Abstract
Knowledge about how the COVID-19 pandemic can affect aquatic wildlife is still extremely limited, and no effect of SARS-CoV-2 or its structural constituents on invertebrate models has been reported so far. Thus, we investigated the presence of the 2019-new coronavirus in different urban wastewater samples and, later, evaluated the behavioral and biochemical effects of the exposure of Culex quinquefasciatus larvae to two SARS-CoV-2 spike protein peptides (PSPD-2002 and PSPD-2003) synthesized in our laboratory. Initially, our results show the contamination of wastewater by the new coronavirus, via RT-qPCR on the viral N1 gene. On the other hand, our study shows that short-term exposure (48 h) to a low concentration (40 μg/L) of the synthesized peptides induced changes in the locomotor and the olfactory-driven behavior of the C. quinquefascitus larvae, which were associated with increased production of ROS and AChE activity (cholinesterase effect). To our knowledge, this is the first study that reports the indirect effects of the COVID-19 pandemic on the larval phase of a freshwater invertebrate species. The results raise concerns at the ecological level where the observed biological effects may lead to drastic consequences.
Keywords: SARS-CoV-2, Peptides, Insects, Contamination, Water, Developmental toxicity
Graphical abstract

1. Introduction
SARS-CoV-2 is a single-stranded RNA virus and the coronaviral genome encodes the spike (S) protein a heavily glycosylated type I membrane protein located on the virion surface, which is essential for attachment to the host receptor (Schoeman et al., 2019). The entry receptor utilized by SARS-CoV-2 is Angiotensin-Converting Enzyme 2 (ACE-2) (Li et al., 2003; Chan et al., 2020). These proteins have two subunits, one composed of highly conserved polypeptides associated with the envelope and the other subunit composed of a single polypeptide that contains the host cell's binding domain (Chan et al., 2020; Hoffmann et al., 2020). This virus causes a disease, the Coronavirus Disease 2019 (COVID-19) with clinical symptoms similarly to severe acute respiratory syndrome (SARS) and was therefore named by the International Committee on Taxonomy of Viruses as SARS-CoV-2 (Lu et al., 2020; A. Wu et al., 2020a). The COVID-19 disease, which started in 2019, became a worldwide pandemic in the year 2020 with a rapid increase in the number of cases in worldwide. This unprecedented pandemic has led to the death of millions of people and has therefore represents a worldwide public health problem (Abduljalil & Abduljalil, 2020; Tang et al., 2020).
The main route of transmission of the SARS-CoV-2 to man is through the inhalation of droplets generated when an infected person coughs, sneezes or exhales (Sunkari et al., 2021). However, recent studies have shown that SARS-CoV-2 can be found in the feces and urine of infected patients (Foladori et al., 2020; Pan et al., 2020; Randazzo et al., 2020). Different studies have shown that SARS-CoV-2 infectious particles actively replicate in the enterocytes of the human intestine and are subsequently eliminated in the feces. The brush border of intestinal enterocytes is the region where there is the greatest expression of ACE2 (SARS-CoV-2 cell receptor, angiotensin-converting enzyme 2) in the human body (Lamers et al., 2020; Qi et al., 2020). All these piece of evidence suggested that SARS-CoV-2 can be detected in wastewater mainly in human areas, can receive a viral input from urban and hospital sewage (Medema et al., 2020; Y. Wu et al., 2020b).
Although SARS-CoV-2 disease has been extensively studied in recent months, the virus effects, or parts of it, in animals at early stages of exclusively aquatic development have not been well explored. Some researchers described that the early stages of development of different organisms are extremely sensitive to water contaminants and pollutants (Pašková & Hilscherová, 2011; Schweizer et al., 2018, Malafaia et al., 2020a, Malafaia et al., 2020b, Malafaia et al., 2020c). Smaller larvae of aquatic insects have a higher uptake of contaminants than larger larvae because of a relatively higher body surface area to body mass ratio (Buchwalter et al., 2002, 2004; Wiberg-Larsen et al., 2016). Thus, it is questionable whether the presence of SARS-CoV-2 viral particles in aquatic ecosystems also poses an additional threat to the health of non-target organisms. Although the image of mosquitoes is commonly associated with the transmission of diseases and damage to human health, these animals serve the purpose of more than just buzzing in our ears, being intrinsic to our ecology. Mosquitoes are considered important pollinating agents of several plant species, in addition to acting in the population control of several wild species (Fang, 2010; Lahondère et al., 2020; Peach et al., 2021). Larval mosquitoes contribute to aquatic food chains by serving food sources for many predators, including fish and birds. In the absence of their larvae, hundreds of animal species would have to change their diet to survive. The larvae themselves eat microscopic organic matter in the water, helping to recycle nutrients back into the ecosystem (Souza et al., 2019). Therefore, the importance of mosquitoes (larvae and adults) in the functioning of natural ecosystems is undeniable (Fang, 2010). Thus, an assessment of the presence of contaminants/pollutants in the aquatic environment and its effects on the biology of species that live in that environment is of utmost importance in the environmental and ecological spheres and can have numerous consequences for environmental health.
In this context, the possibility of detecting the SARS-CoV-2 virus (via RT-qPCR on the viral N1 gene) was initially evaluated in wastewater samples from a Brazilian municipality with a high incidence of COVID-19 (in 2020). Subsequently, the hypothesis that the presence of SARS-CoV-2 spike protein peptides on the aquatic environment induces behavioral changes, cholinesterase effect and REDOX imbalance in the investigated model system (C. quinquefasciatus) was tested. To our knowledge, this is the first report on the impact of SARS-CoV-2 peptides on a species of freshwater invertebrate and, therefore, joins the list of studies that warn about the indirect risk of COVID-19 on wildlife.
2. Materials and methods
2.1. Investigation of the presence of SARS-CoV-2 in wastewater
2.1.1. Sample collection
The sewage samples were obtained from the Primary Treatment Unit of the São José do Rio Preto Sewage Treatment Plant (São Paulo, Brazil), where 98–99 % of all sewage generated in the municipality is processed. Samples were collected in a HACH Sigma SD900 AWRS refrigerated automatic sampler. The collection flow was adjusted to acquire 1 sample for every 3000 m3 processed (equivalent to approximately 36 sampling events/day). After collection, the samples were kept below 4 °C and sent to the Sewage Treatment Plant of São José do Rio Preto/Brazil, where the decanting and filtering processes were carried out. Briefly, the samples were decanted for 10 min and their filtration took place under vacuum using PALL CORPORATION 47 mm 1 μm glass fiber membranes. Subsequently, these samples were taken to the Laboratory of Genomic Studies at UNESP Rio Preto/Brazil. Sewage samples were collected (200 mL) three times a week during the period from July 15, 2020 to August 19, 2020. All samples were kept at 4 °C and processed within 24 h of collection.
2.1.2. Viral precipitation, RNA extraction, reverse transcription, and quantitative PCR
The filtered samples were centrifuged at 40,000×g for 3 h at 4 °C. Viral pellets were resuspended in 1 mL of DEPC-treated water and stored at −80 °C. RNA extraction of 200 μL of each sample was performed using Trizol reagent (Thermo Fisher Scientific, USA) according to manufacturer's instructions. cDNA was synthesized by reverse transcription (RT) using High-capacity cDNA Reverse Transcription kit (Applied Biosystems, USA) based on the manufacturer's protocol. The quantitative PCR (qPCR) was performed with TaqMan assay (ThermoFisher Scientific, USA) and N1 primers and probes from 2019-nCoV CDC EUA Kit (IDT Technologies, USA). Briefly, TaqMan® Universal PCR Master Mix (ThermoFisher Scientific, USA) reaction was set up as follows: 10 μL reactions included 2.5 μL of Nuclease-free water, 1.5 μL of Primer and Probe mix, 5 μL of Master Mix and 1 μL of cDNA from the template. Nuclease-free water was used as negative template control (NTC). The qPCR reaction was carried out for 45 cycles using ABI QuantStudio 12 K Flex PCR System based on the following program: polymerase activation (95 °C for 10 min), PCR (45 cycles, denature at 95 °C for 15 s, and anneal/extend at 60 °C for 1 min). To quantify viral genome copy numbers in the samples, standard curves for N1 were generated using a dilution series of a positive template control (PTC) plasmid (IDT Technologies, USA) with concentrations ranging from 10 to 105 copies per reaction. Three technical replicates were performed at each dilution. The detection limit was determined at 10 copies of the control plasmid. The NTC showed no amplification over the 45 cycles of qPCR. The qPCR experiments were performed in triplicates.
