Abstract
Small bowel obstruction (SBO) remains a common problem for surgeons and nonsurgeons alike. Management of SBO has shifted from primarily being surgical to a nonoperative approach, which can be attributed to a multitude of reasons, including better understanding of the pathophysiology of SBO, the advent of laparoscopy, and improvement in diagnostic imaging. But given the nature of SBO, the need for surgical consultation continues to remain a necessity. This article will review the etiology, diagnosis, and management of SBO.
Keywords: small bowel obstruction, nasogastric tube, adhesive disease
Despite recent advancements in surgical technique and more frequent use of minimally invasive (laparoscopic, robotic, hand-assist, etc.) approaches, small bowel obstruction (SBO) remains a common problem for surgeons and nonsurgeons alike. Over the last decade, there has been an increased interest in understanding the indications for and timing of surgical intervention for SBO. As such, the classic surgical dogma of “never let the sun set on a small bowel obstruction” has been challenged with a renewed focus on effective nonoperative management.
The most common causes of SBO are herniation, malignancy, and postoperative or inflammatory adhesive disease. Internationally, hernias are the most common cause of SBO, while in developed countries, including the United States, adhesions from previous abdominal and pelvic procedures cause 65 to 75% of all SBO. 1 2 3 Hernias and neoplasms (benign and malignant) are the next most common etiologies at 10 and 5%, respectively. 2 Although less common, intussusception, Crohn's disease, gallstone ileus, intestinal volvulus, internal hernia, foreign bodies, and bezoars must be considered in the differential as well. Overall mortality may be as high as 30% when complications such as bowel ischemia occur. 2
In a post-mortem study of 752 patients in a single European hospital over a 9 month period, Weibel et al found that approximately 65% of patients with history of abdominopelvic surgery were found to have presence of adhesive disease while roughly 25% of patients without a surgical history had evidence of adhesions. 4 Among those with adhesive disease, the frequency of adhesive disease was seen to be highest (93%) in those with multiple operations and major laparotomies (72%), when compared with minor procedures, such as appendectomy (51%). However, not all patients with the presence of adhesions were symptomatic.
In a review of a Scottish national database of more than 12,500 patients who underwent lower abdominal surgery in 1 year, 5.1% of patients were admitted with a mean of 2.2 times over the course of a 10 year period, with two-thirds of patients requiring surgical intervention for adhesive-related symptoms with the highest incidence in patients following colon or rectal surgery. 5 In another review of nearly 62 studies and 450,000 patients, the range of adhesion-related admissions varies from <1.0 to 24% stratified by procedure and approach. 6 Laparoscopy was seen to decrease the rate of admissions due to adhesions and in another systematic review was found to decrease the risk of adhesion formation by 45%, 1 while colorectal resections were, again, seen to have an increased rate. 6 Furthermore, operative intervention in adhesive SBO ranges from 20 to 30%. 3 Interestingly, less than 10% of surgeons routinely discuss with patients the risk of postoperative adhesions and their consequences during informed consent. 7
Ultimately, patients who present with a SBO should undergo surgical evaluation. If left untreated, an SBO can progress to luminal perforation, bowel ischemia, profound physiological derangement, sepsis, and death. The following article is an overview of the evaluation and management of SBO in the 21st century.
Evaluation
Initial evaluation of suspected SBO should include a focused history and physical examination, paying particular focus to any previous abdominopelvic surgeries and the presence of hernias. Patients with a history of malignancy need to be queried on their current known status of disease and any relevant treatment to assess for recurrence and/or metastatic disease. Concomitantly, one must quickly determine the clinical status of the patient to determine if they are in extremis . A focus on the degree of volume depletion, metabolic derangements, and vital signs are critical to determine if prompt surgical intervention is necessary.
Symptoms of SBO include nausea, vomiting, bloating, crampy, colicky abdominal pain, with minimal or complete absence of flatus and bowel movements. It is important to characterize the quality of any emesis (gastric, bilious, feculent, etc.), duration of symptoms, intensity of pain, and timing of any definitive bowel function. It is also wise to not be fooled by intermittent diarrhea or stool, as this can be seen with partial obstructions.
Physical examination should focus on pathophysiologic evidence of dehydration and/or shock, including tachycardia, hypotension, wide pulse pressures, characteristics, and frequency of urine (clear, yellow, amber, etc.), with a focus on the abdominal and inguinal exams. The abdominal exam should assess for distension, tympany, pain, and evidence of focal or generalized peritonitis. Surgical scars should be noted along with any hernias (umbilical, incisional, inguinal, etc.). Laboratory values may show leukocytosis, hemoconcentration, metabolic derangements (acidosis versus alkalosis), renal dysfunction, and/or lactic acidosis.
