Abstract
This article reviews the diagnosis and treatment of infection with severe acute respiratory syndrome coronavirus 2, which causes coronavirus disease 2019, as well as a new inflammatory syndrome after severe acute respiratory syndrome coronavirus 2 infection, called multisystem inflammatory syndrome in children.
Keywords: COVID-19, Coronavirus, Multisystem inflammatory syndrome in children (MIS-C), Pediatric inflammatory multisystem syndrome temporally related to SARS CoV-2 (PIMS-TS)
Key points
-
•
Coronavirus disease 2019 in children is typically milder than adults; symptoms commonly include headache, fever, and cough.
-
•
Severe coronavirus disease 2019 has been reported in the pediatric population, typically in children with underlying conditions.
-
•
Multisystem inflammatory syndrome in children is a postinfectious sequela of severe acute respiratory syndrome coronavirus 2 infection that has prominent cardiovascular and gastrointestinal symptomatology.
-
•
There are patients with features of both severe acute coronavirus disease 2019 and multisystem inflammatory syndrome in children, suggesting that further work is needed to characterize this overlap and refine disease definitions.
Coronavirus disease 2019 in children and adolescents
In 2019, a novel coronavirus emerged called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19). Initially identified in Wuhan, China, COVID-19 spread internationally and became a global pandemic. Most pediatric COVID-19 cases were milder than in adults, but in the early spring of 2020, a new inflammatory syndrome emerged in children who had evidence of prior SARS CoV-2 infection, called multisystem inflammatory syndrome in children (MIS-C). This article describes the features, diagnosis, and treatment of pediatric COVID-19 and MIS-C based on the data available at the time of publication.
Incidence and Mortality Rates
As of March 2021, there were approximately 2,592,619 cases of COVID-19 in people under 18 in the United States and 300 deaths.1 Of all American cases, 2.1% were in children aged 0 to 4 years old, and another 10.2% were in those aged 5 to 17.1 Prevalence varies by age, with estimates ranging from 17% for children under 2 years old to 25% of children ages 6 to 10 years old, and 23% in 10 to 14 years old.2
The severity of the disease is generally lower for children, with only 1% to 5% of pediatric cases qualifying as severe versus to 10% to 20% in adults.3 This finding is thought to reflect the lower levels of angiotensin-converting enzyme 2 expression in alveolar cells, which is the mechanism by which SARS-CoV-2 enters cells.3 Likewise, mortality rates are estimated at 0.3% (95% confidence interval, 0.1–0.4) in patients under 21 years of age,2 in comparison with 5.8% for American adults.4 Being older than 12 years and having a high initial C-reactive protein (CRP) are risk factors for admission to a pediatric intensive care unit, and high CRP, leukocytosis, and thrombocytopenia are risk factors for organ dysfunction.5 Viral load and young age, specifically children under 1 year of age, are other risk factors for more severe disease.6
Clinical Features
Presenting symptoms in pediatric COVID-19 cases are variable (Table 1 ).2 Estimates of asymptomatic infection range from 13% to 50% of pediatric cases.2 The median time from exposure to onset of symptoms is 7 days.7 Of symptomatic cases, headache occurs in approximately two-thirds and fever and cough in about one-half.2 Gastrointestinal symptoms, sore throat, and rhinorrhea are rare,2 although patients with more severe COVID-19 experience gastrointestinal and upper respiratory symptoms.8
Table 1.
