STRUCTURED ABSTRACT:
Objective:
To determine how body mass index (BMI) affects the follicular fluid cytokine milieu and investigate how this inflammatory environment impacts cumulus signaling.
Design:
Experimental study
Setting:
Tertiary hospital based research laboratory
Patient(s):
Women with normal (18.5 to 24.9 kg/m2) and obese (35 to 42 kg/m2) BMI undergoing controlled ovarian stimulation for intracytoplasmic sperm injection (ICSI).
Intervention(s):
Cumulus cell treatment with obese follicular fluid, interleukin (IL) 10, and IL-1β
Main outcome measure(s):
Follicular fluid cytokine concentrations between normal and obese women were compared using multiplex bead assay. Differential cumulus cell gene expression of GREM1, HAS2, PTGS2, and VCAN were measured using quantitative reverse polymerase chain reaction (RT-qPCR) while protein levels were determined by flow cytometry and confocal microscopy.
Results:
Compared to women with normal BMI, women with BMI ≥35 kg/m2 undergoing ICSI had higher follicular concentrations of IL-10 (9.46 pg/mL [0.59–19.16] vs 53.39 pg/mL [14.97–236.37], p=0.004) and IL-1β (1.92 pg/mL [1.92–5.18] vs 5.18 pg/mL [1.92–16.33], p=0.017), as well as decreased relative cumulus cell expression of GREM1 (1.01 [0.66–1.40] vs 0.51 [0.38–0.74], p=0.03), a surrogate marker of positive ICSI outcomes. Furthermore, elevated IL-10 and IL-1β appear to be responsible for decreasing GREM1 expression in women with BMI ≥35 kg/m2.
Conclusion:
Our findings suggest that follicular inflammation associated with obesity impacts cumulus cell signaling. At a molecular level, derangements to the immune system resulting in decreased GREM1 expression may be a partial explanation for the suboptimal ICSI outcomes observed with obesity.
Keywords: obesity, cumulus cells, immunology, intracytoplasmic sperm injection, gremlin 1
Capsule:
Cytokines IL-10 and IL-1β, present at high concentrations in the follicular fluid of obese women, decrease GREM1 expression in cumulus cells.
Introduction:
Intracytoplasmic sperm injection (ICSI) is the most common form of assisted reproductive technology (ART) used to help infertile couples conceive a biological child (1). However, suboptimal outcomes in obese women have been recognized. Retrospective studies report lower pregnancy rates and higher miscarriage risks in overweight and obese women compared to women with normal body mass index (BMI) undergoing ART (2–5). Additionally, a recent meta-analysis found the probability of live birth after ART was lower in obese women with BMI ≥30 kg/m2 compared to women with normal BMI (6). The etiology for these outcomes is likely multifactorial; however, follicular impairment due to obesity related chronic inflammation is a mechanism gaining recognition (7, 8).
Obesity associated intracellular lipid accumulation in adipose tissue can result in oxidative stress and increased systemic inflammation (7). The oxidative stress is the result of adipocyte hypertrophy causing increase in cytokine production. Similar to adipose tissue, evidence suggests that lipid accumulation can also occur in the ovary (9). Murine studies have demonstrated lipid accumulation and induction of stress markers in the cumulus oocyte complex of mice fed a high-fat diet (10). Additionally, evaluation of inflammatory markers in the follicular fluid of obese women has found increased levels of C-Reactive protein and other pro-inflammatory cytokines, suggesting obesity related follicular inflammation (11, 12). Elevated free fatty acid levels in the follicular fluid results in poor morphology of the cumulus oocyte complex (13). Therefore, derangements to cumulus cell function and oocyte metabolism may arise from the culmination of lipid build up in the follicular fluid, resulting in lipotoxicity and inflammation (7, 8). Such changes to the humoral response can lead to disturbances in folliculogenesis and fertility (14, 15). As the follicular fluid provides the microenvironment for the oocyte, cytokines may negatively influence the developing oocyte and potentially affect embryo development; however, how the inflammation in the follicular fluid of obese women affects signaling within the cumulus oocyte complex has yet to be explored.
Oocyte development and maturation occurs in a highly coordinated process involving communication through gap junctions and paracrine signaling between the maturing oocyte and the surrounding cumulus cells (16, 17). Thus, cumulus cells may help identify biomarkers related to oocyte competence (18, 19). Oocyte secreted growth differentiation factor 9 (GDF9) and bone morphogenic protein 15 (BMP15) help regulate cumulus cell differentiation and ovarian folliculogenesis (16, 20, 21). Absence of GDF9 or mutations in BMP15 results in sterility, proving the importance of these signaling molecules for normal folliculogenesis (22, 23).
As cumulus cells share an intimate relationship with the oocyte and are discarded following oocyte retrievals, they can potentially serve as an ideal surrogate marker for embryo quality. Cumulus cell expression of genes downstream of GDF9, including gremlin 1 (GREM1), hyaluronan synthase 2 (HAS2), and prostaglandin-endoperoxide synthase 2 (PTGS2 or COX2) were found to be over-expressed in cumulus cells surrounding oocytes that subsequently developed into high grade embryos compared to low grade embryos following ICSI (18). Additionally, versican (VCAN) and PTGS2 expression were higher in cumulus cells surrounding oocytes that resulted in live birth (24). Several other studies have reported similar associations between these markers and positive ICSI outcomes (25–28). With limited information available on obesity related inflammatory changes in women undergoing controlled ovarian stimulation, we sought to explore how BMI affects the cytokine milieu of the follicular fluid. Additionally, GREM1, HAS2, PTGS2, and VCAN, as surrogate markers of positive ICSI outcomes, were measured to identify how obesity induced inflammation impacts cumulus cells.
Materials and Methods:
Patient selection
Recruitment took place at Mayo Clinic in Rochester, Minnesota from July 2018 to June 2019 following Institutional Review Board approval (protocol 18–004771). Written consent forms were obtained from women between the ages of 18 to 45 years who were undergoing controlled ovarian stimulation and planning to utilize ICSI. Women were excluded from the study if their cycle was cancelled prior to the oocyte retrieval or if ICSI was not planned.
Controlled ovarian stimulation
The controlled ovarian stimulation protocol was individualized based on ovarian reserve and patient history. The stimulation was started either randomly or following a course of combination oral contraceptive, progesterone only pill, or oral estradiol priming. Women were stimulated with gonadotropin releasing hormone (GnRH) antagonist, GnRH agonist down-regulation, or GnRH agonist flare protocol. Recombinant follicle stimulating hormone (Gonal-F or Follistim AQ) and menotropins (Menopur) were administered until at least 2 lead follicles were 18 mm in size and at least half of the follicles were above 15 mm by transvaginal ultrasound. The women then self-administered either 10,000 IU of urine-derived human chorionic gonadotropin (hCG, Pregnyl or Novarel) or a combination of leuprolide 4 mg (Lupron) with 1,500 IU of urine-derived hCG for trigger. Transvaginal oocyte retrieval was performed 36 hours following the trigger injection.
Patient and cycle variables collected
Patient demographics, cycle characteristics, and cycle outcomes were collected on women with normal BMI (18.5 to 24.9 kg/m2) and BMI ≥35 kg/m2 (35 to 42 kg/m2). Demographic information included female age at retrieval, race, parity, ovarian reserve measures, and etiology for treatment. For those who had embryos created, number of pronuclear and blastocyst embryos were recorded. If an embryo transfer was performed, cycle outcomes following the transfer were obtained. For those who did not have a fresh transfer, cycle outcomes were collected on the first frozen embryo transfer following the retrieval. Clinical pregnancy was defined as the presence of a gestational sac on transvaginal ultrasound at approximately 7 weeks gestation. Biochemical loss was defined as positive beta hCG without evidence of a clinical pregnancy on transvaginal ultrasound. Miscarriage was defined as a first trimester pregnancy loss. Live birth was defined as delivery of a viable infant. Cycle outcomes are reported per transfer.
