Abstract
Ionic liquids, also called molten salts, are mixtures of cations and anions that melt below 100 °C. Typical ionic liquids are dialkylimidazolium cations with weakly coordinating anions such as [MeOSO3] or [PF6]. Advanced ionic liquids such as choline citrate have biodegradable, less expensive and less toxic anions and cations. Deep eutectic solvents are also included in the advanced ionic liquids. Deep eutectic solvents are mixtures of salts such as choline chloride and uncharged hydrogen bond donors such as urea, oxalic acid, or glycerol. For example, a mixture of choline chloride and urea in 1:2 molar ratio liquifies to form a deep eutectic solvent. Their properties are similar to those of ionic liquids. Water-miscible ionic liquids as cosolvents with water enhance the solubility of substrates or products. Although traditional water-miscible organic solvents also enhance solubility, they often inactivate enzymes, while ionic liquids do not. The enhanced solubility of substrates can increase the rate of reaction and often increases the regio- or enantioselectivity. Ionic liquids can also be solvents for non-aqueous reactions. In these cases, they are especially suited to dissolve polar substrates. Polar organic solvent alternatives inactivate enzymes, but ionic liquids do not even when they have similar polarities. Besides their solubility properties, ionic liquids and deep eutectic solvents may be greener than organic solvents because ionic liquids are non-volatile and can be made from non-toxic components. This review covers selected examples of enzyme catalyzed reaction ionic liquids that demonstrate their advantages and unique properties and point out opportunities for new applications. Most examples involve hydrolases, but oxidoreductases and even whole cell reactions have been reported in ionic liquids.
Keywords: ionic liquids, deep eutectic solvents, hydrolases, oxidoreductases, polymerization
IONIC LIQUIDS AND DEEP EUTECTIC SOLVENTS
Ionic liquids, also called molten salts, are mixtures of cations and anions that melt below 100 °C. The low melting point stems from a mismatch in the size of the anion and cation that prevents crystallization. Changing the structures of the anions or cations can tune the polarities and other properties. Even though they are salts, they are only moderately polar with polarities similar to ethanol. Ionic liquids have negligible vapor pressure. Ionic liquids are viscous, typically 0.1 Pa s or more, which is similar to glycerol or honey.
The first ionic liquid - ethylammonium nitrate (mp 12 °C) - was reported in 1914 [1], but attracted limited use. The first generation of widely studied ionic liquids used the dialkylimmidazolium and related cations reported in 1982 [2], Figure 1. Varying the cation structure varied the properties of the ionic liquid. The anions were chloroaluminate or other metal halide anions, which react with water and thus are not suited for biotransformations. The second generation of ionic liquids, discovered a decade later [3], replaced the water reactive anions with halides or weakly coordinating anions such as [BF4] or [PF6]. These ionic liquids are stable to water and air and are the best-studied ionic liquids [4–11]. The first reports of enzyme-catalyzed reactions in ionic liquids [12–16] and most of the subsequent work used these liquids. These IL’s are moderately polar (similar to ethanol) and tolerate air and water. Spreading the negative charge of the anion over multiple atoms weakens any hydrogen bonds between protein and solvent making it less likely that the solvent will denature the enzyme. Most are hydrophobic so they are immiscible with water. These ionic liquids are largely inert solvents, presumably because strong interactions between the components reduces their reactivity.
Figure 1.

Ionic liquids are salts that melt below 100°C likely due to a mismatch in the size of the anion and cation. Deep eutectic solvents are physical mixtures of salts and hydrogen bond donors that melt at low temperature.
The third generation of ionic liquids - called advanced ionic liquids in this review - retain the moderate polarity, stability, and distributed negative charge of the second generation, but use biodegradable, readily available, and lower toxicity cations and/or anions. For example, the cation may be choline and the anions may be sugars or sugar analogs, amino or organic acids, alkylsulfates, or alkylphosphates. These ionic liquids tend to be more hydrophilic than second generation ionic liquids, and are often water-miscible. Also included in these advanced ionic liquids are deep eutectic solvents, which are physical mixtures of salts such as choline chloride and uncharged hydrogen bond donors such as urea, oxalic acid, or glycerol [17–19]. Deep eutectic solvents contain an uncharged component so they are not entirely ionic. The uncharged hydrogen bond donor likely hydrogen bonds to the anion of the salt [19]. At the eutectic ratio, typically 1–4 molecules of hydrogen donor per molecule of salt, the mixture forms a liquid at room temperature. These eutectics are similarly stable and have low vapor pressure like cation/anion pair ionic liquids and are usually water-miscible. Although these advanced ionic liquids are the most promising class for applications, most examples in this review involve the second generation ionic liquids. The advanced ionic liquids are newer and fewer examples have been reported. We believe that many of the conclusions about second generation ionic liquids will also apply to the advanced ionic liquids.
The main role in biocatalysis of advanced ionic liquids is to replace polar organic solvents like acetone, methanol or DMSO in enzyme-catalyzed reaction mixtures. Polar organic solvents usually denature enzymes, but ionic liquids do not, even when their polarity is similar to the polar organic solvents. Replacing the polar organic solvents with ionic liquids allows substrates to dissolve without deactivating enzymes.
The three types of applications of ionic liquids are 1) as cosolvents with water, 2) as a second phase in water-ionic liquid mixture and 3) as non-aqueous solvents. Their role as cosolvents is to help dissolve non-polar substrates in aqueous solutions or to reduce the activity of water. The advantage of ionic liquids is that enzymes tolerate these solvents more than ordinary polar organic solvents. In the second type of application, ionic liquids are used a second phase mainly in whole cell reactions. Ionic liquids disrupt membranes less than ordinary water-immiscible solvents. In the third type of application, ionic liquids serve as non-aqueous solvents. When they replace a non polar ordinary solvent like toluene, the main advantage of the ionic liquid is that it is non volatile. There is a bigger advantage when they replace a polar organic solvent like dimethylsulfoxide or ethanol. Enzymes denature in these polar solvents, but they do not denature in ionic liquids with similar polarity. This feature makes ionic liquids an ideal non-aqueous solvent for polar substrates like sugars.
The main disadvantage of second generation ionic liquids is high cost. Current price targets from Solvent Innovation GmbH (Cologne, Germany) for ionic liquids are approximately 10 – 20 USD/kg on the ton scale, which is at least ten fold higher than many organic solvents. This high cost stems from 1) the high cost of components and 2) purification required in the preparation. Materials to make the dialkylimmidazolium cation and fluorine-containing anions are expensive. The second generation ionic liquids typically have toxicities similar to chlorinated and aromatic solvents [20].
The advanced ionic liquids, especially deep eutectic solvents, promise to be less expensive and cost similar to organic solvents. First, the components for the advanced ionic liquid are less expensive. Second the deep eutectic solvents do not require purification. Ionic liquids are typically prepared by mixing two salts to form two new salts. One is the ionic liquid and the other, often sodium chloride, must be removed. This removal can be complex, but is essential for most applications with enzymes because even trace impurities of halides or synthesis reagents can inhibit enzyme catalysis [21]. Preparation of deep eutectic solvents requires only stirring the components with gentle warming. No purification is needed because no new salt forms. The purity of the starting materials determines the final purity. As a result, deep eutectic solvents are the least expensive alternative solvent and their cost is similar to organic solvents.
HYDROLASE-CATALYZED REACTIONS
Hydrolases are the most commonly used enzymes in biocatalysis [22] and most examples with ionic liquids also use hydrolases. The first reports of enzyme activity in ionic liquids involved proteases [16] or lipases [13–15, 23]. As a rule of thumb anions that spread their negative charge over multiple atoms are more stabilizing than those that have the negative charge on a single atom. For example, stabilizing ionic liquids often contain anions like bis(trifluoromethane)sulfonimide ([Tf2N]), hexfluorophosphate ([PF6]), and tetrafluororborate ([BF4]). Ionic liquids with halide or acetate anions usually denature enzymes and even traces of halide in an ionic liquid can inactivate an enzyme. Presumably strong hydrogen bonds between anion and enzyme promote unfolding, which leads to irreversible aggregation and precipitation of the protein.
Ionic liquids as cosolvents
As cosolvents, ionic liquids serve to increase the solubility of organic substrates and to decrease the activity of water. In choosing an ionic liquid for use as a cosolvent, one should avoid fluoride-containing anions like [PF6] or [BF4] because these anions can hydrolyze in water to hydrofluoric acid, which inactivates or denatures enzymes. Some examples below nevertheless used ionic liquids containing these anions.
Lipases
Reactions involving lipases do not normally use cosolvents to enhance substrate solubility because lipases accept water-insoluble substrates. However, cosolvents may be needed to dissolve crystalline substrates. The resolution of a a pharmaceutical intermediate for synthesis of Lotrafiban used t-butanol to dissolve it. Replacing the t-butanol cosolvent with 1-butyl-3-methylimidazolium (BMIM) [PF6] increased the stability of the lipase Candida antarctica lipase B (CALB) [24], Figure 2a. The increased stability allowed researchers to raise the temperature from 50 °C to 75 °C, which increased the reaction rate four fold without sacrificing yield or selectivity. The ionic liquid could be reused ten times.
Figure 2.
