Abstract
Nearly one-quarter of bowel obstructions occur in the large bowel. As with all bowel obstructions, large bowel obstructions have three defining characteristics: partial or complete, intrinsic or extrinsic, benign or malignant. The work-up for a large bowel obstruction should focus on the etiology of the obstruction as well as severity. Management strategy is contingent on the previous characteristics and can include endoscopy, diversion, or resection. This chapter will discuss common and rare etiologies of large bowel obstructions as well as management strategies for clinical guidance.
Keywords: large bowel obstruction, adhesion, hernia, endometriosis, retroperitoneal fibrosis, stricture
Bowel obstructions (BOs) are a frequent cause of hospital admission and surgical intervention across the world. They can be separated into two categories—small and large bowel obstructions. Of the two, large bowel obstructions (LBOs) are less common, resulting in 20 to 25% of all bowel obstructions encountered. 1 This chapter will focus on various etiologies of LBOs and their management strategies.
LBOs are more common in elderly individuals. 2 Etiology can range from idiopathic, malignant, and the result of surgery and trauma. It is important to consider the cause of LBOs, as this will often determine the best treatment option. The type of obstruction can be categorized as either a functional disorder of the colon or a mechanical obstruction, both requiring different treatment strategies.
The most common cause of LBOs in the United States is adenocarcinoma of the colon and rectum. 1 2 3 This represents over 50 to 60% of LBOs. 3 4 Diverticular disease represents approximately 10 to 20% of cases. 3 4 Large bowel volvulus represents approximately 10 to 15% of LBOs. 3 4 The remaining LBOs are caused by rarer conditions such as inflammatory bowel disease, hernia, adhesion, endometriosis, intussusception, and functional disorders of the colon. This chapter will focus on the rarer etiologies. Colon cancer, Ogilvie's syndrome, inflammatory bowel disease, and volvulus are discussed in other chapters.
Clinical Evaluation
Patients presenting with LBO may have signs and symptoms similar to those presenting with small bowel obstructions. 1 A careful history and examination can help to raise the suspicion of LBO and potentially an etiology. However, when evaluating the patient with an LBO, history and physical examination alone will often be insufficient to identify the etiology, given the similarities in presentation to other conditions.
The history should focus on potential colorectal problems. One should ascertain any prior episodes of rectal bleeding and weight loss as these may suggest colon cancer. A history of multiple prior bouts of diverticulitis should raise the concern for diverticular stenosis. A history of institutionalization and rapid distention is suspicious for volvulus. Digital rectal exam should always be performed, as distal masses and stool impaction can result in obstruction. Other specific findings will be discussed below with each individual condition.
Attention to the presenting complaints is vital to establishing a working diagnosis of an LBO. Patients may report a timeline of abrupt or slow change in their bowel movements ultimately resulting in constipation or obstructive symptoms. This change can be associated with gradual abdominal distention and cramping or colicky pain. Furthermore, distal lesions may result in a more rapid onset of obstruction as a result of a thinner diameter of bowel and more solid stool. A more proximal lesion will not obstruct as easily due to the wider diameter of bowel and less solid stool. 3
Nausea and vomiting, which are prevalent in small bowel obstructions, may not present if the patient has a competent ileocecal valve. 4 A competent ileocecal valve places the patient at risk for a closed loop obstruction as contents of the colon are unable to move forward or backward. In the case of an incompetent ileocecal valve, the colon can decompress into the small bowel mimicking the presentation of a small bowel obstruction with earlier nausea and vomiting.
Systemic signs and symptoms are of great importance in the evaluation and management of LBOs. Attention should be paid to any history of fevers, chills, or significant pain. On exam, fever, tachycardia, and peritonitis may indicate ischemia and/or perforation. In such circumstances, the patient should be prepared for urgent surgical intervention.
Examination findings are typically consistent across all forms of LBO excluding cases of ischemia or perforation. This makes the diagnosis of LBO difficult. These findings typically consist of distention, pain, and obstipation with or without bilious or feculent emesis. Further work-up is needed to help with diagnosis and management strategies.
Laboratory Assessment
Laboratory assessment should include appropriate management of electrolytes and renal function. Consideration should be given to nutritional assessment based on history and exam findings. Systemic signs of infection and ischemia should be assessed with a complete blood count and lactic acid level.
Diagnosis
The main imaging modality for a suspected LBO may include abdominal plain film and computed tomography (CT). Abdominal plain films are readily available at most Western institutions. They may show single or multiple dilated loops of bowel. Determining whether small bowel or large bowel is affected can be difficult, particularly if dilation results in the loss of haustra. Even if an LBO is diagnosed on plain films, the etiology can be difficult to determine apart from an obvious volvulus. Other findings that must be assessed on plain films are air fluid levels, pneumoperitoneum, and portal venous air.
A CT scan has several advantages in the assessment of an LBO. First, they can differentiate between large and small bowel obstructions. Furthermore, CT imaging can often reliably confirm the etiology of the LBO 3 ( Fig. 1 ). When possible, IV contrast should be administered to best delineate an etiology. 4 If there are contraindications to ionizing radiation, magnetic resonance imaging (MRI) can be used as an alternative. While a diagnosis of volvulus, hernia, intussusception, and retroperitoneal fibrosis can be made with CT or MRI alone, bowel wall spasm or peristalsis, strictures, diverticular stenosis, and obstructing colon cancer can be difficult to differentiate from each other. Other diagnostic modalities may be needed to diagnose the specific cause of an LBO.
