Abstract
Functional movement disorders (FMD) are a common and disabling neuropsychiatric condition, part of the spectrum of functional neurological/conversion disorder. FMD represent one of the most enigmatic disorders in the history of medicine. However, in the twenty years after the first report of distinctive abnormal brain activity associated with functional motor symptoms, there have been tremendous advances in the pathophysiologic understanding of these disorders. FMD can be characterized as a disorder of aberrant neurocircuitry interacting with environmental and genetic factors.
These developments suggest that research on FMD could be better served by an integrative, neuroscience-based approach focused on functional domains and their neurobiological substrates. This approach has been developed in ‘Research Domain Criteria’ (RDoC) project, which promotes a dimensional approach to psychiatric disorders. Here, we use the RDoC conceptualization to review recent neuroscience research on FMD, focusing on the domains most relevant to these disorders. We discuss how the adoption of a similar integrative framework may facilitate the identification of the mechanisms underlying FMD and could also have potential clinical applicability.
Keywords: Functional movement disorders, Research domain criteria, Neurocircuitry, Neuroimaging, Mechanisms, Brain-behavior dimensions
1. Introduction
Functional movement disorders (FMD) are a disabling neuropsychiatric condition, part of the wide spectrum of functional neurological disorders (FND). With an annual incidence of 4–12 cases per 100,000 (Carson and Epidemiology, 2016), this spectrum of disorders represent the second commonest category of referrals to neurology outpatient clinics after headache (Stone et al., 2010). The clinical manifestations characterizing FMD are complex and heterogeneous, and include symptoms affecting motor functions (hyperkinetic movements ranging the gamut from dystonia to tremor and myoclonus, gait abnormalities), alterations in cognitive/executive functioning processes (attention, attribution of agency, prediction error), and perturbations in emotion regulation (emotional hyper-responsiveness) (Espay et al., 2018a; Edwards et al., 2012). Furthermore, patients with FMD may also suffer from other co-occurring FND, such as functional non-epileptic seizures (FNES), as well as from comorbid ‘organic’ movement disorders (e.g., Parkinson’s disease, dystonia), and psychiatric disorders (mood and anxiety disorders, PTSD) (Erro et al., 2016; Wissel et al., 2018).
As with many diseases characterized by considerable within- and between-group heterogeneity, the mechanisms and causes of FMD and other FND have been difficult to delineate. In recent years, neuroimaging research and renewed clinical interest have provided important insights into the neurobiological bases of these disorders, leading to a critical shift in the approach to FMD (Espay et al., 2018a; Edwards et al., 2013). In particular, the historically influential emphasis on emotional trauma and other stressors as etiologic factors of FMD has been progressively replaced by a disease model grounded in brain circuit-behavior/motor relationships (Perez et al., 2015; Ludwig et al., 2018). This shift has prompted substantial changes in classification, diagnostic criteria and therapeutic management of FMD (Espay et al., 2018a). However, despite these advances, we are still far from a complete understanding of the nature of these disorders, our clinical assessment focuses on a limited number of manifestations, we lack an integrated set of measures capturing the broad range of FMD symptoms, and we have not yet identified disease biomarkers, which can be useful for guiding diagnosis and evaluating treatment efficacy.
We propose that these gaps can be addressed by adopting an integrative, neuroscience-based, transdiagnostic approach to FMD, in line with the Research Domain Criteria (RDoC) initiative launched by the National Institute of Mental Health (NIMH) (Insel et al., 2010; Cuthbert and Insel, 2013). The RDoC was developed to provide a heuristic research framework aimed at improving our current understanding of heterogeneous psychiatric “syndromes” by shifting the focus from symptoms to basic constructs that span a full range of human behavior (i.e., from normal to abnormal) and are linked to specific neural circuits (Insel et al., 2010; Cuthbert, 2014). These constructs can be observed across different diagnostic categories and may be studied at different level of analysis, from genes and molecules to physiology, behavior and self-report.
In this article, we explore how the RDoC framework might be applied to the study of FMD. To this extent, we first provide a brief overview of the landmark developments in the history of FMD and other FND that have impacted our understanding, classification and management of these disorders. We then review recent neuroscience research on FMD and illustrate how alterations characterizing this disorder at the clinical, behavioral and circuit-level are strongly conceptually tied to RDoC domains and constructs, including the sensorimotor domain, the cognitive domain and the negative affect domain. We also consider constructs relevant to FMD not yet investigated and provide suggestions for future studies. Finally, we discuss how adopting a neuroscience-based framework may not only advance research on FMD but may also have potential clinical applicability for phenotyping of heterogeneity and identification of disease biomarkers.
2. Functional Movement Disorders: from a wondering uterus to neural circuits
From the earliest medical records to the present day, FMD and other FND have represented one of the most enigmatic and controversial disorders in the history of medicine. When first described by the Egyptians over 4000 years ago, these disorders were attributed to abnormal movement of the uterus, with Hippocrates ultimately coining the term “hysteria” (from the Greek word for uterus) to describe such cases. The concept of a wondering uterus causing functional symptoms influenced medicine for centuries, leaving little room for alternative hypotheses [for a review see (Tasca et al., 2012; Trimble and Reynolds, 2016).
