Abstract
It is essential for the colon and rectal surgeon to understand the evaluation and management of patients with both small and large bowel obstructions. Computed tomography is usually the most appropriate and accurate diagnostic imaging modality for most suspected bowel obstructions. Additional commonly used imaging modalities include plain radiographs and contrast imaging/fluoroscopy, while less commonly utilized imaging modalities include ultrasonography and magnetic resonance imaging. Regardless of the imaging modality used, interpretation of imaging should involve a systematic, methodological approach to ensure diagnostic accuracy.
Keywords: bowel obstruction, small bowel obstruction, large bowel obstruction, imaging, computed tomography, abdominal radiography, contrast enema, small bowel follow-through, ultrasound, magnetic resonance imaging
Intestinal obstruction is a clinical scenario commonly encountered by colorectal surgeons, 1 and it is important to understand the evaluation and management of patients with both suspected small bowel obstruction (SBO) and large bowel obstruction (LBO). Approximately 75% of all mechanical bowel obstructions occur in the small bowel. 2 3 SBO occurs in 10% of patients within 3 years following colectomy 4 and in up to 25% of patients after restorative proctocolectomy. 5 Multiple colorectal surgery–related pathologies can result in SBO, including postoperative adhesions, Crohn's disease, diverticulitis, and parastomal hernias, among others. Evaluation and management of LBO can be a complex problem that challenges even the most experienced clinicians. 6 Common etiologies of both SBO and LBO are listed in Table 1 .
Table 1. Causes of intestinal obstruction 6 90 .
| Small bowel obstruction | Large bowel obstruction |
|---|---|
| Adhesions Hernias (external and internal) Neoplasm (extraintestinal and primary) Crohn's disease Gallstones Malrotation Duplication cysts Diverticulitis Infection (tuberculosis, intestinal parasites, etc.) Hematoma Ischemic stricture Intussusception Endometriosis Radiation Foreign body |
Colorectal cancer Diverticulitis Volvulus Crohn's disease Noncolorectal malignancy Endometriosis Ischemic stricture Radiation Hernia Adhesions Fecal impaction Foreign body |
Diagnostic imaging is an essential aspect of the modern management of both LBO and SBO. While history and physical exam remain the backbone of evaluation, clinical assessment alone lacks accuracy for bowel obstruction diagnosis and guidance of management. 7 8 9 Imaging helps answer a variety of key questions in patients with suspected obstruction, including the following: 10 11
Is obstruction present and at what level?
What is the cause of the obstruction?
Is severe or complicated obstruction present?
Answers to these key questions help guide the surgeon to make critical judgments about both operative and nonoperative management. Given the importance of imaging in the evaluation of suspected bowel obstruction, the colorectal surgeon must strive to be adept in the interpretation of all available modalities of bowel obstruction imaging. The surgeon should personally review all imaging and discuss points of ambiguity with the radiologist. This collaboration can result in mutual edification and improvement in patient care, combining the surgeon's intimate clinical knowledge of the patient with the radiologist's advanced radiographic expertise. This article will review the available modalities for the evaluation of suspected mechanical intestinal obstruction in the context of the three questions above.
Plain Radiographs
Traditionally, plain abdominal radiographs have been recommended as the initial imaging modality for suspected obstructions due to the speed of acquisition, low cost, wide availability, and low radiation exposure. Plain X-rays can in many cases quickly provide the diagnosis of an obstruction, distinguish SBO versus LBO, rule out pneumoperitoneum, and, in a subset of cases, identify the cause of obstruction, such as colonic volvulus or gallstone ileus.
However, the accuracy of plain radiographs in the diagnosis of bowel obstruction ranges from only 50 to 80%. 12 Additionally, in only a minority of cases do plain abdominal radiographs provide a clear etiology of an obstruction. Plain radiographs are poor at identifying closed loop or strangulated obstructions in the setting of SBO, and the specificity of plain radiographs for LBO is only moderate, in part due to mimicry of acute colonic pseudoobstruction causing false positives. 13 Therefore, even when plain abdominal radiographs appear to definitively establish a diagnosis, obtaining additional information via computed tomography (CT) is often still necessary.
Abdominal radiographs can be obtained as singular images or as a series of films. It has been shown that imaging in both dependent (supine or prone) and nondependent (upright or decubitus) positions increases the accuracy of abdominal radiographs. 14 When the possibility of perforation is considered, an upright chest X-ray or lateral decubitus abdominal film should be obtained to evaluate for pneumoperitoneum. Of note, obtaining multiple views can significantly increase patient radiation exposure. For an average patient, the radiation dose from a singular abdominal X-ray is 0.7 mSv, which is 35 times the dose of a singular chest X-ray (0.02 mSv), 15 but still significantly less than the average noncontrast CT abdomen/pelvis (15 mSv). 16
Is Obstruction Present and at What Level?
When reviewing plain abdominal radiographs, it is critical to note the orientation of the patient, as the interpretation differs for images obtained in dependent versus nondependent positions. 11 Small bowel versus large bowel can be distinguished on plain radiographs based on appearance and location. Small bowel has characteristic circular folds (plicae circulares or valvulae conniventes) which appear radiographically as thin lines that span the entire diameter of the small bowel ( Fig. 1 ). In contrast, large bowel has haustral folds that do not span the entire diameter of the bowel ( Fig. 1 ). Generally, small bowel tends to be more centrally located while colon is peripheral, though this is often difficult to delineate when there is obstructive pathology present. While the ascending and descending colon and rectum are usually fixed in location, the transverse and sigmoid colon are mobile; the transverse colon can loop caudally into the pelvis, and the sigmoid colon can loop into the right iliac fossa. Additionally, the anatomic position of bowel often differs after surgery or due to congenital malrotation.
Fig. 1.
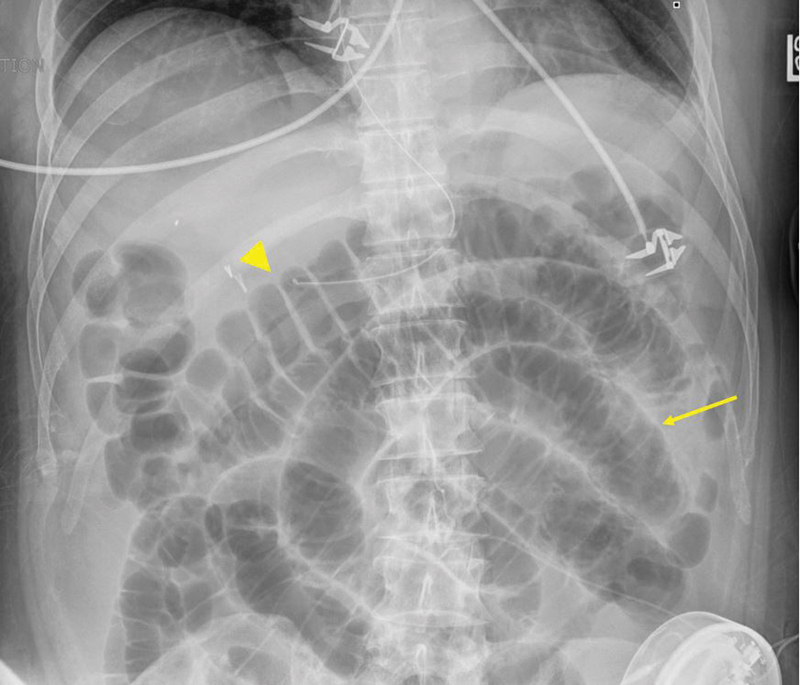
Postoperative ileus with distended small bowel with plicae circulares (thin arrow) and large bowel with haustra (large arrowhead).
The characteristic findings of SBO on plain radiographs are small bowel dilation (diameter > 2.5–3 cm), lack of colonic dilation (colon diameter < 6 cm and cecum diameter < 9 cm), and a relative paucity of colonic gas 11 ( Fig. 2 ). The characteristic radiographic findings of LBO include colonic and cecal dilation (> 6 and > 9 cm, respectively), relative paucity of gas in the rectum, and a proximal colonic fecal burden ( Fig. 3A, B ). 17 In the setting of LBO, small bowel dilation may or may not be present depending on the duration of obstruction, and whether there is a closed loop, competent ileocecal valve, or colonic volvulus. When a closed loop is present, lack of proximal decompression risks progressive segmental colonic dilation, ischemia, and perforation. When the ileocecal valve is incompetent in the setting of a distal LBO, both diffuse small and large bowel distension can mimic the appearance of an ileus on the plain film.
Fig. 2.
Supine abdominal X-ray of small bowel obstruction: dilated small bowel and paucity of colonic gas.
Fig. 3.
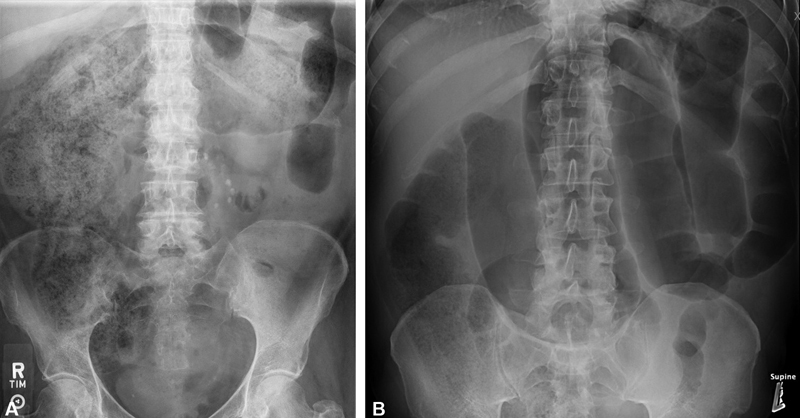
( A ) Abdominal X-ray of large bowel obstruction (LBO), demonstrating significant proximal colonic fecal load. ( B ) Abdominal X-ray of LBO, demonstrating significant transverse colon redundancy and distention.