Follicular fluid and cumulus cell collection
The transvaginal oocyte retrieval was performed under ultrasound guidance utilizing a 16-gauge single lumen aspiration needle. Follicular fluid from the single most accessible follicle was aspirated first to minimize blood contamination and stored at −80°C for future use. Approximately 4 hours after the retrieval, the cumulus cells were denuded from the oocytes with 40 U/mL recombinant human hyaluronidase (ICSI Cumulase, Origio, Denmark) at 37°C. The cumulus cells from individual women were collected and washed with 1:1 Dulbecco Modified Eagle Medium (DMEM)/F12, transferred to freezing media containing 92% fetal bovine serum (FBS) with 8% dimethylsulfoxide (DMSO), and stored in liquid nitrogen for future use.
Follicular fluid cytokine measurement
Follicular fluid cytokines from 46 women were measured in duplicate using a 25-plex multiplex magnetic bead assay per manufacturer’s instruction (EMD Millipore, Temecula, CA). Measured cytokines included: interleukin (IL)-17F, granulocyte-macrophage colony stimulating factor (GM-CSF), interferon (IFN)-ɣ, IL-10, chemokine ligand 20/macrophage inflammatory protein-3 (CCL20/MIP-3α), IL-12, IL-13, IL-15, IL-17A, IL-22, IL-9, IL-1β, IL-33, IL-2, IL-21, IL-4, IL-23, IL-5, IL-6, IL-17E/IL-25, IL-27, IL-31, tumor necrosis factor (TNF)-α, TNF-β, and IL-28A. Briefly, follicular fluid was incubated with antibody coated magnetic beads overnight at 4°C. The beads were washed with buffer and incubated with detection antibodies for 30 minutes at room temperature, followed by 30 minutes with streptavidin-phycoerythrin. Cytokine concentrations were determined by Luminex 200 (Luminex Corporation, Austin, TX) using a 7-point standard curve. Concentrations were confirmed using enzyme-linked immunosorbent assays (ELISA) from R&D Systems (Minneapolis, MN).
Cumulus cell culture
Cumulus cells from women with normal BMI and BMI ≥35 kg/m2 were utilized for comparison. The cells were thawed and cultured overnight in cumulus cell media containing DMEM/F12 supplemented with 10% FBS, 1x penicillin and streptomycin, 5μg/mL bovine insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite, and 1.2 μM 4-androstene-3, 17-dione (Sigma-Aldrich, St. Louis, MO). The average number of cumulus cells collected per woman was 1.4 million and approximately 350,000 cells were plated in a 24-well plate for each experiment.
For quantitative reverse transcription polymerase chain reaction (RT-qPCR) experiments, 6 women with normal BMI and 6 women with BMI ≥35 kg/m2 were cultured in either cumulus cell media alone (untreated group), media plus 10 ng/mL of IL-10 (R&D Systems), or media plus 1 ng/mL of IL-1β (EMD Millipore) at 37°C with 5% CO2 for 4 hours. Conditioned media cultures were performed with cumulus cells from 7 women with normal BMI by treating the cells with either cumulus cell media alone (untreated group) or with media plus 5% obese follicular fluid. Three different obese follicular fluid samples were used for the conditioned media experiments which included the following BMI: 35.6 kg/m2, 41.7 kg/m2, and 42.0 kg/m2. Cumulus cells were incubated for 4 hours following conditioned media treatment or media alone (control) at 37°C with 5% CO2 prior to RT-qPCR.
For the flow cytometry experiments, the same conditioned media culture treatments were performed on cumulus cells from 5 different women with normal BMI. This time, protein levels were measured and compared between the untreated and obese follicular fluid treated groups following incubation for 24 hours. The conditioned media culture treatments were similarly performed for the confocal microscopy experiments utilizing cumulus cells from 3 different women with normal BMI.
RNA isolation and RT-qPCR
Total RNA was extracted from cumulus cells using RNeasy Micro Kit (Qiagen, Germany). After extraction, complementary DNA (cDNA) was synthesized using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Nucleic acids were quantified by NanoDrop (Thermo Fisher Scientific, Waltham, MA) and 250 ng/μL was used for RT-qPCR. Forward and reverse oligonucleotide primers for GREM1, HAS2, PTGS2, VCAN, and GAPDH were used (Integrated DNA Technologies, Coralville, IA; Supplemental Table 1). In each reaction, 1 μL of cDNA, 5 μL of SYBR Green master mix (Applied Biosystems), 0.5 μL forward and reverse primers, and 3.5 μL of nuclease free water were added for a final volume of 10 μL. PCR cycling conditions were 50°C for 2 minutes and 95°C for 2 minutes, followed by 40 amplification cycles at 95°C for 15 seconds, 55°C for 15 seconds, and 72°C for 1 minute. Samples without cDNA were added as negative controls. All samples were run in duplicate and gene expression profiles were determined by using the ΔΔCt method. GAPDH was used as a control. For each gene of interest, melt curves were generated after each RT-qPCR run. Amplification was validated by melt curves and confirmed by running PCR products on a 1.2% agarose gel.
Flow cytometry
Following treatment with media or 5% obese follicular fluid for 24 hours, cumulus cells were fixed with 2% paraformaldehyde in a 96-well plate and washed with flow buffer containing phosphate-buffered saline (PBS), 0.1% bovine serum albumin, and 0.5% sodium azide. Saponin was used for cell permeabilization and immunostaining was performed with 25 μg/mL goat anti-human/mouse gremlin antibody (R&D Systems). Incubation with secondary antibody was performed using 4 μg/mL Alexa Fluor-647 conjugated rabbit anti-goat antibody (Invitrogen, Carlsbad, CA). Incubations were performed at 4°C for 30 minutes and cells were washed two times. Controls included negative and isotype matched antibodies. Samples and analyses were performed using the Guava easyCyte HT cytometer (EMD Millipore). The flow cytometry gating strategy is demonstrated in Supplemental Figure 1.
Immunofluorescence cell staining and confocal microscopy
Following treatment with media alone or 5% obese follicular fluid, cumulus cells were plated on coverslips and incubated overnight at 37°C. The cells were fixed with 4% paraformaldehyde for 15 minutes and incubated with 1 μg/mL Alexa Fluor-488 conjugated wheat germ agglutinin (WGA, Thermo Fisher Scientific) to stain the plasma membrane for 30 minutes at 37°C. Cell permeabilization was performed with 0.1% Triton X-100 for 15 minutes. After washing the cells with Dulbecco PBS (DPBS, Corning, Corning, NY), immunostaining for GREM1 was performed with 25 μg/mL goat anti-human/mouse gremlin antibody (R&D Systems) overnight at 4°C. The cells were washed with DPBS and incubated with secondary antibody using 4 μg/mL Alexa Fluor-647 conjugated rabbit anti-goat antibody (Invitrogen) at room temperature for one hour. Following another wash with DPBS, the cells were mounted on ProLong Gold Antifade Mountant with 4’,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific). Imaging was performed with an upright resonant laser scanning confocal microscope (Nikon Fast A1R+, 60x objective). For each woman, we obtained 9–12 images per treatment group. Fluorescence intensity of GREM1 per cell was measured by Icy image processing software.
Statistical analyses
Patient demographics, cycle characteristics, and cycle outcomes were compared using Wilcoxon rank sum and Fisher exact test for continuous and categorical variables, respectively. Relative gene expression was compared between normal BMI and BMI ≥35 kg/m2 using Mann Whitney U test. Cytokine concentrations in the follicular fluid were also compared between the BMI groups in a similar manner. Values are reported as medians with first and third interquartile ranges. Changes in gene and protein expression between the untreated and treated groups were analyzed by paired t tests and are reported as means with standard deviations. Two sided alpha was set at 0.05. Clinical outcomes with missing data were not included in the analyses. Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA) and JMP Pro 14.1.0 (SAS Institute, Cary, NC).
