Abstract
Background
Disease modifying therapies for multiple sclerosis (MS) can impair the specific immune response to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Specifically, it is recognized that ocrelizumab reduces or abrogates anti-SARS-CoV-2 antibody production after natural infection or vaccination, while very little is known about T-cell responses.
Methods
We developed an interferon (IFN)-γ release assay (IGRA) to detect T-cell responses specific to SARS-CoV-2 after overnight stimulation of whole blood with peptide libraries covering the immunodominant sequence domains of the Spike glycoprotein (S) and the Nucleocapsid phosphoprotein (N).
Results
Five patients with MS receiving ocrelizumab treatment for at least 1 year and recovered from SARS-CoV-2 infection were enrolled in the study. Despite the absence or the very low concentration of anti-S antibodies, a T-cell response was detectable in all the five MS patients. These results are in accordance with the marked reduction of peripheral B-lymphocyte absolute counts induced by ocrelizumab, that, conversely, did not affect peripheral blood T-lymphocyte subset absolute and relative counts and CD4/CD8 ratio.
Conclusions
The detection of specific T-cell responses to SARS-CoV-2 in patients receiving B-cell depleting therapies represents a useful tool to improve the diagnostic approach in SARS-CoV-2 infection and to accurately assess the immunological response after natural infection or vaccination.
Keywords: Disease modifying therapies; T-lymphocyte, peptides; Stimulation; Interferon-gamma; IGRA; CD20
1. Background
We investigated specific T-cell responses towards severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in patients receiving B-cell depleting treatment with ocrelizumab for multiple sclerosis (MS), who recovered from coronavirus disease-2019 (COVID-19). Little is known about cell-mediated responses in patients receiving B-cell depleting therapies after SARS-CoV-2 natural infection or vaccination (Asplund Högelin et al., 2021), while several case reports have demonstrated the absence of detectable anti-SARS-CoV-2 antibodies (Ab) (Iannetta et al., 2020; Khayat-Khoei et al., 2021; Smets et al., 2021; Thornton and Harel, 2020). Recently, Achiron et al. showed poor seroconversion rates after SARS-CoV-2 vaccination in MS patients receiving B-cell depleting treatments (Achiron et al., 2021). The role of T-cell responses in terms of protection against SARS-CoV-2 infection and re-infection needs to be further elucidated.
Interferon (IFN)-γ release assay (IGRA) represents a useful tool to identify individuals with specific cellular immunity for SARS-CoV-2 (Murugesan et al., 2020).
2. Methods
The study was approved by the local Ethic Committees (number 46.20). All patients signed a written informed consent. Anti-Spike Ab specific for SARS-CoV-2 were detected with a commercial electrochemiluminescence immunoassay (Elecsys® Anti-SARS-CoV-2 S, Roche Diagnostics). Peripheral blood lymphocyte subsets were assessed with a lyse no wash standardized protocol, using the BD® Multitest 6-Color TBNK Reagent and trucount tubes (BD Biosciences). T-lymphocyte specific response was assessed with an interferon (IFN)-γ release assay (IGRA) after overnight stimulation with SARS-CoV-2 peptide libraries. Pools of lyophilized peptides, consisting mainly of 15-mer sequences with 11 amino acids overlap, covering the immunodominant sequence domains of the Spike glycoprotein (S) (GenBank MN908947.3, Protein QHD43416.1) and the Nucleocapsid phosphoprotein (N) (GenBank MN908947.3, Protein QHD43423.2) of SARS-CoV-2 were purchased from Milteny Biotec. Briefly, 500µl of fresh heparinized blood were incubated overnight at 37°C and 5% CO2, after stimulation with PepTivator® S1, S, and N (3 conditions for each peptide library plus 1 condition with pooled peptides) at a final concentration of 1µg/ml. For each patient a negative and positive (phytohemagglutinin 5µg/ml) condition was also included. After 18 hours, supernatants were collected and stored at -80°C. IFN-γ production was assessed with a commercial enzyme linked immunosorbent assay (ELISA) kit (Human IFN-γ DuoSet ELISA, R&D Systems), following manufacturer's instructions. SARS-CoV-2 anti-Spike antibodies and T-lymphocyte specific responses were assessed on the same day.