Radiographic evaluation may begin with an abdominal X-ray series which includes an upright chest, abdominal supine, and lateral decubitus radiographs. The chest X-ray can demonstrate aspiration or pneumoperitoneum, while the abdominal radiographs may show gastric distension, air-fluid levels, dilated loops of small bowel, and a paucity of colonic/rectal gas ( Fig. 1 ). Placement of a nasogastric (NG) tube for gastric decompression should be prompt in an actively vomiting patient or if there is evidence of gastric dilatation, especially in an elderly or altered patient who may not be able to effectively protect their airway.
Fig. 1.

Upright plain abdominal radiograph demonstrating air-fluid levels in the stomach and intestines ( arrows ) and dilated loops of small bowel.
A contrasted computed tomography (CT) scan of the abdomen and pelvis is generally obtained ( Figs. 2 and 3 ). The addition of water-soluble oral and intravenous contrast can aid in better defining the relevant gastrointestinal anatomy to determine the location, degree, and cause of the obstruction ( Fig. 4 ). The CT scan has largely replaced the small bowel series as the initial diagnostic imaging modality of choice as it can better define luminal wall thickening, bowel perfusion, free peritoneal fluid, pneumatosis intestinalis, pneumoperitoneum, masses, or any other relevant abdominal pathologies. Administration of oral contrast via NG tube is an option for patients who are unable to tolerate oral contrast or who have a tube already in place. The use of oral contrast should be calculated, however. If there are plans to perform a therapeutic small bowel follow through series (to be discussed later) after bowel rest and decompression, oral contrast from a CT scan on admission may affect interpretation or efficacy of future studies. When used in the postoperative setting, a CT scan can also identify abscesses or confirm the absence of a mechanical/anatomical irregularity suggesting a more global, nonobstructive, paralytic ileus. Trauma, electrolyte abnormalities, extra-abdominal sepsis, and medication side effects may result in nonobstructive conditions that may mimic SBO and should be considered as well.
Fig. 2.

Axial image of a CT abdomen, with proximally dilated bowel ( circled ) and distally collapsed bowel ( arrow ).
Fig. 3.

Coronal image of a CT abdomen, with a distended stomach ( star ) proximally dilated bowel ( circled ) and distally collapsed bowel ( arrow ).
Fig. 4.

CT abdomen/pelvis with oral contrast that abruptly stops, with proximal bowel dilation and gastric distenstion, demonstrating a high-grade obstruction in the left upper quadrant in the proximal jejunum ( arrow ).
Management
Nonoperative/Conservative Management
Appropriate patient selection for a nonoperative approach to a SBO is critical. In the absence of physiologic decline, low-grade, partial SBOs are more likely to resolve with nonoperative, conservative management. This strategy employs the use of bowel rest, fluid resuscitation, electrolyte replacement with or without NG tube decompression. Clinical SBO resolution, although seemingly binary in outcome (return of bowel function), also requires improvement in abdominal exams, correction of metabolic and electrolyte derangements, and progression to a baseline diet that meets nutritional needs. Patients with prior episodes of SBO with spontaneous resolution, or those with a history of multiple abdominal surgeries, should be given a trial of nonoperative management whenever possible in an effort to avoid surgery on a potentially hostile abdomen.
Many patients with SBO present with significant electrolyte disturbances as a result of decreased oral intake and/or intractable vomiting. Classically, as a result of prolonged emesis and the kidney's response to fluid losses, a patient will be found to have a hypochloremic, hypokalemic metabolic alkalosis with paradoxical aciduria. As such, obtaining proper intravenous access with prompt fluid resuscitation and correction of electrolytes to normal values should be a part of the initial therapeutic strategy. A Foley catheter should be placed to monitor urine output in response to resuscitation.
Bowel rest is a mainstay of nonoperative management. Effective gastric decompression via NGT and keeping a patient nil per os decreases gastric distension, thus providing symptomatic relief for the patient while decreasing the likelihood of aspiration and its sequelae ( Fig. 5 ). If the NG tube is not properly maintained or functioning, air and fluid can re-accumulate within the stomach and reflux through the esophageal sphincters which are now stented open by the tube, increasing the risk of aspiration ( Fig. 6 ). The head of the bed should be maintained at greater than 30 degrees.
