Abstract
Background:
Acquired idiopathic stiffness (AIS) remains a common failure mode of contemporary TKAs. The current study investigated the incidence of AIS and manipulation under anesthesia (MUA) at a single institution over time, determined outcomes of MUAs, and identified risk factors associated with AIS and MUA.
Methods:
We identified 9,771 patients (12,735 knees) who underwent primary TKAs with cemented, modular metal-backed, posterior-stabilized implants from 2000 – 2016 using our institutional total joint registry. Mean age was 68 years, 57% were female, and mean BMI was 33 kg/m2. Demographic, surgical, and comorbidity data were investigated via univariate Cox proportional hazard models and fit to an adjusted multivariate model to access risk for AIS and MUA. Mean follow-up was 7 years.
Results:
During the study period, 456 knees (3.6%) developed AIS and 336 knees (2.6%) underwent MUA. Range of motion (ROM) increased a mean of 34° after the MUA; however, ROM for patients treated with MUA was inferior to patients without AIS at final follow-up (102° vs. 116°, p<0.0001). Significant risk factors included younger age (HR 2.3, p<0.001), increased tourniquet time (HR 1.01, p<0.001), general anesthesia (HR 1.3, p=0.007), and diabetes (HR 1.5, p=0.001).
Discussion:
Acquired idiopathic stiffness has continued to have an important adverse impact on the outcomes of a subset of patients undergoing primary TKAs. When utilized, MUA improved mean ROM by 34°, but patients treated with MUA still had decreased ROM compared to patients without AIS. Importantly, we identified several significant risk factors associated with AIS and subsequent MUA.
Keywords: Arthrofibrosis, Contracture, Stiffness, Manipulation Under Anesthesia (MUA), Range of Motion (ROM)
INTRODUCTION
Despite advancements in surgical technique, implant design, and perioperative management strategies such as pain control and mitigation of blood loss, acquired idiopathic stiffness (AIS) continues to be one of the most common complication following primary total knee arthroplasty (TKA) affecting approximately 4% of patients.[1–4] Initial management of patients with AIS includes physical therapy and manipulation under anesthesia (MUA).[5, 6] Unfortunately, some recalcitrant cases go on to more involved procedures including operative lysis of scar tissue (either arthroscopic or open) or revision arthroplasty to reduce pain and restore knee function.[5, 7]
Acquired idiopathic stiffness development likely constitutes host and perioperative factors.[4, 8, 9] While some risk factors such as limited preoperative motion, prior knee surgery, and suboptimal rehabilitative efforts have been consistently reported as increasing the risk of AIS, other patient factors (e.g. age and sex) and comorbidities (e.g. diabetes) have been associated inconsistently.[4–6, 8, 10–12] As the projected incidence of TKA procedures continues to rise, a greater understanding of the incidence and risk factors associated with this complication is desirable.[13–15]
The aims of the current study were to investigate the incidence of AIS and subsequent need for MUA at a single institution over time, describe the outcomes of MUA, and determine the risk factors associated with both AIS and need for MUA.
PATIENTS AND METHODS
Patients
A retrospective review of patients undergoing primary TKA from 1990 to 2016 was performed using our institutional total joint registry.[16] Patients included had undergone primary TKA with cemented, modular metal-backed, posterior-stabilized components, and all patellae were resurfaced. Primary TKAs performed for neoplastic causes were excluded. Patients were divided into contemporary (2000 – 2016) and historical (1990 – 1999) cohorts. The contemporary cohort was utilized to determine study outcomes, while the historical cohort was used only for a longitudinal comparison of the incidences of AIS and MUA, respectively. Institutional review board approval was obtained prior to initiation of the study.
During the contemporary study period, 9,771 patients (12,735 knees) met inclusion criteria. Within 2 years of the primary TKA, 236 patients died, 173 patients were revised, and 270 (3%) patients had less than 2 years of follow-up and were considered lost to follow-up. Among the remaining 9,092 patients, mean follow-up was 7 years (range, 2 – 20 years). Mean age was 68 years (range, 19 – 96 years), 57% were female, and mean body mass index (BMI) was 33 kg/m2 (range, 14 – 69 kg/m2). The three most frequent indications for primary TKA were osteoarthritis (88%), post-traumatic arthritis (10%), and arthritis associated with rheumatologic diseases (1.4%) (Table 1). Prior knee surgery was reported in 2,495 cases (20%). The three most commonly reported prior knee surgeries were meniscectomy in 713 cases (29%), open debridement for non-infectious indications in 531 cases (21%), and arthroscopic debridement in 512 cases (21%).
Table 1.
Study cohort demographics
| Variables | AIS (n=456) | No AIS (n=12,279) |
|---|---|---|
| Age at TKA (years), mean (SD) | 62 (11) | 68 (10) |
| BMI (kg/m2), mean (SD) | 32 (6) | 33 (7) |
| Female, No. (%) | 261 (57) | 7035 (58) |
| Indication for TKA, No. (%) | ||
| Osteoarthritis | 362 (7981.5) | 10851 (88.4) |
| Post-traumatic arthritis | 83 (18) | 1185 (9.6) |
| Rheumatoid arthritis | 3 (1) | 180 (1.5) |
| Other | 8 (2) | 67 (0.5) |
| Manipulation under anesthesia, No. (%) | 336 (74) | 0 (0) |
| Death within 2 years, No. (%) | 5 (1) | 231 (2) |
| Lost to follow-up before 2 years, No. (%) | 14 (3) | 298 (2) |
| Follow-up (years), mean | 7 | 7 |
AIS = acquired idiopathic stiffness; SD = standard deviation; BMI = body mass index; TKA = total knee arthroplasty
Total knee systems utilized included: Press Fit Condylar (P.F.C.) Sigma fixed-bearing (DePuy-Synthes; Warsaw, Indiana) in 6,007 TKAs (47%), Triathlon (Stryker; Mahwah, New Jersey) in 3,032 TKAs (24%), NexGen (Zimmer-Biomet; Warsaw, Indiana) in 1,503 TKAs (12%), P.F.C. Sigma Rotating Platform (DePuy-Synthes) in 1,116 TKAs (9%), Attune (DePuy-Synthes) in 459 TKAs (4%), Persona (Zimmer- Biomet) in 418 TKAs (3%), Genesis II (Smith & Nephew; Memphis, Tennessee) in 88 TKAs (1%), iBalance (Arthrex; Naples, Florida) in 60 TKAs (<1%), Vanguard (Zimmer- Biomet) in 39 TKAs (<1%), and Empowr (DJO Global; Dallas, Texas) in 13 TKAs (<1%).