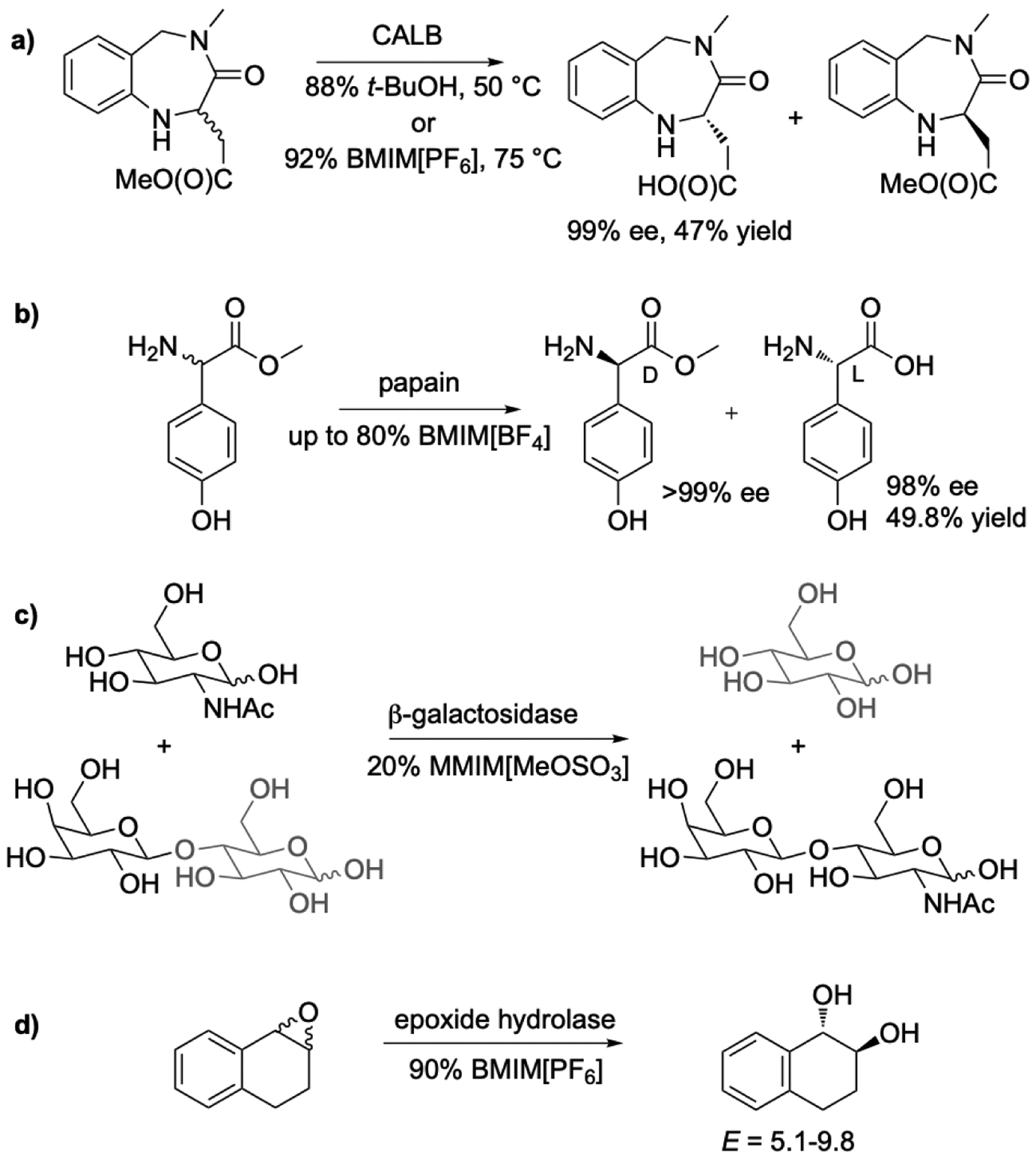
Ionic liquids as cosolvents for hydrolase catalyzed reactions increase the solubility of substrate and decrease the activity of water. a) CALB-catalyzed resolution of an intermediate for synthesis Lotrafiban, a platelet aggregation inhibitor. CALB was more stable in BMIM[PF6] as compared to t-butanol, which allowed the temperature to be increased from 50 °C to 75 °C. The hydrolysis was four time faster and the yield and enantioselectivity remained high. b) Papain catalyzed kinetic resolution of D,L-(p-hydroxyphenyl)glycine methyl ester. The reaction rate increased in solutions containing ionic liquid up to 10-fold likely due to increase solubility of the substrate. c) β-galactosidase-catalyzed synthesis of L-N-acetyllactosamine by transglycosylation. The ionic liquid cosolvent increased the yield from 30 to 60% by minimizing competing hydrolysis of the product. d) Epoxide hydrolase-catalyzed kinetic resolution of an epoxide. Ionic liquid reduces spontaneous non-enantioselective hydrolysis of the substrate epoxide and in this manner increases the enantiomeric purity of the product diol.
Proteases and esterases
The natural role of proteases is hydrolysis and some applications exploit this ability. Papain, a cysteine protease, catalyzed the enantioselective hydrolysis of hydroxyphenylglycine methyl ester [25–27] in 1-alkyl-3-methylimidazolium[BF4] ionic liquids containing >20 vol% water, Figure 2b. The role of the ionic liquid was to increase the solubility of the substrate, but it also increased the enantioselectivity from E = 2 in buffer to E = 100 in 20% water/80% ionic liquid. In other case, addition of 10–25 vol% 1:2 choline chloride: glycerol cosolvent increased the rate of hydrolysis of p-nitrophenyl acetate up to 3-fold for pig liver esterase and Rhizopus oryzae esterase [28]. Pig liver esterase catalyzed the enantioselective hydrolysis of diethyl malonate derivatives with several fold faster with 1% Ammoeng 100 (a mixture quaternary ammonium dimethylphosphate ionic liquids based on alkyl and hydroxylated ether side chains) than in buffer only or with 10% isopropanol added. However, some ionic liquids were not useful. For example, with 10 vol% BMIM[PF6] as the ionic liquid the rate was slower than in buffer [29]. An noted above, fluoride containing anions may hydrolyze in water releasing hydrogen fluoride, so these anions should be avoided as cosolvents with water.
Many applications of proteases use them as catalysts for the reverse reaction - the formation of esters and amides. In these reactions it is important both to dissolve the substrate and to reduce the activity of water to reduce competing hydrolysis of the substrate or product. The first report of ionic liquids as solvent for and enzyme catalyzed reaction used thermolysin, a zinc metalloprotease, to make the dipeptide aspartame in a mixture of 95% BMIM[PF6] and 5% water [16]. The protease was more stable in the ionic liquid than in the traditional solvent ethyl acetate.The proteases trypsin, α-chymotrypsin, and V8-protease catalyzed the synthesis of peptides in up to 30% 1,3-dimethylimidazolium (MMIM) [(MeO)2PO2] in MOPS buffer [30]. The yields were >78% in 30% ionic liquid as compared to 0–45% in buffer alone. The ionic liquid suppressed both hydrolysis of the peptide product and proteolytic side reactions on the fragments by reducing available water to the protease. Subtilisin catalyzed the esterification of N-acetylphenylalanine in 2 M 1-ethyl-3-methylimidazolium (EMIM) [OAc] and showed 14-fold higher enantioselectivity compared to in 2 M acetonitrile [31].
Cross-linked enzyme aggregates (CLEAs) of feruloyl esterase catalyzed the condensation of glycerol and sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) to produce glycerol sinapate, an antioxidant. The enzyme was most active in 80–90% 1-hydroxyethyl-3-methylimidazolium (HEMIM) [PF6] and 10–20% water, but lost activity after ~150 h [32].
Glycosidases
Glycosidases catalyze hydrolysis, but, as with proteases, most synthetic applications use them to catalyze the reverse reaction. The glycoside catalyzed linking of sugars is either a condensation (reverse of hydrolysis) or a transglycoslyation (transfer of a glycosyl group from a donor to an alcohol acceptor). The condensation approach requires non-aqueous conditions, see below, but transglycosylations usually use water with a cosolvent. β-Galactosidase from B. circulans catalyzed the transfer of the galactosyl group from lactose to N-acetylglucosamine in 20% MMIM[MeOSO3]-80% water [33]. The yield of N-acetyllactosamine increased from 30% in buffer to 60%, Figure 3, because the ionic liquid suppressed hydrolysis of product. Similarly, using another glycosidase - β-glycosylhydrolase CelB from Pyroccocus furiosus - 45 vol% of MMIM[MeOSO3] increased the yield of galactosyl transfer from lactose to glycerol by 10% [34].
Figure 3.

Hydrolase-catalyzed reactions in ionic liquids as non-aqueous solvents. a) In a model transesterification catalyzed by CALB at 40 °C, reaction in BMIM[BF4] and BMIM[PF6] gave comparable conversion compared to those in 1-butanol or t-butanol. b) Ammoniolysis of ethyl ocatanoate catalzyed by CALB at 40 °C, reaction in BMIM[BF4] gave lower conversion compared to one in t-butanol. c) Kinetic resolution of secondary alcohols in ionic liquids by acetylation with vinyl acetate. Enantioselectivity for these resolution, listed in Table 2, are comparable or improved in the ionic liquid as compared to organic solvent. d) Acetylation of glucose by vinyl acetate. Ionic liquids that dissolve glucose well also minimize formation of the diacyl side product. e) α-Chymotrypsin requires a small amount of water or supercritical CO2 (scCO2) for activity in non-aqueous solvents. In this example, α-chymotrypsin catalyzed the transesterification of an N-acetylphenylalanine ethyl ester in a mixture of ionic liquid and. This yield is low, but twice as high as the 9% yield in pure supercritical CO2.
Epoxide hydrolases
Epoxide hydrolases catalyze the enantioselective hydrolysis of epoxides to diols, which can be used as synthetic intermediates. Ionic liquids as cosolvents help dissolve hydrophobic substrates and suppress spontaneous hydrolysis, but without inactivating the epoxide hydrolases [37, 38]. Traditional organic solvent can reduce the activity of epoxide hydrolases [35, 36]: 10 vol% ethanol in buffer reduced the activity of epoxide hydrolase from Rhodococcus sp. NCIMB 11216 in half and 10 vol% DMF in buffer reduced the activity by one third [34]. Some epoxide hydrolases remain active in ionic liquids, but there are no comparisons of organic solvent with ionic liquid for the same epoxide hydrolase. The rate of hydrolysis of β-methylstyrene oxide catalyzed by a mammalian epoxide hydrolase-catalyzed was similar in both buffer and in 90 vol% BMIM[PF6], BMIM[Tf2N], and BMIM[BF4] [37]. The ionic liquids enhanced not only the solubility of the epoxide substrates, but also suppressed spontaneous hydrolysis of water sensitive epoxides [38], leading to higher apparent enantioselectivity (Eapparent = 1 in buffer, 5.1–9.8 in 90% ionic liquid), Figure 2c. Deep eutectic solvents as additives also enhance the activity of epoxide hydrolases. Addition of 25 vol% choline chloride: glycerol enhanced the conversion of styrene oxide to styrene glycol by epoxide hydrolase AD1 from Agrobacterium radiobacter by 20-fold compared to in buffer alone [28]. In contrast, adding 25 vol% DMSO or acetonitrile as the cosolvent decreased activity 2–6 fold.
Ionic liquids as non-aqueous solvents
Non-aqueous reaction conditions for hydrolase-catalyzed reactions can reverse a hydrolysis making it a condensation reaction, or allow reaction intermediates to react with added nucleophiles such as alcohols or amines instead of water. Although some of these reactions also proceed in water-cosolvent mixtures, non-aqueous conditions are needed when the selectivity between water and the desired nucleophile is poor or when the condensation is thermodynamically less favorable.
Typical non-aqueous reaction conditions are a suspension of enzyme powder in a non-polar organic solvent such a toluene. Polar organic solvents such as ethanol or DMSO cannot be used because they usually denature enzymes, likely by disrupting the intramolecular hydrogen bonds in the protein [39]. In contrast, many enzymes tolerate ionic liquids even when their polarity is similar to the non-tolerated organic solvents. Enzyme powders usually do not dissolve in ionic liquids, but remain as suspensions, as they do in organic solvents. In one case, CALB dissolved in an ionic liquid, but this dissolution eliminated catalytic activity [40]. Immobilized enzymes can be suspended in ionic liquids as in organic solvents. In some cases, researchers have linked poly(ethylene glycol) to enzymes. This tailoring allows them to dissolve and remain active in organic solvents like toluene. The same approach allowed proteases to dissolve and remain active in ionic liquids [41, 42].
Lipases - General aspects
A variety of lipases are active in ionic liquids, but the most important lipase for organic chemists is CALB. This lipase is the first one reported active in the ionic liquids BMIM[PF6] and BMIM[BF4], Figure 3 [17]. The activity of CALB in ionic liquids is often comparable to that in organic solvent, but can be higher or lower. The yields for a model transesterification reaction were similar in both ionic liquids and in alcohols solvents, Figure 3a, but the yields for an ammoniolysis were lower in the ionic liquid, Figure 3b. The activity for transesterification of 1-butanol with vinyl butyrate to form butyl butanoate was 2–4-fold higher in BMIM[PF6] than in butanol and hexane [43]. CALB is stable in a wide variety of ionic liquids, including those based on the [BF4], [PF6], and [Tf2N] anions, but it has less activity in ionic liquids containing [SbF6] or [CF3SO3] [15]. While the [Tf2N] anion contains two [CF3SO2] moieties, the amide nitrogen link allows the negative charge to spread over five atoms as compared to three atoms in [CF3SO3]. This more delocalized charge in [Tf2N] may be why CALB tolerates this ion better than [CF3SO3]. CALB, Burkholderia cepacia lipase (BCL), and Candida antarctica lipase A (CALA) are also active in deep eutectic solvents [28], Table 1. CALB had activity comparable to in toluene in many DES’s, while CALA and BCL were most active in choline chloride/glycerol.
