Abstract
We describe the time course of neutralizing antibody (NtAb) titer in a cohort of health care workers with mild or asymptomatic severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection. NtAb levels decreased over time; however, serum neutralizing activity remained detectable after a median of 7 months from SARS-CoV-2 diagnosis in the majority of cases.
Keywords: follow-up, health care workers, mild or asymptomatic infection, neutralizing antibody titer, SARS-CoV-2
Despite an impressive number of clinical trials and evidence-based data [1], no antiviral treatment is recommended in subjects with coronavirus disease 2019 (COVID-19) and no or mild symptoms [2].
Neutralizing antibodies (NtAb) hamper viral replication, lowering viral load and favoring viral clearance [3]: these characteristics justify their inclusion among treatment options for COVID-19 patients [2]. However, the clinical importance of viral neutralization is wide-ranging, and titration of NtAb remains the gold standard for identifying immunized people, as a result of both naturally occurring infection and artificial immunization [4].
We aimed to analyze the NtAb kinetics in a cohort of Italian health care workers (HCWs) who experienced a mild or asymptomatic infection in the early phase of the pandemic in one of the most affected countries in Europe. This subset of subjects is an ideal nonexperimental model to study the time course of NtAb because the date of infection can be estimated with accuracy, as the HCWs were regularly screened, and an adequate follow-up is warranted.
METHODS
The study population included HCWs who had severe acute respiratory syndrome coronavirus (SARS-CoV-2) RNA detected on a nasopharyngeal swab because of clinical suspicion or in the context of weekly surveillance conducted in the Veneto area from March 1, 2020, to May 30, 2020. All subjects were asymptomatic or had mild disease according to the World Health Organization definition [5] and had negative reverse transcription polymerase chain reaction tests at the time of NtAb testing. Enrollment was on a voluntary basis, and no exclusion criteria were applied. A written informed consent was obtained from all HCWs to be enrolled in a study of NtAb kinetics, as approved by the Comitato per la Sperimentazione Clinica di Treviso e Belluno.
Serum Neutralization Assay
SARS-CoV-2 (lineage B, kindly provided by Luigi Sacco University Hospital, Milan, Italy) was propagated and titrated in VERO E6 cells grown in Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose (Euroclone) supplemented with 1% penicillin-streptomycin mixture (Euroclone) and 10% fetal bovine serum (FBS; Euroclone) at 37°C in a 5% CO2 humidified incubator. Serum samples were heat-inactivated for 40 minutes at 56°C before use. To titer NtAb, 2-fold serial dilutions of serum, from 1:4 to 1:512, were mixed with 100 TCID50 of virus, incubated for 1 hour at 37°C, and added to a semiconfluent VERO E6 cell monolayer in duplicate. After 72 hours, cell viability was determined through the commercial kit CellTiter-Glo 2.0 (Promega) following the manufacturer’s instructions. Each run included an uninfected control, an infected control, and a known SARS-CoV-2 neutralizing serum yielding a median (interquartile range [IQR]) titer of 69 (59.3–69.9) in 5 independent runs. In each plate, virus back titration was performed to confirm the virus inoculum (100 TCID50 – 50% tissue culture infectious dose). The luminescent signal generated by infected cells was compared with that generated by control cells and used to determine the reciprocal value of the serum dilution corresponding to 50% cell viability (EC50). Serum titers were validated when the duplicate values were within 40% variability and the known control serum yielded a titer within 2-fold of the reference median value. Sera with EC50 titers ≥4 were defined as SARS-CoV-2 neutralizing, and sera with EC50 <4 were defined as negative and scored as 2 for statistical analysis. Sera with EC50 >512 were further diluted and reanalyzed to yield a quantitative result.
