A 64-year-old female nonsmoker with a history of hypertension, obesity, type II diabetes, and recurrent urinary tract infections secondary to chronic kidney stones presented to an outpatient pulmonology clinic with new onset shortness of breath and cough. Her pulmonary history was unremarkable, with no history of asthma, tuberculous, emphysema or pulmonary fibrosis. She denied any farm or factory exposure and reported no exposure to other potential respiratory irritants such as bird or mould; she had no pets. Her home was a townhouse with a forced hot air system and she had worked in an office for the length of her career. Her family history was similarly noncontributory; her parents had passed away from nonrespiratory causes. She was one of 11 children, none of which had a history of asthma, COPD, pulmonary fibrosis, or tuberculous. Her two grown children also had no history of pulmonary disease.
Short abstract
Nitrofurantoin is a cause of drug-induced pneumonitis and can result in clinically significant respiratory symptoms manifesting as interstitial lung disease on chest CT, even if the patient has been taking the drug chronically without side-effects https://bit.ly/3v2m29h
A 64-year-old female nonsmoker with a history of hypertension, obesity, type II diabetes, and recurrent urinary tract infections secondary to chronic kidney stones presented to an outpatient pulmonology clinic with new onset shortness of breath and cough. Her pulmonary history was unremarkable, with no history of asthma, tuberculous, emphysema or pulmonary fibrosis. She denied any farm or factory exposure and reported no exposure to other potential respiratory irritants such as bird or mould; she had no pets. Her home was a townhouse with a forced hot air system and she had worked in an office for the length of her career. Her family history was similarly noncontributory; her parents had passed away from nonrespiratory causes. She was one of 11 children, none of which had a history of asthma, COPD, pulmonary fibrosis, or tuberculous. Her two grown children also had no history of pulmonary disease.
Task 1
What additional history would you want to obtain?
Answer 1
Information about medications she is taking and a focused history of similar episodes.
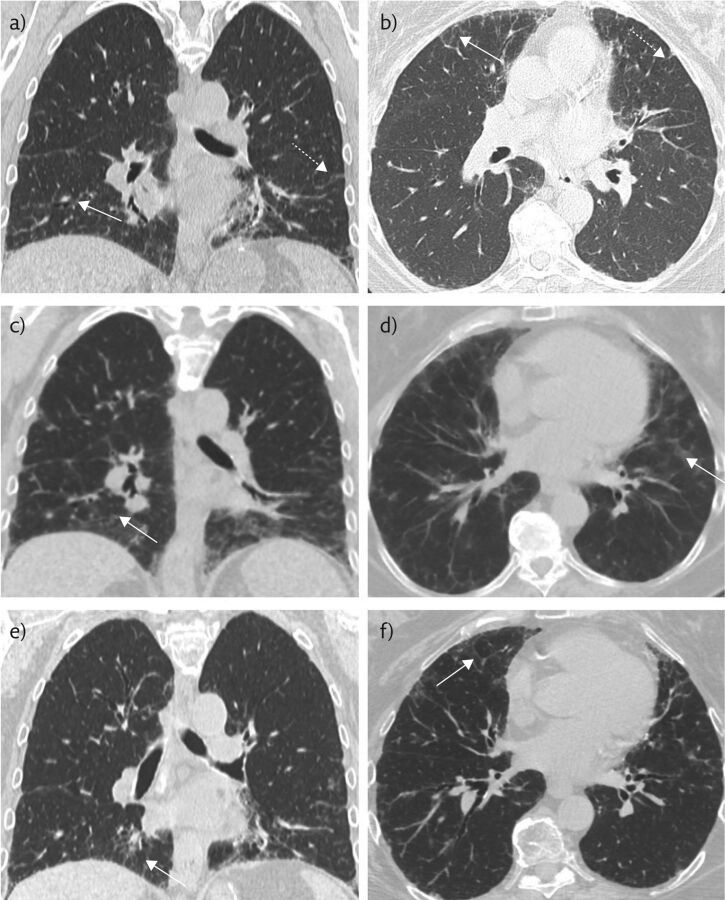
She was taking losartan, atorvastatin, salbutamol and nitrofurantoin for her ongoing medical problems. Of note, the patient had been continuously on nitrofurantoin for 5 years prior to this episode with no periods of discontinuation. 8 months prior to presentation, the patient had presented with milder similar symptoms and a chest computed tomography (CT) scan revealed patchy bibasilar ground-glass opacities (GGOs) (figure 1a and b). The patient did not respond to several courses of antibiotics for pneumonia and was subsequently treated with a short course 20 mg oral prednisone twice daily, which provided only transient amelioration of symptoms.
