Abstract
Background
Few studies have assessed the association between hypertension and risk of detailed causes of death. We investigated the association between hypertension and all-cause mortality and 67 causes of death in a large cohort.
Methods
Multivariable Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for self-reported hypertension vs. no hypertension and mortality. Adults aged ≥18 years (n = 213798) were recruited in 1997-2004 and followed through December 31, 2006.
Results
During 5.81 years of follow-up, 11254 deaths occurred. Self-reported hypertension vs. no hypertension was associated with increased risk of all-cause mortality (HR = 1.25, 95% CI: 1.19-1.31) and mortality from septicemia (HR =1.66, 1.06-2.59), other infectious parasitic diseases (HR = 2.67, 1.09-6.51), diabetes mellitus (HR = 1.97, 1.45-2.67), circulatory disease (HR = 1.49, 1.37-1.61), hypertensive heart disease (HR = 3.23, 2.00-5.20), ischemic heart disease (HR = 1.35, 1.23-1.49), acute myocardial infarction (HR = 1.50, 1.27-1.77), other chronic ischemic heart diseases (HR = 1.35, 1.17-1.56), all other forms of heart disease (HR = 1.51, 1.21-1.89), primary hypertension and renal disease (HR = 3.11, 1.82-5.30), cerebrovascular disease (HR = 1.64, 1.37-1.97), other circulatory system diseases (HR = 1.71, 1.09-2.69), other chronic lower respiratory diseases (HR = 1.39, 1.12-1.73), other chronic liver disease (HR = 1.89, 1.06-3.37), renal failure (HR = 1.91, 1.33-2.74), motor vehicle accidents (HR = 1.60, 1.07-2.37), and all other diseases (HR =1.30, 1.10-1.54), but with lower risk of uterine cancer (HR = 0.37, 95% CI: 0.15-0.90) and Alzheimer's disease (HR = 0.65, 95% CI: 0.47-0.92).
Conclusion
Hypertension was associated with increased risk of all-cause mortality and 17 out of 67 causes of death, with most of these being circulatory disease outcomes, however, some of the remaining associations are unlikely to be causal. Further studies are needed to clarify associations with less common causes of death and potential causality across outcomes.
1. Introduction
Elevated blood pressure is a major risk factor for several cardiovascular disease outcomes [1, 2] and is the leading cause of death globally with an estimated 10.4 million deaths attributable to elevated systolic blood pressure in 2017 [3]. A positive association has been observed between elevated blood pressure or hypertension and a range of cardiovascular outcomes including ischemic heart disease [2], stable and unstable angina [1], myocardial infarction [1], sudden cardiac death [1, 4], ischemic stroke (1; 2), hemorrhagic stroke [1, 2], hypertensive heart disease [2], peripheral arterial disease [1], heart failure [1, 2], atrial fibrillation [5], abdominal aortic aneurysm [1, 2, 6], aortic dissection [7], and pulmonary embolism [2], as well as kidney disease [8, 9].
Whether hypertension is related to other diseases is less clear and has been less studied. Some studies have suggested associations between hypertension and certain cancers including endometrial [10] and kidney cancer [11–16], while a pooled analysis of Norwegian, Swedish, and Austrian cohort studies with 577000 participants suggested positive associations between elevated blood pressure and a range of cancers including cancers of the oral cavity and pharynx, esophagus, colon, liver, lung and larynx, corpus uteri, kidney, and melanoma as well as overall cancer [17]. Similar results were recently observed among 307000 participants in the EPIC study where an increased risk was observed for cancers of the mouth and pharynx, larynx, esophagus, lung, kidney, breast, and corpus uteri with elevated systolic blood pressure and/or diastolic blood pressure [18]. Some studies have also suggested hypertension may increase the risk of kidney disease [19].
Most previous studies on hypertension and chronic disease risk have focused on specific diseases or groups of diseases (such as cardiovascular diseases, cancer, or kidney disease), and we are not aware of any previous studies on hypertension and risk of very detailed causes of death across different disease groups. For this reason, we examined the association between self-reported hypertension and risk of mortality from all causes and 67 specific causes of death in the National Health Interview Study, to provide a more complete assessment of the potential adverse effects of hypertension across different causes of death.
2. Methods
2.1. Study Population
The National Health Interview Survey (NHIS) is a national cross-sectional survey, conducted annually by the National Center for Health Statistics in collaboration with the US Census Bureau since 1957. The study used a multistage sample design to monitor the health of the US civilian noninstitutionalized population. A total of 242952 men and women aged ≥18 years participating in the 8 waves conducted during 1997 to 2004 (linked to mortality data through December 2006) were included in the study. We excluded 360 respondents with missing data on hypertension and 28794 participants with history of coronary heart disease, stroke, or cancer at baseline, leaving 213798 participants for inclusion in the final analysis sample (Supplementary Figure 1). All data were based on self-reports and obtained via household roster section of the questionnaire completed by the participants. The design of the NHIS has been reviewed and approved by the Institutional Review Board at the Centers for Disease Control and Prevention. Written informed consent was obtained from all subjects. The current study was based on secondary analyses of publicly available and deidentified data [20].
2.2. Mortality
The outcomes included all-cause mortality and cause-specific deaths and were classified using the 10th revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-10). Deaths were identified by linkages to the National Death Index through 2006 [21]. Definitions of each outcome examined according to ICD-10 codes are found in Supplementary Table 1.
2.3. Assessment of Hypertension
Self-reported hypertension was assessed at the baseline questionnaire which included a question “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?”
2.4. Covariates
Covariates were selected a priori based on previous literature [22–28] and availability in the dataset. Baseline characteristics associated with hypertension and mortality were included as covariates from the survey and included demographic variables included age, sex, race (Hispanic, non-Hispanic White, non-Hispanic Black, and Other), education level (less than high school degree, high school degree, more than high school degree) and income level (low, middle, high), and lifestyle factors including BMI (<25, 25-<30, ≥30 kg/m2), leisure-time physical activity (inactive, insufficient, sufficient), smoking status (never, former, and current cigarette smokers), and alcohol intake (lifetime abstainer, former drinker, current drinker).
