Abstract
Memories for events that happen early in life are fragile—they are forgotten more quickly than expected based on typical adult rates of forgetting. Although numerous factors contribute to this phenomenon, data show one major source of change is the protracted development of neural structures related to memory. Recent empirical studies in early childhood reveal that the development of specific subdivisions of the hippocampus (i.e., the dentate gyrus) are related directly to variations in memory. Yet the hippocampus is only one region within a larger network supporting memory. Data from young children have also shown that activation of cortical regions during memory tasks and the functional connectivity between the hippocampus and cortex relate to memory during this period. Taken together, these results suggest that protracted neural development of the hippocampus, cortex, and connections between these regions contribute to the fragility of memories early in life and may ultimately contribute to childhood amnesia.
Keywords: childhood amnesia, memory development, brain development, hippocampus
You have to begin to lose your memory, if only in bits and pieces, to realize that memory is what makes our lives. Life without memory is no life at all… Our memory is our coherence, our reason, our feeling, even our action. Without it we are nothing. (Buñuel (1984, p.17).
How Does the Ability to Remember Change Across Development?
The ability to remember details from events in life is critical for functioning and a personal sense of self. Why is it then that, as adults, we recall so little from our childhood? This inability to remember, termed infantile amnesia or childhood amnesia, is one of the most robust and replicable phenomena in developmental psychology (Freud, 1910; Newcombe, Lloyd, & Ratliff, 2007; Pillemer & White, 1989). Although it was originally thought that early experiences were simply not encoded into memory (Piaget & Inhelder, 1969), research with young children has repeatedly documented that this is not the case. In fact, even very young children can form memories for events (see Bauer, 2006, for a review). However, the same research suggests that early memories are extremely fragile and prone to being forgotten, especially when they include the details of events (e.g., Bauer, 2015; Bauer & Larkina, 2014, 2016).
In one of the earliest studies on autobiographical memories, although children as young as 3 years could recall a family vacation to Disneyworld after six to 12 months, the older the children were during the trip, the more details they remembered (Hamond & Fivush, 1991). Building on this landmark study, a sizable empirical literature now documents accelerated rates of forgetting for autobiographical memories across childhood (e.g., Bauer & Larkina, 2014, 2016; Peterson, Warren, & Short, 2011). For example, when researchers track young children’s memories over time, results suggest they grow into their amnesia; this means that although 3- and 4-year-olds initially recall details of events, these details are later forgotten. When rates of forgetting are assessed empirically, 4- to 8-year-olds forget more rapidly than adults (Bauer & Larkina, 2016). Moreover, among children, 4- to 6-year-olds forget more rapidly than 8-year-olds, particularly during open-ended recall of autobiographical memories. Thus, although childhood amnesia may extend through childhood, after about the sixth year, the stability and consistency of memories increase dramatically (e.g., Bauer & Larkina 2014, 2016; Peterson et al., 2011).
Studies examining children’s memories for real-life events have high ecological validity. However, the events and details recalled vary considerably among children. Because of this variability, it is often challenging to manipulate these events parametrically to probe mechanisms underlying changes in children’s ability to recall them. As a result, some researchers have turned to controlled, laboratory-based episodic memory paradigms. Similar to real-world events, in these laboratory paradigms, children are presented with events that are rich in contextual details (i.e., specific items encountered at particular times and places) and then asked to recall these details after a delay. The advantage of this approach is that lab-based events can be designed to be manipulated experimentally. Thus, although autobiographical and lab-based memories differ, they share critical overlapping core features since both require memories for the details of previous experiences.
Lab-based studies identify a developmental timeline of memory that is similar to that identified by naturalistic studies. These studies suggest that the ability to remember details of events improves dramatically across early childhood and becomes robust around the sixth year. In one study, 4-year-olds, 6-year-olds, and adults were tested on their ability to recall isolated parts of pictures as well as combinations of these parts (Sluzenski, Newcombe, & Kovacs, 2006). For example, participants were shown a tiger at a playground and then asked to determine whether they had previously seen the animal (the tiger), the location (the playground), or the animal in the location (the tiger at the playground). Participants’ memory for the animal and the location in isolation were similar across all three age groups. However, their memory for the combinations (i.e., animals in locations) increased between 4-year-olds and 6-year-olds but not between 6-year-olds and adults. Moreover, the ability to remember combinations was related to children’s memory for details from a more naturalistic memory task (recalling a story after a delay). On the basis of these results, the authors argued that memory for details (i.e., memory for items bound to contexts) “may be near or at adult levels by about the age of 6 years” (Sluzenski et al., 2006, p. 98).