A variety of additional radiographic signs for the diagnosis of SBO have been described and are summarized in Table 2 . 11 On supine radiographs, gaseous dilation can stretch and outline the plicae circulares causing the small bowel to be more distinctly visible (termed the “stretch sign”). Obstructed or pathologic bowel can vary in appearance on plain radiographs, depending on the quantity of fluid and gas within the lumen. Completely fluid-filled, dilated loops of small bowel may be invisible on plain radiographs, creating a “gasless abdomen” ( Fig. 4 ). On upright or decubitus radiographs, multiple air–fluid levels, air–fluid levels wider than 2.5 cm, and two air–fluid levels within the same bowel loop at different heights (sometimes called a step-ladder pattern 18 ) are findings suggestive of obstruction 11 14 ( Fig. 5 ). When the bowel is fluid-filled, small pockets of gas trapped between the plicae circulares can create multiple small air–fluid levels that appear as a “string-of-beads.” The greater the number of these individual signs present, the higher the specificity for obstruction. 14 19 It has been suggested that the greater the height difference between two air–fluid levels within the same bowel loop, the more likely there is obstruction rather than ileus. A cutoff height difference of ≥2 cm has a ≥95% specificity for obstruction; however, this sign demonstrates poor sensitivity and overall accuracy, and therefore should not be used in isolation or to exclude obstruction. 20
Table 2. Radiographic diagnostic signs for small bowel obstruction and large bowel obstruction 11 .
| Small bowel obstruction Supine or prone: 1. Dilated small bowel > 2.5–3 cm 2. Paucity of colorectal gas 3. Stretch sign 4. Gasless abdomen 5. Dilated stomach Upright or decubitus: 1. Multiple air–fluid levels 2. Air–fluid level wider than 2.5 cm 3. Air–fluid levels in the same small bowel loop of unequal heights 4. String-of-beads sign |
| Large bowel obstruction 1. Dilated colon > 6 cm or cecum > 9 cm 2. Paucity of rectal gas 3. +/− small bowel dilation depending on duration and presence of closed loop |
Fig. 4.
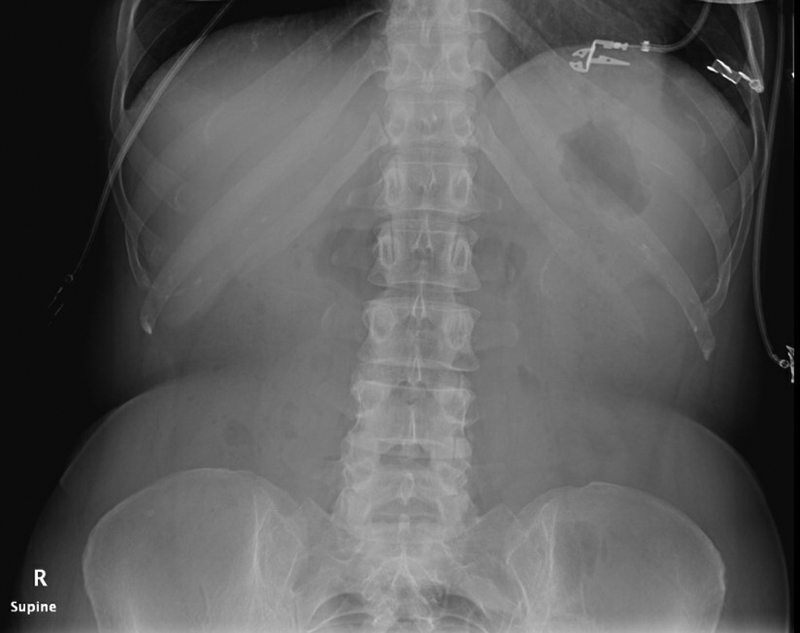
Supine abdominal X-ray: gasless abdomen with relative paucity of gas. The patient had a CT on the same day that demonstrated small bowel obstruction.
Fig. 5.
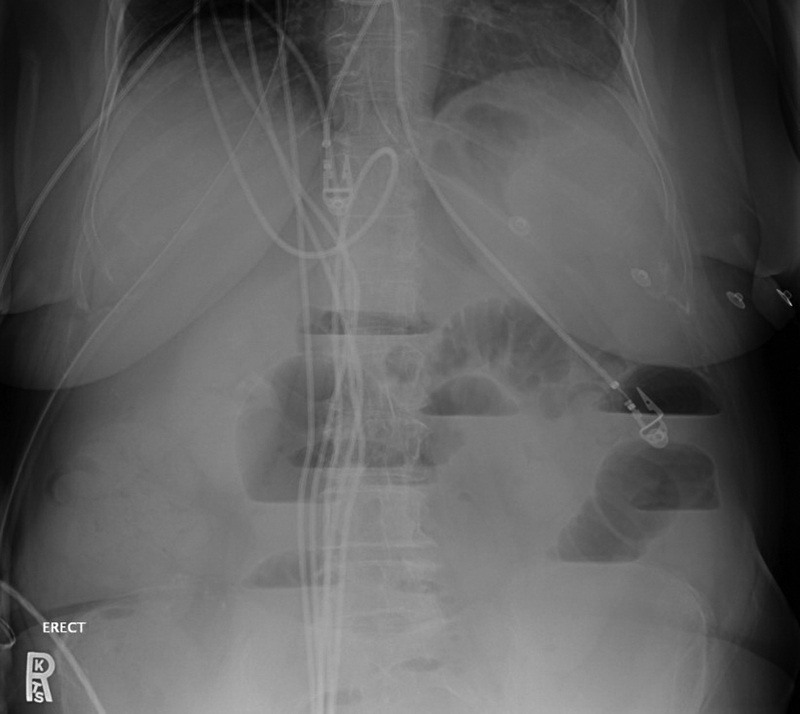
Upright abdominal X-ray of small bowel obstruction: Multiple air–fluid levels, including multiple within the same bowel loop.
Important mimics of mechanical obstruction include paralytic ileus, colonic pseudoobstruction (Ogilvie's syndrome), and toxic megacolon. Relative absence of distal rectal gas and decompression of the distal colon is a frequent feature of colonic pseudoobstruction that can be easily mistaken for mechanical obstruction on plain radiographs. 21 22 Similarly, paralytic ileus can appear without radiographic evidence of colonic dilation, mimicking SBO. Conversely, in the setting of low-grade SBO, distal colorectal gas may be present, leading to an erroneous diagnosis of ileus or pseudoobstruction. Very distal mechanical obstructions can also sometimes mimic a functional obstruction radiographically. Toxic megacolon in the setting of infectious or inflammatory colitis can also mimic LBO due to the presence of colonic dilation. 13
What Is the Cause of Obstruction?
Identification of a clear etiology of SBO is usually not possible on plain radiographs. Approximately 75% of SBOs are caused by intra-abdominal adhesions that cannot be visualized on plain X-ray. 17 Occasionally, incarcerated inguinal hernias can be seen as gas inferior to the inguinal ligament, but these are certainly better diagnosed by physical exam or CT. Obstruction from a gallstone that has traversed a cholecystic-enteric fistula and lodged in the terminal ileum can also be identified radiographically by the triad of dilated small bowel, opacity in the right lower quadrant (RLQ), and pneumobilia (Rigler's triad).
Identification of a specific cause of LBO based on plain radiographs is possible in a small proportion of cases. The three most common causes of LBO are colorectal cancer (CRC), diverticulitis, and volvulus, which together account for more than 95% of all LBO in modern epidemiologic data from a developed nation. 3 CRC and diverticulitis cannot typically be diagnosed via plain radiographs alone, while volvulus can present with distinct appearance on plain radiographs, depending on the site. The sigmoid colon is the most common site of volvulus, followed by the cecum. 23 The twisting of the colon around a narrow, elongated mesenteric pedicle results in a “ U -” or “ C -”shaped appearance, or a “coffee bean” appearance, with the concavity pointing to the site of twist, classically toward the left lower quadrant for sigmoid volvulus and toward the right lower quadrant for cecal volvulus ( Fig. 6 ). 11 17 22 However, this classic appearance is not always present, 6 and one retrospective review found plain abdominal radiographs to be insufficient for diagnosis of 85% of cecal volvulus and 49% of sigmoid volvulus. 24 Additional etiologies of LBO that can be diagnosed on plain radiograph include foreign body obstruction and fecal impaction.
Fig. 6.
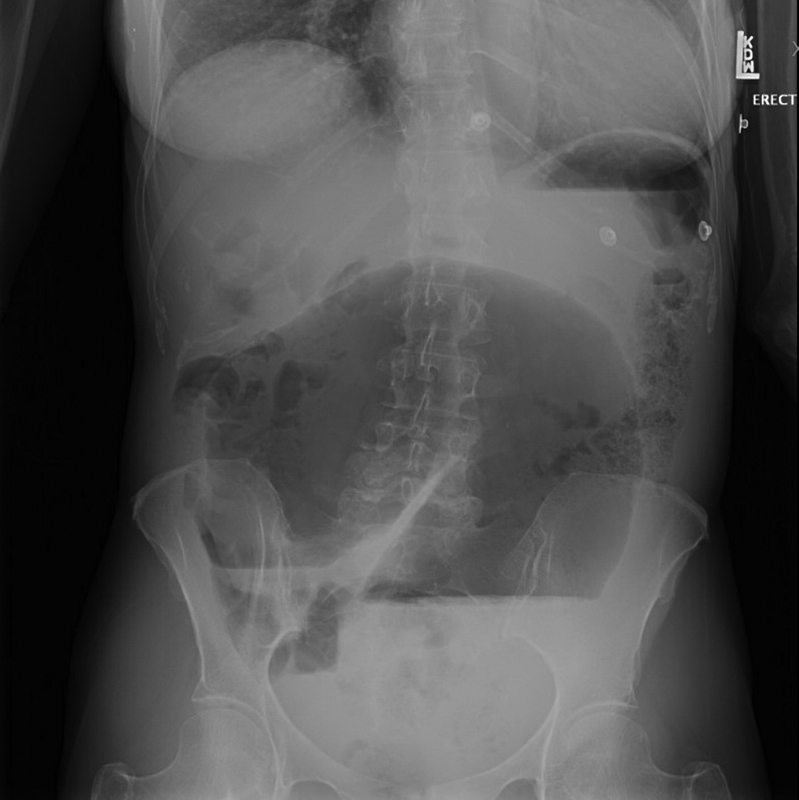
Upright abdominal X-ray of cecal volvulus demonstrating “C” loop with concavity pointing to right lower quadrant.
Is Severe or Complicated Obstruction Present?
Assessment for pneumoperitoneum on supine films can be difficult. Therefore, when perforation is suspected, an erect chest X-ray, which can detect as little as 1 mL of intraperitoneal gas, should be obtained. 17 When interpreting supine abdominal radiographs, subtle findings that may suggest the presence of pneumoperitoneum include lucency on both sides of the bowel (Rigler's sign), lucency outlining the falciform ligament or the liver, or geometrically shaped lucencies, such as triangles of gas trapped between bowel loops. 17 Large-volume pneumoperitoneum on supine radiographs can be observed as a large ovoid central lucency (football sign). 17 A gas-filled colon interposed between the liver and right hemi-diaphragm can mimic pneumoperitoneum (Chilaiditi sign).