2.5. Statistical Analysis
Analyses accounted for the complex survey design employed in NHIS by utilizing sample weights, primary sampling, and clustering units via the Taylor series (linearization) method. Person-years of follow-up were calculated for each participant from the recruitment date to the date of death or end of the study period (31 December 2006). Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between hypertension and all-cause and cause-specific mortality were calculated using multivariable Cox proportional hazards regression model with adjustment for age, sex, race, education, income, BMI, leisure-time physical activity, alcohol, smoking status, and survey year. Missing data were handled by creating missing variable categories. The number of participants with missing data was low (1-4%) (Table 1). Analyses stratified by age, sex, ethnicity/race, alcohol, smoking status, and BMI were conducted to better be able to rule out residual confounding by these risk factors. Further sensitivity analyses were conducted by excluding the first 2 years of follow-up. The analyses were conducted using Stata 13.0 statistical software and R-version 3.3.3. All p values refer to two-tailed tests, and statistical significance was set at p < 0.05.
Table 1.
Baseline characteristics of participants with hypertension vs. participants without hypertension.
| No hypertension | Hypertension | ||||
|---|---|---|---|---|---|
| N | Percentage | N | Percentage | ||
| Total | 167105 | 80 | 46693 | 20 | |
| Age | 40.89 | 56.07 | |||
| Sex | |||||
| Men | 74619 | 49 | 18,627 | 45 | |
| Women | 92486 | 51 | 28,066 | 55 | |
| Race/ethnicity | |||||
| Hispanics | 31687 | 12 | 6,005 | 8 | |
| Non-Hispanic white | 107944 | 72 | 30,377 | 73 | |
| Non-Hispanic black | 20983 | 11 | 9,138 | 16 | |
| Non-Hispanic other | 6491 | 5 | 1,173 | 3 | |
| Education | |||||
| Less than high school | 30042 | 16 | 12,088 | 22 | |
| High school degree | 46781 | 29 | 14,645 | 33 | |
| More than high school | 89317 | 55 | 19,634 | 45 | |
| Missing | 965 | 1 | 326 | 1 | |
| Income | |||||
| Low | 25921 | 12 | 7,919 | 12 | |
| Middle | 83726 | 49 | 24,882 | 52 | |
| High | 57458 | 40 | 13,892 | 36 | |
| BMI | |||||
| <25 | 76423 | 46 | 11,926 | 25 | |
| 25- <30 | 55691 | 34 | 16,421 | 35 | |
| ≥30 | 29397 | 17 | 16,647 | 36 | |
| Missing | 5594 | 3 | 1,699 | 4 | |
| Physical activity | |||||
| Inactive | 61276 | 34 | 21,802 | 44 | |
| Insufficient activity | 31286 | 19 | 9,298 | 20 | |
| Sufficient activity | 69210 | 43 | 14,222 | 33 | |
| Missing | 5333 | 3 | 1,371 | 3 | |
| Smoking status | |||||
| Never | 94949 | 57 | 24,351 | 51 | |
| Former | 29891 | 18 | 12,691 | 28 | |
| Current | 41284 | 24 | 9,426 | 20 | |
| Missing | 981 | 1 | 225 | 0 | |
| Alcohol intake | |||||
| Never | 37249 | 22 | 12,336 | 24 | |
| Former | 20156 | 12 | 9,351 | 19 | |
| Current | 107249 | 65 | 24,462 | 55 | |
| Missing | 2451 | 1 | 544 | 1 | |
3. Results
From a total sample size of 242952 participants, we excluded 360 participants with missing data on hypertension and 28794 participants with prevalent coronary heart disease, stroke, or cancer at baseline. This left 213798 participants (92931 men and 120089 women) aged 18-85 years for inclusion in the current analysis (Table 1). Participants with self-reported hypertension had lower education, lower income, higher BMI, lower levels of physical activity, a higher prevalence of smokers, and lower prevalence of current drinkers when compared to participants without hypertension (Table 1).
During 1419143 person-years of follow-up (a mean [median] follow-up of 5.81 [5.88] years), 11254 deaths occurred. The most common causes of death were circulatory disease (n = 3821), cancer (n = 2361), ischemic heart disease (n = 2050), other chronic ischemic heart diseases (n = 957), acute myocardial infarction (n = 777), lung cancer (n = 733) and cerebrovascular disease (n = 618) (Table 2).
Table 2.
Hazard ratios and 95% confidence intervals (CIs) of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension.