Research has documented similar age-related improvements in memory for details across early childhood using a variety of other paradigms, including memory for pairs of items or words (e.g., Yim, Dennis, & Sloutsky, 2013), the source of novel facts (e.g., Drummey & Newcombe, 2002; Riggins, 2014), and the spatial location in which an item was originally encountered (e.g., Bauer et al., 2012). Closely related research suggests that early childhood is a time when children’s ability to form very detailed memories and discriminate between them also improves (Canada, Ngo, Newcombe, Geng, & Riggins, 2018; Ngo, Newcombe, & Olson, 2017b). Taken together, findings from lab-based paradigms support the suggestion that an important transition in children’s ability to form and recall detailed memories occurs during early childhood.
Why Does Memory Change Across Development?
Researchers have proposed many reasons why memories for details become more robust toward the end of early childhood than during other developmental periods. First, developmental psychologists have long noted changes in the nature of cognition between 5 and 7 years. This shift marks the transition from Piaget’s preoperational stage to the concrete operational stage, and signifies increased sophistication of children’s thinking across numerous domains of cognition (e.g., categorical reasoning, perspective taking, metamemory, strategy use; Piaget & Inhelder, 1969). Second, developments in language, theory of mind, executive function and self-concept (e.g., increases in self-knowledge and the capacity for self-source monitoring) also occur and relate to improvements in autobiographical memory (e.g., Ross, Hutchison, & Cunningham, 2019). Third, studies suggest that the purpose of memory (i.e., what children need to remember) may change during this period. Specifically, infants and young children initially benefit from extracting generalities across items and situations. Only after this initial foundational knowledge is laid down does retaining specific details become important (Newcombe et al., 2007). Fourth, in many societies, formal schooling is introduced at this age, and schooling affects both cognitive ability and brain development (Brod, Bunge, & Shing, 2017).
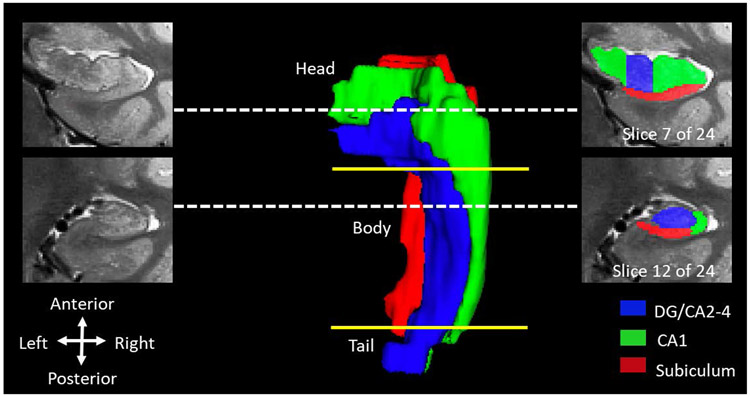
Finally, theories of memory and data from animal models suggest that brain development may underlie this shift in memory (e.g., Bauer, 2006, 2014, 2015; Lavenex & Banta Lavenex, 2013; Nadel & Zola-Morgan, 1984; Pillemer & White, 1989). Specifically, researchers have hypothesized that postnatal changes in the hippocampus, a neural structure critical for memory in adults, underlie age-related improvements in children’s ability to recall past events (Madsen & Kim, 2016; Nadel & Zola-Morgan, 1984). The term hippocampus is of Greek origin and roughly translates to seahorse because of its shape. The hippocampus has specific subdivisions (termed subregions or subfields) that can be examined independently or in relation to each other. Subregions include the head, body, and tail of the hippocampus, which show differential connectivity to surrounding cortical regions via white matter tracts. Subfields refer to the functional subunits of the hippocampus (dentate gyrus, cornu ammonis [CA]1-4, subiculum; Yushkevich et al., 2015; see Figure 1). Although the subfields are anatomically distinct, MRI scans’ low spatial resolution makes it difficult to delineate them individually. To circumvent this issue, researchers often combine smaller subfields (e.g., CA2-4) with larger regions (e.g., the dentate gyrus).
Figure 1. Hippocampal volume from one representative participant.