Pneumatosis intestinalis is the presence of gas within the bowel wall and can be an indicator of bowel ischemia. In the setting of LBO, pneumatosis in the cecum associated with cecal dilation is concerning for impending perforation. 13 A cecal diameter of greater than 12 cm is typically felt to be concerning for impending perforation, but no absolute diameter should be used, as symptoms and duration of distension may be just as important as absolute diameter. 6 Pneumatosis is typically visible as gas in the dependent portion of the bowel. Pseudopneumatosis, in which gas is trapped within feces against the wall, can mimic true pneumatosis on abdominal radiographs and is better distinguished from true pneumatosis with CT. 25 While the presence of pneumatosis in the setting of bowel obstruction should be suspected as evidence of ischemia, it is notable that there are also several benign causes of pneumatosis which do not require operative intervention. 26 Portal venous gas is a potential sign of ischemia in the setting of bowel obstruction; when the volume of portal venous gas is large enough to be visualized on plain radiographs, it is a particularly ominous sign with historical mortality of up to 75%. 27
Computed Tomography
As CT technology has improved, the gap in cost, speed, and availability between CT and plain X-ray has diminished. CT accuracy for the diagnosis of both SBO and LBO is greater than 95%. 28 29 30 This has led some to question the traditional approach of using of plain radiographs as the initial imaging modality in the evaluation of patients with suspected bowel obstruction. 8 31 The “Appropriateness Criteria for Suspected Small-Bowel Obstructions,” published by the American College of Radiology (ACR), states that for initial imaging of the patient with suspected SBO, CT abdomen/pelvis is “usually appropriate” while plain radiographs “may also be appropriate.” 32 The use of a multidetector CT is essential, as multiplanar reconstructions and review of axial, coronal, and sagittal images have been shown to increase the accuracy of interpretation. 33 34
When CT is performed to evaluate suspected bowel obstruction, intravenous (IV) iodinated contrast should be administered unless contraindicated. 32 35 The use of IV contrast has not been shown to significantly change the sensitivity of CT for the detection of bowel obstructions, but it can improve assessment for bowel wall ischemia. 36 Enteric contrast should also be administered in certain clinical scenarios. Enteric contrast agents used for CT can be categorized as positive (radiodensity > water), neutral (radiodensity ≈ water), and negative (radiodensity < water; e.g., gas). Most enteric contrast used for CT is water-soluble iodinated-based contrast but dilute barium preparations for CT do exist. 37 Standard barium cannot be used for CT due to its high radiodensity that creates artifacts obscuring images.
Indications for administration of enteric contrast for CT evaluation of suspected bowel obstructions differ depending on the clinical suspicion. In cases of a suspected high-grade SBO, oral contrast prior to CT should generally be avoided. In this setting, retained fluid within the distended small bowel acts as a natural neutral contrast agent allowing for the same diagnostic accuracy as with oral contrast. 36 In fact, positive oral contrast administration may actually decrease the ability to assess for bowel wall ischemia in this setting. 32 The aforementioned ACR Appropriateness Criteria state that “oral contrast used in a known or suspected high-grade SBO does not add to diagnostic accuracy and can delay diagnosis, increase patient discomfort, and increase the risk of complications, particularly vomiting and aspiration.” 32 In patients with suspected low-grade SBO obstruction, the use of positive oral contrast is appropriate and may increase the sensitivity of diagnosis. 32 In patients with indolent or chronic intermittent obstructive symptoms, the use of large volume neutral contrast administered orally or via a nasoenteric tube can also improve accuracy as part of a specific protocol. In cases of suspected LBO, there is a paucity of published data regarding whether oral and/or rectal contrast should be administered, 29 and no apparent society guidelines exist. Administration of oral contrast alone has the disadvantage of a prolonged waiting period prior to opacifying the colon. While rectal contrast may increase patient discomfort, it may help delineate the site of an obstruction or more definitively rule out mechanical obstruction. Rectal contrast should not be administered if there is any suspicion for perforation. If initial CT is obtained without rectal contrast and diagnosis is in question or further clarity regarding the lesion is needed, CT can be repeated with water-soluble rectal contrast 13 without repeat IV contrast or a water-soluble contrast enema can be obtained.
Is Obstruction Present and at What Level?
The major diagnostic finding for bowel obstruction on CT is proximal dilation with distal decompression. Intestinal dilation on CT is considered to be present at a small bowel diameter greater than 2.5 cm, colon diameter greater than 6 cm, and cecal diameter greater than 9 cm. 11 38 39 In addition to providing high diagnostic accuracy, cross-sectional imaging often allows precise anatomic localization of the site of obstruction by the identification of a transition zone (TZ) where dilated proximal bowel transitions to nondilated distal bowel ( Fig. 7 ).
Fig. 7.
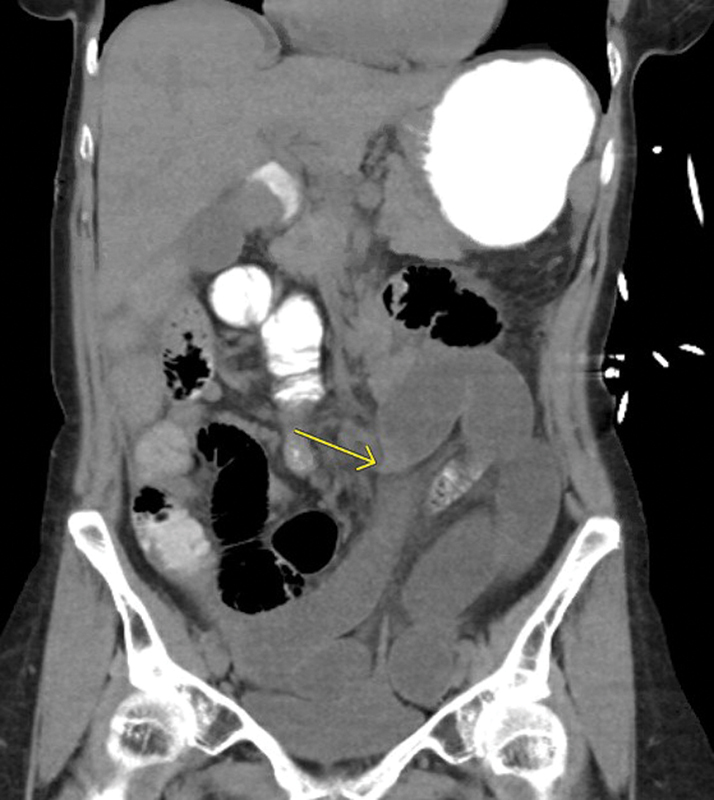
CT without IV contrast demonstrating a transition point (arrow) in the midabdomen with a smooth taper due to a postoperative adhesive small bowel obstruction.
Review of abdominopelvic CT for suspected bowel obstruction should be performed systematically. One proposed method is to begin at the anus and trace the bowel proximally. 38 40 This allows for complete inspection of the large bowel and early identification of LBO, particularly when there is concomitant small bowel dilation due to an incompetent ileocecal valve. In the setting of LBO, identification of a TZ on CT is typically possible, while the ability to identify a distinct TZ in the setting of SBO is less reliable, with reported ranges of 63 to 93%. 11 In instances of a more proximal SBO, identification of a TZ can be aided by tracing antegrade starting from the stomach. 38 It should be noted that the ability to detect a clear TZ in SBO is not absolutely necessary for diagnosis, and the ability to identify a TZ does not necessarily correlate with outcome. 41
Localizing a TZ in the setting of SBO can be aided by identification of a “small bowel feces sign” or “small bowel fecalization,” which is seen when a mixture of intraluminal particulate material and gas bubbles within the small bowel creates an appearance on CT similar to that colonic stool ( Fig. 8 ). First described by Mayo-Smith, 42 this finding has been ascribed various levels of clinical importance. Initial reports described its presence as uncommon (∼7%) 43 on CT images diagnostic for SBO, while later reports have reported it to be present in 37 to 55.9% of patients with CT evidence of SBO. 44 45 This finding can help readily identify a TZ; it has been reported that in 93% of cases in which the “feces sign” is present in the setting of SBO, it is seen immediately proximal to the TZ. Of note, the administration of positive oral contrast may obscure this finding.
Fig. 8.
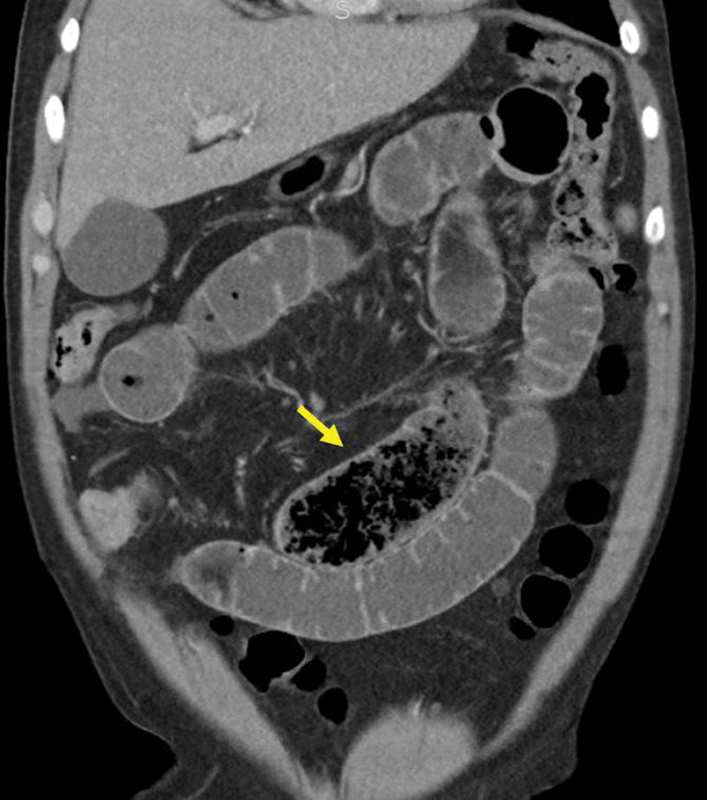
Coronal CT: “small bowel feces sign” (arrow). Although not easily visualized on this image, this is immediately proximal to a Crohn's stricture.
As with plain radiographs, there are several important mimics of SBO and LBO on CT. Low-grade SBO without a distinct TZ can mimic the appearance of an adynamic ileus. Spasm at any level of the normal colon, particularly the flexures and sigmoid colon, can mimic a fixed narrowing, giving the appearance of LBO. 46 Acute and chronic colonic pseudoobstruction can also cause proximal dilation with distal decompression. In the setting of pancreatitis, isolated gaseous distension of the ascending colon and hepatic flexure (termed the “colon cutoff sign”) can also mimic LBO. 47
What Is the Cause of Obstruction?
