ABSTRACT
Cyclophilin A is increased in the plasm of diabetic patients, while its effects on high glucose (HG)-stimulated pancreatic β-cells are still pending. The aim of this research is to investigate the effects of cyclophilin A inhibition on HG-challenged pancreatic β-cells. For investigating the effects of cyclophilin A decrease on HG-induced pancreatic β-cells, the cells were separated into normal glucose (NG), Mannitol, HG, HG + shRNA-NC, and HG + shRNA-Cyclophilin A-1 groups. The protein and mRNA expression were detected via Western blot and qRT-PCR. CCK-8 assay and flow cytometry were employed for assessing cell viability and apoptosis. The levels of oxidative stress, inflammation, and insulin secretion were detected by corresponding kits. The cyclophilin A was higher in HG group. Knockdown of cyclophilin A was able to increase insulin secretion, decrease cell apoptosis, and alleviate inflammation as well as oxidant stress in HG-treated pancreatic β-cells via MAPK/NF-kb pathway. Taken together, Cyclophilin A, highly expressed in pancreatic β-cells induced by HG, is a promising therapeutic target for diabetes. Knockdown of cyclophilin A has protective effects against HG-challenged pancreatic β-cells via regulation of MAPK/NF-kb pathway. The findings in this study provided a new strategy for diabetic treatment and paved the way for future researches on diabetes treatment.
KEYWORDS: Cyclophilin A, apoptosis, inflammation, oxidant stress, pancreatic β-cells, MAPK/NF-kb signaling pathway
Graphical Abstract
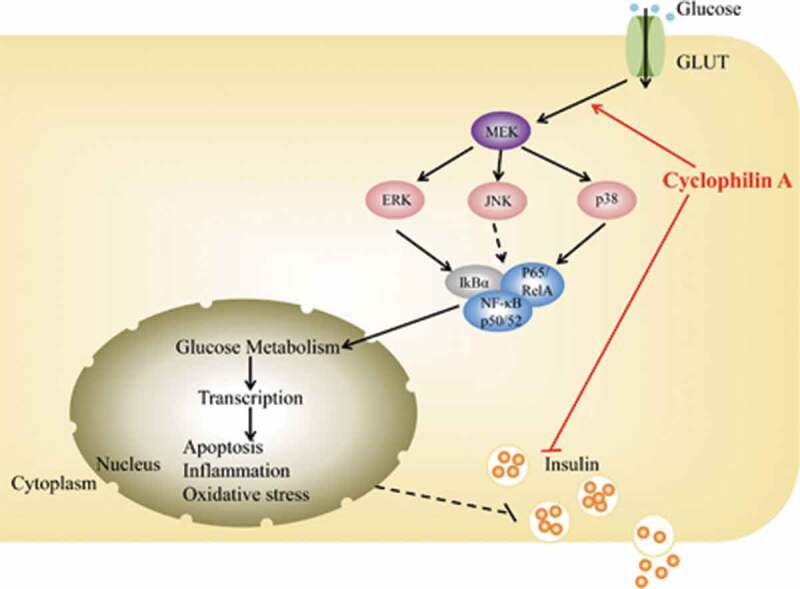
Introduction
Diabetes mellitus has been a major public health problem worldwide [1,2]. The number of the patients with diabetes is on the surge and it is projected that the diabetes patients will reach about 600 million twenty years later [3,4]. Diabetes can result in organ dysfunctions and even a series of complications, such as endothelial dysfunction, diabetic retinopathy, nephropathy, and so forth [5–9]. Therefore, it is urgent to explore the mechanism, occurrence, and development of diabetes and reveal the possible effective targets or agents for therapy of diabetes.
Type 1 diabetes (T1D) and type 2 diabetes (T2D) are the major forms of diabetes [10,11]. T1D is attributed to destruction of the pancreatic β cells or inhibition of glucose uptake [12,13]. T2D is due to pancreatic β cells function decline together with insulin resistance [14]. Ninety-five percent of diabetic patients suffered from T2D [15,16]. Hence, searching for the effective therapeutic target in T2D is beneficial for the majority of diabetic patients.
Cyclophilin A, a highly conserved protein, mainly exists in cytoplasm, regulating protein folding, transport, and cytokine secretion [17]. Besides, cyclophilin A acts as an crucial player in multiple human diseases such as atherosclerosis, hepatitis virus, cancer, cardiac diseases, and so forth [18].
The secretion of cyclophilin A can be provoked by some pathological conditions including oxidative stress, hypoxia, and inflammation [19–21]. Oxidative stress and inflammation are the major causes of diabetes. Cyclophilin A level in plasma is reported to be elevated in patients suffering from T2D, indicating the occurrence of vascular disease [22]. Diabetes is characterized by high glucose (HG) level. Cyclophilin A secretion is increased by HG and oxidant stress in diabetic nephropathy [23]. Moreover, cyclophilin A expression is also increased in HG-induced macrophages. However, the exact function of cyclophilin A in glucose-treated pancreatic β-cells remains unknown.
In the current research, our objective was to explore the possible impacts of cyclophilin A on pancreatic β-cells with HG induction and investigate its underlying mechanism. Our findings revealed that inhibition of cyclophilin A promoted insulin secretion, attenuated cell apoptosis rate, and alleviated inflammation as well as oxidant stress in pancreatic β-cells stimulated by HG. Hence, cyclophilin A may be a potential target for diabetes treatment.