Fig. 5.
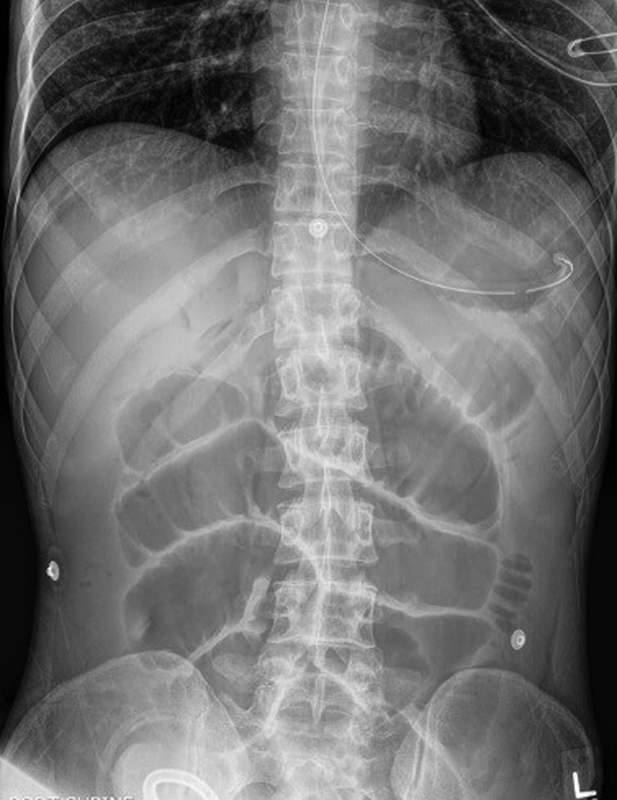
Plain abdominal radiograph demonstrating gastric decompression via NGT and dilated loops of small bowel from a partial bowel obstruction at the site of a loop ileostomy. NGT, nasogastric tube.
Fig. 6.

Plain abdominal radiograph demonstrating an NGT that is correctly positioned in the stomach but not functioning properly, resulting in gastric distension. NGT, nasogastric tube.
The small bowel series (small bowel follow through) after a period of NG decompression has become an increasingly utilized strategy in the SBO management toolbox. In 2017, Zielinski et al performed a prospective, multi-institutional observational study to assess the utility of using a gastrografin (diatrizoate meglumine and diatrizoate sodium; Bracco Diagnostics, Inc., Monroe Township, NJ) small bowel follow through gastrointestinal series to a resolve SBO resulting from adhesive disease and avoid surgical intervention. 8 Gastrografin, a hyperosmolar contrast dye, had been previously shown to hasten resolution of SBO in a prospective randomized control trial when compared with traditional conservative management alone by means of decreasing bowel wall edema. 9 Forty-four percent (44.1%) of patients who were in the control arm ultimately required surgical exploration while only 20.8% required surgery in the gastrografin cohort and, as a result, had a shorter hospital stay. Obtained images also can be used to determine patient anatomy and the location of the obstruction, which may be helpful if operative intervention is needed ( Fig. 7 ).
Fig. 7.

Gastrografin small bowel follow through demonstrating dilated loops of small bowel ( arrow ) and delayed transit to the colon, consistent with a partial bowel obstruction originating in the right lower quadrant.
Failure of improvement in symptoms after a trial of conservative management should prompt a review of the patient's overall clinical status to determine if operative intervention is necessary. The presence of contrast in the colon accompanied by a decrease in NG tube output, improvement in abdominal distension, and increase in bowel function are all signs of SBO resolution.
Early postoperative obstruction should be carefully assessed as the window of opportunity to effectively return to the operating room is limited and etiologies differ from delayed or chronic postoperative SBO. Intramural hematomas or mesenteric hematomas are not infrequent causes of postoperative obstructive symptoms with the jejunum and ileum as the most common sites in the small bowel 10 and can also be seen following abdominal trauma, particularly blunt trauma. 11 12 13 Patients on anticoagulation or antiplatelet therapy, or with conditions such as hemophilia or thrombocytopenia, have also been described to have spontaneous hematomas. 10 14 15 16 Even connective tissue disorders, such as Ehlers–Danlos syndrome, may increase the risk of spontaneous intramural hematomas. 15 Symptoms may range from abdominal pain to obstructive symptoms with PO (per oral) intolerance and nausea/vomiting. Rare cases may exhibit signs of peritonitis from bowel wall necrosis leading to perforation and peritonitis. The majority of these cases may be managed nonoperatively with the need for parenteral nutrition depending on the duration of obstruction.
