Abstract
The deep sea comprises more than 90% of the ocean; therefore, understanding the controlling factors of biodiversity in the deep sea is of great importance for predicting future changes in the functioning of the ocean system. Consensus has recently been increasing on two plausible factors that have often been discussed as the drivers of deep‐sea species richness in the contexts of the species‐energy and physiological tolerance hypotheses: (i) seafloor particulate organic carbon (POC) derived from primary production in the euphotic zone and (ii) temperature. Nonetheless, factors that drive deep-sea biodiversity are still actively debated potentially owing to a mirage of correlations (sign and magnitude are generally time dependent), which are often found in nonlinear, complex ecological systems, making the characterization of causalities difficult. Here, we tested the causal influences of POC flux and temperature on species richness using long-term palaeoecological datasets derived from sediment core samples and convergent cross mapping, a numerical method for characterizing causal relationships in complex systems. The results showed that temperature, but not POC flux, influenced species richness over 103–104-year time scales. The temperature–richness relationship in the deep sea suggests that human-induced future climate change may, under some conditions, affect deep-sea ecosystems through deep-water circulation changes rather than surface productivity changes.
Keywords: climate, deep-sea, biodiversity
1. Introduction
Understanding the driving factors of biodiversity is fundamental to predicting, conserving and maintaining the functions and services of ecosystems [1,2]. Most of the planet's surface is covered by deep sea (greater than 200 m water depth, in general), but because of its remoteness, our understanding of the factors that control deep-sea biodiversity remains limited [3], compared with those of shallow-marine areas [4] that cover a smaller portion of Earth's surface. The controlling factors of deep-sea biodiversity have been debated for many decades [3,5–10], and recently, two factors have emerged as most important [8,10–12]: (i) sinking organic materials derived from primary production in the euphotic zone, known as particulate organic carbon (POC) flux, which is the main food source for organisms in deep-sea environments without sunlight penetration and photosynthesis, and thus is critically important energy available to deep-sea biodiversity [8,12], and (ii) water temperature that may affect species richness through the physiological tolerance or metabolic effects on species coexistence [10,11]. There are increasing numbers of studies testing these two hypotheses. Some support the notion of temperature control [10,13] and some do not [14–16]. The studies supporting the notion of POC control are all based on static spatial macroecological data. By contrast, those supporting the notion of temperature control tend to be based on palaeo time-series data. Recently, Jöst et al. [13] found evidence supporting the notion of temperature control based on spatial macroecological data of the same taxonomic group used for palaeo time-series studies, Ostracoda [13,17,18]. Thus, the temperature hypothesis is now supported both by present-day macroecological and palaeo time-series data. However, the relative importance of temperature and POC flux is still controversial [3,8,10], at least partly because all previous discussions were based on the ‘correlation’ of the relationships, and not the ‘causation’ [3,8,10]. For example, ‘significantly positive linear correlations' between species richness and bottom-water temperature could support the temperature hypothesis. However, in nonlinear systems such as natural ecosystems, such correlations may occur even if two variables are uncoupled [19]. Thus, correlations may not provide sufficient evidence for acceptance/rejection of a hypothesis.
Recently, researchers have developed a time-series analytical framework that is specifically designed to analyse nonlinear dynamical systems, called empirical dynamic modelling (EDM) [19–21]. In the EDM methods, convergent cross mapping (CCM) is a numerical technique that can be used to characterize causal relationships in dynamic systems based on observational data [19]. While CCM has false positive and negative rates that vary depending on the levels of processing and/or observation noise based on simulated data [22], CCM may provide more reliable evidence of causal relationships than simple linear correlation.
Microfossils are small, microscopic-sized organisms with high fossilization potential, such as benthic foraminifera and ostracods. These fossilized organisms preserved in marine sediment cores are valuable for palaeoecological reconstructions, e.g. past biodiversity dynamics, and are the only resource that provides long-term deep-sea species richness time-series data beyond a few decades [18,23]. Indeed, several studies have investigated micropalaeontological time-series data to compare species richness to temperature and POC flux, and these studies support the temperature hypothesis [10,11,24,25]. Once again, these studies are based on ‘correlation’ (e.g. regression models), and the factor causally influencing deep-sea species richness is not yet understood.