Material and methods
Cell culture and treatment
The INS-1 cell line was procured from Xinzhou Biotechnology Company (Shanghai, China). The cells (1 × 105 cells/well) were grown in a 96-pore plate in RPMI 1640 medium with FBS (10%), β-mercaptoethanol (50 μM), streptomycin 100 (μg/ml) as well as penicillin (100 U/ml). The cells were cultivated in an incubator of 5% CO2 [24]. For evaluating the levels of cyclophilin A, the cells were divided into three different groups. In normal glucose (NG) group, 5 mM glucose (normal glucose concentration) was used; in Mannitol group, 33 mM Mannitol was utilized and in HG group, 33 mM glucose was applied. Cells from each group were incubated for 48 h with or without HG treatment [25]. The NG served as control and Mannitol group as an osmotic control. For screening the efficacy of plasmid downregulating cyclophilin A expression, the cells were designed into HG, HG + shRNA-NC, HG + shRNA-Cyclophilin A-1, and HG + shRNA-Cyclophilin A-2 groups. For evaluating the effects of cyclophilin A depletion on HG-treated pancreatic β-cells, the cells were designed into NG, MG, HG, HG+shRNA-NC, and HG+shRNA-cyclophilin A groups.
Cell transfection
The cells were seeded in a 6-well plate in RPMI 1640 medium (Sigma, Darmstadt, Germany). The cells were transfected with shRNA-NC, shRNA-Cyclophilin A-1 and shRNA-Cyclophilin A-2 in accordance with the manufacturer’s protocols. shRNA-NC, shRNA-Cyclophilin A-1 (5′-GCCATCGAGAAGGTTGGATCA-32032), and shRNA-Cyclophilin A-2 (5′-GGTGCAAACACGAACGGATCT-3′) mentioned above were purchased from Shanghai GenePharma company.
Western blot
The cells of different groups were lysed to collect the total proteins, as previously described [26]. The Bradford assay was performed to evaluate the protein concentrations. The proteins were separated with application of 10% SDS-polyacrylamide gel electrophoresis and next transferred onto PVDF membranes (Millipore, USA). Five percent skim milk was employed to block the membranes and subsequently the membranes were subjected to culture with primary antibodies against Cyclophilin A (#ab41684, Abcam), bcl2 (#ab196495, Abcam), Bax (#ab32503, Abcam), cleaved caspase3 (#ab49822, Abcam), caspase3 (#ab13847, Abcam), Cleaved Caspase-9 (#9509, Cell Signaling Technology), caspase 9 (#ab202068, Abcam), JNK (#ab199380, Abcam), P-JNK (#ab47337, Abcam), ERK (#ab32537, Abcam), P-ERK (#ab131438, Abcam), P38 (#ab170099, Abcam), P-P38 (#ab4822, Abcam), p-NF-KB P65 (#ab28856, Abcam), p-IKBα (#ab24783, Abcam), NF-KB P65 (#ab16502, Abcam), and IKBα (#ab32518, Abcam). Thereafter, the membranes were co-incubated with the horseradish peroxidase-conjugated secondary antibody (Cell Signal Technology). The enhanced chemiluminescence kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was utilized to detect the protein blots.
Detection by quantitative real-time PCR (qRT-PCR)
The total RNAs were harvested through TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNA was performed utilizing the PrimeScript RT Master Mix kit (Takara Bio, Inc., Otsu, Japan). ABI7900 Real-time PCR System (Applied Biosystems, Foster City, USA) was applied for qRT-PCR assay. The conditions of PCR were shown as follows: 95°C for 6 min, with 40 cycles of 95°C for 10 s, 60°C for 25 s, 70°C for 30s. The primers for cyclophilin A were: Forward 5′-CAAGGTCCCAAAGACAGCAGA-3′ and Reverse 5′-AAGATGCCAGGACCCGTATGC-3′. 2–ΔΔCT method was applied for calculation of the relative expression [27].
Cell counting kit-8 (CCK-8) assay
The cells (1 × 105) were placed on a 96-well culture dish. After treatment in the corresponding groups, the cells were incubated with CCK-8 agent (DOJINDO, Laboratories, Kumamoto, Japan) for 1 h at 37°C. Next, cell viability was determined through measuring the absorbance at 450 nm [28]. For this procedure, the microplate reader (Bio-Tek, Winooski, USA) was used.
Assessment of insulin secretion
The cells of different groups, after sonication in acid methanol, were centrifuged for 8 min at 4°C for the supernatant collection. Insulin ELISA kit (Thermo Fisher Scientific) was utilized for detecting the insulin levels, as per the manufacturer’s guidance.
Detection of inflammation factors and oxidant stress level
IL-1β, TNF-a, and IL-18 as inflammatory indicators and ROS, MPO, and SOD as indicators of oxidant stress were evaluated herein. The cells of different groups were lysed and centrifuged for collecting the supernatants. The levels of inflammation and oxidant stress were detected via the corresponding kits, following the manufacturer’s guideline.
Assessment of cell apoptosis
Flow cytometry (BD Biosciences, New Jersey, USA) was applied to detect the cell apoptosis in different groups as previously described [29]. Briefly, the cells of different groups were harvested and rinsed with PBS. Then the cells were incubated with annexin V-FITC (5 μL) and PI (5 μL) for another 20 min away from the light. After staining, the cell apoptosis was evaluated by flow cytometry.
Statistical analysis
All the experiments were repeated thrice and the obtained results were presented as mean ± SD. The GraphPad Prism 5.0 software (GraphPad Software, Inc, La Jolla, CA) was applied for data analysis. As for differences, one-way or two-way ANOVA followed by Tukey’s multiple comparison tests was conducted.