| Total | No hypertension | Hypertension | ||||
|---|---|---|---|---|---|---|
| N (deaths) | N (deaths) | HR | N (deaths) | HR (95% CI) | p value | |
| All-cause mortality | 11254 | 6119 | 1.00 | 5135 | 1.25 (1.19-1.31) | <0.0001 |
| Infections | ||||||
| Septicemia | 140 | 66 | 1.00 | 74 | 1.66 (1.06-2.59) | 0.03 |
| Viral hepatitis | 28 | 18 | 1.00 | 10 | 1.54 (0.59-4.07) | 0.38 |
| Human immunodeficiency virus | 72 | 47 | 1.00 | 25 | 1.49 (0.66-3.36) | 0.33 |
| Other infectious parasitic disease | 31 | 13 | 1.00 | 18 | 2.67 (1.09-6.51) | 0.03 |
| Cancers | ||||||
| All cancers | 2310 | 1347 | 1.00 | 963 | 1.05 (0.95-1.17) | 0.36 |
| Oral cavity, pharynx, lip | 27 | 17 | 1.00 | 10 | 0.92 (0.40-2.14) | 0.85 |
| Esophagus | 55 | 31 | 1.00 | 24 | 1.37 (0.71-2.65) | 0.35 |
| Stomach | 53 | 32 | 1.00 | 21 | 0.94 (0.49-1.80) | 0.85 |
| Colon, rectum, anus | 215 | 125 | 1.00 | 90 | 1.07 (0.78-1.48) | 0.67 |
| Liver and bile ducts | 68 | 42 | 1.00 | 26 | 0.85 (0.45-1.61) | 0.62 |
| Pancreas | 150 | 81 | 1.00 | 69 | 1.06 (0.71-1.59) | 0.78 |
| Larynx | 20 | 13 | 1.00 | 7 | 0.80 (0.28-2.32) | 0.68 |
| Lung, trachea, bronchus | 733 | 424 | 1.00 | 309 | 1.20 (0.99-1.46) | 0.06 |
| Malignant melanoma | 25 | 17 | 1.00 | 8 | 0.62 (0.21-1.82) | 0.38 |
| Breast (females) | 128 | 79 | 1.00 | 49 | 0.83 (0.53-1.31) | 0.43 |
| Cervix uteri (females) | 19 | 11 | 1.00 | 8 | 0.88 (0.32-2.40) | 0.80 |
| Uterus (females) | 32 | 23 | 1.00 | 9 | 0.37 (0.15-0.90) | 0.03 |
| Ovaries (females) | 62 | 36 | 1.00 | 26 | 0.88 (0.50-1.55) | 0.66 |
| Prostate (males) | 86 | 49 | 1.00 | 37 | 0.89 (0.55-1.44) | 0.63 |
| Kidney and renal pelvis | 58 | 32 | 1.00 | 26 | 1.46 (0.72-2.95) | 0.30 |
| Bladder | 48 | 35 | 1.00 | 13 | 0.54 (0.25-1.14) | 0.11 |
| Brain, nervous system | 42 | 23 | 1.00 | 19 | 1.37 (0.66-2.82) | 0.40 |
| Hodgkin's disease | 8 | 6 | 1.00 | 2 | 0.91 (0.18-4.56) | 0.90 |
| Non-Hodgkin's lymphoma | 67 | 34 | 1.00 | 33 | 1.40 (0.77-2.54) | 0.27 |
| Leukemia | 88 | 57 | 1.00 | 31 | 0.77 (0.46-1.29) | 0.31 |
| Multiple myeloma | 37 | 20 | 1.00 | 17 | 0.77 (0.40-1.49) | 0.45 |
| All other and unspecified neoplasms | 289 | 160 | 1.00 | 129 | 1.19 (0.87-1.61) | 0.27 |
| In situ and benign neoplasms | 51 | 30 | 1.00 | 21 | 0.72 (0.39-1.33) | 0.29 |
| Endocrine, nutritional, metabolic diseases | ||||||
| Anaemia | 17 | 10 | 1.00 | 7 | 0.99 (0.24-4.15) | 0.99 |
| Diabetes mellitus | 319 | 113 | 1.00 | 206 | 1.97 (1.45-2.67) | <0.0001 |
| Malnutrition | 14 | 9 | 1.00 | 5 | 0.39 (0.12-1.28) | 0.12 |
| Nervous system | ||||||
| Parkinson's disease | 61 | 34 | 1.00 | 27 | 1.16 (0.66-2.05) | 0.61 |
| Alzheimer's disease | 207 | 128 | 1.00 | 79 | 0.65 (0.47-0.92) | 0.01 |
| Circulatory disease | ||||||
| All circulatory diseases | 3821 | 1756 | 1.00 | 2065 | 1.49 (1.37-1.61) | <0.0001 |
| Primary hypertension, renal disease | 115 | 36 | 1.00 | 79 | 3.11 (1.82-5.30) | <0.0001 |
| Hypertensive heart disease | 134 | 46 | 1.00 | 88 | 3.23 (2.00-5.20) | <0.0001 |
| Hypertensive heart and renal disease | 17 | 4 | 1.00 | 13 | 2.23 (0.64-7.77) | 0.21 |
| Ischemic heart disease | 2050 | 991 | 1.00 | 1059 | 1.35 (1.23-1.49) | <0.0001 |
| Acute myocardial infarction | 777 | 359 | 1.00 | 418 | 1.50 (1.27-1.77) | <0.0001 |
| Other acute ischemic heart diseases | 15 | 5 | 1.00 | 10 | 2.88 (0.85-9.72) | 0.09 |
| Atherosclerotic cardiovascular disease | 301 | 175 | 1.00 | 126 | 0.95 (0.72-1.27) | 0.74 |
| Other chronic ischemic heart diseases | 957 | 452 | 1.00 | 505 | 1.35 (1.17-1.56) | <0.0001 |
| Heart failure | 235 | 110 | 1.00 | 125 | 1.17 (0.85-1.61) | 0.33 |
| All other forms of heart disease | 480 | 226 | 1.00 | 254 | 1.51 (1.21-1.89) | <0.0001 |
| Cerebrovascular disease | 618 | 266 | 1.00 | 352 | 1.64 (1.37-1.97) | <0.0001 |
| Atherosclerosis | 30 | 14 | 1.00 | 16 | 1.64 (0.69-3.92) | 0.26 |