Knee Society scores (KSSs) were obtained preoperatively and at 3 months, 2 years, 5 years, and every 5 years postoperatively thereafter per our institutional protocol. Acquired idiopathic stiffness was defined as arc of motion ≤ 90° persisting for ≥ 12 weeks as determined at clinical follow up within 1 year of the index TKAs or as motion ≤ 90° requiring MUA prior to 12 weeks.[4] Patients with pre-existing limitations of motion ≤ 90° were not characterized as having AIS. To provide a longitudinal comparison of AIS and MUA rates, the incidence of AIS and MUA were determined in the same manner for patients in the preceding decade of 1990 to 1999 at our institution. Patients that did not meet AIS criteria were considered the control cohort.
Statistical Analysis
Statistical analysis was performed using Statistical Analysis System (SAS) version 9.2 (SAS Institute Inc.; Cary, North Carolina). Group characteristics including the 17 variables that comprise the Charlson Comorbidity Index (CCI) were collected. Severity and age-weighted CCI was computed for each patient.[17] Categorical variables were summarized as counts and percentages. Continuous variables were summarized as means and standard deviations. Continuous variables were analyzed with unpaired Student’s t-tests. Categorical variables were analyzed with Chi-square tests. Individual associations between baseline characteristics of interest and AIS development were investigated by fitting several univariate Cox proportional hazards models. Hazard ratios (HRs) pertaining to time were evaluated at 1-minute increments for tourniquet and operative times. A select combination of these variables was chosen via best subset selection and used to fit an adjusted multivariate model containing the following variables: age, sex, BMI, unilateral versus bilateral TKA, prior knee surgery, smoking status, primary underlying diagnosis, tourniquet time, operative time, anesthesia type, hypertension, diabetes, peripheral vascular disease, moderate/severe liver disease, and metastatic solid tumor. This survival analysis process was repeated for MUA within 1 year as the event of interest, the selected multivariate model for which contained many of the same variables as for the AIS outcome. The only difference was that moderate/severe renal disease was included as a factor in the model for MUA, while moderate/severe liver disease and metastatic solid tumor were not used. The potential for multicollinearity between the selected variables was assessed with the variance inflation factor (VIF), and no evidence of serious multicollinearity was found with all VIF values less than 3. Significance was set at p< 0.05.
RESULTS
Incidence of AIS and MUA
From 2000 to 2016, 456 of 12,735 knees (3.6%) developed AIS. In the preceding decade (1990 – 1999), 129 of 3,141 knees (4.1%) were affected. There was no difference in the incidence of AIS between the contemporary and historical cohorts (p=0.16). MUA was utilized in 336 of 12,735 TKAs (2.6%) from 2000 to 2016. In the decade prior (1990 – 1999), MUA was performed in 89 of 3141 TKAs (2.8%). There was no difference in the incidence of MUA between the contemporary and historical cohorts (p=0.54).
Preoperatively, patients who went on to develop AIS had a small and non-clinically relevant reduction in ROM compared to patients that did not go on to develop AIS (104° ± 17° vs. 108° ± 16°, respectively; p=0.01). Postoperatively, patients diagnosed with AIS demonstrated decreased ROM compared to those without AIS at most recent follow-up (100° ± 20° vs. 116° ± 12°, respectively; p<0.0001).
The KSSs in patients who developed AIS increased from 39 ± 19 preoperatively to 79 ± 18 postoperatively (p<0.0001) and from 40 ± 19 preoperatively to 86 ± 12 postoperatively (p<0.0001) in patients without AIS. The KSSs for patients with AIS were inferior to those without at most recent follow-up (p<0.0001).
There was no difference in the preoperative range of motion among patients with AIS treated with MUA compared to those with AIS not treated with MUA (105° ± 17° vs. 102° ± 17°, p=0.10). For patients treated with MUA, mean pre-MUA ROM was 67° ± 16° which improved a to a mean of 102° ± 19° at most recent follow-up. Range of motion for patients treated with MUA was inferior to that of controls at final follow-up (102° ± 19° vs. 116° ± 12°, respectively; p<0.0001). MUA was performed at a mean of 6 weeks (range, 1 – 32 weeks) postoperatively. A second MUA was utilized in 16 patients at a mean of 5 weeks following the first intervention (range, 1 – 15 weeks). Mean ROM prior to the second MUA was 68° ± 21° and ROM at most recent follow-up was 105° ± 11° in this subset of patients. Likewise, ROM among patients treated with a second MUA was inferior to that of controls at final follow-up (105° ± 11° vs. 116° ± 12°, respectively; p<0.0001). Overall, MUA was successful in restoring the arc of motion to ≥ 90° in 295 of 336 patients (88%). For the entire MUA cohort, KSSs improved from a mean of 41 ± 20 preoperatively to a mean of 79 ± 19 postoperatively (p <0.0001). Similar to those with AIS, the KSSs for patients who were treated with an MUA were inferior to those not treated with an MUA (79 ± 19 vs. 85 ± 12, respectively; p<0.0001). There were no complications such as periprosthetic fractures or extensor mechanism disruptions associated with any MUAs.