Crohn's disease may present with symptoms of SBO, either from acute inflammation or chronic/recurrent inflammation that has resulted in strictured small bowel segments ( Fig. 8 ). Up to a quarter of patients with Crohn's disease will develop at least one small bowel stricture which may be inflammatory, fibrotic, or mixed depending on chronicity and current or past medical treatment strategies. 17 18 The terminal ileum is the most frequent site of stricturing disease. Many of these patients should be trialed with conservative management and initiation of corticosteroids or biologics prior to surgical intervention whenever feasible.
Fig. 8.

CT abdomen/pelvis with a stricture secondary to Crohn's disease.
Operative Management
Patients with complete obstructions (high-grade SBO) or who fail to resolve their obstruction with conservative management require surgical intervention. Patients who develop a fever, peritonitis, leukocytosis, lactic acidosis, or hemodynamic instability despite appropriate resuscitation also warrant surgical exploration. Elderly patients, those with an altered mental status, or immunocompromised patients, such as those with diabetes or on chemotherapy, may not have a reliable physical exam. In these patients, hemodynamic assessment with serial laboratory evaluation will play a major role in clinical monitoring.
As discussed, patients with a history of prior abdominopelvic surgeries likely develop SBO as a result of adhesive disease. Though the use of laparoscopy is not absolutely contraindicated, dilated loops of bowel may minimize adequate working space and visualization despite pneumoperitoneum, as well as increase the possibility of iatrogenic bowel injury. As such, a midline laparotomy may be necessary to safely obtain access to the abdominal cavity. Careful adhesiolysis should be completed so that the entire small bowel is freely mobile.
Strategies to prevent adhesion formation have been extensively studied. Several products are commercially available that are proposed to help reduce adhesions. One of the most studied, Seprafilm (Baxter, Deerfield, IL), is a synthetic bioresorbable membrane made of sodium hyaluronate-based carboxymethylcellulose (HA/CMC) that comes in thin, transparent sheets. The sheets must lie in contact with the targeted tissue and are left in the abdomen to prevent adhesions. Several systematic reviews have concluded that HA/CMC reduces the extent and severity of adhesions; however, there was no reduction in the incidence of obstruction or need for surgical intervention. 19 20 A retrospective study by Tsuruta et al determined that a multilayered application of barriers at different depths provided the best antiadhesion prophylaxis, with the lowest rate of postoperative ileus seen in that group compared with controls. 21
In patients without a history of abdominal surgery who have obstructive symptoms, differential diagnoses should include incarcerated hernias or tumors. Unreducible incarcerated hernias, especially those with evidence of bowel strangulation by laboratory values or physical exam (i.e., skin changes), warrant prompt surgical intervention. For strangulated inguinal hernias, many surgeons recommend an initial approach by a lower midline laparotomy incision as there is a high likelihood of requiring a bowel resection and adequate exposure would be necessary. Repair of the inguinal hernia should then be completed to address the etiology of the SBO.
Obstructions caused by tumors can present a more complex problem. If possible, in the preoperative setting, review of available imaging and relevant history may help to better understand the burden of disease. Being able to differentiate between one or several lesions that are amenable to surgical resection versus the presence of carcinomatosis will assist in surgical planning. Malignant bowel obstructions can be managed in several ways, depending on patient comorbidities, anatomy, prognosis, and goals of care with the aim of addressing the cause of the obstruction balanced with the risks and benefits of invasive intervention. Surgical management may include resection, diversion, or bypass. A nonoperative palliative approach is an option especially if the extent of the disease makes surgical intervention a heroic undertaking. Palliative gastric decompression with an NG tube or percutaneous gastrostomy for comfort is also an option. In an optimal setting, oncologic bowel resection to negative margins would be the ideal situation without rendering the patient with clinically significant short bowel physiology. Surgical cytoreduction with tumor debulking and hyperthermic intraperitoneal chemotherapy has been shown to have survival benefit in select patients. 22 In the setting of unresectability, a diverting ostomy may be a viable option if there remains sufficient bowel proximally to prevent malabsorption. However, too proximal of an enterostomy will lead to high output with subsequent electrolyte abnormalities and dehydration. Unresectable tumors that have unobstructed healthy bowel proximally and distally may maintain bowel continuity through the creation of an enteroenterostomy to bypass the site of obstruction. Several studies have shown that there are fewer complications and better overall survival with resection compared with bypass, though this could merely be a reflection of the likely increase in disease burden in the bypass cohort. 23 24
While adhesive disease can lead to internal hernias, they are more commonly discussed in the context of bariatric surgery. In the United States, 45% of bariatric surgeries consist of laparoscopic Roux-en-Y gastric bypasses (LRYGB). 25 With an incidence of 0.5 to 11%, internal hernias through mesenteric defects are a complication of LRYGB that typically require operative intervention. 26 Closure of mesenteric defects at the index operation appears to decrease the incidence of internal hernias. 26 27 28
A rare, but commonly tested cause of bowel obstruction is gallstone ileus which occurs when a fistulous connection forms between the gallbladder and small intestine, typically the duodenum, and is more commonly seen in elderly women. 29 30 31 Radiographic interrogation will typically reveal pneumobilia and gallstones within the lumen of the bowel, classically found in the right lower quadrant and causing obstruction at the level of the ileocecal valve leading to obstructive symptoms. Surgical management is necessary to relieve the obstruction and includes creating an enterotomy proximal to the gallstone and guiding the stone retrograde until it is retrieved. If there is evidence of bowel compromise, a bowel resection may be necessary. Care should be taken to inspect the entire length of small bowel for additional stones.