Symptoms in pediatric COVID-19 and MIS-C
| Symptom | Pediatric COVID-19 | MIS-C |
|---|---|---|
| Asymptomatic | 13%2 | 0% by definition |
| Fever | 55%2 | 100% by definition |
| Respiratory | Cough in 45%, dyspnea in 19%2 | 14%2 |
| Cardiovascular | N/Aa | 71%2 Shock in 35%, cardiac dysfunction in 40%, hypotension in 50%41 |
| Gastrointestinal | 6%2 | 87%2 Abdominal pain, vomiting, and diarrhea |
| Mucocutaneous | N/Ab | 73%2 Rash in 53%, conjunctivitis in 48%, mucocutaneous lesions in 35%41 |
| Neurologic | Headache in 67%2 | 22%2 |
The definition of severe COVID-19 in children varies, but includes requiring inpatient care and having at least 1 severe organ system manifestation and a positive reverse transcriptase polymerase chain reaction test for SARS CoV-2 infection.8 Among severe cases described in the United States Overcoming COVID-19 network, the majority (71%) had severe respiratory disease, whereas less than 3% had severe cardiovascular involvement and 9% had severe cardiorespiratory involvement. Of these patients, one-half required some form of respiratory support, including 15% on mechanical ventilation and 1.4% on extracorporeal membrane oxygenation.8 Neurologic manifestations were noted in 20% of patients in the same cohort.9 When considering both patients with severe COVID-19 and MIS-C with neurologic manifestations, 12% had potentially life-threatening complications, including encephalopathy, stroke, cerebral edema, demyelination, and Guillain–Barré syndrome.9
Comorbidities
Previously healthy children are susceptible to a severe COVID-19 course, including death.10 However, the majority of severe and/or hospitalized cases had comorbidities, such as asthma, immunosuppression, and neurologic disease.10, 11, 12 A history of prematurity, asthma, or diabetes; an immunocompromised state; and gastrointestinal disease are associated with an increased odds of admission.13 Furthermore, those with asthma and gastrointestinal disease are more likely to require respiratory support.13 Obesity is associated with a higher risk of severe COVID-19.12 Patients with chronic conditions may have a concomitant worsening of their underlying disease, including diabetic ketoacidosis and acute chest syndrome.5
Coinfection with other viruses and bacteria is an important consideration. A meta-analysis found that 5.6% of pediatric patients had a coinfection; within this group, 58% had Mycoplasma pneumoniae, 11.1% had influenza A or B, 9.7% had respiratory syncytial virus; the remainder had other common types of viral and bacterial infections.11
Thrombotic complications from COVID-19 such as deep vein thrombosis and pulmonary embolism were seen in 2.1% of pediatric cases in a multicenter retrospective cohort study in the United States, sometimes despite thromboprophylaxis.14
Laboratory evaluations
In a meta-analysis of pediatric cases, the most common laboratory findings were high ferritin and procalcitonin in approximately 25% and high CRP in approximately 20% of patients.2 The mean CRP among pediatric cases of COVID-19 is estimated at 9.4 mg/L.11 In contrast with adult cases, the leukocyte count was normal in around 70% of cases, with 15% each having leukopenia or leukocytosis.15 d-Dimer, IL-6, and creatinine kinase may also be elevated.11
Imaging
In reported pediatric cases, the chest radiographs were normal in roughly one-third, and another one-third showed focal consolidations; the remainder demonstrated ground glass opacities.2 , 11 A systematic review of chest computed tomography (CT) scans in pediatric cases found that 61.5% showed either consolidations or ground glass opacities; 26.5% were normal.16
Treatment
Patients with mild or moderate symptoms of COVID-19 often do well with supportive care alone. However, therapies such as monoclonal antibodies, antiviral therapy, glucocorticoids (GC), and immunosuppression may be indicated.
Monoclonal antibodies, such as bamlanivimab–etesevimab and casirivimab–imdevimab, have emergency use authorization through the US Food and Drug Administration in pediatric patients who present with mild to moderate disease and who are at high risk for progression to severe disease.17 , 18 High-risk conditions include obesity, chronic respiratory disease, chronic kidney disease, and immunocompromised states, such as those with rheumatologic disease who are on immunosuppressive therapy.
Antiviral therapy with remdesivir should be considered for patients with a positive SARS-CoV-2 polymerase chain reaction test and severe or critical manifestations of either COVID-19.
GC may be an option for pediatric patients who require respiratory support, but data are lacking.19 GC may also be considered for patients with concurrent acute respiratory distress syndrome, septic shock, or adrenal insufficiency.
Convalescent plasma has an emergency use authorization from the US Food and Drug Administration for adult patients who are critically ill with COVID-19,20 but its use has not been well-studied in pediatric patients with COVID-19.21 If considered, it should be administered early in the course of illness, especially if there is no improvement after remdesivir and GC, and for those patients with impaired humoral immunity.