Table 1.
Lipase-catalyzed transesterification of ethyl valerate with 1-butanol in deep eutectic solvents.a
| Solvent | Conversion (%) | ||
|---|---|---|---|
| CALB | CALA | BCL | |
| Toluene | 92 | 76 | 5 |
| ChCl:Acetamide (1:2) | 96 | 0.5 | 0 |
| ChCl:Glycerol (1:2) | 96 | 70 | 22 |
| ChCl:Malonate (1:1) | 58 | 0.7 | 0 |
| ChCl:Urea (1:2) | 99 | 1.6 | 0.8 |
| EAC:Acetamide (2:3) | 92 | 2.7 | 0 |
| EAC:Glycerol (2:3) | 91 | 2.1 | 0.5 |
Reaction is shown in Figure 3a; conditions: 60 °C, 24 h. ChCl = choline chloride, EAC = ethylammonium chloride
Many lipase-catalyzed reactions use vinyl esters as acyl donors because they are effectively irreversible donors, for example, Figure 3c,d. The vinyl alcohol released tautomerizes to acetaldehyde. One problem with vinyl esters in ionic liquids is the accumulation of inhibitory acetaldehyde oligomers. Even mildly acid protons, such as the one in the 2-position of the dialkylimidazolium cation, can catalyze oligomerization of acetaldehyde. Replacing that hydrogen in BMIM[BF4] with a methyl group avoided this problem [44]. BCL catalyzed the acetylation of 5-phenyl-1-penten-3-ol with vinyl acetate in the modified ionic liquid with rate and enantioselectivity comparable to in organic solvents and without the formation any oligomeric byproducts. BCL could be reused 10 times without loss of activity in the new ionic liquid, whereas it lost most of its activity after one run in BMIM[BF4]. Another good ionic liquid for lipase-catalyzed reactions is the phosphonium salt MeEtBu3P[Tf2N] because it lacks acidic protons. Resolutions of secondary alcohols in this solvent with immobilized BCL were about two to fourfold faster that with immidazolium salts and even slightly faster than in diisopropyl ether [45].
The ionic liquid EMIM[CF3SO3] enhanced CALB-catalyzed biodiesel production [46] from triglycerides and methanol. Methanol deactivates lipases, so lipases are not good catalysts for this reaction. One solution is to dilute the methanol with t-butanol, but a better solution may be diluting with EMIM[CF3SO3] because the yields were ~20% higher [46]. Addition of BMIM[Tf2N] in a biodiesel synthesis catalyzed by BCL similarly enhanced the yield. In the ionic-liquid-containing reaction, the fatty acid methyl ester separates as new phase as the reaction proceeds and this separation provide a driving force that leads to higher yields [47].
Lipases - Enhanced enantioselectivity
Lipases CALB and BCL are already highly enantioselective in the acetylation of secondary alcohols with vinyl acetate in organic solvent, but this enantioselectivity increases further in ionic liquids. The enantioselectivity of the BCL-catalyzed resolution of 1-phenylethanol and several similar reactions was up to ten fold higher in 1-alkyl-3-methylimidazolium [BF4]- and [PF6]-based ionic liquids (E ~100 to E ~1000), Figure 3c, Table 2 [23, 48, 49]. For most of these secondary alcohols there is little practical advantage of the higher enantioselectivity in ionic liquids since the enantioselectivity in organic solvents is already high enough to cleanly separate the enantiomers.
Table 2.
Lipase-catalyzed kinetic resolution of secondary alcohols by acetylation with vinyl acetate.a
| Enzyme | Substrate | Enantioselectivity | ||||
|---|---|---|---|---|---|---|
| X | R | THF | Toluene | EMIM[BF4] | BMIM[PF6] | |
| CALB | H | Bn | 140 | 200 | >600 | >900 |
| CALB | H | −CH2C(O)OBn | 26 | 200 | >600 | 150 |
| BCL | Cl | Ph | 56 | 160 | 180 | >400 |
| BCL | Cl | OPh | 150 | 85 | 170 | >1000 |
Reaction is in Figure 3c. Enantioselectivity is the ratio of the rate of reaction of the fast-reacting enantiomer over the rate for the slow reacting enantiomer.
In other cases the enantioselectivity is low in organic solvents, so any increased enantioselectivity in ionic liquids offers a practical advantage. Candida rugosa lipase (CRL) shows only low to moderate enantioselectivity in the resolution of 2-aryl propanoic acids, a class of non-steroidal anti-inflammatory drugs. The enantioselectivity for hydrolysis of the methyl ester of ibuprofen or methyl ester of naproxen increased from an E of 7.2 and 33, respectively, in water-saturated isooctane to 24 and >200 in ionic liquids [50, 51]. The enantioselectivity of BCL increased from 10–40 to >200 after coating the lipase with 1-butyl-2,3-dimethylimidazolium (BMMIM) [poly(oxyethylene)alkyl sulfate] [52]. The enantioselectivity of porcine pancreatic lipase (PPL) in the hydrolysis of methyl or ethyl esters of N-acetyl amino acids increased up to ten-fold in 15% N-ethylpyridinium[CF3COO] as compared to acetonitrile: from E = 2.3 to E = 23 for threonine methyl ester [53].
Lipases - Regioselective acylation of sugars
Increased substrate solubility in ionic liquids is a big advantage for the regioselective acylation of sugars. Sugar esters are potentially useful as green, biodegradable surfactants. Sugars dissolve poorly in conventional, non-polar organic solvents, but upon acylation the product acyl sugar is more soluble. The high ratio of sugar mono ester to sugar in solution makes it likely that the sugar mono ester will undergo subsequent undesired acylations. Polar organic solvents dissolve sugars better, but usually inactivate enzymes. Ionic liquids are even better than polar organic solvents at dissolving sugars [54, 55], but maintain the activity of hydrolases. The higher sugar concentration makes the initial acylation faster and make the subsequent acylations less likely, Figure 3d. Two good ionic liquids for dissolving glucose are BMIM dicyanamide ([DCA]), which dissolves 145 g/L at 25 °C [54], and EMIM[MeOSO3], which dissolves 90 g/L at 25 °C [55]. Supersaturated solutions of glucose in ionic liquid can be prepared by mixing an aqueous sugar solution with an ionic liquid followed by removal of water. The glucose concentration in BMIM[CF3SO3] can be up to ten times higher than a saturated solution [56]. The rates of esterification and transesterification of glucose were approximately 10-fold faster in these supersaturated solutions. Unfortunately, lipase CALB showed poor stability in in BMIM[CF3SO3], so a 1:1 mixture of BMIM[CF3SO3] and BMIM[TF2N] was a compromise between enzyme stability and glucose solubility [57].
Increased sugar substrate solubility also accounts for the higher regioselectivity of CALB-catalyzed glucose acetylation by vinyl acetate in EMIM[BF4] and 1-methoxyethyl-3-methylimidazolium (MOEMIM) [BF4] as compared to organic solvent. The acetylation yielded 99% mono-acetyl product in EMIM[BF4] (50% conversion in 36 h) and 93% mono acetyl in MOEMIM[BF4] (99% conversion) compared to only 53% in THF (99% conversion) and 76% in acetone (72% conversion). The diacetyl product was the major side product. Glucose was several times more soluble in EMIM[BF4] [54] and approximately one hundred times more soluble in MOEMIM[BF4] than in the organic solvents. The increased solubility in EMIM[BF4] increased the regioselectivity and the even higher solubility in MOEMIM[BF4] gave both high regioselectivity and high conversion. CALB also catalyzed the acylation of glucose with vinyl myristate to the 6-O-myristic acid ester in 89% yield in 60% BMIM[BF4]-40% t-butanol and with vinyl laurate in BMIM[PF6] [58]. CALB also catalyzed the acylation of glucose with palmitic acid to the 6-O-palmitic acid ester in 48% yield. Replacing the expensive vinyl ester with the free acid reduces the cost. BMIM[DCA] dissolved >200 g l−1 sucrose at 60 °C, enabling CALB to catalyze the acylation of sucrose with dodecanoic acid. The authors did not report the regioselectivity [54], but it likely favors the primary alcohol positions (6-O and 6’-O).
Although ionic liquids such as BMIM[Cl] and EMIM[OAc] can dissolve cellulose, lipases are not active in these solvents. Zhao and coworkers designed ionic liquids that dissolve cellulose, but do not inactivate hydrolases [59]. One example is Me(OCH2CH2)3-NEt3[OAc], where the acetate anion dissolves the cellulose while the polyether moiety in the cation stabilizes the enzyme. In this solvent, the CALB-catalyzed acylation of cellulose by methyl methacrylate at 60 °C showed a loss of approximately 60% of its activity in the first 20 minutes, but retained its activity thereafter. FTIR showed an 89% conversion to the 6-O-esters after 72 h with no side reactions.
The solubility behavior of acyl nucleosides is similar to the acyl sugars, but acyl nucleosides are used as antiviral or antitumor agents. CALB catalyzed the acylation of 1-β-D-arabinofuranosylcytosine with vinyl propionate 20% faster in an IL-containing solvent (70% THF-10% BMIM[PF6]-20% pyridine) than in a conventional solvent (28% hexane in pyridine) [60]. The regioselectivity was high in both solvents, likely because both contained pyridine to dissolve the nucleoside substrate.
Ionic liquids that dissolved glucose best also gave the highest regioselectivity for the 6-position in the CALB-catalyzed acetylation of glucose [14]. Similarly, ionic liquids that best dissolved polyhydroxylated flavonoid glucosides (e.g., BMIM[BF4]) gave the best regioselectivity for the 6” position in a CALB-catalyzed acylation with vinyl butyrate [61].
Lipases - Polyester formation
The potential advantage of using ionic liquids as solvents to form polyesters is that the higher solubility of polymer in the ionic liquid would lead to higher molecular weights than in organic solvent. This potential advantage has not yet been realized; polyester formation in ionic liquids gives molecular weights comparable to some reports in organic solvents, but lower than the highest reported molecular weights. This lower molecular weight may be due to difficulties in drying ionic liquids, since achieving high molecular weights in organic solvents required special efforts to dry the solvent and remove water during the polymerization [62].