Of the 336 patients who underwent an MUA, four patients later were treated with a revision TKA for AIS at a mean of 2 years after the primary TKA (range, 0.5 – 5 years). There were two modular revisions combined with a lysis of adhesions (LOA), whereas the other two patients had non-modular revisions and a LOA, including one femoral component revision, and one both component revision. At final follow-up, the mean ROM in these four patients treated with a revision TKA was 80° (range, 60° – 90°).
Univariate Analysis
Demographics, intraoperative factors, and comorbidities were compared among patients with and without AIS by univariate cox hazard analysis. The risk of AIS development was associated with a number of factors (Table 2). Patient factors associated with an increased risk of AIS included younger age at time of primary TKA (HR 2.5; 95% confidence interval (CI) 2.0 – 3.0; p<0.001), smoking (HR 1.7; 95% CI 1.2 – 2.4; p=0.004), and a history of prior knee surgery (HR 1.6; 95% CI 1.4 – 2.0; p<0.001). Additionally, risk of AIS development was associated with intraoperative variables including longer tourniquet times (p<0.001) and longer operative time (p<0.001). Of the operative factors evaluated, administration of general anesthesia was associated with greatest risk for AIS (HR 1.5; 95% CI 1.2 – 1.8; p<0.001). Patients with increased medical complexity, as evident by CCI score, were associated with reduced risk of AIS development (p<0.001) (Table 2).
Table 2.
Univariate and multivariate analysis of variables associated with acquired idiopathic stiffness
| Variable | Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Patient Characteristics | ||||||
| Sex | ||||||
| Female | 1.00 | 0.83 – 1.21 | 0.99 | 1.07 | 0.87 – 1.32 | 0.53 |
| Male (reference) | ||||||
| Age | ||||||
| < 65 years | 2.46 | 2.04 – 2.96 | <0.001 | 2.34 | 1.86 – 2.93 | <0.001 |
| ≥ 65 years (reference) | ||||||
| Body mass index | ||||||
| ≥ 30 kg/m2 | 0.93 | 0.77 – 1.12 | 0.43 | 0.80 | 0.64 – 0.99 | 0.04 |
| < 30 kg/m2 (reference) | ||||||
| Indication for TKA | ||||||
| Post-traumatic arthritis | 2.09 | 1.65 – 2.66 | <0.001 | 1.37 | 1.02 – 1.83 | 0.04 |
| Osteoarthritis (reference) | ||||||
| Smoking status | ||||||
| Current | 1.67 | 1.18 – 2.36 | 0.004 | 0.94 | 0.62 – 1.42 | 0.76 |
| Never (reference) | ||||||
| Prior knee surgery | ||||||
| Yes | 1.64 | 1.35 – 1.99 | <0.001 | 1.08 | 0.85 – 1.37 | 0.53 |
| No (reference) | ||||||
| Surgical Characteristics | ||||||
| Unilateral or Bilateral TKAs | ||||||
| Simultaneous bilateral | 0.66 | 0.46 – 0.96 | 0.03 | 0.78 | 0.70 – 0.88 | <0.001 |
| Staged bilateral | 0.45 | 0.33 – 0.61 | <0.001 | – | – | – |
| Unilateral (reference) | ||||||
| Anesthesia | ||||||
| General | 1.47 | 1.22 – 1.77 | <0.001 | 1.34 | 1.08 – 1.65 | 0.007 |
| Neuraxial (reference) | ||||||
| Tourniquet time, min | 1.01 | 1.01 – 1.01 | <0.001 | 1.01 | 1.00 – 1.01 | <0.001 |
| Operative time, min | 1.00 | 1.00 – 1.00 | <0.001 | 1.00 | 1.00 – 1.00 | 0.76 |
| Comorbidities^ | ||||||
| Diabetes | 1.17 | 0.94 – 1.45 | 0.17 | 1.53 | 1.20 – 1.96 | 0.001 |
| Hypertension | 0.57 | 0.47 – 0.69 | <0.001 | 0.78 | 0.62 – 0.97 | 0.03 |
| Peripheral vascular disease | 0.60 | 0.46 – 0.77 | <0.001 | 0.76 | 0.57 – 1.02 | 0.07 |
| Liver disease† | 1.29 | 0.73 – 2.29 | 0.38 | 1.61 | 0.90 – 2.87 | 0.11 |
| Metastatic solid tumor | 0.77 | 0.45 – 1.31 | 0.33 | 1.05 | 0.60 – 1.84 | 0.86 |
| Congestive heart failure | 0.61 | 0.42 – 0.88 | 0.01 | – | – | – |
| Renal disease† | 0.64 | 0.45 – 0.91 | 0.01 | – | – | – |
| Rheumatologic disease | 0.82 | 0.59 – 1.14 | 0.24 | – | – | – |
| Myocardial infarct | 0.75 | 0.51 – 1.09 | 0.13 | – | – | – |
| Other cancer | 0.84 | 0.66 – 1.07 | 0.15 | – | – | – |
| Cerebrovascular disease | 0.79 | 0.58 – 1.06 | 0.12 | – | – | – |
| Dementia | 0.66 | 0.34 – 1.28 | 0.22 | – | – | – |
| Chronic pulmonary disease | 0.99 | 0.80 – 1.22 | 0.90 | – | – | – |
| Mild liver disease | 1.14 | 0.85 – 1.51 | 0.39 | – | – | – |
| Diabetes with organ damage | 0.93 | 0.63 – 1.36 | 0.70 | – | – | – |
| Hemiplegia | 0.79 | 0.37 – 1.65 | 0.52 | – | – | – |
| Ulcer | 0.82 | 0.57 – 1.17 | 0.27 | – | – | – |
| CCI | 0.45 | 0.37 – 0.54 | <0.001 | – | – | – |
HR = Hazard Ratio; CI = Confidence Interval; TKA = Total Knee Arthroplasty; CCI = Charlson Comorbidity Index
Absence of disease served as reference for comorbidity data
Moderate or severe disease
Univariate analysis demonstrated that the risk of MUA was associated with many of the same risk factors as AIS including smoking at the time of primary TKA (p=0.04), longer tourniquet time (p<0.001), longer operative time (p=0.005), and utilization of general anesthesia (p<0.001). The largest unadjusted risk for MUA was younger age (HR 2.8; 95% CI 2.2 – 3.5; p<0.001) (Table 3). Patients with increasing CCI were associated with reduced risk of MUA (p<0.0001) (Table 3).