Statistical Analysis
NtAb titers were analyzed both as a continuous variable and as a categorical variable (positive vs negative). Continuous variables were expressed as the median (IQR), whereas categorical variables were indicated as the absolute number and frequency. The Mann-Whitney U test, the Wilcoxon signed rank sum test, the chi-square test, and the Fisher exact test were employed as appropriate. Spearman’s rank correlation was applied to determine the degree of relationship between the variables. All calculated P values were 2-tailed and compared with a significance level of .05. Statistical analyses were performed using MedCalc Statistical Software, version 19.7 (MedCalc Software Ltd; https://www.medcalc.org).
RESULTS
Study Populations
One hundred four patients participated in the study, including 34 males and 70 females, with a median (IQR) age of 49 (40–56) years (all Caucasian). Most patients (69, 66.3%) had asymptomatic infection. The median time from diagnosis to first NtAb titration (T1) was 65 (39–86) days. For a subset of 67 patients, NtAb titers were measured at a second time point (T2). In this group, 42 subjects (62.7%) had asymptomatic infection, the median interval between diagnosis and T1 was 70 (45–87) days, the median interval between T1 and T2 was 140 (120–189) days, and the median interval between diagnosis and T2 was 223 (172–262) days. A detailed description of the subjects with NtAb quantified only at T1 and at both T1 and T2 is reported in Table 1.
Table 1.
Main Characteristics of the Health Care Workers Evaluated at T1 and T2 According to the Absence or Presence of Mild Symptoms
| Whole Data Set | Subset With Both T1 and T2 Measurements | |||||
|---|---|---|---|---|---|---|
| Asymptomatic | Mild Disease | P | Asymptomatic | Mild Disease | P | |
| Male, No. (%) | 21 (30.4) | 13 (37.1) | .4929 | 13 (31) | 11 (44) | .285 |
| Age, median (IQR), y | 49 (38–56) | 49 (44–54) | .7255 | 50 (39–57) | 49 (46–55) | .4755 |
| Days from diagnosis to T1, median (IQR) | 70 (39–88) | 61 (42–75) | .3371 | 76 (44–89) | 63 (46–84) | .2905 |
| Days from diagnosis to T2, median (IQR) | n.a. | n.a. | n.a. | 228 (210–268) | 178 (163–225) | .0059 |
| Subjects with undetectable NtAb at T1, No. (%) | 14 (20.3) | 4 (11.4) | .41 | 4 (9.5) | 1 (4) | .643 |
| Subjects with undetectable NtAb at T2, No. (%) | n.a. | n.a. | n.a. | 13 (31) | 0 | .001166 |
| NtAb titer at T1, median (IQR) | 14.5 (7–32.7) | 28 (14.3–100.4) | .0125 | 15.5 (8.5–37) | 57.5 (15.1–121) | .0069 |
| NtAb titer at T2, median (IQR) | n.a. | n.a. | n.a. | 13 (2–29) | 31 (13.6–57.1) | .0147 |
Significant P values are indicated in bold.
Abbreviations: IQR, interquartile range; n.a., not available; T1, first NtAb titration; T2, interval between diagnosis and the second titration.
NtAb Quantification at T1
Overall, 18 (17.3%) patients had a negative result. The median NtAb titer in the remaining 86 was 24.9 (14.0–68.1) EC50. There was no correlation either between NtAb titer and time passed since diagnosis (rho = –0.0783; P = .4343) or between NtAb titer and patient age (rho = 0.0838; P = .4022). However, NtAb titers were significantly higher in subjects experiencing mild disease as compared with those with asymptomatic infection (28 [14.3–100.4] vs 14.5 [7–32.7]; P = .0125).