Kobayashi’s group [63] was the first to make polyesters in ionic liquids either by condensation or by ring-opening polymerization, Figure 4. Condensation polymerization of dicarboxylic acid diesters and 1,4-butanediol catalyzed by CALB produced polymers of Mn up to ~1500, while ring-opening polymerization of ε-caprolactone produced polymers of Mn up to ~4200. In both types of polymerizations, solubility of the polymer can limit molecular weight. For example, the BCL-catalyzed condensation polymerization of diethyl adipate and 1,4-butanediol in BMIM[PF6] [64] gave polyester with a molecular weight of only ~2000 at room temperature with a polydispersity of ~1.05. At 60 °C, where the polymer was more soluble, the molecular weight was more than twice as high: ~5000 with a polydispersity of ~1.25. These values are after precipitation of the polymer with methanol, which leaves lower molecular weight oligomers in solution. The polydispersity in the reaction mixture will be broader. Using a better acyl donor – divinyladipate – did not give higher molecular weights. CALB catalyzed condensation of divinyl adipate and three diols in BMIM[PF6] at room temperature to give molecular weights (Mn) between 1000 and 2900 [65]. The similarity of these molecular weights to those using the diethyl ester suggests that polymer solubility limits the molecular weight. Lipases TLL and MML also catalyzed the polymerizations, but the molecular weights were lower, likely because of lower activity of these lipases in the ionic liquid compared to CALB.
Figure 4.
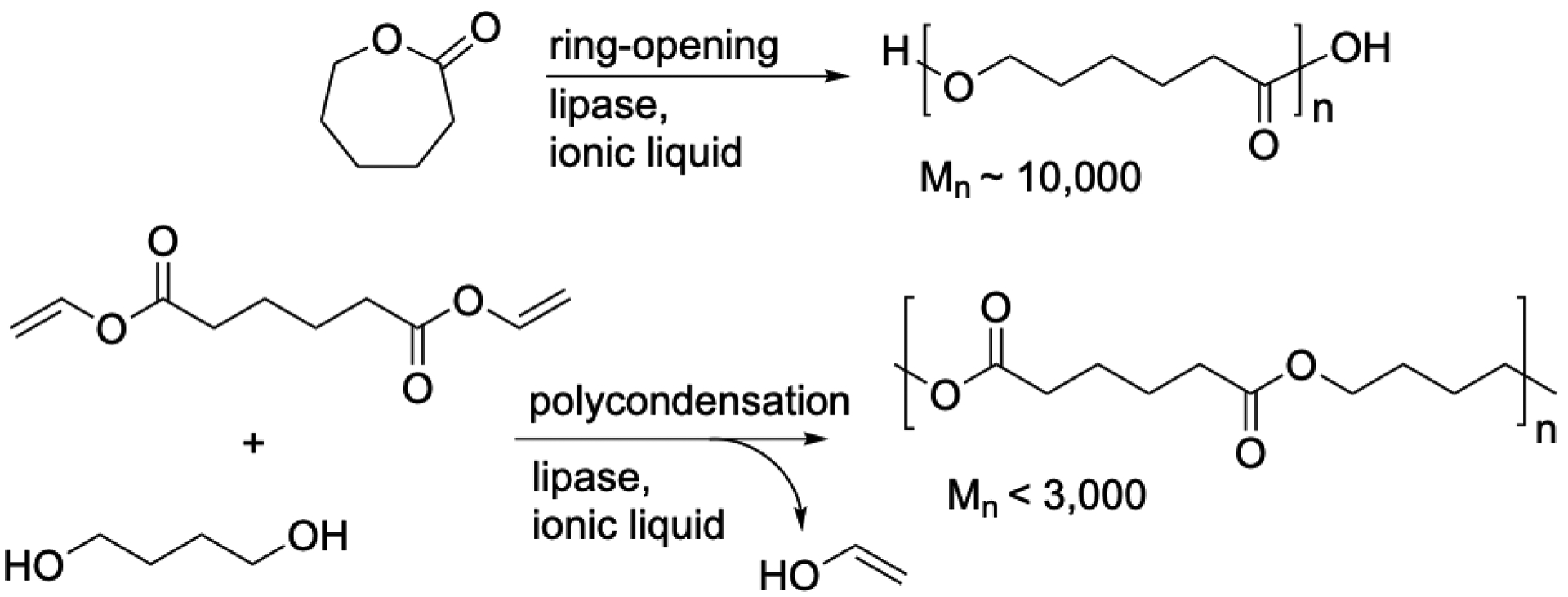
Lipase-catalyzed polyesterifications in ionic liquids. Typically lipases are CALB for ring-opening polymerization and CALB or BCL for condensation polymerization.
Condensation polymerizations are step-growth polymerizations, which reach high molecular weights only at high conversion. Small amount of water associated with the enzyme or release of water or alcohol during polymerization make it difficult to reach high conversion and thus, high molecular weights. In contrast, ring opening polymerization is a chain-growth polymerization with reaches high molecular weights even at incomplete conversions.
Heise’s group [66] compared poly(ε-caprolactone) produced in three ionic liquids by CALB-catalyzed polymerization either by condensation of the hydroxy acid or by ring-opening of ε-caprolactone. Condensation polymerization yielded polymer with a molecular weight of 5,500 with polydispersities up to 1.7; ring-opening polymerization yielded polymer with molecular weights approaching 9,000 with polydispersities of 2.3–2.4 before fractionation. For comparison, the ring-opening polymerization in toluene yielded molecular weights of 13,000 with a polydispersity of 2.4. The lower molecular weight in ionic liquid may be due to the lower solubility of polymer or due to more water in the ionic liquid. Although the authors dried the ionic liquids extensively, they may be more difficult to dry than organic solvents. Ring-opening polymerization of β-propiolactone and ε-caprolactone in BMIM[Tf2N] catalyzed by CALB gave molecular weight ~10,000, but other lactones gave only low molecular weight oligomers [67] similar to the result in organic solvents. CALB also catalyzed the polymerization of ε-caprolactone in deep eutectic solvents [68].
Proteases and esterases
α-Chymotrypsin, a serine protease, catalyzed formation of an amide link in Leu-enkephalin peptide fragment in MOEMIM[PF6] [69] and the transesterification of N-acetylamino acid esters in 1-methyl-3-octylimidazolium (OMIM) [PF6] or BMIM[PF6] [70]. This protease required either >0.5% water or supercritical carbon dioxide for good activity [70], Figure 3e. Subtilisin usually has low activity in ionic liquids with low water content and the activity depend strongly on the enzyme preparation. Changing the purification procedure [71] or modifying subtilisin by covalent attachment of comb-shaped poly(ethylene glycol) (PEG) [41, 42] enhanced activity up to 10,000 fold. The PEG-modified subtilisin dissolved in the ionic liquids, which may also contribute to the higher activity. The modified subtilisin catalyzed hydrolysis of p-nitrophenyl butyrate in EMIM[Tf2N] three times faster than in toluene. The modified enzyme were not active in more polar organic solvents such as DMSO.
PFE catalyzed the transesterification of ethyl valerate to methyl valerate in BMIM[Tf2N] and BMIM[PF6] at 60 °C, although activity in the latter was diminished several fold [67]. Esterases from B. subtilis and B. stearothermophilus catalyzed the acetylation of 1-phenylethanol in BMIM[Tf2N], BMIM[PF6], and BMIM[BF4], but only when immobilized on Celite [72].
Glycosidases
Formation of glycoside links by condensation is thermodynamically more difficult than the formation of amide or ester links, so the yields are often disappointing. Although the ability to dissolve sugars is a big advantage of ionic liquids, most glycosidases lose activity in ionic liquids. For example, β-galactosidase from B. circulans was not catalytically active in MMIM[MeOSO3], but, encouragingly, it also did not denature after 60 h at room temperature in this ionic liquid. Adding 0.6% water to the ionic liquid allow the galactosidase to be active and catalyze the synthesis of lactose by condensation of glucose and galactose. The yield of lactose was only 18% [73], but this low yield is largely due to the less favorable equilibrium for formation of glycosides. The requirement to add 0.6% water for galactosidase activity further decreased the equilibrium amount of product.
OXIDOREDUCTASE-CATALYZED REACTIONS
After the hydrolases, the oxidoreductases are the next most widely studied. Biocatalysis often uses the oxidoreductases that do not require additional enzymes to regenerate the cofactor: peroxidases, chloroperoxidases, laccases, and amino acid oxidases. The redox active group in the active site is regenerated during the reaction without leaving the active site. These oxidoreductases have been the main ones used in ionic liquids.
Peroxidases and chloroperoxidases use hydrogen peroxide as the oxidant. The peroxidases mentioned below all contain a heme-iron prosthetic group. These enzymes catalyze the oxidation of sulfides to sulfoxides and olefins to epoxides. Laccases and amino acid oxidases use oxygen as the oxidant and generate hydrogen peroxide as the product. Laccase contain copper in the active site and catalyze the oxidation of phenol substrates, while amino acid oxidase contain a flavin cofactor in the active site and catalyze the oxidation of amino acids to α-keto acids and ammonia.
Dehydrogenases are more complex to use for biocatalysis. They use NAD(P)H as a cofactor and require an additional enzyme and cosubstrate to recycle the NAD(P)H. There are fewer reports of dehydrogenase-catalyzed reaction in ionic liquids, see below. P450 monooxygenases are even more complex since they require another enzyme to reduce the iron in the active site to complete the catalytic cycle. No one has reported P450 monooxygenase activitiy in pure ionic liquids, but they are active in water with small amounts of ionic liquids as cosolvents. Even small amounts of immidazolium chlorides inhibit P450 monooxygenases [74,75].
Ionic liquids as cosolvent for aqueous phase reactions
The first reports of soybean and horseradish peroxidase activity in ionic liquids showed 10-fold lower activity, but later experiments with different conditions showed higher activity and higer stability in ionic liquids. In 25% BMPyr[BF4] and BMIM[PF6] these peroxidase were 10-fold less active as compared to in 20% tert-butanol [76]. HPO was three fold more stable at 80 °C in 5–10% BMIM[BF4] as compared to phosphate buffer [77]. In BMIM[PF6]/~10% water, HPO could be reused five times as compared to only twice in water for the oxidation of veratryl alcohol, a model lignin compound, to veratryl aldehyde [78]. Microemulsions of Aerosol OT/water/OMIM[Tf2N] enhanced the rate of oxidation of pyrogallol to purpurogallin more than ten fold as compared to only the ionic liquid or 1-hexanol in water [79]. Peroxidase from Coprinus cinereus (mushroom), catalyzed the asymmetric oxidation of phenyl methyl- and 2-naphthyl methyl sulfides to sulfoxides in BMIM[PF6] with 10% water [80]. Although the enantioselectivity (63–92% ee) and yields (<32%) were similar to those in water, the reaction workup was easier because ionic liquids and the extraction solvent did not form emulsions. Hydrogen peroxide was generated continuously in situ using glucose oxidase to minimize deactivation of the peroxidase with hydrogen peroxide.
HPO was immobilized by dissolving it in a hydrophobic ionic liquid BMIM[Tf2N]. After adding a second phase of water/aniline and hydrogen peroxide, the HPO catalyzed the oxidation to polyaniline [81], Figure 5a. The resulting polyaniline had conductivities comparable to polyaniline produced from immobilized HPO in organic solvents, ~10−3 S cm−1 [82]. Although water can extract HPO from the ionic liquid, the water/aniline reaction mixture does not and HPO remains in the ionic liquid.
Figure 5.