Results:
Patient demographics, cycle characteristics, and cycle outcomes between normal BMI and BMI ≥35 kg/m2 women
Baseline demographics and cycle characteristics between women with normal BMI and BMI ≥35 kg/m2 are demonstrated in Table 1. The age at retrieval was similar between the groups, with the median age being 32 years in the normal BMI group and 34 years in the BMI ≥35 kg/m2 group. However, compared to women with a normal BMI, women with BMI ≥35 kg/m2 were more likely to be nulliparous (17.1% vs 54.6%, p=0.02) and have lower anti-müllerian hormone levels (4.5 ng/mL [2.6–7.6] vs 2.0 ng/mL [0.9–3.0], p=0.02). Other measures of ovarian reserve and reason for undergoing ICSI were similar between the groups. Additionally, there were no significant differences in cycle characteristics.
Table 1.
Patient demographics and cycle characteristicsa
| Normal BMI N=35 |
BMI ≥35 kg/m2 N=11 |
P-value | |
|---|---|---|---|
| Patient demographics | |||
| Age, years | 32 (29–35) | 34 (32–41) | 1.00 |
| White race, n (%) | 26 (74.3) | 10 (90.9) | 0.41 |
| Nulliparous, n (%) | 6 (17.1) | 6 (54.6) | 0.02 |
| Follicle stimulating hormone, IU/L | 7.0 (6.0–8.8) | 6.5 (5.8–7.4) | 0.24 |
| Missing, n | 2 | 1 | |
| Anti-müllerian hormone, ng/mL | 4.5 (2.6–7.6) | 2.0 (0.9–3.0) | 0.02 |
| Missing, n | 1 | 0 | |
| Antral follicle count, n | 21 (11–29) | 18 (10–23) | 0.72 |
| Diminished ovarian reserve, n (%) | 2 (5.7) | 2 (18.2) | 0.24 |
| Endometriosis, n (%) | 1 (2.9) | 0 | 1.00 |
| Polycystic ovarian syndrome, n (%) | 6 (17.1) | 1 (9.1) | 1.00 |
| Unexplained, n (%) | 11 (31.4) | 3 (27.3) | 1.00 |
| Male factor, n (%) | 11 (31.4) | 6 (54.6) | 0.28 |
| Preimplantation genetic testing, n (%) | 8 (22.9) | 4 (36.4) | 0.44 |
| Cycle characteristics | |||
| Combination oral contraceptive priming, n (%) | 32 (91.4) | 10 (90.9) | 1.00 |
| Antagonist protocol, n (%) | 33 (94.3) | 11 (100.0) | 1.00 |
| Total stimulation days, n | 11 (10–13) | 11 (10–12) | 0.99 |
| Total gonadotropin dose, units | 2325 (2200–3900) | 3075 (2250–4500) | 0.06 |
| hCG trigger, n (%) | 29 (82.9) | 11 (100.0) | 0.31 |
| Estradiol on day of trigger, pg/mL | 1993 (1468–3097) | 2080 (1289–3312) | 0.78 |
| Total oocytes retrieved, n | 15 (10–21) | 16 (7–28) | 0.99 |
| Mature oocytes retrieved, n | 11 (9–14) | 13 (7–19) | 0.57 |
| Oocyte maturity rate, % | 78.6 (66.7–87.5) | 82.1 (66.7–92.9) | 0.47 |
| Pronuclear embryos, n | 7 (6–10) | 6 (1–15) | 0.71 |
| Missing, n | 3 | 0 | |
| Fertilization rate, % | 61.3 (51.0–80.8) | 66.7 (25.0–88.2) | 0.97 |
| Missing, n | 3 | 0 | |
| Blastocyst embryos, n | 3 (2.0–4.8) | 2 (0–5.0) | 0.40 |
| Missing, n | 3 | 0 | |
| Blastulation rate, % | 41.4 (26.8–65.6) | 33.3 (0–53.3) | 0.32 |
| Missing, n | 3 | 0 |
Values are reported as medians with first and third interquartile ranges unless otherwise specified. Data were compared by Wilcoxon rank sum test and Fisher exact test for continuous and categorical variables, respectively.
A total of 27 women with normal BMI and 9 women with BMI ≥35 kg/m2 underwent an embryo transfer (Table 2). The clinical pregnancy rate was approximately 15% lower for obese women, although this difference was not significant (37.0% vs 22.2%, p=0.69). Additionally, the live birth rate was 34.6% in the normal BMI group while none had a live birth in the BMI ≥35 kg/m2 group (p=0.07).
Table 2.
Cycle outcomes following fresh or frozen embryo transfera
| Normal BMI N=27 |
BMI ≥35 kg/m2 N=9 |
P-value | |
|---|---|---|---|
| Cycle outcomes | |||
| Fresh embryo transfer, n (%) | 15 (55.6) | 5 (55.6) | 1.00 |
| Frozen embryo transfer, n (%) | 12 (44.4) | 4 (44.4) | 1.00 |
| Positive beta hCG, n (%) | 13 (48.1) | 3 (33.3) | 0.70 |
| Clinical pregnancy, n (%) | 10 (37.0) | 2 (22.2) | 0.69 |
| Biochemical loss, n (%) | 3 (11.1) | 1 (12.5) | 1.00 |
| Missing, n | 0 | 1 | |
| Miscarriage, n (%) | 0 | 0 | - |
| Ectopic pregnancy, n (%) | 0 | 1 (12.5) | 0.23 |
| Missing, n | 0 | 1 | |
| Live birth, n (%) | 10 (34.6) | 0 | 0.07 |
| Missing, n | 0 | 1 |
Cycle outcomes are reported per transfer. Data were compared by Fisher exact test.
GREM1 gene expression is decreased in women with BMI ≥35 kg/m2
To investigate differential gene expression between cumulus cells from women with normal BMI versus women with BMI ≥35 kg/m2, relative mRNA levels were measured by RT-qPCR following culture of cumulus cells with media alone. Compared to women with normal BMI, the relative expression of GREM1 was significantly lower in women with BMI ≥35 kg/m2 (1.01 [0.66–1.40] vs 0.51 [0.38–0.74], p=0.03; Figure 1A). There was no difference in the relative expression of HAS2 (1.06 [0.65–1.78] vs 0.73 [0.49–1.17], p=0.39; Figure 1B), PTGS2 (0.58 [0.47–4.19] vs 1.54 [1.09–3.11], p=0.22; Figure 1C), or VCAN (0.93 [0.63–1.56] vs 0.88 [0.61–1.56], p=0.82; Figure 1D) in the normal BMI group compared to the BMI ≥35 kg/m2 group. These results indicate that compared to women with normal BMI, GREM1 expression is significantly decreased in women with BMI ≥35 kg/m2 undergoing controlled ovarian stimulation.
Figure 1. Relative gene expression of cumulus cells isolated from women with normal BMI and BMI ≥35 kg/m2.