3. Results
Five patients (4 males, median age 33 years, range 27-50) with a relapsing remitting (RR) MS, receiving ocrelizumab treatment, who experienced SARS-CoV-2 infection, were enrolled in this study. All patients had been exposed to ocrelizumab for at least 1 year. Median expanded disability status scale (EDSS) was 0.5 (range 0-3). SARS-CoV-2 infection was diagnosed through SARS-CoV-2 RNA molecular detection on nasopharyngeal swab. Two patients needed hospitalization because of COVID-19 pneumonia, and were classified as severe. Patients were sampled after a median of 89 days from COVID-19 onset (range: 66-176) and 89 days from the last ocrelizumab infusion (range: 36-196). Clinical data are reported in Table 1 .
Table 1.
Demographic, clinical and laboratory characteristics of the 5 RRMS patients under ocrelizumab treatment.
| ID | Age | Sex | EDSS | Disease duration (years) | N# of OCRE infusions | Last OCRE COVID-19 (days) | Last OCRE-Sampling (days) | COVID-19 Sampling (days) | COVID-19 Severity | COVID-19 hospital | NPhS-POS NPhS-NEG (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OCRE1 | 33 | M | 0 | 9 | 4 | 184 | 89 | 154 | Mild | No | 7 |
| OCRE2 | 33 | M | 0 | 6 | 3 | 17 | 83 | 66 | Severe | Yes | 31 |
| OCRE3 | 27 | M | 1 | 2 | 5 | 36 | 125 | 89 | Mild | No | 14 |
| OCRE4 | 34 | F | 2.5 | 6 | 4 | 20 | 196 | 176 | Severe | Yes | 25 |
| OCRE5 | 50 | M | 3 | 5 | 5 | 175 | 36 | 85 | Mild | No | 24 |
| ID | Anti-Spike Ab (U/ml) |
CD19% | CD19# cells/µl |
CD3% | CD3# cells/µl |
CD4% | CD4# cells/µl |
CD8% | CD8# cells/µl |
CD4/CD8 ratio | |
| OCRE1 | 0.54 | 0 | 0 | 64.29 | 1337 | 47.3 | 984 | 15.23 | 317 | 3.05 | |
| OCRE2 | <0.40 | 0.08 | 1 | 75.99 | 922 | 38.2 | 464 | 27.54 | 334 | 1.38 | |
| OCRE3 | <0.40 | 0.04 | 1 | 72.74 | 1140 | 35.85 | 562 | 29.54 | 463 | 1.21 | |
| OCRE4 | <0.40 | 0.96 | 15 | 90.88 | 1376 | 53.64 | 812 | 34.19 | 518 | 1.57 | |
| OCRE5 | 155.6 | 0 | 0 | 69.81 | 895 | 48.02 | 615 | 18.47 | 237 | 2.53 | |
Demographic and clinical characteristics of the 5 RRMS patients under ocrelizumab treatment. MS: multiple sclerosis; RR: relapsing-remitting; OCRE: ocrelizumab; Last OCRE COVID-19: days from last ocrelizumab infusion to COVID-19 symptoms onset; Last OCRE Sampling: days from last ocrelizumab infusion to the blood sampling day for T-cell stimulation; COVID-19 Sampling: days from COVID-19 symptom onset to the blood sampling day for T-cell stimulation and anti-Spike antibody quantification; COVID-19 hospital: hospitalization because of COVID-19; NphS-pos NPh-S neg: days from the first SARS-CoV-2 positive nasopharyngeal swab to the first negative nasopharyngeal swab. Ab: antibodies; Anti-Spike Ab limit of detection: 0.40 U/ml
Anti-S Ab were detectable only in two subjects (OCRE1: 0.54 U/ml and OCRE5: 155.6 U/ml) at low concentrations. Flow cytometry analysis of peripheral blood showed a reduction in CD19+ B-lymphocyte absolute and relative counts (median: 1 cell/µl, range 0-15; median 0.04%, range 0-0.96; respectively). Conversely, CD4+ and CD8+ T-cell absolute and relative counts were within normal ranges, and CD4/CD8 ratio was above 1 for all the subjects (Table 1).