Fig. 1.
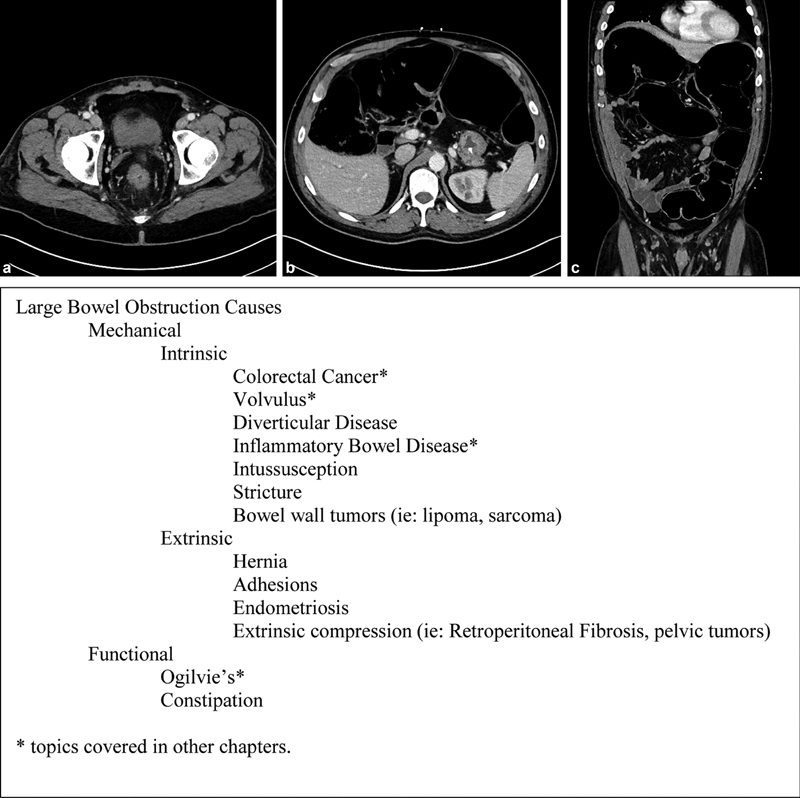
(a–c) Major etiologies of large bowel obstruction.
Contrasted enemas can be used to further characterize and diagnose the cause of bowel obstructions. They aid with the diagnosis of volvulus and can differentiate mechanical from functional obstructions. 4 Water soluble contrast is the preferred contrast as it is better absorbed by the peritoneum in case of a perforation and does not hamper visibility on subsequent CT scans.
On radiographic studies, the bowel can be measured to assess for dilation. The normal diameter of the large bowel is 3 to 6 cm with the cecum being the widest portion. 4 This measurement can be performed using abdominal plain films, CT, and MRI. Bowel diameter over 10 cm is associated with an increase in the risk of perforation. 3
Colonoscopy can have both diagnostic and therapeutic benefits. 3 Colonoscopy can demonstrate mucosal or submucosal lesions that can contribute to an LBO. With the addition of biopsies, colonoscopies provide the needed information to establish a pathologic diagnosis. The addition of endoscopic ultrasonography can help visualize extrinsic masses and provide further information with additional biopsies. Therapeutic benefits of endoscopy will be discussed later in this chapter.
Management
Initial management of patients presenting with LBOs begins with resuscitation in the form of isotonic intravenous fluids and correction of metabolic derangements. Initiation of antibiotics should be reserved for cases of sepsis, ischemia, and perforation. Typically, this initial management process is taking place during the work up of the etiology of the LBO. 3
Consideration for gastric decompression should be based on the patient. A patient with a competent ileocecal valve may not have emesis. The degree of abdominal distention, prior or current emesis, and radiographic findings of a dilated stomach or small bowel can help determine the utility of gastric decompression.
LBOs are mostly the result of a mechanical problem. 1 2 3 Unlike small bowel obstructions, where adhesions predominate as the mechanical cause, LBOs are less likely to be the result of adhesions. Therefore, LBOs are prone to failing conservative management. Depending on the etiology of the LBO endoscopy or surgical intervention may be needed to relieve the obstruction.
Endoscopy
Endoscopy can be used to dilate or stent colonic strictures and obstructing cancers. Stenting and dilation come with the risk of bowel perforation, bleeding, and the need for repeated procedures. Stenting is associated with stent migration and ingrowth or incorporation of the stent into the bowel wall. 5
The FDA has approved the use of self-expandable metal stents (SEMS) for malignant colon obstructions but not for benign obstructions. 5 Patients with obstructions secondary to inflammatory bowel disease may undergo balloon dilation without stenting for management. 6 Dilation has proven safety in this population but comes with risks and the likely need for repeated procedures. 6
Off label use of SEMS may be considered for benign disease. However, there is concern for migration, need for multiple procedures, ingrowth or incorporation of the stent into the bowel wall. 5 These complications have likely been evaluated and documented more in the benign setting because these patients are followed for longer periods of time compared with the cancer population. Whether stenting is used for benign or malignant disease, there may be benefit in using SEMS as a bridge to surgery and as a palliative treatment option. 3 5
Another strategy that has been developing over the past decade is endoscopic laser coagulopathy. This has been used to bypass high-grade strictures to allow for dilation and/or SEMS placement. 7 There is also some growing evidence for fluoroscopic and percutaneous stent placement in the medical literature. 5 As endoscopic and other therapeutic strategies develop, we may see a greater role for endoscopy in the management of LBOs.