Table 3.
Univariate and multivariate analysis of patient variables associated with need for manipulation under anesthesia
| Variable | Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Patient Characteristics | ||||||
| Sex | ||||||
| Female | 1.06 | 0.85 – 1.31 | 0.63 | 1.13 | 0.88 – 1.44 | 0.34 |
| Male (reference) | ||||||
| Age | ||||||
| < 65 years | 2.79 | 2.24 – 3.47 | <0.001 | 2.81 | 2.16 – 3.64 | <0.001 |
| ≥ 65 years (reference) | ||||||
| Body mass index | ||||||
| ≥ 30 kg/m2 | 0.88 | 0.71 – 1.09 | 0.23 | 0.74 | 0.57 – 0.95 | 0.02 |
| < 30 kg/m2 (reference) | ||||||
| Indication for TKA | ||||||
| Post-traumatic arthritis | 2.69 | 2.08 – 3.48 | <0.001 | 1.68 | 1.25 – 2.27 | <0.001 |
| Osteoarthritis (reference) | ||||||
| Smoking status | ||||||
| Current | 1.54 | 1.01 – 2.34 | 0.04 | – | – | – |
| Never (reference) | ||||||
| Prior knee surgery | ||||||
| Yes | 1.79 | 1.44 – 2.24 | <0.001 | – | – | – |
| No (reference) | ||||||
| Surgical Characteristics | ||||||
| Unilateral or Bilateral TKAs | ||||||
| Simultaneous bilateral | 0.66 | 0.46 – 0.96 | 0.03 | 0.78 | 0.70 – 0.88 | <0.001 |
| Staged bilateral | 0.45 | 0.33 – 0.61 | <0.001 | – | – | – |
| Unilateral (reference) | ||||||
| Anesthesia | ||||||
| General | 1.49 | 1.20 – 1.85 | <0.001 | 1.36 | 1.06 – 1.73 | 0.02 |
| Neuraxial (reference) | ||||||
| Tourniquet time, min | 1.01 | 1.01 – 1.01 | <0.001 | 1.01 | 1.00 – 1.01 | <0.001 |
| Operative time, min | 1.00 | 1.00 – 1.00 | 0.005 | 1.00 | 1.00 – 1.00 | 0.55 |
| Comorbidities^ | ||||||
| Diabetes | 1.10 | 0.88 – 1.42 | 0.49 | 1.49 | 1.11 – 1.99 | 0.01 |
| Hypertension | 0.51 | 0.41 – 0.63 | <0.001 | 0.74 | 0.57 – 0.97 | 0.03 |
| Renal disease† | 0.43 | 0.26 – 0.69 | 0.001 | 0.57 | 0.34 – 0.95 | 0.03 |
| Peripheral vascular disease | 0.60 | 0.44 – 0.81 | 0.001 | – | – | – |
| Liver disease† | 1.46 | 0.78 – 2.73 | 0.24 | – | – | – |
| Metastatic solid tumor | 0.90 | 0.50 – 1.59 | 0.71 | – | – | – |
| Congestive heart failure | 0.49 | 0.30 – 0.78 | 0.003 | – | – | – |
| Rheumatologic disease | 0.75 | 0.50 – 1.12 | 0.16 | – | – | – |
| Myocardial infarct | 0.67 | 0.43 – 1.06 | 0.09 | – | – | – |
| Other cancer | 0.80 | 0.60 – 1.06 | 0.12 | – | – | – |
| Cerebrovascular disease | 0.72 | 0.50 – 1.04 | 0.08 | – | – | – |
| Dementia | 0.58 | 0.26 – 1.30 | 0.19 | – | – | – |
| Chronic pulmonary disease | 0.91 | 0.71 – 1.16 | 0.44 | – | – | – |
| Mild liver disease | 1.13 | 0.81 – 1.57 | 0.49 | – | – | – |
| Diabetes with organ damage | 0.90 | 0.57 – 1.41 | 0.63 | – | – | – |
| Ulcer | 0.83 | 0.55 – 1.26 | 0.38 | – | – | – |
| CCI | 0.40 | 0.32 – 0.49 | <0.001 | – | – | – |
HR = Hazard Ratio; CI = Confidence Interval; TKA = Total Knee Arthroplasty; CCI = Charlson Comorbidity Index
Absence of disease served as reference for comorbidity data
Moderate or severe disease
Multivariate Analysis
Multivariate analysis was performed to account for confounding biases (Table 2). Following these adjustments, current smoking and prior knee surgery were no longer associated with AIS. Younger age (p<0.001), post-traumatic arthritis (p=0.04), general anesthesia (p=0.007), and diabetes (HR 1.5; 95% CI 1.2 – 2.0; p=0.001) were found to be independent risk factors for AIS in the multivariate model. Reduced risk of AIS was associated with lower BMI (HR 0.80; 95% CI 0.64 – 0.99; p=0.04) and diagnosis of hypertension (HR 0.76; 95% CI 0.62 – 0.97; p=0.03) (Figure 1).
Figure 1.
