Abstract
Using alcohol-based disinfectants is an effective method for preventing the spread of COVID-19. However, non-traditional manufacturers of alcohol-based disinfectants, such as ethanol plants, need to undergo additional treatment to curb their impurities to limits set by the Food and Drug Association (FDA) to produce alcohol-based disinfectants. To transform them to disinfectant-grade alcohol, 17 process streams in a dry-mill ethanol plant were analyzed to determine the quality parameters for acetaldehyde, acetal, propanol, methanol, and water, including chemical oxygen demand, total suspended solids, and nutrients. Results suggest that the process stream generated by the distillation column requires further treatment because the acetaldehyde and acetal concentrations are significantly higher than the impurity limit set by the FDA. The addition of a second distillation column could be a potential method for addressing impurities and it will have minimal influence on hazardous air pollutant generation and water use.
Keywords: Hand sanitizer production, Impurities, Hazardous air pollutants, Process streams, SARS-CoV-2
Graphical abstract

1. Introduction
The COVID-19 pandemic has affected nearly every country across the globe. Disinfection of hands and surfaces can help prevent its spread. Alcohol-based sanitizers have become a standard for disinfecting frequently touched surfaces. They serve as a substitute when soap and water are not readily available (Bloomfield et al., 2007). Alcohol-based sanitizers are 90%–99.9% effective depending on the target pathogen (Tuladhar et al., 2015). Disinfection products were in high demand at the start of the pandemic (March 2020), and suppliers were struggling to keep up owing to a lack of raw materials. Demand for alcohol-based disinfectants are still high to this day and the market for disinfectants is growing exponentially across the globe (Research Dive, 2021).
The Food and Drug Association (FDA) has strict limits on impurity concentrations in hand sanitizers but has temporarily raised said limits and associated regulations to allow nontraditional manufacturers to enter the market and meet the growing demand (FDA, 2020). The interim standards were instituted on January 31, 2020, with the declaration of a public health emergency by the Secretary of Health and Human Services (HHS). These interim standards will cease and revert to the traditional standards once the public health emergency is over. Interim and normal impurity limits are listed in Table S1.
Ethanol plants, a nontraditional manufacturer of alcohol-based disinfectants, began producing alcohol-based disinfectants during the COVID-19 pandemic (Voegele, 2020). This is because as the demand for alcohol-based disinfectants increased as the demand for ethanol fuel has decreased. Some US ethanol plants restructured to provide ethanol production for hand sanitizers (Kelly, 2020; Reed, 2020). The World Health Organization has two formulations of alcohol based disinfectants with an active ingredient of either isopropyl alcohol or ethanol the latter in which is produced by ethanol plants (World Health Organization, 2009).
The production of ethanol from corn starch biomass is a multistep process. The process begins with the cleaning, grinding, liquefaction, and saccharification of corn starch to turn it into a fermentable mash. Dry mill ethanol plants use corn as the raw material for production. Once the corn is converted into mash, the corn will go through the fermentation process where yeast is added. The fermentation process produces beer containing 10% alcohol which is sent to a distillation tower to separate the alcohol from the solids and water where they will be treated and disposed of (Eidman, 2007). Impurities generated from the fermentation of corn starch include esters, organic acids, and higher alcohols (Onuki et al., 2015). The final product produced by distillation contains 95% alcohol and 5% water.
Incorporation of additional treatment methods is necessary for ethanol plants to address the impurities generated during the fermentation process because several fermentation byproducts may be present at concentrations above the specified FDA limits. Tests have been conducted on the final byproducts (Habe, 2013); however, there is little data in the literature concerning the concentrations of these compounds within ethanol-plant process streams. The incorporation of additional treatment methods may affect the amount of hazardous air pollutants (HAPs) and greenhouse gas (GHG) emissions in a negative manner. In addition, there is little data in the literature characterizing the streams within an ethanol plant in terms of their overall water quality parameters; this data is useful when considering potential treatment options and understanding the potential implications of accidental releases for specific streams.