Surgery
Compared with small bowel obstruction, patient with LBO will more frequently require operative intervention. Surgical exploration, in the form of laparotomy or laparoscopy, should be the first line of treatment for patients with peritonitis, pneumoperitoneum, and/or sepsis. For those patients who are not in need of urgent surgical intervention, there is time for careful consideration of the appropriate timing of surgery.
Attention should be given to optimizing the patient's condition including providing bowel decompression if necessary and optimizing medical comorbidities, nutritional status, and functional status. These efforts will improve postoperative outcomes. Ultimately, almost all LBOs will require some form of intervention. That intervention will be based heavily on the patient's underlying diagnosis.
In general, segmental resection and primary anastomosis can be used in the elective setting if no contamination is present or if the patient is hemodynamically stable. The use of on table colonic lavage has been debated for decades. Theoretically, reducing proximal colonic burden decreases the risk of anastomotic leakage but can increase the risk of abdominal cavity contamination. To date, there is no consensus on whether the benefits outweigh the risks on morbidity and mortality. 8 9 10 Proximal diversion or end colostomies with mucous fistulas are options to consider if concerned for contamination, patient stability, advanced malignancy, or the condition of the bowel itself. 3 In the urgent setting, these may be the safest options. Exact method of surgical intervention is influenced by the etiology of the LBO.
Mechanical—Extrinsic Large Bowel Obstructions
Mechanical LBOs may be intrinsic or extrinsic. Intrinsic obstruction results from pathology of the bowel wall from the serosa to mucosa. Extrinsic causes of bowel obstructions include hernias, adhesive disease, endometriosis, retroperitoneal fibrosis, and compression from extrinsic tumors. Each will be discussed with details regarding etiology, pathophysiology, presentation, and management.
Hernias
Hernias are defects in the abdominal wall, mesentery, or diaphragm. They result from acquired defects, trauma, surgical procedures, and congenital etiologies. As with the small bowel, the large bowel can become obstructed and ischemic after becoming incarcerated within a hernia. The incidence of LBOs resulting from herniation is not known as the literature is restricted to case reports and single institution experiences.
As nearly half of the large bowel is tethered to the retroperitoneum, it is not as mobile or compared with small bowel. These segments can still herniate but are more likely to result in Richter's hernias where only a portion of the small bowel is a component of the hernia sac. While the incidence of LBOs resulting from hernias is likely lower than that seen with small bowel obstructions, they must be considered when evaluating a patient presenting with obstructive symptoms due to hernia.
Examination findings that are particular to strangulated hernias include overlying skin color changes or erythema which can indicate compromised underlying bowel. An abdominal CT scan is the most appropriate modality to determine bowel obstruction secondary to hernia. This will identify the presence of obstructed large bowel within the hernia sac.
When bowel is present within an incarcerated hernia, a patient may have a leukocytosis or lactic acidosis. These, in addition to exam findings, are concerning for ischemia secondary to strangulation. Incarcerated hernias without clear signs of ischemia should have reduction attempted. If successful, repeated evaluation for a leukocytosis and lactic acidosis should be done as potentially strangulated bowel is now reintroduced into circulation. Operative exploration is required for hernias that are unable to be reduced, strangulated, or when lactate and white blood cell counts rise after reduction.
Acquired Hernias
Acquired hernias are a result of laxity to the abdominal wall due to genetic disorders like Ehler–Danlos or as a result of chronic increases in abdominal pressure due to frequent straining from weightlifting or coughing as seen in chronic obstructive pulmonary disease. The pathophysiology of acquired hernias can be attributed to disorders in collagen metabolism, changes in fibroblast function over time due to chronic increases in pressure, or an association with risk factors like cigarette smoking. 11
Traumatic Hernias
Traumatic abdominal hernias can occur along the anterior abdominal wall, flank, inguinal, and lumbar regions. While they are seen at varying rates institutionally, currently reported cases account for less than 1% of blunt trauma results in herniation. Prior to the increased utilization of CT scans, traumatic abdominal wall hernias were less likely to be identified at initial presentation. Currently, there is no set standard for management. Immediate surgical repair with or without mesh has been described for patients requiring surgical repair for other intra-abdominal injuries. The frequency of recurrence is noted to be higher for those undergoing immediate repair, up to 50% has been documented. Delayed surgical repair has been shown in various studies to decrease the recurrence rate. Furthermore, nonoperative management can be an effective strategy provided careful counselling and monitoring are provided. 12
Traumatic diaphragmatic hernias should be considered when evaluating a patient after blunt thoracoabdominal trauma. They are more common after high velocity accidents and their incidence ranges from 1 to 6% of blunt thoracic trauma. 13 Most of these hernias occur on the left side with the liver serving as a protective factor from herniation on the right. 14 Chest plain films and CT scan assist in the diagnosis but despite the advances in radiography, these traumatic hernias are not always easily identified at the time of injury. These hernias may become symptomatic days to weeks or even months to years after presentation. Treatment should include surgical repair with or without mesh. Laparotomy is the preferred approach over laparoscopy. 3
Iatrogenic Hernias
Iatrogenic hernias, which include incisional and internal hernias, are due to previous surgical interventions. The pathophysiology behind incisional hernias is considered to be due to a break down in the normal healing process. This can be due to poor surgical technique, hematoma formation, wound sepsis, or an abnormality in collagen metabolism. 11 While the incidence of LBOs in this category of hernia is not known, they have been documented in case reports.