| Other diseases of the circulatory system | 142 | 63 | 1.00 | 79 | 1.71 (1.09-2.69) | 0.02 |
| Aortic aneurysm and dissection | 74 | 32 | 1.00 | 42 | 1.58 (0.89-2.80) | 0.12 |
| Other diseases of arteries or capillaries | 48 | 22 | 1.00 | 26 | 1.73 (0.72-4.15) | 0.22 |
| Other disorders of the circulatory system | 20 | 9 | 1.00 | 11 | 2.58 (0.71-9.30) | 0.15 |
| Respiratory diseases | ||||||
| Pneumonia | 239 | 137 | 1.00 | 102 | 0.94 (0.70-1.26) | 0.66 |
| Emphysema | 62 | 36 | 1.00 | 26 | 1.18 (0.65-2.13) | 0.59 |
| Other chronic lower respiratory diseases | 492 | 258 | 1.00 | 234 | 1.39 (1.12-1.73) | 0.003 |
| Pneumonitis from solids and liquids | 74 | 47 | 1.00 | 27 | 0.72 (0.40-1.28) | 0.26 |
| Other respiratory system diseases | 141 | 74 | 1.00 | 67 | 1.16 (0.75-1.80) | 0.49 |
| Digestive diseases | ||||||
| Alcoholic liver disease | 80 | 51 | 1.00 | 29 | 1.65 (0.88-3.08) | 0.12 |
| Other chronic liver diseases | 87 | 46 | 1.00 | 41 | 1.89 (1.06-3.37) | 0.03 |
| Cholelithiasis, gallbladder disease | 14 | 5 | 1.00 | 9 | 1.56 (0.42-5.80) | 0.51 |
| Urinary tract disease | ||||||
| Kidney failure | 186 | 71 | 1.00 | 115 | 1.91 (1.33-2.74) | 0.001 |
| Abnormal clinical, lab findings | 116 | 65 | 1.00 | 51 | 1.12 (0.75-1.68) | 0.58 |
| Transport injuries | ||||||
| Motor vehicle accidents | 173 | 126 | 1.00 | 47 | 1.60 (1.07-2.37) | 0.02 |
| Unintentional injuries | 1.00 | |||||
| Falls | 81 | 51 | 1.00 | 30 | 1.00 (0.54-1.87) | 0.99 |
| Other nontransport accidents combined | 186 | 130 | 1.00 | 56 | 1.20 (0.82-1.75) | 0.36 |
| Self-harm, interpersonal violence | 1.00 | |||||
| Suicide | 151 | 115 | 1.00 | 36 | 1.03 (0.62-1.73) | 0.90 |
| Homicide | 56 | 46 | 1.00 | 10 | 0.56 (0.25-1.26) | 0.16 |
| All other diseases (residual) | 910 | 464 | 1.00 | 446 | 1.30 (1.10-1.54) | 0.002 |
| All other causes/all unknown causes | 1135 | 826 | 1.00 | 309 | 1.00 (0.85-1.18) | 0.99 |
Multivariable adjustment for age, sex, education, race, income, alcohol, smoking status, BMI, physical activity, and survey year.
Participants with hypertension vs. no hypertension were at increased risk of all-cause mortality (HR = 1.25, 95% CI: 1.19-1.31) and mortality from septicemia (HR = 1.66, 1.06-2.59), other infectious parasitic diseases (HR = 2.67, 1.09-6.51), diabetes mellitus (HR = 1.97, 1.45-2.67), circulatory disease (HR = 1.49, 95% CI: 1.37-1.61), hypertensive heart disease (HR = 3.23, 95% CI: 2.00-5.20), ischemic heart disease (HR = 1.35, 1.23-1.49), acute myocardial infarction (HR = 1.50, 1.27-1.77), other chronic ischemic heart diseases (HR = 1.35, 1.17-1.56), all other forms of heart disease (HR = 1.51, 1.21-1.89), primary hypertension, renal disease (HR = 3.11, 1.82-5.30), cerebrovascular disease (HR = 1.64, 1.37-1.97), other diseases of the circulatory system (HR = 1.71, 1.09-2.69), other chronic lower respiratory diseases (HR = 1.39, 1.12-1.73), other chronic liver diseases (HR = 1.89, 1.06-3.37), renal failure (HR = 1.91, 1.33-2.74), motor vehicle accidents (HR = 1.60, 1.07-2.37), and all other diseases (HR = 1.30, 1.10-1.54), while inverse associations were observed for mortality from uterine cancer (HR = 0.37, 95% CI: 0.15-0.90) and Alzheimer's disease (HR = 0.65, 95% CI: 0.47-0.92) (Figure 1, Table 2). No significant association was observed between hypertension and risk of any other outcome; although, nonsignificant positive associations were observed for mortality from lung cancer (HR = 1.20, 95% CI: 0.99-1.46), other ischemic heart diseases (HR = 2.88, 95% CI: 0.85-9.72), aortic aneurysm and dissection (HR = 1.58, 95% CI: 0.89-2.80), and alcoholic liver disease (HR = 1.65, 95% CI: 0.88-3.08) (Figure 1, Table 2).
Figure 1.
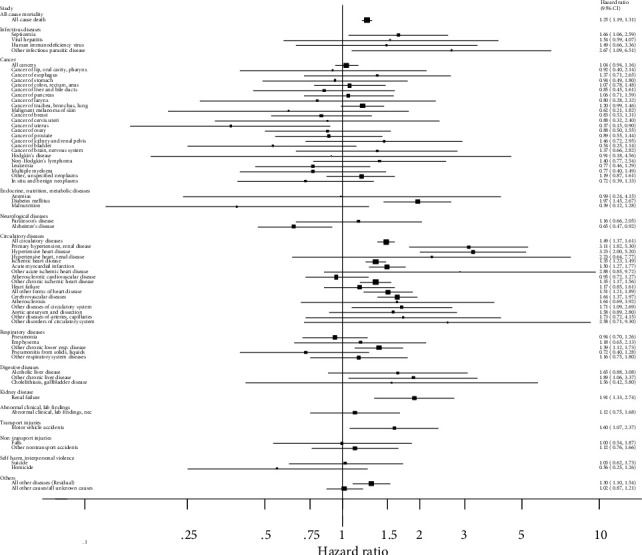
Hazard ratios and 95% confidence intervals for different causes of mortality in participants with hypertension vs. no hypertension.
