Abstract
The management of respiratory diseases requires various levels of care: multidisciplinary teams, educational and behavioural interventions, self-management and home-based technical support are vital to ensure adequate care management. However, it is often difficult to access these networks due to fragmentation of patient care and treatment burden. Care coordination aims to ensure patients have a central role and that there is continuity of care among various levels and professionals involved. Moreover, the coronavirus disease pandemic has caused strain on the global healthcare system, with care coordination becoming increasingly important in increasing the resilience of health systems, supporting healthcare professionals and ensuring the right treatment and adequate level of care for these patients.
Short abstract
Care coordination is fundamental in treating respiratory disease. Although it involves considerable effort it ensures professionals to work together to reduce the treatment burden for respiratory patients. https://bit.ly/2Qrrpz0
Definition and aims of care coordination
Coordinated care management is fundamental to high-quality healthcare systems [1]. There is a growing consensus that fragmented and poorly coordinated healthcare management may compromise the quality of care with adverse impacts, including therapeutic errors, avoidable repeated hospitalisations, and increased healthcare costs [1, 2]. The concept of “care coordination” was introduced recently, though there is as yet no agreed definition of the term [3]. McDonald et al. [4] described care coordination as “the deliberate organisation of patient care activities between two or more participants (including the patient) involved in a patient's care to facilitate the appropriate delivery of healthcare services. Organising healthcare involves the marshalling of personnel and other resources needed to carry out all required patient care activities, and it is often managed by the exchange of information among participants responsible for different aspects of care”. Following this, in 2018 the World Health Organization (WHO) defined care coordination as “a proactive approach to bringing together care professionals and providers to meet the needs of service users to ensure that they receive integrated, person-focused care across various settings” [5]. Therefore, in summary, care coordination is a health system strategy in which many figures are involved, in which the patient should play the leading role through self-management, active collaboration and sharing of information. To be effective, care coordination should also provide appropriate healthcare at the right time, filling the gaps among various levels of care, reducing unnecessary hospitalisations and their related costs [4].
Care coordination in the field of respiratory diseases
Chronic respiratory diseases
The most common chronic respiratory diseases (CRD) are asthma, COPD, obstructive sleep apnoea syndrome (OSAS) and interstitial lung diseases (ILDs). They account for 8.3% of all chronic diseases due to exacerbations, related complications, the need for multidisciplinary treatments, disability, and comorbidities [5, 6]. The management of these patients is complex and requires a comprehensive approach across different care settings from the home to primary care to specialist care in hospital. These layers of care include the following. 1) Multidisciplinary teams (including patients and professionals), general practitioners, specialist clinicians, nurses, social workers, physiotherapists, psychologists, occupational therapists, and technologists, etc. 2) Highly specialised care with home technology support (i.e. telehealth). 3) Patients' educational and behavioural programmes. 4) Self-management patient education. 5) Support for families and non-professional caregivers [7, 8].
A comprehensive holistic approach has been shown to improve the quality of care of patients with COPD, decrease healthcare costs, reduce hospitalisations, and promote higher quality of life [9, 10]. Nevertheless, according to the WHO, the management of CRD is fragmented and dysfunctional with a lack of integration between layers of care [6]. In particular, coordination is often not well-developed between the inpatient (acute exacerbation phase) and outpatient (chronic phase) facilities [11]. Some studies on patients with COPD, asthma or pneumonia highlighted that inadequate coordination between these facilities might be responsible for repeat hospitalisations [12, 13], while care coordination programmes have been shown to reduce readmission risk for COPD patients by 30 days [14]. Respiratory patients often report lack of information sharing between hospitals and primary care [13], while other studies describe excessive delays in accessing secondary care and poor coordination in the healthcare team, with a lack of clear roles among the professionals involved, and practical problems with computer systems/programmes used to exchange clinical information [15, 16]. Equally importantly, neuromuscular patients and those with chronic respiratory insufficiency are often unable to access outpatient care because of poor building design [17].
Therefore, care coordination is an indispensable component of a comprehensive approach to promote high-quality care among health services and to stimulate the creation of more effective management plans. Indeed, it has been widely demonstrated that lower CRD mortality and morbidity are closely correlated with more effective care coordination plans [6]. On a global level, the WHO Chronic Respiratory Diseases Programme, in association with the Global Alliance against Chronic Respiratory Diseases, created an international alliance to promote an integrated and coordinated care approach and to provide tools and guidelines to prevent and control CRD, in an effort to reduce the global burden of respiratory disease [6, 9]. To date the majority of studies focused on care coordination in asthma and COPD. In these studies the pillars of solid care coordination were: 1) “patient-centered” systems where patients are adequately informed about their disease and therapeutic plans, so they may become the main protagonist in clinical care decisions; 2) presence of a team leader who ensures effective coordination; 3) effective information exchange systems (i.e. e-Health records, etc.); 4) vertical coordination between primary, secondary and tertiary levels of care, so that specialist care becomes easily accessible and vice versa; 5) horizontal coordination among health, educational and social services [18–20].