Incisional hernias are readily apparent on exam and require repair of the hernia. Careful consideration is required to decide the type of mesh needed. If there is contamination, ischemia or necrosis primary repair or biologic mesh is a consideration. If there is no evidence of contamination, synthetic mesh is appropriate. Internal hernias require urgent operative intervention which include reduction of contents and closure of the mesenteric defect.
Congenital Hernias
Congenital hernias are a result of failure of closure of the abdominal wall during development at locations like the umbilicus and internal inguinal rings. Typically, the inguinal ring closes prior to birth. If found on exam in a newborn or infant, repair is recommended. The umbilicus, however, may not close until years after birth. If present after 5 years or there is a defect over 2 cm, repair is indicated. Adult surgeons are less likely to manage hernias due to congenital issues. If present into adulthood, they are likely mistaken for acquired hernias without an in-depth history. 15
Adhesive Disease
Adhesive disease is a rare cause of LBOs described only with case reports. Peristalsis occurs throughout the healthy colon and with the presence of adhesions can result in kinking of segments of the bowel which leads to obstruction. The more mobile and redundant the colon is the more predisposed it is to adhesive obstruction. 16 Adhesions can be congenital, idiopathic, or acquired. 17 Idiopathic adhesions can occur from various sources of abdominal inflammation, for example inflammation of appendices epiploicae or salpingitis. 17 Acquired adhesions occur as a result of the normal healing process after iatrogenic or traumatic insult. Inflammation can result in the colon being tethered to other segments of bowel or the abdominal wall which in turn can kink and obstruct the bowel during peristalsis.
Diagnosing adhesions as the cause of an LBO can be difficult. Patients have no other symptoms apart from the typical findings of an LBO. Barium enemas may show a sharp or localized narrowing of the bowel. 18 Likewise, CT scans may show a narrowed segment or transition point—neither of which is specific to adhesive disease. Treatment of obstruction secondary to adhesive disease should begin with resuscitative measures mentioned at the start of this chapter. A thorough work-up should be performed to rule out other causes of LBO. The current recommendation for adhesive colonic obstruction is surgical intervention with lysis of adhesions and other procedures as needed. 17 If time does not allow based on patient presentation, urgent laparotomy with lysis of adhesion and potential resection should be performed to relieve the obstruction.
Endometriosis
Endometriosis is a rare and benign cause of LBO. It predominantly affects the pelvic organs, i.e., uterus, ovaries, and associated ligaments. When other organs are involved, the bowel is affected 3 to 12% of the time particularly at rectosigmoid junction. 19
The etiology and pathophysiology of endometriosis are not fully understood. Typical complaints of endometriosis include dyspareunia, irregular menstruation, cyclic pelvic pain, and dysmenorrhea. 19 When endometriosis involves the bowel, signs and symptoms can expand to cyclical pain with defecation, rectal bleeding, nonspecific abdominal pains, bowel irregularity, and finally obstruction. 19 Support from gynecology should be obtained to help with earlier diagnosis and management.
Due to the variability in signs and symptoms, the diagnosis of endometriosis may be difficult to make. Plain films and CT will show evidence of obstruction but may not give a clear diagnosis of endometriosis. Instead, a suspicion for colon cancer may be raised with evidence of a mass on CT. Colonoscopy to sample the mass may be nondiagnostic depending on the depth of the implants invasion. 20 If mucosal invasion is present then endometriosis can be diagnosed more easily, otherwise the biopsy depth will impact the ability to make diagnosis. There are cases where MRI has been able to show similar appearance of an obstructing lesion to endometrial implants elsewhere in the patient's body. 19
When the diagnosis of endometrial implants cannot be established, malignancy must be considered. After failed attempts at endoscopy, surgical intervention becomes the next diagnostic modality provided the patient can tolerate an operation. Pathology will confirm the diagnosis and the patient should be referred to gynecology for the management of their endometriosis.
In the case where the diagnosis of endometriosis has been made, endoscopic stenting or surgery is the treatment option to discuss with patients. 19
Extrinsic Compression
Extrinsic compression of the colon or externally caused bowel obstructions can occur for a variety of reasons. There are multiple benign and malignant causes, for example: large bladder calculi, uterine fibroids, pregnancy, and pelvic malignancy all play a factor is LBOs. Retroperitoneal fibrosis is another rare extramural cause of LBOs.
An LBO resulting from extrinsic forces will result in similar presentation as mentioned at the start of this chapter. Other symptoms may include hematuria for bladder calculi, weight loss for pelvic tumors, or menstrual irregularities with large fibroids or uterine masses. The acuity of the presentation will vary depending on the degree of the compression.