In sensitivity analyses excluding first 2 years of follow-up, the association between hypertension and all-cause mortality was slightly strengthened (HR = 1.28, 95% CI: 1.21-1.35), and the associations with heart failure 1.51 (95% CI: 1.05-2.16), aortic aneurysm and dissection (HR = 2.47, 95% CI: 1.18-5.15), and kidney failure (HR = 2.23, 95% CI: 1.51-3.28) became stronger, while a few associations lost significance (septicemia, other infectious parasitic diseases, other chronic liver diseases, motor vehicle accidents, Alzheimer's disease) (Supplementary Table 2).
When results were stratified by age, the associations with all-cause mortality and mortality from circulatory disease, hypertensive heart disease, ischemic heart disease, acute myocardial infarction, other chronic ischemic heart diseases, heart failure, all other forms of heart disease, other diseases of the circulatory system, other diseases of arteries or capillaries, other diseases of the circulatory system, kidney failure, and all other diseases were stronger in the younger subjects (<65 years) than in the older participants (≥65 years) (Supplementary Table 3).
There were few differences of the association between hypertension and mortality when analyses were stratified by sex (Supplementary Table 4). The association between hypertension and all-cause mortality was slightly stronger in whites than in other ethnicities (Hispanics, non-Hispanic black, non-Hispanic others combined) (Supplementary Table 5).
The association between hypertension and all-cause mortality was stronger in more highly educated participants compared to participants with low or medium education (Supplementary Table 6). This was also observed for mortality from ischemic heart disease and for other chronic ischemic heart diseases, but for all other forms of heart disease, cerebrovascular disease, and other chronic liver diseases, the opposite was observed (Supplementary Table 6). The association between hypertension and all-cause mortality was stronger in participants with high income compared to participants with a low or medium income, but there were few differences for other outcomes (Supplementary Table 7). The association between hypertension and mortality from diabetes, renal failure, and motor vehicle accidents was restricted to overweight and/or obese participants (Supplementary Table 8). There were little differences in the association between hypertension and mortality when analyses were stratified by physical activity (Supplementary Table 9) and smoking status (Supplementary Table 10).
4. Discussion
In this analysis of 213798 participants from the National Health Interview Survey, we found statistically significant positive associations between hypertension and all-cause mortality and mortality from 17 out of 67 causes of death examined. Half of these causes of death were circulatory diseases, and in addition, increased risks were observed for mortality from certain infectious diseases, liver disease, respiratory disease, diabetes, kidney failure, motor vehicle accidents, and a group of all other diseases combined. Overall, the associations with all-cause mortality were stronger in younger than older participants, but similar in men and women, and slightly weaker in whites than in all other ethnicities combined. Associations were also stronger in participants with higher education and income, while few differences were observed when analyses were stratified by BMI, physical activity, and smoking status.
The current findings support some previous observations that elevated blood pressure is associated with increased risk of a large range of cardiovascular disease outcomes (1; 2; 4; 6) and all-cause mortality [29], but also raises the possibility that there may be associations with additional causes of death. The positive association between hypertension and all-cause mortality is probably to a large degree driven by the increased risk of multiple circulatory diseases, but some other causes of death may also have contributed to an elevated overall mortality.
Our findings of a 66% increased risk of mortality from septicemia among participants with hypertension are consistent with results from the REGARD study which found a 49% increased risk [30]. We also found an increased risk of mortality from other infectious parasitic diseases among hypertensive participants, but are not aware of previous studies on this outcome.
Our finding of no association between hypertension and cancer mortality is consistent with a Japanese cohort [29], but in contrast to some recent cohort studies which found positive associations between blood pressure and hypertension and incidence of cancer overall and of specific cancers (17; 18). However, we may also have had too low power to detect moderate or weak associations as most of the confidence intervals were wide. The HR for total cancer mortality was 1.04 (95% CI: 0.94-1.16) comparing hypertensives with non-hypertensives which is comparable to the findings from the EPIC study which reported a more precise HR of 1.03 (95% CI: 1.01-1.05) for total cancer incidence [18].
Our finding that hypertension is associated with increased mortality from diabetes is consistent with several other cohort studies [31–33], but not with a recent MR analysis which found no evidence for a causal association between hypertension and diabetes incidence [34]. Since hypertension is strongly associated with BMI [35] and since the association between hypertension and diabetes mortality was restricted to overweight and obese participants in the NHIS (HRs were 1.29, 2.31, and 2.85 for normal weight, overweight, and obese participants, respectively), the observed association between hypertension and diabetes mortality could be confounded by BMI.
In the current analysis, we found increased risk of all circulatory diseases combined, hypertensive heart disease, ischemic heart disease, acute myocardial infarction, other chronic ischemic heart diseases, all other forms of heart disease, primary hypertension/renal disease, cerebrovascular disease, and other diseases of the circulatory system and additionally for heart failure and aortic aneurysm and dissection after exclusion of the first 2 years of follow-up. For several other circulatory disease outcomes, associations were in the direction of increased risk, but did not reach statistical significance, possibly due to low numbers of deaths. These findings are consistent with previous studies which have consistently shown that hypertension increases the risk of a large number of circulatory diseases [1, 2, 4–7].
There was a positive association between hypertension and chronic lower respiratory disease mortality in the current analysis however, we are not aware of any previous studies which have investigated this association and given the lack of association with mortality from other respiratory diseases and the lack of plausible mechanisms; it is possible that this could have been a chance finding.
Although several studies have found that liver disease may increase blood pressure [36–38], other studies found that hypertension or elevated blood pressure was associated with increased risk of liver disease [39–41], and the latter results are consistent with our finding of an association between hypertension and increased risk of liver disease mortality [39]. Our finding of a positive association between hypertension and kidney disease mortality is consistent with several previous studies [8, 9]. The positive association between hypertension and mortality from motor vehicle accidents was restricted to obese participants, which might suggest confounding by obesity explains this association, as obesity has been associated with increased risk of fatal motor vehicle crashes [42]. The latter association was also attenuated and no longer significant after exclusion of the first two years of follow-up. The positive association with all other diseases could be due to additional diseases being linked to hypertension, misclassification of underlying cause of death, or simply be a chance finding.