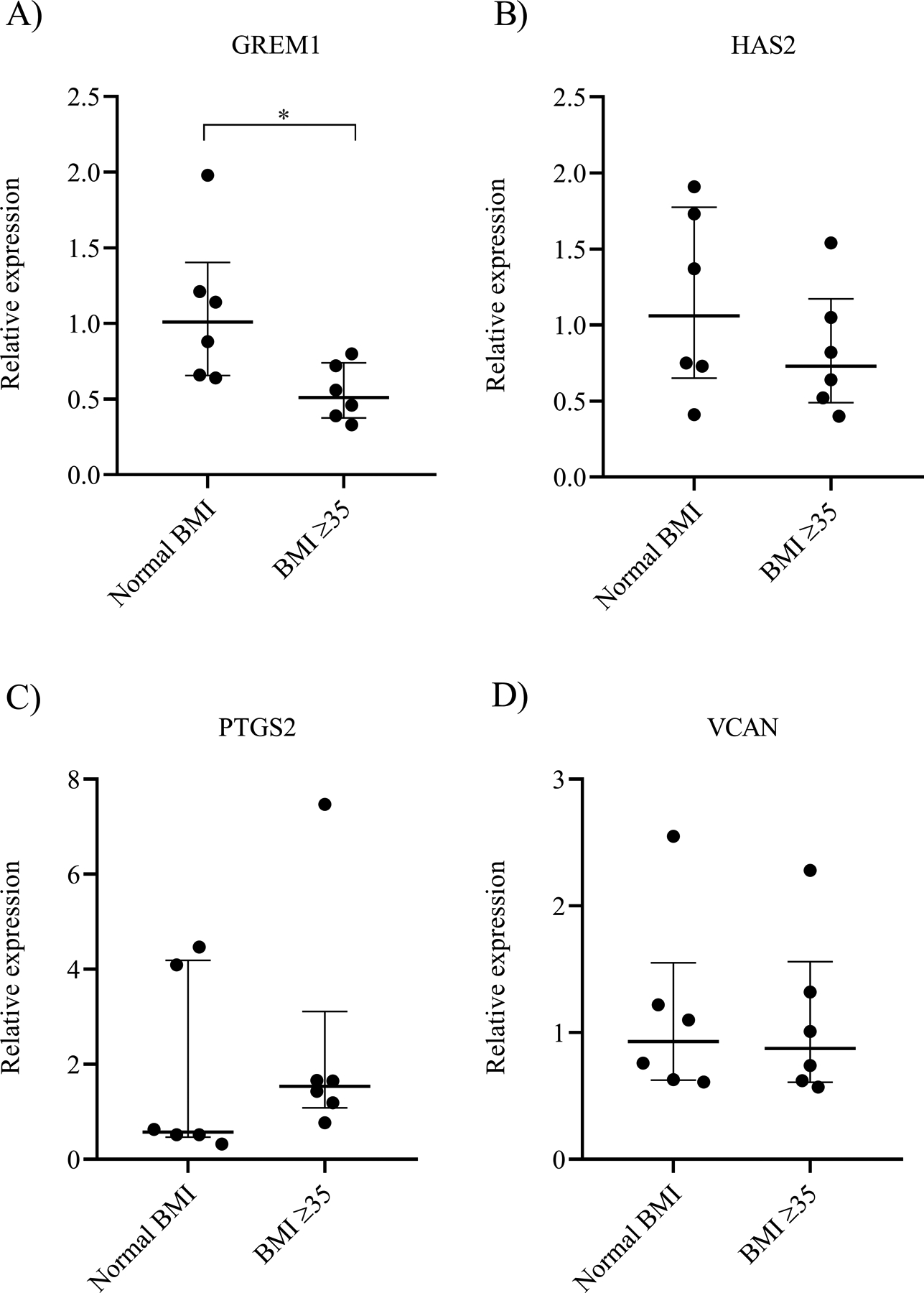
Gene expression levels of A) GREM1, B) HAS2, C) PTGS2, and D) VCAN in each cohort. The graphs represent the median value with first and third interquartile ranges. Data were compared by Mann Whitney U test (N=6 women/group). * indicates p<0.05.
GREM1 gene expression decreases following conditioned media treatment
To examine how cumulus cell expression may be affected when exposed to an obese follicular fluid environment, conditioned media cultures were performed by treating cumulus cells isolated from normal BMI women with follicular fluid from women with BMI ≥35 kg/m2. The relative gene expression cultured with or without treatment was measured with RT-qPCR and compared. Following treatment, the relative expression of GREM1 from cumulus cells of normal BMI women significantly decreased (1.11±0.62 vs 0.51±0.22, p=0.014; Figure 2A). The relative expression of HAS2 (1.11±0.47 vs 1.57±0.81, p=0.08; Figure 2B), PTGS2 (1.64±1.75 vs 3.40±3.75, p=0.10; Figure 2C), and VCAN (1.14±0.72 vs 1.32±1.35, p=0.53; Figure 2D) did not change consistently with and without treatment of obese follicular fluid. From this experiment, we demonstrated that GREM1 gene expression in women with normal BMI can be suppressed when exposed to an obese follicular environment. This suggests that factors present in the follicular fluid of obese women may be responsible for the differential expression of GREM1 observed between women with normal BMI and BMI ≥35 kg/m2.
Figure 2. Relative gene expression of cumulus cells isolated from women with normal BMI following treatment with obese follicular fluid.
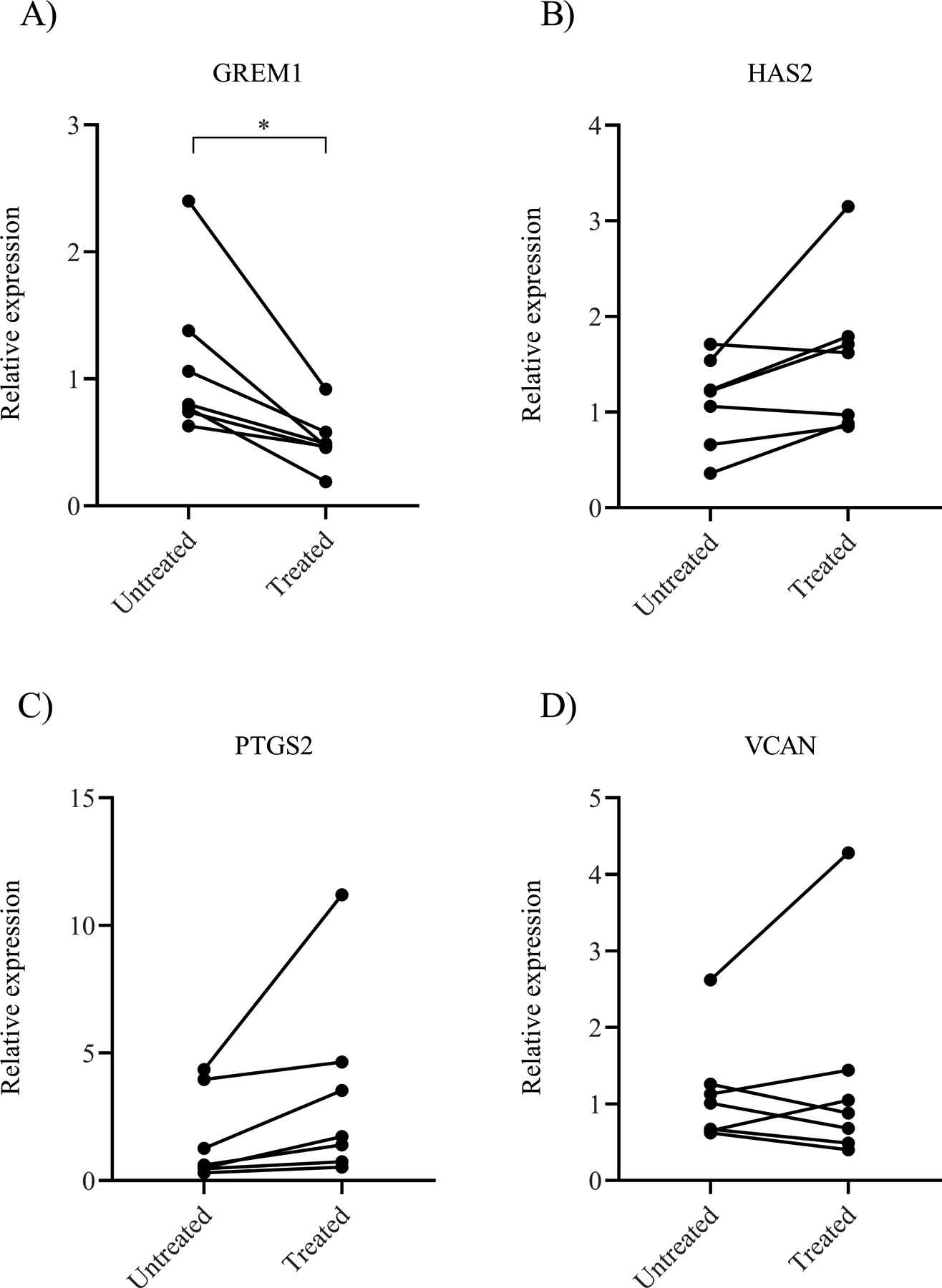
Gene expression levels of A) GREM1, B) HAS2, C) PTGS2, and D) VCAN in untreated compared to treated samples. Data were compared by paired t test (N=7 women). * indicates p<0.05.