2.2. Viral peptides
The synthesis, cleavage, purification, and characterization of the peptides of the SARS-CoV-2 Spike protein was performed according to methods described recently in detail by Charlie-Silva et al., 2021a, Charlie-Silva et al., 2021b. The synthesis of the Spike S protein was conducted using the solid phase peptide synthesis method (SPPS) following the Fmoc strategy (Raibaut et al., 2014; Behrendt et al., 2016). The resins used in this process were Fmoc-Cys-Wang, Fmoc-Thr-Wang and Fmoc-Asn-Wang for the Arg-Val-Tyr-Ser-Ser-Ala-Asn-Asn-Cys-COOH peptides; Gln-Cys-Val-Asn-Leu-Thr-Thr-Arg-Thr-COOH and Asn-Asn-Ala-Thr-Asn-COOH, respectively. At the end of the synthesis, these resins made it possible to obtain peptides with the carboxylated C-terminal end. After coupling all the amino acid residues of the peptide sequences, the chains were removed from the solid support by means of acid cleavage using trifluoroacetic acid (TFA), similarly to Guy & Fields (1997). The crude compounds were purified by high performance liquid chromatography (HPLC) with a reverse phase column using different purification methods according to the retention time obtained in a gradient program of 5–95 % in 30 min (exploration gradient) in Analytical HPLC [similarly to Klaassen et al. (2019)]. Only compounds with purity equal to or greater than 95 % were considered for in vivo evaluation, following the rules determined by the National Health Surveillance Agency (ANVISA/Brazil) and Food and Drug Administration (FDA/USA). The similarities between the PSPD2002 peptides (sequence: Gln-Cys-Val-Asn-Leu-Thr-Thr-Arg-Thr-COOH; MW: 1035.18 g/mol) and PSPD2003 (sequence: Asn-Asn-Ala-Thr- Asn-COOH; MW: 532.51 g/mol) synthesized in the present study were tested using the CLUSTAL W software version 1.83 [Higgins et al. (1996); Pais et al. (2014) - http://www.ebi.ac.uk/clustalw/]. The results of the processes described above can be seen in Charlie-Silva et al., 2021a, Charlie-Silva et al., 2021b and the structural models of peptides PSPD2002 and PSPD2003 that were synthesized in the present study are shown in Fig. 1 .
Fig. 1.
Molecular models of the active conformations of peptides (A) PSPD2002 and (B) PSPD2003 that were synthesized in the present study.
It is noteworthy that these peptides were chosen because they showed relevant biological effects on the infected host cell (see details in Fernandes et al. (2021) - bioRxiv preprint https://doi.org/10.1101/2020.10.20.346262). Considering that this in silico method mimics possible degradation of spike protein in the environment, an evaluation of its effects on animals that use this medium to develop has become relevant.
2.3. In vivo evaluation
2.3.1. Model organism and experimental design
To assess the toxicity of SARS-CoV-2 proteins on aquatic biota, we used larvae of a species distributed worldwide (Samy et al., 2016) and highly anthropophilic, Culex quinquefasciatus (Vieira et al., 2020). In addition, the species has recognized importance in public health, as it acts as the main vector of the periodic Wuchereria bancrofti (which causes lymphatic filariasis) (Shenoy, 2008; WHO, 2010; Samy et al., 2016; King, 2020), and it is a secondary vector of different human arboviruses (Lima-Camara, 2016; Pesko & Mores, 2009; Romero-Alvarez et al., 2018).
The larvae of C. quinquefasciatus used in this study were obtained in a semi-natural breeding place maintained at the Bioterium of the Biological Research Laboratory of the Federal Goiano Institute - Campus Urutaí (GO, Brazil), according to procedures recommended by Gerberg et al. (1994) which were also used by Alves et al. (2018) and Alves et al. (2020). Briefly, the females lay on plastic trays (50 cm × 40 cm x 25 cm) covered with Sombrite®60 % mesh, containing 25 L of deionized water and 20 g of feed used to feed mice. The trays were prepared about 10 days before the first laying and left under natural conditions of temperature and photoperiod. Upon reaching the 4th instar, the larvae were distributed in different experimental groups, whose experimental design was completely randomized into three groups, consisting of ten replicates/each, with 10 larvae of fourth instar/replica, totaling 100 larvae/experimental group. The groups “PSPD-2” and “PSPD-3” were composed of larvae exposed to the respective peptides at a concentration of 40 μg/L, respectively, diluted in water, which simulates the presence of viral particles in the aquatic environment in low concentration. The control group was composed of larvae kept in purified water free from any viral protein.
The static exposure system was used over a period of 48 h, defined based on the Guidelines for Laboratory and Field testing of Mosquito larvicides from the World Health Organization (No. WHO/CDS/WHOPES/GCDPP/2005.13 – WHO, 2005).
During this period, the larvae were kept in beakers containing 50 mL of dechlorinated water (containing or not the peptides). The larvae were not fed to avoid energetic carry-over effects due to changes in food intake during the exposure period to the post-exposure period, similarly to the procedures adopted by Malafaia et al., 2020a, Malafaia et al., 2020b, Malafaia et al., 2020c. All groups were kept in an experimental room with controlled luminosity (light/dark cycle of 12 h–100 lux) and temperature (25 °C ± 1 °C). At the end of the experiment, the larvae were weighed and separated in microtubes previously cleaned for later storage in an ultra-freezer (−80 °C). Biochemical analyzes were performed on the day after the end of the experiment.
2.3.2. Behavioral assessment
After 48 h of exposure, the larvae of C. quinquefasciatus were subjected to two different behavioral tests, in which possible effects of the peptides on the locomotor activity and olfactory capacity of the animals were evaluated.
2.3.2.1. Evaluation of the locomotive activity of the larvae
To evaluate the locomotor activity of the animals, we followed the protocol described by Juliano & Reminger (1992) with modifications. Briefly, 18 larvae from each experimental group were placed randomly and individually in glass beakers (height: 5 cm; diameter: 3.5 cm) containing 25 mL of dechlorinated water. The behavior of this larva was filmed for 5 min. During the behavioral test, the time and frequency of fluctuation, the time of “swim wriggle”, the locomotor activity index (determined by the ratio between “swim wriggle” and immobility time) and the traveled distance (cm) were recorded for each larva. The fluctuation behavior was recorded when the larva remained positioned at the surface of the water, with the respiratory siphon attached to the air-water interface and the body hanging obliquely into the water column. The “swim wriggle” is the characteristic wriggling motion that mosquito larvae make when they swim. It consists of flexing and unflexing movements that have been described previously (Walter & Merritt, 1991). We also recorded the time (s) of residence of each larva in the superficial positions (zone 1, when the spiracular siphon of larvae was in contact with the water's surface) and at the bottom of the beakers (zone 2, when the larvae were 1 mm from the container bottom) (Fig. 2 A). All parameters described above were registered with the aid of the PlusMZ software.
Fig. 2.