(age 4.54 years), including subregions (head, body, tail) and subfields (CA1-4, subiculum, dentate gyrus or DG). Note the disproportionate distribution of subfields along the longitudinal axis. Dotted lines indicate exact location of coronal slices. Yellow lines indicate approximate boundaries between subregions.
Neuroanatomical data from nonhuman primates show that age-related changes in specific subfields and the connections between them persist until 5 to 7 years (Lavenex & Banta Lavenex, 2013; Serres, 2001). One of these subfields, the dentate gyrus, is critical for adultlike memory formation. Thus, researchers have proposed that the prolonged developmental trajectory may underlie the immature profile of memory during this period (i.e., poor ability to recall details and accelerated forgetting, Bauer, 2006, 2014; Lavenex & Banta Lavenex, 2013; Nadel & Zola-Morgan, 1984; Pillemer & White, 1989).
In addition, in recent studies with animals, changes in rates of generating new neurons (neurogenesis) contributed to an observed shift in young rodents’ ability to remember (Josselyn & Frankland, 2012). In these studies, the decline of postnatal neurogenesis corresponded with the ability to form long-term memories. The authors suggest that high levels of neurogenesis prohibit the formation of stable memories, likely by replacing synaptic connections in preexisting hippocampal memory circuits (Josselyn & Frankland, 2012). Thus, animal models clearly support the notion that neural development, particularly development of the hippocampus, influences memory early in life.
Yet the hippocampus is only one piece within the memory network. In research with adults and school-aged children, cortical areas (e.g., the prefrontal and posterior parietal cortices) are recruited during the formation and retrieval of detailed memories (Ghetti & Bunge, 2012; Ofen, 2012). In fact, these cortical regions are often credited for age-related changes in memory later in childhood and adolescence (e.g., Tang, Shafer, & Ofen, 2018). In particular, the prefrontal cortex is thought to be necessary for the strategic part of memory, which includes cognitive control mechanisms that aid and regulate memory (Shing, Werkle-Bergner, Li, & Lindenberger, 2008). These findings are consistent with research with both animals (e.g., Huttenlocher & Dabholkar, 1997) and humans (e.g., Giedd et al., 1999) that shows protracted development of cortical regions, particularly the prefrontal cortex. However, how these cortical regions contribute to memory early in life has been studied less.
Evidence for Relations Between Neural Development and Memory in Early Childhood
As a result of the challenges of obtaining neuroimaging data from children younger than age 8, empirical evidence exploring the hypothesis that brain development is related to the ability to form long-term, detailed memories has emerged only recently.1 These studies have examined age-related differences in both brain structure and function, and how these differences relate to memory ability. Specifically, development of brain structure, function, and the functional connections between brain regions are all linked with developmental improvements in memory. Thus, neural development during this period is multifaceted, which may be why dramatic changes in memory are observed near the end of early childhood.
Brain Structure
Building on behavioral research in early childhood and neuroimaging studies in school-aged children, the first study to examine links between memory and the hippocampus early in life explored relations between detailed memories and the hippocampus. The study compared 4- and 6-year-olds’ capacity to recall details of a past lab-based event (i.e., where an object was previously encountered) and the size (i.e., volume) of subregions of the hippocampus (Riggins, Blankenship, Mulligan, Rice, & Redcay, 2015). Better memory was related to larger hippocampal head volume for 6-year-olds, but not for 4-year-olds. These results suggest that relations between brain and behavior fluctuate across early development (consistent with reports in school-aged children; DeMaster, Pathman, Lee, & Ghetti, 2013), and may emerge during early childhood.