During the production of ethanol from the fermentation of corn, volatile HAPs are generated and potentially released. The major gaseous HAPs generated from ethanol production are formaldehyde, acetaldehyde, and acrolein (Brady and Pratt, 2007). The Environmental Protection Agency regulates the quantity of HAPs released by each plant into the environment (USEPA, 2018). HAP emissions from ethanol plants are measured in a tons/year basis and have specific limits set by the National Emission Standards for HAP (NESHAP). Carbon dioxide (CO2) scrubbers and thermal oxidizers are often used to treat these HAPs (US Environmental Protection Agency, 2021). Exposure to HAPs could cause negative impacts to the immune system, fertility, neurological problems in addition to the increased cancer risk.
In addition to meeting emission standards set by NESHAP, many ethanol facilities are seeking methods to reduce their GHG footprint to increase their market share and profitability. An example of this trend is California's low-carbon fuel standard (LCFS) carbon credit system, which incentivizes creating low-carbon intensity fuels (California Air Resources Board, 2020). US ethanol producers can generate credits, which can be exchanged for monetary value, by reducing CO2 emissions in production processes (Batres-Marquez, 2017). Unfortunately, the addition of treatment processes to meet impurity standards for hand sanitizer may complicate the reduction of GHG emissions.
The goal of this study is to support the global fight against COVID-19 by assisting the ethanol industry in producing of alcohol-based hand sanitizers by identifying the concentrations of harmful fermentation byproducts at different plant locations and to provide information to researchers examining the influences of this transition. Understanding the impurity concentrations within the process streams of an ethanol plant can help with respect to developing new treatment methods, optimize unit operations, and understand the associated impacts on air and water emissions from the impurities and possible treatment processes. These data may be helpful in identifying possible streams that could be used as an input to innovative waste treatment processes developed for the ethanol industry, such as bio-scrubbers for air emissions (Duerschner, 2020). This study examines data from one dry-mill ethanol plant (hereafter referred to as the plant), and although operations in other facilities will not be the same, it is believed that the general concentration and mass flow rates for the impurities will be similar to other dry-mill ethanol plants.
2. Materials and methods
2.1. Ethanol plant description and potential locations for impurity generation
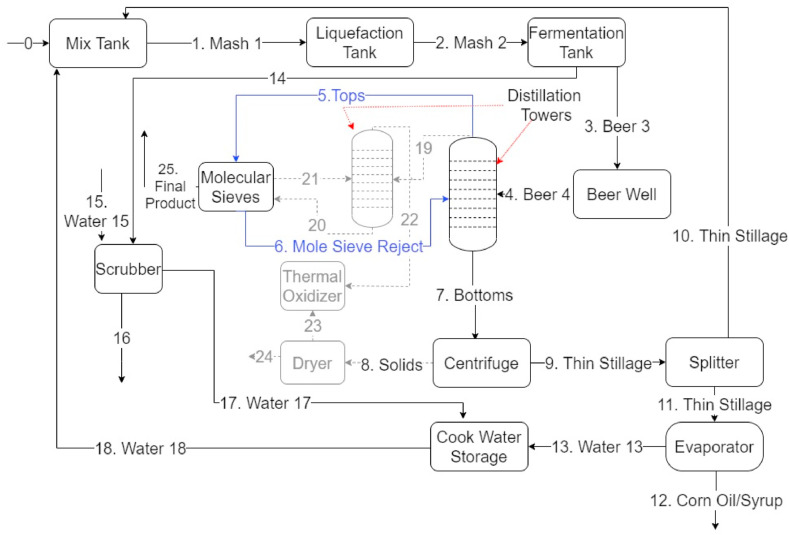
A process flow diagram of the plant, representing most US dry-mill ethanol plants, is shown in Fig. 1 . Seventeen samples were acquired from locations across the plant.
Fig. 1.
Generalized process flow diagram of the plant.
The plant operates based on the process streams with solid lines. Unlike some ethanol plants, the plant omits the use of a thermal oxidizer for the treatment of HAPs and does not contain a second distillation tower. The treatment of ethanol is solely dependent on molecular sieving after initial distillation.
Unlike the purification process used by the plant, another common design method to purify ethanol is based on the solid lines in combination with the gray dashed lines indicated in Fig. 1. The black streams in the diagram are streams that nearly all ethanol plants share. Accordingly, it is assumed that the stream compositions examined herein are similar to most ethanol plants.