Schematic representation of the apparatus used in the behavioral tests to which the larvae of Culex quinquefasciatus were subjected. (A) Apparatus used in the evaluation of the general locomotive activity of the larvae and (B) the olfactory-driven behavior.
2.3.2.2. Evaluation of olfactory-driven behavior
The evaluation of the possible effect of the peptides on the larvae's ability to respond to chemical stimuli, the olfactory-driven behavior to identify a range of odorant-specific responses of C. quinquefasciatus larvae was carried out. We used the methodology proposed by Xia et al. (2008), with some modifications. For this, 50 larvae from each experimental group were carefully placed in a polyethylene box (40 cm long x 15 cm high x 33 cm wide) with white walls containing 2 L of dechlorinated water. After 15 min of acclimatization, a layer of 1.5 % agarose (thickness: 1 mm; diameter: 3 cm) prepared in a 5 % phenol solution, chosen as an odoriferous chemical stimulus, was inserted on one side of the apparatus, as previously evaluated by Millar et al. (1992). On the opposite side, a layer of agarose free of phenol solution was placed (see details in Fig. 2B). Real-time images were acquired every 30 s in a 60 min trial. The number of larvae in the odoriferous zone (radius of 30 mm from the center of the layer of agarose) and control (layer without phenol) was recorded in all time points. From there, the response index (RI) of the larvae was determined, according to the following equation (Heimbeck et al., 1999; Gonzalez et al., 2015).
where the “#odorant” indicates the number of larvae in the test zone (odoriferous) (≤30 mm from the center of the odor source) and the “#control” indicates the number in the control zone (≤30 mm from the center of the layer of agarose phenol free). Positive RIs indicate attraction; negative RIs indicate avoidance; and RI = 0 indicates indifferent behavior.
2.3.3. Biochemical assessment
To assess the effects of peptides on different biochemical parameters, samples were prepared based on the study by Guimarães et al. (2021a) and the toxicity biomarkers were evaluated according to different previous studies (see details in Table 1 ).
Table 1.
General information about sample preparation and toxicity biomarkers evaluated.
| Sample preparation | |
|---|---|
| Number of samples analyzed/group: n = 8 | Each sample analyzed was composed of a pool of 4 larvae - totaling 32 animals/group |
| Maceration | Maceration of the animals' bodies in phosphate buffered saline (1 mL), followed by centrifugation |
| Centrifugation | 13,000 rpm, for 5 min at 4 °C |
| Sample used |
Supernatant |
|
Oxidative stress biomarkers |
References (methods) |
| Nitrite [(μmol/L)/g protein] | Griess colorimetric reaction, described in Bryan and Grisham (2007) |
| Reactive oxygen species (ROS) [(relative fluorescence)/g protein] | Maharajan et al. (2018) |
| Hydrogen peroxide (H2O2) [(mmol/L)/g protein] | Elnemma et al. (2004) |
| Malondialdehyde (MDA) (nmol MDA/g protein) |
Sachett et al. (2020) |
|
Antioxidant response biomarkers |
References (methods) |
| Catalasea (mmol/g protein) | Sinha et al. (1972) [see details in Montalvão et al. (2021)] |
| Superoxide dismutase (SOD)a (units/mg protein) |
Del-Maestro & McDonald (1987) |
|
Cholinesterase effect |
References (methods) |
| Acetylcholinesterase (AChE) [(mmol/min/mL)/g protein] |
Ellman et al. (1961) |
|
Total protein |
References (methods) |
| Total protein (g/dL) | Commercial kit (Bioténica Ind. Com. LTD, Varginha, MG, Brasil. CAS number: 10.009.00), according to Gornall et al. (1949). |
These molecules are considered first-line antioxidants defenses that are important for preventing physiological oxidative stress.
2.4. Anti-contamination procedures
For all assays, we adopt procedures to avoid potential procedural contamination. In synthesis, all equipment/materials used, such as glassware, metal instruments and aquariums (in the bioassay) were rinsed in purified water and 70 % ethyl alcohol before use and were immediately covered with parafilm until use. In addition, personal protective equipment (such as lab coats, masks, and gloves) was used by researchers during the assays. Gloved hands were cleaned with purified water and 70 % ethyl alcohol before performing each step. The work areas were also kept sanitized with disinfectants and 70 % ethyl alcohol was used to clean all laboratory benches. During the execution of the experimental procedures, the air circulation in the work areas was turned off and the traffic of people was restricted to the researchers involved in the study.
2.5. Data analysis
GraphPad Prism Software Version 8.0 (San Diego, CA, USA) was used to perform the statistical analyses. Data were initially checked for deviations from variance normality and homogeneity before the analysis. Data normality was assessed through Shapiro-Wilks test, and variance homogeneity was assessed through Bartlette's test. Multiple comparisons were performed based on one-way ANOVA, Tukey's post-hoc analysis (for parametric data) or Kruskal-Wallis test, with Dunn's post-hoc (for non-parametric data). Correlation analysis was performed based on the Pearson's method. Regression analysis was performed when significant differences were detected between different treatments. Significance levels were set at Type I error (p) values lower than 0.05.
3. Results
3.1. SARS-CoV-2 genomes were detected in wastewater
Initially, our data showed that almost all processed samples scored positive for the presence of SARS-CoV-2 genomes as assessed by RT-qPCR on the viral N1 gene, except in August 2020 (Fig. 3 A). SARS-CoV-2 RNA was found in a range from 9 × 104 to 1,1 × 105 genome copies/L with two peaks at days 07/17/20 and 07/27/20. Although there was no significant correlation between these results and other epidemiological parameters analyzed (Fig. 6B — F, Table 1S), in some evaluation days (especially on July 20 and 29, 2020), the high concentrations of SARS-CoV-2 RNA identified in the urban wastewater samples coincided with a high number of cases registered in the last 24 h in the municipality of São José do Rio Preto (São Paulo, Brazil) (Fig. 3B).
Fig. 3.
(A) SARS-CoV-2 RNA (copy/L) identified in wastewater. (B) Reported COVID-19 cases (last 24 h). (C) Reported COVID-19 deaths (last 24 h). (D) Number of people hospitalized (intensive care unit + medical wards). (E) COVID-19 incidence coefficient. (F) Cumulative cases of COVID-19. The data contained in “B–F” were obtained from the Influenza Surveillance Information System (SIVEP-Gripe) and the e-SUS-VE System (epidemiological surveillance), both from the Brazilian Ministry of Health. The reference municipality was São José do Rio Preto (São Paulo, Brazil) and the epidemiological data collected are from the same wastewater collection dates (2020). The dashed lines represent the overlap of the data related to the SARS-CoV-2 RNA and each epidemiological parameter analyzed.
Fig. 6.
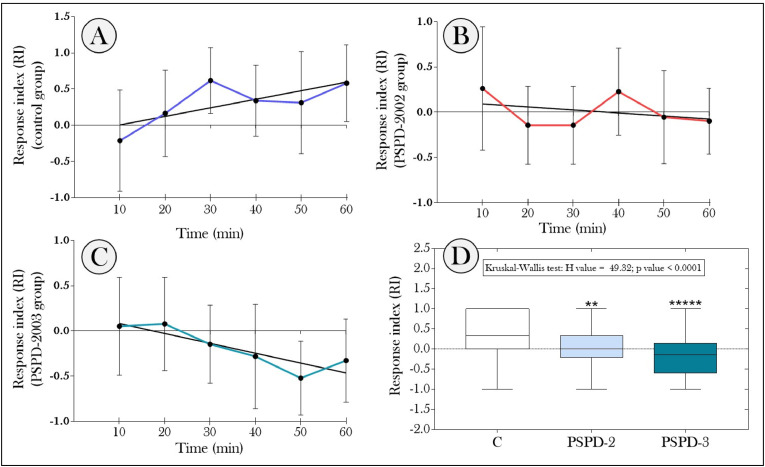
Response index (RI) of larvae of C. quinquefasciatus to the olfactory-driven behavior test. (A–C) RI recorded during the behavioral test for the control group, PSPD-2 and PSPD-3; and (D) RI calculated considering the average of the values obtained in the evaluated times. In “D”, asterisks denote significant difference among the treated groups and the control group. C: control group; PSPD-2: group exposed to SARS-CoV-2 spike protein peptide 2002; PSPD-3: group exposed to SARS-CoV-2 spike protein peptide 2003. [n = 18 larvae/group; p-value < 0.01 (*), 0.001 (**), 0.0001 (***), <0.0001 (****)].