Under the circumstances, applying CCM analysis to late Quaternary fossil records would provide us with a unique opportunity to test the causality between deep-sea species richness and environmental variables (i.e. temperature and POC flux) in geological, but still ecological (i.e. with few, if any, speciations or extinctions [10,11]) time scales. In this study, we use CCM to test the causal influences of temperature and POC flux on species richness using four long-term palaeoecological datasets of benthic foraminifera compiled from the deep-sea sediment core data used by Hunt et al. [11].
2. Material and methods
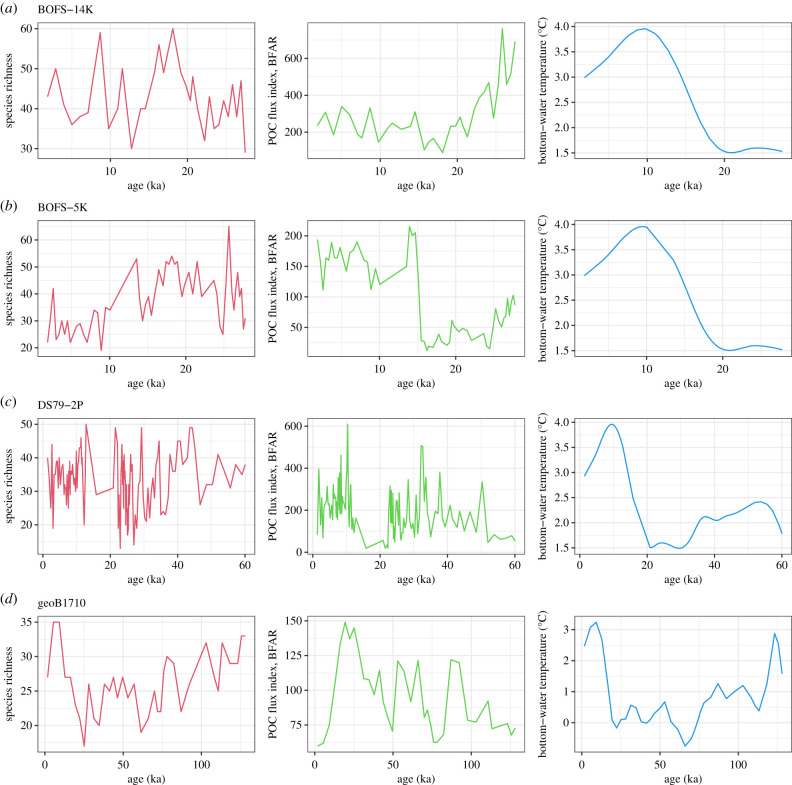
For CCM analysis, we selected four time-series datasets out of the 10 used by Hunt et al. [11] that included more than 20 time points after correction of time intervals (see the method below). The details to obtain richness, temperature and POC can be found in the electronic supplementary material, figure S1 and the data in electronic supplementary material, table S1. The analysed cores were BOFS-14 K (location: 58.6 N 19.4 W, time span: last approximately 30 000 years, mean sampling interval: 800 years, water depth: 1756 m), BOFS-5 K (50.7 N 21.9 W, last approximately 30000 years, 499 years, 3547 m), DS79-2P (58.9 N 30.4 W, last approximately 60000 years, 473 years, 1685 m) and geoB1710 (23.4 S 11.7 E, last approximately 130 000 years, 3500 years, 2987 m).
We used CCM to test the hypotheses that temperature and POC are causally related to species richness. Cross-map skills were evaluated by a correlation coefficient (ρ) or forecasting error (e.g. mean absolute error or root mean square error) between observed and predicted variables (electronic supplementary material, figure S2). As the number of points in the state space increases (i.e. the time-series, or library, becomes longer), the trajectory defining the attractor fills in, resulting in closer nearest neighbours and decreasing estimation error (a higher cross-map skill) if variables are causally coupled. Therefore, if the two variables belong to the same dynamic system (i.e. causally coupled), the cross-map skill should increase with library length (i.e. the number of points in the reconstructed state space of an effect variable). Cross mapping was performed from one variable to another using simplex projection [26]. More detailed algorithms for simplex projection and CCM have been described in previous studies [19,20,27,28]. rEDM v. 0.7.4 was used for simplex projection and CCM, and ggplot2 v. 3.1.1 for some graphics in R v. 3.6.0 [29].