In the 16th century, Thomas Willis first introduced the idea that functional disorders were a mental illness, although it is only around the turn of the last century that Charcot, influenced by Briquet’s work on hysteria, and then Freud, made the revolutionary step of recognizing FND as disorders of the mind. In particular, Freud postulated that psychological stressors induced by traumatic events were “converted” into neurologic symptoms (a phenomenon he ultimately described as “hysterical conversion”) (Anna, 2004). The influence of this theory on the terminology, diagnosis, treatment and research agenda of FND has been profound and can still be recognized: the diagnostic label of conversion disorder currently used retains the original idea that psychological stressors trigger these disorders. Similarly, other clinical definers, including psychogenic, psychosomatic, medically unexplained, and nonorganic, suggested that FMD and other FND lack any biological cause or, more subtly, that patients may be feigning their symptoms (Demartini et al., 2016). Indeed, early attempts, using electroencephalography and later structural imaging, failed to identify brain abnormalities in FND patients, thus reinforcing the idea that these disorders had no biological explanations.
Confined at the borderland between psychiatry and neurology, during the 20th century FMD and other FND were largely neglected in both research and clinical service development. This lack of interest left these disorders far behind the neurobiological understanding of other neuropsychiatric conditions until about two decades ago, when Spence and colleagues (Spence et al., 2000) conducted a positron emission tomography study demonstrating abnormal activation in prefrontal areas implicated in volition in patients with functional weakness compared to controls with simulated weakness. These findings were confirmed in a subsequent study (Stone et al., 2007), which identified distinct patterns of functional activation in cortical and subcortical motor areas between patients and actors instructed to mimic weakness. These pioneering studies led to a tectonic shift in the approach to FND since they clearly indicated that patients were not faking their symptoms and that brain activation abnormalities were associated with functional neurological symptoms. Consequently, the research interest in these disorders revamped, although their classification, diagnosis and treatment were still firmly anchored in psychoanalytic theories.
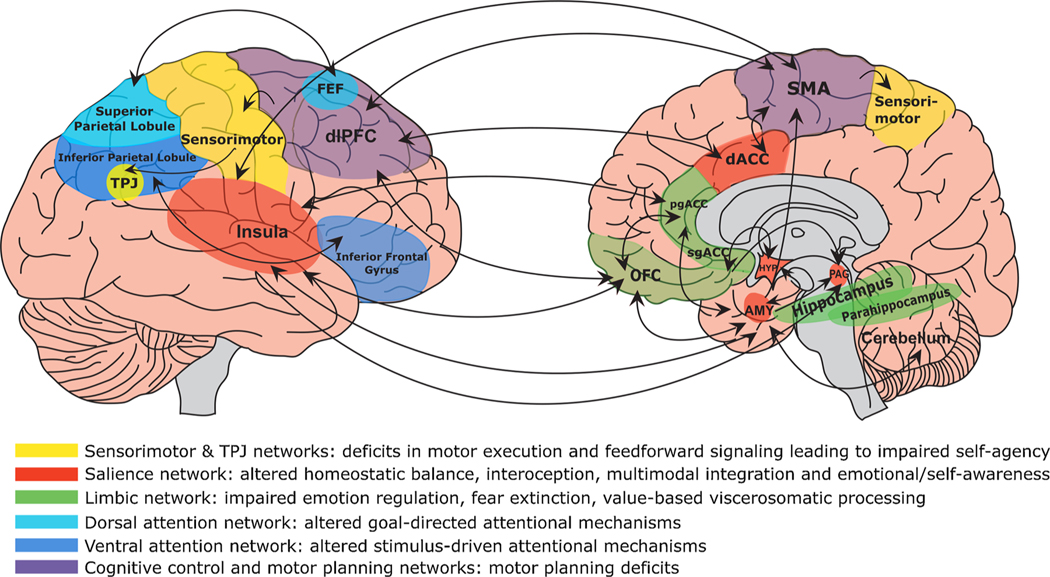
The number of neurophysiological and imaging studies carried out in FMD patients as well as in patients with other FND grew exponentially in the last decade [for a comprehensive review of functional and structural neuroimaging findings in FND the reader is referred to (Bègue et al., 2019; Roelofs et al., 2019; Voon, 2014; Voon et al., 2016). These investigations provided extensive evidence of alterations in activity and connectivity in neural circuits mediating motor planning and coordination (supplementary motor area, cerebellum), emotional processing, regulation, and awareness (anterior cingulate and ventromedial prefrontal cortices, insula, amygdala, vermis), cognitive control and motor inhibition (dorsal anterior cingulate, dorsolateral prefrontal, inferior frontal cortices), and self-referential processing and perceptual awareness (posterior parietal cortex, temporoparietal junction, precuneus) [Fig. 1]. Functional alterations in these circuits were also mirrored by structural abnormalities, with studies showing increased grey matter volume in subcortical motor (basal ganglia), cerebellum, thalamus and limbic/paralimbic cortical structures; and decreased volume in precentral and postcentral gyri and primary motor cortex in patients with FMD (Bègue et al., 2019). However, to date there is no clear evidence that these volumetric alterations underlie the onset of FMD or are the results of functional brain abnormalities observed in these patients.
Fig. 1.