Finally, antiplatelet and anticoagulation therapy should be considered to prevent thrombotic complications. Experts have recommended prophylactic low molecular weight heparin in children hospitalized for COVID-19 or MIS-C who are at higher risk for thrombosis, including elevated d-dimer, risk factors for severe SARS-CoV-2–related disease, or risk factors for venous thromboembolism, such as a family history, obesity, and chronic inflammatory conditions.22
Treatment resistance and complications
A subset of adult patients develop high levels of inflammation around the second week of COVID-19, termed COVID-19–associated hyperinflammatory syndrome.23 Frequent manifestations include fever, high ferritin, liver injury, hematologic abnormalities, coagulopathy, and high levels of inflammatory cytokines, specifically CRP and IL-6. There have been several adult trials for COVID-19–associated hyperinflammatory syndrome, primarily with tocilizumab, with conflicting results.24 , 25 The use of tocilizumab in pediatric patients with COVID-19 has not been studied formally. The American College of Rheumatology (ACR) has issued guidelines for pediatric patients with COVID-19 who develop hyperinflammation; recommendations include anakinra and consideration of other therapies with less evidence, including GC and tociliuzumab.26
Outcomes and Long-Term Recommendations
Data are still emerging on the long-term outcomes after COVID-19 in children. In a small case series from China, a repeat CT scan approximately 30 days after discharge showed that one-half of pediatric patients had imaging abnormalities, but dyspnea scores were all mild and improving, and no patients required oxygen.27
In adults, long COVID has been described with symptoms of headache, fatigue, dyspnea, and anosmia lasting for weeks to months after infection.28 The full spectrum of long COVID, or postacute sequelae of SARS-CoV-2 infection, has not yet been fully characterized in children, but efforts are underway.
Currently, messenger RNA vaccines against SARS-CoV-2 have been approved for patients age 12 and older; large pediatric trials are assembled with results expected by summer 2021. Vaccination is recommended for eligible people who have had COVID-19, but delaying vaccination for 90 days after the acute illness is recommended.
COVID-19 in Pediatric Rheumatic Diseases
The ACR has issued guidance regarding medication management in children with rheumatologic disease in the setting of the SARS-CoV-2 pandemic.29 Pediatric patients with chronic rheumatologic disease do not uniformly seem to be at a higher risk of COVID-19. In adults, there is a higher odds of death in those with moderate to high rheumatologic disease activity and certain medications, including GC, rituximab, and sulfasalazine.30 Similar data have not been reported in pediatric rheumatic diseases, but are being collected. To date, specific guidance has not been issued regarding SARS-CoV-2 vaccination in pediatric rheumatology patients, but the ACR has recommended holding certain immunosuppressives for adults with rheumatic disease around vaccination.31
Multisystem Inflammatory Syndrome in Children
MIS-C is a potentially life-threatening condition that can have acute, severe cardiovascular symptoms. It is presumably a post infectious phenomenon following SARS-CoV-2 infection and32 , 33 is considered on the spectrum of postacute sequelae of SARS-CoV-2 infection manifestations. This syndrome was first described with a cluster of children presenting with hyperinflammatory shock in London in mid-April of 2020.34 Soon thereafter, cases rose globally, prompting the Centers for Disease Control and Prevention (CDC) to define MIS-C in May.35 The case definition for MIS-C from the CDC, the World Health Organization (WHO),36 and Royal College of Pediatrics and Child Health37 are outlined in Table 2 .
Table 2.
Definitions of MIS-C and PIMS-TS
| Organization | Centers for Disease Control and Prevention | World Health Organization | Royal College of Pediatrics and Child Health |
|---|---|---|---|
| Population | Individuals aged <21 y | Individuals aged <20 y | Children |
| Clinical symptoms |
|
|
|
| Laboratory evidence of inflammation |
|
|
|
| SARS-CoV-2 testing |
|
|
|
| Evaluation of other diagnoses |
|
|
|
Abbreviations: ESR, erythrocyte sedimentation rate; RT-PCR, reverse transcriptase polymerase chain reaction test.
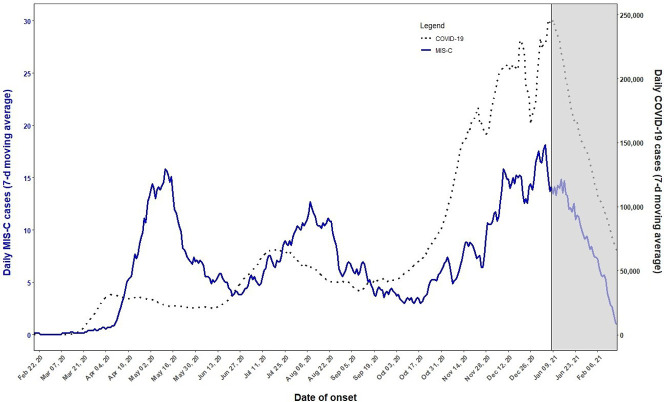
The timing of symptom onset is typically 3 to 6 weeks after exposure to SARS-CoV-2 (Fig. 1 ).38 The majority of patients with MIS-C did not have significant illness at the time of the inciting SARS-CoV-2 infection. Of note, this time frame is longer than that seen for hyperinflammatory states from COVID-19.