GREM1 protein levels decrease following conditioned media treatment
To explore whether the observed decrease in GREM1 transcripts following conditioned media treatment also implied decreased translation, GREM1 protein levels were measured from cumulus cells of women with a normal BMI following treatment with obese follicular fluid. GREM1 protein levels were measured utilizing flow cytometry. Following treatment, GREM1 protein levels significantly decreased in women with a normal BMI. This was evident by the decrease in mean fluorescence intensity (MFI) of GREM1 when comparing untreated to treated groups (12.5 103±0.77 103 vs 10.7 103±0.51 103, p<0.001; Figure 3A) and a decrease in GREM1 percent positivity (0.68±0.05 vs 0.52±0.09, p=0.002; Figure 3B). The decrease in GREM1 signal intensity can be appreciated in the representative flow cytometry histogram (Figure 3C). Immunofluorescent staining further validated changes in GREM1 following treatment with obese follicular fluid. Figure 4A depicts a representative confocal microscopy image demonstrating visualization of the GREM1 decrease at a cellular level. Figure 4B demonstrates the difference in normalized MFI of GREM1 between the untreated and obese follicular fluid treated groups (1.0±0.16 vs 0.25±0.26, p<0.001). These results indicate that factors present in the follicular fluid of obese women are capable of decreasing both GREM1 transcription and translation.
Figure 3. GREM1 protein levels in cumulus cells isolated from women with normal BMI following treatment with obese follicular fluid.
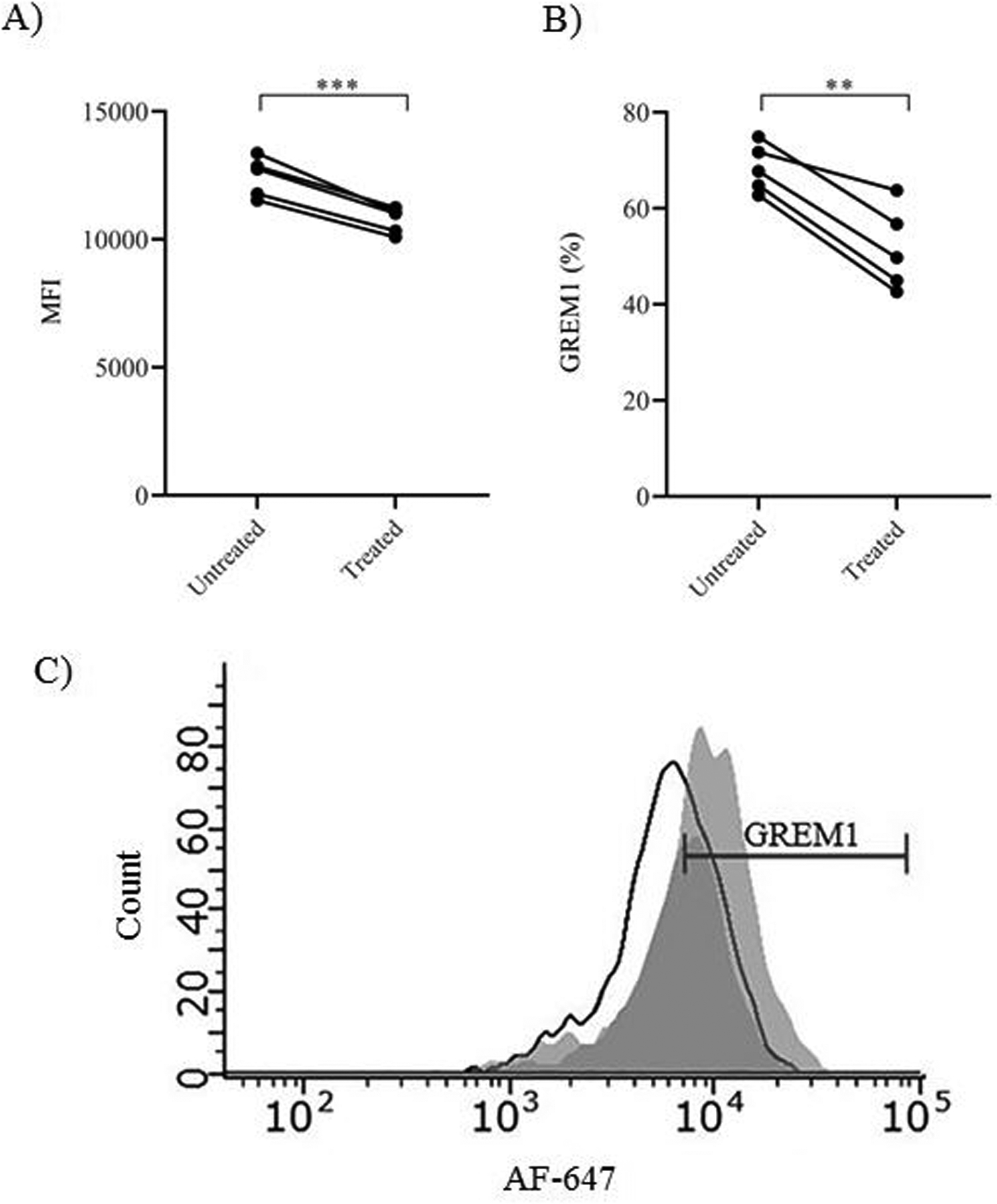
A) MFI of GREM1. B) Percentage of GREM1 positive cells. C) Representative flow cytometry histogram of GREM1. Dark gray peak represents treatment with obese follicular fluid, light gray peak represents no treatment, and black outline represents isotype. Data were compared by paired t test (N=5 women). ** indicates p<0.01. *** indicates p<0.001.
Figure 4. Confocal microscopy of GREM1 in cumulus cells isolated from women with normal BMI following treatment with obese follicular fluid.
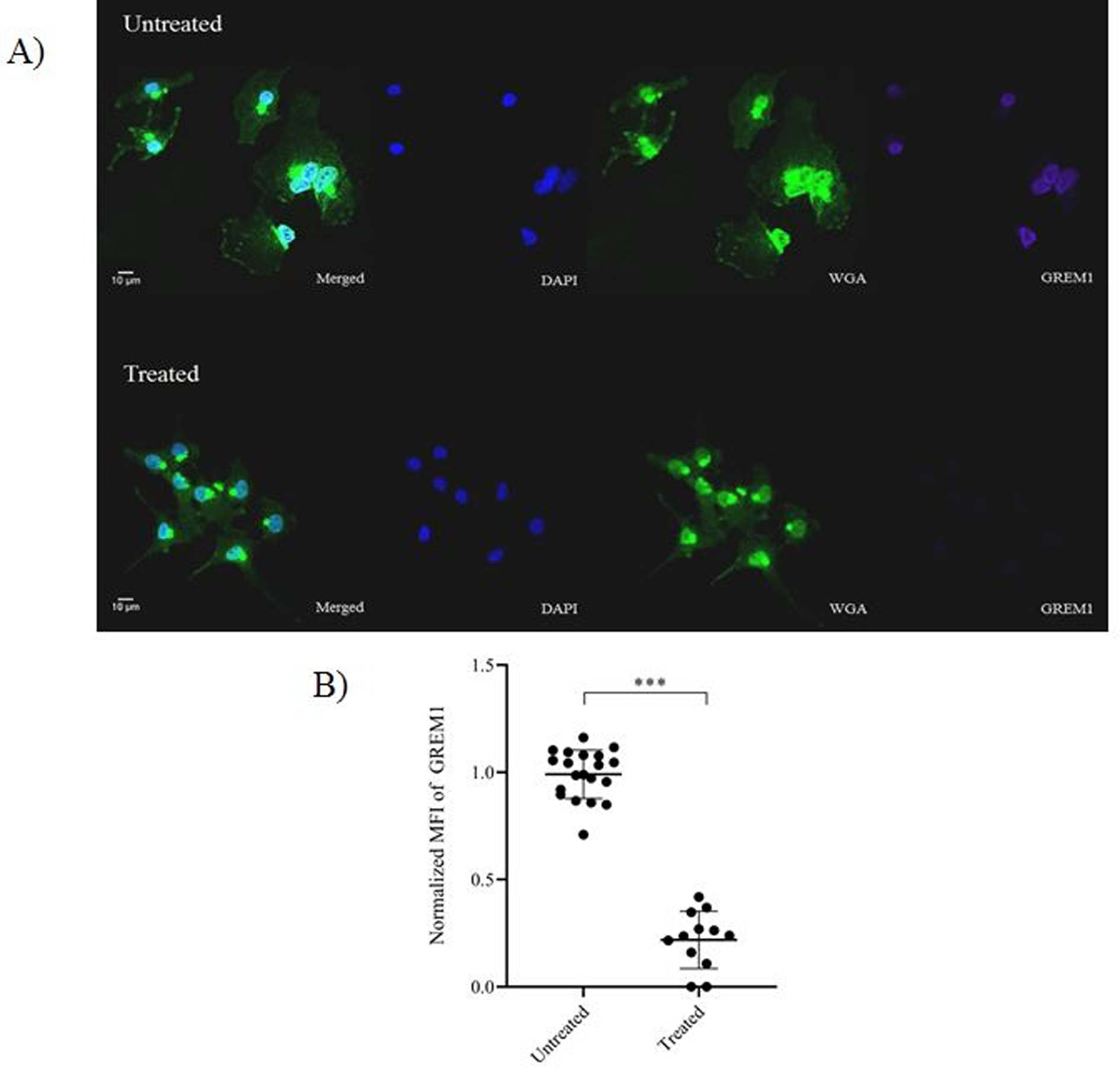
A) Representative image depicting GREM1 intensity in cumulus cells following treatment with obese follicular fluid. Nucleus was stained with DAPI (blue). Glycocalyx on the plasma membrane was stained with Alexa Fluor-488 conjugated wheat germ agglutinin (green). GREM1 was stained with Alexa Fluor-647 (purple). The bar indicates 10 μm. B) Comparison of normalized MFI of GREM1 between untreated and obese follicular fluid treated cumulus cells. Data were compared by paired t test (N=3 women with 9–12 images per treatment condition). *** indicates p<0.001.