Figure 1.
a, b) Non-contrast chest CT 8 months prior to presentation, when the patient was mildly symptomatic, in the coronal and axial planes, respectively. These show mild basilar-predominant peripheral reticulations (dashed white arrows) and minimal GGOs (solid white arrows). c, d) Representative coronal and axial images during acute exacerbation, the solid white arrows indicate GGOs. e, f) Representative coronal and axial images after discontinuation of nitrofurantoin and initiation of steroid therapy, with near-complete resolution of GGOs and peripheral reticulations.
Task 2
What is the next step for both diagnosis and treatment?
Answer 2
The patient should undergo non-contrast CT imaging of the chest to identify if new findings correlate with the presenting symptoms and undergo pulmonary function tests. Steroids should be initiated to decrease inflammation given this probably represents exacerbation of an underlying condition.
Subsequent to this, she was treated with umeclidinium bromide, a long-acting muscarinic antagonist used for treatment of COPD and combination fluticasone furoate and vilanterol also without long-term relief of symptoms.
On presentation, her resting oxygen saturation was 92% with desaturations as low as 84% with ambulation. A chest CT was obtained and showed interval worsening of bibasilar predominant ground-glass and reticular opacities (figure 1c and d). Pulmonary function studies showed a forced vital capacity (FVC) of 1.78 L (56% of expected), forced expiratory volume in 1 s (FEV1) of 1.83 L (62%), and a diffusing capacity of the lungs for carbon monoxide (DLCO) of 10.14 mL·min−1·mmHg−1 (49%). She had a resting oxygen saturation of 97–98% with a drop to 93–94% with ambulation (table 1). Given her long history of nitrofurantoin use, the strong clinical suspicion was for drug-induced pneumonitis. As such, steroid therapy was initiated and nitrofurantoin was discontinued. Although blood tests were not pursued during her previous exacerbation, extensive blood tests were obtained at this time to rule out other aetiologies of her pulmonary symptoms. These included tests for C-reactive protein, a hypersensitivity pneumonitis panel, immunoglobulin IgE, aldolase, anti-neutrophil cytoplasmic antibodies (P-ANCA and C-ANCA), anti-nuclear antibodies (ANA), anti-DSDNA antibody, anti-centromere antibodies, anti-cyclic citrulline (CCP) antibodies, anti-Smith antibodies, rheumatoid factor (RF), erythrocyte sedimentation rate, a quantitative immunoglobulin test, anti-Jo antibodies, anti-histone, and anti-SCL-70 antibodies. In light of the patient's ongoing symptoms, she was treated by discontinuation nitrofurantoin with a working diagnosis of drug-induced pneumonitis. Results were sent out and not available at the time of clinical decision making.
Table 1.
Pulmonary function tests before and after discontinuation of nitrofurantoin
| FVC | FEV1 | DLCO mL·min−1·mmHg−1 | Tiffeneau Index | |
| Initial encounter | 1.78 L (56%) | 1.83 L (62%) | 10.14 (49%) | 87% |
| 6-week follow-up | 2.25 L (71%) | 1.7 L (69%) | 13.4 (65%) | 76% |
Bronchoalveolar lavage (BAL) is frequently employed to assess the aetiology of pulmonology pathology. In this case, clinical suspicion was high for drug-induced pneumonitis given the patient's longstanding history of nitrofurantoin use. Discontinuation of the drug was the first step in the diagnostic pathway and had this not resolved symptoms BAL would have been pursued.
Task 3
How would you describe the new findings on chest CT (figure 1c and d)?
Answer 3
CT at the time of presentation demonstrated a marked increase in lower lobe predominant GGOs.
Given ongoing concern for nephrolithiasis, the patient underwent abdominal CT and was found to have a new obstructive right-sided renal calculus with associated hydroureteronephrosis and was scheduled for surgery to remove the calculus (figure 2a and b).
Figure 2.