Display of brain circuits (and related functions) that are emerging as relevant in the pathophysiology of Functional Movement Disorders (FMD). As depicted, FMD is a brain disorder characterized by abnormalities within and across neural circuits implicated in self-agency, emotion processing, attention, interoception, self-referential processing and perceptual awareness, multimodal integration, and cognitive/motor control, among other functions. Circuits are described by their related dysfunction in the pathophysiology of FMD. TPJ indicates temporoparietal junction; FEF, frontal eye fields; dlPFC, dorsolateral prefrontal cortex; pgACC, perigenual anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; OFC, orbitofrontal cortex; SMA, supplementary motor area; AMY, amygdala; HYP, hypothalamus; PAG, periaqueductal gray. Figure modified from Dranes et al., 2020 CNS Spectrum (Drane et al., 2020).
Neuroimaging findings converged with results of clinical studies showing impairment in several motor-behavioral domains linked to the neurocircuits implicated in FMD (Perez et al., 2015), thus strengthening the view of FMD and other FND as primarily disorders of circuit function, or “circuitopathies”. The concept of circuitopathies is not new as it has already been adopted to explain the pathophysiology of several neurological conditions, including PD and Alzheimer’s disease (AD) (Lozano and Lipsman, 2013) and, more recently, has also been applied to FND. Indeed, in a pivotal review published by the American Neuropsychiatric Association, the authors proposed a model of FND as a disorder resulting from impairments in higher-order and bottom-up (limbic) mechanisms, and in their interaction with sensory processing and motor functions (Voon et al., 2016). Importantly, they highlighted how this model provided neurobiological substrates to the variety of clinical manifestations characterizing FND, while also suggesting possible common mechanisms shared across these disorders and other neuropsychiatric disorders, including functional syndromes.
In parallel with these developments, clinical and epidemiological studies in FMD patients also contributed to advance the field, by providing robust evidence that not all individuals affected by these disorders reported a history of traumatization or recent stressful events (Ludwig et al., 2018). This knowledge, together with the budding understanding of the biological bases of FMD, prompted a further tectonic shift in the approach to FMD and other FND, as indicated by recent changes in classification, terminology and diagnostic criteria. Specifically, in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the definition of Functional Neurological Symptom Disorder/Conversion Disorder has been introduced, replacing the term psychogenic with functional (American Psychiatric Association, 2013). Furthermore, diagnosis by DSM-5 no longer requires a temporal relationship between exposure to a stressor and disease onset. Conversely, FMD is diagnosed by identifying positive neurological signs that are specific to these disorders (e.g. Hoover’s sign (Ziv et al., 1998)), rather than being an exclusionary diagnosis. The improved understanding of the pathophysiology of FMD has also expanded the range of therapeutic interventions for this disorder, with multidisciplinary, integrated programs combining physical and cognitive-behavioral therapies showing promising results (LaFaver, 2020; Perez, 2020).
However, the current diagnostic approach to FMD and other FND is based on a categorical organization of the disease. A categorical approach may lead to a systematic under appreciation of the importance of variations in overt symptoms and in underlying mechanisms from individual to individual. Furthermore, it may limit our ability to identify potential shared disease mechanisms and substrates across commonly comorbid conditions. This ultimately may hamper research on the etiology and pathophysiology of heterogenous conditions and may hinder the development of tailored treatments. Conversely, a dimensional perspective may allow for more fine-grained research into complex disorders and could also help informing categorical treatment decisions.
3. Can a RDoC-like approach be applied to FMD research?
A first step in addressing this question requires identifying the RDoC domains and constructs most relevant to FMD. The RDoC matrix is constructed around six major domains of human functioning, which are further divided into constructs and subconstructs (Table 1). These brain-behavior domains are studied across different disorders and can be linked to specific symptom clusters. FMD and other FND can be conceptualized as multifactorial disorders characterized by neurocircuitry dysfunctions, although motor and behavioral manifestations of FMD have not yet been mapped to specific brain-behavior domains. Toward this goal, we selectively reviewed the extant literature on FMD, focusing on studies that have used neuroimaging and/or neurobehavioral tasks, to elucidate the neurobehavioral domains of dysfunctions that lead to FMD symptoms.
Table 1.
RDoC Systems and Constructs.
| Negative Valence Systems | Positive Valence Systems | Cognitive Systems | Systems for Social Processes | Arousal and Regulatory Systems | Sensorimotor Systems |
|---|---|---|---|---|---|
| Acute Threat (“Fear”) | Reward Responsiveness | Attention | Affiliation and Attachment | Arousal | Motor Actions |
| Potential Threat (“Anxiety”) | Reward Learning | Perception | Social Communication | Circadian Rhythms | Agency and Ownership |
| Sustained Threat | Reward Valuation | Declarative Memory | Perception and Understanding of Self | Sleep-Wakefulness | Habit - Sensorimotor |
| Loss | Language | ||||
| Frustrative Nonreward | Cognitive Control Working Memory | Perception and Understanding of Others | Innate Motor Patterns |
3.1. Negative valence system
The Negative Valence Systems domain refers to systems that respond to aversive stimuli, situations, or context, such as threat (National Institute on Mental Health, 2020a). The constructs included in this domain are mainly related to fear, anxiety, avoidance and loss. In the FMD literature, this domain has received significant attention, particularly in the areas of acute threat (fear circuitry mediating intense defensive behaviors in response to imminent threats) and sustained threat (e.g., early life adversities, chronic stress), with some evidence related to the potential threat construct (anxiety).