There are several different treatment options for reducing impurities in ethanol. The common method for treating impurities, in particular acetaldehyde, is the use of a rectifying column. A rectifying column is a second distillation column that further purifies the ethanol (Summers, 2006). Stream 19 in Fig. 1 feeds into a rectifying column. A degassing system, which is a part of the rectifying column, can enhance acetaldehyde reduction within the rectifying column, allowing a small amount of vapor to escape from the last condenser to eliminate acetaldehyde content (Batista and Meirelles, 2009). However, degassing systems have the drawback of bioproduct loss. A common method for treating fuel-grade ethanol to produce food-grade ethanol is ozonation, followed by activated carbon adsorption and then gas stripping with CO2 (Onuki et al., 2015). The location for the treatment system of these impurities is dependent on the process streams wherein impurities are generated. The treatment system can be placed after the molecular sieve to treat Final Product 25.
2.2. Impurity analysis
Seventeen stream samples were analyzed for acetaldehyde, propanol, methanol, and acetal concentrations. Streams that had high solid content (Mash 1 & 2, Beer 3 & 4, Thin Stillage 9/10/11, Bottoms 7, and Corn Oil/Syrup 12) were centrifuged, and the supernatant was analyzed. The solid portion of the centrifuged samples was separately analyzed for the same compounds. The concentration of each compound was normalized by the concentration of total suspended solids (TSS).
Impurity analysis was accomplished by the Entech 5800 SPDU vacuum-assisted sorbent extraction (VASE) headspace sorbent pens (Entech, 2020a) in conjunction with the Agilent 7820A gas chromatography system with mass spectrometry detector (Santa Clara, CA) For liquid samples, a 2-mL aqueous sample was placed in a glass vial. For solid samples, a 1.0-g solid material was placed in a glass vial. A VASE pin was inserted into the vial, and then a 30-mm mercury vacuum was applied to the pin, which ensured that volatile organic compounds in the headspace were adsorbed by the pin. The sample was then placed in a Entech 5600-SPES Sorbent Extraction System for 3 h at 70 °C and 200 rpm (Entech, 2020b). After incubation, the samples were placed in a cold tray for 10 min. VASE pin samples were then examined via GC–MS to analyze impurities formed in the vial headspace.
After the solid samples were prepared for VASE extraction, the glass vial containing the solids was placed in an oven at 100 °C for 2 h. The glass vial was weighed subsequent to heating to calculate the total mass of the solids.
2.3. Analysis of major water properties
Total phosphorous (TP) was determined using TNTplus method 10209 (Hach, 2013a). Total nitrogen (TN) was determined using TNTplus method 10208 (Hach, 2018). The chemical oxygen demand (COD) was determined using TNT 820 vials and the reactor digestion method (Hach, 2017). All vials were analyzed using a Hach DR2800 (Hach, 2013b). TSS was analyzed using standard wastewater analysis techniques (Baird et al., 2017).
3. Results
All 17 stream samples were analyzed for acetaldehyde, propanol, methanol, acetal, and COD concentrations as a measure of the overall organic matter. Three of the five compounds measured are regulated in the final product of alcohol-based sanitizers. The results of the liquid and solid analysis are presented in Table 1 . Fig. 2 (A) presents the flow throughout the plant in terms of the impurity concentration and flow rate of the liquid samples. Fig. 2(B) presents impurity concentrations in terms of COD as well as the overall COD concentration for the liquid samples. High concentrations of impurities were found in the following streams: Beer & 4, Column Tops 5, and Mole Sieve Reject 6. The relation between Column Tops 5 and Mole Sieve Reject 6 is important because any mass lost between the two streams is found in the final ethanol product. Impurities found in the ethanol are either available in the feedstock or they are byproducts of incomplete fermentation. In previous studies, it was determined that the types of impurities depend mainly on the type of the feedstock used (Habe, 2013). Final product was not provided by the plant for proprietary reasons, and consequently was not tested for impurities.
Table 1.