Small Bowel Obstruction
As previously discussed, approximately 75% of SBOs are caused by adhesions. On CT imaging, the diagnosis of adhesive SBO is presumptive, based on the presence of obstruction, the patient's clinical history, and exclusion of other findings. Careful study of the TZ is essential for identifying a potential etiology of SBO on CT, as radiographic clues will often be immediately in proximity to the TZ. Many nonadhesive etiologies of SBO can also be identified on CT, including hernia (external and internal), small bowel masses, gallstones, strictures (e.g., related to Crohn's disease), intussusception, and small bowel volvulus, or closed loop obstruction. Benign small bowel masses such as lipomas and gastrointestinal stromal tumors causing obstruction can often be identified on CT. Small bowel primary malignancies are rare but can sometimes be identified as masses at the TZ. Small intestinal or ileocecal intussusception can often be visualized as a “target sign.”
Large Bowel Obstruction
CRC is the cause of greater than 60% of LBO, 3 13 with more than 75% of obstructing CRCs occurring distal to the splenic flexure. CT findings suggestive of CRC include an enhancing soft-tissue mass or short-segment asymmetric thickening of the colonic wall occurring at the TZ. 22 Diagnostic suspicion for CRC may also be heightened by identification of associated lymphadenopathy or lesions in the liver or lungs suspicious for metastatic disease. Colonoscopy and direct visualization/biopsy of the colonic lesion is necessary for a definitive diagnosis of CRC, though this is not always possible prior to surgical intervention in the patient who presents with complete or near-complete LBO and impending perforation.
Diverticulitis is responsible for approximately 3.6 to 10% of LBO. 3 22 In comparison to LBO due to CRC, these lesions typically appear on CT as longer, more symmetric segmental colonic thickening at the TZ. 22 Associated findings with active disease include mesenteric fat stranding, abscess, and phlegmon, but these findings are often difficult to differentiate from perforated CRC, highlighting the need for follow-up colonoscopy following resolution of an episode of acute diverticulitis. 48 Additional CT findings with chronic diverticular disease include progressive colonic wall thickening, fibrosis, and stricture formation. The presence of air in the bladder or vagina associated with a chronically diseased segment of sigmoid colon on CT suggests the presence of a colovesical or colovaginal fistula.
Colonic volvulus most commonly occurs at the level of the sigmoid colon (70%), followed by the cecum (25%) and transverse colon (5%). 21 On CT imaging, the segment of redundant colon is often massively elongated and dilated, and is typically associated with twisting of the mesocolon, known as a “whirl sign” ( Fig. 9 ). The proximal and distal limbs of the loop can also be seen to taper in a “bird's beak” fashion. Additional CT signs of colonic volvulus include the “X-marks-the-spot” sign which is the visualization of two TZs crossing in opposite directions at the same location, and the “split-wall” sign in which the colonic wall appears separated by mesenteric fat due to folding occurring with the twist. 49 50
Fig. 9.
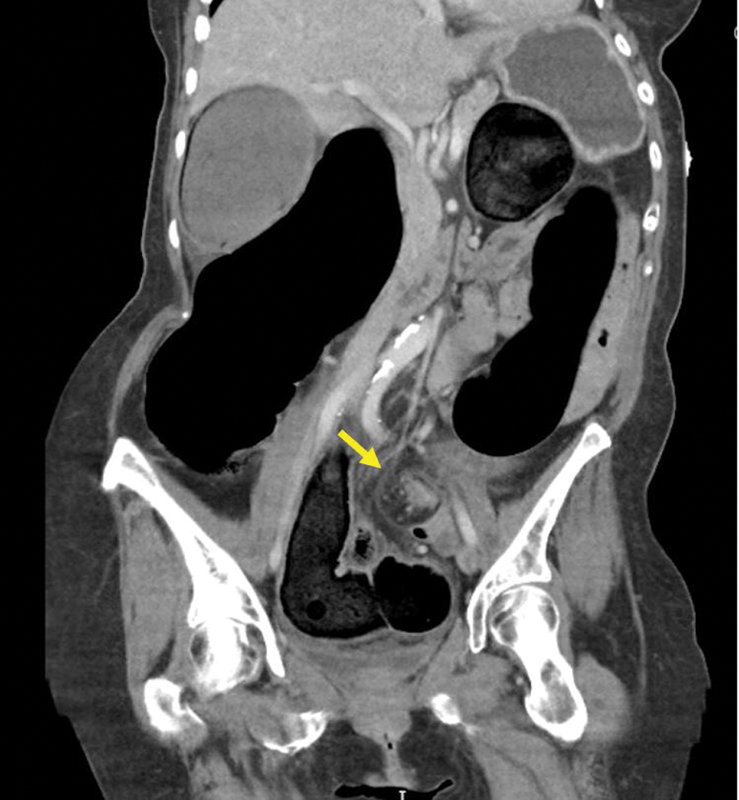
Coronal CT with “whirl sign” (arrow) in the setting of sigmoid volvulus.
Additional causes of LBO are much less common, but still occasionally seen on CT imaging. Colocolonic intussusception is rare, and most frequently occurs due to colonic neoplasm. CT signs of intussusception includes the “target sign” when viewed perpendicular to the lumen and the “sausage pattern” when viewed parallel to the lumen 22 ( Fig. 10A, B ). Fecal impaction, most commonly in the rectosigmoid, can result in LBO. This diagnosis should be considered when focal stool is present that is equal to or greater than the upstream colonic diameter without any associated soft-tissue mass. Inflammatory bowel disease can also cause LBO as a result of colonic strictures. 49 Crohn's disease causes strictures of the colon more frequently than ulcerative colitis due to the transmural nature of inflammation. On CT, IBD-related strictures typically appear as focal colonic wall thickening with enhancement and can be difficult to differentiate from CRC, again highlighting the need for endoscopic visualization and biopsy.
Fig. 10.
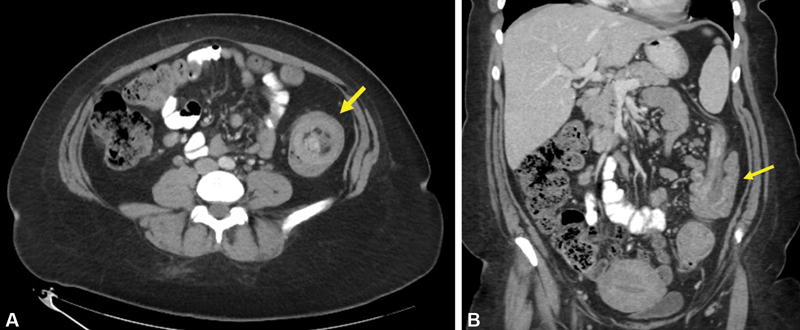
( A ) Axial CT showing colonic intussusception with “target sign” (arrow). ( B ) Coronal CT showing colonic intussusception with “sausage sign” (arrow).
Is Severe or Complicated Obstruction Present?
CT is also particularly useful for assessing the severity of obstruction (partial vs. complete or low-grade vs. high-grade) and for identifying potential complications that can guide decisions regarding the need and timing for operative intervention. When positive oral contrast is administered, failure of contrast to progress past the TZ in 3 to 24 hours can be diagnostic of complete obstruction. 38 51 However, oral contrast is often either not administered or insufficient time has elapsed at the time of imaging to allow passage of contrast for definitive diagnosis of complete obstruction. 51 In this scenario, a subjective judgment of the severity of obstruction can be made. Factors used to grade the severity of SBO include a subjective grading of the quantity of gas/fluid in the distal small bowel and/or the ascending colon, and quantitative comparison of the proximal and distal luminal diameters, with a difference of ≥50% felt to be suggestive of high-grade obstruction. 38 Terminology can be ambiguous, with the term “high-grade” often being used synonymously with “complete obstruction,” while partial obstructions are frequently subcategorized into high-grade partial and low-grade partial obstruction.
Complications related to intestinal obstruction that can be identified on CT include perforation, closed loop obstruction, internal hernia, volvulus, and intestinal ischemia. These are frequently identified only after careful inspection of the TZ on multiple reconstructed CT views. 38 CT allows for extremely sensitive identification of pneumoperitoneum and free fluid. Free intraperitoneal fluid is present in over one-third of patients with acute SBO and is nonspecific for need of operative intervention. In one study, fluid with a Hounsfield unit density greater than 10 was associated with a positive predictive value and negative predictive value of ≥75% in predicting the need for operative intervention. 52
Closed loop SBO can be identified on CT but requires a high index of suspicion. Closed loop SBO often results from a single constricting lesion such as an adhesive band that occludes both proximally and distally and can be associated with volvulus. 11 Another common etiology of closed loop SBO is an internal hernia caused by congenital or iatrogenic mesenteric defects. Knowledge of the patient's surgical history is essential in evaluating imaging studies in the setting of suspected intestinal obstruction. Patients who have undergone prior Roux-en-Y gastric bypass are at risk for SBO due to internal hernia, with potential sites of mesenteric defects at the jejunojejunostomy, transverse mesocolon (if a retrocolic roux limb was created), and beneath the gastrojejunostomy (Peterson's hernia). On CT, a closed loop obstruction or volvulus typically appears as a “C” or “U” with the concavity pointing to the site of obstruction, when the loop is within the plane of imaging. At the site of tethering, the ends of the loop narrow to a “beak” configuration. When there is coexistent volvulus, the mesentery will be twisted and the “whirl sign” can be present similar to colonic volvulus. 11 It is important to assess the images in multiple reconstructed views, as a closed loop may sometimes be most apparent on a coronal or sagittal view. 11
CT is highly specific for the detection of intestinal ischemia, but it lacks sensitivity. Therefore, one must have a high index of suspicion for intestinal ischemia based on the patient's clinical presentation. Radiographic signs of bowel ischemia are listed in Table 3 . In one study that compared prospective and retrospective review of CT for the detection of intestinal ischemia, the prospective interpretation showed a sensitivity of only 14.8% and a specificity of 94.1%. When the imaging studies were re-reviewed retrospectively by two additional blinded, independent gastrointestinal radiologists, the sensitivity was still only 51.9% and the specificity was 88.2%. 53 The administration of IV contrast can increase the sensitivity of ischemia detection by demonstrating relative hypoenhancement or heterogenous enhancement of strangulated bowel compared with adjacent nonischemic bowel. In noncontrast CT images, hemorrhagic infarcted or ischemic bowel will often appear hyperattenuated. 11 Portal venous gas on CT is a particularly worrisome finding in the setting of intestinal obstruction. Portal venous gas should not be confused with pneumobilia which can occur with gallstone-related obstruction from a cholecysto-enteric fistula, as well as in patients who have undergone recent ERCP or prior surgery with a biliary-enteric anastomosis. Portal venous gas appears in a tubular and branching pattern extending peripherally (within 2 cm of the liver capsule), whereas pneumobilia appears as isolated bubbles and is located centrally (> 2 cm from the capsule). 54
Table 3. Radiographic signs suggestive of bowel ischemia in the setting of small bowel obstruction 11 .
| Wall hypoenhancement or heterogenous enhancement (with IV contrast) Hyperattenuation without IV contrast Wall thickening (> 3 mm) Mesenteric edema Engorged or occluded mesenteric vessels Pneumatosis intestinalis Portal venous or mesenteric venous gas Free intraperitoneal fluid Whirl sign Closed loop obstruction |
Contrast Imaging/Fluoroscopy
While CT and plain X-ray are typically the most appropriate initial imaging modalities for patients with suspected bowel obstruction, contrast imaging/fluoroscopy studies are important common adjuncts that can help clarify specific clinical questions and guide therapeutic intervention.