The significance of CCM was judged using two methods. First, the cross-map skill (ρ) of original and twin surrogate time-series were compared [30]. The algorithm used for the twin surrogate method is provided in the electronic supplementary material and the custom R implementation is available at https://zenodo.org/record/1181937. A total of 1000 surrogate time-series were generated for one original time-series. Then, cross-map skills were calculated for the original and each surrogate time-series. If the number of surrogates with a higher cross-map skill as well as a higher convergence (i.e. the difference in cross-map skills between minimum and maximum library sizes) is less than 50 (i.e. 5% of the surrogates; this is a one-sided test because we are not interested in low cross-map skills), the cross mapping was judged as significant.
Second, to test the significant convergence of cross-map skill (ρ), we calculated the differences of cross-map skill (ρ) at minimum and maximum library sizes (Δρ). A simple heuristic threshold Δρ = 0.1 was set for the significance, and the forecasting skill was judged to have increased with library length if Δρ exceeded the threshold, with regard to Ushio et al. [30].
In addition to CCM, simple linear regressions were performed for the relationship of species richness to temperature and POC flux in the cores by the ‘lm’ function.
3. Results and discussion
Our results, using deep-sea sediment core datasets, partly support temperature as the controlling factor of deep-sea species richness. The CCM analysis using palaeo time-series data (figure 1; electronic supplementary material, figure S1) showed the potential causal relationship between richness and temperature (in two cores out of four; figure 2). Some cores showed no linear relationship between temperature and richness (see electronic supplementary material, figure S1 and table S2) despite the significant CCM results (figure 2). This may be because CCM tests nonlinear coupling in a dynamic system.
Figure 1.
The time-series of deep-sea benthic foraminifera species richness (left-hand column), POC flux proxy of BFAR (benthic foraminifera accumulation rate) (middle column) and bottom-water temperature in sediment cores (right-hand column); (a) BOFS-14 K, (b) BOFS-5 K, (c) DS79-2P and (d) geoB1710. ka, thousands of years ago.
Figure 2.
The cross-map skill (ρ) of CCM with library size in sediment cores (a) BOFS-14 K, (b) BOFS-5 K, (c) DS79-2P and (d) geoB1710. y-axis indicates the cross-map skills (i.e. the correlation coefficients between observed and predicted values by cross mapping [19]). x-axis indicates the library size (i.e. the size of data that were used to reconstruct the attractor). The lines indicate the cross-map skill of the original core data (only positive values shown; see electronic supplementary material, figure S3). The grey shading indicates the confidence interval based on cross-map skills of the twin surrogate data (95% of 1000 surrogate time-series). The causality was judged as significant if the cross-map skill (ρ, line) of the original time-series was higher than the upper limit of the confidence interval (shading) and the convergence of cross-map skill (ρ) (i.e. the cross-map skill at the maximum library length minus that at the minimum library length) was larger than 0.1. See electronic supplementary material, figure S3 for a detailed explanation about how the significant and non-significant cross-map skills were distinguished. rich, species richness; temp, temperature; POC, particulate organic carbon flux. Arrows indicate direction of causality.
The cross-map skills between richness and POC flux were not significant according to those of the 95% surrogate data in almost all cores (figure 2), except for core geoB1710, where the cross-map skill of POC flux and richness was higher than that of the 95% surrogate data (figure 2d). However, the cross-map skill did not show signs of convergence based on our criterion (Δρ < 0.1), suggesting that the influences were not strong enough to exclude the possibility of a pseudo-correlation (see fig. 4B in Sugihara et al. [19]). Therefore, in contrast with the influences of temperature, no strong linkage between species richness and POC flux based on CCM was found. This supports the regression method-based conclusion of Hunt et al. [11]: benthic foraminiferal diversity tended to increase with increasing bottom-water temperature and did not show any significant linear relationship with POC. Further, Hunt et al. [11] did not consider the possibility of a nonlinear relationship between richness and POC flux. As CCM can characterize causality in nonlinear systems, our results suggest that deep-sea species richness and POC may not be causally linked (either linearly or nonlinearly) (see potential issues of our data in the Material and methods, such as the number of data points). Although POC flux is the main food source for deep-sea organisms [3] and has been considered an important driving factor of deep-sea benthic biomass [31], we found no causal relationship between species richness and POC flux, at least at the time interval and/or time scale of this study. In addition, small organisms account for the vast majority of marine biodiversity [32] and are considered to be more sensitive to climatic change than larger organisms [33]. Thus, our results based on benthic foraminifera are likely to reflect the temperature control on the majority of deep-sea biodiversity that is sensitive to climate change, in considering space-for-time substitution and the fact that benthic foraminifera tend to show large-spatial scale diversity patterns that are consistent with other deep-sea organisms that do not have fossil records [3,10].