Plant process stream COD and impurity concentrations.
| Process Streams |
Liquid Portion |
Solid Portion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | Name | COD (g/L) | Acetalde-hyde (mg/L) | Prop-anol (mg/L) | Meth-anol (mg/L) | Acetal (mg/L) | Acetalde-hyde (mg/L) | Meth-anol (mg/L) | Prop-anol (mg/L) | Acetal (mg/L) |
| 1 | Mix Tank Mash | 310 | <DL | <DL | <DL | <DL | 11 | <DL | 3.6 | <DL |
| 2 | Liquefaction Tank Mash | 290 | <DL | <DL | <DL | <DL | 360 | 33 | <DL | <DL |
| 3 | Ferm Tank Beer | 230 | 130 | 14 | 390 | <DL | 220 | 860 | <DL | 2.0 |
| 4 | Beer Well | 280 | 260 | 12 | 170 | <DL | 160 | 990 | 5.0 | 2.0 |
| 5 | Column Tops | 1200 | 610 | 130 | 380 | 130 | NS | NS | NS | NS |
| 6 | Mole Sieve Reject | 1200 | 580 | 13 | 1700 | 10 | NS | NS | NS | NS |
| 7 | Column Bottoms | 120 | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 3.0 |
| 9,10,11 | Thin Stillage | 150 | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 1.3 |
| 12 | Corn Oil/Syrup | 52 | <DL | 10 | <DL | <DL | <DL | <DL | <DL | 2.7 |
| 13 | Evaporated Water | 5 | <DL | <DL | <DL | <DL | NS | NS | NS | NS |
| 15 | Well Water | 14 | <DL | <DL | <DL | <DL | NS | NS | NS | NS |
| 17 | CO2 Scrubber Water | 40 | <DL | <DL | 77 | <DL | NS | NS | NS | NS |
| 18 | Recycled Cook Water | 17 | <DL | <DL | <DL | <DL | NS | NS | NS | NS |
| * | Cooling Tower Blow Down | 1 | <DL | <DL | <DL | <DL | NS | NS | NS | NS |
| * | Cooling Tower Blow Down + RO Reject | <DL | <DL | <DL | <DL | <DL | NS | NS | NS | NS |
NS = no solids in the process stream and <DL = under detection limit. DL is as follows: COD = 1.0 mg/l; acetaldehyde = 7.5 mg/l; propanol = 9 mg/l; methanol = 9 mg/l; and acetal = 5 mg/l.
Fig. 2.
Plant diagram with (A) impurity flow and concentration and (B) impurities in terms of COD. Note: “<DL” is less than detection limits.
3.1. Acetaldehyde and acetal
Acetaldehyde and acetal are two of the impurities regulated in hand sanitizers by the FDA. The concentration of acetaldehyde and acetal must be treated if the combined concentration in the final ethanol product is over the interim impurity limit of 50 mg/L. Acetaldehyde appears in Mash 1, Mash 2, Beer 3, Beer 4, Column Tops 5, and Mole Sieve Reject 6, the concentrations of which are larger than the impurity limit for all samples except Mash 1. There is an acetaldehyde mass-flow difference of 80 g/min between Column Tops 5 and Mole Sieve Reject 6. This 80-g/min difference is retrieved in Final Product 25. Further treatment of Column Tops 5 or Final Product 25 is necessary to reduce acetaldehyde to below FDA limits.
The concentration of acetal is first detected in the solid portion of Beer 3 and Beer 4 streams. After distillation, some of the acetal remains in the solid portions of Bottoms 7, Thin Stillage 9/10/11, and Corn Oil/Syrup 12 streams but at concentrations below 3 mg/L. The acetal concentration of Column Tops 5 is well-above the FDA limit at 130 mg/L. There is a mass difference of 21.5 g/min acetal between Column Tops 5 and Mole Sieve Reject 6, which will be retrieved in Final Product 25. Both acetaldehyde and acetal concentrations must be reduced in either Column Tops 5 or Final Product 25 to meet the impurity limits set by the FDA.
