Background:
Food insecurity, or the lack of sufficient healthy food to sustain an active, healthy lifestyle, is associated with greater body weight in adults,1 especially underserved patients.2 However, the influence of food insecurity on the effectiveness of behavioral weight loss interventions is unknown.
Objective:
To examine if food security status modified patient responses to a high-intensity, lifestyle-based weight loss intervention via post-hoc analysis.
Methods and Findings:
The Promoting Successful Weight Loss in Primary Care in Louisiana (PROPEL) trial was a two-year cluster-randomized, two-arm pragmatic trial conducted in 18 primary care clinics across Louisiana to test the effectiveness of a high intensity, lifestyle-based obesity treatment program in an underserved population.3 After stratification by health system, clinics were randomized (1:1) to either an intensive lifestyle intervention (ILI) group or a usual care (UC) group.4 Patients were recruited from participating clinics and enrolled patients received the intervention to which their clinic was assigned. The ILI was delivered by trained health coaches embedded in the primary care clinics and consisted of weekly in-person sessions for the first 6 months followed by monthly sessions. The UC group received usual care from their primary care team. Full eligibility criteria, intervention descriptions, and assessment procedures for PROPEL have been described elsewhere.4 The Pennington Biomedical Research Center Institutional Review Board approved the study protocol and all patients provided written informed consent.
The modeled outcome was body weight (kilograms [kg]) at 6, 12, 18, and 24 months. Food insecurity was assessed using a 6-item instrument (Supplement); we categorized patients with two or more affirmative responses as food insecure. To assess intervention group differences in body weight between food secure and food insecure patients, a repeated-measures linear mixed-effects multilevel model with random cluster (clinic) effects was estimated. The model included intervention group, assessment time, food security status, a 3-way interaction term between intervention group*assessment time*food security status, baseline body weight, age, sex, race, and income as covariates. Analyses used SAS 9.4 (SAS Institute).
The Table presents baseline characteristics by food security status and study arm for 803 randomized patients (678 [84%] female; 540 [67%] African American). Among patients, 247 (31%) reported being food insecure, with 129 (29%) and 118 (34%) food insecure patients in the ILI and UC groups, respectively. The Figure shows body weight by food security status and study arm at each assessment time. The 3-way interaction term indicated effect modification (P<0.001) of food insecurity on body weight. At 24 months, subjects randomized to the ILI lost more weight than the UC group regardless of food security status; however, the ILI appeared less effective among those who were food insecure. The mean absolute weight difference between ILI and UC groups was 5.2 kg (95% CI 3.7, 6.8; P<0.001) among food secure patients and 2.7 kg (0.7, 4.8; P=0.009) among food insecure patients. The mean absolute weight difference between the ILI and UC groups was 2.5 kg (0.2, 4.8; P=0.030) lower among food insecure patients than food secure patients.
Table.
Baseline Characteristics of PROPEL Patients by Food Security Status and Usual Care and Intensive Lifestyle Intervention Groups
| Food Secure | Food Insecure | |||
|---|---|---|---|---|
| UC | ILI | UC | ILI | |
| No. of patients, mean (SD) | 233 | 323 | 118 | 129 |
| Body weight (kg), mean (SD) | 102.0 (17.2) | 101.0 (16.5) | 104.0 (16.6) | 103.1 (16.2) |
| BMI (kg/m2), mean (SD) | 36.9 (4.7) | 36.9 (4.5) | 37.9 (4.9) | 38.0 (4.7) |
| Age, mean (SD) | 51.2 (13.8) | 49.0 (13.0) | 48.1 (13.0) | 48.2 (11.9) |
| Female, n (%) | 177 (76.0) | 283 (87.6) | 103 (87.3) | 115 (89.2) |
| African American, n (%) | 121 (51.9) | 239 (74.0) | 87 (73.7) | 93 (72.1) |
| Health Literacy, n (%) | ||||
| ≤8th grade | 59 (25.3) | 91 (28.2) | 47 (39.8) | 50 (38.8) |
| ≥9th grade | 174 (74.7) | 232 (71.8) | 71 (60.2) | 79 (61.2) |
| Educationb, n (%) | ||||
| Less than HS | 13 (5.6) | 26 (8.1) | 11 (9.3) | 11 (8.5) |
| HS | 38 (16.4) | 69 (21.4) | 32 (27.1) | 39 (30.2) |
| Some college | 95 (41.0) | 121 (37.5) | 60 (50.9) | 58 (45.0) |
| Bachelor’s degree | 52 (22.4) | 58 (18.0) | 11 (9.3) | 13 (10.1) |
| Postgraduate degree | 34 (14.7) | 49 (15.2) | 4 (3.4) | 8 (6.2) |
| Income (annual family)c, n (%) | ||||
| < $10,000 | 29 (12.8) | 45 (14.2) | 41 (35.7) | 41 (31.8) |
| $10,000-$19,999 | 44 (19.5) | 61 (19.3) | 29 (25.2) | 34 (26.4) |
| $20,000-$39,999 | 47 (20.8) | 77 (24.4) | 32 (27.8) | 35 (27.1) |
| $40,000-$59,999 | 39 (17.3) | 55 (17.4) | 9 (7.8) | 14 (10.9) |
| ≥$60,000 | 67 (29.7) | 78 (24.7) | 4 (3.5) | 5 (3.9) |
| Marital Status, n (%) | ||||
| Married | 91 (39.1) | 146 (45.2) | 28 (23.7) | 35 (27.1) |
| Divorced/separated | 50 (21.5) | 73 (22.6) | 41 (34.8) | 47 (36.4) |
| Never married | 68 (29.2) | 82 (25.4) | 41 (34.8) | 40 (31.0) |
| Widowed | 24 (10.3) | 22 (6.8) | 8 (6.8) | 7 (5.4) |
UC: Usual care; ILC: Intensive lifestyle intervention.