3.1.1. Acute threat
In one of the first studies examining threat response in FMD, Seignourel and colleagues (Seignourel et al., 2007) assessed eyeblink amplitude to positive and negative valenced images paired with an acoustic startle reflex paradigm, in FMD patients and controls. The eyeblink component of the startle response is modulated by affective states: it is potentiated by fear, anxiety, and other aversive states, and attenuated by positive affective states (Grillon and Baas, 2003). This expected pattern of startle modulation was observed in healthy controls, while FMD patients exhibited increased eyeblink amplitude during exposure to both positive and negative stimuli. These results indicated that individuals with FMD were characterized by emotional hyper-responsiveness, which may reflect increased amygdala reactivity to emotional stimuli, since the magnitude of the startle amplitude is directly related to amygdala activity (Davis, 2006).
Indeed, this hypothesis was confirmed in a subsequent fMRI study investigating fear circuitry in FMD, which showed increased right amygdala reactivity and decreased habituation in response to both positive and negative emotional stimuli in FMD patients compared to controls (Voon et al., 2010a). Furthermore, while viewing emotional salient stimuli, patients exhibited greater functional connectivity between the right amygdala and the right supplementary motor area than controls. These findings confirmed a state of emotional hyperarousal in individuals with FMD and suggested that impairment in emotion processing influenced movement initiation in these patients.
Further fMRI studies provided evidence of enhanced limbic reactivity, particularly in response to negatively valenced stimuli, alongside increased connectivity between amygdala and motor planning/control areas in FMD patients compared to controls (Aybek et al., 2015; Hassa et al., 2017). Additionally, alterations in other brain regions implicated in emotion processing including periaqueductal gray, insula, anterior cingulate cortex and paracingulate gyrus have been reported during negative stimuli exposure (Aybek et al., 2015; Aybek et al., 2014; Espay et al., 2018b; Espay et al., 2018c)
Taken together, these observations demonstrate dysregulations in fear circuitry in FMD, which are linked to alterations in motor pathways, thus suggesting an interaction between the negative valence systems and sensorimotor domain. Future research adopting fear conditioning/extinction paradigms could better characterize alterations in the construct of acute threat. Furthermore, it will be important to assess whether FMD-specific stimuli may aggravate this dysregulation, as preliminary evidence has suggested (Hassa et al., 2017).
3.1.2. Potential threat
Far fewer studies have focused on potential threat (“anxiety”) other than examining comorbidity with anxiety disorders and symptoms in FMD patients. Indeed, individuals with FMD have a higher frequency of anxiety disorders (69.1 %) and score higher in validated clinical scales assessing anxious symptomatology (Feinstein et al., 2001; Kranick et al., 2011a; Ekanayake et al., 2017), but little is known about the mechanisms underlying this association. One study investigating attentional threat biases found that children and adolescents with mixed FND showed faster reaction time in identifying negative vs happy faces, compared to controls, suggesting increased vigilance to potential threats (Kozlowska et al., 2021). These findings parallel studies in patients with functional non-epileptic seizures (FNES), who also display preferential allocation of attention to threatening cues compared to other stimuli (Bakvis et al., 2009). Consistent with this, a further study found that FMD patients exposed to emotional stimuli of different valence during a motor task, exhibited a more pronounced influence of aversive stimuli on voluntary force control, while positive and neutral stimuli presentation was associated with a decay in force output (Blakemore et al., 2016). These findings support the presence of attentional threat biases in FMD patients and confirm the association between heightened reactivity to negative stimuli and abnormal motor functions. Future studies should assess whether increased threat attention observed in FMD patients is also associated with elevated threat expectancy and to maladaptive responses to threat uncertainty (threat probabilistic task). These processes are closely related and have been shown to influence both cognitive (e.g. decision-making) and motor functions (e.g., inhibition of motor actions) (Grupe and Nitschke, 2013).
3.1.3. Sustained threat
This RDoC construct is defined as ‘an aversive emotional state caused by prolonged exposure to internal or external stimuli that are adaptive to escape or avoid’ (National Institute on Mental Health, 2020a). Research on this construct has mainly focused on chronic stress and early life adversities, which historically have been considered important factors in FMD pathogenesis, as discussed above. Currently, childhood maltreatment is recognized as a predisposing factor for FMD and other FND (Ludwig et al., 2018), and stress-diathesis models of FND have been theorized to explain how repeated exposure to even minimal stressors may increase vulnerability to these disorders (Keynejad et al., 2019; Ejareh Dar and Kanaan, 2016). However, the majority of research on early-life, chronic stress and FND, appears to have focused on confirming or refuting the relevance of this association on the basis of epidemiological data, such as rates of stressful events in FND samples compared to controls (Stone et al., 2004; Kranick et al., 2011b; Nicholson et al., 2016).
Several clinical studies have suggested that exposure to childhood maltreatment in FMD patients is associated with worse symptom severity (Roelofs et al., 2002; Roelofs et al., 2005), emergence of distinct attachment styles (Williams et al., 2019), and poorer treatment outcomes (Van der Feltz-Cornelis and Allen, 2020), thus suggesting that early life adversities modulate the phenotypical manifestations of FMD. These findings parallel results from imaging studies, which have provided preliminary evidence of distinct brain features in FMD patients with history of childhood maltreatment (Perez et al., 2017; Diez et al., 2020; Maurer et al., 2018).