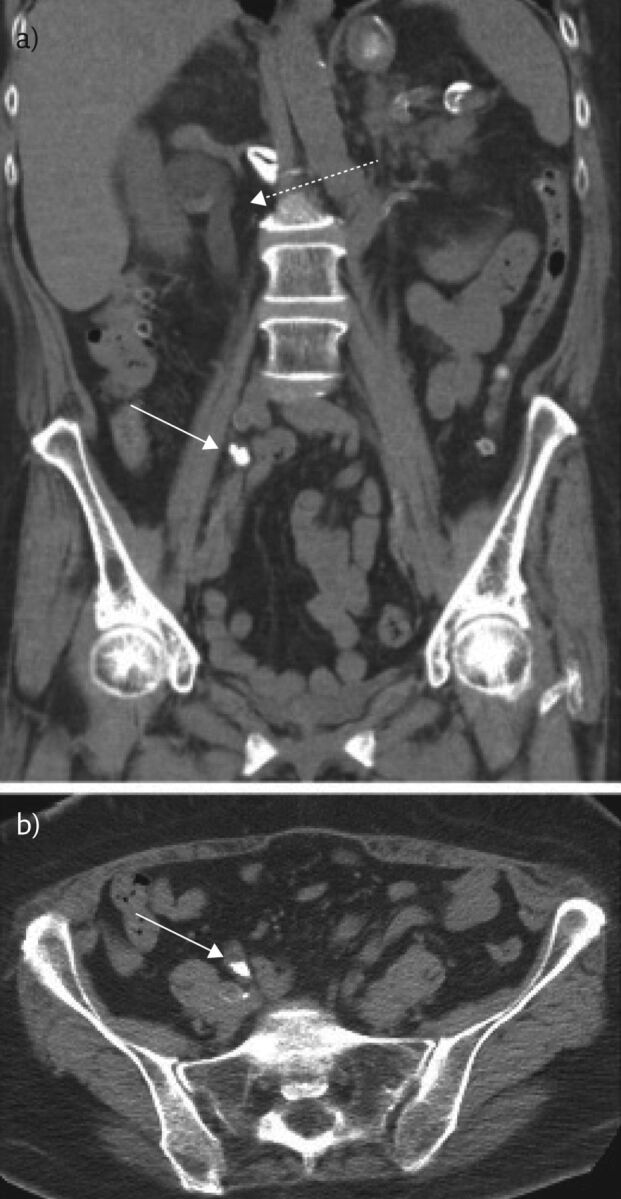
Representative a) coronal and b) axial images from a non-contrast chest CT of the abdomen and pelvis of this 64-year-old female obtained to evaluate for renal calculus. An obstructive calculus is seen in the distal right ureter (solid white arrow) with proximal hydroureteronephrosis (dotted white arrow).
Follow-up 6 weeks later revealed marked improvements in pulmonary function tests with a FVC of 2.25 L (71%), FEV1 of 1.7 L (69%), and a DLCO of 13.4 mL·min−1·mmHg−1 (65%). Her oxygen saturation improved to 98% at rest and 97% with walking (table 1). Repeat chest CT 4 weeks later showed decreased conspicuity of GGOs compatible with resolving disease (figure 1e and f). It was recommended that another follow-up CT be obtained 3 months later to ensure resolution. Ultimately, the results from the blood tests found an elevated IgG and IgA, an elevated erythrocyte sedimentation rate of 72 mm∙h−1, anti-SCL-70 antibody level of 2.5 AU∙mL−1, positive ANA, anti-histone antibodies of 5.2 units, and was positive exposure to Saccharomonospora viridis.