3.2. SARS-CoV-2 spike protein peptides alters the behavior of Culex quinquefasciatus larvae
Since the presence of SARS-CoV-2 genomes in urban wastewater samples was detected, we exposed the larvae of C. quinquefasciatus to the synthesized peptides, which represents the presence of 2019-new coronavirus virus particles in the aquatic environment. Our data reveal that the larvae exposed to the peptide PSPD-2002 exhibited altered behavior. It was noticed that these animals exhibited a greater time and frequency of fluctuation behavior (Fig. 4 A–B, respectively), “swim wriggle” (Fig. 4C), locomotor activity index (Fig. 4D) and distance covered (Fig. 4E). This suggests increased animal mobility induced by exposure to PSPD-2002 when they were not exhibiting fluctuation behavior.
Fig. 4.
Boxplot's parameters of (A) fluctuation time, (B) fluctuation frequency, (C) “swim wriggle” time, (D) locomotor activity index and (E) distance traveled of Culex quinquefasciatus larvae exposed or not to SARS-CoV Spike protein peptides, submitted to the locomotor activity test (see details in item “2.3.2.1”). Asterisks denote significant difference among the treated groups and the control group. C: control group; PSPD-2: group exposed to SARS-CoV spike protein peptide 2002; PSPD-3: group exposed to SARS-CoV spike protein peptide 2003. Bars indicate the mean ± standard deviation (n = 18 larvae/group). [p-value < 0.01 (*), 0.001 (**), 0.0001 (***), <0.0001 (****)].
Regarding the position of the animals during the locomotor activity test, we also observed that the exposure to PSPD-2002 led the larvae to stay longer in surface of the apparatus than in bottom (Fig. 5 A–B, respectively). Again, the group exposed to PSPD-2003 did not change and presented data similar those of the control group. Considering the olfactory-driven behavior, the RIs of the animals in the control group increased throughout the test (Fig. 6A), suggesting an attractive response of these animals to the odorous stimulus (Fig. 6D). However, the same behavioral pattern was not observed in animals exposed to the peptides. In the PSPD-2002 group, there was a tendency for the RI to decrease over the evaluation period (Fig. 6B), with most of the values obtained at the evaluated times being negative. Although the mean RI value obtained for this group was positive (Fig. 6D), the animals' behavior throughout the temporal evaluation suggests a repulsion to the odorous stimulus provided by the phenol agarose slide, when compared to the animals in the control group. On the other hand, we observed that exposure to PSPD-2003 of the larvae induced a repulsion to the odorous stimulus from the first minutes of evaluation, showing a greater accentuation at the end of the test (Fig. 6C–D). The mean RI's for the PSPD-2 and PSPD-3 groups were 69.5 % and 157.8 % lower than the response of the animals in the control group (Fig. 6D).
Fig. 5.
Boxplot's of the time spent on (A) the surface and (B) bottom of the apparatus used in the locomotor activity test of Culex quinquefasciatus larvae, exposed or not to the SARS-CoV Spike protein peptides and subjected to the locomotor activity test (see details in item “2.3.2.1”). Asterisks denote significant difference among the treated groups and the control group. C: control group; PSPD-2: group exposed to SARS-CoV-2 spike protein peptide 2002; PSPD-3: group exposed to SARS-CoV-2 spike protein peptide 2003. [n = 18 larvae/group; p-value < 0.01 (*), 0.001 (**), 0.0001 (***), <0.0001 (****)].
3.3. SARS-CoV-2 spike peptides induces RODOX imbalance
To understand the cause of the behavioral changes observed in the larvae of C. quinquefasciatus, we evaluated the relationship between these changes and the induction of REDOX imbalance by the SARS-CoV-2 spike protein peptides. Initially, as biochemical analyses have shown that concentrations of nitrite and malondialdehyde not differ between the groups exposed to SARS-CoV-2 spike protein peptides and control group (Fig. 7 A–B, respectively). However, it was observed that the treatments induced a differentiated effect on the ROS and H2O2 levels. There was a significant increase in ROS level in groups exposed to PSPD-2002, but not in PSPD-2003 (Fig. 7C), and a decrease in H2O2 levels was found after exposure to PSPD-2003, but not by PSPD-2002 (Fig. 7D). The statistical analyzes reveal that the increase in the production of ROS was correlated with all the behavioral parameters evaluated except for the “distance covered” (Fig. 1S). On the other hand, the antioxidant activity parameters were also altered after exposure to SARS-CoV-2 spike protein peptides (Fig. 8 ). Both peptides induced an increase in SOD levels, showing even more significant effects in the animals exposed to PSPD-2003 (Fig. 8A). However, the catalase activity was decreased in larvae of PSPD-2202 group, whereas PSPD-2003 led an increase (Fig. 8B), highlighting once more the specific response of the peptides on the physiology of the model organisms evaluated.
Fig. 7.
Boxplot of nitrite (A), malondialdehyde (B), reactive oxygen species (C) and hydrogen peroxide (D) levels in Culex quinquefasciatus larvae exposed or not to SARS-CoV-2 Spike protein peptides. In “C” e “D”, asterisks denote significant difference among the treated groups and the control group. C: control group; PSPD-2: group exposed to SARS-CoV-2 spike protein peptide 2002; PSPD-3: group exposed to SARS-CoV-2 spike protein peptide 2003. [n = 32 larvae/group; p-value < 0.01 (*), 0.001 (**), 0.0001 (***), <0.0001 (****)].
Fig. 8.
Boxplot of the activity of the enzymes superoxide dismutase (A) and catalase (B) in Culex quinquefasciatus larvae exposed or not to SARS-CoV-2 Spike protein peptides. Asterisks denote significant difference among the treated groups and the control group. C: control group; PSPD-2: group exposed to SARS-CoV-2 spike protein peptide 2002; PSPD-3: group exposed to SARS-CoV-2 spike protein peptide 2003. [n = 32 larvae/group; p-value < 0.01 (*), 0.001 (**), 0.0001 (***), <0.0001 (****)].
3.4. SARS-CoV-2 spike peptides increase AChE activity
The SARS-CoV-2 peptides also affected the animals' cholinesterase system. An increased in AChE activity was shown in the treated groups compared to the controls. This result being more evident in PSPD-2002 group than in PSPD-2003 group (Fig. 9 ). These results were positively correlated with the production of ROS in the larvae of C. quinquefasciatus.
Fig. 9.
(A) Boxplot of the activity of the enzyme acetylcholinesterase of larvae of Culex quinquefasciatus exposed or not to SARS-CoV-2 spike protein peptides and (B) correlation analysis with the production of reactive oxygen species. In “A”, asterisks denote significant difference among the treated groups and the control group. C: control group; PSPD-2: group exposed to SARS-CoV-2 spike protein peptide 2002; PSPD-3: group exposed to SARS-CoV-2 spike protein peptide 2003. [n = 32 larvae/group; p-value < 0.01 (*), 0.001 (**), 0.0001 (***), <0.0001 (****)].