A number of biological mechanisms could explain the positive association observed between hypertension and increased circulatory disease mortality. Hypertension induces endothelial dysfunction, exacerbates the atherosclerotic process, and makes the atherosclerotic plaque more unstable [43]. Hypertension also increases risk of left ventricular hypertrophy, which is a risk factor for coronary heart disease [44], heart failure [45], atrial fibrillation [46], and stroke [47]. Hypertension increases the risk of stroke by altering the endothelium and smooth muscle function in the intracerebral arteries, and the endothelial damage and altered blood cell endothelium interaction can lead to local thrombi formation and ischemic lesions.
Hypertension accelerates the atherosclerotic process, increasing the likelihood for cerebral lesions related to stenosis and embolism originating from large extracranial vessels, the aortic arch, and from the heart [48]. High blood pressure contributes to kidney failure by causing damage to the blood vessels that deliver blood to the kidneys, causing the arteries to narrow, weaken, or harden and ultimately failing in delivering sufficient blood to the kidneys [49].
The main limitation of our study is that we relied on self-reported data at baseline regarding hypertension status rather than measured blood pressure, and we were therefore not able to assess these associations across the full range of blood pressure in the population or take into account changes in hypertension status during follow-up. This is likely to have led to misclassification of the exposure, but given the prospective design of the study, such misclassification is most likely to have been non-differential and most likely would have led to attenuation toward the null or underestimation of the observed associations. Although we are not aware of a validation study of the self-reported hypertension data in the NHIS, a recent publication found a similar prevalence of hypertension in the NHIS as in the National Health and Nutrition Examination Survey, which had clinical measurements of blood pressure [50]. Although we adjusted for a range of important confounding factors including age, sex, education, income, alcohol, smoking, BMI, physical activity, and survey year, we cannot exclude the possibility of residual confounding or that additional confounders (e.g., dietary factors) could have influenced the results. We found that many of the observed associations persisted across categories of smoking, BMI, and physical activity, which might suggest that several of the observed associations are likely independent of these risk factors. However, the associations between hypertension and mortality from diabetes, renal failure, and motor vehicle accidents were only observed in the overweight and/or obese participants, and given previously documented associations between adiposity and these outcomes [42, 51, 52], it is possible that confounding by adiposity could explain these results. The duration of follow-up was relatively short, and the number of deaths was modest; so, we may not have had sufficient power to detect significant associations across all causes of death. In addition, given the multiple causes of death investigated, some of the observed associations could have been due to chance. However, the findings are still important as an indicator of outcomes that may need further study in other cohorts.
Strengths of this study include the prospective design and large nationally representative sample which allowed for analyses of detailed causes of death, many of which have been minimally studied previously. We adjusted for important confounding factors, and the results persisted in a number of stratified analyses. Many of the observed results, particularly for cardiovascular outcomes, are consistent with previous studies, lending some credibility to the observed associations.
In conclusion, we found that hypertension was associated with increased risk of all-cause mortality as well as mortality from 17 out of 67 specific causes of death that were examined. Approximately half of these causes were circulatory diseases and the remaining largely metabolic, renal and infectious diseases. Further studies with measured blood pressure are needed to confirm these findings and to assess causality across causes of death, but these results reinforce the important impact of elevated blood pressure across several causes of death.
Acknowledgments
This work has been supported by funding from the South-East Regional Health Authorities of Norway.
Data Availability
This study used publicly available data from the National Health Interview Survey, and the data are available online https://www.cdc.gov/nchs/nhis/index.htm and https://nhis.ipums.org/nhis/.
Disclosure
The funding sources had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
YW, WH, and JN had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis. DA and YW performed the study concept and design. DA, WH, JN, and YW performed the acquisition, analysis, or interpretation of data. Da performed the drafting of the manuscript. DA, WH, JN, and YW performed the critical revision of the manuscript for important intellectual content. DA and YW contributed to the obtaining funding.
Supplementary Materials
Supplementary Table 1: list of ICD codes for cause-specific mortality. Supplementary Table 2: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension excluding first 2 years of follow-up. Supplementary Table 3: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension stratified by age. Supplementary Table 4: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension stratified by sex. Supplementary Table 5: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension stratified by race/ethnicity. Supplementary Table 6: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension stratified by education. Supplementary Table 7: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension stratified by income. Supplementary Table 8: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension stratified by BMI. Supplementary Table 9: hazard ratios of all-cause mortality and cause-specific mortality participants with hypertension vs. no hypertension, stratified by physical activity. Supplementary Table 10: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension, stratified by smoking status.
