In this JCB issue, work from Yang et al. highlights how the ESCRT pathway localizes to the vacuole surface to execute protein sorting of its resident proteins.
Abstract
Lysosomes (vacuoles in yeast) are master regulators of metabolism and protein turnover, but how they degrade their own resident proteins is unclear. Recently, multiple models have been proposed explaining yeast vacuole protein sorting, but the role of the ESCRT pathway was unclear. In this JCB issue, work from Yang et al. (https://doi.org/10.1083/jcb.202012104) highlights how the ESCRT pathway localizes to the vacuole surface to execute protein sorting of its resident proteins.
Lysosomes are key metabolic organelles that influence nutrient sensing, protein trafficking, lipid homeostasis, and catabolic metabolism (1). Because of their many roles, how lysosomes receive and degrade proteins has been a pervasive question in cell biology, and has driven the discovery of multiple trafficking pathways that deliver proteins from different regions of the cell to the lysosome for turnover. In budding yeast, the vacuole (functionally equivalent to the lysosome) is a superb model to dissect the mechanisms of endolysosomal trafficking. However, how the vacuole/lysosome senses and degrades its own resident proteins has remained mysterious. This is a critical question, since the vacuole/lysosome surface is home to many nutrient and lipid transporters, which must be selectively maintained or degraded in response metabolic cues to enable cell homeostasis.
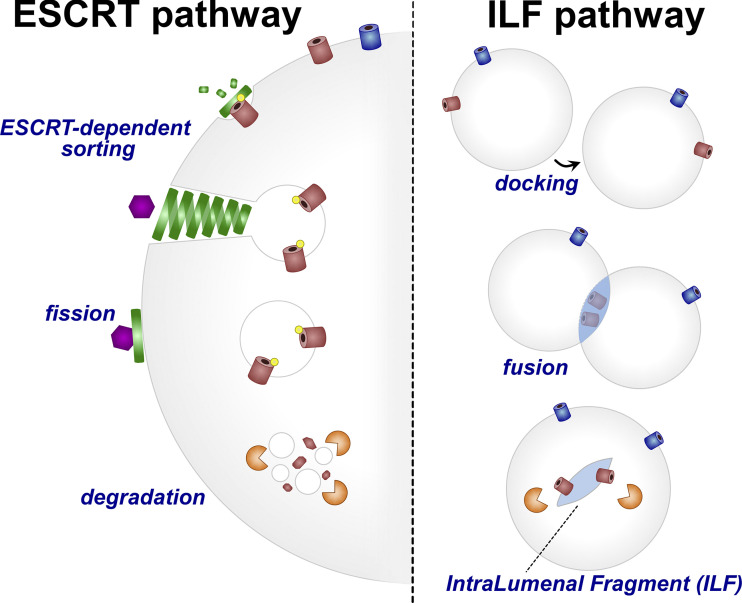
Recently, a flurry of papers were published with models explaining how the turnover of resident vacuole proteins is achieved. Two opposing models emerged (Fig. 1). In one, the ESCRT (endosomal sorting complexes required for transport) proteins, which are traditionally known to sort cargoes on the endosome surface into intralumenal vesicles that push into the endosome, were proposed to localize to the vacuole surface and execute a topologically similar protein sorting mechanism (albeit now on a different organelle; 2, 3, 4). However, another model called the intralumenal fragment (ILF) model proposed a radically different mechanism. Here, the homotypic fusion of the vacuole with itself could create a bubble-like fragment inside, containing vacuole surface proteins to be degraded. Critically, the formation of this fragment would be ESCRT-independent but require other membrane trafficking machinery like Rab7 (5, 6, 7). Paradoxically, both models were implicated in sorting the same vacuole proteins. Whether these two models were mutually exclusive, and how they related to one another, was unclear.
Figure 1.
Cartoon schematic of the models for resident vacuole protein turnover. Left: ESCRT-dependent sorting of vacuole proteins via recruitment of ESCRTs to ubiquitinated surface proteins, which are sorted into a vesicle that protrudes into the vacuole lumen and is degraded. Right: ILF pathway showing an ESCRT-independent selective sorting of proteins into a fragment, which via homotypic vacuole fusion is deposited into the vacuole lumen and then degraded.
In a recent paper published by JCB, Yang and colleagues (8) shed new light on the mystery of how resident vacuole proteins are degraded and resolve several of the issues between these two contrasting models. To begin, Yang et al. used Western blotting and a microfluidics-based imaging system to monitor GFP-tagged vacuole proteins. Capitalizing on a tetracycline (TET)-OFF system that enabled them to essentially conduct pulse-chase assays, they could monitor each GFP-tagged protein and its turnover kinetics following treatment with rapamycin, which initiated the degradation process. They learned that some proteins like Zrt3-GFP (a zinc transporter) were first sorted into foci on the vacuole surface, then a short time later accumulated in the vacuole lumen, consistent with a sorting process that enabled Zrt3-GFP breakdown by vacuolar proteases. Indeed, Western blotting revealed free GFP accumulation over time as the Zrt3-GFP was degraded, indicating the fusion protein was being broken down in the vacuole lumen, leaving behind soluble GFP.
Using this experimental setup, they next examined a vacuole protein previously reported to be a substrate of the ILF pathway, Fth1 (5). Fth1 had been proposed to be selectively sorted via the ILF pathway, whereas its binding partner Fet5 was not. However, Yang et al. were able to show that these proteins coimmunoprecipitated together, suggesting they exist in a complex and were unlikely to be separated. Indeed, deletion of Fth1 caused Fet5 to be trapped at the ER, consistent with it needing Fth1 for stability. Furthermore, time-lapse imaging indicated that Fth1 turnover was very slow, in contrast to earlier reports that Fth1 was constitutively turned over via the ILF pathway. Motivated by these observations, they next examined other ILF cargoes reported to be degraded by the ILF pathway following exposure of yeast to heat or the drug cycloheximide. Again capitalizing on their time-lapse imaging system and Western blotting, they failed to observe significant turnover of these proteins, in contrast to previous work.