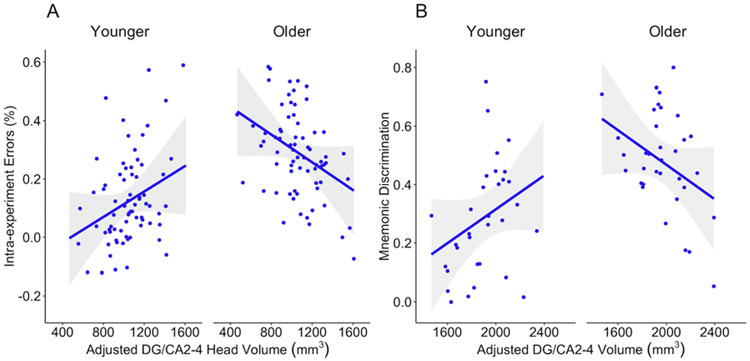
Another study, of 4- to 8-year-olds, provided further evidence of differential relations between brain and behavior using more precise measurements of the hippocampus (Riggins et al., 2018). The study used a similar memory paradigm and performance was related to subfields of the hippocampus. Again, relations between brain and behavior varied across development. Specifically, within the head of the hippocampus, relations between children’s aptitude for recalling fine-grained details was related to volume of the dentate gyrus/CA2-4 subfields. However, this association was moderated by age: In younger individuals, smaller volumes were associated with less detailed memories; in older individuals, smaller volumes were associated with more detailed memories, as reflected by the type and number of errors made on the task (see Figure 2A). This finding is not only consistent with previous research, but also extends prior studies to implicate the dentate gyrus/CA2-4 subfields as the subdivisions related to developmental improvements in precision of memory.
Figure 2. Relations between dentate gyrus/CA2-4 subfields.
and A) memory for details (as measured by the number of intra-experimental errors on a source memory task) in the head of the hippocampus (Riggins et al., 2018) and B) precision of memories (as measured by mnemonic discrimination) in the head and body of the hippocampus (Canada et al., 2018). In both studies, age moderated the association so that in younger children, larger volumes were associated with better performance, whereas in older children, smaller volumes were associated with better performance.
A third studyprobed the association between memory for details and the dentate gyrus more specifically. Researchers examined hippocampal subfields in relation to young children’s ability to discriminate between two similar events from memory (Canada et al., 2018). Developmental differences in relations between the precision of memories and the volume of the dentate gyrus/CA2-4 subfields appeared; in younger individuals, smaller volumes were associated with less precise memories, but in older children, smaller volumes correlated with more precise memories (see Figure 2B). These results further support the hypothesis that age-related differences in the hippocampus (specifically, the dentate gyrus/CA2-4 subfields) are related to developmental improvements in children’s ability to form and retain detailed memories during this transitional period (see Keresztes et al., 2017, for similar findings in 6- to 14-year-olds and adults).
Finally, structural connections between brain regions via axonal pathways (i.e., white matter tracts) implicated in memory in school-aged individuals and adults also vary by age (e.g., Lebel & Beaulieu, 2011). White matter pathways have been related to differences in memory performance across early childhood. Specifically, the integrity of the white matter fiber bundles between the hippocampus and the inferior parietal lobule (a region important for memory in adults) was associated with 4- and 6-year-olds’ performance on two lab-based memory tasks. These findings suggest that not only is development of hippocampal structure important, but also the connections between the hippocampus and cortical regions (Ngo et al., 2017a).
Brain Function
Task-based functional magnetic resonance imaging (fMRI). 2
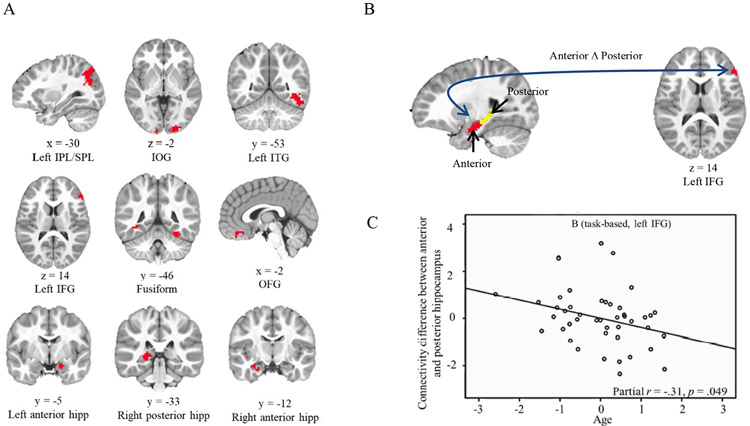
Task-based fMRI is challenging to do with young children because of the constraints of the MRI environment (lying still in a scanner while performing a cognitively challenging task for an extended period). One study of young children examined patterns of activation during the formation of memories for associations between an item (e.g., a banana) and a character (e.g., Mickey Mouse; Geng, Redcay, & Riggins, 2019). During successful memory formation, the hippocampus and several cortical regions showed increased activity (see Figure 3A). Increased activity in some of these cortical regions (e.g., the inferior/superior parietal lobule) was expected since studies of older individuals have reported similar results (Ghetti & Bunge, 2012). However, the increases in other cortical regions (e.g., the orbital frontal gyrus) were unexpected since they have been reported infrequently in studies of older individuals; this suggests that younger children may rely on a wider or more distributed network of brain regions to encode detailed memories successfully. In addition, connectivity between the hippocampal subregions (the head versus the body/tail) and the cortex (i.e., the inferior frontal gyrus) varied as a function of age, implying increased specialization of connectivity of the hippocampus along the anterior-to-posterior axis to cortical regions across development (see Figures 3B and 3C). Finally, activation of the hippocampus and several cortical regions varied as a function of both age and performance. These findings suggest it is neither maturation nor task demands alone that contribute to activation differences during development, but that both are important.