Water-Soluble Contrast Enema
The use of the fluoroscopic unprepped contrast enema historically played a significant role in the initial diagnosis of acute LBO. 55 However, with the advent and wide availability of multidetector CT, the indications for contrast enema are now more limited and it is largely reserved for complimenting CT findings. 13 22 28 55 A review by Jacob et al 55 demonstrated a one-third decrease in contrast enema use for the evaluation of suspected LBO from the years 2000–2006 with a concomitant increase in the use of multidetector CT for the same indication.
Contemporary indications for diagnostic contrast enema include evaluation of equivocal cases of LBO, where it can help distinguish LBO from acute colonic pseudo-obstruction, 13 56 and the evaluation of equivocal cases of colonic volvulus. 13 In the setting of suspected LBO, the examination should be performed with water-soluble contrast, with low pressure, and without inflation of the catheter balloon. 13 Barium should be avoided in the setting of suspected LBO because of the risk of intraperitoneal contamination with perforation and the potential interference with subsequent cross-sectional imaging or colonoscopy. 37 Complete examination does require patient rotation on the fluoroscopy table and therefore the exam can be limited in patients who are unable to participate in these maneuvers. 13
Is Obstruction Present and at What Level?
Diagnosis of LBO is made by failure of contrast to progress proximal to the level of obstruction in the setting of complete or near-complete LBO, or by the presence of nondistensile narrowing in the setting of partial LBO, such as that seen with the classic “apple core” narrowing of an annular colorectal carcinoma. LBO is ruled out if contrast progresses the extent of the colon without luminal irregularity or narrowing.
What Is the Cause of Obstruction?
Identification of a specific cause of LBO is often possible with contrast enema. Findings suggestive of a neoplastic process, as described earlier, can be seen. Distinguishing diverticulitis from CRC used similar criteria as CT; neoplasms are typically associated with short, eccentric narrowing (< 10 cm), acute shouldering of the lesion rather than gradual tapering, and absence of diverticula in the affected segment. 57 Sigmoid volvulus characteristically appears as a “bird's beak” sign with progressive narrowing of the proximal and distal bowel loops ending at the site of torsion. 23
Is Severe or Complicated Obstruction Present?
Water-soluble contrast enema is usually not the most appropriate initial study to rule out perforation or assess for other complications in the setting of suspected obstruction. A modern role for water-soluble contrast enema is for pretreatment planning prior to colonic stent placement. A contrast study (either contrast enema fluoroscopy or rectal contrast CT) should always be performed prior to attempted colonic stent placement to identify the location, length, and caliber of the obstructing lesion and also rule out perforation ( Fig. 11A–C ). 58 Additionally, fluoroscopy is typically used during stent deployment and for confirmation that the guidewire has successfully traversed the lesion by exchanging the wire with a catheter and injecting water-soluble contrast. 58
Fig. 11.
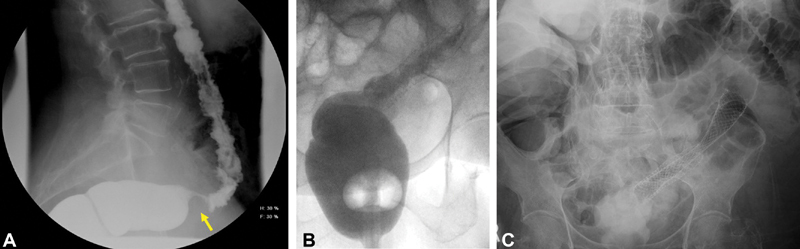
( A ) Water-soluble contrast enema in a patient with obstructing sigmoid colon cancer demonstrating an “apple core lesion” (arrow). ( B ) Water-soluble contrast enema showing in-stent stenosis after prior colonoscopic stent placement. ( C ) Supine abdominal radiograph in the same patient status post–repeat colonic stent placement within prior stent.
Small Bowel Contrast Studies
Formal small bowel follow-through (SBFT) is usually not performed as an initial test for acute obstruction, but it has a role in the evaluation of low-grade and chronic SBOs. 11 37 SBFT is performed with barium because water-soluble contrast is inadequate for delineation of small bowel anatomy and dilution of the contrast within the small bowel with progression typically does not allow identification of a more distal TZ. 37 59 Barium can be safe to administer in suspected low-grade SBO when there is no suspicion of perforation, but it should be avoided in high-grade SBO. 37 Barium can also interfere with subsequent cross-sectional imaging by creating artifact. It is important to rule out LBO prior to administration of oral barium because when barium is trapped proximal to a colonic lesion, progressive water absorption can lead to barium inspissation (“barolith”) with risk of worsening obstruction, ulceration, ischemia, and perforation. 60
SBFT can be performed by a variety of protocols, but it generally involves serial-wide field-of-view radiographs and focused fluoroscopic examinations every 15 to 45 minutes by a real-time radiologist who performs manual palpation to splay out individual bowel loops and rotates the patient to obtain multiple views. 37 61 The location and width of the small bowel is assessed with progression of contrast and any abnormal luminal protrusions or depressions are noted. 37
Enteroclysis is similar to SBFT except that instead of oral contrast administration, enteral contrast is rapidly administered via a postpyloric enteric tube. Contrast media include singular or combination barium, methylcellulose, and/or air. Enteroclysis has greater sensitivity than SBFT for subtle obstructing lesions as a result of over distending the bowel proximal to a lesion. This examination is less commonly performed than SBFT due to both patient discomfort and logistics of nasoenteric tube placement. Another variation of the SBFT is a peroral pneumocolon. In this examination, a standard SBFT is performed and then an air enema is administered per rectum with manual manipulation of air into the terminal ileum to result in a distal small bowel double-contrast study to help assess terminal ileum pathology.
Is Obstruction Present and at What Level?
SBFT and enteroclysis can diagnose obstruction and provide accurate determination of the level of obstruction in many cases. Criteria for obstruction on enteroclysis includes jejunal distension to greater than 4 cm and ileal distension to greater than 3 cm. 37
What Is the Cause of Obstruction?
The test can be useful to identify chronic adhesion-related strictures, small bowel tumors, and strictures in the setting of Crohn's disease. 61 The real-time visualization of contrast progression and outline of the bowel facilitated by a radiologist can identify small bowel pathology not visible on a stand CT.
Is Severe or Complicated Obstruction Present?
SBFT has limited ability to diagnose ischemia or perforation. 32 However, one use of small bowel contrast imaging in the acute setting is to distinguish complete from partial obstruction. In the special case of adhesion-related SBO, water-soluble contrast administration has been advocated in the form of an “abbreviated” SBFT also known as a “Gastrografin challenge” for this purpose. Protocols vary, but typically one to four plain abdominal radiographs are obtained between 4 and 24 hours post water-soluble contrast administration to assess whether the contrast reaches the colon. 62 A meta-analysis by Abbas et al reported that water-soluble contrast in the colon within 24 hours predicts nonoperative resolution of obstruction with 97% sensitivity and 96% specificity. 63 Use has been demonstrated to reduce hospital length of stay for those not requiring surgical intervention. 63 More controversial is a reputed therapeutic benefit with use accelerating the resolution of obstructive symptoms. Multiple randomized trials have reported accelerated resolution of symptoms in patients treated nonoperatively, 64 65 but a meta-analysis was unable to support this therapeutic role. 62 It is important to emphasize that studies do not demonstrate a reduced percentage of patients requiring operative intervention with administration of water-soluble contrast. 62 63
Ultrasound
Ultrasound has traditionally played a very limited role in the evaluation of patients in the United States with suspected bowel obstruction, except in children where it is frequently used for targeted evaluation for pyloric stenosis and intussusception. However, studies with use of point-of-care ultrasound (POCUS) in adults have shown both a high sensitivity and specificity for SBO and have even reported POCUS to perform better as a screening exam than abdominal radiographs. 66 Ultrasound has the advantages of dynamic assessment and lack of radiation exposure. However, ultrasound is limited by user dependency, limited ability to identify the level of obstruction, and limited ability to assess for evidence of complications at the site of obstruction. Given this, ultrasound has not gained mainstream use in the evaluation of suspected adult bowel obstructions, though POCUS use by emergency department physicians may be increasing.
Is Obstruction Present and at What Level?
Typical probe placement patterns utilized for POCUS include specific sites (bilateral paracolic gutters, epigastric, and suprapubic regions) versus complete abdominal survey performed in sequential horizontal rows. Criteria for SBO include small bowel diameter greater than 2.5 cm and bidirectional or “to-and-fro” peristalsis. 67 Additional diagnostic signs include the “piano key” sign, which describes the ultrasound appearance of dilated plicae circularis, and the “tanga sign” of free fluid between bowel loops. 67 Occasionally, a specific TZ can be detected, depending on the availability of a fluid-filled acoustic window. A TZ is more readily identified when it is located superficially or when the bowel is fluid filled without gas, as the presence of bowel gas can obscure deeper imaging due to reflection of sound waves at the fluid–air interface. Ultrasound has also been specifically evaluated for the diagnosis of LBO with a sensitivity of approximately 85% in one study. 68 In this study, ultrasound even identified some cases of LBO misdiagnosed as SBO by abdominal radiograph.
What Is the Cause of the Obstruction?
Occasionally, a specific cause of intestinal obstruction can be identified, such as a tumor, hernia, intussusception, or Crohn's disease. 38 69 Again, the presence of bowel gas can limit the available sonographic window and reduce the likelihood of identifying a specific cause. Intussusception can typically be seen via ultrasound as a classic “target sign.” Ultrasound is also useful for confirming the site of an obstruction due to an incarcerated external hernia, as the superficial location allows for tracing of the bowel within the hernia and identification of both the hernia neck and TZ. 38 Ultrasound is poor at distinguishing causes of LBO; Ogata et al reported on its inability to specifically diagnosis sigmoid volvulus. 68
Is Severe or Complicated Obstruction Present?