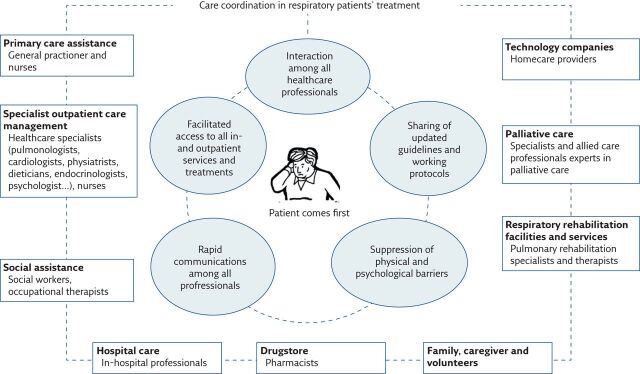
Figure 1 summarises the parties involved in the care coordination of patients affected by respiratory diseases.
Figure 1.
The parties involved in and the main roles of care coordination in patients with respiratory diseases.
Neuromuscular diseases
Similarly to the CRD, and in accordance with current guidelines, a multidisciplinary team approach is also the gold standard for healthcare in patients with neuromuscular diseases (NMD). In particular, those with chronic respiratory insufficiency requiring noninvasive ventilation [21, 22]. It proved to be effective in improving outcomes, quality of life, avoiding waste of resources and staff burnout [23]. In several nations dedicated multi-specialist clinical teams and other allied care professionals work together in the same location to ensure patients with NMD and their caregivers receive efficient multi-disciplinary support. Sometimes, the main challenge of care coordination in this field is facilitating access to this support. Since multidisciplinary clinics are generally located in main city centres, the geographic distance for many disabled patients remains a barrier, especially during the coronavirus disease pandemic. To avoid disrupting patient care, telehealth has been successfully used to fill the gap with high levels of acceptance and satisfaction among patients and caregivers [24, 25].
Telemonitoring
Telehealth has also been used effectively in CRD. Recent studies show that telehealth can be used to initiate and monitor noninvasive ventilation in patients with CRD and NMD [21–26].
Respiratory care coordination was first initiated in the field of lung cancer by respiratory and palliative specialist clinicians, surgeons and oncologists joining forces. The benefits of this have been described in the literature as extremely positive and satisfying by many patients [27, 28].
Unfortunately, some other CRD patients (for instance OSAS, ILD and rare lung diseases) still experience fragmented care among healthcare professionals. Further research in this field is warranted [29].
The burden of respiratory treatments
The treatment of respiratory patients requires a multi-specialist and comprehensive approach. When this is absent the treatment offered may be suboptimal. Improved care coordination is needed to overcome this treatment burden [30]. In this section, the most prominent issues about the current organisation of respiratory treatments and the related care coordination solutions will be described.
Inhaled therapy
Long-term management of asthma and COPD is centred on inhaled short- and long-acting bronchodilators and steroids [31], but these therapies are not always fully effective and do not always achieve their goals. The main reasons for this are as follows. 1) Adherence to inhalers: good outcomes largely depend on a patient's adherence to inhalers. However, inhaler use is often incorrect and adherence to therapy is suboptimal (e.g. in asthma and COPD it is 50–60%) [31]. The main reasons for this are: complex therapeutic regimens, poor patient knowledge on disease and treatments, and high cost of inhalers [32]. 2) Inhaler technique: incorrect inhaler technique is a major problem, especially among infants and the elderly. Other factors include the complexity of devices, the simultaneous use of several different devices, using devices not suited to the patient's abilities, mental or physical limitations, low education level and lack of proper technique training by healthcare professionals [31–34].
These factors contribute to the global burden of CRD, increasing the number of exacerbations, hospitalisations, deaths and healthcare costs [35, 36].
Better management programmes can help reduce these problems [16] through correct choice of device, effective patient training and careful monitoring over time [37, 38]. Moreover, families and caregivers should be educated in self-management [36] and different professional figures (pulmonologists, general practitioners, paediatricians, nurses, pharmacists and physiotherapists) should be involved [39, 40]. However, to date, such programmes are not widely implemented. Patients complain that they are not properly informed about their disease, not instructed on proper inhalation technique, or told about the follow-up programmes [13]. Care coordination is missing. Finally, since many clinicians were not able to demonstrate inhalation techniques, care coordination should also ensure adequate monitoring of healthcare professionals continuing educational programmes [34].
Exacerbation phases
Very often CRD, especially asthma, COPD and ILD, are characterised by exacerbations with worsening of symptoms and decline in respiratory function, with adverse economic consequences on society [41]. More than one-third of patients hospitalised for COPD exacerbations are readmitted to hospital within 90 days [42]. A possible explanation might be poor adherence to guidelines in managing these events. In particular, the misuse of antibiotics in the acute phase and poor adherence to rehabilitation in the post-acute phase have been considered as the main causes of quick readmission to hospital, leading to increased antibiotic resistance, long hospital stays and increased healthcare costs [43–45]. As the Global Alliance against Chronic Respiratory Diseases states, coordinated and integrated approaches should continually share updated guidelines, thus lifting this care burden [9]. Moreover, early recognition of disease symptoms, quickly establishing home therapy and the adequate long-term adherence to inhalation therapy are essential to reduce the number of exacerbations. In this, self-management plays a pivotal role [46, 47]. Educating patients and carers to recognise early signs of exacerbation should allow the patient to recognise and manage the acute phase together with the help of healthcare providers (i.e. clinicians, nurses and physiotherapists) [42, 48]. This is possible only in a “patient-centred” system, with strong care coordination [20].