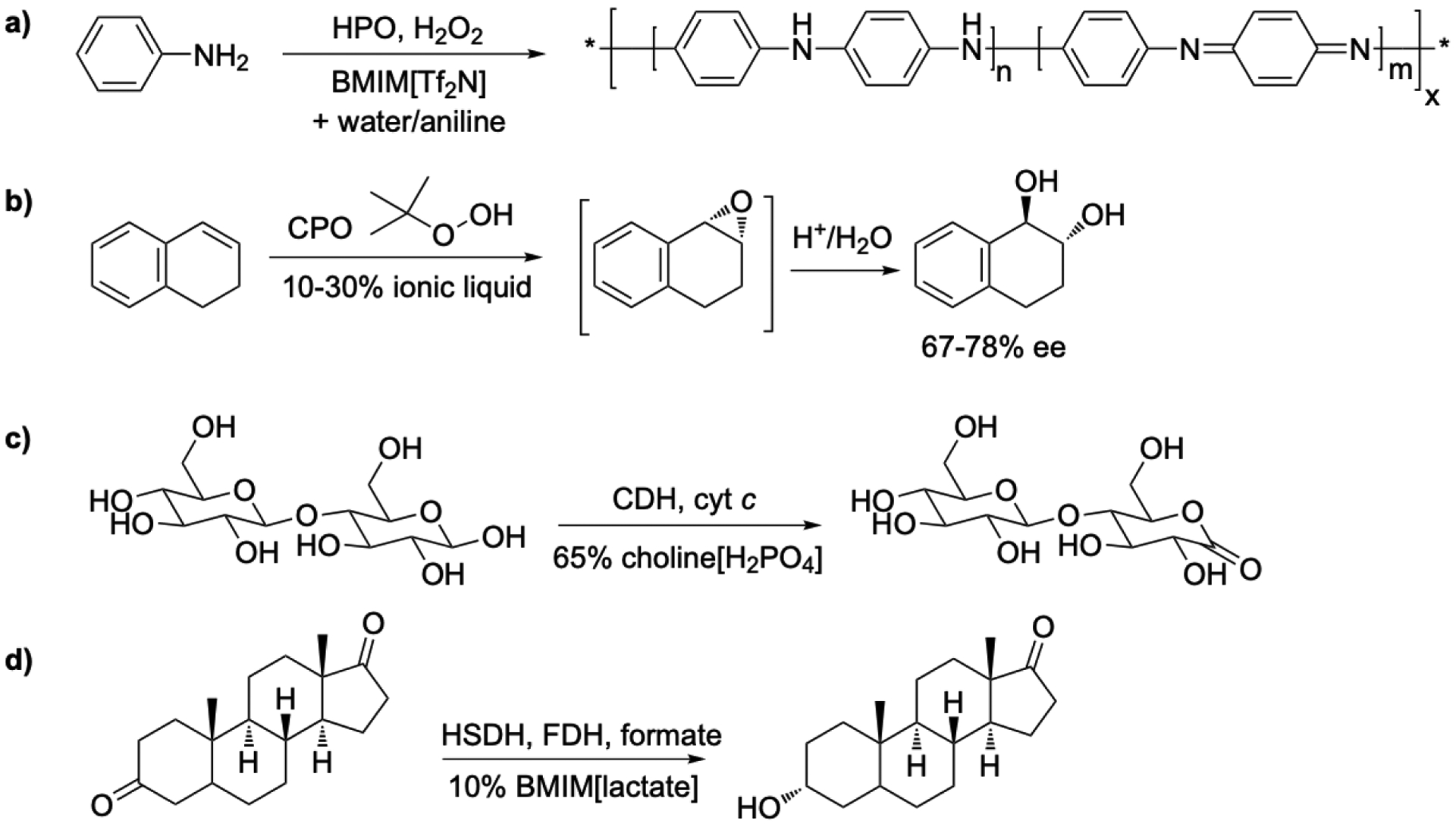
Ionic liquids as cosolvents with water for peroxidase- and dehydrogenase-catalyzed reactions. a) HPO-catalyzed synthesis of polyaniline in a two phase mixture of ionic liquid and aniline/water. HPO dissolved in the ionic liquid phase. b) CPO-catalyzed oxidation of 1,2-dihydronaphthalene. Ionic liquids as additives gave either improved enantioselectivity or conversion as compared to added organic solvents. c) Oxidation of cellobiose to cellobiolactone for use in a biofuel cell. The ionic liquid did not inhibit enzyme activity, unlike typical high-salt electrolyte solutions. CDH: choline dehydrogenase; cyt c: cytochrome c. d) Reduction of androstandione to androsterone. Adding 10 vol% ionic liquid to the aqueous phase increased the yield from 60 to 80%, likely due to increase solubility of the substrate. HSDH: 3-α-hydroxysteroid dehydrogenase, FDH: formate dehydrogenase.
Chloroperoxidase from Caldariomyces fumago catalyzed enantioselective oxidation of 1,2-dihydronaphthalene to the corresponding epoxide in 10–30 vol% MMIM[MeOSO3] and BMIM[MeOSO3], Figure 5b.The activity was comparable to acetone/ water and tert-butanol/water mixtures, but lower and less enantioselective than in pure citrate buffer [83]. Enantioselective sulfoxidation of thioanisole in up to 70% choline[citrate], choline[acetate] or MMIM[(MeO)2PO2] showed less over oxidation to to the sulfone (from 89% to >99% sulfoxide) compared to buffer and up to twofold higher conversion at 30–50% ionic liquid [84].
Laccases typically do not tolerate organic solvent additives, but they do tolerate ionic liquids. Laccase C from Trametes species gave up to 30-fold higher conversion in 25% ionic liquid BMPyr[BF4] as compared to in 20% tert-butanol [76]. The reaction was a mediator-assisted oxidation of anthracene or veratryl alcohol. These reactions required the use of poorly soluble substrates or mediators, and the reactions in the ionic liquid typically gave higher conversions than those in the organic solvent due to increased solubility. The commercial laccase DeniLite base II had activity comparable to in buffer in a standard dye oxidation assay (ABTS assay) in 10–50 vol% of three different ionic liquids at pH 5–9 [85]. Although the laccase tolerated similar amounts of DMSO or acetonitrile at pH 7, it did not tolerate these solvents at pH 5 or pH 9. Adding 10–20% BMIM[Br] or 50–60% BMIM[DCA] increased the laccase-catalyzed oxidation of catechol to benzoquinone [86].
D-amino acid oxidase (DAAO) is used industrially in the production of 7-aminocephalosporanic acid from cephalosporin As a model reaction Lutz-Wahl and coworkers [87] used the oxidative resolution of rac-phenylalanine to phenylpyruvic acid and L-phenylalanine. The ionic liquid did not give any clear advantage. They used immobilized DAAO and free catalase (to destroy the product hydrogen peroxide) in 20% MMIM[(MeO)2PO2] or 40% BMIM[BF4]. Higher proportions of ionic liquid inactivated either the DAAO or the catalase. The time needed for complete reaction was the same in the BMIM[BF4] mixture as in water, but it was 25% faster in the MMIM[(MeO)2PO2]. A reaction analogous to the first step of industrial cephalosporin C production in was 25% slower in 20% MMIM[(MeO)2PO2] than in buffer.
Dehydrogenases tend to lose activity upon addition of increasing amounts of ionic liquid. Yeast ADH showed 25% of its water activity in 50 vol% 1:2 choline chloride: glycerol/50% buffer (100 mM CHES, pH 9), but was not active a higher concentrations of deep eutectic solvent (unpublished results). Horse liver alcohol dehydrogenase (ADH) was slightly more active 15 wt to vol% BMIM[Cl] than in buffer alone, but activity decreased with higher amounts of ionic liquid [88].
Cellobiose dehydrogenase (CDH) catalyzed the oxidation of cellobiose, the major disaccharide product of cellulose hydrolysis, to cellobiolactone in 65% choline phosphate [89], Figure 5c. The goal was to extract electrons from sugars as a potential biofuel cell. The electron acceptor was an either cytochrome c or 2,6-dichloroindophenolate. Although high salt-content aqueous systems, such as those used in certain electrodes, inhibited the electron transfer reaction, the ionic liquid did not. The authors attributed the slower reaction in the ionic liquid to the higher viscosity.
De Gonzalo and coworkers [90] also used hydroxyl-functionalized ionic liquids as cosolvents for the asymmetric reduction of ketones catalyzed by crude ADH A from R. ruber. The dehydrogenase was active in 90 vol% tris-(2-hydroxyethyl)-methylammonium [MeOSO3]/10% buffer and in Ammoeng 100, 101, 102 (quaternary ammonium salts containing polyethyleneglycol substituents). The enantioselectivity remained high as all eight ketones tested yielded products with 99% ee. At more than 90 vol% ionic liquid, the activity of the dehydrogenase decreased.
Ionic liquids added to the aqueous phase of an octane-water system enhanced the solubility of androstandione [91], Figure 5d. 3-α-Hydrosteroid dehydrogenase (HSDH) catalyzed its reduction and formate dehydrogenase (FDH) and formate regenerated the NADH. While HSDH tolerated 10 vol% of a number of ionic liquids, FDH denatured in solutions containing EMIM[OTf], BMIM[OTf], and BMIM[BF4]. Both enzymes were active in with BMIM[lactate], which the authors suggested was due to its ability to form the strongest hydrogen bonds. Using 5 vol% BMIM[lactate] gave a higher yield after 8 h: 80% as compared to 60% in buffer alone.
Ionic liquids as nonaquesous solvents
Unlike for hydrolases, there is no general advantage to using oxidoreducatases in nonaqueous solvents. One special case was the synthesis of oxycodone, where the use of non-aqueous media prevented reaction of the intermediate codienone with water, Figure 6. Walker and Bruce [92] used an ionic liquid with a hydroxylated cation HPMIM[glycolate] containing as little as <100 ppm water. The hydroxyalkyl moiety on the HPMIM cation stabilized the dehydrogenase at these low water contents while still dissolving the protein. The ionic liquid prevented reaction of the intermediate with water and allowed the final step to continue with an overall 14% yield based on the starting material. In a subsequent report [93], the researchers added glucose dehydrogenase and gluconolactone to regenerate the cofactor NADP+. The first step, oxidation of codeine to codienone, gave 20% yield as compared to 10% yield in water.
Figure 6.