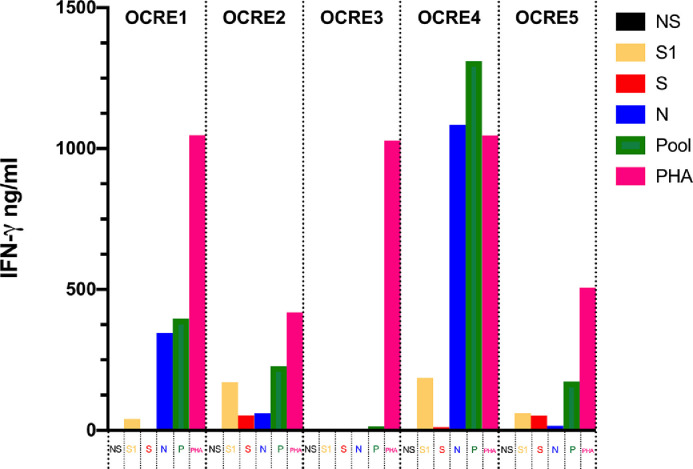
Whole blood stimulation showed detectable levels of IFN-γ upon PepTivator® S1 and N stimulation in all patients with the exception of subject OCRE3. After stimulation with PepTivator® S, IFN-γ was undetectable in supernatants of 2 subjects (OCRE1 and OCRE3). Interestingly, after pooling together the three peptide libraries (S1, S and N), an IFN-γ production was detectable in all the subjects, including subject OCRE3 (Fig. 1 ).
Fig. 1.
Interferon-gamma release assay for the detection of T-cell responses to SARS-CoV-2 in 5 multiple sclerosis patients treated with ocrelizumab.
T-cell specific response to SARS-CoV-2 was assessed with an IFN-γ release assay after overnight stimulation with SARS-CoV-2 peptide libraries (PepTivator® SARS-CoV-2 Prot_S1, Prot_S and Prot_N). PepTivator® SARS-CoV-2 Prot_S1 covers the N-terminal S1 domain of the spike protein (amino acids [aa] 1–692). PepTivator® SARS-CoV-2 Prot_S covers selected immunodominant sequence domains of the spike protein (aa 304–338, 421–475, 492–519, 683–707, 741–770, 785–802, and 885–1273). PepTivatorSARS-CoV-2 Prot_N covers the complete sequence of the N phosphoprotein of SARS-CoV-2.
For each subject, 6 conditions were set up: unstimulated (NS); stimulation with PepTivator® SARS-CoV-2 Prot_S1 [1µg/ml] (S1); stimulation with PepTivator® SARS-CoV-2 Prot_S [1µg/ml] (S); stimulation with PepTivator® SARS-CoV-2 Prot_N [1µg/ml] (N); stimulation with pooled peptides, S1 + S + N [1µg/ml] (P); stimulation with phytohemagglutinin 5µg/ml (PHA). After overnight stimulation, IFN-γ concentration (ng/ml) in supernatants was assessed with a commercial ELISA KIT. Each sample was tested in duplicate. A 7-point standard curve was included in each ELISA session. IFN-γ response was defined as peptide or PHA stimulated condition minus unstimulated condition.
Results from three healthy controls are reported in supplementary table 1, showing the consistency of the stimulation.
4. Discussion
In this case collection of 5 MS patients under ocrelizumab treatment who recovered from COVID-19, despite the absence of anti-S antibody production, a specific T-cell response was detectable with a SARS-CoV-2 specific IGRA test. This immunological profile is consistent with the alterations observed in peripheral blood lymphocyte count of the enrolled patients, showing very low B-lymphocyte absolute counts and normal T-lymphocyte subset absolute counts. Whole blood stimulation with the same type of SARS-CoV-2 peptides libraries used in our experiment, has shown high sensitivity and specificity to diagnose and distinguish different SARS-CoV-2 exposures (Murugesan et al., 2020). SARS-CoV-2 IGRA assay could be a valuable tool for implementing the accuracy of diagnosis of COVID-19, especially in those subjects receiving B-cell depleting treatments. Moreover, it may represent an integrative approach to correctly establish the efficacy of vaccination in patients receiving immunosuppressants targeting the B-cell compartment.