Fig. 1.
CDC daily MIS-C cases and COVID-19 cases (7-day moving average). The graph shows the 7-day average number of MIS-C and COVID-19 cases with MIS-C date of onset between February 19, 2020, and February 21, 2021. The grayed-out area on the right side represents the most recent 6 weeks of data, for which reporting of MIS-C cases is still incomplete.
(From Centers for Disease C. Daily MIS-C Cases and COVID-19 Cases (Seven-Day Moving Average). https://www.cdc.gov/mis-c/cases/index.html Published 2021. Accessed March 1, 2021.)
Incidence and Mortality Rates
As of early April 2021, there were 3185 cases of MIS-C in the United States and 36 deaths reported to the CDC.35 The incidence is low, with an estimated 2 in 200,000 people under 21 years of age.39 In a systematic review of global cases including 665 patients, 11 patients (1.7%) died.40
MIS-C is more commonly seen in males (59%) than females.38 The median age is between 7.3 and 10.0 years,40 , 41 and a similar illness can rarely be seen in adults.42 Patients with MIS-C aged 6 to 12 and 13 to 20 years were more likely than those aged 0 to 5 years to require intensive care.43 There is a greater incidence among patients identifying as racial or ethnic minorities, especially people with African, Afro-Caribbean, and Hispanic ancestries.26 , 40 In a meta-analysis, between 31% and 62% of patients identified as Black or Afro-Caribbean, and 36% to 39% as Hispanic.38 Intensive care unit admission is also more common among patients identifying as non-Hispanic Black, compared with non-Hispanic White.43 It is not clear whether these race-based differences are due to genetics, inequities in social determinants of health impacting exposure to SARS-CoV-2, or both.38 , 41 Obesity is common in patients with MIS-C, with roughly one-half of patients qualifying as overweight or obese based on body mass index.44
Clinical Features
Patients with MIS-C have fever and involvement of at least 2 organ systems. The most common symptoms are gastrointestinal, mucocutaneous, and cardiovascular (see Table 1).38 The mucocutaneous features and rash in MIS-C have invoked comparisons to Kawasaki disease.45 The rash is variable, and mucocutaneous symptoms occur quickly, within a mean of 2.7 days after start of fever.46 Roughly one-quarter to one-half of patients with MIS-C also met the criteria for Kawasaki disease, most commonly the incomplete presentation.26 , 40 Nearly one-quarter of patients have myocarditis, making hypotension and shock common presenting symptoms.40 Other cardiac complications include arrhythmia, left ventricular (LV) dysfunction, and coronary artery ectasia or aneurysms.6 Neurologic symptoms occur in 22%, including headache, altered mental status, and aseptic meningitis.9 , 40 In contrast with patients with acute COVID-19, rhinorrhea and cough are only seen in 13% and 7% of patients, respectively.44
The ACR has issued guidelines for a tiered diagnostic evaluation of MIS-C (Table 3 ).26 Hospitalization is recommended for patients with potential MIS-C, because patients are at risk for rapid evolution to severe illness. Hypotension, driven by either decreased LV function and/or vasodilatory shock, can require urgent intervention.8 , 32
Table 3.
ACR guidelines for diagnostic evaluation of MIS-C
| Patient Population | Testing |
|---|---|
|
|
|
|
Abbreviation: ESR, erythrocyte sedimentation rate.
Data from Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19. Version 2. Arthritis Rheumatol. 2020.