As with any constellation of signs and symptoms, it was important to consider the differential diagnosis for the patient's pulmonary complaints to ensure that she received appropriate treatment. Leading the differential diagnosis was drug-induced pneumonitis; however, the blood tests raised the spectre of underlying autoimmune disease. Specifically, positive anti-SCL-70 can be seen in the setting of diffuse systemic scleroderma; positive anti-histone antibody and ANA are suggestive of autoimmune disease but are less specific. Given the broad nature of these antibodies, differential considerations included Sjögren syndrome, systemic lupus erythematous, scleroderma, and mixed connective tissue disorder. Additional rheumatological diseases that may have positive ANA include rheumatoid arthritis, polymyositis and autoimmune liver disease. Further, some patients with hepatic cirrhosis or neoplasm also may have positive ANA. Exposure to Saccharomonospora viridis suggested hypersensitivity pneumonitis (HP) as a possible underlying cause. For this reason, referral for rheumatology consultation was critically important for our patient and in any patient where blood work suggests possible rheumatological disease to exclude this as an underlying cause of pulmonary symptoms. Indeed, our patient did follow-up with a rheumatologist; however, no unifying rheumatological diagnosis was reached. While underlying rheumatological disease cannot be entirely excluded, given that the patient's symptoms had resolved with withdrawal of nitrofurantoin and brief steroid treatment had only provided transient symptomatic relief, clinicians concluded that the aetiology of this patient's pulmonary disease was nitrofurantoin-induced pneumonitis. The patient continues to follow-up with pulmonology to monitor for any future exacerbations which may warrant additional clinical investigation.
Discussion
Here, we present a rare case of nitrofurantoin-induced pneumonitis which occurred after chronic use of the drug, although pulmonary symptoms occurred acutely. Drug-induced lung disease is a well-known entity with a broad range of offending agents. A number of pathological manifestations are seen with drug-induced lung disease including acute respiratory distress syndrome, pulmonary oedema, pulmonary hypertension, pulmonary vasculitis and interstitial lung disease (ILD) [1]. Patterns of drug-induced ILD include HP and the idiopathic interstitial pneumonias, such as usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organising pneumonia and diffuse alveolar damage (DAD) [2]. Diagnosis is often difficult because symptoms occur gradually and ILD may arise from many aetiologies; therefore, drug-induced ILD is a diagnosis of exclusion. Table 2 summarises the clinical and radiographic characteristics of patients with drug induced pneumonitis. Table 3 presents a differential diagnosis for patients presenting with respiratory complaints, diminished pulmonary function tests and GGOs on imaging.
Table 2.
Drug-induced pneumonitis clinical and radiographic features
| Aetiology | Many drugs, most commonly chemotherapy agents (bleomycin, busulfan, cyclophosphamide, methotrexate, thalidomide), immunosuppressive agents (sirolimus), amiodarone, antibiotics (nitrofurantoin, amphotericin B), pembrolizumab |
| Incidence | Overall incidence unknown (varies depending on agent) |
| Sex ratio | Unknown |
| Age predilection | Varies depending on agent (e.g. bleomycin-induced lung injury risk is increased in the elderly) Median age for acute and chronic nitrofurantoin-induced pulmonary reactions was 59 and 68 years, respectively |
| Risk factors | Varies depending on agent (e.g. marked increased risk of bleomycin lung injury if cumulative dose exceeds 450 units) |
| Treatment | Cessation of offending agent Corticosteroid therapy is often used, although evidence is lacking |
| Prognosis | Varies widely, from complete clinical recovery and resolution of imaging findings to respiratory failure and death |
| Findings on imaging | CT findings vary among different aetiologies, includes multifocal GGOs with intralobular interstitial thickening, patchy GGO, centrilobular nodules |
Table 3.
Differential diagnosis for presenting symptoms of this patient
| Differential diagnosis | Radiographic findings | CT findings |
| Hypersensitivity pneumonitis | Low sensitivity, with many patients having normal radiographs Findings may include: nodular opacities, GGOs or consolidation, fine reticulation, eventual fibrosis with honeycombing |
Mid and upper lobe predominance, centrilobular nodules, bilateral and symmetric airspace opacities (ground glass or consolidation), air trapping with mosaic pattern, head cheese sign, fibrosis with honeycombing |
| Pulmonary interstitial oedema | Peribronchial cuffing, septal (Kerley B) lines, interlobular fissure thickening | GGOs, interlobular septal thickening, bronchovascular bundle thickening |
| Sarcoidosis | Mid and upper lobe predominant opacities, lymphadenopathy, calcified lymph nodes | Mid and upper lobe predominant reticulation, honeycombing, traction bronchiectasis, lymphadenopathy, calcified lymph nodes |
| Coronavirus disease 2019 (COVID-19) | Bilateral lower lobe predominant airspace opacities | Bilateral lower lobe predominant ground glass and consolidative opacities |
| Mycoplasma pneumoniae | Unilateral or bilateral patchy consolidation, nodular or reticular opacities | Patchy or ground-glass opacities, centrilobular nodules, tree-in-bud, bronchial wall thickening |
Distinguishing the pattern of ILD in the setting of suspected drug-induced ILD is difficult as signs, symptoms and imaging features often overlap. NSIP is the most commonly reported pattern in association with nitrofurantoin [2, 3]. Imaging features indicative of UIP [4–6], DAD and organising pneumonia [7] have all been reported with nitrofurantoin use. HP is also commonly reported [8] and there have been rare cases of chronic eosinophilic pneumonia [9] and giant cell interstitial pneumonia [10, 11].