Long-term noninvasive ventilation
CRD such as COPD, NMD, obesity hypoventilation syndrome and OSAS may require long-term noninvasive ventilation [49–53]. However, noninvasive ventilation may fail due to poor adherence. Although NIV should be performed for an adequate number of hours, many studies have shown suboptimal adherence in a non-negligible percentage of cases [54, 55]. The main causes of poor adherence are: interface-related discomfort; unintentional leaks; patient–ventilator asynchrony; patient reluctance; lack of support from the family; absent or delayed follow-up by the healthcare team; absence of symptom improvement; lack of knowledge of therapeutic goals; psychological issues; and low socioeconomic status [56, 57]. To detect and solve all these problems, clinical and instrumental monitoring, solid support of the caregiver, and educational, psychological and behavioural interventions are strongly recommended [58, 59]. However, these interventions are not available in most clinical practices and can only be found in a few specialised care locations. Care coordination should work to ensure adequate delivery and management of all these interventions. In the past few years, a big step forward has been made with the introduction of telemonitoring and technological strategies that allow remote assistance and provide a supporting network among patients and all involved professionals [60]. Indeed, recent studies have showed the effectiveness of telehealth in improving access to care and patient's outcomes [61–63].
Long-term home oxygen
Patients with chronic hypoxemic respiratory failure need long-term home oxygen but this is also burdened by management issues, which adversely affect patients’ adherence to therapy [64, 65]. Patients report problems concerning oxygen supply, low portability of equipment and lack of experienced and dedicated assistance. Moreover, physicians report issues related to long-term home oxygen prescriptions [66]. In particular, prescriptive models rely on values of arterial oxygen tension to assess oxygen demand, without considering a real titration according to oxygen saturation measured by pulse oximetry. This leads to possible under/over-estimation of oxygen need with negative clinical impacts such as disease exacerbations and emergency department admissions, and adverse economic consequences [67]. Finally, due to the current bidding system, oxygen providers cut their financial resources, equipment and staff, in order to survive in a very competitive market [66]. Consequently, the oxygen on offer in countries may be limited, causing discomfort for patients affected by chronic respiratory failure [65]. An American Thoracic Society workshop referred to this as the “oxygen supplementation crisis” and recommended using a coordinated multidisciplinary and data integration approach to change the current policy, and improve assistance to patients and outcomes. More research is needed to understand how to solve this “crisis” and guide clinical practice [66].
Pulmonary rehabilitation
During both stable periods and following exacerbations, CRD patients with moderate-to-severe disease and dyspnoea often require rehabilitation [68, 69]. In line with the latest European Respiratory Society/American Thoracic Society recommendations pulmonary rehabilitation is a multidisciplinary, comprehensive intervention, which requires coordination among pulmonologists, physiatrists, cardiologists, physiotherapists, nutritionists and psychologists. Despite the importance of this therapy, several studies report that many patients have not accessed the treatment [70, 71]. This might be due to a shortage of pulmonary rehabilitation services or because patients are unaware of the benefits, are poorly motivated or face other barriers. There are significant variations in rehabilitation rates among countries, and among patients with different types of CRD. There is a need to improve care coordination among healthcare professionals and to increase the availability of services [68]. Home-based pulmonary telerehabilitation has been promoted in recent years. It has been shown to improve functional exercise capacity, outcomes, adherence rates and access to pulmonary rehabilitation, possibly because it does not require patients to travel and is easier to perform [71–73]. Healthcare professionals may now closely follow-up patients at home via virtual reality, augmented reality and artificial intelligence [74–76]. The use of pulmonary telerehabilitation was expanded during the coronavirus disease 2019 (COVID-19) pandemic because standard rehabilitation programmes were suspended and to respond to the specialist rehabilitation needs of COVID-19 survivors following hospital discharge [77]. However, some significant issues have emerged including: some patients lack access to computers; methods and tools for programme evaluation are not standardised; and training and resources for health professionals is inadequate [78]. To overcome these problems, researchers have proposed models of pulmonary telerehabilitation in respiratory diseases patients, and rapidly implemented their use in clinical practice [79].
Comorbidities
CRD patients often suffer from comorbidities such as cardiovascular diseases, metabolic disorders, osteoporosis, skeletal muscle dysfunction, anxiety/depression, cognitive impairment, gastrointestinal diseases and chronic cough [80–82]. One or more comorbidity was present in up to 97% of COPD patients and four or more comorbidities in 53% of COPD patients [83]. These multi-morbidities require a complex, multidisciplinary interventions and are usually associated with worse outcomes and increased health costs. The simultaneous management of these conditions represents a significant challenge which may lead to a sub-optimal treatment experience. The different specialists involved may not share information effectively and may differ in their views on how best to treat the patient. This may lead to patients losing trust in health professionals’ competence [80, 84]. Although recent research indicates that care coordination significantly improves the burden of care in patients affected by CRD [14], health systems still need to improve coordination and integration of these patients’ care. E-health may play an important role in facilitating healthcare professionals and patients to access information [85].
Palliative care
The advanced/end stages of all respiratory diseases may require palliative care. Unfortunately, many barriers to palliative care still exist, including lack of skilled professionals, patients’ fear of moving to end-of-life care and the lack of adequate financial and other resources. New integrated palliative care models have been shown to be effective but they require multidisciplinary cooperation and coordination. In particular, robust integration between respiratory palliative care clinicians, specialist nurses, physiotherapists, psychologists and social workers proved to be effective in ensuring high-quality standards for care were met in the field of CRD [86, 87], with many patients with end-stage respiratory diseases reporting significant improvement in their quality of life and approach to the disease. There is a growing literature consensus that palliative care should be considered more for severe disease symptoms management and earlier initiation of palliative care over the course of the chronic respiratory disease would result in a remarkable improvement in the quality of life of patients with ILD and lung cancer. Indeed, as the patients report; timely palliative care was able to “take away their breathlessness and fears” [88, 89].