4. Discussion
This work shed light important and pioneering knowledge about the toxicity of SARS-CoV-2 protein spike peptides in C. quinquefasciatus larvae. Peptides from the SARS-CoV-2 spike protein generate behavioral alterations, increase in ROS production, changes in antioxidant responses and, finally, increase in the rates of acetylcholinesterase in C. quinquefasciatus larvae, suggesting a possible absorption of these peptides from water. Our results demonstrate that viruses can be found in aquatic environments and go beyond the indication of an ecological imbalance caused by peptides, but they also show the importance of this species as a bioindicator of aquatic contamination.
The SARS coronavirus has been detected in wastewater during outbreaks (Bivins et al., 2020; Guerrero-Latorre et al., 2020; Odih et al., 2020; Randazzo et al., 2020). Odih et al. (2020) described that the environmental surveillance of SARS-CoV-2 in wastewater and efforts to mitigate the entry of the virus into unprotected domestic water sources, should be a priority in environments without basic sanitation. Fecal-oral transmission can occur if it is high and sustained in densely populated cities and with inadequate sanitation. Bivins et al. (2020) assessed the infectivity of SARS-CoV-2 and its RNA in wastewater. Their results pointed to a 90 % reduction in viable SARS-CoV-2 in wastewater and tap water at room temperature in around 1.5 days. However, the SARS-CoV-2 RNA was shown to be more persistent than the infectious SARS-CoV-2, it was detected up to around 4 days. This suggests that this virus may be disassembled in the aquatic environment, which reinforces our tests with peptides from the coronavirus spike protein.
In the work of Fernandes et al. (2021) (bioRxiv preprint https://doi.org/10.1101/2020.10.20.346262), three peptides from the SARS-CoV-2 spike protein were generated after a phagolysosomal proteolysis memorization pattern using the virtual proteolytic cleavage tool. Two of them, PSPD-2002 and PSPD-2003, showed relevant biological effects on the infected host cell. Considering that this in silico method mimics possible degradation of spike protein in the environment, an evaluation of its effects on animals that use this medium to develop has become relevant.
This is the first work that demonstrates the effects of SARS-CoV-2 spike protein peptides on the behavior of C. quinquefascitus. Behavioral analysis plays an important role in assessing the effects of stressors in animals. Some studies have shown that different contaminants induce behavioral changes in mosquito larvae such as unusual movement, excitation, horizontal and aggressive vertical movements (Choochote et al., 2005; Kembro et al., 2009; Ragavendran et al., 2019). Fluctuation analyzes, for example, appear as an effective tool in eliminating trends in the data. This test detects subtle changes in the pattern of behavior that may be imperceptible in other behavioral analyzes (Kembro et al., 2009; Rutherford et al., 2003). Our results show that fluctuation patterns were altered when exposed to PSPD-2002. This result is an indication that this peptide can cause certain damage to the larvae. Further, the results of this work also follow this trend of changes in swim wriggle time, distance traveled and locomotor activity in animals that were exposed to PSPD-2002. Interestingly, peptide 3 (PSPD-2003) did not promote any behavioral changes in these animals, showing its low toxicity when evaluating the behavior of the larvae. Modifications in locomotion can compromise some activities of the larvae such as food acquisition, finding shelter from adverse environmental conditions, anti-predator responses and trying to avoid mechanical shocks by raindrops (Brackenbury, 2001; Tuno et al., 2004). The work of Kembro et al. (2009) highlights that behavioral analyzes in Culex quinquefasciatus are very useful in toxicological tests where the patterns of locomotion and fluctuation are modified. These changes have also been accompanied by changes in biochemical (ROS, H2O2, SOD, catalase) and neurological data (acetylcholinesterase) (Kembro et al., 2009; Malafaia et al., 2020a, Malafaia et al., 2020b, Malafaia et al., 2020c).
On the other hand, the sensitivity and the ability to respond to a wide range of olfactory stimulus are essential for many behavioral processes that mediate the biology of several species of aquatic invertebrates, including C. quinquefasciatus. Our data suggest that SARS-CoV-2 spike protein peptides may have interfered, in a still unknown way, with the function of the larvae's olfactory system, such as in the transduction and coding of the olfactory signal emitted by the phenol present in the agarose slides. Alternatively, we cannot rule out the hypothesis that these peptides have altered the behavior of the larvae from changes in the level of odorant receptors (OR). The behavior of Drosophila melanogaster larvae in relation to different odorous stimuli was associated with the performance and functioning of more than 20 ORs that are expressed in 21 olfactory receptor neurons (ORNs) in each of the two dorsal organs (Fishilevich et al., 2005; Kreher et al., 2005; Couto et al., 2005). In C. quinquefasciatus larvae, the neural basis that regulates the olfactory-driven behavior is still unknown. However, studies involving larvae of other mosquito species provide insights that reinforce our hypothesis that changes in these receptors (eg: ORs and ORNs), induced by SARS-CoV-2 spike protein peptides could explain the different olfactory-driven display behavior compared to the larvae of the control group (Xia et al., 2008; Andersson et al., 2015; Clark & Ray, 2016; Ruel et al., 2019).
ROS are produced in many normal aerobic cellular metabolic processes. Superoxide and hydrogen peroxide are types of species that, at altered levels, induce oxidative stress causing damage such as cell death, mutations, chromosomal aberrations, and carcinogenesis. To reverse the increase in ROS, cells have a large number of antioxidants to prevent or repair damage caused by reactive oxygen species. SOD convert the superoxide radical into hydrogen peroxide and molecular oxygen and the catalase convert hydrogen peroxide into water. In this way, the cell is able to eliminate two potentially harmful species, superoxide and hydrogen peroxide by producing water (Weydert & Cullen, 2010). In this work was shown that PSPD-2002 was able to induce oxidative stress in animals, which can be affect the normal functioning of several physiological systems. The high level of ROS found in larvae exposed to PSDP-2002 was associated with an increase in SOD activity and a decrease in catalase. These results demonstrate that the antioxidant activity of SOD could be compromised since the levels of ROS were increased and the levels of hydrogen peroxide were not altered. Contrary responses have been shown in animals exposed to PSPD-2003, in which SOD activity was effective since there was no increase in ROS levels and the decrease in H2O2 probably occurred due to the high activity of catalase.
This study also showed changes in AChE activity, which plays a key role in the central nervous system of insects. In addition to its hydrolytic function over acetylcholine at synapses and neuromuscular junctions, AChE is also involved in other cellular processes such as apoptosis, modulation of cellular interactions, cell adhesion and synaptogenesis. During the larval development of insects, the expression of the AChE gene has been reported in glial cells and in nervous tissue (Bicker et al., 2004; Zhang et al., 2002). Kumar et al. (2009) suggested new roles for AChE in the growth and development of insect larvae. Through the silencing of this gene, they demonstrated increased mortality, inhibition of larvae growth, reduced pupal weight, malformation and drastically reduced fertility compared to control larvae.
SARS-CoV-2 has neurotoxic effects already shown in different animal models. Its neurotoxicity is due in part to its connection to the nicotinic acetylcholine receptor (Azevedo-Pereira et al., 2011; Charlie-Silva et al., 2021a-bioRxiv preprint doi: https://doi.org/10.1101/2021.01.11.425914; Oliveira et al., 2021). On the other hand, behavioral parameters have also shown relevant correlations with the activity of AChE. In both situations, a significant increase in AChE activity can occur. Azevedo-Pereira et al. (2011) demonstrated that the exposure to the insecticide imidacloprid was associated with changes in AChE activity and the behavior parameters ventilation and locomotion. Our results showed that behavioral parameters and the activity of AChE was increased after exposure to PSPD-2002. Despite the possibility that SARS-CoV-2 peptides may have an indirect effect on AChE activity, the behavioral outcome seems to have a more direct correlation with AChE activities. AChE was also increased in animals exposed to PSPD-2003, however, it showed no significant behavioral changes and biochemical responses did not indicate oxidative imbalance. It is not unlikely, that this response may be linked to neurotoxic effects by binding to the nicotinic acetylcholine receptor. In this perspective, future studies will show if the binding of SARS-CoV-2 peptides to the acetylcholine receptor could induce an increase in acetylcholine in the synaptic fissure and therefore, through a compensatory mechanism, promote an increase in AChE.