Figure 3. Memory-related activation and hippocampal functional connectivity during task and task-free conditions in 4- to 8-year-old children.
A) Brain regions showing greater activation during memory formation when details are subsequently recalled.
IPL/SPL: inferior/superior parietal lobule; IOG: inferior occipital gyrus; ITG: inferior temporal gyrus; IFG: inferior frontal gyrus; hipp: hippocampus; OFG: orbital frontal gyrus.
B) Functional connectivity differences between anterior and posterior (body and tail) hippocampus to left IFG were associated with age during memory formation.
C) Scatterplot showing relation between functional connectivity differences between anterior and posterior hippocampus and left IFG and age during memory formation.
Task-free fMRI.
Given the challenges of obtaining task-based fMRI data from young children, many researchers have begun to explore functional connectivity between regions in the absence of an overt task (e.g., Vanderwal, Kelly, Eilbott, Mayes, & Castellanos, 2015). These measures of functional connectivity are thought to arise from co-activation of brain regions that builds up over time (Fox & Raichle, 2007). Thus, although not measured during an overt task, the strength of functional connectivity between regions can be used as an estimate of the integrity or maturity of the memory system.
Two studies have explored relations between task-free hippocampal functional connectivity and memory ability assessed outside the MRI scanner in young children (Geng et al., 2019; Riggins, Geng, Blankenship, & Redcay, 2016). Findings from both studies were similar to those from task-based fMRI studies. Specifically, they revealed that functional connectivity between the hippocampus and cortical regions was influenced by age and performance. These studies also suggested that functional connectivity between the hippocampus and regions not typically thought to relate to memory formation in adults decreased developmentally (i.e., the orbital frontal gyrus and left and right middle temporal gyrus in Geng et al., 2019; the right inferior frontal gyrus in Riggins et al., 2016).
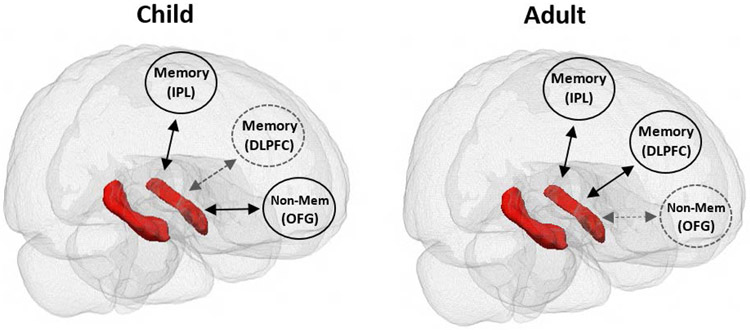
Overall, findings from both task-based and task-free fMRI studies are in line with the interactive specialization framework, which suggests that, with age, the hippocampus becomes functionally integrated with cortical regions that are part of the hippocampal memory network in adults, and also becomes functionally segregated from regions not related to memory in adults (Johnson, 2011). Thus, both integration and segregation are critical for developmental improvements in memory (see Figure 4).
Figure 4. Depiction of interactive specialization framework.
The hippocampus is depicted in red and cortical regions are depicted in circles. Double-sided arrows represent functional connections. Solid black arrows represent connections that are present and gray arrows represent connections that are weak or absent. Interactive specialization suggests that changes occur both in the integration and the segregation of brain regions over development. In this depiction, there are functional connections between the hippocampus and memory regions that are present in children and in adults (e.g., inferior parietal lobule, IPL). There are also functional connections with other memory regions that are weak or absent in children, but present in adults (e.g., dorsolateral prefrontal cortex, DLPFC) as the hippocampus becomes more functionally integrated with memory regions. Finally, there are functional connections with nonmemory regions in children (e.g., orbital frontal gyrus, OFG) that are weakened or absent in adults, as the hippocampus becomes more segregated from nonmemory regions. Not pictured are relations between cortical regions, which also likely change across development. For illustrative purposes, lines are indicated as present or absent; however, the strength of these functional connections likely varies with age.