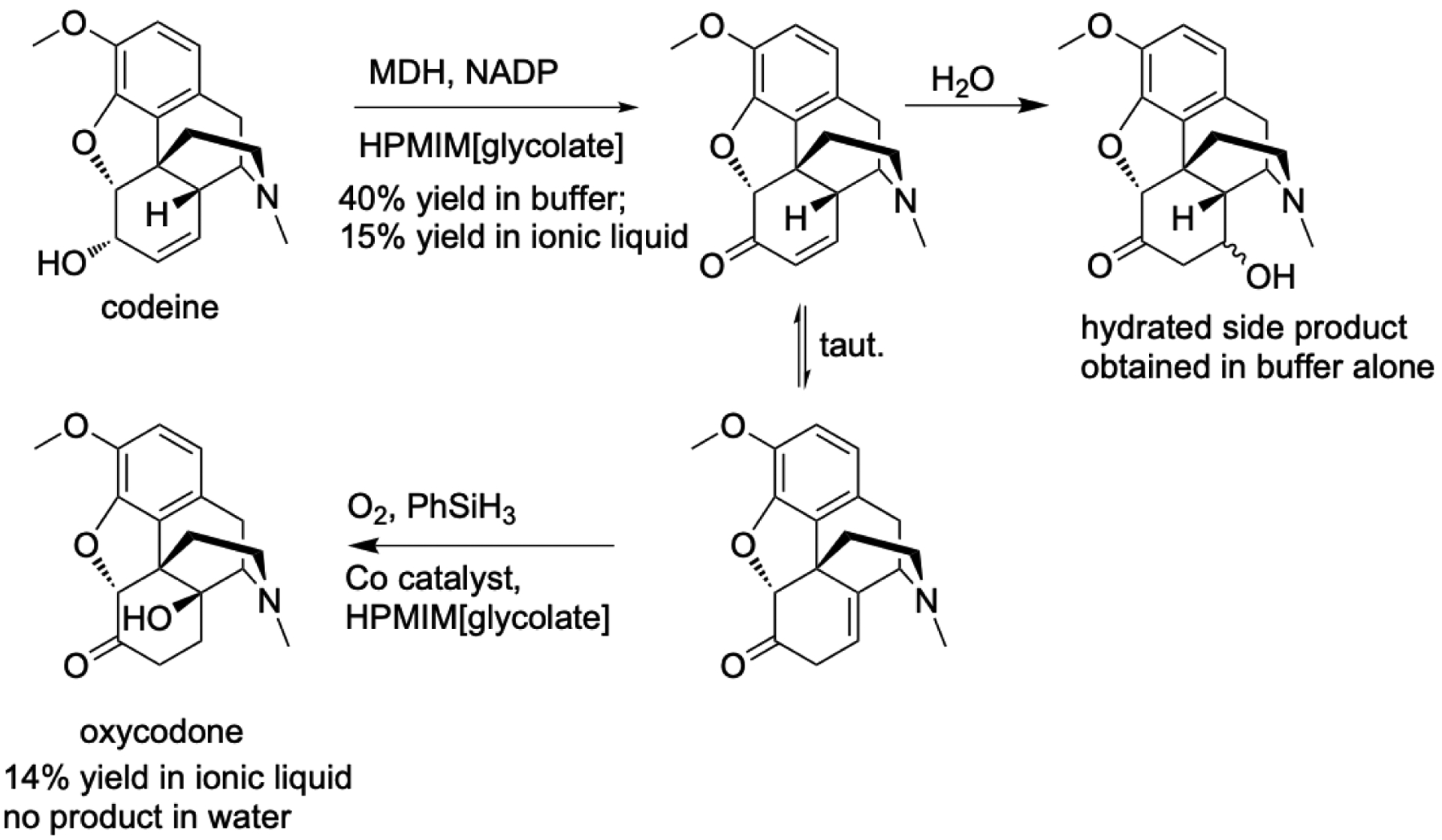
Synthesis of oxycodone by combined enzymatic and chemical catalysis. The intermediate reacts with water, so one-pot synthesis is not possible in water. NADP: nicotinamide adenine dinucleotide phosphate, MDH: morphine dehydrogenase.
Whole-cell catalyzed reactions
Many cofactor-dependent reactions use whole cells rather than purified proteins to exploit innate cofactor recycling ability in cells.
Ionic liquids as cosolvents
Water-miscible ionic liquids are usually toxic to microorganisms, but some microorganisms tolerate small amounts of water miscible ionic liquids. Adding only 1% BMIM[BF4] to the culture medium completely inhibited the growth of P. pastoris, B. cereus, and E. coli [94]. Ionic liquids containing chloride anions completely inhibited the growth of E. coli MG1655 [87], which is not surprising, as chloride-containing ionic liquids inactivated hydrolases. P. membranaefaciens tolerated 2.5% BMIM[BF4] and even improved the yield and selectivity of the reduction of ethyl acetoacetate to ethyl-(R)-3-hydroxybutyate as compared to buffer [95]. Immobilized baker’s yeast in 10 vol% BMIM[BF4] catalyzed the reduction of acetyltrimethylsilane to (S)-1-trimethylsilylethanol [96]. The initital reduction rate was ten times fasters ionic liquid system than in aqueous buffer only, likely due to increased substrate solubility.
Ionic liquids as an immiscible second phase
Ionic liquids are useful as second phases to minimize toxic effects of reactants or products and can also simplify product separation. Unlike organic solvents which usually disrupt cell membranes, ionic liquids do not typically disrupt cell membranes. We hypothesize that the strong intermolecular interaction within the ionic liquid makes them less soluble in the non-polar membranes.Whole cells tolerate water immiscible ionic liquids more readily than water miscible ones, but the effects vary. A second phase of BMIM[PF6] did not inhibit the growth of Pichia pastoris, inhibited Bacillus cereus by about 50% and completely inhibited growth of E. coli [94].
The first report of whole cell biocatalysis using an ionic liquid second phase were whole cells of Rhodococcus R312. They contained nitrile hydratase, which catalyzed the hydration of 1,3-dicyanobenzene to 3-cyanobenzamide [12]. Reactions involving nitrile hydratase usually use whole cells because the isolated enzyme is unstable. Using a second phase of 20 vol% BMIM[PF6] further stabilized the nitrile hydratase, while adding toluene did not. The initial rate of reaction was slower in ionic liquid than in toluene, likely due to slower mass transfer in the viscous ionic liquid, but the final yield was similar for both ionic liquid and toluene. The nitrile hydratase activity remained constant in the ionic liquid mixture, but decreased 50–90% in the toluene mixture.
In another example, adding a second phase of ionic liquid (23 vol% methyltrioctylammonium (OMA) [Tf2N] or trihexyltetradecaphosphonium[Tf2N]) protected E. coli cells from the toxic effects of the substrate toluene [97]. The whole cells contained a dioxygenase that catalyzed the oxidation of toluene to toluene cis-diol. Although the ionic liquid second phase inhibited growth by 26–39%, the highest specific yield (11.7 mmol/g cell dry weight) was 2.5-fold higher than in buffer. The higher yield is likely due to reduced toxicity of the substrate toluene because adding the ionic liquid increased the tolerance of the cells for toluene eight-fold.
Whole cells of Lactobacillus kefir with a second phase of ionic liquid catalyzed the asymmetric reduction of chloroacetophenone to (R)-1-(4-chlorophenyl)ethanol [98]. The yield and enantioselectivity improved BMIM[PF6], BMIM[Tf2N], and OMA[Tf2N] as a second phase instead of MTBE. The membrane integrity was nearly ten fold higher with the ionic liquids than with several organic solvents, suggesting that the ionic liquids do not partition into membranes like organic solvents. Similar experiments with Saccharomyces cerevisiae FasB His6and Escherichia coli K12 also showed higher membrane integrity in biphasic ionic liquid systems compared to organic solvents [99].
Conclusions
Ionic liquids are a large class of nonvolatile, moderately polar solvents. Their properties vary with structure and they can be water miscible or immiscible. In many cases, enzymes tolerate ionic liquids as cosolvents for water, as second phases or even as non aqueous solvents. Even when enzymes are inactivated or denatured by polar organic solvents, they may tolerate an ionic liquid with similar polarity. In addition, the low vapor pressure of ionic liquids eliminates release of volatile solvent. The enhanced solubility of the substrate can increases the rate of reaction and often increases the regio- or enantioselectivity. Hydrolases and oxidoreductases are particularly well-studied in ionic liquids. Precisely why enzymes tolerate ionic liquids, but not organic solvents of comparable polarity, is still unknown. Most of the research has used second generation ionic liquids, which may be too expensive for commercial applications. The availability of advanced ionic liquids – greener, inexpensive, and biodegradable – increases the likelihood that they will find commercial use in biocatalysis.
Acknowledgements
RJK thanks the Chemical and Biological Engineering Department at Seoul National University for their warm hospitality during his stay as WCU professor (grant R32-10213). This review was written during this stay. The research on advanced ionic liquids at the University of Minnesota was supported by the Institute for Renewable Energy and the Environment and the National Institutes of Health (Biotechnology Training Grant 5T32GM008347).
Abbreviations
- ABTS
2,2-O-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt
- Aliquat
cation of Aliquat 336, a commercial phase transfer catalyst composed of a mixture of methyl tri(octyl or decyl) ammonium chlorides
- Ammoeng
a series of commercial quaternary ammonium ionic liquids with one methyl group, two short chains (4–25 units total) of poly(ethylene glycol), and a cocos (100 and 101), tallow (102), or other natural group; and a methylsulfate (100), chloride (101), ethylsulfate (102) or similar anion
- BCL
Burkholderia (formerly Pseudomonas) cepacia lipase
- BSE
Bacillus subtilis esterase
- BSteE
Bacillus stearothermophilus esterase
- BMIM
1-butyl-3-methylimidazolium
- BMMIM
1-butyl-2,3-dimethylimidazolium
- CALA
Candida antarctica lipase A
- CALB
Candida antarctica lipase B
- CLEA
cross-linked enzyme aggregate
- CRL
Candida rugosa lipase
- DAAO
D-amino acid oxidase
- DCA
dicyanamide
- E
enantioselectivity, the relative rate of reaction of the fast-reacting enantiomer as compared to the slow-reacting enantiomer
- EMIM
1-ethyl-3-methylimidazolium
- HEMIM
1-(2-hydroxyethyl)-3-methylimidazolium
- HPMIM
1-(3-hydroxypropyl)-3-methylimidazolium
- HPO
horseradish peroxidase
- MMIM
1,3-dimethylimidazolium
- MML
Mucor miehei lipase
- MOEMIM
1-methoxyethyl-3-methylimidazolium
- MTEOA
methyltri(2-hydroxyethyl)ammonium
- NAD(H)
(reduced) nicotinamide adenine dinucleotide
- OMA
methyltrioctylammonium
- OMIM
1-methyl-3-octylimidazolium
- PEG
poly(ethylene glycol)
- PFE
Pseudomonas fluorescens esterase
- RMIM
1-alkyl-3-methylimidazolium
- TLL
Thermomyces languinosus lipase
- Tf2N
bis(trifluoromethane)sulfonimide
REFERENCES
- (1).Walden P (1914) Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. (St. Petersburg) 405–422. [Google Scholar]
- (2).Wilkes JS, Levisky JA, Wilson RA, and Hussey CL (1982) Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem 21: 1263–1264. [Google Scholar]
- (3).Wasserscheid P and Welton T. (2008) Ionic liquids in synthesis, 2nd Ed. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- (4).Dominquez de María P (2008) “Nonsolvent” applications of ionic liquids in biotransformations and organocatalysis. Angew. Chem. Int. Ed 47: 6960–6968. [DOI] [PubMed] [Google Scholar]
- (5).Park S and Kazlauskas RJ (2003) Biocatalysis in ionic liquids – advantages beyond green technology. Curr. Opin. Biotechnol 14: 432–437. [DOI] [PubMed] [Google Scholar]
- (6).Pinto PCAG, Saraiva M.Lúcia M. F. S., and Lima JLFC (2008) Oxidoreductase behavior in ionic liquids: A review. Anal. Sci 24: 1231–1238. [DOI] [PubMed] [Google Scholar]
- (7).Sheldon RA, Madeira Lau R, Sorgedrager MJ, van Rantwijk, and Seddon KR (2002) Biocatalysis in ionic liquids. Green Chem. 4: 147–151. [Google Scholar]
- (8).Welton T (1999) Room-temperature ionic liquids. solvents for synthesis and catalysis. Chem. Rev 99: 2071–2072–2083. [DOI] [PubMed] [Google Scholar]
- (9).Kragl U, Eckstein M, and Kaftzik N (2002) Enzyme catalysis in ionic liquids. Curr. Opin. Biotechnol 13: 565–571. [DOI] [PubMed] [Google Scholar]
- (10).van Rantwijk F and Sheldon RA (2007) Biocatalysis in ionic liquids. Chem. Rev 107: 2757–2785. [DOI] [PubMed] [Google Scholar]
- (11).Yang Z and Pan W (2005) Ionic liquids: Green solvents for nonaqueous biocatalysis. Enz. Microb. Technol 37: 19–28. [Google Scholar]