Differential diagnosis
A broad differential, including infection and malignancy, should be entertained when considering MIS-C, because many features are nonspecific and overlap with sepsis.26 Patients with MIS-C may have superimposed bacterial infections and should be covered empirically with antibiotics when indicated clinically.6
The presentation of MIS-C has similarities to other inflammatory conditions including Kawasaki disease, toxic shock syndrome, and macrophage activation syndrome. Among patients with Kawasaki-like features, 26% experienced shock, which is much higher than the 5% typically seen in US patients who present with Kawasaki disease shock syndrome.47 A British study compared pediatric patients with MIS-C with patients with Kawasaki disease with or without shock, and toxic shock syndrome.48 Patients with MIS-C were older (median age, 9.0 years vs 2.7 years), had more hematologic abnormalities, and higher elevations in CRP, troponin, and fibrinogen compared with those with Kawasaki disease. Compared with patients with toxic shock syndrome, patients with MIS-C were older with more profound anemia and higher CRP and alanine aminotransferase levels. Both patients with macrophage activation syndrome and MIS-C have cytopenias, hyperferritinemia, coagulopathy, and high soluble IL-2 receptor.49 However, increases in ferritin, high soluble IL-2 receptor, IL-18, and CXCL9 are less marked in MIS-C as compared with macrophage activation syndrome.49
Laboratory features
Inflammatory markers are elevated, including the erythrocyte sedimentation rate, CRP, and ferritin.40 The median initial CRP is higher among patients requiring pediatric intensive care unit admission and in those who develop organ dysfunction.5 Lymphopenia is common (58%), and approximately 20% have neutrophilia, anemia, or thrombocytopenia.40 A higher median initial white blood cell count and lower median initial platelet count were seen in patients who develop organ dysfunction.5 As for cardiac markers, around 40% have elevated B-type natriuretic peptide and more than 30% have high troponin.40 High B-type natriuretic peptide and troponin, and low platelets and lymphocytes are associated with need for intensive care.43 Coagulation studies commonly show elevations in d-dimer and fibrinogen. Cytokine levels are typically elevated for IL-6, IL-10, soluble IL-2 receptor, and tumor necrosis factor.26 , 49 , 50
After the initial evaluation, the erythrocyte sedimentation rate, CRP, B-type natriuretic peptide, and troponin T levels should be trended closely to monitor for development of sometimes rapidly progressive cardiac involvement and ongoing inflammation.26
Approximately 75% to 100% of patients have positive SARS-CoV-2 antibodies (IgG or IgM), whereas reverse transcriptase polymerase chain reaction testing is more variable, ranging from 13% to 69%.26 , 38 In 1 study, 7% had positive antibodies and reverse transcriptase polymerase chain reaction test, whereas another 15% had negative testing but had been exposed to family members who were positive.40
Imaging
Reports of lung imaging abnormalities have varied, with a meta-analysis demonstrating that 13.7% of patients with MIS-C had findings on radiographs or CT scans,40 which is less than in acute COVID-19. A recent study comparing severe acute COVID-19 versus MIS-C in a single large cohort (n = 1116) revealed similarities in rates of infiltrates on chest radiography, with 37% of acute severe COVID-19 versus 38% of patients with MIS-C having the finding.8
Echocardiography can reveal a decreased LV ejection fraction and coronary artery ectasia or aneurysm. Arrythmias have been reported, usually during the acute illness,51 and ACR guidelines recommend obtaining electrocardiograms every 48 hours during hospitalization and at follow-up visits, with escalation to telemetry and possible outpatient Holter monitoring for those with conduction abnormalities.26 Echocardiograms are likewise recommended at diagnosis and during subsequent follow-up, including 1 to 2 weeks and 4 to 6 weeks after presentation at a minimum.26 A cardiac CT scan may be needed to identify distal coronary artery aneurysms not seen on an echocardiogram. Cardiac MRI may be indicated for those with significant or persistent LV dysfunction.26
Treatment
The ACR guidelines for treatment of MIS-C recommend26 intravenous immunoglobulin (IVIG, 2 g/kg per ideal body weight), which may need to be administered slowly for those with abnormal cardiac function and/or volume overload. For those with moderate to severe symptoms, IVIG with GC is the first tier of therapy.26 GC can also be given for refractory disease at low-to-moderate doses of 1 to 2 mg/kg/d, with a taper over 2 to 3 weeks. For patients refractory to first-line therapy, pulse doses of methylprednisolone (10–30 mg/kg/dose) and/or anakinra can be considered. Observational studies have suggested that giving IVIG and GC together was superior to IVIG alone in regard to the resolution of fever within 2 days, need for second-line therapy or hemodynamic support, acute LV dysfunction after the initial therapy, duration of intensive care unit stay,52 and time to improvement of cardiac function.53
Antiplatelet therapy and anticoagulation may be indicated for patients with MIS-C.26 The ACR recommends low-dose aspirin of 3 to 5 mg/kg/d up to a maximum dose of 81 mg in those with features of Kawasaki disease, coronary artery aneurysm, or thrombocytosis. Anticoagulation should be started for patients with coronary artery aneurysm with a z-score of more than 10, and considered for those with LV ejection fraction of less than 35%. If vasopressor agents are required, epinephrine followed by norepinephrine is recommended; dobutamine may also be effective for those with severe myocardial dysfunction.6
Short-term outcomes and complications
The median hospital stay for patients with MIS-C is 7.9 ± 0.6 days.44 A study early in the pandemic noted that 80% of patients required intensive care unit admission.33 In a systematic review of 505 cases, more than one-half of patients (57%) required vasopressor agents, 26% required mechanical ventilation, and 5% required extracorporeal membrane oxygenation.54 Nearly 12% had acute kidney injury. Thromboses were rare, in only 3.5% of patients, but this finding was in the setting of more than one-half of patients receiving anticoagulation.