The incidence of LBOs resulting from retroperitoneal fibrosis is not known as the literature is restricted to case reports. The disease itself varies depending on the cause, primary or secondary retroperitoneal fibrosis. Primary or idiopathic retroperitoneal fibrosis has many similarities to systemic autoimmune disorders like systemic lupus erythematosus and can be noted in multiple organ systems and areas in the body. Secondary retroperitoneal fibrosis has been associated with medications, prior medical interventions, nearby infection, or malignancy. Surgical considerations include prior radiotherapy or surgery to the retroperitoneum, notably colon and rectum surgeries. Similarly, inflammation from nearby malignancy or infection can lead to retroperitoneal fibrosis. 21 22
Typical manifestations of retroperitoneal fibrosis include back, flank, and abdominal pain along with systemic symptoms like fatigue, weight loss, and fevers. Obstructive retroperitoneal fibrosis has been described affecting retroperitoneal structures like the testicular vessels, ureters, and major blood vessels. 22 23 In the case of LBOs, retroperitoneal fibrosis can affect colonic anastomosis and segments where adenocarcinoma is present. Work-up must include a CT scan and attempts should be made to rule out malignancy before determining retroperitoneal fibrosis as the cause of LBO.
Treatment of retroperitoneal fibrosis includes steroids and immunomodulators. Unfortunately, the presence of an LBO warrants intervention as medical management will not usually treat the fibrosis fast enough to relieve the obstruction. As previously mentioned, the urgency of surgical intervention is based on patient presentation.
Mechanical—Intrinsic
Mechanical LBOs can also result from the bowel wall itself. These intrinsic sources can be the result of inflammation of the bowel wall, benign and malignant tumors as well as iatrogenic sources. This chapter will focus on inflammatory lesions with the exception of Crohn's disease, intussusception, and strictures. Each will be discussed with details regarding etiology, pathophysiology, presentation, and management.
Strictures
Causes of colonic strictures include malignancy, ischemia, and inflammation ( Fig. 2 ). Inflammatory strictures include Crohn's and tuberculosis (TB)-related strictures. Strictures can also result from anastomoses, trauma, and radiation. Malignant and Crohn's-related strictures are discussed in other chapters.
Fig. 2.
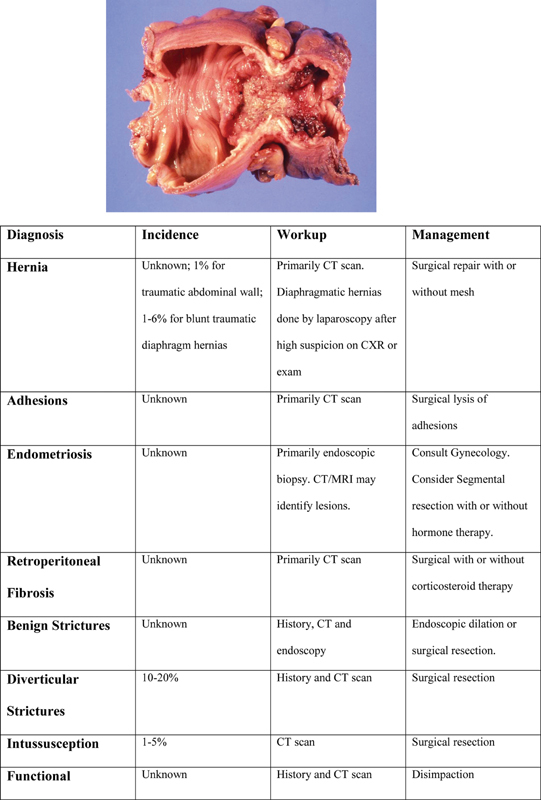
Diagnosis and incidence with recommended work-up and management.
Patients with strictures that result in LBOs will usually endorse obstructive symptoms that develop over a varied period of time. There is often a report of changes in bowel habits that have been ongoing for days to weeks to even months. Some patients seek medical attention early and do not have overt signs of obstruction. Varied exam and imaging findings will be detailed in each category below. Imaging does not easily differentiate malignant from benign causes. Endoscopy can assist in the diagnosis.
LBO secondary to strictures requires intervention. Management strategies involve some mixture of medical, endoscopic, and/or surgical treatment. A level of diagnostic certainty is needed prior to proceeding with therapeutic endoscopic procedures as malignancy must be ruled out. Some centers are using laser coagulation to incise and bypass high-grade strictures to perform dilations. 7 24
Diverticular Strictures
Diverticular strictures are identified as the second most common cause of LBO in the United States (10–20%). The inflammation of acute diverticulitis leads to scar formation and fibrosis which gradually narrows the lumen of the sigmoid colon resulting in an intrinsic compression of the lumen. Obstruction caused by diverticulitis is usually an insidious process. Patients with have a history of multiple bouts of diverticulitis and report gradual onset of constipation, abdominal bloating, narrowed stools and potentially diarrhea. An endoscopic history of diverticulosis or subclinical narrowing is common.