Although Woolley et al. [34] indicated that deep-sea diversity is primarily controlled by temperature in depths less than 2000 m but by POC flux in depths greater than 2000 m based on regression modelling, our study found evidence that temperature influences deep-sea diversity both above and below 2000 m, at least at some locations, and no influence by POC in either depth range. However, further studies will need to perform more comprehensive testing with wider coverages of geographic regions, time scales and water depth ranges (of both shallower and deeper than 2000 m) to draw more general conclusions. Together, our results provide statistical evidence of the significant influence of temperature on deep-sea biodiversity. On the other hand, previously proposed POC controls on biodiversity [8,12] were not statistically supported by our analysis. These previous cases for POC control are correlation-based studies that used static spatial data, which may not necessarily indicate causality. Nonetheless, POC is known to be important for many aspects of deep-sea ecosystems [8], and further testing with time-series data of different time scales and POC ranges and analyses with nonlinear perspectives may improve our understanding of the role of POC on the deep-sea ecosystem.
The relationships between biodiversity and ecosystem function in the deep sea are largely unknown [23]. In fact, Yasuhara et al. [18] suggested that environmental factors and mechanisms might better explain community dynamics than the biodiversity–ecosystem functions relationship in long-term deep-sea time-series records. EDM, and especially CCM, analyses on palaeoecological records can be applied to biodiversity and community aspects other than species richness, e.g. functional diversity, evenness, abundance and biomass. CCM would help to improve our understanding of the relationship between biodiversity–ecosystem functions at long-time scales and large-spatial scales where it is difficult to test links experimentally or observationally [35].
Acknowledgements
We thank Gene Hunt for providing the dataset used in the study.
Contributor Information
Hideyuki Doi, Email: hideyuki.doi@icloud.com.
Moriaki Yasuhara, Email: yasuhara@hku.hk.
Masayuki Ushio, Email: ong8181@gmail.com.
Ethics
This study was conducted using previously published datasets and did not require any ethical approval or permits.
Data accessibility
All data are available in Hunt et al. (2005) [11] and the electronic supplementary material, table S1 (Doi_sediment_coredata.csv) (doi:https://doi.org/10.6084/m9.figshare.c.5506728.v1).
The data are provided in the electronic supplementary material.
Authors' contributions
H.D., M.Y. and M.U. designed the study; H.D. and M.U. analysed the data and interpreted the results; H.D., M.Y. and M.U. wrote the manuscript.
All authors meet all four of these conditions: (i) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (ii) drafting the article or revising it critically for important intellectual content; (iii) final approval of the version to be published and (iv) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
The authors declare no competing financial or non-financial interests.
Funding
This study was partly supported by a grant from the JST-CREST (JPMJCR13A2) and the Environment Research and Technology Development Fund (JPMEERF20204004) to H.D., a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (project code: HKU 17311316); the Seed Funding Programme for Basic Research of the University of Hong Kong (project code: 201711159057); and the Faculty of Science RAE Improvement Fund of the University of Hong Kong to M.Y. M.U. is supported by the Hakubi Project at Kyoto University.