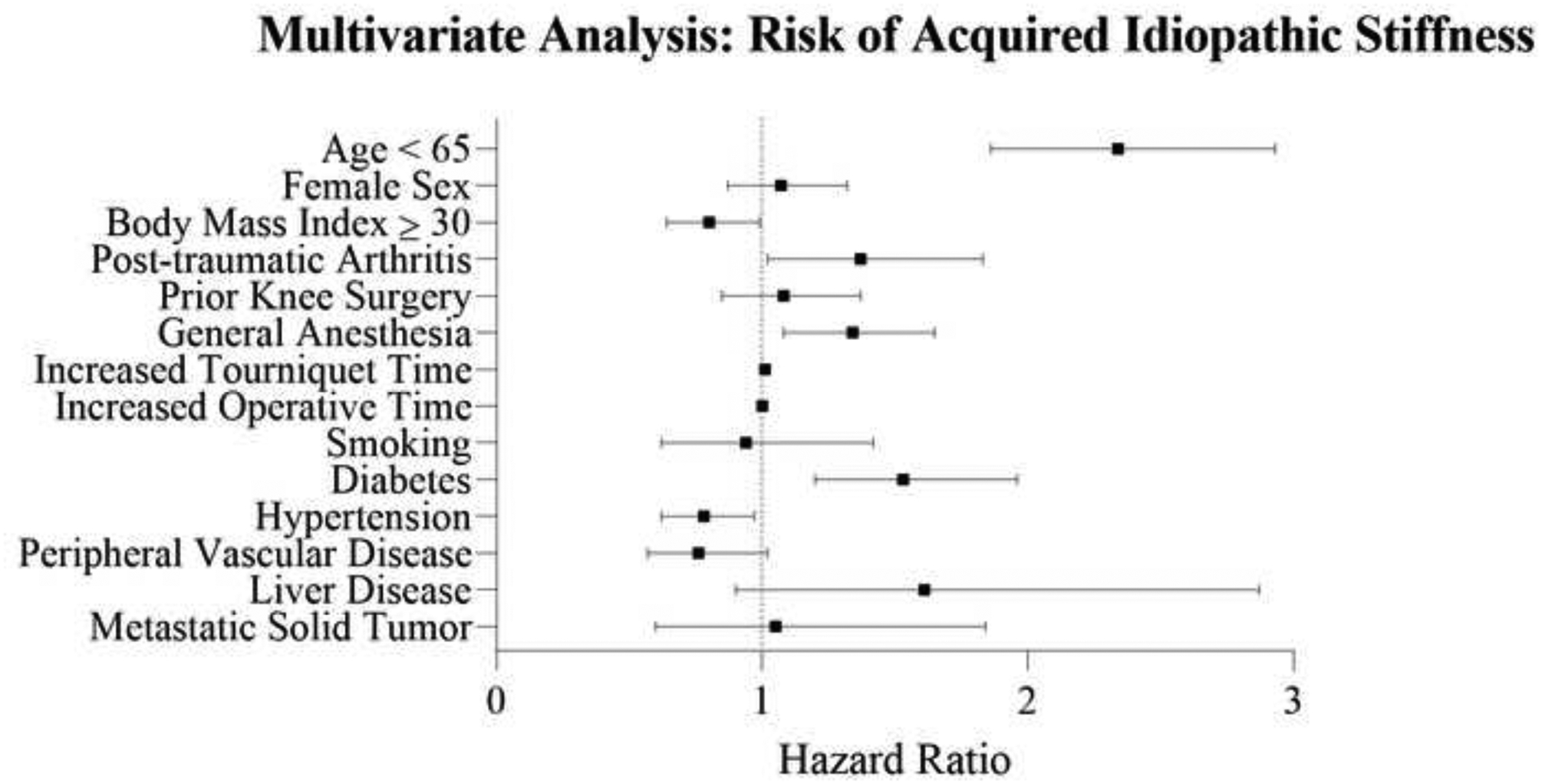
Forest plot demonstrating the hazard ratios and confidence intervals of patient demographics, surgical characteristics, and comorbidities associated with risk of acquired idiopathic stiffness as determined by multivariate analysis.
Multivariate analysis also was performed to account for confounding biases regarding risk of MUA (Table 3). Following adjustments, need for MUA was associated with increased tourniquet time (p<0.001), younger age (p<0.001), and a diagnosis of diabetes (HR 1.5; 95% CI 1.1 – 2.0; p=0.01). Reduced risk was associated with lower BMI (HR 0.74; 95% CI 0.57 – 0.95; p=0.02), hypertension (HR 0.74; 95% CI: 0.57 – 0.97; p=0.03), and moderate to severe renal disease (HR 0.6; 95% CI 0.35 – 0.97; p=0.03) (Figure 2).
Figure 2.
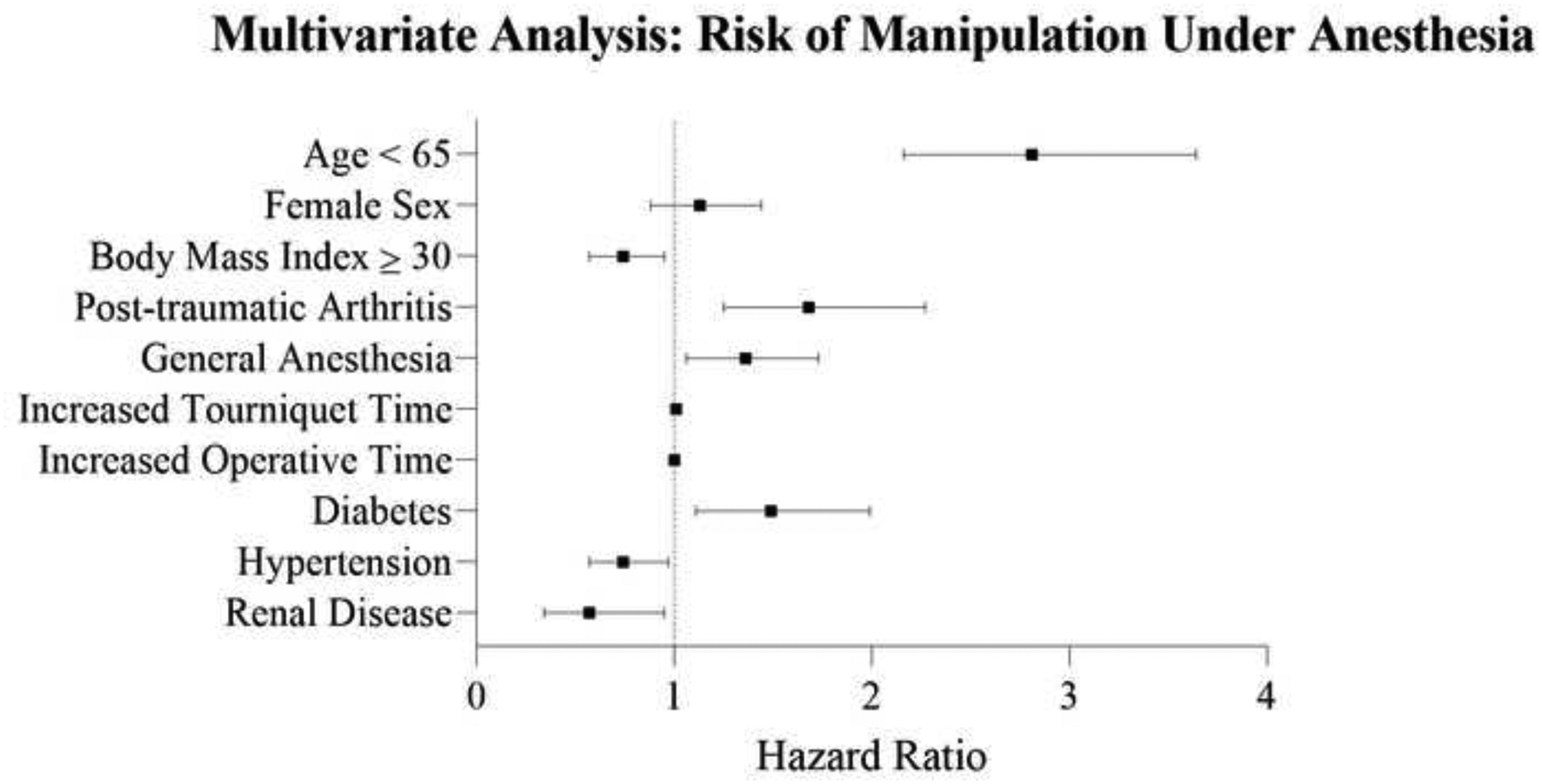
Forest plot demonstrating the hazard ratios and confidence intervals of patient demographics, surgical characteristics, and comorbidities associated with manipulation under anesthesia as determined by multivariate analysis.
DISCUSSION
AIS is a disabling and common complication following primary TKA.[8] Despite advances in contemporary TKAs, the pathogenesis, risk factors, and true incidence of AIS are not fully understood. In a high volume, academic practice, we found the incidence of AIS and subsequent need for MUA following primary TKA has remained relatively unchanged over the past 25 years with incidences in contemporary practice of 3.6% and 2.8%, respectively.
Our group has previously used the term AIS and defined it as an arc of motion ≤ 90° that persists beyond 12 weeks post-operatively as measured by goniometer at clinical follow-up in patients without prior limitations in knee range of motion.[4] Using this definition, we report the incidence of AIS to be 3.6% of primary TKAs in our contemporary cohort and 4.1% in our historical cohort. Historically, “postoperative stiffness” has been defined by a variety of measures including the maximum flexion, arc of motion, as well as the degree of flexion contracture observed.[12, 18, 19] These differences have led to discrepancies in the reported incidence of AIS with values ranging from < 1% to 38%.[4, 12] Notably, a recent meta-analysis demonstrated the aggregate incidence of AIS to be approximately 4% which is comparable to our institution’s incidence over the previous three decades.[4]
The incidence of MUA has been reported to range from 0.5% – 10% in both historical and contemporary primary TKA cohorts.[6, 11, 20–22] To date, to our knowledge, no study has evaluated the incidence of MUA over time at a single institution. At our institution from 1990 – 2016, MUA was offered to patients with AIS who failed to improve with conservative management following primary TKAs. Utilizing this indication, the incidence of MUA was 2.6% in our contemporary cohort and 2.8% in the historical cohort.