COVID-19 pandemic
Recently, the global healthcare system suffered an enormous challenge due to the COVID-19 pandemic. The world is witnessing a global healthcare crisis with huge demands being placed on over-stretched national healthcare systems. The surge in capacity of staff, space, supplies, systems and most importantly communications has been vital in making it possible for healthcare systems to cope with the disaster. Also, the importance of effective care coordination has become particularly clear. Despite massive efforts to face the emergency, the COVID-19 tsunami flooded the worlds healthcare systems, highlighting their frailties [90, 91]. Older patients affected by CRD, those with multiple comorbidities dependant on caregivers, oncologic and immunocompromised patients are more likely to be infected, become seriously ill after infection, be isolated, have worse access to medical care and clinical follow-up, and die [92, 93]. Unfortunately, many patients interrupted their usual outpatient follow-up visits because of a fear of being infected, incomplete cover of telehealth facilities and because there was a shortage of physicians and nurses diverted to COVID intensive care units [94, 95]. Despite this, patient-centred care, social care networks and public health have, in some places, resulted in a more integrated approach of care [96]. Clinical guidelines have been introduced which emphasise “crisis care coordination” in all medical fields, especially respiratory disease [96]. Implementation guidelines have emphasised re-organisation of all levels of inside and outside hospital care. Health systems have risen to the occasion and established new ways of working. For example, intensive care unit staff have been supported by doctors and nurses from other specialties, including advanced practice practitioners’ working in the intensive care unit and anaesthesiologists working as intensive care unit flexible physicians; using tele-critical and tele-outpatient care models to screen for emergency admission or necessary respiratory follow-up visits, decentralising the hub of care [97, 98].
Telemedicine
Different applications of telehealth technologies have been used in the care of respiratory patients, including telemonitoring, teleconsultations, tele-education, and telehealth-pulmonary rehabilitation [99]. This has expanded during the last year due to the COVID-19 pandemic, with many healthcare services forced to rely on telemedicine to follow-up patients who cannot visit clinics. Unfortunately, many hospitals and advanced care facilities are yet not fully equipped to provide the support needed. However, some nations such as France and China have already approved telehealth programme networks partially or fully covering the related costs while others have not done so as yet. The use and funding of digital health technologies should be carefully evaluated and monitored by governments which should play a more active role in surveying its real outcomes on the health system [100–103]. It must be recognised that telemedicine may also carry ethical and legal problems that are still not recognised internationally [104].
Summary and future vision
Care coordination in the field of CRD still offers some heterogeneity of practice. The future of care coordination in the field of respiratory disease holds the improvement of all described networks and their management. Some CRD have already reached a better care coordination network setting than others. However, the incredible support given to the hospitals, the speed and the strength demonstrated in optimising the healthcare forces has begun to bear fruits. Patients feedback is already positive but robust literature on the CRD is still missing [14, 99, 100]. It is indeed a topic of current interest for the scientific community, therefore, more research will be warranted to further expand it.
In summary, the burden of respiratory treatment related to the lack of care coordination is still high but progress has been made to fill the gap and to continue to improve, aiming to offer the best possible integrated care for patients affected by CRD.
Self-evaluation questions
What does “care coordination” stand for?
Why is care coordination useful in the field of respiratory diseases?
What is the treatment burden of respiratory patients related to?
How could care coordination reduce the treatment burden of respiratory patients?
Could care coordination have a key role in patient management during the COVID-19 pandemic?
Suggested answers
Care coordination characterises high-quality healthcare systems filling the gaps between various levels of care facilities. Its main aims are to provide appropriate and efficient care within the correct time-frame, reducing costs and hospitalisations.
The management of respiratory diseases is complex and needs a comprehensive approach and continuous attention. Care coordination is useful in providing continuity among all healthcare professionals and facilities involved.
The treatment of patients with chronic respiratory diseases involves education, monitoring, clinical follow-up, rehabilitation, palliative care and telemedicine. Due to many inter-connected layers and inefficient care coordination among them the result may be sub-optimal leading to a burden that is added to the disease load.
Care coordination should facilitate patients' access to treatments, educational programmes, therapy monitoring systems and sharing of updated guidelines. It should also provide logistic support and adequate information among all healthcare professionals inside and outside hospitals and primary care, to promote social health and educational services.
Yes. It could help ensure a strong, collaborative, effective response and a consistent standard of care to patients affected by COVID-19 during the global pandemic.
Key points
Treatment of respiratory diseases may be complex and often requires multidisciplinary approaches.
Care coordination is an important aspect of high-quality healthcare systems in ensuring optimal care of respiratory patients and reducing hospitalisations and costs.
Care coordination should guarantee the coordination of multidisciplinary care and the access to effective levels of care (i.e. acute, chronic, in- and outpatients, rehabilitation and home care).
During the COVID-19 pandemic, care coordination has an important role to play in ensuring correct management and safeguarding adequate treatment for all respiratory patients.
Footnotes
Conflict of interest: P. Pierucci has nothing to disclose.
Conflict of interest: C. Santomasi has nothing to disclose.
Conflict of interest: N. Ambrosino has nothing to disclose.
Conflict of interest: A. Portacci has nothing to disclose.
Conflict of interest: F. Diaferia has nothing to disclose.
Conflict of interest: K. Hansen has nothing to disclose.