Due to missing values, the denominators for waist circumference are 232 for Food Secure UC, 322 for Food Secure ILI, and 117 for Food Insecure UC.
Due to missing values, the denominator for education is 232 for Food Secure UC.
Due to missing values, the denominators for income are 226 for Food Secure UC, 316 for Food Secure ILI, and 115 for Food Insecure UC, respectively.
Figure.
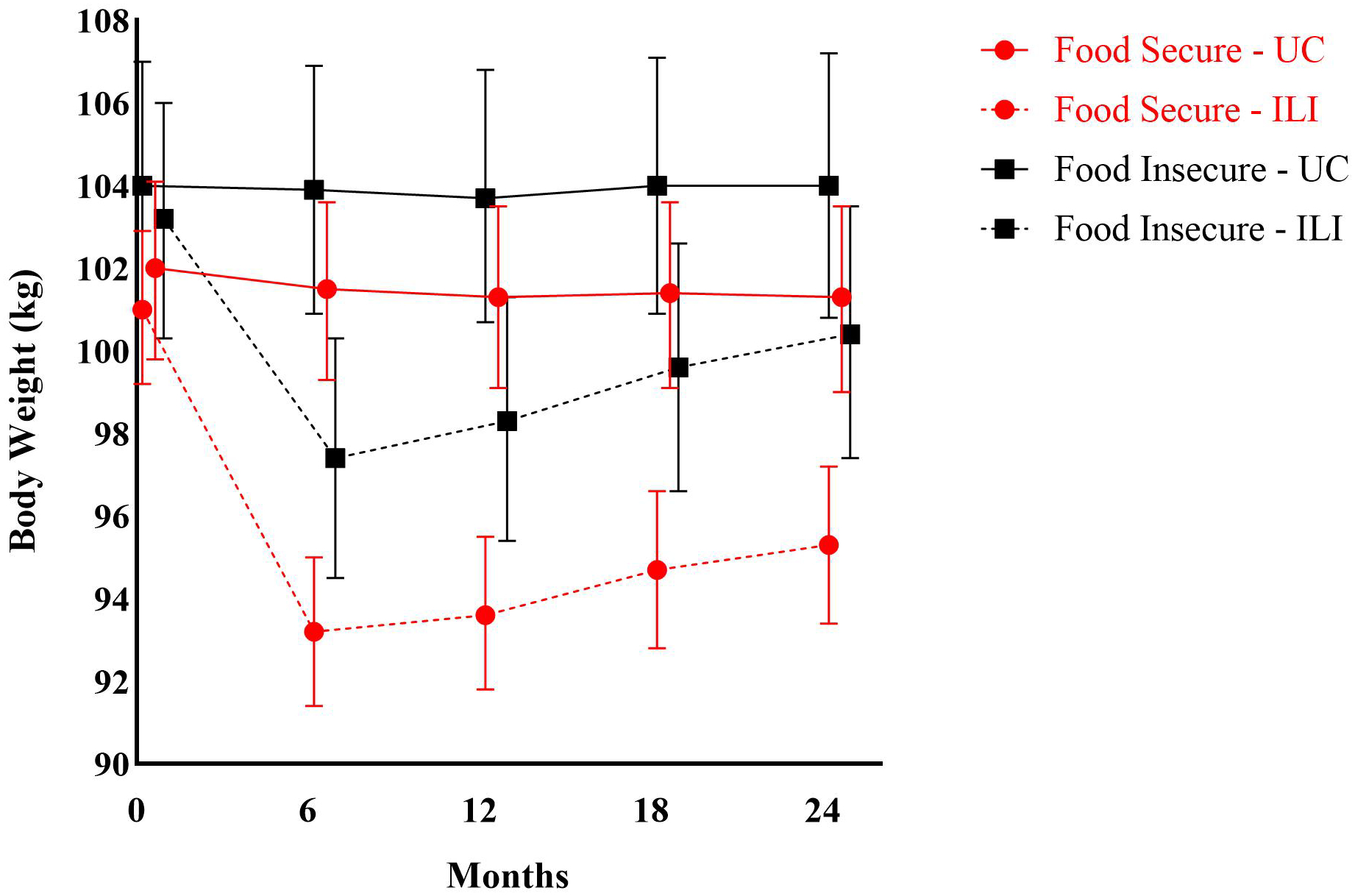
Body Weight of PROPEL Patients over Two Years by Food Security Status and Usual Care and Intensive Lifestyle Intervention Groups.