Ultrasound can be used to detect pneumoperitoneum, which appears on ultrasound as a bright line at the fluid–gas interface with shadowing that obscures the underlying anatomy. 70 The location of the gas varies depending on position similar to plain radiographs and a variety of signs have been described for these specific ultrasound findings. 70 Studies have revealed that the overall accuracy of ultrasound detection of pneumoperitoneum is actually similar to plain radiography. 70 Free abdominal fluid can be readily seen on abdominal ultrasound, but this has very low specificity.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is an alternative cross-sectional modality for intestinal obstruction that offers advantages of no ionizing radiation, better soft-tissue contrast, and the ability to obtain dynamic information, including bowel motility and distensibility. 71 The lack of radiation exposure makes MRI particularly useful for children, pregnant women, and patients with Crohn's disease who are at risk for high lifetime radiation exposure from serial imaging. 37 The main limitations of MRI are its significant cost, lack of widespread availability, decreased spatial resolution, and variability of examination quality. 71 Depending on the sequences acquired, the examination can be time consuming and require patient participation with breath holding and lying still. Combined, these aspects make it unlikely that MRI will replace CT as the principal modality for the evaluation of bowel obstruction in the near future. 69
Is Obstruction Present and at What Level?
MRI has excellent accuracy for detecting intestinal obstruction, and it can be performed rapidly when done for pure diagnostic purposes. A prospective study utilizing a rapid protocol with only HASTE (half-Fourier acquisition single-shot turbo spin-echo) sequence MRI without IV or oral contrast demonstrated a sensitivity of 95% and a specificity of 100% for the detection of intestinal obstruction with better accuracy than helical CT. 72 The study noted that all participants completed the imaging process from arrival to departure in ≤ 10 minutes. 72 Diagnostic criteria for MRI are similar to CT criteria, namely, dilated proximal bowel and decompressed distal bowel. 69 A TZ can often be determined. On HASTE sequence MRI, the small bowel lumen appears bright, while the colonic lumen appears dark; diagnosis of LBO is more difficult than SBO. 72 The sensitivity for SBO diagnosis can be enhanced by administration of oral contrast agents to distend the proximal bowel and exaggerate the TZ. However, this rapid protocol utilizing only HASTE sequences is limited by a decreased ability to assess colonic obstruction and difficulty assessing complications of obstruction, such as inflammation and bowel viability, when compared with other MRI sequences or CT. 73
What Is the Cause of the Obstruction?
The acquisition of multiplanar cross-sectional imaging of MRI can allow for the visualization of specific causes of obstruction. 69 HASTE sequence imaging is limited by the presence of dark motion artifacts at areas of intraluminal fluid flow and it has no ability to visualize the mesentery. 74 However, even with these limitations, HASTE sequence can have high accuracy for detecting the cause of obstruction but with poor visualization. 72 75 Additional complimenting sequences can be added to improve delineation of the specific etiology. Overall, MRI provides better soft-tissue contrast compared with CT and can better diagnose soft-tissue masses compared with CT. 71 A specific postoperative adhesion causing SBO can sometimes be visualized as a high-signal intensity soft-tissue band on T2-weighted images. 71 However, the better soft-tissue contrast of MRI compared with CT is tempered by less spatial resolution, which results in objects appearing to have less distinct edges and difficulty distinguishing smaller objects with similar signal intensity.
Is Severe or Complicated Obstruction Present?
HASTE sequences are particularly poor at detecting inflammation or bowel viability/ischemia and the mesentery is not visualized. 72 74 75 Neither pneumoperitoneum nor pneumatosis can reliably be detected. Additional sequences with longer acquisition time can improve detection of inflammation and bowel viability; however, the time-sensitive nature of the exam and more favorable features (time, cost, ease of image interpretation, etc.) of other modalities make the use of MRI impractical for the routine evaluation of complications resulting from intestinal obstruction.
CT/MR Enterography and Enteroclysis
CT enterography (CTE), MR enterography (MRE), and CT/MR enteroclysis involve specific techniques which allow better visualization of the intestine compared with conventional CT or MRI. They are used mostly in the setting of Crohn's disease, but can also be utilized in the evaluation of chronic or recurrent low-grade SBOs of unclear etiology. The overall accuracy of diagnosis of Crohn's disease is similar between the two modalities. 76
CTE differs from traditional CT of the abdomen/pelvis in its use of large volume neutral contrast, specific timing of IV contrast to image the enteric or portal venous phase, and the routine reconstruction of thin (3 mm or less) multiplanar images. The volume of oral contrast is significant and can be a factor in the patient successfully completing the examination. CT enteroclysis is performed in a similar fashion, though the neutral contrast is administered via a nasoenteric tube that allows for rapid enteral contrast administration and improved sensitivity. 37 77
MRE involves the administration of oral contrast agents (which differ from CT agents) along with specific MR sequences to obtain both static and dynamic images. The exact protocols and sequences are detailed and beyond the scope of this text. In addition to the various static sequences with and without contrast, MR fluoroscopic sequences can also be obtained to assess the overall bowel caliber, mobility, and transit time of enteral contrast to the ascending colon. 37 Fluoroscopic pulse sequences can be obtained to view the distensibility of suspicious specific regions of narrowing. 37
With both CTE and MRE, findings suggestive of Crohn's disease include bowel wall thickening, enhancement, increased vascularity of the vasa recta (comb sign), adenopathy, skip lesions, fistulas, and abscess ( Fig. 12A–C ). 76 The normal thickness of the wall of the small intestine is 1 to 2 mm and that of the colon is 3 mm when the bowel is distended. 74 Bowel wall thickening is the most consistent feature of Crohn's disease on cross-sectional imaging. 74 Mural hyperenhancement is the most sensitive finding for the detection of active disease. 37 The diagnostic accuracy of CTE and MRE for small bowel Crohn's disease is similar. In a prospective blinded study of patients receiving both CTE and MRE for direct comparison in the diagnosis of Crohn's disease, the sensitivity rates for CTE and MRE were 74 and 83%, and the specificity rates were 80 and 70%, respectively. 78 The sensitivity for stenosis was 70% for CTE and 55% for MRE. 78
Fig. 12.
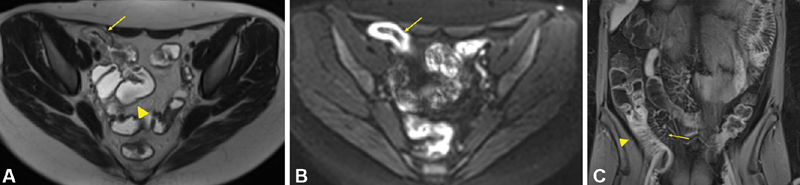
( A ) MRE T2 axial HASTE sequence image in a patient with Crohn's ileitis revealing ileal stricture (thin arrow) and upstream small bowel dilation (large arrowhead). ( B ) MRE trace diffusion-weighted sequence image in the same patient revealing high-signal intensity in the ileus (arrow) consistent with active Crohn's disease. ( C ) MRE T1 coronal VIBE Dixon method sequence image post contrast in the same patient with Crohn's ileitis revealing ileal mural hyperenhancement (thick arrow), thickening, and “comb sign” (thin arrow).
Special Populations
Patients with a History of Intravenous Contrast Reactions
The incidence of allergy-like reactions to modern iodinated and gadolinium-based contrast medium is 0.6% 79 and 0.01 to 0.22%, 80 respectively. The exact mechanism of hypersensitivity is not fully understood, but there is an association with direct histamine release rather than IgE-mediated allergy and antibody formation. 81 Severe reactions have been seen to occur with the first exposure without apparent prior exposure. 35 Premedication reduces the risk of reaction, even for average risk individuals, 82 and is believed to reduce the risk in high-risk individuals, though this has not been studied in a randomized fashion, since all patients with known sensitivity typically receive premedication. 35 Premedication does not prevent all reactions. An example of a standard premedication regimen for elective imaging is 50 mg of prednisone at 13 hours, 7 hours, and 1 hour prior to the scan in addition to 50 mg of diphenhydramine 1 hour prior. 35
Chronic Kidney Disease
Contrast-induced nephropathy (CIN) is kidney injury caused by iodinated IV contrast administration. The greatest risk factor is preexisting chronic kidney disease. 83 There is no specific agreed upon creatinine or glomerular filtration rate (GFR) cutoff for which iodinated contrast cannot or should not be given, and administration should always be based on risk–benefit analysis. 35 A survey by Elicker et al 84 of radiologists' choice for creatinine cutoff in patients without other risk factors varied from 1.5 to 2.0 mg/dL. GFR may be a better assessment of risk than creatinine, and the best apparent evidence is for a GFR cutoff of approximately 30 mL/min/1.73 m 2 . 85 In terms of prevention, the strongest evidence is for preprocedure IV crystalloid fluid administration, and there is insufficient evidence to recommend routine preimaging administration of sodium bicarbonate or N-acetylcysteine to prevent CIN. 35 Iodinated contrast can be administered to patients on dialysis with low risk of harm, and there is no need for emergent dialysis following imaging. 86 Gadolinium-based contrasts are contraindicated in patients with GFR less than 30 mL/min/1.73 m 2 , including patients on dialysis, and risk/benefit of gadolinium administration should be weighed for patients with GFR 50 to 30 mL/min/1.73 m 2 due to the chance of developing nephrogenic systemic sclerosis. 87
Pregnancy
Bowel obstruction during pregnancy is rare but is associated with a high incidence of fetal loss. 88 Both CT and MRI are potentially appropriate diagnostic imaging modalities in this population, with MRI having the advantage of no radiation exposure. 88 There is no evidence that MRI can be detrimental to an unborn fetus. 89 POCUS may also serve as a useful initial screening exam in pregnancy. Traditional teaching dictates that treating the mother is the safest way to protect the fetus, and diagnostic imaging should not be withheld. IV gadolinium contrast should not be administered in pregnancy. 32 The most common cause of SBO in pregnancy remains postoperative adhesions. 88 Nonoperative management is typically employed with success in the absence of worrisome findings after cross-sectional imaging, but there should be a low threshold for operative intervention when indicated. 88
Conclusion
Imaging plays a central role in the modern evaluation and management of suspected intestinal obstruction. CT is the imaging modality of choice for the majority of patients with suspected intestinal obstruction, as it is practical to obtain, accurate for diagnosis, and provides substantial information to help determine the cause of obstruction and identify complications. Plain radiographs may serve as an initial screening modality and can be useful for serial imaging examinations in the setting of nonoperative management. Contrast enema and SBFT are important adjuncts to CT for equivocal cases. Water-soluble oral contrast challenge for adhesive SBO is a tool that can predict those who will succeed nonoperative management and potentially reduce length of hospital stay. Ultrasound is useful in children and pregnant women, and POCUS is an alternative screening exam for adults. MRI is an alternative cross-sectional modality that can exceed the accuracy of CT, but practical considerations significantly limit its use. The routine and systematic review of all radiographic images by the surgeon may improve patient outcomes and improve the surgeon's skill at image interpretation in the evaluation of the patient with suspected intestinal obstruction.