3.2. Methanol, propanol, and chemical oxygen demand
Methanol is one of the impurities regulated by the FDA for hand sanitizers. The FDA limit for methanol is 630 mg/L under interim conditions and 200 mg/L under normal standards. Methanol concentrations are over the normal FDA limit in Beer 3, Column Tops 5, and Mole Sieve Reject 6. An interesting occurrence is the accumulation of methanol between the distillation column and the molecular sieve owing to the properties of methanol. First, methanol is extremely volatile has a lower boiling point than ethanol (O'Neil, 2013; Haynes, 2015), and the majority of the methanol should be found at the top of the distillation column (Column Tops 5). Methanol also has a small molecule size, which is comparable to water, and thus the molecular sieve can filter and send it back to the column. As methanol is filtered out by the molecular sieve, the concentrations of methanol in Final Product 25 should be below the specified FDA limits.
Propanol is not one of the regulated impurities in hand sanitizer. Propanol concentrations are low throughout the plant streams except Column Tops 5, wherein the concentration is 130 mg/l. COD concentrations are approximately 300 mg/L for the first four process streams. The spike in Column Tops 5 and Mole Sieve Reject 6 streams owing to the high concentration of ethanol being released from the distillation-column top. COD concentrations dropped in subsequent streams coming out of the distillation-column bottom.
4. Discussion
The samples used herein were collected in July 2020 prior to changes in the processing to reduce impurity concentrations. These samples indicate the concentrations of regulated compounds as well as ethanol and propanol prior to the production of alcohol-based disinfectants. Ethanol plants had to undergo additional treatment to reduce the concentration of regulated compounds, such as acetaldehyde, methanol, and acetal. Generally, the treatment method would be incorporated to treat the Column Tops 5 process, which releases a stream from the top of the first distillation column. In Column Tops 5, 610 mg/L of acetaldehyde and 130 mg/L of acetal can be observed, both of which are well-above the interim (50 mg/l) and standard (10 mg/l) limits set by the FDA. Column Tops 5 has a methanol concentration of 380 mg/L that meets the FDA interim limit (600 mg/l) but would need to be further treated to meet the normal limit (200 mg/l).
Acetal formation in the distillation column is caused by the acid catalyzed reaction of ethanol and acetaldehyde (McMurry, 2019). This reaction causes acetal concentration to rise from below detection limit to 130 mg/L in the Column Tops 5. The pH in the distillation column should be controlled to limit the formation of acetal. The addition of an oxidant able of cleaving C C bonds such as permanganate salts, ozone, nitrates, etc. could potentially be used to eliminate impurities. Any oxidant to be used need to show minimal impact on the produced ethanol (Schmidt et al., 2020).
Ethanol plants can incorporate a second distillation column to reduce the concentration of regulated compounds. Double distillation produced fractions with lower impurities concentration (Balcerek et al., 2017). Acetal was correctly calculated and the fractions removed were accurately estimated in columns (Haaz et al., 2021). Unfortunately, this would increase the operating costs and natural gas use, and it would also require capital costs for the construction and instillation of the column. Second distillation column in combination with a degassing system can be used to remove levels of acetaldehyde in the 2–2.2 mg/l range (Batista and Meirelles, 2009). In many cases, an oxidant may also be used to help remove acetaldehydes and acetal (Marcus, 1985). The second distillation column is unlikely to significantly increase HAP generation or water usage. However, the increase in natural gas from the second distillation column would increase GHG emissions owing to the combustion of natural gas to heat the column. Adding a second distillation column could increase GHG emissions by about 5%–10%, and occasionally, by 100% depending on the configuration of the reboiler and condenser systems (Tgarguifa et al., 2018; Baeyens et al., 2015). The plant currently produces 0.4–0.5 kg of CO2 per liter of ethanol. These calculations are based on the annual electricity and natural gas usage standardized by the volume of ethanol produced each year by the plant. The amount of CO2 currently produced by the plant does not account for production from the fermentation tank, which many ethanol plants collect and sell in the industrial gas market.
To put GHG emissions into context, an estimate can be made for the CO2 emitted during ethanol production for a travel-sized disinfectant bottle. The standard size for a travel-sized disinfectant bottle is 30 mL, which contains 70% ethanol (GOJO US, 2021). The CO2 emitted is estimated to be 0.01 kg per bottle of disinfectant. Calculations for this value can be found in the supplementary materials.