Results
The cyclophilin A level was increased by HG in pancreatic β-cells
The cyclophilin A level in NG group was nearly the same to that in Mannitol group, indicating that the cyclophilin A level was not influenced by Mannitol (Figure 1). Compared with NG group and Mannitol group, the cyclophilin A level was higher in HG group, demonstrating that the secretion of cyclophilin A was increased by HG and its inhibition may protect against HG-caused injury in pancreatic β-cells.
Figure 1.
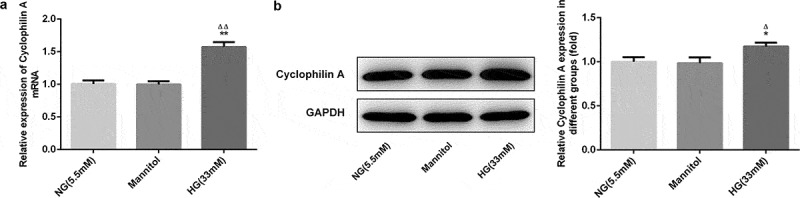
The cyclophilin A level evaluated by qRT-PCR (a) and Western blot (b) in pancreatic β-cells.
*P < 0.05 and **P < 0.01 vs. NG group, ΔP < 0.05 and ΔΔP < 0.01 vs. Mannitol group.
Knockdown of cyclophilin A increased the cell viability of HG-stimulated pancreatic β-cells
As examined by qRT-PCR, the cyclophilin A level is not alerted by shRNA-NC but downregulated efficiently by transfection of shRNA-Cyclophilin A-1 (Figure 2(a and b)). No significant changes of cell viability between NG group and Mannitol group were found, suggesting that high concentration of Mannitol had no effect on cell viability. The cell viability was reduced significantly in HG group in contrast to NG group, indicating that HG resulted in cell injury (Figure 2(c)). After knockdown of cyclophilin A, the cell viability of pancreatic β-cells in HG group was increased, confirming that knockdown of cyclophilin A have promoting effect on cell viability in pancreatic β-cells treated by HG.
Figure 2.
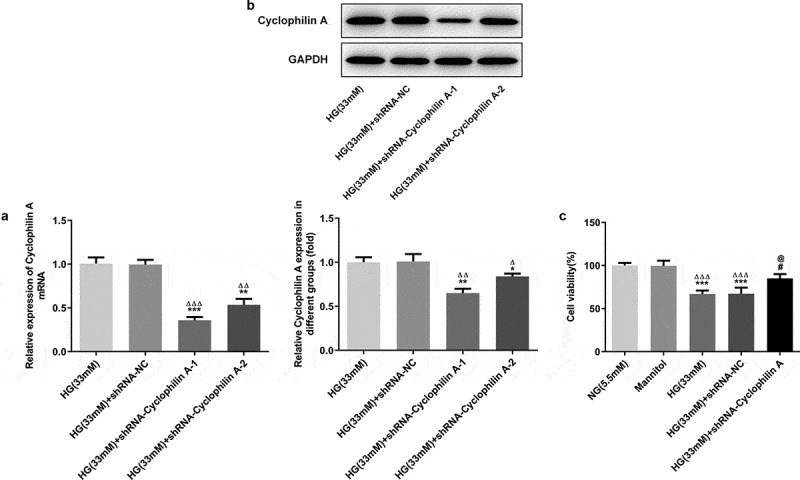
The effects of cyclophilin A silencing on the cell viability in HG-treated pancreatic β-cells.
The cyclophilin A level evaluated by PCR (a) and Western blot (b) in the study groups. The cell viability level in the study groups (c).*P < 0.05 and **P < 0.01 and ***P < 0.01 vs. HG (33 mM) group, ΔP < 0.05 and ΔΔP < 0.01 and ΔΔΔP < 0.01 vs. HG (33 mM) +shRNA-NC group (A and B); ***P < 0.001 vs. NG group, ΔΔΔP < 0.001 vs. Mannitol group, #P < 0.05 vs. HG (33 mM) group, @P < 0.05 vs. HG (33 mM) + shRNA-NC group (C)
Knockdown of cyclophilin A increased insulin secretion in HG-damaged pancreatic β-cells
As shown in Figure 3(a and b), the insulin secretion was not affected by either Mannitol or shRNA-NC. The insulin secretion was inhibited obviously by HG in contrast to NG group. Moreover, the insulin secretion decreased by HG induction was increased after cyclophilin A was knocked down. The results demonstrated that knockdown of cyclophilin A was able to increase the HG-reduced insulin content.
Figure 3.
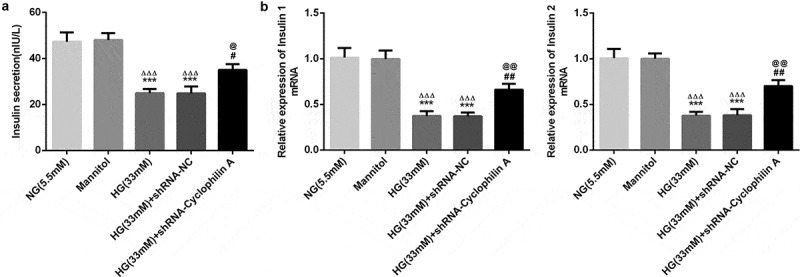
The effects of cyclophilin A silence on insulin secretion in pancreatic β-cells exposed to HG.
Insulin secretion level evaluated by insulin ELISA kit (a) and PCR (b) in the study groups.***P < 0.001 vs. NG group, ΔΔΔP < 0.001 vs. Mannitol group, #P < 0.05 and ##P < 0.01 vs. HG (33 mM) group, @P < 0.05 and @@P < 0.01 vs. HG (33 mM) + shRNA-NC group.