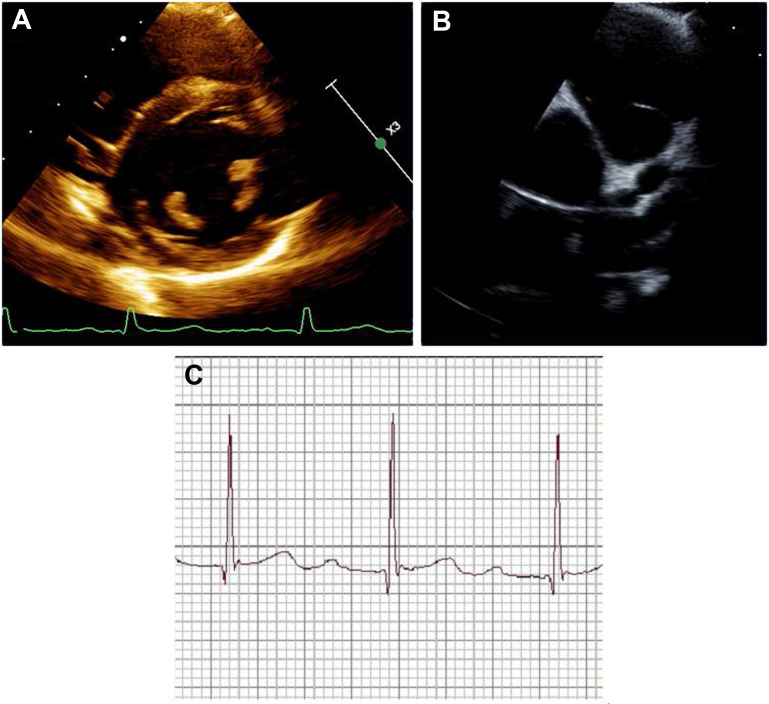
The cardiovascular burden in MIS-C is significant. In a European cohort study, the most common findings in MIS-C included shock, arrhythmias, pericardial effusion, and coronary artery aneurysm.32 More than one-half of the patients had a depressed LV ejection fraction and the majority had high troponin. A recent CDC report found that elevated CRP, ferritin, d-Dimer, and markers of cardiac injury (troponin, B-type natriuretic peptide, and pro B-type natriuretic peptide) were associated with decreased cardiac function in a cohort of more than 1000 children with MIS-C.43 Rates of coronary artery abnormalities have ranged from 13%8 to almost 19%.41 The majority of aneurysms reported to date have been small with z scores of 2.5 to 5.0 or greater,8 , 32 , 33 with very few giant aneurysms reported.48 First-degree atrioventricular block has also been noted in approximately 20% of patients with MIS-C in several small retrospective cohort studies,51 , 55 and can progress to second- or third-degree atrioventricular block.51 Short-term follow-up has not been studied extensively yet, but Feldstein and colleagues8 reported that decreased LV systolic function resolved in 91% (156 of 172 patients), as did the coronary artery aneurysms in 79% (45 of 57 patients) at 30 days of follow-up. An example of cardiac dysfunction on echocardiogram and first degree atrioventricular block on electrocardiogram are shown in Fig. 2 .
Fig. 2.
Cardiac dysfunction in MIS-C. (A) Short axis view of the bilateral ventricles. (B) Parasternal short axis showing the aortic valve and coronary aneurysms of the left main coronary artery and proximal left anterior descending artery. (C) Electrocardiogram with first-degree atrioventricular block and a PR interval of 280 ms.