Similar to previous reports, the current study demonstrated significant improvement in ROM after MUA.[23] While MUA was effective in restoring ROM to > 90° in the majority of patients, mean ROM in patients requiring MUA remained inferior to others at long-term follow-up.[24–26] Additionally, although patients who were treated with MUA demonstrated significant improvements in KSSs, these scores were inferior to patients not treated with an MUA.[27]
The pathogenesis of AIS and subsequent need for MUA is multifactorial and incompletely understood. Regarding host-specific variables, our study identified younger age, BMI < 30 mg/kg2, smoking, diabetes, and lower CCI as significant risk factors for AIS and MUA. These risk factors are consistent with previous individual reports, but the first time these were comprehensively identified from a single data source.[11, 26, 28]
Interestingly, reduced risk of AIS as well as MUA was associated with both increasing number of comorbidities as well as individual diagnoses such as hypertension, history of myocardial infarction, peripheral vascular disease, congestive heart failure, renal disease and rheumatologic disease (Table 2). This observation may be related to the anti-myofibroblastogenesis impacts of medications these patients take for their co-morbidities combined with the fact that some of these patients are low demand and simply cannot tolerate an incremental reoperation and/or revision.[29, 30] Translational work has attempted to elucidate the role of pharmacologic agents utilized to treat the comorbidities and their effect on excessive fibrotic tissue development.[30–35] However, to date the usage of these medications in the clinical space have been limited.[36, 37] Furthermore, investigation into the medical and perioperative management of these patients may identify factors modulating the pathogenesis of disease. Likewise, stratification of patients based on comorbidity status may provide insights into patients’ predisposition to AIS.
In addition to host-specific variables, our study identified surgical factors that increased the risk of AIS and MUA including utilization of general anesthesia and increased tourniquet time. Many investigations have shown the benefits of neuraxial anesthesia in regards to perioperative pain control and achievement of postoperative milestones (e.g. time to mobilization and hospital discharge).[38, 39] Similarly, tourniquet utilization has been reported in some but not all previous studies to result in increased postoperative pain, early reductions in postoperative knee ROM, and quadriceps strength after TKA.[40–42] It is also possible that these findings are associations and not related to causation; use of general anesthesia and longer tourniquet times may have been proxies for case complexity.