Knockdown of cyclophilin A alleviated inflammatory response and oxidative stress in pancreatic β-cells under exposure to HG
IL-18, TNF-a, and IL-1β, three indicators of inflammation, were evaluated herein. As shown by Figure 4(a), the inflammation level was not alerted by Mannitol and shRNA-NC. But there was a significant increase in inflammation of HG group compared with NG group, indicating that HG increased the inflammation level. Whereas the HG-increased inflammation was decreased by knockdown of cyclophilin A, confirming that knockdown of cyclophilin A had inhibitory effects on HG-promoted inflammation in pancreatic β-cells. After that, SOD, ROS, and MPO as indicators of oxidative stress were evaluated (Figure 4(b)). Mannitol and shRNA-NC had no effects on oxidative stress level. Furthermore, the levels of ROS and MPO were increased and that of SOD was decreased by HG. The effects of HG on these indicators were reversed by knockdown of cyclophilin A. Together, all the findings uncovered that knockdown of cyclophilin A had suppressive effects on levels of inflammation and oxidative stress in HG-stimulated pancreatic β-cells.
Figure 4.
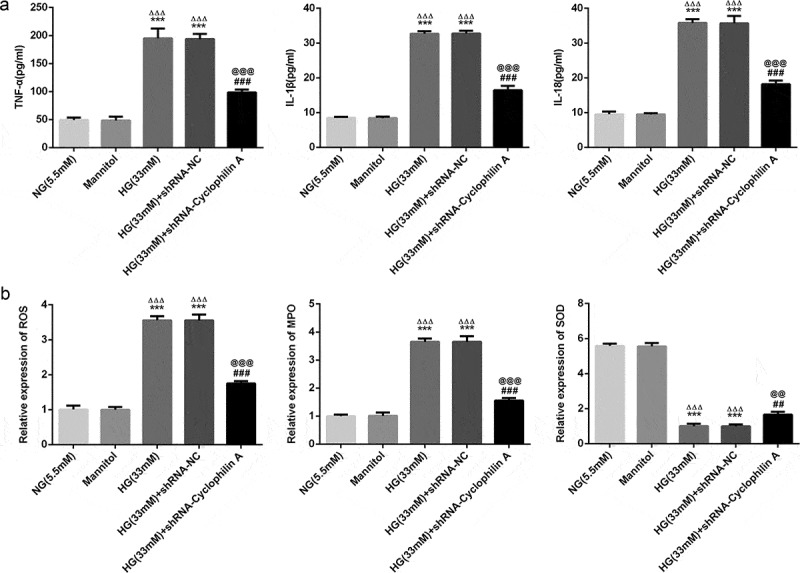
The effects of cyclophilin A decrease on inflammation and oxidative stress in HG-stimulated pancreatic β-cells.
The inflammation level (a) and oxidative stress level (b) in the study groups.***P < 0.001 vs. NG group, ΔΔΔP < 0.001 vs. Mannitol group, ##P < 0.01 and ###P < 0.001 vs. HG (33 mM) group, @@P < 0.01 and @@@P < 0.001 vs. HG (33 mM) + shRNA-NC group.
Knockdown of cyclophilin A reduced the cell apoptosis in HG group
As estimated by flow cytometry, the apoptosis was not changed by Mannitol and shRNA-NC (Figure 5). The cell apoptosis was increased after HG exposure and this effect was restrained by silencing of cyclophilin A, suggesting the attenuative effect of cyclophilin A knockdown on cell apoptosis. The bcl2, bax, cleaved caspase3 and cleaved caspase 9 as apoptosis-related proteins were also evaluated. As seen in Figure 6, the Mannitol and shRNA-NC exerted no effects on expression levels of apoptosis-related proteins. The levels of bcl-2 as an anti-apoptosis protein were decreased and levels of cleaved caspase 3/9 as pro-apoptosis proteins were increased in HG-induced pancreatic β-cells. This result consisted with the finding of flow cytometry, confirming that HG had promoting effects on cell apoptosis. The effects of HG on apoptosis-related proteins were further abrogated by knockdown of cyclophilin A. All the findings confirmed that knockdown of cyclophilin A was able to lessen cell apoptosis activated by HG.
Figure 5.
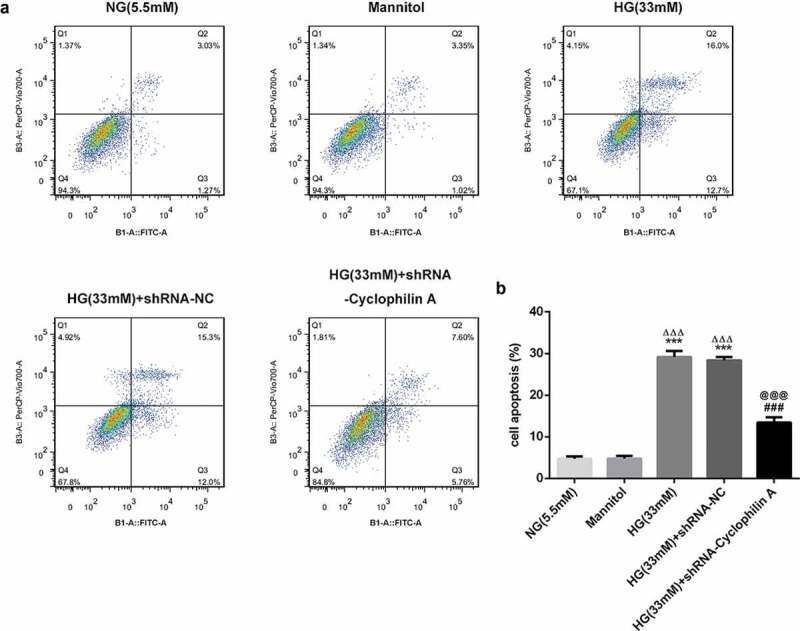
The effects of cyclophilin A silencing on cell apoptosis in HG-treated pancreatic β-cells.