(Courtesy of Audrey Dionne)
There are reports of venous thromboembolism in 6.5% of patients with MIS-C, compared with 2.1% with symptomatic and 0.7% of asymptomatic COVID-19 cases.14 This finding is thought to be related to abnormalities in the coagulation cascade. Risk factors include age greater than 12 years, cancer, and central venous catheters.14
Outcomes and Long-Term Recommendations
Studies are needed on the long-term outcomes and recommendations for monitoring for patients with a history of MIS-C. Patients should be followed by rheumatology, infectious disease, cardiology, hematology, and other subspecialties as needed based on disease manifestations.
Future directions
In May of 2020, the CDC definition of MIS-C was made deliberately broad to capture all cases of a newly emergent syndrome as well as the spectrum of illness in MIS-C. However, the definition likely needs to be refined and narrowed. Increasing seroprevalence to SARS CoV-2 owing to natural infection in addition to vaccination will also complicate the ability to make the diagnosis going forward. Last, the cause of the disproportionate burden of MIS-C in Black and Hispanic children and adolescents requires further investigation.
Summary and Discussion
The manifestations of COVID-19 are typically milder in children than adults, although severe cases can occur in otherwise healthy children and more commonly in those with underlying conditions. MIS-C is a postinfectious hyperinflammatory syndrome occurring weeks after SARS-CoV-2 exposure that has distinct clinical features including gastrointestinal, mucocutaneous, and cardiac symptoms, and is characterized by high levels of inflammation. More data is needed to define optimal treatments of both pediatric COVID-19 and MIS-C, as well as long-term outcomes.
Clinics care points
-
•
COVID-19 infection in children can be mild or asymptomatic. Patients at a greater risk for severe disease or complications include children under 1 year of age, as well as those with obesity, other chronic conditions and Black or Hispanic race/ethnicity.
-
•
Consider MIS-C in patients with previous COVID-19 or exposure who present with fever, high levels of inflammation, and gastrointestinal, mucocutaneous, and cardiovascular symptoms.
-
•
The criteria for MIS-C are broad. Alternative and comorbid diagnoses should be considered, including hyperinflammatory response to acute COVID-19, toxic shock syndrome and other bacterial infections, autoimmune disease, and malignancy.
Disclosure
The authors have nothing to disclose.
References
- 1.Centers for Disease Control . 2021. Demographic trends of COVID-19 cases and deaths in the US reported to CDC.https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fcases-in-us.html#demographics Available at: Accessed April 7, 2021. [Google Scholar]
- 2.Badal S., Thapa Bajgain K., Badal S., et al. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J Clin Virol. 2020;135:104715. doi: 10.1016/j.jcv.2020.104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabeerdoss J., Pilania R.K., Karkhele R., et al. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41(1):19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loomba R.S., Aggarwal G., Aggarwal S., et al. Disparities in case frequency and mortality of coronavirus disease 2019 (COVID-19) among various states in the United States. Ann Med. 2021;53(1):151–159. doi: 10.1080/07853890.2020.1840620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisler G., Izard S.M., Shah S., et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10(1):171. doi: 10.1186/s13613-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel N.A. Pediatric COVID-19: systematic review of the literature. Am J Otolaryngol. 2020;41(5):102573. doi: 10.1016/j.amjoto.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78(5):536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oualha M., Bendavid M., Berteloot L., et al. Severe and fatal forms of COVID-19 in children. Arch Pediatr. 2020;27(5):235–238. doi: 10.1016/j.arcped.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang A., Chorath K., Moreira A., et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsankov B.K., Allaire J.M., Irvine M.A., et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graff K., Smith C., Silveira L., et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40(4):e137–e145. doi: 10.1097/INF.0000000000003043. [DOI] [PubMed] [Google Scholar]
- 14.Whitworth H.B., Sartain S.E., Kumar R., et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138(2):190–198. doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry B.M., Lippi G., Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;58(7):1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 16.Katal S., Johnston S.K., Johnston J.H., et al. Imaging findings of SARS-CoV-2 infection in pediatrics: a systematic review of coronavirus disease 2019 (COVID-19) in 850 patients. Acad Radiol. 2020;27(11):1608–1621. doi: 10.1016/j.acra.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federal Drug Administration: Silver Spring, Maryland, USA . 2020. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab [press release] [Google Scholar]
- 18.Federal Drug Administration: Silver Spring, Maryland, USA . 2020. Fact sheet for health care providers emergency use authorization (EUA) of casirivimab and imdevimab [press release] [Google Scholar]