Patients with LBO due to diverticular stricture present with abdominal distension, obstipation, and potentially nausea progressing to vomiting. Diagnosis is usually made with a CT scan revealing a transition point in the sigmoid colon with upstream dilation of the proximal colon. Barium enema is an alternative imaging modality. Obstruction due to diverticular stricture can be difficult to differentiate from strictures due to carcinoma. Carcinoma is best differentiated from mass-like diverticular disease by the absence of diverticula in the affected segment and the presence of shoulder phenomenon (bulging, acute edge [90 degrees], or lack of tapering at either the proximal or distal edge of the obstruction). 25
Surgical management should follow the basic principles laid out above. If the patient is appropriate for a resection and primary anastomosis, great care should be taken to ensure that the distal resection margin extends to the top of the rectum to decrease the risk of recurrence. 26 Endoscopic stenting for the management of diverticular obstructions remains an unsettled question. Compared with malignant lesions, diverticular strictures tend to be longer and more tortuous, making stenting technically difficult. Though still feasible, complications appear to be greater with poorer outcomes. 27 28 The long-term use of stents in this instance are associated with perforations, fistulae, and pain. Based on the current body of evidence, stenting for diverticular strictures is not currently recommended. 29
Ischemic Strictures
Ischemic strictures are commonly short segment strictures of the colon that develop at watershed areas. Strictures usually develop within weeks of the inciting ischemic event. There are three known mechanisms of bowel ischemia, arterial, venous and nonocclusive ischemia. Hypercoagulable disorders will typically affect younger patients. Otherwise, bowel ischemia is more common in the elderly or those with multiple comorbidities. 30
Thorough history and chart review can help make the diagnosis particularly if the patient cannot recall the events surrounding their bowel ischemia episode as is common in the critically ill population. Segmental resection or stenting should be based on the patient's condition at the time of presentation.
Inflammatory Strictures
Inflammatory strictures can result from diverticulosis, infections such as TB or chronic diseases like Crohn's. Often the diagnosis of TB strictures can be difficult to differentiate from other causes of strictures for example Crohn's disease. A history of weight loss associated with typical signs of LBO can help assist with the diagnosis. The diagnosis of inflammatory strictures is best made with endoscopy and biopsy. Once the biopsy is submitted to pathology, Crohn's disease, TB, ischemia, and colonic malignancy can be identified. TB-related strictures should be treated with antituberculosis medications and endoscopic or surgical intervention. 3 31
Anastomotic Strictures
After creation of a colorectal anastomosis the incidence of stricture ranges from 2 to 20%. 32 33 The number of strictures that progress to an LBO is unknown.
The various factors that are implicated in the cause of anastomotic structuring include: ischemia, tension, recurrent malignancy, collagen overproduction, abnormality in the healing process, use of circular staplers, radiotherapy, and anastomotic leakage. A recent meta-analysis found that stapled colorectal anastomosis was associated with an increased risk of stricture formation compared with a hand-sewn anastomosis. 34 In a prospective observational study, the risk of developing a stenosis following a colorectal anastomosis was 2.4 times greater in men (25%) compared with women (14%). 35 This may reflect the increased technical difficulty of operating in the narrow male pelvis. Of note, endoscopic evaluation should be performed to sample the stricture for malignancy prior to finalizing management. 36
Treatment of anastomotic strictures has changed over the past few decades with the advancement of endoscopic techniques. Previously surgery was recommended for all strictures, benign and malignant. Now, endoscopy and dilation with or without stenting are the preferred first-line management strategy for benign cases. Timing of stenting is of concern as the risk to a new anastomosis must be considered. True structuring takes time to develop and is unlikely to occur within weeks of surgery. Six months to 1 year is the mean time period; however, there have been reports of strictures in as little time as 3 months. 32 33
Intussusception
Intussusception is rare in adults. Overall, intussusception is responsible for 1 to 5% of all adult bowel obstructions. 37 The percent of LBOs due to intussusception is not known but is considered less common than obstructions from small bowel intussusception given the most common type of adult intussusception is small bowel in origin. 38 In the colon, adenocarcinoma is the lead point in over 50% of the cases. Other less common etiologies include, lymphoma, sarcoma, adenoma, polyps, and lipomas. 37
Adult patients presenting with intussusception do not have the common constellation of symptoms known in children: intermittent pain, bloody stool, and a palpable mass. Symptoms are often nonspecific and imitate a bowel obstruction but can be a mix of abdominal pain, fever, nausea, vomiting, bowel changes or obstipation, distention, bloody stool, and palpable masses. 38 Without a consistent constellation of symptoms, diagnosis relies heavily on imaging.
The diagnostic modality preferred in the adult population is a CT scan. Expected findings that indicate an intussusception are the “target” sign and or a sausage-shaped lesion with two layers of bowel wall present within. Ultrasound, barium enema, and colonoscopy have not been as sensitive in various case reports. 37 38
Treatment of colon intussusception is primarily surgical given the high rate of malignant lead points. 39 Resection of the lesion and primary anastomosis should be sufficient given no concern for gross contamination or ischemia. There is no consistent decision on whether the lesion should be reduced prior to resection.
Functional
Functional LBOs are equally capable of causing a bowel obstruction as mechanical causes. Stool impaction or constipation and Ogilvie's syndrome are considered causes of functional bowel obstructions. Ogilvie's will be discussed in a separate chapter in this book.