In gallstone ileus, debate usually ensues regarding the management of the biliary-enteric fistula at the time of surgery. Several options exist: (1) stone extraction alone, (2) a two-stage approach consisting of stone extraction alone followed by delayed cholecystectomy and fistula repair weeks to months later, and (3) a single-stage approach consisting of stone extraction, cholecystectomy, and fistula repair at the index surgery. 29 31 32 33 34 Stone extraction alone has been associated with decreased morbidity and mortality when compared with stone extraction plus fistula repair at the index operation. 29 31 While ideally a cholecystectomy and repair of the fistula would occur at the index operation, the patient's clinical status must be taken into consideration. Patients who are not stable enough to undergo more extensive surgery should be limited to enterolithotomy.
While more known for representing a cause of bowel obstruction in the pediatric patient, intussusception that occurs in adults should immediately raise alarm for a tumor acting as a lead point. While idiopathic intussusception typically presents between 6 months and 3 years of age, there is nearly a 25% chance of a pathologic entity acting as a lead point. 35 With the use of barium or air enemas, successful nonoperative radiographic reduction of intussusception in the pediatric population is high. In adults, however, intussusception is secondary to a pathologic cause in up 90% of cases, with 22 to 40% due to malignant causes, usually metastatic carcinoma. 36 37 38 39 As such, intussusception in adults should prompt surgical exploration ( Fig. 9 ).
Fig. 9.
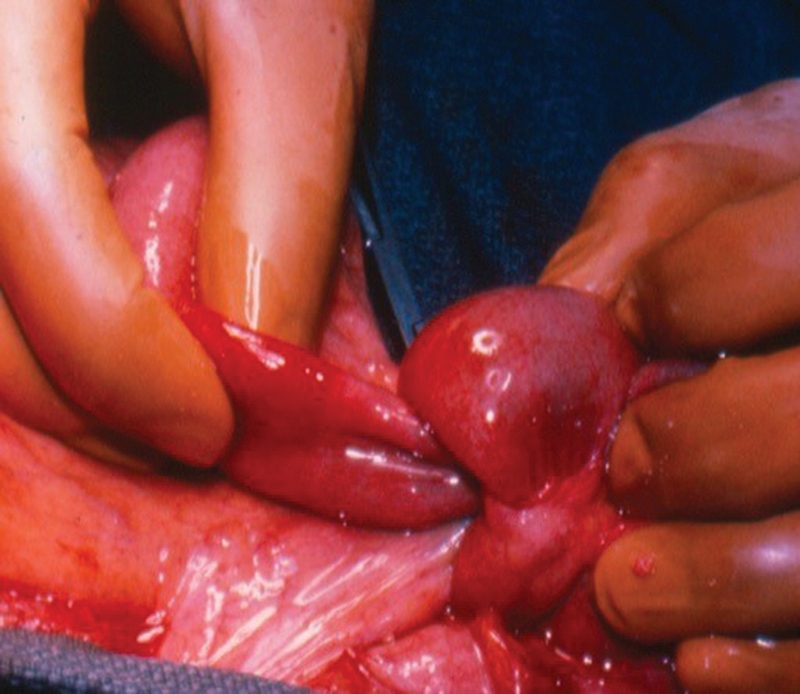
Intussusception secondary to a tumor.