There are limitations to the current study. Foremost, radiographs were not reviewed. Instead, a contemporary cohort of patients with similar prostheses was analyzed to determine the incidence of AIS and MUA at our institution. Moreover, there were patients with flexion less than 90° who refused or were not recommended to have MUA, which altered the incidence and results of the intervention. Finally, multiple surgeons participated in the study. Although this introduced variability in terms of surgical technique and implant utilization, it increases the generalizability and allowed nearly 10,000 patients to be analyzed.
In conclusion, the incidence of AIS (3.6%) and MUA (2.6%) following primary TKA at our institution has remained stable over the past 25 years. Although MUA resulted in improved motion in a majority of patients by a mean of 34°, motion and clinical outcomes at final follow-up were inferior to those unaffected by AIS. Continued investigation into the multiple intrinsic (i.e. genetic) and extrinsic (i.e. surgical technique and implant design) factors that contribute to AIS may reduce the incidence of this problematic complication and promote preventative strategies of care.
ACKNOWLEDGMENTS
We thank Dirk R. Larson, M.S. and Meredith C. Hyun, M.S. for their statistical expertise and contributions to this study. Additionally, we thank Youlonda A. Loechler and the members of the Mayo Clinic Total Joint Registry for their contributions to this study.
REFERENCES
- 1.Amundson AW, Johnson RL, Abdel MP, Mantilla CB, Panchamia JK, Taunton MJ, Kralovec ME, Hebl JR, Schroeder DR, Pagnano MW, Kopp SL. A Three-arm Randomized Clinical Trial Comparing Continuous Femoral Plus Single-injection Sciatic Peripheral Nerve Blocks versus Periarticular Injection with Ropivacaine or Liposomal Bupivacaine for Patients Undergoing Total Knee Arthroplasty. Anesthesiology 126(6): 1139, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Hines JT, Petis SM, Amundson AW, Pagnano MW, Sierra RJ, Abdel MP. Intravenous Tranexamic Acid Safely and Effectively Reduces Transfusion Rates in Revision Total Knee Arthroplasties. The Journal of bone and joint surgery American volume 102(5): 381, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Abdel MP, Chalmers BP, Taunton MJ, Pagnano MW, Trousdale RT, Sierra RJ, Lee YY, Boettner F, Su EP, Haas SB, Figgie MP, Mayman DJ. Intravenous Versus Topical Tranexamic Acid in Total Knee Arthroplasty: Both Effective in a Randomized Clinical Trial of 640 Patients. The Journal of bone and joint surgery American volume 100(12): 1023, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Tibbo ME, Limberg AK, Salib CG, Ahmed AT, van Wijnen AJ, Berry DJ, Abdel MP. Acquired Idiopathic Stiffness After Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. The Journal of bone and joint surgery American volume 101(14): 1320, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitta M, Esposito CI, Li Z, Lee YY, Wright TM, Padgett DE. Failure After Modern Total Knee Arthroplasty: A Prospective Study of 18,065 Knees. The Journal of arthroplasty 33(2): 407, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman ET, Herschmiller TA, Attarian DE, Vail TP, Bolognesi MP, Wellman SS. Risk Factors, Outcomes, and Timing of Manipulation Under Anesthesia After Total Knee Arthroplasty. The Journal of arthroplasty 33(1): 245, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Bingham JS, Bukowski BR, Wyles CC, Pareek A, Berry DJ, Abdel MP. Rotating-Hinge Revision Total Knee Arthroplasty for Treatment of Severe Arthrofibrosis. The Journal of arthroplasty 34(7s): S271, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Cheuy VA, Foran JRH, Paxton RJ, Bade MJ, Zeni JA, Stevens-Lapsley JE. Arthrofibrosis Associated With Total Knee Arthroplasty. The Journal of arthroplasty 32(8): 2604, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Bayram B, Limberg AK, Salib CG, Bettencourt JW, Trousdale WH, Lewallen EA, Reina N, Paradise CR, Thaler R, Morrey ME, Sanchez-Sotelo J, Berry DJ, van Wijnen AJ, Abdel MP. Molecular pathology of human knee arthrofibrosis defined by RNA sequencing. Genomics 112(4): 2703, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walton NP, Jahromi I, Dobson PJ, Angel KR, Lewis PL, Campbell DG. Arthrofibrosis following total knee replacement; does therapeutic warfarin make a difference? The Knee 12(2): 103, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ritter MA, Lutgring JD, Davis KE, Berend ME, Pierson JL, Meneghini RM. The role of flexion contracture on outcomes in primary total knee arthroplasty. The Journal of arthroplasty 22(8): 1092, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Nelson CL, Lotke PA. Stiffness after total knee arthroplasty. Prevalence of the complication and outcomes of revision. The Journal of bone and joint surgery American volume 86(7): 1479, 2004 [PubMed] [Google Scholar]
- 13.Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res 467(10): 2606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. The Journal of bone and joint surgery American volume 100(17): 1455, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. The Journal of bone and joint surgery American volume 89(4): 780, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Berry DJ, Kessler M, Morrey BF. Maintaining a hip registry for 25 years. Mayo Clinic experience. Clinical orthopaedics and related research (344): 61, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology 45(6): 613, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Rutherford RW, Jennings JM, Levy DL, Parisi TJ, Martin JR, Dennis DA. Revision Total Knee Arthroplasty for Arthrofibrosis. The Journal of arthroplasty 33(7s): S177, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Donaldson JR, Tudor F, Gollish J. Revision surgery for the stiff total knee arthroplasty. Bone Joint J 98-B(5): 622, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Kelly MP, Prentice HA, Wang W, Fasig BH, Sheth DS, Paxton EW. Reasons for Ninety-Day Emergency Visits and Readmissions After