units (f.u.); two stable structures with space group Cmcm (<182 GPa) and C2/m (>182 GPa) were found. Phonon dispersions calculations of the two structures do not give any imaginary frequencies and therefore this verifies their dynamic stabilities (Fig. 4). The superconducting Tc was estimated using the Allen and Dynes modified McMillan equation [34] with a typical choice of 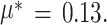 The electron–phonon coupling constant λ of the Cmcm structure is only 0.59 (Table 1) at 100 GPa, and a superconducting Tc of 13 K was obtained. A relatively large λ value of 1.39 was found for the C2/m structure at 200 GPa, and the superconducting Tc was estimated to be 67 K. As summarized in Table 1, the estimated Tc agrees with the values measured by Drozdov et al., suggesting that P4H6 could be responsible for the superconductivity.
The electron–phonon coupling constant λ of the Cmcm structure is only 0.59 (Table 1) at 100 GPa, and a superconducting Tc of 13 K was obtained. A relatively large λ value of 1.39 was found for the C2/m structure at 200 GPa, and the superconducting Tc was estimated to be 67 K. As summarized in Table 1, the estimated Tc agrees with the values measured by Drozdov et al., suggesting that P4H6 could be responsible for the superconductivity.
Figure 4.
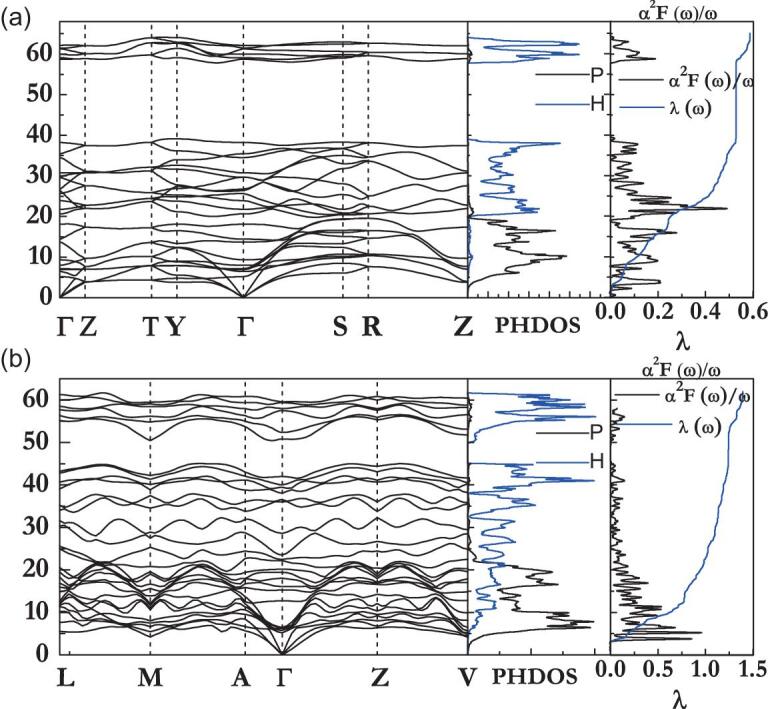
Phonon dispersions, phonon density of states projected onto atoms (PHDOS), the spectral functions α2F(ω)/ω and electron–phonon coupling integration of λ(ω) for the (a) Cmcm structure at 100 GPa and (b) C2/m structure at 200 GPa, respectively.
Table 1.
The calculated electron–phonon coupling constants (λ), the logarithmic average phonon frequency (ωlog) and the Tc with μ* = 0.13.
| Phases | Pressure (GPa) | λ | ω log | T c (μ* = 0.13) |
|---|---|---|---|---|
| Cmcm | 100 | 0.59 | 889 | 13 K |
| C2/m | 200 | 1.39 | 700 | 67 K |
Similar to H2S, PH3 is unstable at high pressure. Instead of becoming more hydrogen-enriching, it dehydrogenates through a series of polymerization/decomposition processes upon compression. This could be one of the critical factors that limit the maximum Tc near 100 K, at the same pressure where the H–S system has a Tc up to 180 K. These phenomena from H2S and PH3 highlight that avoiding the pressure-induced dehydrogenation or becoming more hydrogen-enriched is vital for a superconducting hydride with a high Tc.
CONCLUSION
In summary, we determined the stability of PH3 under high pressure. At room temperature, two steps of polymerization were obtained. P2H4 and P4H6 were the reaction products of the first and second step dimerization, respectively. Above 35 GPa, the generated P4H6 completely decomposed into elemental phosphorus. However, at low temperature, P4H6 could remain up to 205 GPa. Vibrational measurements and theoretical simulation confirmed the formation of P2H4 and P4H6, which enriches the phase diagram of the P–H system under high pressure. Our work proves that the P4H6 phase can be generated under high pressure and suggests that it might be responsible for the reported superconducting transition.