References
- 1.Mace GM, Norris K, Fitter AH. 2012. Biodiversity and ecosystem services: a multilayered relationship. Trends Ecol. Evol. 27, 19-26. ( 10.1016/j.tree.2011.08.006) [DOI] [PubMed] [Google Scholar]
- 2.Bennett EM, et al. 2015. Linking biodiversity, ecosystem services, and human well-being: three challenges for designing research for sustainability. Curr. Opin. Env. Sust. 14, 76-85. ( 10.1016/j.cosust.2015.03.007) [DOI] [Google Scholar]
- 3.Rex MA, Etter RJ. 2010. Deep-sea biodiversity: pattern and scale. Cambridge, MA: Harvard University Press. [Google Scholar]
- 4.Tittensor DP, Mora C, Jetz W, Lotze HK, Ricard D, Berghe EV, Worm B. 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098-1101. ( 10.1038/nature09329) [DOI] [PubMed] [Google Scholar]
- 5.Sanders HL, Hessler RR. 1969. Ecology of the deep-sea benthos. Science 163, 1419-1424. ( 10.1126/science.163.3874.1419) [DOI] [PubMed] [Google Scholar]
- 6.Rex MA, Stuart CT, Hessler RR, Allen JA, Sanders HL, Wilson GDF. 1993. Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature 365, 636-639. ( 10.1038/365636a0) [DOI] [Google Scholar]
- 7.Levin LA, Etter RJ, Rex MA, Gooday AJ, Smith CR, Pineda JÃ, Stuart CT, Hessler RR, Pawson D. 2001. Environmental influences on regional deep-sea species diversity. Ann. Rev. Ecol. Syst. 32, 51-93. ( 10.1146/annurev.ecolsys.32.081501.114002) [DOI] [Google Scholar]
- 8.Mcclain CR, Allen AP, Tittensor DP, Rex MA. 2012. Energetics of life on the deep seafloor. Proc. Natl Acad. Sci. USA 109, 15 366-15 371. ( 10.1073/pnas.1208976109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers AD. 2015. Environmental change in the deep ocean. Ann. Rev. Environ. Resour. 40, 1-38. ( 10.1146/annurev-environ-102014-021415) [DOI] [Google Scholar]
- 10.Yasuhara M, Danovaro R. 2016. Temperature impacts on deep-sea biodiversity. Biol. Rev. 91, 275-287. ( 10.1111/brv.12169) [DOI] [PubMed] [Google Scholar]
- 11.Hunt G, Cronin TM, Roy K. 2005. Species–energy relationship in the deep sea: a test using the Quaternary fossil record. Ecol. Lett. 8, 739-747. ( 10.1111/j.1461-0248.2005.00778.x) [DOI] [Google Scholar]
- 12.Tittensor DP, Rex MA, Stuart CT, Mcclain CR, Smith CR. 2011. Species–energy relationships in deep-sea molluscs. Biol. Lett. 7, 718-722. ( 10.1098/rsbl.2010.1174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jöst AB, Yasuhara M, Wei CL, Okahashi H, Ostmann A, Martínez Arbizu P, Mamo B, Svavarsson J, Brix S. 2019. North Atlantic Gateway: test bed of deep-sea macroecological patterns. J. Biogeogr. 46, 2056-2066. ( 10.1111/jbi.13632) [DOI] [Google Scholar]
- 14.Wei C-L, Rowe GT. 2019. Productivity controls macrofauna diversity in the deep northern Gulf of Mexico. Deep Sea Res. Part I. 143, 17-27. ( 10.1016/j.dsr.2018.12.005) [DOI] [Google Scholar]
- 15.Wei C-L, Chen M, Wicksten M, Rowe GT. 2020. Macrofauna bivalve diversity from the deep Northern Gulf of Mexico. Ecol. Res. 35, 343-361. ( 10.1111/1440-1703.12077) [DOI] [Google Scholar]
- 16.Ashford OS, Kenny AJ, Barrio Froján CR, Horton T, Rogers AD. 2019. Investigating the environmental drivers of deep-seafloor biodiversity: a case study of peracarid crustacean assemblages in the Northwest Atlantic Ocean. Ecol. Evol. 9, 14 167-14 204. ( 10.1002/ece3.5852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyson RV. 2001. Sedimentation rate, dilution, preservation and total organic carbon: some results of a modelling study. Org. Geochem. 32, 333-339. ( 10.1016/S0146-6380(00)00161-3) [DOI] [Google Scholar]
- 18.Yasuhara M, Doi H, Wei CL, Danovaro R, Myhre SE. 2016. Biodiversity–ecosystem functioning relationships in long-term time series and palaeoecological records: deep sea as a test bed. Phil. Trans. R Soc. B 371, 1471-2970. ( 10.1098/rstb.2015.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugihara G, May R, Ye H, Hsieh C-h, Deyle E, Fogarty M, Munch S. 2012. Detecting causality in complex ecosystems. Science 338, 496-500. ( 10.1126/science.1227079) [DOI] [PubMed] [Google Scholar]