Conflict of Interest None declared.
Disclosures
The authors have no relevant financial disclosures.
References
- 1.Yeo H L, Lee S W. Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg. 2013;17(11):2007–2012. doi: 10.1007/s11605-013-2343-x. [DOI] [PubMed] [Google Scholar]
- 2.Markogiannakis H, Messaris E, Dardamanis D. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13(03):432–437. doi: 10.3748/wjg.v13.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drożdż W, Budzyński P. Change in mechanical bowel obstruction demographic and etiological patterns during the past century: observations from one health care institution. Arch Surg. 2012;147(02):175–180. doi: 10.1001/archsurg.2011.970. [DOI] [PubMed] [Google Scholar]
- 4.Parikh J A, Ko C Y, Maggard M A, Zingmond D S. What is the rate of small bowel obstruction after colectomy? Am Surg. 2008;74(10):1001–1005. [PubMed] [Google Scholar]
- 5.Aberg H, Påhlman L, Karlbom U. Small-bowel obstruction after restorative proctocolectomy in patients with ulcerative colitis. Int J Colorectal Dis. 2007;22(06):637–642. doi: 10.1007/s00384-006-0215-5. [DOI] [PubMed] [Google Scholar]
- 6.3rd ed. New York, NY: Springer Science+Business Media; 2016. The ASCRS Textbook of Colon and Rectal Surgery. [Google Scholar]
- 7.Eskelinen M, Ikonen J, Lipponen P. Contributions of history-taking, physical examination, and computer assistance to diagnosis of acute small-bowel obstruction. A prospective study of 1333 patients with acute abdominal pain. Scand J Gastroenterol. 1994;29(08):715–721. doi: 10.3109/00365529409092499. [DOI] [PubMed] [Google Scholar]
- 8.OPTIMA Study Group . Laméris W, van Randen A, van Es H W. Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ. 2009;338:b2431. doi: 10.1136/bmj.b2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breum B M, Rud B, Kirkegaard T, Nordentoft T. Accuracy of abdominal auscultation for bowel obstruction. World J Gastroenterol. 2015;21(34):10018–10024. doi: 10.3748/wjg.v21.i34.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gore R M, Silvers R I, Thakrar K H. Bowel obstruction. Radiol Clin North Am. 2015;53(06):1225–1240. doi: 10.1016/j.rcl.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Paulson E K, Thompson W M. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology. 2015;275(02):332–342. doi: 10.1148/radiol.15131519. [DOI] [PubMed] [Google Scholar]
- 12.Suri S, Gupta S, Sudhakar P J, Venkataramu N K, Sood B, Wig J D. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999;40(04):422–428. doi: 10.3109/02841859909177758. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe T, Thompson W M. Large-bowel obstruction in the adult: classic radiographic and CT findings, etiology, and mimics. Radiology. 2015;275(03):651–663. doi: 10.1148/radiol.2015140916. [DOI] [PubMed] [Google Scholar]
- 14.Thompson W M, Kilani R K, Smith B B. Accuracy of abdominal radiography in acute small-bowel obstruction: does reviewer experience matter? AJR Am J Roentgenol. 2007;188(03):W233–W238. doi: 10.2214/AJR.06.0817. [DOI] [PubMed] [Google Scholar]
- 15.Mettler F A, Jr, Huda W, Yoshizumi T T, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(01):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Bindman R, Lipson J, Marcus R. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James B, Kelly B. The abdominal radiograph. Ulster Med J. 2013;82(03):179–187. [PMC free article] [PubMed] [Google Scholar]
- 18.Musson R E, Bickle I, Vijay R K.Gas patterns on plain abdominal radiographs: a pictorial review Postgrad Med J 201187(1026):274–287. [DOI] [PubMed] [Google Scholar]
- 19.Maglinte D D, Reyes B L, Harmon B H. Reliability and role of plain film radiography and CT in the diagnosis of small-bowel obstruction. AJR Am J Roentgenol. 1996;167(06):1451–1455. doi: 10.2214/ajr.167.6.8956576. [DOI] [PubMed] [Google Scholar]
- 20.Harlow C L, Stears R L, Zeligman B E, Archer P G. Diagnosis of bowel obstruction on plain abdominal radiographs: significance of air-fluid levels at different heights in the same loop of bowel. AJR Am J Roentgenol. 1993;161(02):291–295. doi: 10.2214/ajr.161.2.8333364. [DOI] [PubMed] [Google Scholar]
- 21.Taourel P, Kessler N, Lesnik A, Pujol J, Morcos L, Bruel J M. Helical CT of large bowel obstruction. Abdom Imaging. 2003;28(02):267–275. doi: 10.1007/s00261-002-0038-y. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan S, Ojili V, Vassa R, Nagar A. Large bowel obstruction in the emergency department: imaging spectrum of common and uncommon causes. J Clin Imaging Sci. 2017;7:15. doi: 10.4103/jcis.JCIS_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingold D, Murrell Z. Management of colonic volvulus. Clin Colon Rectal Surg. 2012;25(04):236–244. doi: 10.1055/s-0032-1329535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swenson B R, Kwaan M R, Burkart N E. Colonic volvulus: presentation and management in metropolitan Minnesota, United States. Dis Colon Rectum. 2012;55(04):444–449. doi: 10.1097/DCR.0b013e3182404b3d. [DOI] [PubMed] [Google Scholar]
- 25.Wang J H, Furlan A, Kaya D, Goshima S, Tublin M, Bae K T. Pneumatosis intestinalis versus pseudo-pneumatosis: review of CT findings and differentiation. Insights Imaging. 2011;2(01):85–92. doi: 10.1007/s13244-010-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres U S, Fortes C DFM, Salvadori P S, Tiferes D A, Ippolito G D. Pneumatosis from esophagus to rectum: a comprehensive review focusing on clinico-radiological differentiation between benign and life-threatening causes. Semin Ultrasound CT MR. 2018;39(02):167–182. doi: 10.1053/j.sult.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Liebman P R, Patten M T, Manny J, Benfield J R, Hechtman H B. Hepatic--portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg. 1978;187(03):281–287. doi: 10.1097/00000658-197803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frager D, Rovno H D, Baer J W, Bashist B, Friedman M. Prospective evaluation of colonic obstruction with computed tomography. Abdom Imaging. 1998;23(02):141–146. doi: 10.1007/s002619900307. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey E M, Addley H C, Shaw A S.The use of computed tomography in the detection and characterisation of large bowel obstruction N Z Med J 2009122(1305):57–73. [PubMed] [Google Scholar]
- 30.Pongpornsup S, Tarachat K, Srisajjakul S. Accuracy of 64 sliced multi-detector computed tomography in diagnosis of small bowel obstruction. J Med Assoc Thai. 2009;92(12):1651–1661. [PubMed] [Google Scholar]
- 31.Gans S L, Stoker J, Boermeester M A. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525–533. doi: 10.2147/IJGM.S17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ros P R, Huprich J E. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol. 2006;3(11):838–841. doi: 10.1016/j.jacr.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Hong S S, Kim A Y, Byun J H. MDCT of small-bowel disease: value of 3D imaging. AJR Am J Roentgenol. 2006;187(05):1212–1221. doi: 10.2214/AJR.04.1762. [DOI] [PubMed] [Google Scholar]
- 34.Hodel J, Zins M, Desmottes L. Location of the transition zone in CT of small-bowel obstruction: added value of multiplanar reformations. Abdom Imaging. 2009;34(01):35–41. doi: 10.1007/s00261-007-9348-4. [DOI] [PubMed] [Google Scholar]
- 35.ACR Manual on Contrast Media: 2020 VersionAvailable at:https://www.acr.org/~/media/acr/files/clinical-resources/contrast_media.pdf2020
- 36.Atri M, McGregor C, McInnes M. Multidetector helical CT in the evaluation of acute small bowel obstruction: comparison of non-enhanced (no oral, rectal or IV contrast) and IV enhanced CT. Eur J Radiol. 2009;71(01):135–140. doi: 10.1016/j.ejrad.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Gore R M, Levine M S. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015. Textbook of Gastrointestinal Radiology. [Google Scholar]
- 38.Silva A C, Pimenta M, Guimarães L S. Small bowel obstruction: what to look for. Radiographics. 2009;29(02):423–439. doi: 10.1148/rg.292085514. [DOI] [PubMed] [Google Scholar]
- 39.Ha H K, Kim J S, Lee M S. Differentiation of simple and strangulated small-bowel obstructions: usefulness of known CT criteria. Radiology. 1997;204(02):507–512. doi: 10.1148/radiology.204.2.9240545. [DOI] [PubMed] [Google Scholar]
- 40.Khurana B, Ledbetter S, McTavish J, Wiesner W, Ros P R. Bowel obstruction revealed by multidetector CT. AJR Am J Roentgenol. 2002;178(05):1139–1144. doi: 10.2214/ajr.178.5.1781139. [DOI] [PubMed] [Google Scholar]
- 41.Colon M J, Telem D A, Wong D, Divino C M. The relevance of transition zones on computed tomography in the management of small bowel obstruction. Surgery. 2010;147(03):373–377. doi: 10.1016/j.surg.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Mayo-Smith W W, Wittenberg J, Bennett G L, Gervais D A, Gazelle G S, Mueller P R. The CT small bowel faeces sign: description and clinical significance. Clin Radiol. 1995;50(11):765–767. doi: 10.1016/s0009-9260(05)83216-7. [DOI] [PubMed] [Google Scholar]
- 43.Catalano O. The faeces sign. A CT finding in small-bowel obstruction. Radiologe. 1997;37(05):417–419. doi: 10.1007/s001170050231. [DOI] [PubMed] [Google Scholar]
- 44.Khaled W, Millet I, Corno L. Clinical relevance of the feces sign in small-bowel obstruction due to adhesions depends on its location. AJR Am J Roentgenol. 2018;210(01):78–84. doi: 10.2214/AJR.17.18126. [DOI] [PubMed] [Google Scholar]
- 45.Lazarus D E, Slywotsky C, Bennett G L, Megibow A J, Macari M. Frequency and relevance of the “small-bowel feces” sign on CT in patients with small-bowel obstruction. AJR Am J Roentgenol. 2004;183(05):1361–1366. doi: 10.2214/ajr.183.5.1831361. [DOI] [PubMed] [Google Scholar]