When operating on an obstructed patient, exposure and visualization can be limited by the dilated distended loops of bowel. Luminal decompression is an intraoperative option that will allow for easier manipulation of the bowel and better exposure for adhesiolysis, improve perfusion to the distended bowel wall to decrease the chance of necrosis, and aid in abdominal wall closure. Several techniques for luminal decompression can be utilized. After confirming a well-placed and well-functioning NG tube, luminal gas and succus can be carefully and methodically squeezed in a retrograde fashion toward the duodenum to aid in removal and decompression. If manual shifting of fluid is difficult, a small enterotomy may be made to pass a catheter or suction tip into the bowel lumen for decompression, making sure this is done with complete control of the enterotomy so as to minimize spillage of enteric contents. If a bowel resection is undertaken, decompression can be done after resection via the proximal limb prior to anastomosis.
If a bowel resection is necessary, viability assessment of the remaining bowel may be difficult at times, particularly if the bowel has been chronically distended. In addition to the gross assessment of bowel viability, adjuncts such as indocyanine green angiography (ICG-A) may help to determine appropriate resection margins. The use of ICG-A to assess tissue perfusion has been described in bowel surgery to evaluate resection margins prior to anastomosis, thus decreasing anastomotic leak rates. 40 41 42
A series of 108 patients by Boni et al showed the safety of the intraoperative use of ICG in different laparoscopic procedures without any adverse effects. 43 The typical dose in laparoscopic surgery is 0.4 mg/mL/kg, which is well below toxic levels. 44 If ICG-A is unavailable, standard Doppler flow assessment is an alternative. In the case of borderline bowel perfusion, maneuvers should include making sure the patient is normotensive and normothermic, such as bathing the abdominal cavity with warm irrigation, prior to resection. If there is still question of viability despite using various methods, a decision should be made to either resect a short segment of bowel or return to the operating room for a second look within 24 hours. This may be necessary if the patient's clinical status does not improve, especially if there was a longer segment of bowel in question.
Conclusion
Small bowel obstructions remain a common entity in the 21st century, particularly with the volume of abdominal procedures that are being performed. A surgical consultation should always be obtained to prevent delay in care in the patient who is not a candidate for or who has failed conservative management.
Footnotes
Conflict of Interest The authors have no conflict of interest to declare.
References
- 1.Ouaïssi M, Gaujoux S, Veyrie N. Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012;149(02):e104–e114. doi: 10.1016/j.jviscsurg.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Rami Reddy S R, Cappell M S. A systematic review of the clinical presentation, diagnosis, and treatment of small bowel obstruction. Curr Gastroenterol Rep. 2017;19(06):28. doi: 10.1007/s11894-017-0566-9. [DOI] [PubMed] [Google Scholar]
- 3.Ten Broek R PG, Krielen P, Di Saverio S. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2017 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg. 2018;13:24. doi: 10.1186/s13017-018-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weibel M A, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg. 1973;126(03):345–353. doi: 10.1016/s0002-9610(73)80123-0. [DOI] [PubMed] [Google Scholar]
- 5.Parker M C, Ellis H, Moran B J.Postoperative adhesions: ten-year follow-up of 12,584 patients undergoing lower abdominal surgery Dis Colon Rectum 20014406822–829., discussion 829–830 [DOI] [PubMed] [Google Scholar]
- 6.Barmparas G, Branco B C, Schnüriger B, Lam L, Inaba K, Demetriades D. The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg. 2010;14(10):1619–1628. doi: 10.1007/s11605-010-1189-8. [DOI] [PubMed] [Google Scholar]