5. Conclusions
Ethanol plants transitioning into the production of alcohol-based disinfectants must meet impurity limits as set by the FDA. For the plant, the Column Tops 5 or the Final Product 25 processes streams will need to be further treated to meet impurity limits as set by the FDA. The acetaldehyde and acetal concentrations in Column Tops 5 are well over the limits set by the FDA. Concentration of methanol in Column Tops 5 is below the interim impurity limits but will need to be lowered further to meet limits under normal conditions.
Based on communications with multiple ethanol plants, the installation and operation of a second distillation column will result in increased plant operations costs and natural gas usage. The addition of the second distillation column would increase CO2 emissions, however, the second distillation column will not contribute to a significant increase in HAP generation or water usage.
Credit author statement
Gabriel Cohen: Validation, Formal analysis, Investigation, Writing - Original Draft, Visualization, Nathan Kreutzer: Validation, Investigation, Katie Mowat: Validation, Investigation, Ashraf Aly Hassan: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition, Bruce Dvorak: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Nebraska Center for Energy Sciences Research (NCESR), Cycle 13.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jenvman.2021.113329.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Baeyens J., et al. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015;47(Apr):60–88. [Google Scholar]
- Baird R., et al. American Public Health Association; 2017. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Balcerek M., Pielech-Przybylska K., Patelski P., Dziekońska-Kubczak U., Strąk E. The effect of distillation conditions and alcohol content in ‘heart’ fractions on the concentration of aroma volatiles and undesirable compounds in plum brandies. J. Inst. Brew. 2017;123:452–463. doi: 10.1002/jib.441. [DOI] [Google Scholar]
- Batista Fabio R.M., Meirelles A.J.A. A strategy for controlling acetaldehyde content in an industrial plant of bioethanol. IFAC Proceedings Volumes. 2009;42(11):928–933. [Google Scholar]
- Batres-Marquez S.P. 2017. Ethanol Leads Credit Generation for California Low Carbon Fuel Standard | Agricultural Marketing Resource Center.https://www.agmrc.org/renewable-energy/renewable-energy-climate-change-report/renewable-energy-climate-change-report/june-2017-report/ethanol-leads-credit-generation-for-california-low-carbon-fuel-standard ≤. [Google Scholar]
- Bloomfield S.F., et al. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am. J. Infect. Contr. Dec. 2007;35(10):S27–S64. [Google Scholar]
- Brady D., Pratt G.C. Volatile organic compound emissions from dry mill fuel ethanol production. J. Air Waste Manag. Assoc. 2007;57(9):1091–1102. doi: 10.3155/1047-3289.57.9.1091. [DOI] [PubMed] [Google Scholar]
- California Air Resources Board Low carbon fuel standard. https://ww2.arb.ca.gov/our-work/programs/low-carbon-fuel-standard/about
- Duerschner C., et al. Biofiltration of acetaldehyde resulting from ethanol manufacturing facilities. Chemosphere. Feb. 2020;241:124982. doi: 10.1016/j.chemosphere.2019.124982. [DOI] [PubMed] [Google Scholar]
- Eidman V.R. USDA. University of Illinois; 2007. Ethanol Economics of Dry Mills in Corn-Based Ethanol in Illinois and the US. Ch 3. [Google Scholar]
- Entech . 2020. 5800 SPDU VASE and Headspace Sorbent Pen.https://www.entechinst.com/support/5800spdu-for-vase-headspace-sorbent-pens Simi Valley, CA. [Google Scholar]
- Entech . 2020. 5600 Sorbent Pen Extraction System.https://www.entechinst.com/store/headspace-extraction-analysis/vacuum-assisted-sorbent-extraction/agitation-extraction-water-management/storeheadspace-extraction-analysis5600-sorbent-pen-extraction-system-3/ Simi Valley, CA. [Google Scholar]