Conflict of interest: M. Odemir has nothing to disclose.
Conflict of interest: S. Jones has nothing to disclose.
Conflict of interest: G.E. Carpagnano has nothing to disclose.
References
- 1.National Quality Forum . 2021. https://www.qualityforum.org/Home
- 2.Agency for Healthcare Research and Quality . 2021. www.ahrq.gov/ncepcr/care/coordination.html [DOI] [PubMed]
- 3.Schultz EM, Pineda N, Lonhart J, et al. A systematic review of the care coordination measurement landscape. BMC Health Serv Res 2013; 13: 119. doi: 10.1186/1472-6963-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Agency for Healthcare Research and Quality, 2007. [Google Scholar]
- 5.World Health Organization . Continuity and coordination of care: a practice brief to support implementation of the WHO Framework on integrated people-centred health services. 2018. https://apps.who.int/iris/handle/10665/274628
- 6.World Health Organization . Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. 2007. www.who.int/gard/publications/GARD_Manual/en/
- 7.San Ko FW, Chan KP, Cheong Hui DS. Comprehensive care for chronic obstructive pulmonary disease. J Thorac Dis 2019; 11: Suppl. 17, S2181–S2191. doi: 10.21037/jtd.2019.06.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano I, Duenas-Espìn I, Hernandez C, et al. Protocol for regional implementation of community-based collaborative management of complex chronic patients. NPJ Prim Care Respir Med 2017; 27: 44. doi: 10.1038/s41533-017-0043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaltaev N. GARD, a new way to battle with chronic respiratory diseases, from disease oriented programmes to global partnership. J Thorac Dis 2017; 9: 4676–4689. doi: 10.21037/jtd.2017.11.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CX, Hwang CH, Tan WS, et al. Effectiveness of a chronic obstructive pulmonary disease integrated care pathway in a regional health system: a propensity score matched cohort study. BMJ Open 2018; 8: e019425. doi: 10.1136/bmjopen-2017-019425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nici L, ZuWallack R, American Thoracic Society Subcommittee Integrated Care of the COPD Patient . An Official American Thoracic Society workshop report: the integrated care of the COPD patient. Proc Am Thorac Soc 2012; 9: 9–18. doi: 10.1513/pats.201201-014ST [DOI] [PubMed] [Google Scholar]
- 12.Russo AN, Sathiyamoorthy G, Lau C, et al. Impact of a post-discharge integrated disease management program on COPD hospital readmissions. Respir Care 2017; 62: 1396–1402. doi: 10.4187/respcare.05547 [DOI] [PubMed] [Google Scholar]
- 13.Jo HS, Jeong S, Kim WJ, et al. Development of a transitional care model program for patients with pneumonia, asthma, and chronic obstructive pulmonary disease: in-depth interviews with readmitted patients. J Korean Med Sci 2020; 35: e352. doi: 10.3346/jkms.2020.35.e352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo AN, Sathiyamoorthy G, Lau C,et al. The impact of a COPD care coordination program and its components on COPD 30-Day readmission. Am J Respir Crirt Care Med 2016; 193: A115. [Google Scholar]
- 15.Waibel S, Vargas I, Aller M-B, et al. The performance of integrated health care networks in continuity of care: a qualitative multiple case study of COPD patients. Int J Integr Care 2015; 15: e029. doi: 10.5334/ijic.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pike KC, Levy ML, Moreiras J, et al. Managing problematic severe asthma: beyond the guidelines. Arch Dis Child 2018; 103: 392–397. doi: 10.1136/archdischild-2016-311368 [DOI] [PubMed] [Google Scholar]
- 17.Fradgley EA, Paul CL, Bryant J. A systematic review of barriers to optimal outpatient specialist services for individuals with prevalent chronic diseases: what are the unique and common barriers experienced by patients in high income countries? Int J Equity Health 2015; 14: 52. doi: 10.1186/s12939-015-0179-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amalakuhan B, Adams SG. Improving outcomes in chronic obstructive pulmonary disease: the role of the interprofessional approach. Int J Chron Obstruct Pulmon Dis 2015; 10: 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanfleteren LEGW, van‘t Hul AJ, Kulbacka-Ortiz K, et al. Challenges to the application of integrated, personalized care for patients with COPD – A vision for the role of clinical information. J Clin Med 2020; 9: 1311. doi: 10.3390/jcm9051311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yawn BP, Wechsler ME. Severe asthma and the primary care provider: identifying patients and coordinating multidisciplinary care. Am J Med 2017; 130: 1479. doi: 10.1016/j.amjmed.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Shah NM, Murphy PB, Kaltsakas G. The adult multidisciplinary respiratory neuromuscular clinic. Breathe 2020; 16: 200121. doi: 10.1183/20734735.0121-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierucci P, Crimi C, Carlucci, et al. ERS International Survey on REstrictive thoracic diseases IN long term home noninvasive VENTilation: REINVENT. ERJ Open Res 2021; 7: 00911-2020. doi: 10.1183/23120541.00911-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paganoni S, Nicholson K, Leigh F, et al. Developing multidisciplinary clinics for neuromuscular care and research. Muscle Nerve 2017; 56: 848–858. doi: 10.1002/mus.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard I, Potts A. Interprofessional care for neuromuscular disease. Curr Treat Options Neurol 2019; 21: 35. doi: 10.1007/s11940-019-0576-z [DOI] [PubMed] [Google Scholar]