Finally, it is necessary acknowledging some limitations of the current study, which can be used as starting point for future research. One of the limitations of the work was to find similar and more in-depth studies on this subject, which somewhat limited our discussion. Also, studies with an approach regarding the viability time of this virus in sewage and how they are digested and disassembled in these polluted waters are scarce. The effects observed in this study cannot be extrapolated to adult individuals of the investigated species or be similar in both sexes. Although individuals were sexually randomized, future investigations should explore gender-specific susceptibility to the effects of PSPD-2002 and PSPD-2003. It is also necessary assuming that the behavioral responses of native, or endemic, species to SARS-CoV-2 peptides polluting sources may be different from the ones observed in the present study, since these effects may be more comprehensive in, and harmful to, these species. In addition, a more physiologically comprehensive investigative approach can help elucidating many action mechanisms of these peptides, besides providing an important basis for the assessment of the ecotoxicological risks posed by SARS-CoV-2 peptides to aquatic biota.
5. Conclusion
SARS-CoV-2 peptides can alter important behavioral, biochemical, and neurological parameters in C. quinquefasciatus larvae. Our results showed that peptide 2 (PSPD-2) mediates severe effects on the larvae, such as oxidative imbalance and important behavioral changes associated with an increase in AChE activity. This study also suggests the ecological and environmental importance of assessing the presence and damage caused by SARS-CoV-2 in the aquatic environment. Also, several avenues have been opened for future investigations of the effects of SARS-CoV-2 peptides in animals with aquatic larval development. However, given the pioneering nature of the present study, it is possible that our data are showing only the “tip of the iceberg” that represents the ecotoxicological risks associated with the presence of SARS-CoV-2 or its viral particles in environmental freshwater ecosystems. Therefore, further studies are warranted to investigate the impact of SARS-CoV-2 on the aquatic environment with the aim of understanding the real magnitude of the possible impacts caused by the COVID-19 pandemic on aquatic wildlife.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by São Paulo Research Foundation (FAPESP 2020/05761–3; 19/19939–1), Brazilian National Research Council (CNPq) (426531/2018–3) and Goiano Federal Institute for financial support (23219.000340.2021-13). Malafaia G. holds productivity scholarship from CNPq (307743/2018–7).
Footnotes
This paper has been recommended for acceptance by Da Chen.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2021.117818.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All experimental procedures were carried out in compliance with ethical guidelines on animal experimentation. Meticulous efforts were made to assure that animals suffered the least possible and to reduce external sources of stress, pain, and discomfort. The current study did not exceed the number of animals necessary to produce trustworthy scientific data. This article does not refer to any study with human participants performed by any of the authors.
Author contributions
Autor: Juliana Moreira Mendonça-Gomes. Contributed data or analysis tools, Performed the analysis, Wrote the paper.
Autor: Ives Charlie-Silva, Contributed data or analysis tools, Wrote the paper.
Autor: Abraão Tiago Batista Guimarães, Collected the data, Contributed data or analysis tools, Autor: Marilia Freitas Calmon, Collected the data, Wrote the paper.
Autor: Rafael Nava Miceli, Collected the data.
Autor: Paulo R. S. Sanches, Collected the data.
Autor: Cíntia Bittar, Collected the data.
Autor: Paula Rahal, Collected the data.
Autor: Eduardo M. Cilli, Collected the data.
Autor: Fernanda Neves Estrela, Collected the data.
Autor: Mohamed Ahmed Ibrahim Ahmed, Contributed data or analysis tools, Wrote the paper.
Autor: Christoph F. A. Vogel, Contributed data or analysis tools, Wrote the paper.
Autor: Guilherme Malafaia, Conceived and designed the analysis, Collected the data, Contributed data or analysis tools, Performed the analysis, Wrote the paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abduljalil J.M., Abduljalil B.M. Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes and New Infections. 2020;35:100672. doi: 10.1016/j.nmni.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves K.F., Caetano F.H., Garcia I.J.P., Santos H.L., Silva D.B., Siqueira J.M., et al. Baccharis dracunculifolia (Asteraceae) essential oil toxicity to Culex quinquefasciatus (Culicidae) Environ. Sci. Pollut. Control Ser. 2018;25(31):31718–31726. doi: 10.1007/s11356-018-3149-x. [DOI] [PubMed] [Google Scholar]
- Alves S.N., Pujoni D.G., Mocelin G., Melo A.L., Serrão J.E. Evaluation of Culex quinquefasciatus wings asymmetry after exposure of larvae to sublethal concentration of ivermectin. Environ. Sci. Pollut. Control Ser. 2020;27(3):3483–3488. doi: 10.1007/s11356-019-06963-5. [DOI] [PubMed] [Google Scholar]
- Andersson M.N., Löfstedt C., Newcomb R.D. Insect olfaction and the evolution of receptor tuning. Frontiers Ecol. Evol. 2015;3:53. [Google Scholar]
- Azevedo-Pereira H.M.V.S., Lemos M.F.L., Soares A.M.V.M. Effects of imidacloprid exposure on Chironomus riparius Meigen larvae: linking acetylcholinesterase activity to behaviour. Ecotoxicol. Environ. Saf. 2011;74(5):1210–1215. doi: 10.1016/j.ecoenv.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Behrendt R., White P., Offer J. Advances in Fmoc solid‐phase peptide synthesis. J. Pept. Sci. 2016;22(1):4–27. doi: 10.1002/psc.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G., Naujock M., Haase A. Cellular expression patterns of acetylcholinesterase activity during grasshopper development. Cell Tissue Res. 2004;317(2):207–220. doi: 10.1007/s00441-004-0905-7. [DOI] [PubMed] [Google Scholar]
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7(12):937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury J. Locomotion through use of the mouth brushes in the larva of Culex pipiens (Diptera: Culicidae) Proc. Biol. Sci. 2001;268(1462):101–106. doi: 10.1098/rspb.2000.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007;43(5):645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalter D.B., Jenkins J.J., Curtis L.R. Respiratory strategy is a major determinant of [3H]water and [14C]chlorpyrifos uptake in aquatic insects. Can. J. Fish. Aquat. Sci. 2002;59(8):1315–1322. doi: 10.1139/f02-107. [DOI] [Google Scholar]
- Buchwalter D.B., Sandahl J.F., Jenkins J.J., Curtis L.R. Roles of uptake, biotransformation, and target site sensitivity in determining the differential toxicity of chlorpyrifos to second to fourth instar Chironomous riparius (Meigen) Aquat. Toxicol. 2004;66(2):149–157. doi: 10.1016/j.aquatox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlie-Silva I., Araujo A., Guimaraes A., Veras F., Braz H., Pontes L., et al. 2021. An Insight into Neurotoxic and Toxicity of Spike Fragments SARS-CoV-2 by Exposure Environment: A Threat to Aquatic Health?. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlie-Silva I., Araujo A., Guimaraes A., Veras F., Braz H., Pontes L., Jorge R., Belo M., Fernandes B., Nobrega R.H., Galdino G., Condino-Neto A., Galindo-Villegas J., Machodo-Santelli G., Sanches P., Rezende R., Cilli E., Malafaia G. An insight into neurotoxic and toxicity of spike fragments SARS-CoV-2 by exposure environment A threat to aquatic health. 2021. https://biorxiv.org/cgi/content/short/2021.01.11.425914 BioRxiv. [DOI] [PMC free article] [PubMed]