- 7.Schreinemacher M H, ten Broek R P, Bakkum E A, van Goor H, Bouvy N D. Adhesion awareness: a national survey of surgeons. World J Surg. 2010;34(12):2805–2812. doi: 10.1007/s00268-010-0778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EAST SBO Workgroup: Martin D. Zielinski, MD ; Nadeem N. Haddad, MD ; Asad J. Choudhry, MBBS ; Daniel C. Cullinane, MD ; Kenji Inaba, MD ; Agustin Escalante ; D. Dante Yeh, MD ; Salina Wydo, MD ; David Turay, MD ; Andrea Pakula, MD ; Therese M. Duane, MD ; Jill Watras, MD ; Kenneth A. Widom, MD ; John Cull, MD ; Carlos J. Rodriguez, DO ; Eric A. Toschlog, MD ; Valerie G. Sams, MD ; Joshua P. Hazelton, DO ; John Christopher Graybill, MD, Ruby Skinner, MD, Ji-Ming Yune, MD . Zielinski M D, Haddad N N, Cullinane D C. Multi-institutional, prospective, observational study comparing the Gastrografin challenge versus standard treatment in adhesive small bowel obstruction. J Trauma Acute Care Surg. 2017;83(01):47–54. doi: 10.1097/TA.0000000000001499. [DOI] [PubMed] [Google Scholar]
- 9.Assalia A, Schein M, Kopelman D, Hirshberg A, Hashmonai M. Therapeutic effect of oral Gastrografin in adhesive, partial small-bowel obstruction: a prospective randomized trial. Surgery. 1994;115(04):433–437. [PubMed] [Google Scholar]
- 10.Khan K, Saeed S, Alothman S, Iqbal F, Ramcharan A, Donaldson B. Warfarin induced mesenteric and intestinal hematoma requiring surgical resection to relieve small bowel obstruction: a case report. Int J Surg Case Rep. 2018;52:111–113. doi: 10.1016/j.ijscr.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauterive A H, Flancbaum L, Cox E F. Blunt intestinal trauma. A modern-day review. Ann Surg. 1985;201(02):198–203. doi: 10.1097/00000658-198502000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamidian Jahromi A, Johnson L, Youssef A M. Delayed small bowel perforation following blunt abdominal trauma: a case report and review of the literature. Asian J Surg. 2016;39(02):109–112. doi: 10.1016/j.asjsur.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 13.North M, Aveyard N, Diya O, Berger J, Al-Whouhayb M. A case of small bowel obstruction and enterocutaneous fistulation resulting from a mesenteric haematoma following blunt abdominal trauma. Case Rep Surg. 2017;2017:7.639265E6. doi: 10.1155/2017/7639265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang E A, Han S J, Chun J. Clinical features and outcomes in spontaneous intramural small bowel hematoma: cohort study and literature review. Intest Res. 2019;17(01):135–143. doi: 10.5217/ir.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano K, Bando T, Osawa S, Shimizu T, Okumura T, Fujii T. Spontaneous mesenteric hematoma of the sigmoid colon associated with rivaroxaban: a case report. Int J Surg Case Rep. 2018;44:33–37. doi: 10.1016/j.ijscr.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delibegovic M, Alispahic A, Gazija J, Mehmedovic Z, Mehmedovic M. Intramural haemorrhage and haematoma as the cause of ileus of the small intestine in a haemophiliac. Med Arh. 2015;69(03):206–207. doi: 10.5455/medarh.2015.69.206-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg H I, Caruthers S B, Jr, Nelson J A, Singleton J W.Radiographic findings of the National Cooperative Crohn's Disease Study Gastroenterology 197977(4 Pt 2):925–937. [PubMed] [Google Scholar]
- 18.Rieder F, Zimmermann E M, Remzi F H, Sandborn W J. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62(07):1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Wong P F, Leaper D J. Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after non-gynaecological abdominal surgery. Cochrane Database Syst Rev. 2009;(01):CD005080. doi: 10.1002/14651858.CD005080.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Robb W B, Mariette C. Strategies in the prevention of the formation of postoperative adhesions in digestive surgery: a systematic review of the literature. Dis Colon Rectum. 2014;57(10):1228–1240. doi: 10.1097/DCR.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruta A, Itoh T, Hirai T, Nakamura M. Multi-layered intra-abdominal adhesion prophylaxis following laparoscopic colorectal surgery. Surg Endosc. 2015;29(06):1400–1405. doi: 10.1007/s00464-014-3813-2. [DOI] [PubMed] [Google Scholar]
- 22.Bakrin N, Cotte E, Golfier F. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19(13):4052–4058. doi: 10.1245/s10434-012-2510-4. [DOI] [PubMed] [Google Scholar]
- 23.Helyer L, Easson A M. Surgical approaches to malignant bowel obstruction. J Support Oncol. 2008;6(03):105–113. [PubMed] [Google Scholar]