The RDoC approach may represent an ideal framework to investigate the mechanisms by which childhood trauma and other stressors may produce distinct changes at the molecular, circuit and behavioral level, which may uniquely drive both risk for and severity of FMD in exposed individuals. Consistent with this, a recent study has demonstrated that childhood trauma, interactively with a polymorphism on the serotonin-related gene TPH2, is associated with worse symptom severity in FMD patients. Because both early life adversities and TPH2 polymorphisms influence serotoninergic transmission, these findings suggest a potential neurobiological mechanism modulating functional motor symptoms (Spagnolo et al., 2020). Furthermore, exposure to childhood trauma in FMD patients is associated with DNA methylation changes at the level of genes implicated in neurodevelopment and neurotransmission (Spagnolo et al., personal communication).
Since exposure to early life adversities and chronic stress have been associated with alterations in stress response systems (e.g., glucocorticoids, endocannabinoids) (van Bodegom et al., 2017; Morena et al., 2016; Herman et al., 2016), future studies should investigate whether such dysregulations are also observed in FMD patients and, importantly, whether they underlie distinct disease features. In this regard, in a study evaluating HPA-axis functioning in FMD, baseline cortisol levels did not differ between patients and controls (Maurer et al., 2015), whereas increased basal cortisol levels have been observed in individuals with FNES [for a review see (Sundararajan et al., 2016)]. However, given the dynamic nature of the stress response, the question remains open as to whether differences in neuroendocrine response may emerge during stress-eliciting procedures (e.g., Trier social stress test (Kirschbaum et al., 1995), Maastricht acute stress task (Smeets et al., 2012), and as a function of stress exposure during childhood and adulthood. In Table 2, we provide some examples of task-based measures, questionnaires and scales, which can be performed to assess this and other constructs in the Negative Valence Systems domain.
Table 2.
Proposed measures for a neuroclinical assessment of FMD.
| Measure | Time to Complete | Type of Task |
|---|---|---|
| Negative Valence System | ||
| Approach Avoidance Task (AAT) (Rinck and Becker, 2007) | 10 minutes | Behavioral |
| This task measures approach and avoidance tendencies (movements) toward positively and negatively valenced stimuli. | ||
| Trier Social Stress Test (TSST) (Kirschbaum et al., 1995) | 20 minutes | Behavioral |
| The TSST is a well-established procedure evoking a robust psychological and neuroendocrine response to acute stress. It incorporates social evaluation and unpredictability (mock job interview in front of an unresponsive audience and a surprise mental arithmetic test). | ||
| Cold Pressor Task (Lovallo, 1975) | 10 minutes | Behavioral |
| This is a pain tolerance task which also evoke a robust stress response. Pain is often reported by patients with FMD and other FND, thus this task could be a useful tool to investigate the overlap between dysregulated stress response and pain. | ||
| Toronto Alexithymia Scale (TAS) (Bagby et al., 1994) | 5 minutes | Self-Report |
| The TAS is a self-report questionnaire designed to measure the three components of alexithymia: difficulty identifying and distinguishing emotions from bodily sensations; difficulty describing and verbalizing emotions; and externally oriented thinking. Thus, the constructs assessed by this scale span across several RDoC domains (negative valence, social processes). | ||
| Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003) | 5 minutes | Self-Report |
| The CTQ is one of the most widely used self-reports of early adversity used in research settings. This questionnaire measures five types of maltreatment: emotional, physical, and sexual abuse, and emotional and physical neglect. | ||
| Facial Emotion Matching Task (Hariri et al., 2002) | 10 minutes | Neuroimaging |
| This emotional matching paradigm, introduced by Hariri and colleagues in 2000, is a widely used neuroimaging experiment that reliably activates the amygdala. In the classic version of the experiment faces with negative emotional expression and scenes depicting distressing events are compared with geometric shapes instead of neutral stimuli of the same category (i.e., faces or scenes). This paradigm has been previously used in several studies in FMD and other FND patients. | ||
| Beck Depression Inventory (BDI) (Beck et al., 1988a) | 5 minutes | Self-Report |
| This is a self-report rating inventory that measures symptoms of depression. The BDI has been validated in a range of clinical and non-clinical populations and has been included among the recommended outcome measure for FND. | ||
| Beck Anxiety Inventory (BAI) (Beck et al., 1988b) | 5 minutes | Self-Report |
| The BAI is a self-report scale used to assess the severity of anxious symptomatology. As the BDI, it is also included among the recommended outcomes measures for FND. | ||
| Life Events and Difficulties Schedule(LEDS) (Nicholson et al., 2016) | 30–120 minutes | Semi-structured interview |
| The LEDS system employs a semi-structured interview that systematically covers life domains and potential stressors. Next, the interviewer presents the reported events and chronic difficulties to a panel of raters who judge each stressor. | ||
| Fear conditioning/ extinction paradigm (Schiller et al., 2008) | 3– 4 days | Behavioral/Neuroimaging |
| Standard fear conditioning paradigms are commonly used to study the regulation and inhibition of fear memories. They include three phases: 1) Fear acquisition, 2) Extinction and 3) Extinction Retention. | ||