T-cell response is also involved in SARS-CoV-2 infection recovery process (Peng et al., 2020; Sekine et al., 2020) therefore the rapid and easy assessment of this response would allow a better risk stratification and management of immunocompromised patients with SARS-CoV-2 infection.
Very little is known about the protective role of SARS-CoV-2 specific T-cell responses in patients with low or absent anti-SARS-CoV-2 neutralizing antibody titers. However, recently in a rhesus macaques SARS-CoV-2 infection animal model, McMahan K. et al. showed that depletion of CD8+ T cells in convalescent animals partially abrogated the protective efficacy of natural immunity against SARS-CoV-2 re-challenge, suggesting the importance of cellular immunity in the context of waning or sub-protective antibody titers (McMahan et al., 2021).
One limitation of our study is represented by the lack of pre-infection samples to evaluate SARS-CoV-2 specific T-cell responses prior to COVID-19 disease. It has been shown that in some SARS-CoV-2 unexposed donors T-cells display significant reactivity to SARS-CoV-2 antigen peptide pools, due to cross-reactivity with other coronaviruses (Sette and Crotty, 2020).
Further studies are needed, involving larger cohorts of immunocompromised subjects, to confirm the role of T-cell responses during SARS-CoV-2 acute infection and after vaccination.
Credit roles
Marco Iannetta: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data
Doriana Landi: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data
Gaia Cola: Major role in the acquisition of data
Vincenzo Malagnino: Major role in the acquisition of data; Study concept or design
Elisabetta Teti: Study concept or design; Analysis or interpretation of data
Daniela Fraboni: Major role in the acquisition of data; Study concept or design
Francesco Buccisano: Major role in the acquisition of data; Study concept or design
Sandro Grelli: Major role in the acquisition of data
Luigi Coppola: Major role in the acquisition of data; Analysis or interpretation of data
Laura Campogiani: Drafting/revision of the manuscript for content, including medical writing for content; Analysis or interpretation of data
Massimo Andreoni: Drafting/revision of the manuscript for content, including medical writing for content; Analysis or interpretation of data
Girolama Alessandra Marfia: Drafting/revision of the manuscript for content, including medical writing for content; Analysis or interpretation of data
Loredana Sarmati: Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design; Analysis or interpretation of data
Declaration of Competing Interests
M. Iannetta received honoraria for lectures from Biogen Italia, AIM Educational, MICOM srl;
D. Landi received travel funding from Biogen, Merk-Serono, Sanofi-Genzyme, Teva, speaking or consultation fees from Sanofi-Genzyme, Merk-Serono, Teva, Biogen, Novartis, Roche;
G. Cola reports no disclosures relevant to the manuscript;
V. Malagnino received honoraria for lectures from Janssen-Cilag;
E. Teti received honoraria for lectures from Gilead, AbbVie and MSD, and research grants from Gilead, outside the submitted work;
D. Fraboni reports no disclosures relevant to the manuscript;
F. Buccisano received honoraria for lectures from Novartis;
S. Grelli reports no disclosures relevant to the manuscript;
L. Coppola reports no disclosures relevant to the manuscript;
L. Campogiani received honoraria for lectures from MICOM srl;
M. Andreoni reports honoraria for lectures and research grants from Merk, Gilead, Abbvie, Angelini SpA, outside the submitted work;
G.A. Marfia is an Advisory Board member of Biogen Idec, Genzyme, Merck-Serono, Novartis, Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, Roche, Mylan, Teva;
L. Sarmati reports honoraria for lectures and research grants from Merk, Gilead, Abbvie, Angelini SpA, outside the submitted work;
Acknowledgments
Funding source declaration
No specific funding for this work.