Impaction
Obstruction due to fecal impaction can occur in both sexes at any age but are particularly concentrated in three main populations—children, the institutionalized or impaired elderly, and patients with certain psychiatric disorders or medical conditions that predispose to constipation. Patients with LBO due to fecal impaction, especially the infirm and mentally impaired, can present with vague symptoms that make a diagnosis challenging. 40 A history of chronic constipation is frequently observed. Patients commonly present with abdominal distention and pain, fecal incontinence, anorexia, weight loss, intestinal obstruction, and stercoral ulceration with bleeding or colonic perforation. Fecal incontinence is a paradoxical presentation and is due to overflow of loose stool around the obstruction. The first step in diagnosis is a digital rectal exam. The hallmark findings of fecal impaction are firm or clay-like stool in the rectal vault. CT scans can assess the level of obstruction, proximal dilation, and the presence of free air from stercoral perforation. Management centers around disimpaction. This can be accomplished by digital manipulation, enema, or disimpaction under anesthesia. For more proximal impactions, water-soluble contrast enema may be both diagnostic and therapeutic. Following disimpaction, the patient should be started on an aggressive bowel regimen to prevent recurrence. Surgery, in the form of exploratory laparotomy, is rarely required and reserved for complications such as stercoral ulcer perforation. 41
Conclusion
LBOs represent nearly 25% of all bowel obstructions. History and physical exam are rarely enough to make the diagnosis. A combination of readily accessible diagnostic modalities like plain films, CT scans, and endoscopy are necessary for complete evaluation. Surgeons should be ready to intervene surgically on patients who present with a LBO as this condition rarely resolves with medical management and observation alone. Operative intervention must be tailored to the underlying cause of the obstruction.
Footnotes
Conflict of Interest None declared.
References
- 1.Markogiannakis H, Messaris E, Dardamanis D. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13(03):432–437. doi: 10.3748/wjg.v13.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne J J. Large bowel obstruction. Am J Surg. 1960;99(02):168–178. doi: 10.1016/0002-9610(60)90111-2. [DOI] [PubMed] [Google Scholar]
- 3.Farkas N GWT, Welman T JP, Ross T, Brown S, Smith J J, Pawa N. Unusual causes of large bowel obstruction. Curr Probl Surg. 2019;56(02):49–90. doi: 10.1067/j.cpsurg.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe T, Thompson W M. Large-bowel obstruction in the adult: classic radiographic and CT findings, etiology, and mimics. Radiology. 2015;275(03):651–663. doi: 10.1148/radiol.2015140916. [DOI] [PubMed] [Google Scholar]
- 5.Razzak A ARA, Kozarek R A. Cham: Humana Press; 2018. Gastrointestinal Tract Stenting. [Google Scholar]
- 6.Adler D G. Colonic strictures: dilation and stents. Gastrointest Endosc Clin N Am. 2015;25(02):359–371. doi: 10.1016/j.giec.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Bravi I, Ravizza D, Fiori G. Endoscopic electrocautery dilation of benign anastomotic colonic strictures: a single-center experience. Surg Endosc. 2016;30(01):229–232. doi: 10.1007/s00464-015-4191-0. [DOI] [PubMed] [Google Scholar]
- 8.Allen-Mersh T G. Should primary anastomosis and on-table colonic lavage be standard treatment for left colon emergencies? Ann R Coll Surg Engl. 1993;75(03):195–198. [PMC free article] [PubMed] [Google Scholar]
- 9.Awotar G K, Guan G, Sun W. Reviewing the management of obstructive left colon cancer: assessing the feasibility of the one-stage resection and anastomosis after intraoperative colonic irrigation. Clin Colorectal Cancer. 2017;16(02):e89–e103. doi: 10.1016/j.clcc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hsu T C. Comparison of one-stage resection and anastomosis of acute complete obstruction of left and right colon. Am J Surg. 2005;189(04):384–387. doi: 10.1016/j.amjsurg.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Franz M G. The biology of hernias and the abdominal wall. Hernia. 2006;10(06):462–471. doi: 10.1007/s10029-006-0144-9. [DOI] [PubMed] [Google Scholar]
- 12.Coleman J J, Fitz E K, Zarzaur B L.Traumatic abdominal wall hernias: location matters J Trauma Acute Care Surg 20168003390–396., discussion 396–397 [DOI] [PubMed] [Google Scholar]
- 13.Fischer N J, Aiono S.Delayed presentation of a traumatic diaphragmatic hernia presenting as a large bowel obstruction: case report ANZ J Surg 201686(1-2):97–98. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Bagaria D, Ratan A, Gupta A. Missed diaphragmatic injury after blunt trauma presenting with colonic strangulation: a rare scenario. BMJ Case Rep. 2017;2017:bcr-2017-221220. doi: 10.1136/bcr-2017-221220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung D. 20th ed. Philadelphia, PA: Elsevier Saunders; 2017. Sabiston Textbook of Surgery. [Google Scholar]
- 16.Holt R WWR, Wagner R C. Adhesional obstruction of the colon. Dis Colon Rectum. 1984;27(05):314–315. doi: 10.1007/BF02555640. [DOI] [PubMed] [Google Scholar]