Women with BMI ≥35 kg/m2 have higher IL-10 and IL-1β in the follicular fluid
To identify which inflammatory markers in the follicular fluid may be contributing to decreased GREM1 levels in the BMI ≥35 kg/m2 group, cytokine concentrations were measured with multiplex bead assay. When cytokine concentrations were compared between normal BMI and BMI ≥35 kg/m2, we found IL-10 and IL-1β to be significantly elevated with higher BMI (Table 3). Compared to women with normal BMI, women with BMI ≥35 kg/m2 had significantly higher levels of IL-10 (9.46 pg/mL [0.59–19.16] vs 53.39 pg/mL [14.97–236.37], p=0.004) and IL-1β (1.92 pg/mL [1.92–5.18] vs 5.18 pg/mL [1.92–16.33], p=0.017). Based on these results, we focused on the potential role of inflammatory cytokines IL-10 and IL-1β on cumulus cell signaling through GREM1, HAS2, PTGS2, and VCAN expression.
Table 3.
Comparison of follicular fluid cytokines in women with normal BMI and BMI ≥35 kg/m2
| Normal BMI N=35 |
BMI ≥35 N=11 |
P-value | |
|---|---|---|---|
| Cytokine | |||
| IL-10, pg/mLa | 9.46 (0.59–19.16) |
53.39 (14.97–236.37) |
0.004 |
| IL-1β, pg/mLa | 1.92 (1.92–5.18) |
5.18 (1.92–16.33) |
0.017 |
Values are reported as medians with first and third interquartile ranges. Data were compared by Mann Whitney U test.
IL-10 and IL-1β decrease GREM1 gene expression in women with normal BMI
To investigate whether IL-10 or IL-1β in the follicular fluid influences gene expression of GREM1, HAS2, PTGS2, or VCAN in women with BMI ≥35 kg/m2, cumulus cells from women with normal BMI were treated with either IL-10 or IL-1β and compared to cultured, untreated controls. Following IL-10 treatment, the relative gene expression of GREM1 significantly decreased (1.09±0.50 vs 0.70±0.31, p=0.04). The relative expression of HAS2 (1.15±0.61 vs 1.34±1.01, p=0.74), PTGS2 (1.76±1.96 vs 1.46±1.46, p=0.75), and VCAN (1.15±0.73 vs 0.95±0.57, p=0.07) did not significantly change between untreated and IL-10 treated cumulus cells (Figure 5A).
Figure 5. Relative gene expression of cumulus cells isolated from women with normal BMI following treatment with IL-10 or IL-1β.
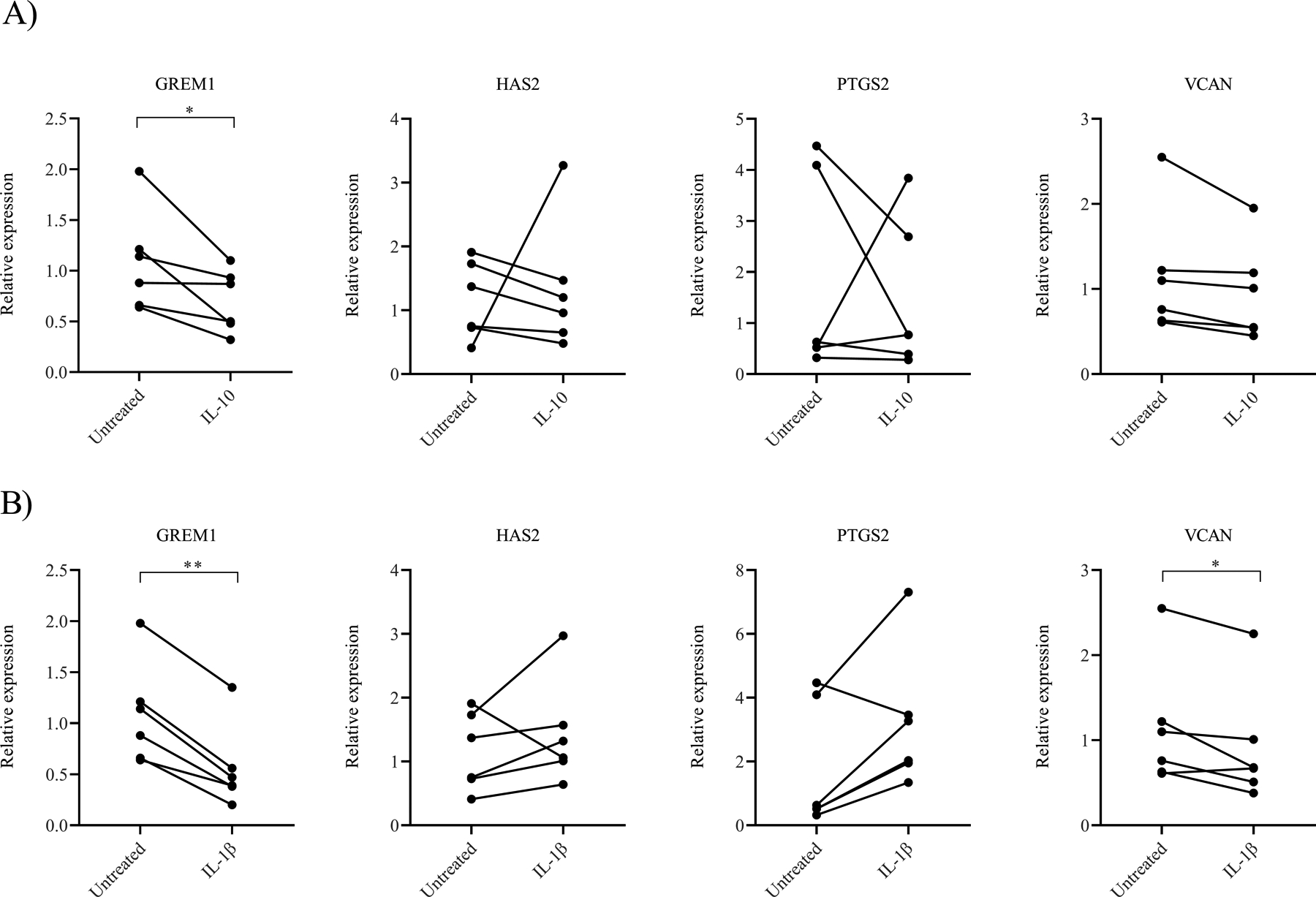
A) Gene expression following IL-10 treatment of cumulus cells from women with a normal BMI. B) Gene expression following IL-1β treatment of cumulus cells from women with a normal BMI. Data were compared by paired t test (N=6 women). * indicates p<0.05. ** indicates p<0.01.