The cell apoptosis level in the study groups (a and b).***P < 0.001 vs. NG group, ΔΔΔP < 0.001 vs. Mannitol group, ###P < 0.001 vs. HG (33 mM) group, @@@P < 0.001 vs. HG (33 mM) + shRNA-NC group.
Figure 6.
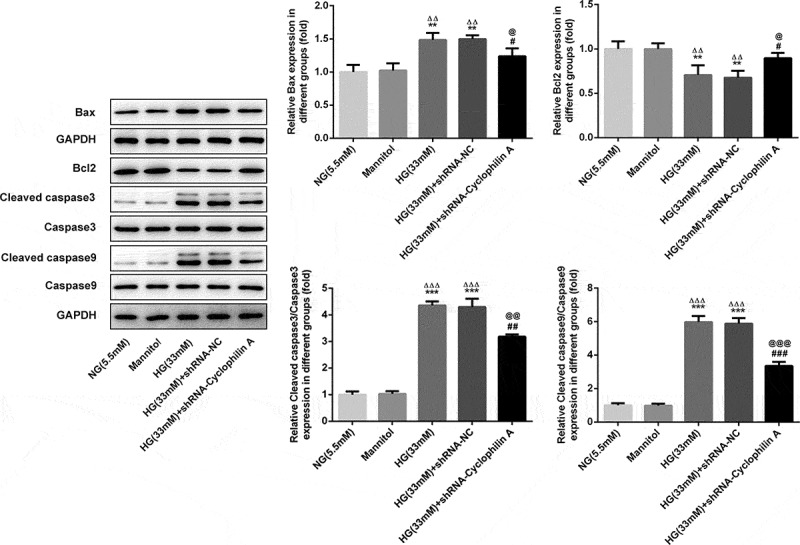
The effects of cyclophilin A decrease on apoptosis-related proteins in HG-induced pancreatic β-cells.
The levels of apoptosis-related proteins in the study groups.**P < 0.01 and ***P < 0.01 vs. NG group, ΔΔP < 0.01 and ΔΔΔP < 0.001 vs. Mannitol group, #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. HG (33 mM) group, @P < 0.05, @@P < 0.01 and @@@P < 0.001 vs. HG (33 mM) + shRNA-NC group.
The effects of knockdown of cyclophilin A on proteins of MAPK/NF-kb signaling pathway
Cyclophilin A is reported to involve in inflammation by activating MAPK/NF-kb signaling [30]. The expression levels of MAPK/NF-kb pathway proteins were also evaluated. As shown in Figure 7, proteins involved in MAPK/NF-kb pathway were unaffected with the treatment of Mannitol and shRNA-NC. The p-JNK, p-ERK, p-P38, p-NF-KB P65, and p-IKBα levels were all increased by HG treatment and these phenomena were further inhibited by knockdown of cyclophilin A. The results demonstrated that protective effects of cyclophilin A knockdown in HG-damaged pancreatic β-cells was realized via inactivating MAPK/NF-kb signaling.
Figure 7.
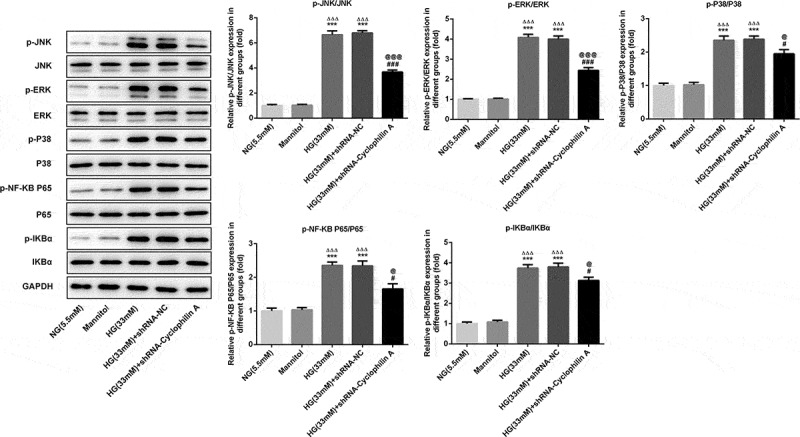
The effects of cyclophilin A downregulation on protein expressions of MAPK/NF-kb signaling pathway in HG-treated pancreatic β-cells.
***P < 0.01 vs. NG group, ΔΔΔP < 0.001 vs. Mannitol group, #P < 0.05 and ###P < 0.001 vs. HG group, @P < 0.05 and @@@P < 0.001 vs. HG (33 mM) + shRNA-NC group.
Discussion
Nowadays, diabetes mellitus remains to be a major health killer and there are no effective therapies for its treatment [31,32]. The general strategies are glucose control and alimentary control, incapable of radical treatment [33,34]. Finding the endogenous targets that exert substantial roles in the occurrence and development of diabetes mellitus may open up new direction for radical treatment of diabetes mellitus.