Longitudinal Analysis
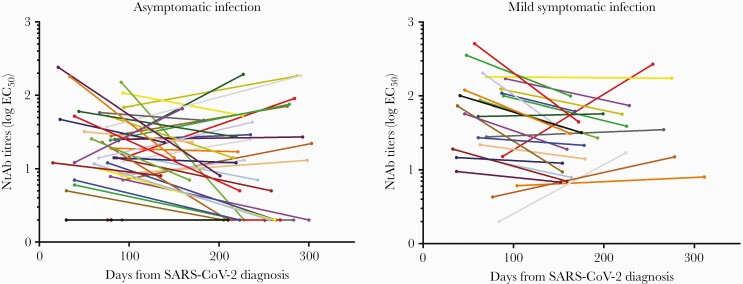
As for T1, there was no correlation between NtAb titers at T2 and time passed since diagnosis (rho = 0.0194; P = .8771). Overall, the median NtAb titer (IQR) decreased significantly from T1 to T2 (24 [11.7–66.1] vs 17 [7–44.2]; P = .0092). Five (7.5%) and 13 (19.4%) HCWs had no detectable NtAb at T1 and T2, respectively (P = .0435). These cases included 4 subjects who were negative at both T1 and T2, 1 subject positive at T2 only, and 9 subjects positive at T1 only. Asymptomatic subjects had comparable NtAb values (IQR) at T1 and at T2 (15.5 [8.5–37] and 13 [2–29], respectively); conversely, patients with mild disease experienced a significant decrease from T1 to T2 (57.5 [15.1–121] vs 31 [13.6–57.1]; P = .0045). The dynamics of NtAb titers are described in Figure 1.
Figure 1.
Dynamics of NtAb titers in the 42 subjects with asymptomatic infection and the 25 subjects with mild symptomatic infection, measured at 2 different time points following SARS-CoV-2 diagnosis. Each line represents 1 subject. Abbreviations: EC50, half maximal serum neutralization titer; NtAb, neutralizing antibody; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T1, first NtAb titration; T2, interval between diagnosis and the second titration.
DISCUSSION
Patient care exposes HCWs to a significantly increased risk of contracting SARS-CoV-2 infection [6]. Indeed, HCWs have undergone regular testing since the beginning of the pandemic to identify infection early and prevent onward transmission within the hospital. This in turn facilitates structured sampling and the study of the kinetics of NtAb response over time.
In Italy, different rates of laboratory-confirmed diagnosis of COVID-19 in HCWs have been reported, depending on different epidemiological settings and cohorts, exceeding 10% in multiple studies [7–9]. In agreement with previously published data [10], we observed a wide distribution of NtAb titers at T1, a timeline encompassing the expected peak of neutralizing activity [11]. The frequency of negative results was slightly higher with respect to that described by Marot et al. [12] after 2 months (11.8% of 17 HCWs tested) but similar to that reported by Schlickeiser et al. [13] (16% after a median interval of 53 days in a cohort of 206 convalescent plasma donors). Possible reasons for this variability include the use of different methods for NtAb detection and the inclusion of different study populations. It must be noted that a rate of negative NtAb similar to that detected in our study has been reported in a study of HCWs with asymptomatic and mild SARS-CoV-2 infection where T-cell responses were documented to complement antibody response [14].
In agreement with this study, a decline of NtAb titers over a limited period of time was reported by Gaebler et al. [15], who demonstrated a 5-fold decrease of NtAb levels from 1.3 months to 6.2 months following diagnosis using a neutralization method based on SARS-CoV-2 spike pseudoparticles. In our study, the last neutralizing capacity assessment was performed >7 months after molecular diagnosis and demonstrated NtAb activity in a proportion of cases (80.6%) similar to that reported by Dan et al. [16] (90%) after a 6/8-month period in a cohort of asymptomatic patients of different ethnicities. All the patients included in our study were Caucasian; thus, we were not able to detect a possible influence of ethnicity on antibody response [17].
The NtAb titer translating into protective immunity from reinfection with SARS-CoV-2 is currently unknown and cannot be extrapolated from other viral diseases where a wide range of values have also been reported [18]. However, low titers have been shown to be protective in primate models [19].
The strengths of this study include the homogeneity of the study population and the use of live-virus neutralization with a SARS-CoV-2 isolate circulating in Italy at HCW enrollment [20]. The main limitation is the availability of a short follow-up in a fraction of subjects. In addition, we acknowledge that the study of neutralizing activity is not possible in all laboratories, as it is time-consuming and expensive. However, we believe it is a fundamental tool to define the kinetics and role of SARS-CoV-2 immunity in HCWs recovering from infection as well as in other patient categories and vaccine recipients.