Nitrofurantoin is an antibiotic medication often used in the treatment of urinary tract infections and is a well-documented cause of lung toxicity. An analysis of 921 reports of adverse reactions to nitrofurantoin showed that 43% of all cases involved acute pulmonary reactions, while <10% involved chronic pulmonary reactions [12, 13]. For our patient, there was a long latency period from initiation of the drug to the onset of symptoms. The severity of adverse reactions range from mild hypersensitivity reactions to diffuse and irreversible pulmonary fibrosis with high mortality rates [14]. In this case, when the patient presented her symptoms were severe. Radiographically, nitrofurantoin-induced ILD manifests as DAD, organising pneumonia, HP, UIP or other less common patterns of lung disease which may share a common final pathway of lung fibrosis [1, 2].
While manifesting symptoms after 5 years of treatment with nitrofurantoin, this patient's symptoms promptly resolved after recognition and discontinuation of the drug combined with corticosteroid therapy. Steroids provided only transient relief. This case underscores the critical importance of considering drug toxicity as a possible aetiology of ILD, even in cases where the patient has been treated for a long period of time with no or minimal pulmonary side-effects. Onset of acute toxicity is typically within 2 weeks of initiation the drug [15], manifesting as nonspecific clinical findings of fever, cough, dyspnoea and peripheral eosinophilia [2]. Chronic lung toxicity is rare, occurring in <1% of patients taking nitrofurantoin, presenting clinically as cough and dyspnoea [16, 17].
A large proportion of drug-induced pneumonitis cases are never definitively diagnosed as a biopsy is rarely performed. For ILD, the gold standard for diagnosis is the multidisciplinary discussion, and given that these cases frequently resolve with discontinuation of the offending drug, they often are not discussed in these conferences [18]. The mainstay of treatment is discontinuation of nitrofurantoin and supportive therapy. In chronic or more severe cases, corticosteroids are often trialled regardless of aetiology. However, high-quality evidence is lacking for the routine use of steroids [14].
Here we present a unique case of nitrofurantoin-induced ILD occurring 5 years after initiation of the drug. This case highlights several key points for radiologists and clinicians: that onset of acute pulmonary symptoms can occur at any point while taking nitrofurantoin, that drug-induced pneumonitis is an important differential consideration, and that treatment by withdrawal of the drug and/or initiation of steroids can result in prompt resolution of symptoms confirming that the pulmonary disease is in fact drug-induced pneumonitis. Clinical history, including medication use, is important for radiologists to consider when diagnosing ILD.
Conclusion
This case of nitrofurantoin-induced ILD highlights the role of the radiologist in the diagnostic interpretation of ILD, which has experienced an overall paradigm shift from the historic “gold standard” of histological diagnosis to a multidisciplinary approach. More specifically, recognition of the often subtle and nonspecific imaging features of ILD in the setting of drug exposure may alert clinicians to consider drug-induced pneumonitis.
Footnotes
Conflict of interest: K.M. Capaccione has nothing to disclose.
Conflict of interest: C.V. Tran has nothing to disclose.
Conflict of interest: J.S. Leb has nothing to disclose.
Conflict of interest: M.M. Salvatore reports grants and other from Genentech (Speaker and Advisory Board), grants and other from Boehringer Ingelheim (Speaker and Advisory Board), outside the submitted work.
Conflict of interest: B. D'souza has nothing to disclose.