| Facial expression detection (via electromyography or software) in response to visual stimuli and/or after stress-evoking procedures (Ree et al., 2019) (Mayo et al., 2020), | 10–15 minutes | Psychophysiological |
| Facial reactions in response to facial expressions or other emotional valenced stimuli represents an index of affective states and can be measured objectively using Electromyography (EMG) or software. | ||
| Cognitive System | ||
| Stop Signal Reaction Task (SSRT) (Verbruggen and Logan, 2008) | 10 minutes | Behavioral |
| The SSRT measures inhibition of a response that has already been initiated (action cancellation). This task elicits an early activation of attentional mechanisms followed by an engagement of motor control processes. | ||
| Go-No Go Task (GNGT) (van Wouwe et al., 2020) | 10 minutes | Behavioral |
| Similar to the SSRT, the GNGT is also used to measure response inhibition, although this task captures action restrain (decision whether to respond or not). The GNGT appears to mainly rely on cognitive control mechanisms. | ||
| Continuous Performance Test (Riccio et al., 2002) | 15 minutes | Behavioral |
| This test is commonly used to assess attention performances in the areas of inattentiveness, impulsivity, sustained attention, and vigilance. | ||
| Beads in a Jar Task (Pareés et al., 2012b) | 5 minutes | Behavioral |
| This is a well-studied probabilistic reasoning paradigm, which is used to measure the ‘jumping to conclusion’ bias, a tendency to make decisions with certainty based on insufficient information. | ||
| Rapid Visual Information Processing Task (Hilti et al., 2010) | 7 minutes | Behavioral/Neuroimaging |
| This task is a serial detection task used to probe visual sustained attention and working memory processes. | ||
| Attentional blink and psychological refractory period paradigm (Dual Task) (Marti et al., 2012) | Variable (minutes) |
Behavioral/Neuroimaging |
| This paradigm is used to test dual-task interference in sensory consolidation (e.g., the attentional blink, AB) and response selection (e.g., the psychological refractory period). Dual-tasking relies on cognitive control and attentional processes. | ||
| Behavior Rating Inventory of Executive Function-Adult Version (Løvstad et al., 2016) | 10–15 minutes | Self-Report |
| This inventory, which include self- and informant-report versions, assesses executive dysfunction in daily life. | ||
| Arousal and Regulatory Systems | ||
| Psychomotor Vigilance Task (PVT) (Loh et al., 2004) | 10 minutes | Behavioral |
| The PVT is a sustained visual vigilance/attention reaction-time test used to measure behavioral alertness, particularly in the context of sleep loss. | ||
| POMS arousal subscale (McNair et al., 1971) | 5 minutes | Self-Reported |
| This is one of the 6 subscales of the Profile of mood state (POMS), a self-report inventory measuring transient and distinct changes in mood. | ||
| Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) | 5 minutes | Self-Reported |
| The PSQI is a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval. | ||
| Electrodermal responding | Variable (minutes) | Psychophysiological |
| The electrodermal response, also known as skin conductance response, occurs in response to either external or internal arousing stimuli. It is an indirect measure of sympathetic autonomic activity that is associated with both emotion and attention. | ||
| Heart rate variability (HRV) (Maurer et al., 2016b) | Variable (minutes) | Psychophysiological |
| HRV is an index of cardiac vagal tone, which is relevant for both physiological and emotional arousal. | ||
| Sensorimotor System | ||
| Action Recognition Paradigm (Synofzik et al., 2010) | 10 minutes | Behavioral/Neuroimaging |
| This experimental paradigm is used to assess the ability of a subject to distinguish whether or not a sensory event has been selfproduced, thus it is considered a measure of the sense of agency. | ||
| Force matching task (Pareés et al., 2014) | Variable (minutes) | Neurophysiological/Neuroimaging |
| This task, in which subjects are asked to match perceived force with self-generated force, is a measure of sensory attenuation (SA), which is the reduction in the perceived intensity of stimuli generated by one’s actions, compared to externally generated stimuli. Thus, SA relates to sense of agency. | ||
| Intentional binding (IB) paradigms (Hughes et al., 2013) | Variable (minutes) | Neurophysiological/Neuroimaging |
| In a standard IB paradigm, subjects are requested to provide estimates, direct numerical judgements or motor responses (pressing a key) corresponding to the perceived temporal interval between the voluntary action and the external sensory consequence. | ||
| Action monitoring task with visual feedbacks of the movement (Mahon et al., 2020) | Variable (minutes) |
Neurophysiological/ Neuroimaging |
| This experimental paradigm is used to investigate how sensory information regarding an action (e.g., visual information about target position) is integrated to monitor the consequences of an action, that is motor control. | ||
| The sense of agency scale (Tapal et al., 2017) | 5 minutes | Self-Reported |
| This scale quantifies perceived control over one’s mind, body, and the immediate environment. | ||
| Interoception Attention Task* (Avery et al., 2014) | 20 minutes | Neuroimaging |
| This fMRI task has been designed to investigate the neural correlates of interoceptive attention toward visceral sensations vs. brain response patterns underlying exteroceptive attention to visually presented targets. | ||
| Multidimensional Assessment of Interoceptive Awareness*(Mehling et al., 2012) | 5 minutes | Self-Reported |
| This scale is a self-report measure of interoceptive body awareness. |
The interoception construct is currently not included in any RDoC domain.