Summary and Conclusion
Taken together, these studies suggest development in multiple neural measures that vary during the early years of life and contribute to age-related differences in young children’s ability to remember details of events. First, relations between hippocampal structure (i.e., the volume of subregions and subfields) and memory vary across development. Second, functional activation of the hippocampus and multiple cortical regions contribute to memory in early childhood, yet vary as a function of age and performance. Finally, structural and functional connectivity between the hippocampus and multiple cortical regions is related to memory but also varies with age and performance. Together, these findings highlight the multifaceted ways in which the brain relates to memory development during this period and may account for why changes in memory at this time are quite dramatic.
These findings provide some of the first empirical support from young children regarding brain-behavior associations in the domain of memory early in life. These data are critical because they provide evidence that supports neural explanations for childhood amnesia. Although the findings we have reviewed focused on lab-based memories, they are consistent with research with 8- to 11-year-olds that used fMRI to investigate recall of autobiographical memories (Bauer, Pathman, Inman, Campanella, & Hamann, 2016). Research on neural bases of memory in early childhood is beginning to provide a bridge and fill a gap in the literature connecting what we know about memory processes early in life versus what we know about these processes later. Making such connections is critical for a comprehensive understanding of memory. Ultimately, this knowledge will help develop interventions targeting memory when they can have the largest impact—early in development, when plasticity is abundant.
Findings regarding the role of brain development in children’s ability to recall events may contribute to the offset of childhood amnesia. Yet changes in the neural correlates of memory must be considered with other factors, including improvements in other areas of cognition and their underlying neural systems (e.g., language, theory of mind, self-concept), changes in the goal of memory during this time, and the context in which children form and retrieve these memories. Researchers should explore to what extent these factors are competitive versus complementary in nature. Although the idea is speculative, development in other cognitive domains, which appear dissimilar on the surface, may converge at the neural level because they may rely on overlapping neural circuitry. For example, the hippocampus plays a role in the development of memory as well as of language (Lee et al., 2015), emotion (Stern, Botdorf, Cassidy, & Riggins, 2019), and spatial navigation (Lavenex & Banta Lavenex, 2013). Moreover, improvements across domains may have additive or interactive effects. For example, simultaneous improvements in memory and self-concept or theory of mind may combine to produce gains in autobiographical memory that exceed what would be expected by either in isolation. Such possibilities provide opportunities for research on the numerous measures that contribute simultaneously to childhood amnesia.
Knowledge regarding why childhood amnesia exists is important to scientists, students, policymakers, and the public for several reasons. First, memory development and brain development are both active areas of scientific inquiry and are of interest to those studying these constructs. Second, autobiographical memory is important for developing self-identity, mental health, and functioning within social contexts, which makes childhood amnesia intriguing to those who are interested primarily in social development. Third, policymakers are particularly interested in information regarding brain development in early childhood since changes occur rapidly during this time; previous research has informed an array of policies, such as those related to early childhood education. Finally, childhood amnesia is a ubiquitous phenomenon—it affects everyone. Understanding why we forget events from our earliest years gives everyone more insight into their minds and the records of their personal pasts.
Acknowledgments
The work described in this article was supported by the National Institute of Health under grant HD079518 (to Tracy Riggins), by the National Science Foundation via a Graduate Research Fellowship Program grant (to Morgan Botdorf), and by the University of Maryland. We also thank two anonymous reviewers for their insightful feedback.
Footnotes
A fair amount of neuroimaging research has been conducted in older children and adolescents, but the youngest children in these studies tend to be 8 years old, which is beyond the period of childhood amnesia, the focus of this article (see Ghetti & Bunge, 2012, for a review).
Although event-related potentials have been used to examine brain function in young children during memory tasks, they lack spatial resolution to test the hypotheses generated from animal models. Therefore, we focus our review on fMRI.