- (12).Cull SG, Holbrey JD, Vargas-Mora V, Seddon KR, and Lye GJ (2000) Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol. Bioeng 69: 227–233. [PubMed] [Google Scholar]
- (13).Madeira Lau R, van Rantwijk F, Seddon KR, and Sheldon RA (2000) Lipase-catalyzed reactions in ionic liquids. Org. Lett 2: 4189–4191. [DOI] [PubMed] [Google Scholar]
- (14).Park S and Kazlauskas RJ (2001) Improved preparation and use of room-temperature ionic liquids in lipase-catalyzed enantio- and regioselective acylations. J. Org. Chem 66: 8395–8401. [DOI] [PubMed] [Google Scholar]
- (15).Itoh T, Akasaki E, Kudo K, and Shirakami S (2001) Lipase-catalyzed enantioselective acylation in the ionic liquid solvent system: Reaction of enzyme anchored to the solvent. Chem. Lett 262–263. [Google Scholar]
- (16).Erbeldinger M, Mesiano AJ, and Russell AJ (2000) Enzymatic catalysis of formation of Z-aspartame in ionic liquid - an alternative to enzymatic catalysis in organic solvents. Biotechnol. Prog 16: 1129–1131. [DOI] [PubMed] [Google Scholar]
- (17).Abbott AP, Capper G, Davies DL, Rasheed RK, and Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem. Commun 70–71. [DOI] [PubMed] [Google Scholar]
- (18).Abbott AP, Boothby D, Capper G, Davies DL, and Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc 126: 9142–9147. [DOI] [PubMed] [Google Scholar]
- (19).Abbott AP, Harris RC, and Ryder KS (2007) Application of hole theory to define ionic liquids by their transport properties. J. Phys. Chem. B 111: 4910–4913. [DOI] [PubMed] [Google Scholar]
- (20).Docherty KM and Kulpa CF (2005) Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 7: 185–189. [Google Scholar]
- (21).Seddon KR, Stark A, and Torres M (2000) Influence of chloride,water,and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem 72: 2275–2287. [Google Scholar]
- (22).Zaks A and Klibanov AM (1984) Enzymatic catalysis in organic media at 100 °C. Science 224: 1249–1251. [DOI] [PubMed] [Google Scholar]
- (23).Kim K, Song B, Choi M, and Kim M (2001) Biocatalysis in ionic liquids: Markedly enhanced enantioselectivity of lipase. Org. Lett 3: 1507–1509. [DOI] [PubMed] [Google Scholar]
- (24).Roberts NJ, Seago A, Carey JS, Freer R, Preston C, and Lye GJ (2004) Lipase catalysed resolution of the lotrafiban intermediate 2,3,4,5-tetrahydro-4-methyl-3-oxo-1H-1,4-benzodiazepine-2-acetic acid methyl ester in ionic liquids: Comparison to the industrial t-butanol process Green Chem. 6: 475–482. [Google Scholar]
- (25).Lou W, Zong M, and Wu H (2005) Enzymic asymmetric hydrolysis of D, L-p-hydroxyphenylglycine methyl ester in aqueous ionic liquid co-solvent mixtures. Biotechnol. Appl. Biochem 41: 151–156. [DOI] [PubMed] [Google Scholar]
- (26).Liu Y, Lou W, Zong M, Xu R, Hong X, and Wu H (2005) Increased enantioselectivity in the enzymatic hydrolysis of amino acid esters in the ionic liquid 1-butyl-3-methyl-imidazolium tetrafluoroborate. Biocatal. Biotransform 23: 89–95. [Google Scholar]
- (27).Lou W, Zong M, Smith TJ, Wu H, and Wang J (2006) Efficient regioselective acylation of 1-β-D-arabinofuranosylcytosine catalyzed by lipase in ionic liquid containing systems. Green Chem. 8: 509–512. [Google Scholar]
- (28).Gorke JT, Srienc F, and Kazlauskas RJ (2008) Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun 1235–1237. [DOI] [PubMed] [Google Scholar]
- (29).Wallert S, Drauz K, Grayson I, Gröger H, Dominguez de Maria P, and Bolm C (2005) Ionic liquids as additives in the pig liver esterase (PLE) catalysed synthesis of chiral disubstituted malonates. Green Chem. 7: 602–605. [Google Scholar]
- (30).Wehofsky N, Wespe C, Cerovsky V, Pech A, Hoess E, Rudolph R, and Bordusa F (2008) Ionic liquids and proteases: A clean alliance for semisynthesis. ChemBioChem 9: 1493–1499. [DOI] [PubMed] [Google Scholar]
- (31).Zhao H, Jackson L, Song Z, and Olubajo O (2006) Using ionic liquid [EMIM][CH3COO] as an enzyme-’friendly’ co-solvent for resolution of amino acids. Tetrahedron Asymmetr. 17: 2491–2498. [Google Scholar]
- (32).Vafiadi C, Topakas E, Nahmias VR, Faulds CB, and Christakopoulos P (2009) Feruloyl esterase-catalysed synthesis of glycerol sinapate using ionic liquids mixtures. J. Biotechnol 139: 124–129. [DOI] [PubMed] [Google Scholar]
- (33).Kaftzik N, Wasserscheid P, and Kragl U (2002) Use of ionic liquids to increase the yield and enzyme stability in the β-galactosidase catalysed synthesis of N-acetyllactosamine. Org. Process Res. Dev: 553–557. [Google Scholar]
- (34).Lang M, Kamrat T, and Nidetzky B (2006) Influence of ionic liquid cosolvent on transgalactosylation reactions catalyzed by thermostable β-glycosylhydrolase CelB from Pyrococcus furiosus. Biotechnol. Bioeng 95: 1093–1100. [DOI] [PubMed] [Google Scholar]
- (35).Mischitz M, Faber K, and Willetts A (1995) Isolation of a highly enantioselective epoxide hydrolase from Rhodococcus sp. NCIMB 11216. Biotechnol. Lett 17: 893–898. [Google Scholar]
- (36).Karboune S, Archelas A, and Baratti J (2006) Properties of epoxide hydrolase from Aspergillus niger for the hydrolytic kinetic resolution of epoxides in pure organic media. Enz. Microb. Technol 39: 318–324. [Google Scholar]
- (37).Chiappe C, Leandri E, Lucchesi S, Pieraccini D, Hammock BD, and Morisseau C (2004) Biocatalysis in ionic liquids: The stereoconvergent hydrolysis of trans-β-methylstyrene oxide catalyzed by soluble epoxide hydrolase. J. Mol. Catal. B: Enz 27: 243–248. [Google Scholar]
- (38).Chiappe C, Leandri E, Hammock BD, and Morisseau C (2007) Effect of ionic liquids on epoxide hydrolase-catalyzed synthesis of chiral 1,2-diols. Green Chem. 9: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chin JT, Wheeler SL, and Klibanov AM (1994) On protein solubility in organic solvents. Biotechnol. Bioeng 44: 140–145. [DOI] [PubMed] [Google Scholar]
- (40).van Rantwijk F, Secundo F, and Sheldon RA (2006) Structureand activity of Candida antarctica lipase B in ionic liquids. Macromolecules 8: 282–286. [Google Scholar]
- (41).Nakashima K, Maruyama T, Kamiya N, and Goto M (2005) Comb-shaped poly(ethylene glycol)-modified subtilisin carlsberg is soluble and highly active in ionic liquids. Chem. Commun 4297–4299. [DOI] [PubMed] [Google Scholar]
- (42).Nakashima K, Maruyama T, Kamiya N, and Goto M (2006) Homogeneous enzymatic reactions in ionic liquids with poly (ethylene glycol)-modified subtilisin. Org. Biomol. Chem 4: 3462–3467. [DOI] [PubMed] [Google Scholar]
- (43).Lozano P, De Diego T, Carrié D, Vaultier M, and Iborra JL (2001) Over-stabilization of Candida antarctica lipase B by ionic liquids in ester synthesis. Biotechnol. Lett 23: 1529–1533. [Google Scholar]
- (44).Itoh T, Nishimura Y, Ouchi N, and Hayase S (2003) 1-butyl-2,3-dimethylimidazolium tetrafluoroborate: The most desirable ionic liquid solvent for recycling use of enzyme in lipase-catalyzed transesterification using vinyl acetate as acyl donor. J. Mol. Catal. B: Enz 26: 41–45. [Google Scholar]
- (45).Abe Y, Kude K, Hayase S, Kawatsura M, Tsunashima K, and Itoh T (2008) Design of phosphonium ionic liquids for lipase-catalyzed transesterification. J. Mol. Catal. B: Enz 51: 81–85. [Google Scholar]
- (46).Ha SH, Lan MN, Lee SH, Hwang SM, and Koo Y-M (2007) Lipase-catalyzed biodiesel production from soybean oil in ionic liquids. Enz. Microb. Technol, 41: 480–483. [Google Scholar]
- (47).Gamba M, Lapis AAM, and Dupont J (2008) Supported ionic liquid enzymatic catalysis for the production of biodiesel. Adv. Synth. Catal 350: 160–165. [Google Scholar]
- (48).Schofer SH, Kaftzik N, Kragl U, and Wasserscheid P (2001) Enzyme catalysis in ionic liquids: Lipase catalyzed kinetic resolution of 1-phenylethanol with improved enantioselectivity. Chem. Commun 425–426. [Google Scholar]
- (49).Kamal A and Chouhan G (2004) Chemoenzymatic synthesis of enantiomerically pure 1,2-diols employing immobilized lipase in the ionic liquid [bmim]PF6. Tetrahedron Lett. 45: 8801–8805. [Google Scholar]
- (50).Hongwei Y, Jinchuan W, and Bun CC (2005) Kinetic resolution of ibuprofen catalyzed by Candida rugosa lipase in ionic liquids. Chirality 17: 16–21. [DOI] [PubMed] [Google Scholar]
- (51).Xin J, Zhao Y, Zhao G, Zheng Y, Ma X, Xia C, and Li S (2005) Enzymatic resolution of (R, S)-naproxen in water-saturated ionic liquid. 23: 353–361. [Google Scholar]
- (52).Itoh T, Matsushita Y, Abe Y, Han S, Wada S, Hayase S, Kawatsura M, Takai S, Morimoto M, and Hirose Y (2006) Increased enantioselectivity and remarkable acceleration of lipase-catalyzed transesterification by using an imidazolium PEG-alkyl sulfate ionic liquid. 12: 9228–9237. [DOI] [PubMed] [Google Scholar]
- (53).Malhotra SV and Zhao H (2005) Enantioseparation of the esters of α-N-acetylamino acids by lipase in ionic liquid. Chirality 17: S240–S242. [DOI] [PubMed] [Google Scholar]
- (54).Liu Q, Janssen MHA, van Rantwijk F, and Sheldon RA (2005) Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem. 7: 39–42. [Google Scholar]
- (55).Lee SH, Nguyen HM, Koo Y, and Ha SH (2008) Ultrasound-enhanced lipase activity in the synthesis of sugar ester using ionic liquids. Proc. Biochem 43: 1009–1012. [Google Scholar]