References
- 1.Rapsomaniki E., Timmis A., George J., et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383(9932):1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H., Hibino M., Kobeissi E., Aune D. Blood pressure, hypertension and the risk of sudden cardiac death: a systematic review and meta-analysis of cohort studies. European Journal of Epidemiology. 2020;35(5):443–454. doi: 10.1007/s10654-019-00593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y. G., Han K. D., Choi J. I., et al. Impact of the duration and degree of hypertension and body weight on new-onset atrial fibrillation: a nationwide population-based study. Hypertension. 2019;74(5):e45–e51. doi: 10.1161/HYPERTENSIONAHA.119.13672. [DOI] [PubMed] [Google Scholar]
- 6.Kobeissi E., Hibino M., Pan H., Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. European Journal of Epidemiology. 2019;34(6):547–555. doi: 10.1007/s10654-019-00510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landenhed M., Engstrom G., Gottsater A., et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. Journal of the American Heart Association. 2015;4(1, article e001513) doi: 10.1161/JAHA.114.001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Seaghdha C. M., Perkovic V., Lam T. H., et al. Blood pressure is a major risk factor for renal death: an analysis of 560 352 participants from the Asia-Pacific region. Hypertension. 2009;54(3):509–515. doi: 10.1161/HYPERTENSIONAHA.108.128413. [DOI] [PubMed] [Google Scholar]
- 9.Gajalakshmi V., Lacey B., Kanimozhi V., Sherliker P., Peto R., Lewington S. Body-mass index, blood pressure, and cause-specific mortality in India: a prospective cohort study of 500 810 adults. The Lancet Global Health. 2018;6(7):e787–e794. doi: 10.1016/S2214-109X(18)30267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aune D., Sen A., Vatten L. J. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Scientific Reports. 2017;7(1):p. 44808. doi: 10.1038/srep44808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow W. H., Gridley G., Fraumeni J. F., Jr., Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. The New England Journal of Medicine. 2000;343(18):1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 12.Everatt R., Virviciute D., Tamosiunas A. Body mass index and other risk factors for kidney cancer in men: a cohort study in Lithuania. Central European Journal of Public Health. 2019;27(4):272–278. doi: 10.21101/cejph.a5080. [DOI] [PubMed] [Google Scholar]
- 13.Gelfond J., Al-Bayati O., Kabra A., Iffrig K., Kaushik D., Liss M. A. Modifiable risk factors to reduce renal cell carcinoma incidence: insight from the PLCO trial. Urologic Oncology. 2018;36(7):340.e1–340.e6. doi: 10.1016/j.urolonc.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Washio M., Mori M., Mikami K., et al. Cigarette smoking and other risk factors for kidney cancer death in a Japanese population: Japan collaborative cohort study for evaluation of cancer risk (JACC study) Asian Pacific Journal of Cancer Prevention. 2014;14(11):6523–6528. doi: 10.7314/apjcp.2013.14.11.6523. [DOI] [PubMed] [Google Scholar]
- 15.Choi M. Y., Jee S. H., Sull J. W., Nam C. M. The effect of hypertension on the risk for kidney cancer in Korean men. Kidney International. 2005;67(2):647–652. doi: 10.1111/j.1523-1755.2005.67137.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim C. S., Han K. D., Choi H. S., Bae E. H., Ma S. K., Kim S. W. Association of hypertension and blood pressure with kidney cancer risk: a nationwide population-based cohort study. Hypertension. 2020;75(6):1439–1446. doi: 10.1161/HYPERTENSIONAHA.120.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocks T., Van Hemelrijck M., Manjer J., et al. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and Cancer project. Hypertension. 2012;59(4):802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 18.Christakoudi S., Kakourou A., Markozannes G., et al. Blood pressure and risk of cancer in the European prospective investigation into cancer and nutrition. International Journal of Cancer. 2020;146(10):2680–2693. doi: 10.1002/ijc.32576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae E. H., Lim S. Y., Jung J. H., et al. Chronic kidney disease risk of isolated systolic or diastolic hypertension in young adults: a nationwide sample based-cohort study. Journal of the American Heart Association. 2021;10(7, article e019764) doi: 10.1161/JAHA.120.019764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blewett L. A., Rivera Drew J. A., King M. L., Williams K. C. W. IPUMS Health Surveys: National Health Interview Survey, Version 6.4. Minneapolis, MN: IPUMS; 2019. [Google Scholar]
- 21.National Center for Health Statistics. Linkage methods and analytical support for NCHS linked mortality data. May 2021, https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm.
- 22.Menotti A., Blackburn H., Kromhout D., Nissinen A., Adachi H., Lanti M. Cardiovascular risk factors as determinants of 25-year all-cause mortality in the seven countries study. European Journal of Epidemiology. 2001;17(4):337–346. doi: 10.1023/A:1012757616119. [DOI] [PubMed] [Google Scholar]
- 23.Jemal A., Thun M. J., Ward E. E., Henley S. J., Cokkinides V. E., Murray T. E. Mortality from leading causes by education and race in the United States, 2001. American Journal of Preventive Medicine. 2008;34(1):1–8.e7. doi: 10.1016/j.amepre.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen L. H., Rehnberg J., Dahl E., et al. Shape of the association between income and mortality: a cohort study of Denmark, Finland, Norway and Sweden in 1995 and 2003. BMJ Open. 2016;6(12, article e010974) doi: 10.1136/bmjopen-2015-010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aune D., Sen A., Prasad M., et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353 doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D. H., Rezende L. F. M., Ferrari G., et al. Physical activity and all-cause and cause-specific mortality: assessing the impact of reverse causation and measurement error in two large prospective cohorts. European Journal of Epidemiology. 2021;36(3):275–285. doi: 10.1007/s10654-020-00707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter B. D., Abnet C. C., Feskanich D., et al. Smoking and mortality--beyond established causes. The New England Journal of Medicine. 2015;372(7):631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 28.Wood A. M., Kaptoge S., Butterworth A. S., et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–1523. doi: 10.1016/S0140-6736(18)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi S. Personal past history and mortality in the Japan collaborative cohort study for evaluation of Cancer (JACC) Asian Pacific Journal of Cancer Prevention. 2007;8(Supplement):9–20. [PubMed] [Google Scholar]