- Choochote Wej, Chaiyasit Dana, Kanjanapothi Duangta, Rattanachanpichai Eumporn, Jitpakdi Atchariya, Tuetun Benjawan, Pitasawat B. Chemical composition and anti-mosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti (Diptera: Culicidae) J. Vector Ecol. 2005;2:302–309. [PubMed] [Google Scholar]
- Clark J.T., Ray A. Olfactory mechanisms for discovery of odorants to reduce insect-host contact. J. Chem. Ecol. 2016;42(9):919–930. doi: 10.1007/s10886-016-0770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A., Alenius M., Dickson B.J. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Del Maestro R., McDonald W. Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mech. Ageing Develop. 1987;41(1–2):29–38. doi: 10.1016/0047-6374(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Ellman G.L., Courtney K.D., Andres V., Jr., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Elnemma E.M. Spectrophotometric determination of hydrogen peroxide by a hydroquinone-aniline system catalyzed by molybdate. Bull. Kor. Chem. Soc. 2004;25(1):127–129. [Google Scholar]
- Fang J. Ecology: a world without mosquitoes. Nature News. 2010;466(7305):432–434. doi: 10.1038/466432a. [DOI] [PubMed] [Google Scholar]
- Fernandes Bianca H Ventura, Feitosa Natália Martins, Barbosa Ana Paula, Bomfim Camila Gasque, Garnique Anali M.B., Gomes Francisco I.F., Nakajima Rafael T., Belo Marco A.A., Eto Silas Fernandes, Fernandes Dayanne Carla, Malafaia Guilherme, Manrique Wilson G., Conde Gabriel, Rosales Roberta R.C., Todeschini Iris, Rivero Ilo, Llontop Edgar, Sgro German G., Umaji Oka Gabriel, Bueno Natalia F, Ferraris Fausto K., de Magalhaes Mariana T.Q., Medeiros Renata J., Gomes Juliana M. M, Souza Junqueira Mara de., Conceição Katia, Pontes Letícia G., Condino-Neto Antonio, Perez Andrea C., Barcellos Leonardo J.G., Correa junior Jose Dias, Dorlass Erick G., Camara Niels O.S, Durigon Edison Luiz, Cunha Fernando Q., Nóbrega Rafael H., Machado-Santelli Glaucia M., Farah Chuck, Veras Flávio P, Galindo-Villegas Jorge, Costa-Lotufo Leticia, Cunha Thiago M., Chammas Roger, Guzzo Cristiane R., Carvalho Luciani R, Charlie-Silva Ives. Zebrafish studies on the vaccine candidate to COVID-19, the Spike protein: production of antibody and adverse reaction. Sci. Rep. 2021 doi: 10.1101/2020.10.20.346262. bioRxiv 2020.10.20.346262. [DOI] [Google Scholar]
- Fishilevich E., Domingos A.I., Asahina K., Naef F., Vosshall L.B., Louis M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 2005;15(23):2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerberg E.J., Barnard D.R., Ward R.A. American Mosquito Control Association, Inc.; 1994. Manual for Mosquito Rearing and Experimental Techniques. [Google Scholar]
- Gonzalez P.V., González Audino P.A., Masuh H.M. Behavioral response of Aedes aegypti (Diptera: Culicidae) larvae to synthetic and natural attractants and repellents. J. Med. Entomol. 2015;52(6):1315–1321. doi: 10.1093/jme/tjv136. [DOI] [PubMed] [Google Scholar]
- Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. (February), 0–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C.A., Fields G.B. [5] Trifluoroacetic acid cleavage and deprotection of resin-bound peptides following synthesis by Fmoc chemistry. Methods Enzymol. 1997;289:67–83. doi: 10.1016/s0076-6879(97)89044-1. [DOI] [PubMed] [Google Scholar]
- Heimbeck G., Bugnon V., Gendre N., Häberlin C., Stocker R.F. Smell and taste perception in Drosophila melanogasterLarva: toxin expression studies in chemosensory neurons. J. Neurosci. 1999;19(15):6599–6609. doi: 10.1523/JNEUROSCI.19-15-06599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D.G., Thompson J.D., Gibson T.J. vol. 266. Academic Press; 1996. [22] Using CLUSTAL for multiple sequence alignments; pp. 383–402. (Methods in Enzymology). [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano S.A., Reminger L. The relationship between vulnerability to predation and behavior of larval treehole mosquitoes: geographic and ontogenetic differences. Oikos. 1992:465–476. [Google Scholar]
- Kembro J.M., Marin R.H., Zygadlo J.A., Gleiser R.M. Effects of the essential oils of Lippia turbinata and Lippia polystachya (Verbenaceae) on the temporal pattern of locomotion of the mosquito Culex quinquefasciatus (Diptera: Culicidae) larvae. Parasitol. Res. 2009;104(5):1119–1127. doi: 10.1007/s00436-008-1296-6. [DOI] [PubMed] [Google Scholar]
- King C.L. Hunter's Tropical Medicine and Emerging Infectious Diseases. 2020. Lymphatic filariasis; pp. 851–858. (Content Repository Only!) [Google Scholar]
- Klaassen N., Spicer V., Krokhin O.V. Universal retention standard for peptide separations using various modes of high-performance liquid chromatography. J. Chromatogr. A. 2019;1588:163–168. doi: 10.1016/j.chroma.2018.12.057. [DOI] [PubMed] [Google Scholar]
- Kreher S.A., Kwon J.Y., Carlson J.R. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46(3):445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kumar M., Gupta G.P., Rajam M.V. Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J. Insect Physiol. 2009;55(3):273–278. doi: 10.1016/j.jinsphys.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lahondère C., Vinauger C., Okubo R.P., Wolff G.H., Chan J.K., Akbari O.S., Riffell J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(1):708–716. doi: 10.1073/pnas.1910589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., van Schayck J.P., Mykytyn A.Z., Duimel H.Q., van Donselaar E., Riesebosch S., Kuijpers H.J.H., Schipper D., van de Wetering W.J., de Graaf M., Koopmans M., Cuppen E., Peters P.J., Clevers H. SARS-CoV-2 productively infects human gut enterocytes. BioRxiv. 2020;3(July):50–54. doi: 10.1101/2020.04.25.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Camara T.N. Arboviroses emergentes e novos desafios para a saúde pública no Brasil. Rev. Saude Publica. 2016;50:36. [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharajan K., Muthulakshmi S., Nataraj B., Ramesh M., Kadirvelu K. Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): a multi biomarker study. Aquat. Toxicol. 2018;196:132–145. doi: 10.1016/j.aquatox.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Malafaia G., da Luz T.M., Guimarães A.T.B., Araújo A.P.C. Polyethylene microplastics are ingested and induce biochemical changes in Culex quinquefasciatus (Diptera: Culicidae) freshwater insect larvae. Ecotoxicol. Environ. Contamination. 2020;15:79–89. doi: 10.5132/eec.2020.01.10. [DOI] [Google Scholar]
- Malafaia G., da Luz T.M., Guimarães A.T.B., da Costa Araújo A.P. Polyethylene microplastics are ingested and induce biochemical changes in Culex quinquefasciatus (Diptera: Culicidae) freshwater insect larvae. Ecotoxicol. Environ. Contamination. 2020;15(1):79–89. [Google Scholar]
- Malafaia G., de Souza A.M., Pereira A.C., Gonçalves S., da Costa Araújo A.P., Ribeiro R.X., Rocha T.L. Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total Environ. 2020;700:134867. doi: 10.1016/j.scitotenv.2019.134867. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Millar J.G., Chaney J.D., Mulla M.S. Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. J. Am. Mosq. Contr. Assoc. 1992;8(1):11–17. [PubMed] [Google Scholar]