References
- 1.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 2: Noncytotoxic drugs. Am Rev Respir Dis 1986; 133: 488–505. doi: 10.1164/arrd.1986.133.3.488 [DOI] [PubMed] [Google Scholar]
- 2.Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. RadioGraphics 2000; 20: 1245–1259. doi: 10.1148/radiographics.20.5.g00se081245 [DOI] [PubMed] [Google Scholar]
- 3.Padley SP, Adler B, Hansell DM, et al. High-resolution computed tomography of drug-induced lung disease. Clin Radiol 1992; 46: 232–236. doi: 10.1016/S0009-9260(05)80161-8 [DOI] [PubMed] [Google Scholar]
- 4.Goemaere NN, Grijm K, van Hal PT, et al. Nitrofurantoin-induced pulmonary fibrosis: a case report. J Med Case Rep 2008; 2: 169. doi: 10.1186/1752-1947-2-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin AJ, Rashid S, Gharibeh TR, et al. Interstitial lung disease secondary to long-term nitrofurantoin use. Am J Case Rep 2020; 21: e920386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A, Singh P, Sidhu US. Reversible interstitial lung disease with prolonged use of nitrofurantoin: do the benefits outweigh the risks? Lung India 2013; 30: 212–214. doi: 10.4103/0970-2113.116271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson C, Nyi PP. Probable nitrofurantoin-induced bronchiolitis obliterans with organising pneumonia. Am J Health Syst Pharm 2009; 66: 1919–1922. doi: 10.2146/ajhp090403 [DOI] [PubMed] [Google Scholar]
- 8.Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med 2003; 168: 952–958. doi: 10.1164/rccm.200301-137OC [DOI] [PubMed] [Google Scholar]
- 9.Martins RR, Marchiori E, Viana SL, et al. Chronic eosinophilic pneumonia secondary to long-term use of nitrofurantoin: high-resolution computed tomography findings. J Bras Pneumol 2008; 34: 181–184. doi: 10.1590/S1806-37132008000300009 [DOI] [PubMed] [Google Scholar]
- 10.Lee B, Balavenkataraman A, Sanghavi D, et al. Recurrent nitrofurantoin-induced giant cell interstitial pneumonia: case report and literature review. Respir Med Case Rep 2015; 14: 49–52. doi: 10.1016/j.rmcr.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magee F, Wright JL, Chan N, et al. Two unusual pathological reactions to nitrofurantoin: case reports. Histopathology 1986; 10: 701–706. doi: 10.1111/j.1365-2559.1986.tb02523.x [DOI] [PubMed] [Google Scholar]
- 12.Holmberg L, Boman G, Böttiger LE, et al. Adverse reactions to nitrofurantoin: analysis of 921 reports. Am J Med 1980; 69: 733–738. doi: 10.1016/0002-9343(80)90443-X [DOI] [PubMed] [Google Scholar]
- 13.Holmberg L, Boman G. Pulmonary reactions to nitrofurantoin. 447 cases reported to the Swedish Adverse Drug Reaction Committee 1966–1976. Eur J Respir Dis 1981; 62: 180–189. [PubMed] [Google Scholar]
- 14.Mir E, Malik JA, Lone SA, et al. Spontaneous resolution of nitrofurantoin-induced chronic pulmonary toxicity presenting with respiratory failure. Adv Respir Med 2017; 85: 333–338. doi: 10.5603/ARM.2017.0057 [DOI] [PubMed] [Google Scholar]
- 15.Boggess KA, Benedetti TJ, Raghu G. Nitrofurantoin-induced pulmonary toxicity during pregnancy: a report of a case and review of the literature. Obstet Gynecol Surv 1996; 51: 367–370. doi: 10.1097/00006254-199606000-00021 [DOI] [PubMed] [Google Scholar]
- 16.Madani Y, Mann B. Nitrofurantoin-induced lung disease and prophylaxis of urinary tract infections. Prim Care Respir J 2012; 21: 337–341. doi: 10.4104/pcrj.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaeffer EM. Re: Nitrofurantoin-induced lung disease and prophylaxis of urinary tract infections. J Urol 2013; 190: 1252. [DOI] [PubMed] [Google Scholar]
- 18.Furini F, Carnevale A, Casoni GL, et al. The role of the multidisciplinary evaluation of interstitial lung diseases: Systematic literature review of the current evidence and future perspectives. Front Med 2019; 6: 246. doi: 10.3389/fmed.2019.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]