Acknowledgments
We would like to thank Alessia Lai for making the SARS-CoV-2 lineage B strain available for this study.
Financial support. This work was supported by University of Padova, grant numbers DOR-2019 and DOR-2020 to S.G.P. and M.B.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. I.V.: performed the laboratory experiments, helped to interpret the findings and to write the paper. A.B.: performed the laboratory experiments. F.G.: managed the patients and collected the samples. R.S.: managed the patients and collected the samples. E.M.: managed the patients and collected the samples. D.Z.: helped to interpret the findings, wrote the paper. M.B.: performed the statistical analysis, helped to interpret the findings, and wrote the paper. F.D.: performed the laboratory experiments. N.B.: performed the laboratory experiments. M.Z.: supervised the laboratory experiments, helped to interpret the findings, performed the statistical analysis, and wrote the paper. S.G.P.: designed and coordinated the study, collected and managed the samples and the data, interpreted the findings, and wrote the paper.
Patient consent. A written informed consent was obtained from all the HCWs to be enrolled in a study of NtAb kinetics, as approved by the Comitato per la Sperimentazione Clinica di Treviso e Belluno.
Prior presentation. Preliminary data from this study were presented as Science Spotlight presentation at CROI 2021 (virtual).
References
- 1. Mussini C, Falcone M, Nozza S, et al. ; Italian Society of Infectious and Tropical Diseases . Therapeutic strategies for severe COVID-19: a position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT). Clin Microbiol Infect 2021; 27:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health. COVID-19 treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 6 March 2021. [PubMed]
- 3. Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 2020; 11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol 2003; 21:515–46. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. Geneva: World Health Organization; 2020. [Google Scholar]
- 6. Nguyen LH, Drew DA, Graham MS, et al. ; COronavirus Pandemic Epidemiology Consortium . Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colaneri M, Novelli V, Cutti S, et al. The experience of the health care workers of a severely hit SARS-CoV-2 referral hospital in Italy: incidence, clinical course and modifiable risk factors for COVID-19 infection. J Public Health (Oxf) 2021; 43:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalla Volta A, Valcamonico F, Pedersini R, et al. The spread of SARS-CoV-2 infection among the medical oncology staff of ASST Spedali Civili of Brescia: efficacy of preventive measures. Front Oncol 2020; 10:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sotgiu G, Barassi A, Miozzo M, et al. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med 2020; 20:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu F, Liu M, Wang A, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med 2020; 180:1356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee WT, Girardin RC, Dupuis AP, et al. Neutralizing antibody responses in COVID-19 convalescent sera. J Infect Dis 2021; 223:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marot S, Malet I, Leducq V, et al. ; Sorbonne Université SARS-CoV-2 Neutralizing Antibodies Study Group . Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun 2021; 12:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlickeiser S, Schwarz T, Steiner S, et al. Disease severity, fever, age, and sex correlate with SARS-CoV-2 neutralizing antibody responses. Front Immunol 2020; 11:628971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reynolds CJ, Swadling L, Gibbons JM, et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol 2020; 5:eabf3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shields AM, Faustini SE, Perez-Toledo M, et al. Serological responses to SARS-CoV-2 following non-hospitalised infection: clinical and ethnodemographic features associated with the magnitude of the antibody response. medRxiv 2020.11.12.20230763 [Preprint]. 16 November 2020. Available at: 10.1101/2020.11.12.20230763. Accessed 18 February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krammer F, Weir JP, Engelhardt O, et al. Meeting report and review: immunological assays and correlates of protection for next-generation influenza vaccines. Influenza Other Respir Viruses 2020; 14:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020; 369:806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vicenti I, Gatti F, Scaggiante R, et al. Single-dose BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibody response in health care workers recovering from asymptomatic or mild natural SARS-CoV-2 infection. Int J Infect Dis 2021; 108:176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]