References
- Bauer PJ (2007). Remembering the times of our lives. Memory in infancy and beyond. New York, NY: Psychology Press. 10.4324/9781315785226 [DOI] [Google Scholar]
- Bauer PJ (2015). A complementary processes account of the development of childhood amnesia and a personal past. Psychological Review, 122, 204–231. doi: 10.1037/a0038939 [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Doydum AO, Pathman T, Larkina M, Güler OE, & Burch M (2012). It’s all about location, location, location: Children’s memory for the “ where” of personally experienced events. Journal of Experimental Child Psychology, 113, 510–522. 10.1016/j.jecp.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, & Larkina M (2014). Childhood amnesia in the making: Different distributions of autobiographical memories in children and adults. Journal of Experimental Psychology: General, 143, 597–611. 10.1037/a0033307 [DOI] [PubMed] [Google Scholar]
- Bauer PJ, & Larkina M (2016). Predicting remembering and forgetting of autobiographical memories in children and adults: A 4-year prospective study. Memory, 24, 1345–1368. doi: 10.1080/09658211.2015.1110595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Pathman T, Inman C, Campanella C, & Hamann S (2016). Neural correlates of autobiographical memory retrieval in children and adults. Memory, 25, 450–466. doi: 10.1080/09658211.2016.1186699 [DOI] [PubMed] [Google Scholar]
- Brod G, Bunge SA, & Shing YL (2017). Does one year of schooling improve children’s cognitive control and alter associated brain activation? Psychological Science. 28, 967–978. 10.1177/0956797617699838 [DOI] [PubMed] [Google Scholar]
- Buñuel L (1984). My Last Sigh, The Autobiography of Luis Buñuel. New York: Vintage Books. [Google Scholar]
- Canada KL, Ngo CT, Newcombe NS, Geng F, & Riggins T (2018). It’s all in the details: Relations between young children’s developing pattern separation abilities and hippocampal subfield volumes. Cerebral Cortex, 29, 3427–3433. 10.1093/cercor/bhy211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, & Ghetti S (2013). Structural development of the hippocampus and episodic memory: Developmental differences along the anterior / posterior axis. Cerebral Cortex, 24, 3036–3045. 10.1093/cercor/bht160 [DOI] [PubMed] [Google Scholar]
- Drummey AB, & Newcombe NS (2002). Developmental changes in source memory. Developmental Science, 5, 502–513. 10.1111/1467-7687.00243 [DOI] [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8, 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Freud S (1910). Three contributions to the sexual theory. Nervous and Mental Disease Monograph Series. New York: Journal of Nervous and Mental Disease Publishing Company. [Google Scholar]
- Geng F, Redcay E, & Riggins T (2019). The influence of age and performance on hippocampal function and the encoding of contextual information in early childhood. NeuroImage, 195, 433–443. 10.1016/j.neuroimage.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, & Bunge SA (2012). Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience, 2, 381–395. 10.1016/j.dcn.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2, 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Hamond NR, & Fivush R (1991). Memories of Mickey Mouse: Young children recount their trip to Disneyworld. Cognitive Development, 6, 433–448. 10.1016/0885-2014(91)90048-I [DOI] [Google Scholar]
- Huttenlocher PR, & Dabholkar AS (1997). Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology, 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2011). Interactive specialization: A domain-general framework for human functional brain development? Developmental Cognitive Neuroscience, 1, 7–21. 10.1016/j.dcn.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, & Frankland PW (2012). Infantile amnesia: A neurogenic hypothesis. Learning & Memory, 19, 423–433. 10.1101/lm.021311.110 [DOI] [PubMed] [Google Scholar]
- Keresztes A, Bender AR, Bodammer NC, Lindenberger U, Shing YL, & Werkle-Bergner M (2017). Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proceedings of the National Academy of Sciences, 114, 9212–9217. 10.1073/pnas.1710654114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, & Banta Lavenex P (2013). Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behavioural Brain Research, 254, 8–21. 10.1016/j.bbr.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Lebel C, & Beaulieu C (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, 31, 10937–10947. 10.1523/jneurosci.5302-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Nordahl CW, Amaral DG, Lee A, Solomon M, & Ghetti S (2015). Assessing hippocampal development and language in early childhood: Evidence from a new application of the automatic segmentation adapter tool. Human Brain Mapping, 36, 4483–4496. doi: 10.1002/hbm.22931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, & Kim JH (2016). Ontogeny of memory: An update on 40 years of work on infantile amnesia. Behavioural Brain Research, 298, 4–14. 10.1016/j.bbr.2015.07.030 [DOI] [PubMed] [Google Scholar]