Acknowledgments
PTV-ID-COVID Group: Lorenzo Ansaldo, Filippo Barreca, Federica Caldara, Marcella Capozzi, Laura Ceccarelli, Davide Checchi, Mirko Compagno, Angela Maria Antonia Crea, Giuseppe De Simone, Andrea Di Lorenzo, Luca Dori, Ludovica Ferrari, Alessandra Lodi, Tiziana Mulas, Pier Giorgio Pace, Benedetta Rossi, Ilaria Spalliera, Simona Tedde, Pietro Vitale, Erika Zampieri, Marta Zordan
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.103157.
Appendix. Supplementary materials
Bibliography
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund Högelin K., Ruffin N., Pin E., Månberg A., Hober S., Gafvelin G., Grönlund H., Nilsson P., Khademi M., Olsson T., Piehl F., Al Nimer F. Development of humoral and cellular immunological memory against SARS-CoV-2 despite B-cell depleting treatment in multiple sclerosis (SSRN Scholarly Paper No. ID 3796531) Social Sci. Research Network. 2021 doi: 10.2139/ssrn.3796531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta M., Cesta N., Stingone C., Malagnino V., Teti E., Vitale P., De Simone G., Rossi B., Ansaldo L., Compagno M., Spalliera I., Di Lorenzo A., Landi D., Nicoletti C.G., Marfia G.A., Andreoni M., Sarmati L. Mild clinical manifestations of SARS-CoV-2 related pneumonia in two patients with multiple sclerosis under treatment with ocrelizumab. Mult. Scler. Relat. Disord. 2020;45 doi: 10.1016/j.msard.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat-Khoei M., Conway S., Rubinson D.A., Jarolim P., Houtchens M.K. Negative anti-SARS-CoV-2 S antibody response following Pfizer SARS-CoV-2 vaccination in a patient on ocrelizumab. J. Neurol. 2021 doi: 10.1007/s00415-021-10463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., Bondzie E.A., Dagotto G., Gebre M.S., Jacob-Dolan C., Li Z., Nampanya F., Patel S., Pessaint L., Van Ry A., Blade K., Yalley-Ogunro J., Cabus M., Brown R., Cook A., Teow E., Andersen H., Lewis M.G., Lauffenburger D.A., Alter G., Barouch D.H. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K., Jagannathan P., Pham T.D., Pandey S., Bonilla H.F., Jacobson K., Parsonnet J., Andrews J.R., Weiskopf D., Sette A., Pinsky B.A., Singh U., Banaei N. Interferon-gamma release assay for accurate detection of SARS-CoV-2 T cell response. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., López-Camacho C., Slon-Campos J., Zhao Y., Stuart D.I., Paesen G.C., Grimes J.M., Antson A.A., Bayfield O.W., Hawkins D.E.D.P., Ker D.-S., Wang B., Turtle L., Subramaniam K., Thomson P., Zhang P., Dold C., Ratcliff J., Simmonds P., de Silva T., Sopp P., Wellington D., Rajapaksa U., Chen Y.-L., Salio M., Napolitani G., Paes W., Borrow P., Kessler B.M., Fry J.W., Schwabe N.F., Semple M.G., Baillie J.K., Moore S.C., Openshaw P.J.M., Ansari M.A., Dunachie S., Barnes E., Frater J., Kerr G., Goulder P., Lockett T., Levin R., Zhang Y., Jing R., Ho L.-P., Oxford Immunology Network Covid-19 Response T cell Consortium, ISARIC4C Investigators. Cornall R.J., Conlon C.P., Klenerman P., Screaton G.R., Mongkolsapaya J., McMichael A., Knight J.C., Ogg G., Dong T. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., Wullimann D.J., Kammann T., Emgård J., Parrot T., Folkesson E., Karolinska COVID-19 Study Group. Rooyackers O., Eriksson L.I., Henter J.-I., Sönnerborg A., Allander T., Albert J., Nielsen M., Klingström J., Gredmark-Russ S., Björkström N.K., Sandberg J.K., Price D.A., Ljunggren H.-G., Aleman S., Buggert M. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat. Rev. Immunol. 2020;20:457–458. doi: 10.1038/s41577-020-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets I., Reyes S., Baker D., Giovannoni G. Blunted vaccines responses after ocrelizumab highlight need for immunizations prior to treatment. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.