- 17.El-Masry N SGR, Geevarghese R. Large bowel obstruction secondary to adhesive bands. J Surg Case Rep. 2015;2015(02):2. doi: 10.1093/jscr/rju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodey P ASD, Schuldt D R, Magnuson A, Esterkyn S. Complete colonic obstruction secondary to adhesions. AJR Am J Roentgenol. 1979;133(05):917–918. doi: 10.2214/ajr.133.5.917. [DOI] [PubMed] [Google Scholar]
- 19.Alexandrino G, Lourenço L C, Carvalho R, Sobrinho C, Horta D V, Reis J. Endometriosis: a rare cause of large bowel obstruction. GE Port J Gastroenterol. 2018;25(02):86–90. doi: 10.1159/000480707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozdech J M. Endoscopic diagnosis of colonic endometriosis. Gastrointest Endosc. 1992;38(05):568–570. doi: 10.1016/s0016-5107(92)70518-5. [DOI] [PubMed] [Google Scholar]
- 21.Vaglio A, Salvarani C, Buzio C.Retroperitoneal fibrosis Lancet 2006367(9506):241–251. [DOI] [PubMed] [Google Scholar]
- 22.Vaglio A, Palmisano A, Corradi D, Salvarani C, Buzio C.Retroperitoneal fibrosis: evolving concepts Rheum Dis Clin North Am 20073304803–817., vi–vii [DOI] [PubMed] [Google Scholar]
- 23.Yan T, Wang Y, Liu Z, Zhang X, Wu Q, Xi M. Idiopathic retroperitoneal fibrosis causing unilateral ureteral and sigmoid colon obstruction: a case report. Medicine (Baltimore) 2017;96(07):e6105. doi: 10.1097/MD.0000000000006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchan K LMA, Muldner A, Manegold B C. Endoscopic treatment of postoperative colorectal anastomotic strictures. Surg Endosc. 2003;17(07):1110–1113. doi: 10.1007/s00464-002-8926-3. [DOI] [PubMed] [Google Scholar]
- 25.Lips L M, Cremers P T, Pickhardt P J. Sigmoid cancer versus chronic diverticular disease: differentiating features at CT colonography. Radiology. 2015;275(01):127–135. doi: 10.1148/radiol.14132829. [DOI] [PubMed] [Google Scholar]
- 26.Thaler K, Baig M K, Berho M. Determinants of recurrence after sigmoid resection for uncomplicated diverticulitis. Dis Colon Rectum. 2003;46(03):385–388. doi: 10.1007/s10350-004-6560-y. [DOI] [PubMed] [Google Scholar]
- 27.Meisner S, Hensler M, Knop F K, West F, Wille-Jørgensen P. Self-expanding metal stents for colonic obstruction: experiences from 104 procedures in a single center. Dis Colon Rectum. 2004;47(04):444–450. doi: 10.1007/s10350-003-0081-y. [DOI] [PubMed] [Google Scholar]
- 28.Paúl L, Pinto I, Gómez H, Fernández-Lobato R, Moyano E. Metallic stents in the treatment of benign diseases of the colon: preliminary experience in 10 cases. Radiology. 2002;223(03):715–722. doi: 10.1148/radiol.2233010866. [DOI] [PubMed] [Google Scholar]
- 29.European Society of Gastrointestinal Endoscopy . van Hooft J E, van Halsema E E, Vanbiervliet G. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46(11):990–1053. doi: 10.1055/s-0034-1390700. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Kahler K H, Sarawate C, Quimbo R, Kralstein J. Assessment of potential risk factors associated with ischaemic colitis. Neurogastroenterol Motil. 2008;20(01):36–42. doi: 10.1111/j.1365-2982.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M PBV, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120(04):305–315. [PubMed] [Google Scholar]
- 32.Dieruf L MPC, Prakash C. Endoscopic incision of a postoperative colonic stricture. Gastrointest Endosc. 2001;53(04):522–524. doi: 10.1067/mge.2001.112369. [DOI] [PubMed] [Google Scholar]
- 33.Weinstock L BBA, Shatz B A. Endoscopic abnormalities of the anastomosis following resection of colonic neoplasm. Gastrointest Endosc. 1994;40(05):558–561. doi: 10.1016/s0016-5107(94)70252-7. [DOI] [PubMed] [Google Scholar]
- 34.Neutzling C B, Lustosa S A, Proenca I M, da Silva E M, Matos D. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst Rev. 2012;(02):CD003144. doi: 10.1002/14651858.CD003144.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bannura G C, Cumsille M A, Barrera A E, Contreras J P, Melo C L, Soto D C. Predictive factors of stenosis after stapled colorectal anastomosis: prospective analysis of 179 consecutive patients. World J Surg. 2004;28(09):921–925. doi: 10.1007/s00268-004-7375-7. [DOI] [PubMed] [Google Scholar]
- 36.Guyton K LHN, Hyman N H, Alverdy J C. Prevention of Perioperative anastomotic healing complications: anastomotic stricture and anastomotic leak. Adv Surg. 2016;50(01):129–141. doi: 10.1016/j.yasu.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsicovetere P, Ivatury S J, White B, Holubar S D. Intestinal intussusception: etiology, diagnosis, and treatment. Clin Colon Rectal Surg. 2017;30(01):30–39. doi: 10.1055/s-0036-1593429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKay R. Ileocecal intussusception in an adult: the laparoscopic approach. JSLS. 2006;10(02):250–253. [PMC free article] [PubMed] [Google Scholar]
- 39.Yalamarthi S, Smith R. Adult intussesception: case reports and review of literature. Postgrad Med J. 2005;81(953):174–177. doi: 10.1136/pgmj.2004.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wald A. Management and prevention of fecal impaction. Curr Gastroenterol Rep. 2008;10(05):499–501. doi: 10.1007/s11894-008-0091-y. [DOI] [PubMed] [Google Scholar]
- 41.Serrano Falcón B, Barceló López M, Mateos Muñoz B, Álvarez Sánchez A, Rey E. Fecal impaction: a systematic review of its medical complications. BMC Geriatr. 2016;16:4. doi: 10.1186/s12877-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