- Nadel L, & Zola-Morgan S (1984). Infantile amnesia: A neurobiological perspective. In Moscovitch M (Ed.), Infant memory (pp. 145–172). New York, NY: Plenum Press. [Google Scholar]
- Newcombe NS, Lloyd ME, & Ratliff KR (2007). Development of episodic and autobiographical memory: A cognitive neuroscience perspective. Advances in Child Development and Behavior, 35, 37–85. 10.1016/B978-0-12-009735-7.50007-4 [DOI] [PubMed] [Google Scholar]
- Ngo CT, Alm KH, Metoki A, Hampton W, Riggins T, Newcombe NS, & Olson IR (2017a). White matter structural connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience, 28, 41–53. 10.1016/j.dcn.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo CT, Newcombe NS, & Olson IR (2017b). The ontogeny of relational memory and pattern separation. Developmental Science, 21, e12556. 10.1111/desc.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N (2012). The development of neural correlates for memory formation. Neuroscience and Biobehavioral Reviews, 36, 1708–1717. 10.1016/j.neubiorev.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C, Warren KL, & Short MM (2011). Infantile amnesia across the years: A 2-year follow-up of children’s earliest memories. Child Development, 82, 1092–1105. 10.1111/j.1467-8624.2011.01597.x [DOI] [PubMed] [Google Scholar]
- Piaget J, & Inhelder B (1969). The psychology of the child. New York, NY: Basic Books. [Google Scholar]
- Pillemer DB, & White SH (1989). Childhood events recalled by children and adults. Advances in Child Development and Behavior, 21, 297–340. 10.1016/S0065-2407(08)60291-8 [DOI] [PubMed] [Google Scholar]
- Riggins T (2014). Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Developmental Psychology, 50, 449–459. 10.1037/a0033622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Blankenship SL, Mulligan E, Rice K, & Redcay E (2015). Developmental differences in relations between episodic memory and hippocampal subregion volume during early childhood. Child Development, 86, 1710–1718. 10.1111/cdev.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, & Redcay E (2016). Hippocampal functional connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience, 19, 58–69. 10.1016/j.dcn.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Botdorf M, Canada K, Cox L, & Hancock GR (2018). Protracted hippocampal development is associated with age-related improvements in memory during early childhood. NeuroImage, 174, 127–137. 10.1016/j.neuroimage.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Hutchison J, & Cunningham SJ (2019). The me in memory: The role of the self in autobiographical memory development. Child Development. Advance online publication. 10.1111/cdev.13211 [DOI]
- Serres L (2001). Morphological changes of the human hippocampal formation from midgestation to early childhood. In Nelson CA & Luciana M (Eds.), Handbook of developmental cognitive neuroscience (pp. 45–58). Cambridge, MA: MIT Press. [Google Scholar]
- Shing YL, Werkle-Bergner M, Li SC, & Lindenberger U (2008). Associative and strategic components of episodic memory: A life-span dissociation. Journal of Experimental Psychology: General, 137, 495–513. 10.1037/0096-3445.137.3.495 [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, & Kovacs SL (2006). Binding, relational memory, and recall of naturalistic events: A developmental perspective, Journal of Experimental Psycholology: Learning, Memory, and Cognition, 32, 89–100. 10.1037/0278-7393.32.1.89 [DOI] [PubMed] [Google Scholar]
- Stern JA, Botdorf M, Cassidy J, & Riggins T (2019). Empathy and hippocampal volume in young children. Developmental Psychology, 55, 1908–1920. doi: 10.1037/dev0000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Shafer AT, & Ofen N (2018). Prefrontal cortex contributions to the development of memory formation. Cerebral Cortex, 28, 3295–3308. 10.1093/cercor/bhx200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Kelly C, Eilbott J, Mayes LC, & Castellanos FX (2015). Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. NeuroImage, 122, 222–232. 10.1016/j.neuroimage.2015.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H, Dennis SJ, & Sloutsky VM (2013). The development of episodic memory: Items, contexts, and relations. Psychological Science, 24, 2163–2172. 10.1177/0956797613487385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RS, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, … Hippocampal Subfields Group (2015). Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. NeuroImage, 111, 526–541. doi: 10.1016/j.neuroimage.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]