Cyclophilin A ubiquitously expressed in varieties of cells has been confirmed as a vital function protein that contributes to diabetic nephropathy, with high level in plasm of patients with diabetes [22,23]. We speculated that cyclophilin A might act as a pivotal role in pancreatic β-cells exposed to HG. Insulin insufficiency resulted from pancreatic β-cell dysfunction is the essential pathogenic factor in diabetes. Based on the theory above, in this research, we first explored the influences of cyclophilin A on HG-treated pancreatic β-cells. The results unmasked that knockdown of cyclophilin A improved insulin secretion, reduced cell apoptosis, and alleviated inflammation as well as oxidant stress in pancreatic β-cells under exposure to HG via repressing MAPK/NF-kb pathway.
HG-challenged pancreatic β-cells are the common diabetic cell model, as reported by many researches [35–37]. We next assessed the level of cyclophilin A, which unveiled that the level of cyclophilin A was higher in diabetic cell model. We speculated that knockdown of cyclophilin A exerted protective effects in pancreatic β-cells exposed to HG. HG has pro-apoptotic property in pancreatic β-cells [38–40]. Consistent with previous researches, the cell viability was decreased, and cell apoptosis was promoted in HG group, suggesting that the diabetic cell model was established successfully. The pro-apoptosis protein level was increased, and antiapoptosis protein level was decreased by HG, which was reversed by introduction of cyclophilin A knockdown plasmids. In a word, knockdown of cyclophilin A increased the cell viability and decreased the cell apoptosis affected by HG. Pancreatic β-cell apoptosis inevitably causes insufficient insulin, which is a vital factor in the initiation and progress of diabetes. We further evaluated the insulin content as the further evidence. The insulin content was decreased by HG treatment, which was possibly associated with the high cell apoptosis rate in HG-induced pancreatic β cells. Previous study reported that insulin secretion was reduced under the environment of HG [41]. Moreover, decreased blood glucose alleviated apoptosis of islet β cells and increased insulin secretion [42]. In present study, knockdown of cyclophilin A increased insulin secretion in HG-damaged pancreatic β-cells. Thus, knockdown of cyclophilin A may be a promising strategy for radical treatment of diabetes. Inflammation and oxidative stress are two major factors contributing to diabetes-induced injuries [43,44]. And inhibition of inflammation and oxidative stress develop protective effects in diabetes [45–48]. Cyclophilin A is confirmed to be secreted by cells under the stimulation of inflammation and oxidative stress [49]. In addition, cyclophilin A serves as the major downstream factor of oxidative stress and inflammatory cytokines [50–53]. In the current work, the levels of inflammation and oxidative stress stimulated by HG was restricted by knockdown of cyclophilin A, portending cyclophilin A as a considerable therapeutic target for diabetes.
The signaling pathway underlying cyclophilin A in diabetes was also explored primarily. MAPK/NF-kb signaling pathway is an inflammation-related pathway and plays a pivotal role in inflammation [54,55]. The MAPK/NF-kb is activated when cyclophilin A partakes in inflammation [56]. In this paper, the MAPK/NF-kb pathway was activated under HG exposure, which may be the mechanism accounting for high inflammation level in HG group. Furthermore, after knockdown of cyclophilin A, the proteins of MAPK/NF-kb pathway induced by HG was markedly down-regulated, hinting that MAPK/NF-kb signaling was hampered by knockdown of cyclophilin A and the protective effects of cyclophilin A silence may be partly attributed to inactivation of the MAPK/NF-kb signaling pathway.
In summary, cyclophilin A was an important function protein in diabetes and its knockdown exerted protective effects on pancreatic β-cells against HG via inhibiting MAPK/NF-kb signaling. Hence, cyclophilin A may be a potential target for diabetes treatment. Our study provided new insights into understanding the pathogenesis of diabetes and presented novel therapeutic strategies for its treatment. Additionally, further experiments will be performed to investigate the specific mechanism underlying the role of cyclophilin A in diabetes progression.
Conclusion
In this research, the results uncovered that knockdown of cyclophilin A possessed protective effects in HG-challenged pancreatic β-cells via MAPK/NF-kb pathway, offering a new strategy for treatment of diabetic. The role of cyclophilin A knockdown in vivo still needs further study. In addition, MAPK/NF-kb signaling may be not the unique signaling implicated in the role of cyclophilin A. In-depth researches are still needed to illuminate the role of cyclophilin A in diabetes.
Acknowledgments
Thanks for all participants.
Funding Statement
This study was supported by Hainan Provincial Key Research and Development Project (ZDYF2018130) and Hainan Medical Research Project (1801320249A2001).