Elective Total Joint Arthroplasty: Results From a US Integrated Healthcare System. J Arthroplasty 33(7): 2075, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Esler CN, Lock K, Harper WM, Gregg PJ. Manipulation of total knee replacements. Is the flexion gained retained? The Journal of bone and joint surgery British volume 81(1): 27, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Thorsteinsson H, Hedstrom M, Robertsson O, Lundin N, A WD. Manipulation under anesthesia after primary knee arthroplasty in Sweden: incidence, patient characteristics and risk of revision. Acta orthopaedica 90(5): 484, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzsimmons SE, Vazquez EA, Bronson MJ. How to treat the stiff total knee arthroplasty?: a systematic review. Clin Orthop Relat Res 468(4): 1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keating EM, Ritter MA, Harty LD, Haas G, Meding JB, Faris PM, Berend ME. Manipulation after total knee arthroplasty. J Bone Joint Surg Am 89(2): 282, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Babis GC, Trousdale RT, Pagnano MW, Morrey BF. Poor outcomes of isolated tibial insert exchange and arthrolysis for the management of stiffness following total knee arthroplasty. J Bone Joint Surg Am 83(10): 1534, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Daluga D, Lombardi AV Jr., Mallory TH, Vaughn BK. Knee manipulation following total knee arthroplasty. Analysis of prognostic variables. J Arthroplasty 6(2): 119, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Miller AJ, Stimac JD, Smith LS, Feher AW, Yakkanti MR, Malkani AL. Results of Cemented vs Cementless Primary Total Knee Arthroplasty Using the Same Implant Design. J Arthroplasty 33(4): 1089, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Issa K, Rifai A, Boylan MR, Pourtaheri S, McInerney VK, Mont MA. Do various factors affect the frequency of manipulation under anesthesia after primary total knee arthroplasty? Clin Orthop Relat Res 473(1): 143, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barlow JD, Morrey ME, Hartzler RU, Arsoy D, Riester S, van Wijnen AJ, Morrey BF, Sanchez-Sotelo J, Abdel MP. Effectiveness of rosiglitazone in reducing flexion contracture in a rabbit model of arthrofibrosis with surgical capsular release: A biomechanical, histological, and genetic analysis. Bone Joint Res 5(1): 11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limberg AK, Tibbo ME, Salib CG, McLaury AR, Turner TW, Berry CE, Jay AG, Carter JM, Bolon B, Berry DJ, Morrey ME, Sanchez-Sotelo J, van Wijnen AJ, Abdel MP. Reduction of arthrofibrosis utilizing a collagen membrane drug-eluting scaffold with celecoxib and subcutaneous injections with ketotifen. J Orthop Res, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel MP, Morrey ME, Barlow JD, Grill DE, Kolbert CP, An KN, Steinmann SP, Morrey BF, Sanchez-Sotelo J. Intra-articular decorin influences the fibrosis genetic expression profile in a rabbit model of joint contracture. Bone Joint Res 3(3): 82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salib CG, Reina N, Trousdale WH, Limberg AK, Tibbo ME, Jay AG, Robin JX, Turner TW, Jones CR, Paradise CR, Lewallen EA, Bolon B, Carter JM, Berry DJ, Morrey ME, Sanchez-Sotelo J, van Wijnen AJ, Abdel MP. Inhibition of COX-2 Pathway as a Potential Prophylaxis Against Arthrofibrogenesis in a Rabbit Model of Joint Contracture. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 37(12): 2609, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranowski A, Schlemmer L, Forster K, Slotina E, Mickan T, Truffel S, Klein A, Mattyasovszky SG, Hofmann A, Ritz U, Rommens PM. Effects of losartan and atorvastatin on the development of early posttraumatic joint stiffness in a rat model. Drug design, development and therapy 13: 2603, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terada S, Ota S, Kobayashi M, Kobayashi T, Mifune Y, Takayama K, Witt M, Vadalà G, Oyster N, Otsuka T, Fu FH, Huard J. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J Bone Joint Surg Am 95(11): 980, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Dixon D, Coates J, del Carpio Pons A, Horabin J, Walker A, Abdul N, Kalson NS, Brewster NT, Weir DJ, Deehan DJ, Mann DA, Borthwick LA. A potential mode of action for Anakinra in patients with arthrofibrosis following total knee arthroplasty. Sci Rep 5: 16466, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langston JR, Ramsey DC, Skoglund K, Schabel K. Angiotensin II blockade had no effect on range of motion after total knee arthroplasty: a retrospective review. Journal of orthopaedic surgery and research 15(1): 48, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen AF, Lee YS, Seidl AJ, Abboud JA. Arthrofibrosis and large joint scarring. Connect Tissue Res 60(1): 21, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Johnson RL, Kopp SL, Burkle CM, Duncan CM, Jacob AK, Erwin PJ, Murad MH, Mantilla CB. Neuraxial vs general anaesthesia for total hip and total knee arthroplasty: a systematic review of comparative-effectiveness research. British journal of anaesthesia 116(2): 163, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Turcotte JJ, Stone AH, Gilmor RJ, Formica JW, King PJ. The Effect of Neuraxial Anesthesia on Postoperative Outcomes in Total Joint Arthroplasty With Rapid Recovery Protocols. The Journal of arthroplasty 35(4): 950, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Dennis DA, Kittelson AJ, Yang CC, Miner TM, Kim RH, Stevens-Lapsley JE. Does Tourniquet Use in TKA Affect Recovery of Lower Extremity Strength and Function? A Randomized Trial. Clin Orthop Relat Res 474(1): 69, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br 81(1): 30, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Goel R, Rondon AJ, Sydnor K, Blevins K, O’Malley M, Purtill JJ, Austin MS. Tourniquet Use Does Not Affect Functional Outcomes or Pain After Total Knee Arthroplasty: A Prospective, Double-Blinded, Randomized Controlled Trial. J Bone Joint Surg Am 101(20): 1821, 2019 [DOI] [PubMed] [Google Scholar]