UC: Usual Care; ILI: Intensive Lifestyle Intervention.
Data are mean body weight (kg) and 95% confidence intervals and are derived from an unadjusted repeated-measures linear mixed-effects multilevel model with random cluster (clinic) effects and a 3-way interaction term between intervention group*assessment time*food security status.
Number of patients contributing data to the analysis at each time point:
0 Months=749 (Food Secure - UC: 226; Food Secure - ILI: 291; Food Insecure - UC: 115; Food Insecure - ILI: 117).
6 Months=726 (Food Secure - UC: 226; Food Secure - ILI: 277; Food Insecure - UC: 114; Food Insecure - ILI: 109).
12 Months=691 (Food Secure - UC: 215; Food Secure - ILI: 261; Food Insecure - UC: 111; Food Insecure - ILI: 104).
18 Months=673 (Food Secure - UC: 212; Food Secure - ILI: 254; Food Insecure - UC: 104; Food Insecure - ILI: 103).
24 Months=670 (Food Secure - UC: 207; Food Secure - ILI: 258; Food Insecure - UC: 101; Food Insecure - ILI: 104).
Discussion:
This study found that food insecurity moderated patient responses to high intensity, lifestyle-based obesity treatment. Relative to food secure patients, food insecure patients who received the ILI versus UC had smaller reductions in body weight over 24 months. While food insecure patients did experience weight loss in response to the ILI, their weight loss was blunted compared to food secure patients. Future research should identify mechanisms (e.g., psychological, physiological) that underlie this compromised response.
This study holds implications for physicians and healthcare practitioners working to address the growing obesity epidemic among US adults, especially among women, minorities, and low-income adults.5 Within the clinical care setting, screeners (e.g., 2-items) can identify food insecure patients. In turn, patients can be referred to support services (e.g., the charitable food system [food banks, food pantries], Federal nutrition assistance programs [Supplemental Nutrition Assistance Program]). Screening for food insecurity can also identify patients who face barriers (e.g., poor nutrition and diet quality, reduced medication adherence) and medical complications (e.g., emergency room visits, hospitalizations) that can compromise chronic disease management. Importantly, both food insecurity and obesity continue to increase in the US.1 In order to address effectively and equitably obesity prevention and treatment, tailored weight loss approaches that simultaneously address food insecurity and obesity are needed.
Supplementary Material
Acknowledgement
The authors would like to acknowledge the patients and stakeholders who are members of the PROPEL Patient Advisory Boards, Community Monitoring Board, and the Project Management Committee (Chris Lodge and Ava Zebrick, MSHCM), who significantly impacted the trial’s design and conduct. The authors are also indebted to the PROPEL patients, assessment technicians and health coaches, without whom this study would not have been possible. The authors gratefully acknowledge the contributions of Willie C. White III, MPH and the David Raines Community Health Centers, Dr. Gary Wiltz and the Teche Action Clinic sites, Michael G. Griffin and Dr. Robert Post and the Daughters of Charity Services of New Orleans, and the Ochsner Health System and Access Health Louisiana clinic sites. The authors also thank our Data and Safety Monitoring Board for monitoring patient safety and the overall conduct of the trial: Robert Ross, PhD (Chair), John Lefante, PhD, Michael Rolfsen, MD, and Chris Lodge.