METHODS
Solidified PH3 was prepared via a cryogenic method and sealed into a symmetric DAC at ∼2 GPa for our in situ high-pressure measurements. T301 stainless steel and tungsten gaskets were used for the room-temperature and low-temperature measurements, respectively. The ruby fluorescence and Raman shifts of the diamond were used to calibrate the pressure. A micro-Raman system (Renishaw, UK) with a 532-nm laser excitation was used to obtain the sample's Raman spectra. The high-pressure in situ IR spectra were collected on a Bruker VERTEX 70v FTIR spectrometer and a custom-built IR microscope. High-pressure XRD measurements were carried out at the BL15U1 beamline of the Shanghai Synchrotron Radiation Facility (λ = 0.6199 Å) [35]. Low temperature was generated by cryostat using liquid nitrogen. Detailed information about each cycle is provided in the Supplementary Materials, available as Supplementary Data at NSR online. The ab initio structure predictions for P4H6 were performed using the particle swarm optimization technique implemented in the CALYPSO code [36,37]. CALYPSO has been used to investigate many materials at high pressures [38–42]. The ab initio structure relaxations were performed using density functional theory with the Perdew–Burke–Ernzerhof generalized gradient approximation implemented in the Vienna ab initio simulation package (VASP) [43]. Details of the simulations are provided in the Supplementary Materials, available as Supplementary Data at NSR online.
Supplementary Material
FUNDING
This work was mainly supported by the National Natural Science Foundation of China (11874076), the National Science Associated Funding (NSAF, U1530402) and the Science Challenging Program (TZ2016001).
REFERENCES
- 1. Onnes HK. Disappearance of the electrical resistance of mercury at helium temperatures. Proc K Ned Akad Wet 1911; 14: 113–5. [Google Scholar]
- 2. Onnes HK. The resistance of pure mercury at helium temperatures. Commun Phys Lab Univ Leiden b 1911; 120. [Google Scholar]
- 3. Ashcroft NW. Hydrogen dominant metallic alloys: high temperature superconductors? Phys Rev Lett 2004; 92: 187002. [DOI] [PubMed] [Google Scholar]
- 4. Eremets MI, Trojan IA, Medvedev SAet al.. Superconductivity in hydrogen dominant materials: silane. Science 2008; 319: 1506–9. [DOI] [PubMed] [Google Scholar]
- 5. Gao G, Oganov AR, Bergara Aet al.. Superconducting high pressure phase of germane. Phys Rev Lett 2008; 101: 107002. [DOI] [PubMed] [Google Scholar]
- 6. Gao G, Wang H, Bergara Aet al.. Metallic and superconducting gallane under high pressure. Phys Rev B 2011; 84: 064118. [Google Scholar]
- 7. Li Y, Gao G, Xie Yet al.. Superconductivity at 100 K in dense SiH4(H2)2 predicted by first principles. Proc Natl Acad Sci USA 2010; 107: 15708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H, Tse JS, Tanaka Ket al.. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc Natl Acad Sci USA 2012; 109: 6463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Hao J, Liu Het al.. Pressure-stabilized superconductive yttrium hydrides. Sci Rep 2015; 5: 9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drozdov AP, Eremets MI, Troyan IAet al.. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015; 525: 73–6. [DOI] [PubMed] [Google Scholar]
- 11. Gao L, Xue YY, Chen Fet al.. Superconductivity up to 164 K in HgBa2Cam−1CumO2m+2+δ (m=1, 2, and 3) under quasihydrostatic pressures. Phys Rev B 1994; 50: 4260–3. [DOI] [PubMed] [Google Scholar]
- 12. Duan D, Liu Y, Tian Fet al.. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci Rep 2015; 4: 6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Wang L, Liu Het al.. Dissociation products and structures of solid H2S at strong compression Yinwei. Phys Rev B 2016; 93: 020103. [Google Scholar]
- 14. Einaga M, Sakata M, Ishikawa Tet al.. Crystal structure of the superconducting phase of sulfur hydride. Nat Phys 2016; 12: 835–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drozdov AP, Eremets MI, Troyan IA. Superconductivity above 100 K in PH3 at high pressures. arXiv: 1508.06224.