- Montalvão M.F., Guimarães A.T.B., de Lima Rodrigues A.S., Malafaia G. Carbon nanofibers are bioaccumulated in Aphylla williamsoni (Odonata) larvae and cause REDOX imbalance and changes of acetylcholinesterase activity. Sci. Total Environ. 2021;756:143991. doi: 10.1016/j.scitotenv.2020.143991. [DOI] [PubMed] [Google Scholar]
- Odih E.E., Afolayan A.O., Akintayo I.O., Okeke I.N. Could water and sanitation shortfalls exacerbate SARS-CoV-2 transmission risks? Am. J. Trop. Med. Hyg. 2020;103(2):554–557. doi: 10.4269/ajtmh.20-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A.S.F., Ibarra A.A., Bermudez I., Casalino L., Gaieb Z., Shoemark D.K., Gallagher T., Sessions R.B., Amaro R.E., Mulholland A.J. A potential interaction between the SARS-CoV-2 spike protein and nicotinic acetylcholine receptors. Biophys. J. 2021:1–11. doi: 10.1016/j.bpj.2021.01.037. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais F.S.M., de Cássia Ruy P., Oliveira G., Coimbra R.S. Assessing the efficiency of multiple sequence alignment programs. Algorithm Mol. Biol. 2014;9(1):4. doi: 10.1186/1748-7188-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pašková V., Hilscherová K. 2011. Pašková2011_Chapter_TeratogenicityAndEmbryotoxicit.pdf [DOI]
- Peach D.A.H., Carroll C., Meraj S., Gomes S., Galloway E., Balcita A., et al. Nectar-dwelling microbes of common tansy are attractive to its mosquito pollinator, Culex pipiens L. BMC Ecol. Evol. 2021;21(1):1–12. doi: 10.1186/s12862-021-01761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko K., Mores C.N. Effect of sequential exposure on infection and dissemination rates for West Nile and St. Louis encephalitis viruses in Culex quinquefasciatus. Vector Borne Zoonotic Dis. 2009;9(3):281–286. doi: 10.1089/vbz.2007.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragavendran C., Manigandan V., Kamaraj C., Balasubramani G., Prakash J.S., Perumal P., Natarajan D. Larvicidal, histopathological, antibacterial activity of indigenous fungus penicillium sp. against Aedes aegypti L and Culex quinquefasciatus (say) (Diptera: Culicidae) and its acetylcholinesterase inhibition and toxicity assessment of zebrafish (Danio re. Front. Microbiol. 2019;10(MAR):1–17. doi: 10.3389/fmicb.2019.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaut L., El Mahdi O., Melnyk O. Protein Ligation and Total Synthesis II. Springer; Cham: 2014. Solid phase protein chemical synthesis; pp. 103–154. [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Alvarez D., Escobar L.E. Oropouche fever, an emergent disease from the Americas. Microb. Infect. 2018;20(3):135–146. doi: 10.1016/j.micinf.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Ruel D.M., Yakir E., Bohbot J.D. Supersensitive odorant receptor underscores pleiotropic roles of indoles in mosquito ecology. Front. Cell. Neurosci. 2019;12:533. doi: 10.3389/fncel.2018.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford K.M.D., Haskell M.J., Glasbey C., Jones R.B., Lawrence A.B. Detrended fluctuation analysis of behavioural responses to mild acute stressors in domestic hens. Appl. Anim. Behav. Sci. 2003;83(2):125–139. doi: 10.1016/S0168-1591(03)00115-1. [DOI] [Google Scholar]
- Sachett A., Gallas-Lopes M., Conterato G.M.M., Benvenutti R., Herrmann A.P., Piato A. Quantification of thiobarbituric acid reactive species (TBARS) optimized for zebrafish brain tissue. protocols.io. 2020. [DOI]
- Samy A.M., Elaagip A.H., Kenawy M.A., Ayres C.F., Peterson A.T., Soliman D.E. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PloS One. 2016;11(10) doi: 10.1371/journal.pone.0163863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Dieterich A., Corral Morillas N., Dewald C., Miksch L., Nelson S., Wick A., Triebskorn R., Köhler H.R. The importance of sediments in ecological quality assessment of stream headwaters: embryotoxicity along the Nidda River and its tributaries in Central Hesse, Germany. Environ. Sci. Eur. 2018;30(1) doi: 10.1186/s12302-018-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy R.K. Clinical and pathological aspects of filarial lymphedema and its management. Kor. J. Parasitol. 2008;46(3):119. doi: 10.3347/kjp.2008.46.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Souza R.S., Virginio F., Riback T.I.S., Suesdek L., Barufi J.B., Genta F.A. Microorganism-based larval diets affect mosquito development, size and nutritional reserves in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae) Front. Physiol. 2019;10:152. doi: 10.3389/fphys.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkari E.D., Korboe H.M., Abu M., Kizildeniz T. Sources and routes of SARS-CoV-2 transmission in water systems in Africa: are there any sustainable remedies? Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.142298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Changcheng Wu, Li X., Song Y., Yao X., Wu X., Dung Y., Zhang H., Wang Y., Qian Z., Cui J., Lu J. On the origin and continuing evolution of SARS-CoV-2. Nat. Sci. Rev. 2020;1–24 doi: 10.1093/nsr/nwaa036. https://academic.oup.com/nsr/advance-article-abstract/doi/10.1093/nsr/nwaa036/5775463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuno N., Miki K., Minakawa N., Githeko A., Yan G., Takagi M. Diving ability of Anopheles gambiae (Diptera: Culicidae) larvae. J. Med. Entomol. 2004;41(4):810–812. doi: 10.1603/0022-2585-41.4.810. [DOI] [PubMed] [Google Scholar]
- Vieira C.J.D.S.P., Thies S.F., da Silva D.J.F., Kubiszeski J.R., Barreto E.S., de Oliveira Monteiro H.A., et al. Ecological aspects of potential arbovirus vectors (Diptera: Culicidae) in an urban landscape of Southern Amazon, Brazil. Acta Trop. 2020;202:105276. doi: 10.1016/j.actatropica.2019.105276. [DOI] [PubMed] [Google Scholar]
- Walker E.D., Merritt R.W. Behavior of larval Aedes triseriatus (Diptera: Culicidae) J. Med. Entomol. 1991;28(5):581–589. doi: 10.1093/jmedent/28.5.581. [DOI] [PubMed] [Google Scholar]
- Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010;5(1):51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg-Larsen P., Graeber D., Kristensen E.A., Baattrup-Pedersen A., Friberg N., Rasmussen J.J. Trait characteristics determine pyrethroid sensitivity in nonstandard test species of freshwater macroinvertebrates: a reality check. Environ. Sci. Technol. 2016;50(10):4971–4978. doi: 10.1021/acs.est.6b00315. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2005. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. No. WHO/CDS/WHOPES/GCDPP/2005.13. [Google Scholar]
- World Health Organization (WHO) World Health Organization; 2010. Progress Report 2000-2009 and Strategic Plan 2010-2020 of the Global Programme to Eliminate Lymphatic Filariasis: Halfway towards Eliminating Lymphatic Filariasis. No. WHO/HTM/NTD/PCT/2010.6. [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Wang G., Buscariollo D., Pitts R.J., Wenger H., Zwiebel L.J. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105(17):6433–6438. doi: 10.1073/pnas.0801007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.J., Yang L., Zhao Q., Caen J.P., He H.Y., Jin Q.H., Guo L.H., Alemany M., Zhang L.Y., Shi Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002;9(8):790–800. doi: 10.1038/sj.cdd.4401034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.