When cumulus cells were treated with IL-1β, the relative gene expression of GREM1 similarly decreased (1.09±0.50 vs 0.56±0.41, p=0.001). The relative expression of VCAN was also significantly lower following IL-1β treatment (1.15±0.73 vs 0.92±0.69, p=0.04). HAS2 (1.15±0.61 vs 1.43±0.82, p=0.36) and PTGS2 (1.76±1.96 vs 3.23±2.16, p=0.06) did not change significantly between the untreated and treated samples (Figure 5B). These results imply that cytokines IL-10 and IL-1β, which are present at high concentrations in the follicular fluid of women with BMI ≥35 kg/m2, have a role in suppressing GREM1 gene expression. Additionally, elevated IL-1β levels may be involved in regulating VCAN gene expression as well. Interestingly, when cumulus cells from women with BMI ≥35 kg/m2 were treated with either IL-10 or IL-1β, we did not see significant changes in gene expression (Supplemental Figure 2A and 2B, respectively). These results suggest that additional exposure to either IL-10 or IL-1β cannot further suppress GREM1 expression in women with BMI ≥35 kg/m2.
Discussion:
Obese women have suboptimal outcomes following ART compared to women with normal BMI (2–6). Despite increasing trends in both ART and obesity in reproductive aged women, very little is known regarding what molecular alterations are responsible for the differences in clinical outcomes observed in obese women undergoing assisted reproduction. The etiology is likely multifactorial, but many hypothesize that aberrations to the immune system in the obese population is a partial explanation for this complex pathophysiology (7, 8). We found higher follicular fluid levels of IL-10 and IL-1β as well as decreased expression of GREM1, a surrogate molecule associated with better quality embryos and higher pregnancy rates following ICSI, in women with BMI ≥35 kg/m2. Furthermore, we demonstrate that exposure to high levels of IL-10 and IL-1β can decrease cumulus cell expression of GREM1 in women with normal BMI. This suggests that inflammation in the follicular fluid of obese women is affecting the cumulus oocyte complex, and this change to the microenvironment significantly impacts GREM1 gene expression. Therefore, these cytokines may play a critical role in cumulus cell signaling and may modulate ICSI outcomes in obese women.
GDF9 is vital to normal oocyte development because it regulates follicular maturation and cumulus expansion (20, 29). Given the integral role of GDF9, dysregulation of its downstream targets may represent an underlying deficiency of the oocyte (18). GREM1 is a BMP antagonist and has been shown to be involved in several different processes including regulating embryonic development (30, 31). The function of GREM1 in the ovary is not well understood, but selective inhibition of BMP signaling may direct GDF9 towards cumulus expansion during ovulation (29). When comparing GREM1, HAS2, and PTGS2, McKenzie et al. found GREM1 to be the single best predictor of oocyte maturity, fertility, and embryo quality (18). Other studies similarly found higher levels of GREM1 transcripts in cumulus cells surrounding oocytes that developed into high quality embryos compared to low quality embryos or oocytes that failed to fertilize (25, 27). Higher expression of GREM1 has also been associated with successful pregnancies following ICSI (26). We found significantly lower levels of GREM1 in women with BMI ≥35 kg/m2 compared to women with normal BMI. Our findings corroborate available literature regarding suboptimal clinical outcomes observed with obesity and with decreased GREM1 expression.
Our study found an association between higher levels of pro-inflammatory cytokine IL-1β and obesity. Additionally, when cumulus cells from women with a normal BMI were treated with high concentrations of IL-1β, the relative gene expression of GREM1 significantly decreased. Interestingly, VCAN expression also significantly decreased following treatment with IL-1β, which could be due to shared signaling pathways with GREM1. IL-1β is essential for mediating ovulation; however, derangements to IL-1β and other inflammatory factors can be detrimental (32, 33). Significantly lower levels of IL-1β have been reported in women with tubal factor infertility, suggesting that irregular IL-1β concentrations may indicate abnormal folliculogenesis (14). Although BMI was not specifically compared, Mendoza et al. found higher follicular fluid IL-1 levels were associated with lower pregnancy success (34). Another study found elevated levels of follicular fluid IL-18 concentrations with increasing BMI (35). Although we did not specifically measure IL-18, this pro-inflammatory cytokine is closely related to IL-1β and shares similar functions (36).
We found high levels of IL-10 also decrease GREM1 expression in cumulus cells. IL-10 is classically considered an anti-inflammatory cytokine, but its role in reproductive processes is not well understood (37). As a potent immunosuppressant molecule, IL-10 can inhibit the synthesis of various pro-inflammatory cytokines including that of IL-1β, TNF-α, and IL-6 (38). One possible explanation for IL-10 increase in obese women may be compensation for inflated pro-inflammatory responses, such as IL-1β release. However, our results suggest excess IL-10 in the follicular fluid is also negatively impacting to GREM1 expression. Contrary to our study, La Vignera et al. included women with BMI up to 39.9 kg/m2 and did not find elevated IL-10 levels in follicular fluid of obese women (12). La Vignera et al included women having a BMI up to 39.9 kg/m2 with a total 6 women having a BMI between 35.0 to 39.9 kg/m2. Our study included women with BMI up to 42.0 kg/m2 with 6 women having BMI between 35.0 to 39.9 kg/m2 and 5 women having BMI between 40.0 to 42.0 kg/m2. Our data demonstrates continuing elevation of IL-10 levels with BMI into the 40’s (39.55 pg/mL [13.76–103.67] in BMI 35.0 to 39.9 kg/m2 vs 236.37 pg/mL [22.81–420.38] in BMI 40.0 to 42.0 kg/m2). Thus, the nearly double increase in sample size and the inclusion of women with BMI beyond 39.9 kg/m2 in our study may explain this difference.
A separate study looking at the association of both IL-10 and IL-1β found that women with significantly higher levels of both cytokines in the follicular fluid were less likely to get pregnant compared to women who had lower levels (39). This study complements our theory that perhaps decreased GREM1, as a result of elevated IL-10 and IL-1β, is partly responsible for the worse clinical outcomes in obese women undergoing ICSI. Although we cannot conclude that elevation of these cytokines is associated with suboptimal pregnancy outcomes given our small sample size, a trend towards worse cycle outcomes following embryo transfer was observed in our obese cohort of women. Importantly, when cumulus cells from women with BMI ≥35 kg/m2 were treated with high levels of either IL-10 or IL-1β, no change to GREM1 gene expression was observed. We hypothesize that the follicular environment in the setting of obesity is already chronically inflamed; therefore, treatment with additional IL-10 or IL-1β may not provoke significant changes to GREM1 expression.
Our study is not without limitations. As human samples were utilized, the cumulus cells are heterogeneous due to inherent patient differences. Therefore, we utilized paired sampling to compare changes before and after treatment for each woman. Another limitation is that the follicular fluid was collected from only the first follicle and may not be representative of the entire oocyte microenvironment in the ovary. However, this strategy limited blood contamination during the collection. Additionally, cumulus cells were collected from all retrieved oocytes in a given patient including both mature and immature oocytes. The rationale for this collection method was to limit deviation from the embryologists’ current clinical protocol at our institution. A limitation of the conditioned media experiments is the exclusion of an additional control group in which cumulus cells from normal BMI women were treated with normal BMI follicular fluid. Due to a limited number of total cumulus cell available per patient, we were unable to perform these experiments. We hypothesize that no change would be observed as cumulus cells from normal BMI women are exposed to normal BMI follicular fluid in vivo as this is the environment these cells are accustom to. Future experiments will address this and include non-biased proteomic analyses which may identify other factors influencing gene expression. Finally, due to the small sample size, we were unable to adjust for possible confounding factors such as nulliparity and AMH. With the small sample size, effects on embryo quality and clinical correlations cannot be concluded. However, given the infancy of our current understanding on the pathophysiology of the immune system and ART outcomes, our findings remain an important addition to the scientific literature.