The PROPEL Research Group includes: Pennington Biomedical Research Center: Peter T. Katzmarzyk, PhD (PI), Robert L. Newton, Jr., PhD (Outcomes Assessment Director), Corby K. Martin, PhD (Intervention Director), John W. Apolzan, PhD (Intervention Co-Director), William Johnson, PhD (Biostatistician), Kara D. Denstel, MPH (Project Manager), Emily F. Mire, MS (Data Manager), Robert K. Singletary, Jr., MHS, Cheryl Lewis, MPH, Phillip Brantley, PhD, Ronald Horswell, PhD, Betty Kennedy, PhD, Dachuan Zhang, MAppStats, Stephanie Authement, RD, LDN, MS, Shiquita Brooks, RDN, LDN, Danielle S. Burrell, M.Ed., MCHES, Leslie Forest-Everage, MA, Angelle Graham Ullmer, RDN, LDN, MS, Laurie Murphy, RDN, LDN, Cristalyn Reynolds, MA, Kevin Sanders, MS, RDN, LDN, Stephen Bower, MS, Daishaun Gabriel, MHA, Hillary Gahagan, MPH, Tabitha K. Gray, MA, Jill Hancock, MPH, Marsha Herrera, Brittany Molinere, Georgia Morgan, MA, Brittany Neyland, Stephanie Rincones, Deanna Robertson, MA, Ekambi Shelton, MPH, Russell J. Tassin, MS, Kaili Williams; Louisiana State University Health Sciences Center at New Orleans: Benjamin F. Springgate, MD; Louisiana State University Health Sciences Center at Shreveport: Terry C. Davis, PhD, Connie L. Arnold, PhD; Ochsner Health System: Eboni Price-Haywood, MD, Carl J. Lavie, MD, Jewel Harden-Barrios, MEd; Tulane University Medical School: Vivian A. Fonseca, MD, Tina K. Thethi, MD (Medical Monitor), Jonathan Gugel, MD; Xavier University: Kathleen B. Kennedy, PhD, Daniel F. Sarpong, PhD, Amina D. Massey. Study data were collected and managed using REDCap electronic data capture tools hosted at Pennington Biomedical. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding/Support:
Research reported in this article was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (OB–1402–10977). The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee. Additional support was provided by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health that funds the Louisiana Clinical and Translational Science Center, and NORC Grant # P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by National Institute of Diabetes and Digestive and Kidney Diseases. Our thanks also go to Health and Nutrition Technology (Carmel, CA) for providing us with the HealthOne formula and Nutrisystem (Fort Washington, PA) for providing us with the meal replacements used in the study. Louisiana State University (LSU), Pennington Biomedical, and Montclair State University have interest in the intellectual property surrounding the weight graph that was used in the intervention and CKM, among others, is an inventor of the technology. Licensing of that technology results in financial benefits to LSU, Pennington Biomedical, Montclair State University and the inventors.
Role of the Funder/Sponsor:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors, or Methodology Committee, and the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: The intervention used in the PROPEL trial included aspects of the SmartLoss® intervention, and the intellectual property associated with SmartLoss® is owned by Louisiana State University (LSU)/Pennington Biomedical Research Center (PBRC) and C. Martin is an inventor of the technology. LSU/PBRC and C. Martin benefit financially from the licensing of SmartLoss®.
Registration: ClinicalTrials.gov Identifier NCT02561221
References
- 1.Myers CA, Mire EF, Katzmarzyk PT. Trends in Adiposity and Food Insecurity Among US Adults. JAMA Netw Open. 2020;3(8):e2012767. Epub 2020/08/09. doi: 10.1001/jamanetworkopen.2020.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers CA, Martin CK, Newton RL Jr., Apolzan JW, Arnold CL, Davis TC, Price-Haywood EG, Katzmarzyk PT. Cardiovascular Health, Adiposity, and Food Insecurity in an Underserved Population. Nutrients. 2019;11(6). Epub 2019/06/30. doi: 10.3390/nu11061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Martin CK, Newton RL, Apolzan JW, Arnold CL, Davis TC, Price-Haywood EG, Denstel KD, Mire EF, Thethi TK, Brantley PJ, Johnson WD, Fonseca V, Gugel J, Kennedy KB, Lavie CJ, Sarpong DF, Springgate B. Weight Loss in Underserved Patients — A Cluster-Randomized Trial. N Engl J Med. 2020;383(10):909–18. Epub 2020/09/03. doi: 10.1056/NEJMoa2007448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzmarzyk PT, Martin CK, Newton RL Jr., Apolzan JW, Arnold CL, Davis TC, Denstel KD, Mire EF, Thethi TK, Brantley PJ, Johnson WD, Fonseca V, Gugel J, Kennedy KB, Lavie CJ, Price-Haywood EG, Sarpong DF, Springgate B. Promoting Successful Weight Loss in Primary Care in Louisiana (PROPEL): Rationale, design and baseline characteristics. Contemp Clin Trials. 2018;67:1–10. Epub 2018/02/07. doi: 10.1016/j.cct.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–50. Epub 2019/12/19. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