Disclosure statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Highlights
The cyclophilin A level was increased by HG in pancreatic β-cells
Knockdown of cyclophilin A increased the cell viability and reduced the cell apoptosis in HG group
Knockdown of cyclophilin A increased insulin secretion in HG-damaged pancreatic β-cells
Knockdown of cyclophilin A suppressed inflammation and oxidative stress in pancreatic β-cells under exposure to HG
References
- [1].Ayelign B, Negash M, Genetu M, et al. Immunological impacts of diabetes on the susceptibility of mycobacterium tuberculosis. J Immunol Res. 2019;2019:6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kretchy IA, Koduah A, Ohene-Agyei T, et al. The association between diabetes-related distress and medication adherence in adult patients with type 2 diabetes mellitus: a cross-sectional study. J Diabetes Res. 2020;2020:4760624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].King H, Aubert RE, Herman WH.. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. [DOI] [PubMed] [Google Scholar]
- [4].Eriksson L, Nystrom T. Antidiabetic agents and endothelial dysfunction - beyond glucose control. Basic Clin Pharmacol Toxicol. 2015;117(1):15–25. [DOI] [PubMed] [Google Scholar]
- [5].Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Selvaraj K, Gowthamarajan K, Karri VV, et al. Current treatment strategies and nanocarrier based approaches for the treatment and management of diabetic retinopathy. J Drug Target. 2017;25(5):386–405. [DOI] [PubMed] [Google Scholar]
- [7].Papatheodorou K, Papanas N, Banach M, et al. Complications of diabetes 2016. J Diabetes Res. 2016;2016:6989453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu SM, Bonventre JV. Acute kidney injury and progression of diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25(2):166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pafili K, Papanas N, Ziegler D. Neuropathy in diabetes: “One cannot begin it too soon”. Angiology. 2018;69(9):752–754. [DOI] [PubMed] [Google Scholar]
- [10].Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang Y, Chan L. Monogenic diabetes: what it teaches us on the common forms of type 1 and type 2 diabetes. Endocr Rev. 2016;37(3):190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12(6):580–591. [DOI] [PubMed] [Google Scholar]
- [13].Ghosh R, Karmohapatra SK, Bhattacharyya M, et al. The appearance of dermcidin isoform 2, a novel platelet aggregating agent in the circulation in acute myocardial infarction that inhibits insulin synthesis and the restoration by acetyl salicylic acid of its effects. J Thromb Thrombolysis. 2011;31(1):13–21. [DOI] [PubMed] [Google Scholar]
- [14].Bhattacharya S, Khan MM, Ghosh C, et al. The role of Dermcidin isoform-2 in the occurrence and severity of Diabetes. Sci Rep. 2017;7(1):8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Banerjee S, Grobelna A. Sodium-glucose cotransporter 2 inhibitors for the treatment of diabetic nephropathy: a review of clinical effectiveness. Ottawa ON: ﺁﺏ 2019 Canadian Agency for Drugs and Technologies in Health; 2019. [PubMed] [Google Scholar]
- [16].Roshanzamir N, Hassan-Zadeh V. Methylation of specific CpG sites in IL-1ﺧ٢ and IL1R1 genes is affected by hyperglycaemia in type 2 diabetic patients. Immunol Invest. 2020;49(3):287–298. [DOI] [PubMed] [Google Scholar]
- [17].Dawar FU, Xiong Y, Khattak MNK, et al. Potential role of cyclophilin A in regulating cytokine secretion. J Leukoc Biol. 2017;102(4):989–992. [DOI] [PubMed] [Google Scholar]
- [18].Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Obchoei S, Sawanyawisuth K, Wongkham C, et al. Secreted cyclophilin A mediates G1/S phase transition of cholangiocarcinoma cells via CD147/ERK1/2 pathway. Tumour Biol. 2015;36(2):849–859. [DOI] [PubMed] [Google Scholar]
- [20].Xue C, Sowden M, Berk BC. Extracellular cyclophilin A, especially acetylated, causes pulmonary hypertension by stimulating endothelial apoptosis, redox stress, and inflammation. Arterioscler Thromb Vasc Biol. 2017;37(6):1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mao M, Yu X, Ge X, et al. Acetylated cyclophilin A is a major mediator in hypoxia-induced autophagy and pulmonary vascular angiogenesis. J Hypertens. 2017;35(4):798–809. [DOI] [PubMed] [Google Scholar]
- [22].Ramachandran S, Venugopal A, Kutty VR, et al. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc Diabetol. 2014;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tsai SF, Hsieh CC, Wu MJ, et al. Novel findings of secreted cyclophilin A in diabetic nephropathy and its association with renal protection of dipeptidyl peptidase 4 inhibitor. Clin Chim Acta. 2016;463:181–192. [DOI] [PubMed] [Google Scholar]
- [24].Kong X, Liu CX, Wang GD, et al. LncRNA LEGLTBC functions as a ceRNA to antagonize the effects of miR-34a on the downregulation of SIRT1 in glucolipotoxicity-induced INS-1 beta cell oxidative stress and apoptosis. Oxid Med Cell Longev. 2019;2019:4010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang C, Zou S, Cui Z, et al. Zerumbone protects INS-1 rat pancreatic beta cells from high glucose-induced apoptosis through generation of reactive oxygen species. Biochem Biophys Res Commun. 2015;460(2):205–209. [DOI] [PubMed] [Google Scholar]
- [26].Zhu K, Hu X, Chen H, et al. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine. 2019;49:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang GG, Li W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iran J Basic Med Sci. 2019;22(10):1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fan G, Gu Y, Zhang J, et al. Transthyretin upregulates long non-coding RNA MEG3 by affecting PABPC1 in diabetic retinopathy. Int J Mol Sci. 2019;20(24):6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gong J, Zhan H, Li Y, et al. Krﺃﺱppelﻗ°∞like factorﺁ 4 ameliorates diabetic kidney disease by activating autophagy via the mTOR pathway. Mol Med Rep. 2019;20(4):3240–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yuan W, Ge H, He B. Pro-inflammatory activities induced by CyPA-EMMPRIN interaction in monocytes. Atherosclerosis. 2010;213(2):415–421. [DOI] [PubMed] [Google Scholar]