Conclusions:
With the paucity of data on how inflammation affects ART outcomes, specifically in the obese population, our study aids in characterizing the interaction between the immune and reproductive systems. Specifically, BMI ≥35 kg/m2 is associated with high levels of cytokines IL-10 and IL-1β in the follicular fluid which alters GREM1 expression in cumulus cells. To our knowledge, this is the first study connecting follicular fluid cytokine levels to cumulus cell gene expression in the obese population. At a molecular level, derangements to the immune system resulting in decreased GREM1 may help explain the suboptimal pregnancy outcomes observed in obese women.
Supplementary Material
Supplemental Figure 1. Flow cytometry gating strategy. Cumulus cells were gated on single cells determined by FSC-A versus FSC-H, then gated on live cells by SSC-A versus FSC-A. Cells that were positive for GREM1 were determined by comparing GREM1-APC by FCS-A.
Supplemental Figure 2. Relative gene expression of cumulus cells isolated from women with BMI ≥35 kg/m2 following treatment with IL-10 and IL-1β. A) Gene expression following IL-10 treatment of cumulus cells from women with BMI ≥35 kg/m2. B) Gene expression following IL-1β treatment of cumulus cells from women with BMI ≥35 kg/m2. Data were compared by paired t test (N=6 women). No statistical differences were seen.
Acknowledgements:
We thank the members of the Division of Reproductive Endocrinology and Infertility and the embryologists at Mayo Clinic, Rochester, MN.
Funding support: This study was financially supported by internal research funding from the Department of Obstetrics and Gynecology at Mayo Clinic (TK) and by a grant from the NIH/NICHD K12 HD065987 (EE). The authors do not have financial interests to disclose.
Footnotes
Disclosures: The authors report no conflict of interests.
References:
- 1.SART National Summary Report 2017.
- 2.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Hum Reprod Update 2007;13:433–44. [DOI] [PubMed] [Google Scholar]
- 3.Arabipoor A, Ashrafi M, Hemat M, Zolfaghari Z. The Effects of Maternal and Paternal Body Mass Index on Live Birth Rate after Intracytoplasmic Sperm Injection Cycles. Int J Fertil Steril 2019;13:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudesia R, Wu H, Hunter Cohn K, Tan L, Lee JA, Copperman AB et al. The effect of female body mass index on in vitro fertilization cycle outcomes: a multi-center analysis. J Assist Reprod Genet 2018;35:2013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasim K, Roshdy A. Body mass index and pregnancy outcome after assisted reproduction treatment. Int J Reprod Med 2014;2014:257974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update 2019. [DOI] [PubMed] [Google Scholar]
- 7.Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol 2011;88:142–8. [DOI] [PubMed] [Google Scholar]
- 8.Boots CE, Jungheim ES. Inflammation and Human Ovarian Follicular Dynamics. Semin Reprod Med 2015;33:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu LL, Norman RJ, Robker RL. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Reprod Fertil Dev 2011;24:29–34. [DOI] [PubMed] [Google Scholar]
- 10.Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010;151:5438–45. [DOI] [PubMed] [Google Scholar]
- 11.Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab 2009;94:1533–40. [DOI] [PubMed] [Google Scholar]
- 12.La Vignera S, Condorelli R, Bellanca S, La Rosa B, Mousavi A, Busa B et al. Obesity is associated with a higher level of pro-inflammatory cytokines in follicular fluid of women undergoing medically assisted procreation (PMA) programs. Eur Rev Med Pharmacol Sci 2011;15:267–73. [PubMed] [Google Scholar]
- 13.Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril 2011;95:1970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Bene MC, de Carvalho Bittencourt M et al. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol 2012;2012:606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimand K, Talja I, Metskula K, Kadastik U, Matt K, Uibo R. Autoantibody studies of female patients with reproductive failure. J Reprod Immunol 2001;51:167–76. [DOI] [PubMed] [Google Scholar]
- 16.Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod 2010;16:531–8. [DOI] [PubMed] [Google Scholar]
- 17.Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev 2002;61:414–24. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod 2004;19:2869–74. [DOI] [PubMed] [Google Scholar]
- 19.van Montfoort AP, Geraedts JP, Dumoulin JC, Stassen AP, Evers JL, Ayoubi TA. Differential gene expression in cumulus cells as a prognostic indicator of embryo viability: a microarray analysis. Mol Hum Reprod 2008;14:157–68. [DOI] [PubMed] [Google Scholar]
- 20.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999;13:1035–48. [DOI] [PubMed] [Google Scholar]
- 21.Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update 2003;9:35–48. [DOI] [PubMed] [Google Scholar]
- 22.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996;383:531–5. [DOI] [PubMed] [Google Scholar]
- 23.Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 2000;25:279–83. [DOI] [PubMed] [Google Scholar]
- 24.Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril 2011;96:47–52 e2. [DOI] [PubMed] [Google Scholar]
- 25.Cillo F, Brevini TA, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction 2007;134:645–50. [DOI] [PubMed] [Google Scholar]
- 26.Wathlet S, Adriaenssens T, Segers I, Verheyen G, Van de Velde H, Coucke W et al. Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod 2011;26:1035–51. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RA, Sciorio R, Kinnell H, Bayne RA, Thong KJ, de Sousa PA et al. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction 2009;138:629–37. [DOI] [PubMed] [Google Scholar]
- 28.Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod 2007;22:3069–77. [DOI] [PubMed] [Google Scholar]
- 29.Pangas SA, Jorgez CJ, Matzuk MM. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem 2004;279:32281–6. [DOI] [PubMed] [Google Scholar]
- 30.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 2003;34:303–7. [DOI] [PubMed] [Google Scholar]
- 31.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1998;1:673–83. [DOI] [PubMed] [Google Scholar]
- 32.Vassiliadis S, Relakis K, Papageorgiou A, Athanassakis I. Endometriosis and infertility: a multi-cytokine imbalance versus ovulation, fertilization and early embryo development. Clin Dev Immunol 2005;12:125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet 2018;35:735–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod 2002;17:1017–22. [DOI] [PubMed] [Google Scholar]
- 35.Gunther V, Alkatout I, Fuhs C, Salmassi A, Mettler L, Hedderich J et al. The Role of Interleukin-18 in Serum and Follicular Fluid during In Vitro Fertilization and Intracytoplasmic Sperm Injection. Biomed Res Int 2016;2016:6379850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–50. [DOI] [PubMed] [Google Scholar]
- 37.Geva E, Lessing JB, Lerner-Geva L, Azem F, Yovel I, Ben-Yosef D et al. Interleukin-10 in preovulatory follicular fluid of patients undergoing in-vitro fertilization and embryo transfer. Am J Reprod Immunol 1997;37:187–90. [DOI] [PubMed] [Google Scholar]
- 38.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991;174:1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar YPS, Khan MA, Husain SA, Prasad S, Sharma S. Ovarian interleukin profile and pregnancy outcome in women undergoing assisted reproduction: A prospective study. Fertil Sci Res 2017;4:93–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow cytometry gating strategy. Cumulus cells were gated on single cells determined by FSC-A versus FSC-H, then gated on live cells by SSC-A versus FSC-A. Cells that were positive for GREM1 were determined by comparing GREM1-APC by FCS-A.
Supplemental Figure 2. Relative gene expression of cumulus cells isolated from women with BMI ≥35 kg/m2 following treatment with IL-10 and IL-1β. A) Gene expression following IL-10 treatment of cumulus cells from women with BMI ≥35 kg/m2. B) Gene expression following IL-1β treatment of cumulus cells from women with BMI ≥35 kg/m2. Data were compared by paired t test (N=6 women). No statistical differences were seen.