- 25.Schwarz SB, Windisch W. Outpatient noninvasive ventilation: can the Dutch setting serve as a blueprint for other countries? Chest 2020; 158: 2255–2257. doi: 10.1016/j.chest.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Hogden A, Foley G, Henderson RD, et al. Amyotrophic lateral sclerosis: improving care with a multidisciplinary approach. J Multidiscip Healthc 2017; 10: 205–215. doi: 10.2147/JMDH.S134992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collett GK. Patients’ experience of lung cancer care coordination: a quantitative exploration. Support Care Cancer 2019; 27: 485–493. doi: 10.1007/s00520-018-4338-3 [DOI] [PubMed] [Google Scholar]
- 28.Linford G. Patient and physician perceptions of lung cancer care in a multidisciplinary clinic model. Curr Oncol 2020; 27: e9–e19. doi: 10.3747/co.27.5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veigh CM. The experience of palliative care service provision for people with non-malignant respiratory disease and their family carers: an all-Ireland qualitative study. J Adv Nurs 2018; 74: 383–394. doi: 10.1111/jan.13453 [DOI] [PubMed] [Google Scholar]
- 30.Dobler CC. Treatment burden is important to patients but often overlooked by clinicians. Breathe 2021; 17: 210031. doi: 10.1183/20734735.0031-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin AN, Ganapathy V, Roughley A, et al. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence 2017; 11: 1205–1212. doi: 10.2147/PPA.S140139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Tarso Roth Dalcin P, Grutcki DM, Paganella Laporte P, et al. Factors related to the incorrect use of inhalers by asthma patients. J Bras Pneumol 2014; 40: 13–20. doi: 10.1590/S1806-37132014000100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duarte-deAraukop A, Teixeira P, Espanhol V, et al. COPD: misuse of inhaler devices in clinical practice. Int J Chron Obstruct Pulmon Dis 2019; 14: 1209–1217. doi: 10.2147/COPD.S178040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis 2012; 7: 495–502. doi: 10.2147/COPD.S32674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virchow JC, Akdis CA, Darba J, et al. A review of the value of innovation in inhalers for COPD and Asthma. J Market Access Health Pol 2015; 3: 28760. doi: 10.3402/jmahp.v3.28760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leiva-Fernández F, Leiva-Fernández J, Zubeldia-Santoyo F, et al. Efficacy of two educational interventions about inhalation techniques in patients with chronic obstructive pulmonary disease (COPD). TECEPOC: study protocol for a partially randomized controlled trial (preference trial). Trials 2012; 13: 64. doi: 10.1186/1745-6215-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2020. www.goldcopd.org
- 38.Global Initiative for Asthma . GINA Report: Global Strategy for Asthma Management and Prevention. 2020. https://ginasthma.org
- 39.Catel-Branco MM, Fontes A, Figueiredo IV. Identification of inhaler technique errors with a routine procedure in Portuguese community pharmacy. Pharm Pract (Granada) 2017; 15: 1072. doi: 10.18549/PharmPract.2017.04.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willard-Grace R, Chirinos C, Wolf J, et al. Lay health coaching to increase appropriate inhaler use in COPD: a randomized controlled trial. Ann Fam Med 2020; 18: 5–14. doi: 10.1370/afm.2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halpin DM, Miravitlles M, Metzdorf N, et al. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis 2017; 12: 2891–2908. doi: 10.2147/COPD.S139470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong CW, Wilkinson TMA. Predicting and preventing hospital readmission for exacerbations of COPD. ERJ Open Res 2020; 6: 00325-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, et al. Antibiotic prescription for COPD exacerbations admitted to hospital: European COPD Audit. PLoS One 2015; 10: e0124374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plantinga NL, Wittekamp BH, van Duijn PJ, et al. Fighting antibiotic resistance in the intensive care unit using antibiotics. Future Microbiol 2015; 10: 391–406. doi: 10.2217/fmb.14.146 [DOI] [PubMed] [Google Scholar]
- 45.Machowska A, Stålsby Lundborg C. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health 2018; 16: 27. doi: 10.3390/ijerph16010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yawn BP, Thomashow B. Management of patients during and after exacerbations of chronic obstructive pulmonary disease: the role of primary care physicians. Int J Gen Med 2011; 4: 665–676. doi: 10.2147/IJGM.S22878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology 2016; 21: 1152–1165. doi: 10.1111/resp.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jardim JR, Nascimento OA. The importance of inhaler adherence to prevent COPD exacerbations. Med Sci 2019; 7: 54. doi: 10.3390/medsci7040054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricciardolo FLM, Carriero V, Bullone M. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020; 202: 4–6. doi: 10.1164/rccm.202004-1216ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banfi P, Pierucci P, Volpato E, et al. Daytime noninvasive ventilatory support for patients with ventilatory pump failure: a narrative review. Multidiscip Respir Med 2019; 14: 38. doi: 10.1186/s40248-019-0202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clini E, Sturani C, Porta R, et al. Outcome of COPD patients performing nocturnal non-invasive mechanical ventilation. Respir Med 1998; 92: 1215–1222. doi: 10.1016/S0954-6111(98)90424-3 [DOI] [PubMed] [Google Scholar]
- 52.Clini E, Vitacca M, Foglio K, et al. Long-term home care programmes may reduce hospital admissions in COPD with chronic hypercapnia. Eur Respir J 1996; 9: 1605–1610. doi: 10.1183/09031936.96.09081605 [DOI] [PubMed] [Google Scholar]