Finally, while much of the work on FMD and early adversity has focused on the first years of life, prenatal exposure to maternal stress can also lead to serious neurodevelopmental consequences and compromised cognitive and social-emotional processing (Wu et al., 2020). Thus, investigating the relationship between prenatal stress exposure and risk for FMD may represent a further area of research.
3.2. Cognitive systems
This domain includes several constructs and subconstructs related to different cognitive processes, from attention to perception and cognitive control, which are fundamental to flexibly respond to the environment and perform goal-directed behaviors. Several studies in FMD patients have investigated dysregulations in this domain, particularly with regard to attention and cognitive control.
3.2.1. Attention
As commonly observed during clinical assessment of patients with FMD, there is a need for explicit attention towards the movement for impairment to manifest and be maintained, and a normalization of movement when attention is diverted. Consistent with this, Schwingenschuh and colleagues (Schwingenschuh et al., 2011) found that patients with functional tremor showed changes in tremor frequency when asked to tap at a different rhythm with another limb, even to the extent of complete entrainment with the frequency of tapping. Likewise, asking patients with functional leg weakness to flex their unaffected hip caused their unattended affected hip to automatically extend (Ziv et al., 1998). Attention dysregulations were specifically assessed in a further study (Kumru et al., 2007) comparing healthy volunteers with patients with functional tremor, essential tremor and PD using a dual task paradigm, which is one of the tools proposed in the RDoC matrix to evaluate this construct. Patients with functional tremor had prolonged reaction time compared to individuals with essential tremor. The delay observed in FMD individuals may indicate an interference during dual task performance, which results from a bottleneck in central processing of attention, thus suggesting that attention is required for maintenance of functional movements. Moreover, a recent study reported that clinical improvement of patients with FMD following physiotherapy is associated with the normalization of the contingent negative variation, a neurophysiological measure of anticipatory attention (Teodoro et al., 2020).
This ‘disease-centered’ attention allocation appears to be done at the expenses of other information, leading to impairments in perception and other cognitive processes (Edwards et al., 2012). Other studies have also indicated that patients with FMD allocate increased attentional resources to their symptoms and selectively monitor somatic information related to the affected body part and/or function. Indeed, it has been observed that patients with functional tremor have greater visual attention toward the trembling limb when performing a motor task compared to patients with organic tremors (van Poppelen et al., 2011). Similarly, in subjects with functional lateralized paresis, simulating movements of the affected hand is associated with increased self-monitoring processes (Roelofs et al., 2006; Vuilleumier, 2005), and with increased engagement of the medial prefrontal cortex and superior temporal cortex (de Lange et al., 2007), which are involved in processing of information related to oneself (Frith U, Frith CD, 2003).
In line with this hypothesis, a study assessing objective (wristwatch-like accelerometer recordings) vs. subjective (self-reporting diaries) perception of tremor, found that patients with functional tremor reported 65 % more tremor than the one registered by actigraphy, compared to 28 % excess for patients with organic tremor (Pareés et al., 2012a). Alterations in perception (a further construct in the Cognitive Systems domain), together with attention dysregulation, may impact on high-order cognitive functions as well as on motor behavior in FMD patients, and future research should evaluate how the interplay between these constructs is linked to the emergence of sensorimotor symptoms. We suggest that a first step in this direction could be represented by conducting imaging studies using tasks such as the dot-probe task, the Rapid Visual Information Processing task, combined with movement detection measures (e.g., accelerometer recordings).
3.2.2. Cognitive control
Few studies have evaluated this construct in individuals with FMD, particularly with regard to response inhibition. To assess this subconstruct, Voon and colleagues (Voon et al., 2013) conducted a study using the Conner’s Continuous Performance Test II task, a computerized 14-minute visual performance task in which the subject responds to rapidly presented nontarget letters (‘go’) and inhibits responding to an infrequently shown target letter (‘no go’). Patients with FMD compared to controls made more commission errors (errors in withholding responding) on the go/no-go task relative to controls, while no differences in executive functioning were observed between the two groups, as measured by a neuropsychological battery. Failure to suppress a response is closely related to impulsivity, and impulsive decision-making has been reported in FMD patients compared to controls during the bead task, a probabilistic reasoning task (Pareés et al., 2012b). However, both patients and controls demonstrated similar levels of cognitive flexibility, as novel information were properly integrated to guide choices. Conversely, a recent study found a deficit in information processing associated with impaired decision-making in individuals with FMD, as indicated by reduced drift rate in the patient group, a parameter that quantifies the quality and rate of information accumulation (Sadnicka et al., 2020).
3.3. Arousal and regulatory system
This domain focuses on physiological responses to internal/external stimuli representing activation of neural systems. Within this domain, the arousal construct is closely related to the Negative Valence Systems, since it represents the sensitivity of an organism to various stimuli, including emotionally negative stimuli, and may vary with the intensity of the stimulus valence.
Few studies have examined the arousal construct outside the context of emotional valence in FMD patients. In a pioneering study, (Lader and Sartorius (1968) showed that patients with mixed FND symptoms had greater baseline arousal levels, as measured by spontaneous fluctuation in skin resistance, compared to both those with anxiety disorders and healthy volunteers. These findings were extended in a further study (Horvath et al., 1980), reporting that patients with remitted mixed functional symptoms had a failure to habituate skin conductance response to repeated acoustic stimuli, compared with controls with trait anxiety, More recently, Maurer and colleagues (Maurer et al., 2015) found that heart-rate variability, which is commonly used as a measure of autonomic arousal, was reduced in FMD patients compared to controls, similar to patients with depression, anxiety and PTSD, who also exhibit low heart-rate variability (Chalmers et al., 2014; Shah et al., 2013; Hartmann et al., 2018).