- [31].Khursheed R, Singh SK, Wadhwa S, et al. Treatment strategies against diabetes: success so far and challenges ahead. Eur J Pharmacol. 2019;862:172625. [DOI] [PubMed] [Google Scholar]
- [32].Xu YXZ, Xi S, Qian X. Evaluating traditional chinese medicine and herbal products for the treatment of gestational diabetes mellitus. J Diabetes Res. 2019;2019:9182595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mohan V, Kalpana N, Lakshmipriya N, et al. A pilot study evaluating the effects of diabetes specific nutrition supplement and lifestyle intervention on glycemic control in overweight and obese Asian Indian adults with type 2 diabetes mellitus. J Assoc Physicians India. 2019;67(12):25–30. [PubMed] [Google Scholar]
- [34].Yu Y, Hu L, Xu Y, et al. Impact of blood glucose control on sympathetic and vagus nerve functional status in patients with type 2 diabetes mellitus. Acta Diabetol. 2020;57(2):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Song HL, Zhang SB. Therapeutic effect of dexmedetomidine on intracerebral hemorrhage via regulating NLRP3. Eur Rev Med Pharmacol Sci. 2019;23(6):2612–2619. [DOI] [PubMed] [Google Scholar]
- [36].Lee JS, Lee HA, Han JS. Sargassum sagamianum extract protects INS-1 pancreatic beta cells against high glucose-induced apoptosis. Cytotechnology. 2019;71(1):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ruan D, Liu Y, Wang X, et al. miR-149-5p protects against high glucose-induced pancreatic beta cell apoptosis via targeting the BH3-only protein BIM. Exp Mol Pathol. 2019;110:104279. [DOI] [PubMed] [Google Scholar]
- [38].Karunakaran U, Elumalai S, Moon JS, et al. Myricetin protects against high glucose-induced beta-cell apoptosis by attenuating endoplasmic reticulum stress via inactivation of cyclin-dependent kinase 5. Diabetes Metab J. 2019;43(2):192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Park JE, Seo Y, Han JS. HM-chromanone isolated from portulaca oleracea L. protects INS-1 pancreatic beta cells against glucotoxicity-induced apoptosis. Nutrients. 2019;11(2). DOI: 10.3390/nu11020404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pillai SS, Mini S. Attenuation of high glucose induced apoptotic and inflammatory signaling pathways in RIN-m5F pancreatic beta cell lines by Hibiscus rosa sinensis L. petals and its phytoconstituents. J Ethnopharmacol. 2018;227:8–17. [DOI] [PubMed] [Google Scholar]
- [41].Zhang YN, Fu DX, Xu JX, et al. The effect of SOX9 on islet ﺧ٢ cells in high glucose environment through regulation of ERK/P38 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(19):8476–8484. [DOI] [PubMed] [Google Scholar]
- [42].Zhang Y, Xiang R, Fang S, et al. Experimental study on the effect of tibetan medicine triphala on the proliferation and apoptosis of pancreatic islet ﺧ٢ cells through incretin-cAMP signaling pathway. Biol Pharm Bull. 2020;43(2):289–295. [DOI] [PubMed] [Google Scholar]
- [43].Jiao Y, Zhang S, Zhang J, et al. Tetramethylpyrazine attenuates placental oxidative stress, inflammatory responses and endoplasmic reticulum stress in a mouse model of gestational diabetes mellitus. Arch Pharm Res. 2019;42(12):1092–1100. [DOI] [PubMed] [Google Scholar]
- [44].Luc K, Schramm-Luc A, Guzik TJ, et al. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70(6). DOI: 10.26402/jpp.2019.6.01 [DOI] [PubMed] [Google Scholar]
- [45].Cheng Y, Yang Z, Shi J, et al. Total flavonoids of epimedium ameliorates testicular damage in streptozotocin-induced diabetic rats by suppressing inflammation and oxidative stress. Environ Toxicol. 2020;35(2):268–276 [DOI] [PubMed] [Google Scholar]
- [46].Azul L, Leandro A, Boroumand P, et al. Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic Biol Med. 2019. DOI: 10.1016/j.freeradbiomed.2019.11.002 [DOI] [PubMed] [Google Scholar]
- [47].Ebrahimi F, Sahebkar A, Aryaeian N, et al. Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes. 2019;12:2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wen W, Lin Y, Ti Z. Antidiabetic, antihyperlipidemic, antioxidant, anti-inflammatory activities of ethanolic seed extract of annona reticulata L. in streptozotocin induced diabetic rats. Front Endocrinol (Lausanne). 2019;10:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li W, Liu W, Chen C, et al. Effect of extracellular cyclophilin A on inflammatory response and anti-inflammatory activity of antibody against cyclophilin A. Sheng Wu Gong Cheng Xue Bao. 2018;34(1):90–101. [DOI] [PubMed] [Google Scholar]
- [50].Su H, Chen T, Li J, et al. Correlations of serum cyclophilin A and melatonin concentrations with hypertension-induced left ventricular hypertrophy. Arch Med Res. 2017;48(6):526–534. [DOI] [PubMed] [Google Scholar]
- [51].Jin ZG, Lungu AO, Xie L, et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24(7):1186–1191. [DOI] [PubMed] [Google Scholar]
- [52].Satoh K, Matoba T, Suzuki J, et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117(24):3088–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Satoh K, Nigro P, Matoba T, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15(6):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Thandavarayan RA, Giridharan VV, Sari FR, et al. Depletion of 14-3-3 protein exacerbates cardiac oxidative stress, inflammation and remodeling process via modulation of MAPK/NF-kB signaling pathways after streptozotocin-induced diabetes mellitus. Cell Physiol Biochem. 2011;28(5):911–922. [DOI] [PubMed] [Google Scholar]
- [55].Zhu X, Wang L, Teng X, et al. N-methyl pyrrolidone (NMP) alleviates lipopolysaccharide (LPS)-induced inflammatory injury in articular chondrocytes. Med Sci Monit. 2018;24:6480–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee MJ, Kim DW, Sohn EJ, et al. Anti-inflammatory effect of transduced PEP-1-cyclophilin A in Raw264.7 cells and 12-O-tetradecanoylphorbol-13-acetate-induced mice. Life Sci. 2011;89(23–24):896–904. [DOI] [PubMed] [Google Scholar]