- 53.Crimi C, Pierucci P, Carlucci Asa, et al. Long-term ventilation in neuromuscular patients: review of concerns, beliefs, and ethical dilemmas. Respiration. 2019; 97: 185–196. 10.1159/000495941 [DOI] [PubMed] [Google Scholar]
- 54.Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung 2019; 197: 115–121. doi: 10.1007/s00408-018-00193-1 [DOI] [PubMed] [Google Scholar]
- 55.Fiorentino G, Annunziata A, Gaeta MA, et al. Continuous noninvasive ventilation for respiratory failure in patients with amyotrophic lateral sclerosis: current perspectives. Degener Neurol Neuromuscul Dis 2018; 8: 55–61. doi: 10.2147/DNND.S170771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ennis J, Rohde K, Chaput J-P, et al. Facilitators and barriers to noninvasive ventilation adherence in youth with nocturnal hypoventilation secondary to obesity or neuromuscular disease. J Clin Sleep Med 2015; 11: 12. doi: 10.5664/jcsm.5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo MY, Lee SH. Compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Sleep Med Res 2020; 11: 7–14. doi: 10.17241/smr.2020.00563 [DOI] [Google Scholar]
- 58.Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med 2019; 15: 335–343. doi: 10.5664/jcsm.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eleonora Volpato E, Banfi P, Francesco Pagnini F. A psychological intervention to promote acceptance and adherence to non-invasive ventilation in people with chronic obstructive pulmonary disease: study protocol of a randomised controlled trial. Trials 2017; 18: 59. doi: 10.1186/s13063-017-1802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamarrón C, Morete E, González F. Telemedicine system for the care of patients with neuromuscular disease and chronic respiratory failure. Arch Med Sci 2014; 10: 1047–1051. doi: 10.5114/aoms.2014.46223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baltaxe E, Embid C, Aumatell E, et al. Integrated care intervention supported by a mobile health tool for patients using noninvasive ventilation at home: randomized controlled trial monitoring. JMIR Mhealth Uhealth 2020; 8: e16395. doi: 10.2196/16395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aardoom JJ, Loheide-Niesmann L, Ossebaard HC, et al. Effectiveness of eHealth interventions in improving treatment adherence for adults with obstructive sleep apnea: meta-analytic review. J Med Internet Res 2020; 22: e16972. doi: 10.2196/16972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto A, Almeida JP, Pinto S, et al. Home telemonitoring of non-invasive ventilation decreases healthcare utilisation in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2010; 81: 1238–1242. doi: 10.1136/jnnp.2010.206680 [DOI] [PubMed] [Google Scholar]
- 64.Almutairi HJ, Mussa CC, Lambert CTM, et al. Perspectives from COPD subjects on portable long-term oxygen therapy devices. Respir Care 2018; 63: 1321–1330. doi: 10.4187/respcare.05916 [DOI] [PubMed] [Google Scholar]
- 65.Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy: results of the American Thoracic Society nursing assembly oxygen working group survey. Ann Am Thorac Soc 2018; 15: 24–32. doi: 10.1513/AnnalsATS.201703-209OC [DOI] [PubMed] [Google Scholar]
- 66.Jacobs SS, Lederer DJ, Garvey CM, et al. Optimizing home oxygen therapy: An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2018; 15: 1369–1381. doi: 10.1513/AnnalsATS.201809-627WS [DOI] [PubMed] [Google Scholar]
- 67.Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol 2018; 11: 279–289. doi: 10.1080/17512433.2018.1421457 [DOI] [PubMed] [Google Scholar]
- 68.Spruit MA, Singh SJ, Garvey C, et al. An Official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 69.Rochester CL, Vogiatzis I, Holland AE, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med 2015; 192: 1373–1386. doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 70.Puhan MA. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 12: CD005305. doi: 10.1002/14651858.CD005305.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Qian H, Yu K, et al. Nonadherence in home-based pulmonary rehabilitation program for COPD patients. Can Respir J 2020; 2020: 5146765. doi: 10.1155/2020/5146765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang R, Bruning J, Morris N, et al. A systematic review of the effects of telerehabilitation in patients with cardiopulmonary diseases. J Cardiopulm Rehabil Prev 2015; 35: 380–389. doi: 10.1097/HCR.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 73.Cox NS, McDonald CF, Alison JA, et al. Telerehabilitation versus traditional centre-based pulmonary rehabilitation for people with chronic respiratory disease: protocol for a randomised controlled trial. BMC Pulm Med 2018; 18: 71. doi: 10.1186/s12890-018-0646-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colombo V, Aliverti A, Sacco M. Virtual reality for COPD rehabilitation: a technological perspective. Pulmonology 2020; in press [ 10.1016/j.pulmoe.2020.11.010]. doi: 10.1016/j.pulmoe.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 75.Angelucci A, Aliverti A. Telemonitoring systems for respiratory patients: technological aspects. Pulmonology 2020; 26: 221–232. doi: 10.1016/j.pulmoe.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 76.Bernocchi P, Vitacca M, La Rovere MT, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age aging 2018; 47: 82–88. doi: 10.1093/ageing/afx146 [DOI] [PubMed] [Google Scholar]