- 24.Shariat-Madar B, Jayakrishnan T T, Gamblin T C, Turaga K K. Surgical management of bowel obstruction in patients with peritoneal carcinomatosis. J Surg Oncol. 2014;110(06):666–669. doi: 10.1002/jso.23707. [DOI] [PubMed] [Google Scholar]
- 25.Ponce J, Nguyen N T, Hutter M, Sudan R, Morton J M. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011-2014. Surg Obes Relat Dis. 2015;11(06):1199–1200. doi: 10.1016/j.soard.2015.08.496. [DOI] [PubMed] [Google Scholar]
- 26.Aghajani E, Nergaard B J, Leifson B G, Hedenbro J, Gislason H. The mesenteric defects in laparoscopic Roux-en-Y gastric bypass: 5 years follow-up of non-closure versus closure using the stapler technique. Surg Endosc. 2017;31(09):3743–3748. doi: 10.1007/s00464-017-5415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geubbels N, Lijftogt N, Fiocco M, van Leersum N J, Wouters M W, de Brauw L M. Meta-analysis of internal herniation after gastric bypass surgery. Br J Surg. 2015;102(05):451–460. doi: 10.1002/bjs.9738. [DOI] [PubMed] [Google Scholar]
- 28.Stenberg E, Szabo E, Ågren G.Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial Lancet 2016387(10026):1397–1404. [DOI] [PubMed] [Google Scholar]
- 29.Halabi W J, Kang C Y, Ketana N. Surgery for gallstone ileus: a nationwide comparison of trends and outcomes. Ann Surg. 2014;259(02):329–335. doi: 10.1097/SLA.0b013e31827eefed. [DOI] [PubMed] [Google Scholar]
- 30.Artioli G, Muri M, Praticò F E. Gallstone ileus: literature review. Acta Biomed. 2016;87 03:40–44. [PubMed] [Google Scholar]
- 31.Ploneda-Valencia C F, Gallo-Morales M, Rinchon C. Gallstone ileus: an overview of the literature. Rev Gastroenterol Mex. 2017;82(03):248–254. doi: 10.1016/j.rgmx.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Berliner S D, Burson L C. One-stage repair for cholecyst-duodenal fistula and gallstone Ileus. Arch Surg. 1965;90:313–316. doi: 10.1001/archsurg.1965.01320080137028. [DOI] [PubMed] [Google Scholar]
- 33.Inukai K, Tsuji E, Takashima N, Yamamoto M. Laparoscopic two-stage procedure for gallstone ileus. J Minim Access Surg. 2019;15(02):164–166. doi: 10.4103/jmas.JMAS_88_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuño-Guzmán C M, Arróniz-Jáuregui J, Moreno-Pérez P A, Chávez-Solís E A, Esparza-Arias N, Hernández-González C I. Gallstone ileus: one-stage surgery in a patient with intermittent obstruction. World J Gastrointest Surg. 2010;2(05):172–176. doi: 10.4240/wjgs.v2.i5.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntoulia A, Tharakan S J, Reid J R, Mahboubi S. Failed intussusception reduction in children: correlation between radiologic, surgical, and pathologic findings. AJR Am J Roentgenol. 2016;207(02):424–433. doi: 10.2214/AJR.15.15659. [DOI] [PubMed] [Google Scholar]
- 36.Honjo H, Mike M, Kusanagi H, Kano N. Adult intussusception: a retrospective review. World J Surg. 2015;39(01):134–138. doi: 10.1007/s00268-014-2759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong K D, Kim J, Ji W, Wexner S D. Adult intussusception: a systematic review and meta-analysis. Tech Coloproctol. 2019;23(04):315–324. doi: 10.1007/s10151-019-01980-5. [DOI] [PubMed] [Google Scholar]
- 38.Zubaidi A, Al-Saif F, Silverman R. Adult intussusception: a retrospective review. Dis Colon Rectum. 2006;49(10):1546–1551. doi: 10.1007/s10350-006-0664-5. [DOI] [PubMed] [Google Scholar]
- 39.Erkan N, Haciyanli M, Yildirim M, Sayhan H, Vardar E, Polat A F. Intussusception in adults: an unusual and challenging condition for surgeons. Int J Colorectal Dis. 2005;20(05):452–456. doi: 10.1007/s00384-004-0713-2. [DOI] [PubMed] [Google Scholar]
- 40.Near-Infrared Anastomotic Perfusion Assessment Network VOIR . Ris F, Liot E, Buchs N C. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg. 2018;105(10):1359–1367. doi: 10.1002/bjs.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc. 2016;30(07):2736–2742. doi: 10.1007/s00464-015-4540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafari M D, Wexner S D, Martz J E. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220(01):82–920. doi: 10.1016/j.jamcollsurg.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Boni L, David G, Mangano A. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29(07):2046–2055. doi: 10.1007/s00464-014-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alander J T, Kaartinen I, Laakso A. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]