- 19.WHO. Rapid evidence appraisal for COVID-19 Therapies Working Group. Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fact sheet for healthcare providers: emergency use authorization (EUA) of COVID-19 convalescent plasma for treatment of hospitalized patients with COVID-19 [press release]; Silver Spring, Maryland, USA:Federal Drug Administration. 2020. [Google Scholar]
- 21.Diorio C., Anderson E.M., McNerney K.O., et al. Convalescent plasma for pediatric patients with SARS-CoV-2-associated acute respiratory distress syndrome. Pediatr Blood Cancer. 2020;67(11):e28693. doi: 10.1002/pbc.28693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldenberg N.A., Sochet A., Albisetti M., et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illness. J Thromb Haemost. 2020;18(11):3099–3105. doi: 10.1111/jth.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb B.J., Peltan I.D., Jensen P., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2(12):e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tocilizumab reduces deaths in patients hospitalised with COVID-19 [press release]; Oxford, United Kingdom: Nuffield Department of Population Health. 2021. [Google Scholar]
- 25.Okoh A.K., Bishburg E., Grinberg S., et al. Tocilizumab use in COVID-19-associated pneumonia. J Med Virol. 2021;93(2):1023–1028. doi: 10.1002/jmv.26471. [DOI] [PubMed] [Google Scholar]
- 26.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Version 2. Arthritis Rheumatol. 2020;2(11):1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C., Huang L., Tang X., et al. Pulmonary sequelae of pediatric patients after discharge for COVID-19: an observational study. Pediatr Pulmonol. 2021 doi: 10.1002/ppul.25239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahezi D.M., Lo M.S., Rubinstein T.B., et al. American College of Rheumatology guidance for the management of pediatric rheumatic disease during the COVID-19 pandemic: version 1. Arthritis Rheumatol. 2020;72(11):1809–1819. doi: 10.1002/art.41455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strangfeld A., Schafer M., Gianfrancesco M.A., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rheumatology ACo:Atlanta, Georgia, USA . 2021. COVID-19 vaccine clinical guidance summary for patients with rheumatic and Musculoskeletal diseases. [Google Scholar]
- 32.Valverde I., Singh Y., Sanchez-de-Toledo J., et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 33.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riphagen S., Gomez X., Gonzalez-Martinez C., et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Health department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States. Centers for Disease Control. 2021. https://www.cdc.gov/mis-c/cases/index.html Available at: Accessed April 4, 2021.
- 36.World Health Organization Multisystem inflammatory syndrome in children and adolescents with COVID-19. 2021. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available at: Accessed April 4th, 2021.
- 37.Royal College of Paediatrics and Child Health Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. 2020. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance Available at: Accessed April 4th, 2021.
- 38.Abrams J.Y., Godfred-Cato S.E., Oster M.E., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushik A., Gupta S., Sood M., et al. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020;39(11):e340–e346. doi: 10.1097/INF.0000000000002888. [DOI] [PubMed] [Google Scholar]
- 41.Godfred-Cato S., Bryant B., Leung J., et al. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrams J.Y., Oster M.E., Godfred-Cato S.E., et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed M., Advani S., Moreira A., et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young T.K., Shaw K.S., Shah J.K., et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol. 2020;157(2):207–212. doi: 10.1001/jamadermatol.2020.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 48.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee P.Y., Day-Lewis M., Henderson L.A., et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130(11):5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diorio C., Henrickson S.E., Vella L.A., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130(11):5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dionne A., Mah D.Y., Son M.B.F., et al. Atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics. 2020;146(5) doi: 10.1542/peds.2020-009704. e2020009704. [DOI] [PubMed] [Google Scholar]
- 52.Ouldali N., Toubiana J., Antona D., et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325(9):855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belhadjer Z., Auriau J., Meot M., et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. 2020;142(23):2282–2284. doi: 10.1161/CIRCULATIONAHA.120.050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aronoff S.C., Hall A., Del Vecchio M.T. The natural history of SARS-Cov-2 related multisystem inflammatory syndrome in children (MIS-C): a systematic review. J Pediatr Infect Dis Soc. 2020;9(6):746–751. doi: 10.1093/jpids/piaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi N.H., Fremed M., Starc T., et al. MIS-C and cardiac conduction abnormalities. Pediatrics. 2020;146(6) doi: 10.1542/peds.2020-009738. e2020009738. [DOI] [PubMed] [Google Scholar]