- 30.Wang H. E., Shapiro N. I., Griffin R., Safford M. M., Judd S., Howard G. Chronic medical conditions and risk of sepsis. PLoS One. 2012;7(10, article e48307) doi: 10.1371/journal.pone.0048307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emdin C. A., Anderson S. G., Woodward M., Rahimi K. Usual blood pressure and risk of new-onset diabetes: evidence from 4.1 million adults and a meta-analysis of prospective studies. Journal of the American College of Cardiology. 2015;66(14):1552–1562. doi: 10.1016/j.jacc.2015.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M. J., Lim N. K., Choi S. J., Park H. Y. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertension Research. 2015;38(11):783–789. doi: 10.1038/hr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y. T., Song L., Liu X. X., et al. Time-cumulated blood pressure exposure and incident impairment of glucose tolerance and diabetes mellitus. BMC Cardiovascular Disorders. 2017;17(1):p. 106. doi: 10.1186/s12872-017-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D., Zhou T., Heianza Y., et al. Type 2 diabetes and hypertension. Circulation Research. 2019;124(6):930–937. doi: 10.1161/CIRCRESAHA.118.314487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayedi A., Rashidy-Pour A., Khorshidi M., Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obesity Reviews. 2018;19(5):654–667. doi: 10.1111/obr.12656. [DOI] [PubMed] [Google Scholar]
- 36.Patel S., Lawlor D. A., Ferreira D. L., et al. The association of nonalcoholic fatty liver disease with central and peripheral blood pressure in adolescence: findings from a cross-sectional study. Journal of Hypertension. 2015;33(3):546–553. doi: 10.1097/HJH.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwimmer J. B., Zepeda A., Newton K. P., et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9(11, article e112569) doi: 10.1371/journal.pone.0112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau K., Lorbeer R., Haring R., et al. The association between fatty liver disease and blood pressure in a population-based prospective longitudinal study. Journal of Hypertension. 2010;28(9):1829–1835. doi: 10.1097/HJH.0b013e32833c211b. [DOI] [PubMed] [Google Scholar]
- 39.Liu P., Tang Y., Guo X., et al. Bidirectional association between nonalcoholic fatty liver disease and hypertension from the Dongfeng-Tongji cohort study. Journal of the American Society of Hypertension. 2018;12(9):660–670. doi: 10.1016/j.jash.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Wu S. J., Zou H., Zhu G. Q., et al. Increased levels of systolic blood pressure within the normal range are associated with significantly elevated risks of nonalcoholic fatty liver disease. Medicine (Baltimore) 2015;94(19, article e842) doi: 10.1097/MD.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Li M., Zhao Z., et al. Ideal cardiovascular health is inversely associated with nonalcoholic fatty liver disease: a prospective analysis. The American Journal of Medicine. 2018;131(12):1515.e1–1515.e10. doi: 10.1016/j.amjmed.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Desapriya E., Giulia S., Subzwari S., et al. Does obesity increase the risk of injury or mortality in motor vehicle crashes? A systematic review and meta-analysis. Asia-Pacific Journal of Public Health. 2014;26(5):447–460. doi: 10.1177/1010539511430720. [DOI] [PubMed] [Google Scholar]
- 43.Escobar E. Hypertension and coronary heart disease. Journal of Human Hypertension. 2002;16(Supplement 1):S61–S63. doi: 10.1038/sj.jhh.1001345. [DOI] [PubMed] [Google Scholar]
- 44.Brown D. W., Giles W. H., Croft J. B. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. American Heart Journal. 2000;140(6):848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 45.Pandey A., Keshvani N., Ayers C., et al. Association of cardiac injury and malignant left ventricular hypertrophy with risk of heart failure in African Americans. JAMA Cardiology. 2019;4(1):51–58. doi: 10.1001/jamacardio.2018.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel N., O'Neal W. T., Whalen S. P., Soliman E. Z. Electrocardiographic left ventricular hypertrophy predicts atrial fibrillation independent of left ventricular mass. Annals of Noninvasive Electrocardiology. 2017;22(3):1–5. doi: 10.1111/anec.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aronow W. S., Ahn C., Kronzon I., Gutstein H. Association of left ventricular hypertrophy and chronic atrial fibrillation with the incidence of new thromboembolic stroke in 2,384 older persons. The American Journal of Cardiology. 1999;84(4):468–469. doi: 10.1016/S0002-9149(99)00336-7. A9. [DOI] [PubMed] [Google Scholar]
- 48.Johansson B. B. Hypertension mechanisms causing stroke. Clinical and Experimental Pharmacology & Physiology. 1999;26(7):563–565. doi: 10.1046/j.1440-1681.1999.03081.x. [DOI] [PubMed] [Google Scholar]
- 49.Bidani A. K., Griffin K. A. Pathophysiology of hypertensive renal damage. Hypertension. 2004;44(5):595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 50.Hsia J., Zhao G., Town M., et al. Comparisons of estimates from the behavioral risk factor surveillance system and other national health surveys, 2011-2016. American Journal of Preventive Medicine. 2020;58(6):e181–e190. doi: 10.1016/j.amepre.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 51.Bhaskaran K., Dos-Santos-Silva I., Leon D. A., Douglas I. J., Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. The Lancet Diabetes and Endocrinology. 2018;6(12):944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garofalo C., Borrelli S., Minutolo R., Chiodini P., De N. L., Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney International. 2017;91(5):1224–1235. doi: 10.1016/j.kint.2016.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: list of ICD codes for cause-specific mortality. Supplementary Table 2: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension excluding first 2 years of follow-up. Supplementary Table 3: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension stratified by age. Supplementary Table 4: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension stratified by sex. Supplementary Table 5: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension stratified by race/ethnicity. Supplementary Table 6: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension stratified by education. Supplementary Table 7: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension compared to participants without hypertension stratified by income. Supplementary Table 8: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension stratified by BMI. Supplementary Table 9: hazard ratios of all-cause mortality and cause-specific mortality participants with hypertension vs. no hypertension, stratified by physical activity. Supplementary Table 10: hazard ratios of all-cause mortality and cause-specific mortality among participants with hypertension vs. no hypertension, stratified by smoking status.
Data Availability Statement
This study used publicly available data from the National Health Interview Survey, and the data are available online https://www.cdc.gov/nchs/nhis/index.htm and https://nhis.ipums.org/nhis/.