- 77.Salawu A, Green A, Crooks MG, et al. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int J Environ Res Public Health 2020; 17: 4890. doi: 10.3390/ijerph17134890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsutsui M, Gerayeli F, Sin D D. Pulmonary rehabilitation in a post-COVID-19 world: telerehabilitation as a new standard in patients with COPD. Int J Chron Obstruct Pulmon Dis 2021; 16: 379–391. doi: 10.2147/COPD.S263031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Selzler A-M, Wald J, Sedeno M, et al. Telehealth pulmonary rehabilitation: a review of the literature and an example of a nationwide initiative to improve the accessibility of pulmonary rehabilitation. Chron Respir Dis 2018; 15: 41–47. doi: 10.1177/1479972317724570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med 2013; 1: 73–83. doi: 10.1016/S2213-2600(12)70060-7 [DOI] [PubMed] [Google Scholar]
- 81.Negewo NA, McDonald VM, Gibson PG. Comorbidity in chronic obstructive pulmonary disease. Respir Investig 2015; 53: 249–258. doi: 10.1016/j.resinv.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 82.Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology 2015; 20: 1160–1171. doi: 10.1111/resp.12642 [DOI] [PubMed] [Google Scholar]
- 83.Vanfleteren LE. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. doi: 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 84.Haggerty JL. Ordering the chaos for patients with multimorbidity. Building continuity of care takes work but earns trust. BMJ 2012; 345: e5915. doi: 10.1136/bmj.e5915 [DOI] [PubMed] [Google Scholar]
- 85.Han MK. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med 2016; 4: 473–526. doi: 10.1016/S2213-2600(16)00094-1 [DOI] [PubMed] [Google Scholar]
- 86.Carlucci A, Vitacca M, Malovini A, et al. End-of-life discussion, patient understanding and determinants of preferences in very severe COPD patients: a multicentric study. COPD 2016; 13: 632–638. doi: 10.3109/15412555.2016.1154034 [DOI] [PubMed] [Google Scholar]
- 87.Ambrosino N, Fracchia C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. Pulmonology 2019; 25: 289–298. doi: 10.1016/j.pulmoe.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 88.Pierucci P, Carlucci A. End-stage respiratory diseases and respiratory support. In: Bausewein C, Currow DC, Johnson MJ, eds. Palliative Care in Respiratory Disease (ERS Monograph). Sheffield, European Respiratory Society; , 2016; pp. 233. [Google Scholar]
- 89.Kreuter M, Bendstrup E, Russell AM, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med 2017; 5: 968–980. doi: 10.1016/S2213-2600(17)30383-1 [DOI] [PubMed] [Google Scholar]
- 90.Hick JL, Hanfling D, Wynia MK, et al. Duty to plan: health care, crisis standards of care, and novel coronavirus SARS-CoV-2. NAM Perspectives. Discussion paper. Washington, National Academy of Medicine; , 2020. [Google Scholar]
- 91.Winck JC, Ambrosino N. COVID-19 pandemic and non invasive respiratory management: every Goliath needs a David. Pulmonology 2020; 26: 213–220. doi: 10.1016/j.pulmoe.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodin G, Zimmermann C, Rodin D, et al. COVID-19, palliative care and public health. Eur J Cancer 2020; 136: 95–98. doi: 10.1016/j.ejca.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perrotta F, Corbi G. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res 2020; 16: 1–10. doi: 10.1007/s40520-020-01631-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhutani M, Hernandez P, Bourbeau J, et al. Key Highlights of the Canadian Thoracic Society's Position Statement on the Optimization of COPD management during the coronavirus disease 2019 pandemic. Chest 2020; 158: 869–872. doi: 10.1016/j.chest.2020.05.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Licskai C, Yang CL, Ducharme FM, et al. Key highlights from the Canadian Thoracic Society position statement on the optimization of asthma management during the coronavirus disease 2019 pandemic. Chest 2020; 158: 1335–1337. doi: 10.1016/j.chest.2020.05.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stein KV, Goodwin N, Miller R. From crisis to coordination: challenges and opportunities for integrated care posed by the COVID-19 pandemic. Int J Integr Care 2020; 20: 7. doi: 10.5334/ijic.5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hussain RS, Kataria TC. Adequacy of workforce – are there enough critical care doctors in the US-post COVID? Curr Opin Anaesthesiol 2021; 34: 149–153. doi: 10.1097/ACO.0000000000000970 [DOI] [PubMed] [Google Scholar]
- 98.Provenzano DA. Clinical and economic strategies in outpatient medical care during the COVID-19 pandemic. Reg Anesth Pain Med 2020; 45: 579–585. doi: 10.1136/rapm-2020-101640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ambrosino N, Vitacca M, Dreher M, et al. Tele-monitoring of ventilator-dependent patients: a European Respiratory Society Statement. Eur Respir J 2016; 48: 648–663. doi: 10.1183/13993003.01721-2015 [DOI] [PubMed] [Google Scholar]
- 100.Hohmann NS, McDaniel CC, Mason, SW, et al. Patient perspectives on primary care and oncology care coordination in the context of multiple chronic conditions: a systematic review. Res Social Adm Pharm 2020; 16: 1003–1016. doi: 10.1016/j.sapharm.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 101.Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015; 9: CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marcelo A, Ganesh J, Mohan J, et al. Governance and management of national telehealth programs in Asia. Stud Health Technol Inform 2015; 209: 95–101. [PubMed] [Google Scholar]
- 103.Ricciardi W, Pita Barros P, Bourek A, et al. How to govern the digital transformation of health services. Eur J Public Health 2019; 29; Suppl. 3: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ambrosino N, Makhabah DN. Tele-medicine: a new promised land, just to save resources? Eur Respir J 2017; 49: 1700410. [DOI] [PubMed] [Google Scholar]