- FDA . 2020. Temporary Policy for Manufacture of Alcohol for Incorporation into Alcohol Based Hand Sanitizer Products during the Public Health Emergency (COVID-19)”.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/temporary-policy-manufacture-alcohol-incorporation-alcohol-based-hand-sanitizer-products-during [Google Scholar]
- GOJO US PURELL hand sanitizer ingredients. 2021. https://www.gojo.com/en/Product-Catalog/Hand-Sanitizer/Ingredient-Transparency
- Haaz E., Fozer D., Toth J.A. Development of anhydrous ethanol purification: reduction of acetal content and vapor–liquid equilibrium study of the ethanol–acetal binary system. ACS Omega. 2021;6(2):1289–1298. doi: 10.1021/acsomega.0c04750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habe H. Chemical analysis of impurities in diverse bioethanol samples. J. Jpn. Petrol. Inst. 2013;56(6):414–422. [Google Scholar]
- Hach . Hach Company; Loveland, CO: 2013. Hach Company TNTplusTM Phosphorus – Spectrophotometric Measurement of Phosphorus in Water and Wastewater. [Google Scholar]
- Hach . Hach Company; Loveland, CO: 2013. DR 2800 User Manual. [Google Scholar]
- Hach . Hach Company; Loveland, CO: 2017. Chemical Oxygen Demand USEPA Method 10211. [Google Scholar]
- Hach . Hach Company; Loveland, CO: 2018. Persulfate Digestion Method. [Google Scholar]
- Haynes W.M. CRC Press LLC; 2015. CRC Handbook of Chemistry and Physics. [Google Scholar]
- Kelly S. Reuters; 20 Apr. 2020. As U.S. Fuel Demand Dries up, More Ethanol Producers Turn to Hand Sanitizer.https://www.reuters.com/article/us-usa-biofuels-sanitizer-idUSKBN22231G [Google Scholar]
- Marcus Y. Recommended methods for the purification of solvents and tests for impurities: methanol and ethanol. Pure Appl. Chem. 1985;57(6):855–864. [Google Scholar]
- McMurry John. ninth ed. Cengage Learning; 2019. Organic Chemistry. [Google Scholar]
- O'Neil M.J. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Royal Society of Chemistry. 2013 [Google Scholar]
- Onuki S., et al. Ethanol purification with ozonation, activated carbon adsorption, and gas stripping. Separ. Purif. Technol. 2015;151(Sept) 165–71. [Google Scholar]
- Reed L. University of Nebraska-Lincoln; 7 Apr. 2020. Hand Sanitizer for Health Care Produced at Nebraska Innovation Campus.https://news.unl.edu/newsrooms/today/article/hand-sanitizerfor- health-care-produced-at-nebraska-innovation-campus/ [Google Scholar]
- Research Dive . 2021. Impact Analysis of COVID-19 on Hand Sanitizer Market.https://www.researchdive.com/covid-19-insights/355/hand-sanitizer-market [Google Scholar]
- Schmidt, Roland, Sebastiano Licciulli, and Shahid Azam. "Process for removing impurities from acetic acid." U.S. Patent No. 10,758,838. 1 Sep. 2020.
- Summers Daniel R. Rectifier design for fuel ethanol plants. AIChE Annual Meeting. Nov. 2006:7. [Google Scholar]
- Tgarguifa A., et al. Energy efficiency improvement of a bioethanol distillery, by replacing a rectifying column with a pervaporation unit. Renew. Energy. July 2018;122:239–250. [Google Scholar]
- Tuladhar E., et al. Reducing viral contamination from finger pads: handwashing is more effective than alcohol-based hand disinfectants. J. Hosp. Infect. July 2015;90(3):226–234. doi: 10.1016/j.jhin.2015.02.019. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency US environmental protection agency RACT/BACT/LAER clearinghouse clean air technology center (2021) 2021. https://www.epa.gov/catc/ractbactlaer-clearinghouse-rblc-basic-information
- USEPA . US Environmental Protection Agency; 2018. Hazardous Air Pollutants.https://www.epa.gov/haps [Google Scholar]
- Voegele E. 8 Apr. 2020. Ethanol Plants Continue to Help Produce Hand Sanitizer.http://ethanolproducer.com/articles/17066/ethanol-plants-continue-to-help-produce-hand sanitizer [Google Scholar]
- World Health Organization . WHO Press; 2009. WHO Guidelines on Hand Hygiene in Health Care. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.