- 20.Ye H, Deyle ER, Gilarranz LJ, Sugihara G. 2015. Distinguishing time-delayed causal interactions using convergent cross mapping. Sci. Rep. 5, 14750. ( 10.1038/srep14750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deyle ER, Maher MC, Hernandez RD, Basu S, Sugihara G. 2016. Global environmental drivers of influenza. Proc. Natl Acad. Sci. 113, 13 081-13 086. ( 10.1073/pnas.1607747113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark AT, Ye H, Isbell F, Deyle ER, Cowles J, Tilman D, Sugihara G. 2015. Spatial ‘convergent cross mapping’ to detect causal relationships from short time-series. Ecology 96, 1174-1181. ( 10.1890/14-1479.1) [DOI] [PubMed] [Google Scholar]
- 23.Yasuhara M, Tittensor DP, Hillebrand H, Worm B. 2017. Combining marine macroecology and palaeoecology in understanding biodiversity: microfossils as a model. Biol. Rev. 92, 199-215. ( 10.1111/brv.12223) [DOI] [PubMed] [Google Scholar]
- 24.Yasuhara M, Hunt G, Cronin TM, Okahashi H. 2009. Temporal latitudinal-gradient dynamics and tropical instability of deep-sea species diversity. Proc. Natl Acad. Sci. USA 106, 21 717-21 720. ( 10.1073/pnas.0910935106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasuhara M, Okahashi H, Cronin TM, Rasmussen TL, Hunt G. 2014. Deep-sea biodiversity response to deglacial and Holocene abrupt climate changes in the North Atlantic Ocean. Glob. Ecol. Biogeogr. 23, 957-967. ( 10.1111/geb.12178) [DOI] [Google Scholar]
- 26.Sugihara G, May RM. 1990. Nonlinear forecasting as a way of distinguishing chaos from measurement error in time series. Nature 344, 734. ( 10.1038/344734a0) [DOI] [PubMed] [Google Scholar]
- 27.Chang CW, Ushio M, Hsieh CH. 2017. Empirical dynamic modeling for beginners. Ecol. Res. 32, 785-796. ( 10.1007/s11284-017-1469-9) [DOI] [Google Scholar]
- 28.Ushio M. 2020. Interaction capacity underpins community diversity. BioRxiv. ( 10.1101/2020.04.08.032524) [DOI]
- 29.R Core Team 2019. 2012. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org. [Google Scholar]
- 30.Ushio M, Hsieh C-h, Masuda R, Deyle ER, Ye H, Chang C-W, Sugihara G, Kondoh M, 2018. Fluctuating interaction network and time-varying stability of a natural fish community. Nature 554, 360-363. ( 10.1038/nature25504) [DOI] [PubMed] [Google Scholar]
- 31.Wei CL, et al. 2010. Global patterns and predictions of seafloor biomass using random forests. PLoS ONE 5, e15323. ( 10.1371/journal.pone.0015323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leray M, Knowlton N. 2015. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl Acad. Sci. USA 112, 2076-2081. ( 10.1073/pnas.1424997112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu WTR, Yasuhara M, Cronin TM, Hunt G, Gemery L, Wei C-L. 2020. Marine latitudinal diversity gradients, niche conservatism and out of the tropics and Arctic: climatic sensitivity of small organisms. J. Biogeogr. 47, 817-828. ( 10.1111/jbi.13793) [DOI] [Google Scholar]
- 34.Woolley SN, Tittensor DP, Dunstan PK, Guillera-Arroita G, Lahoz-Monfort JJ, Wintle BA, Worm B, O'hara TD. 2016. Deep-sea diversity patterns are shaped by energy availability. Nature 533, 393-396. ( 10.1038/nature17937) [DOI] [PubMed] [Google Scholar]
- 35.Van Nes EH, Scheffer M, Brovkin V, Lenton TM, Ye H, Deyle E, Sugihara G. 2015. Causal feedbacks in climate change. Nat. Clim. Change 5, 445-448. ( 10.1038/nclimate2568) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in Hunt et al. (2005) [11] and the electronic supplementary material, table S1 (Doi_sediment_coredata.csv) (doi:https://doi.org/10.6084/m9.figshare.c.5506728.v1).
The data are provided in the electronic supplementary material.