- (56).Lee SH, Dang DT, Ha SH, Chang W-J, and Koo Y-M (2008) Lipase-catalyzed synthesis of fatty acid sugar ester using extremely supersaturated sugar solution in ionic liquids. Biotechnol. Bioeng, 99: 1–8. [DOI] [PubMed] [Google Scholar]
- (57).Lee SH, Ha SH, Hiep NM, Chang W-J, and Koo Y-M (2008), Lipase-catalyzed synthesis of glucose fatty acid ester using ionic liquids mixtures. J. Biotechnol 133: 486–489. [DOI] [PubMed] [Google Scholar]
- (58).Ganske F and Bornscheuer UT (2005) Lipase-catalyzed glucose fatty acid ester synthesis in ionic liquids. Org. Lett 7: 3097–3098. [DOI] [PubMed] [Google Scholar]
- (59).Zhao H, Baker GA, Song Z, Olubajo O, Crittlea T, and Peters D (2008) Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem. 10: 696–705. [Google Scholar]
- (60).Li X, Lou W, Smith TJ, Zong M, Wu H, and Wang J (2006) Efficient regioselective acylation of 1-β-D-arabinofuranosylcytosine catalyzed by lipase in ionic liquid containing systems. Green Chem. 8: 538–544. [Google Scholar]
- (61).Katsoura MH, Polydera AC, Katapodis P, Kolisis FN, and Stamatis H (2007) Effect of different reaction parameters on the lipase-catalyzed selective acylation of polyhydroxylated natural compounds in ionic liquids. Proc. Biochem 42: 1326–1334. [Google Scholar]
- (62).Dong H, Cao S, Li Z, Han S, You D, and Shen J (1999) Study on the enzymic polymerization mechanism of lactone and the strategy for improving the degree of polymerization. J. Polym. Sci. A: Polym. Chem 37: 1265–1275. [Google Scholar]
- (63).Uyama H, Takamoto T, and Kobayashi S (2002) Enzymatic synthesis of polyesters in ionic liquids. Polym. J 34: 94–96. [Google Scholar]
- (64).Nara SJ, Harjani JR, Salunkhe MM, Mane AT, and Wadgaonkar PP (2003) Lipase-catalysed polyester synthesis in 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. 44: 1371–1373. [Google Scholar]
- (65).Kaar JL, Jesionowski AM, Berberich JA, Moulton R, and Russell AJ (2003) Impact of ionic liquid physical properties on lipase activity and stability. J. Am. Chem. Soc 125: 4125–4131. [DOI] [PubMed] [Google Scholar]
- (66).Marcilla R, de Geus M, Mecerreyes D, Duxbury CJ, Koning CE, and Heise A (2006) Enzymatic polyester synthesis in ionic liquids. 42: 1215–1221. [Google Scholar]
- (67).Gorke JT, Okrasa K, Louwagie A, Kazlauskas RJ, and Srienc F (2007) Enzymatic synthesis of poly(hydroxyalkanoates) in ionic liquids. J. Biotechnol 132: 306–313. [DOI] [PubMed] [Google Scholar]
- (68).Gorke JT, Srienc F, and Kazlauskas RJ (2009) Deep eutectic solvents for Candida antarctica lipase B-catalyzed reactions. In ACS Symposium Series, Malhotra SV, Ed., in press. [Google Scholar]
- (69).Xing G, Li F, Ming C, and Ran L (2007) Peptide bond formation catalyzed by α-chymotrypsin in ionic liquids. 48: 4271–4274. [Google Scholar]
- (70).Laszlo JA and Compton DL (2001) α-Chymotrypsin catalysis in imidazolium-based ionic liquids. Biotechnol. Bioeng 75: 181–186. [DOI] [PubMed] [Google Scholar]
- (71).Shah S and Gupta MN (2007) Obtaining high transesterification activity for subtilisin in ionic liquids. 1770: 94–98. [DOI] [PubMed] [Google Scholar]
- (72).Persson M and Bornscheuer UT (2003) Increased stability of an esterase from Bacillus stearothermophilus in ionic liquids as compared to organic solvents. J. Mol. Catal. B: Enzym 22: 21–27. [Google Scholar]
- (73).Kaftzik N, Neumann S, Kula M, and Kragl U. (2003) Enzymatic condensation reactions in ionic liquids. pp. 206–211. In: Rogers RD and Seddon KR (eds.), Ionic Liquids as Green Solvents. American Chemical Society, Washington, D.C. [Google Scholar]
- (74).Chefson A, and Auclair K (2007) CYP3A4 activity in the presence of organic cosolvents, ionic liquids, or water-immiscible organic solvents. ChemBioChem 8: 1189–1197. [DOI] [PubMed] [Google Scholar]
- (75).Tee KL, Roccatano D, Stolte S, Arning J, Jastorff B, and Schwaneberg U (2008) Ionic liquid effects on the activity of monooxygenase P450 BM-3. Green Chem. 10: 117–123. [Google Scholar]
- (76).Hinckley G, Mozhaev VV, Budde C, and Khmelnitsky YL (2002) Oxidative enzymes possess catalytic activity in systems with ionic liquids. Biotechnol. Lett 24: 2083–2087. [Google Scholar]
- (77).Machado MF and Saraiva JM (2005) Thermal stability and activity regain of horseradish peroxidase in aqueous mixtures of imidazolium-based ionic liquids. Biotechnol. Lett 27: 1233–1239. [DOI] [PubMed] [Google Scholar]
- (78).Kumar A, Jain N, and Chauhan SMS (2007) Biomimetic oxidation of veratryl alcohol with H2O2 catalyzed by iron(III) porphyrins and horseradish peroxidase in ionic liquid. Synlett 2007: 411–414. [Google Scholar]
- (79).Moniruzzaman M, Kamiya N, and Goto M (2009) Biocatalysis in water-in-ionic liquid microemulsions: A case study with horseradish peroxidase. Langmuir 25: 977–982. [DOI] [PubMed] [Google Scholar]
- (80).Okrasa K, Guibé-Jampel E, and Therisod M (2003) Ionic liquids as a new reaction medium for oxidase–peroxidase-catalyzed sulfoxidation. Tetrahedron Asymmetr. 14: 2487–2490. [Google Scholar]
- (81).Rumbau V, Marcilla R, Ochoteco E, Pomposo JA, and Mecerreyes D (2006) Ionic liquid immobilized enzyme for biocatalytic synthesis of conducting polyaniline. Macromolecules 39: 8547–8549. [Google Scholar]
- (82).Alvarez S, Manolache S, and Denes F (2003) Synthesis of polyaniline using horseradish peroxidase immobilized on plasma-functionalized polyethylene surfaces as initiator. J. Appl. Polym. Sci 88: 369–379. [Google Scholar]
- (83).Sanfilippo C, D’Antona N, and Nicolosi G (2004) Chloroperoxidase from Caldariomyces fumago is active in the presence of an ionic liquid as co-solvent. Biotechnol. Lett 26: 1815–1819. [DOI] [PubMed] [Google Scholar]
- (84).Chiappe C, Neri L, and Pieraccini D (2006) Application of hydrophilic ionic liquids as co-solvents in chloroperoxidase catalyzed oxidations. Tetrahedron Lett. 47: 5089–5093. [Google Scholar]
- (85).Tavares APM, Rodriguez O, and Macedo EA (2008) Ionic liquids as alternative co-solvents for laccase: Study of enzyme activity and stability. Biotechnol. Bioeng 101: 201–207. [DOI] [PubMed] [Google Scholar]
- (86).Shipovskov S, Gunaratne HQN, Seddon KR, and Stephens G (2008) Catalytic activity of laccases in aqueous solutions of ionic liquids Green Chem. 10: 806–810. [Google Scholar]
- (87).Lutz-Wahl S, Trost E, Wagner B, Manns A, and Fischer L (2006) Performance of D-amino acid oxidase in presence of ionic liquids. J. Biotechnol 124: 163–171. [DOI] [PubMed] [Google Scholar]
- (88).Shi X, Zong M, Meng C, and Guo YH (2005) Catalytic characteristics of horse liver alcohol dehydrogenase in a medium containing ionic liquid [bmim]Cl. Chin. J. Catal 26: 982–986. [Google Scholar]
- (89).Fujita K, Nakamura N, Igarashi K, Samejima M, and Ohno H (2009) Biocatalytic oxidation of cellobiose in a hydrated ionic liquid. Green Chem. 11: 351–354. [Google Scholar]
- (90).de Gonzalo G, Lavandera I, Durchschein K, Wurm D, Faber K, and Kroutil W (2007) Asymmetric biocatalytic reduction of ketones using hydroxy-functionalised water-miscible ionic liquids as solvents. Tetrahedron Asymmetr. 18: 2541–2546. [Google Scholar]
- (91).Okochi M, Nakagawa I, Kobayashi T, Hayashi S, Furusaki S, and Honda H (2007) Enhanced activity of 3-α-hydroxysteroid dehydrogenase by addition of the co-solvent 1-butyl-3-methylimidazolium (L)-lactate in aqueous phase of biphasic systems for reductive production of steroids. J. Biotechnol 128: 376–382. [DOI] [PubMed] [Google Scholar]
- (92).Walker AJ, and Bruce NC (2004) Combined biological and chemical catalysis in the preparation of oxycodone. Tetrahedron 60: 561–568. [Google Scholar]
- (93).Walker AJ, and Bruce NC (2004) Cofactor-dependent enzyme catalysis in functionalized ionic solvents. Chem. Commun 2570–2571. [DOI] [PubMed] [Google Scholar]
- (94).Ganske F, and Bornscheuer U (2006) Growth of Escherichia coli, Pichia pastoris and Bacillus cereus in the presence of the ionic liquids [BMIM][BF4] and [BMIM][PF6] and organic solvents. Biotechnol. Lett 28: 465–469. [DOI] [PubMed] [Google Scholar]
- (95).He J, Zhou L, Wang P, and Zu L (2009) Microbial reduction of ethyl acetoacetate to ethyl (R)-3-hydroxybutyrate in an ionic liquid containing system. Proc. Biochem 44: 316–321. [Google Scholar]
- (96).Lou W, Zong M, and Smith TJ (2006) Use of ionic liquids to improve whole-cell biocatalytic asymmetric reduction of acetyltrimethylsilane for efficient synthesis of enantiopure (S)-1-trimethylsilylethanol. Green Chem. 8: 147–155. [Google Scholar]
- (97).Cornmell RJ, Winder CL, Schuler S, Goodacre R, and Stephens G (2008) Using a biphasic ionic liquid/water reaction system to improve oxygenase-catalysed biotransformation with whole cells. Green Chem. 10: 685–691. [Google Scholar]
- (98).Pfruender H, Amidjojo M, Kragl U, and Weuster-Botz D (2004) Efficient whole-cell biotransformation in a biphasic ionic liquid/water System. Angew. Chem. Int. Ed 43: 4529–4531. [DOI] [PubMed] [Google Scholar]
- (99).Pfruender H, Jones R, and Weuster-Botz D (2006) Water immiscible ionic liquids as solvents for whole cell biocatalysis. J. Biotechnol 124: 182–190. [DOI] [PubMed] [Google Scholar]