- 16. Flores-Livas JA, Amsler M, Heil Cet al.. Superconductivity in metastable phases of phosphorus-hydride compounds under high pressure. Phys Rev B 2016; 93: 020508. [Google Scholar]
- 17. Liu H, Li Y, Gao Get al.. Crystal structure and superconductivity of PH3 at high pressures. J Phys Chem C 2016; 120: 3458–61. [Google Scholar]
- 18. Shamp A, Terpstra T, Bi Tet al.. Decomposition products of phosphine under pressure: PH2 stable and superconducting? J Am Chem Soc 2016; 138: 1884–92. [DOI] [PubMed] [Google Scholar]
- 19. Bi T, Miller DP, Shamp Aet al.. Superconducting phases of phosphorus hydride under pressure: stabilization by mobile molecular hydrogen. Angew Chem Int Ed 2017; 129: 10326–9. [DOI] [PubMed] [Google Scholar]
- 20. Durajski AP. Quantitative analysis of nonadiabatic effects in dense H3S and PH3 superconductors. Sci Rep 2016; 6: 38570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang TH, Decius JC, Nibler JW. Raman and IR spectra of crystalline phosphine in the γ phase. J Phys Chem Solids 1977; 38: 897–904. [Google Scholar]
- 22. Frankiss SG. Vibrational spectrum and structure of solid diphosphine. Inorg Chem 1968; 7: 1931–3. [Google Scholar]
- 23. Odom JD, Wurrey CJ, Carreira LAet al.. Vibrational spectra and structure of biphosphine and biphosphine-d4. Inorg Chem 1975; 14: 2849–53. [Google Scholar]
- 24. Baudler M, Glinka K. Open-chain polyphosphorus hydrides (phosphines). Chem Rev 1994; 94: 1273–97. [Google Scholar]
- 25. Olego DJ, Baumann JA, Schachter R. The microscope structures of amorphous phosphorus. Solid State Commun 1985; 53: 905–8. [Google Scholar]
- 26. Winchester RAL, Whitby M, Shaffer MSP. Synthesis of pure phosphorus nanostructures. Angew Chem Int Ed 2009; 48: 3616–21. [DOI] [PubMed] [Google Scholar]
- 27. Nixon ER. The infrared spectrum of biphosphine. J Phys Chem 1956; 60: 1054–9. [Google Scholar]
- 28. Frisch MJ, Trucks GW, Schlegel HBet al.. Gaussian 09, revision A.02. Gaussian Inc Wallingford CT 2009; 34. [Google Scholar]
- 29. Mao N, Tang J, Xie Let al.. Optical anisotropy of black phosphorus in the visible regime. J Am Chem Soc 2016; 138: 300–5. [DOI] [PubMed] [Google Scholar]
- 30. Sugai S, Shirotani I. Raman and infrared reflection spectroscopy in black phosphorus. Solid State Commun 1985; 53: 753–5. [Google Scholar]
- 31. Wittig J, Matthias BT. Superconducting phosphorus. Science 1968; 160: 994–5. [DOI] [PubMed] [Google Scholar]
- 32. Kawamura H, Shirotani I, Tachikawa K. Anomalous superconductivity in black phosphorus under high pressures. Solid State Commun 1984; 49: 879–81. [Google Scholar]
- 33. Karuzawa M, Ishizuka M, Endo S. The pressure effect on the superconducting transition temperature of black phosphorus. J Phys Condens Matter 2002; 14: 10759–62. [Google Scholar]
- 34. Allen PB, Dynes RC. Transition temperature of strong-coupled superconductors reanalyzed. Phys Rev B 1975; 12: 905–22. [Google Scholar]
- 35. Zhang LL, Yan S, Jiang Set al.. Hard X-ray micro-focusing beamline at SSRF. Nucl Sci Tech 2015; 26: 60101. [Google Scholar]
- 36. Wang Y, Lv J, Zhu Let al.. Crystal structure prediction via particle-swarm optimization. Phys Rev B 2010; 82: 1–8. [Google Scholar]
- 37. Wang Y, Lv J, Zhu Let al.. CALYPSO: a method for crystal structure prediction. Comput Phys Commun 2012; 183: 2063–70. [Google Scholar]
- 38. Li Y, Hao J, Liu Het al.. The metallization and superconductivity of dense hydrogen sulfide. J Chem Phys 2014; 140: 174712. [DOI] [PubMed] [Google Scholar]
- 39. Lv J, Wang Y, Zhu Let al.. Predicted novel high-pressure phases of lithium. Phys Rev Lett 2011; 106: 015503. [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Wang Y, Miao Met al.. Cagelike diamondoid nitrogen at high pressures. Phys Rev Lett 2012; 109: 175502. [DOI] [PubMed] [Google Scholar]
- 41. Errea I, Calandra M, Pickard CJet al.. Quantum hydrogen-bond symmetrization in the superconducting hydrogen sulfide system. Nature 2016; 532: 81–4. [DOI] [PubMed] [Google Scholar]
- 42. Peng F, Sun Y, Pickard CJet al.. Hydrogen clathrate structures in rare earth hydrides at high pressures: possible route to room-temperature superconductivity. Phys Rev Lett 2017; 119: 107001. [DOI] [PubMed] [Google Scholar]
- 43. Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 1996; 54: 11169–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.