A key difference between the two models is dependence on the ESCRT pathway. To further dissect, Yang et al. used imaging to interrogate how loss of ESCRT machinery impacted vacuole protein dynamics and degradation. By monitoring vacuole proteins whose turnover was stimulated by rapamycin, they observed that yeast lacking the ESCRT component Vps4 failed to sort Zrt3 and other cargoes into the vacuole. This argued that their degradation was ESCRT dependent, and likely not through the ILF pathway. Serendipitously, time-lapse imaging experiments also detected occasional ILF-like structures within the vacuole. However, imaging revealed that ILF structures were rare, and often contained the protein Zrc1-mCherry, which is stable and not degraded. Collectively, this argued that ESCRT machinery is required for the turnover of several vacuole proteins, and that the ILF system is likely not the predominant mechanism of resident vacuole protein degradation.
Finally, Yang et al. used their imaging platform to interrogate how ubiquitin and the ESCRTs influence vacuole protein turnover. They took advantage of an inducible degradation system called RapIDeg that uses an FK506-binding protein–FKBP Rapamycin-binding domain system to inducibly attach ubiquitin to a GFP-tagged vacuole protein by adding rapamycin. Strikingly, this revealed that within 10–30 min of ubiquitination, the vacuole protein was sorted into bright foci on the membrane surface that contained ESCRT machinery, strongly suggesting this protein sorting was ESCRT dependent.
Collectively, the data argue that many vacuole proteins are degraded via a ubiquitin and ESCRT-dependent pathway that operates on the vacuole surface in a manner topologically similar to the formation of multi-vesicular bodies at endosomes (Fig. 1). In this model, ubiquitination of vacuole proteins attracts ESCRT machinery, which bend the membrane away from the cytoplasm to create a vesicle within the vacuole, which is subsequently degraded by vacuolar proteases. Although ILFs were observed in this and previous studies, these structures are rare, and appear to lack the ability to selectively sort proteins.
It should be noted that this study and the earlier ILF pathway studies used very different methodologies. The ILF studies relied on purified vacuoles in a cell-free system (5, 7), whereas Yang et al. employed in vivo time-lapse imaging and blotting (8). Since purified vacuoles lack the full cellular ubiquitination machinery, it is feasible that ESCRT-independent membrane remodeling may occur in vitro. Relatedly, it is possible that the ILF system may sort other proteins not examined by Yang et al. However, Yang clearly establishes that several vacuole proteins are sorted via an ESCRT-dependent pathway that acts on the vacuole surface. Second, it is worth noting that ILF structures are observed in several studies of vacuole remodeling. Although likely not a major mechanism for protein sorting, these ILF structures may provide means for the vacuole to control its membrane composition and even size. Third, the work of Yang et al. adds to the continually growing list of roles for ESCRTs in cellular homeostasis. ESCRTs now have established roles at endosomes, the plasma membrane, the nuclear envelope, and yeast vacuole. This versatile machinery is continually used to execute its topologically unique membrane remodeling. This is important to understand, as experiments that perturb or block ESCRT function likely impact multiple cellular processes and have pleiotropic effects.
Given these insights, questions still remain. Although Yang et al. shed light on how the yeast vacuole consumes its proteins, how this system relates to mammalian lysosomes and how distinct resident proteins are selectively degraded or retained on the surface during metabolic cues remains to be explored. It is also unclear whether the ESCRT pathway, which utilizes several distinct complexes, operates completely the same on vacuoles as it does at other organelles. How ESCRTs function on vacuoles/lysosomes is of growing importance since ESCRTs have also been shown to facilitate micro-autophagy, delivering substrates such as lipid droplets into the vacuole lumen (9). These and other questions will no doubt drive further studies of the amazing yeast vacuole and mammalian lysosome, and how they organize and govern the lives of their many resident proteins.
Acknowledgments
I apologize for any references I omitted due to space constraints.
W.M. Henne is supported by funding from the National Institute of General Medical Sciences (GM119768), National Institute of Diabetes and Digestive and Kidney Diseases (DK126887), Welch Foundation (I-1873), the Ara Parseghian Medical Research Fund, and the University of Texas Southwestern Medical Center Endowed Scholars program.
The author declares no competing financial interests.
References
- 1.Lawrence, R.E., and Zoncu R.. 2019. Nat. Cell Biol. 10.1038/s41556-018-0244-7 [DOI] [PubMed] [Google Scholar]
- 2.Li, M., et al. 2015a. J. Cell Biol. 10.1083/jcb.201505062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li, M., et al. 2015b. Mol. Cell. 10.1016/j.molcel.2014.12.012 [DOI] [Google Scholar]
- 4.Zhu, L., et al. 2017. eLife. 10.7554/eLife.26403 [DOI] [Google Scholar]
- 5.McNally, E.K., et al. 2017. Dev. Cell. 10.1016/j.devcel.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 6.Karim, M.A., et al. 2018. Dev. Cell. 10.1016/j.devcel.2018.09.002 [DOI] [Google Scholar]
- 7.McNally, E.K., and Brett C.L.. 2018. Nat. Commun. 10.1038/s41467-018-07734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang, X., et al. 2021. J. Cell Biol. 10.1083/jcb.202012104 [DOI] [Google Scholar]
- 9.Oku, M., et al. 2017. J. Cell Biol. 10.1083/jcb.201611029 [DOI] [Google Scholar]