- 46.Beattie G C, Peters R T, Guy S, Mendelson R M. Computed tomography in the assessment of suspected large bowel obstruction. ANZ J Surg. 2007;77(03):160–165. doi: 10.1111/j.1445-2197.2006.03998.x. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz S, Nadelhaft J. Simulation of colonic obstruction at the splenic flexure by pancreatitis: roentgen features. Am J Roentgenol Radium Ther Nucl Med. 1957;78(04):607–616. [PubMed] [Google Scholar]
- 48.Zaman S, Chapman W, Mohammed I, Gill K, Ward S T. Patients with computed tomography-proven acute diverticulitis require follow-up to exclude colorectal cancer. Intest Res. 2017;15(02):195–202. doi: 10.5217/ir.2017.15.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayakawa K, Tanikake M, Yoshida S. Radiological diagnosis of large-bowel obstruction: nonneoplastic etiology. Jpn J Radiol. 2012;30(07):541–552. doi: 10.1007/s11604-012-0092-5. [DOI] [PubMed] [Google Scholar]
- 50.Levsky J M, Den E I, DuBrow R A, Wolf E L, Rozenblit A M. CT findings of sigmoid volvulus. AJR Am J Roentgenol. 2010;194(01):136–143. doi: 10.2214/AJR.09.2580. [DOI] [PubMed] [Google Scholar]
- 51.Santillan C S. Computed tomography of small bowel obstruction. Radiol Clin North Am. 2013;51(01):17–27. doi: 10.1016/j.rcl.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Matsushima K, Inaba K, Dollbaum R. High-density free fluid on computed tomography: a predictor of surgical intervention in patients with adhesive small bowel obstruction. J Gastrointest Surg. 2016;20(11):1861–1866. doi: 10.1007/s11605-016-3244-6. [DOI] [PubMed] [Google Scholar]
- 53.Sheedy S P, Earnest F, IV, Fletcher J G, Fidler J L, Hoskin T L. CT of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology. 2006;241(03):729–736. doi: 10.1148/radiol.2413050965. [DOI] [PubMed] [Google Scholar]
- 54.Kinoshita H, Shinozaki M, Tanimura H. Clinical features and management of hepatic portal venous gas: four case reports and cumulative review of the literature. Arch Surg. 2001;136(12):1410–1414. doi: 10.1001/archsurg.136.12.1410. [DOI] [PubMed] [Google Scholar]
- 55.Jacob S E, Lee S H, Hill J. The demise of the instant/unprepared contrast enema in large bowel obstruction. Colorectal Dis. 2008;10(07):729–731. doi: 10.1111/j.1463-1318.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 56.Chapman A H, McNamara M, Porter G. The acute contrast enema in suspected large bowel obstruction: value and technique. Clin Radiol. 1992;46(04):273–278. doi: 10.1016/s0009-9260(05)80170-9. [DOI] [PubMed] [Google Scholar]
- 57.Lips L M, Cremers P T, Pickhardt P J. Sigmoid cancer versus chronic diverticular disease: differentiating features at CT colonography. Radiology. 2015;275(01):127–135. doi: 10.1148/radiol.14132829. [DOI] [PubMed] [Google Scholar]
- 58.de Gregorio M A, Mainar A, Rodriguez J. Colon stenting: a review. Semin Intervent Radiol. 2004;21(03):205–216. doi: 10.1055/s-2004-860941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.New York, NY: Springer Berlin Heidelberg; 2018. Emergency General Surgery: A Practical Approach. [Google Scholar]
- 60.Kurer M A, Davey C, Chintapatla S. Intestinal obstruction from inspissated barium (Barolith): a systematic review of all cases from 1950 to 2006. Colorectal Dis. 2008;10(05):431–439. doi: 10.1111/j.1463-1318.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- 61.Bardan E. New York, NY: Springer Science+Business Media; 2017. Gastrointestinal Motility Disorders: A Point-of-Care Clinical Guide. [Google Scholar]
- 62.Koh A, Adiamah A, Chowdhury A, Mohiuddin M K, Bharathan B. Therapeutic role of water-soluble contrast media in adhesive small bowel obstruction: a systematic review and meta-analysis. J Gastrointest Surg. 2020;24(02):473–483. doi: 10.1007/s11605-019-04341-7. [DOI] [PubMed] [Google Scholar]
- 63.Abbas S M, Bissett I P, Parry B R. Meta-analysis of oral water-soluble contrast agent in the management of adhesive small bowel obstruction. Br J Surg. 2007;94(04):404–411. doi: 10.1002/bjs.5775. [DOI] [PubMed] [Google Scholar]
- 64.Burge J, Abbas S M, Roadley G. Randomized controlled trial of Gastrografin in adhesive small bowel obstruction. ANZ J Surg. 2005;75(08):672–674. doi: 10.1111/j.1445-2197.2005.03491.x. [DOI] [PubMed] [Google Scholar]
- 65.Assalia A, Schein M, Kopelman D, Hirshberg A, Hashmonai M. Therapeutic effect of oral Gastrografin in adhesive, partial small-bowel obstruction: a prospective randomized trial. Surgery. 1994;115(04):433–437. [PubMed] [Google Scholar]
- 66.Gottlieb M, Peksa G D, Pandurangadu A V, Nakitende D, Takhar S, Seethala R R. Utilization of ultrasound for the evaluation of small bowel obstruction: a systematic review and meta-analysis. Am J Emerg Med. 2018;36(02):234–242. doi: 10.1016/j.ajem.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 67.Graham A, Carlberg D J. Cham, Switzerland: Springer; 2019. Gastrointestinal Emergencies: Evidence-Based Answers to Key Clinical Questions. [Google Scholar]
- 68.Ogata M, Imai S, Hosotani R, Aoyama H, Hayashi M, Ishikawa T. Abdominal sonography for the diagnosis of large bowel obstruction. Surg Today. 1994;24(09):791–794. doi: 10.1007/BF01636308. [DOI] [PubMed] [Google Scholar]
- 69.Nicolaou S, Kai B, Ho S, Su J, Ahamed K. Imaging of acute small-bowel obstruction. AJR Am J Roentgenol. 2005;185(04):1036–1044. doi: 10.2214/AJR.04.0815. [DOI] [PubMed] [Google Scholar]
- 70.Goudie A. Detection of intraperitoneal free gas by ultrasound. Australas J Ultrasound Med. 2013;16(02):56–61. doi: 10.1002/j.2205-0140.2013.tb00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fidler J. MR imaging of the small bowel. Radiol Clin North Am. 2007;45(02):317–331. doi: 10.1016/j.rcl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Beall D P, Fortman B J, Lawler B C, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57(08):719–724. doi: 10.1053/crad.2001.0735. [DOI] [PubMed] [Google Scholar]
- 73.Eastern Association for the Surgery of Trauma Maung A A, Johnson D C, Piper G L.Evaluation and management of small-bowel obstruction: an Eastern Association for the Surgery of Trauma practice management guideline J Trauma Acute Care Surg 201273(05, Suppl 4):S362–S369. [DOI] [PubMed] [Google Scholar]
- 74.Furukawa A, Saotome T, Yamasaki M. Cross-sectional imaging in Crohn disease. Radiographics. 2004;24(03):689–702. doi: 10.1148/rg.243035120. [DOI] [PubMed] [Google Scholar]
- 75.Regan F, Beall D P, Bohlman M E, Khazan R, Sufi A, Schaefer D C. Fast MR imaging and the detection of small-bowel obstruction. AJR Am J Roentgenol. 1998;170(06):1465–1469. doi: 10.2214/ajr.170.6.9609154. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez P, Mendez R, Matute F, Hernandez P, Mendoza J L. Imaging Crohn disease: MR enterography. J Comput Assist Tomogr. 2014;38(02):219–227. doi: 10.1097/RCT.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 77.Boudiaf M, Jaff A, Soyer P, Bouhnik Y, Hamzi L, Rymer R. Small-bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233(02):338–344. doi: 10.1148/radiol.2332030308. [DOI] [PubMed] [Google Scholar]
- 78.Jensen M D, Kjeldsen J, Rafaelsen S R, Nathan T. Diagnostic accuracies of MR enterography and CT enterography in symptomatic Crohn's disease. Scand J Gastroenterol. 2011;46(12):1449–1457. doi: 10.3109/00365521.2011.613947. [DOI] [PubMed] [Google Scholar]
- 79.Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(03):621–628. doi: 10.1148/radiology.175.3.2343107. [DOI] [PubMed] [Google Scholar]
- 80.Jung J W, Kang H R, Kim M H. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264(02):414–422. doi: 10.1148/radiol.12112025. [DOI] [PubMed] [Google Scholar]
- 81.Trcka J, Schmidt C, Seitz C S, Bröcker E B, Gross G E, Trautmann A. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? AJR Am J Roentgenol. 2008;190(03):666–670. doi: 10.2214/AJR.07.2872. [DOI] [PubMed] [Google Scholar]
- 82.Lasser E C, Berry C C, Talner L B. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med. 1987;317(14):845–849. doi: 10.1056/NEJM198710013171401. [DOI] [PubMed] [Google Scholar]
- 83.Toprak O, Cirit M. Risk factors for contrast-induced nephropathy. Kidney Blood Press Res. 2006;29(02):84–93. doi: 10.1159/000093381. [DOI] [PubMed] [Google Scholar]
- 84.Elicker B M, Cypel Y S, Weinreb J C. IV contrast administration for CT: a survey of practices for the screening and prevention of contrast nephropathy. AJR Am J Roentgenol. 2006;186(06):1651–1658. doi: 10.2214/AJR.05.0407. [DOI] [PubMed] [Google Scholar]
- 85.Davenport M S, Khalatbari S, Cohan R H, Ellis J H. Contrast medium-induced nephrotoxicity risk assessment in adult inpatients: a comparison of serum creatinine level- and estimated glomerular filtration rate-based screening methods. Radiology. 2013;269(01):92–100. doi: 10.1148/radiol.13122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Younathan C M, Kaude J V, Cook M D, Shaw G S, Peterson J C. Dialysis is not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR Am J Roentgenol. 1994;163(04):969–971. doi: 10.2214/ajr.163.4.8092045. [DOI] [PubMed] [Google Scholar]
- 87.Grobner T, Prischl F C. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72(03):260–264. doi: 10.1038/sj.ki.5002338. [DOI] [PubMed] [Google Scholar]
- 88.Webster P J, Bailey M A, Wilson J, Burke D A. Small bowel obstruction in pregnancy is a complex surgical problem with a high risk of fetal loss. Ann R Coll Surg Engl. 2015;97(05):339–344. doi: 10.1308/003588415X14181254789844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kok R D, de Vries M M, Heerschap A, van den Berg P P. Absence of harmful effects of magnetic resonance exposure at 1.5 T in utero during the third trimester of pregnancy: a follow-up study. Magn Reson Imaging. 2004;22(06):851–854. doi: 10.1016/j.mri.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 90.Sabiston D C, Townsend C M. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. [Google Scholar]