While these observations suggest that FMD is characterized by arousal dysregulations, future research is needed to investigate the mechanistic link between these alterations and those in the motor and negative valence domain. Furthermore, neurocircuits regulating arousal interact with those implicated in sleep-wakefulness, which represents a further construct in the Arousal and Regulatory Systems domain, and in motor control (Liu et al., 2020). Thus, evaluating the sleep construct in FMD patients may represent a novel area of investigation. To this extent, examples of objective and self-reported measures assessing this construct are provided in Table 2.
3.4. Sensorimotor systems
This domain has been recently added to the RDoC matrix to underscore the importance of motor symptoms across psychopathology (Garvey and Cuthbert, 2017; National Institute on Mental Health, 2020b). Alterations in motor processes are a common feature of many psychiatric disorders, including schizophrenia, mood disorders and OCD, although they have been traditionally neglected in both clinical practice and research (Peralta and Cuesta, 2017). These alterations, spanning from hypo- to hyperkinetic movements, and impairments in motor coordination and balance, seem to emerge from abnormalities in the reciprocal modulatory effects between cortical regions implicated in cognitive, social, and affective functions and cerebello-thalamo-cortical motor circuits and basal ganglia circuits (Aron et al., 2007; Alexander et al., 1990). These circuits work in close concert through both cortical and direct connections. Specifically, cortical regions mediating cognitive and emotional processes exert a top-down modulatory effects on cortical and subcortical regions implicated in motor function. The reverse modulation also takes place, namely from subcortical motor regions to motor cortex and other regions like the prefrontal cortex (bottom-up modulation) (Alexander et al., 1990; Bernard et al., 2016). Alterations in these modulatory processes have been observed in patients with catatonia, who show both affective-emotional and motor abnormalities (Northoff, 2002), and may also play an important role in the pathophysiology of FMD.
3.4.1. Motor action
This multifaceted construct comprises: ‘the processes that must be engaged during the planning and execution of a motor action’, and includes action planning and selection, sensorimotor dynamics, initiation, execution, inhibition and termination (National Institute on Mental Health, 2020a). Many studies have investigated these processes in FMD patients with the aim to identify where and how functional movements originated.
Clinical observations and neurophysiological studies have demonstrated the integrity of the pathway from the primary motor cortex (M1) to the muscles (Liepert et al., 2008). However, abnormal activity in M1 has been observed when patients with FMD thought about moving or attempted to move the affected limb. In both cases, M1 activity should increase, while decreased activation (Marshall et al., 1997; Cojan et al., 2009; Schrag et al., 2013) or even deactivation of M1 (Matt et al., 2019) has been found in individuals with FMD. Importantly, motor imagery or movement preparation was also associated with abnormal activity in prefrontal areas (Cojan et al., 2009; Matt et al., 2019; Nowak and Fink, 2009), thus suggesting that M1 inhibition may result from dysfunctions in top-down cortical modulation of motor circuits. Consistent with this, decreased activity in motor cortex has been observed in patients with functional dystonia during emotional processing (Espay et al., 2018b).
Further studies have also shown altered activation patterns in areas implicated in motor preparation and suppression of motor plans, such as the supplementary motor area (SMA) and the pre-SMA, both during execution of motor tasks and in the context of emotion-eliciting procedures (Nachev et al., 2007; Boy et al., 2010). These alterations seem to emerge from an abnormal interaction between these areas and prefrontal and subcortical regions. Specifically, there is evidence of decreased functional connectivity between the dlPFC and the SMA (Voon et al., 2011), while increased connectivity between the right amygdala and the right SMA has been observed in patients with FMD during emotional processing, using psychophysiological interaction analysis (Voon et al., 2010a). Limbic modulation of SMA may be mediated by amygdala projections through the basal ganglia and the thalamus to the SMA, as there are no direct connections between the amygdala and the SMA (Groenewegen et al., 1997). Interestingly, a fMRI study comparing pre- and post-rehabilitation brain activity during a Go/No-Go task in FMD patients, found that positive treatment outcomes were correlated with increased connectivity between amygdala and M1, and were associated with engagement of the SMA, caudate and putamen during the task (Faul et al., 2020).
Alterations in subcortical motor network have also been reported in FMD. Specifically, increased activity in bilateral cerebellum, bilateral thalamus and basal ganglia has been observed in patients with functional dystonia compared to normal controls and patients with organic dystonia, using a motor paradigm with the leg at rest, holding a posture and in motion (Schrag et al., 2013). Furthermore, enhanced activation of the cerebellum has been found in patients with functional tremor compared to those with essential tremor, and in patients with functional dystonia during a motor task (Espay et al., 2018b; Espay et al., 2018c).
It will be important to understand how alterations in these subcortical networks may yield to abnormalities in cortical motor areas since, while each motor circuit is active during any given activity, cortical and subcortical motor circuits are inter-related (DeLong and Wichmann, 2007). In addition, it will also be critical to map the extent to which motor circuit abnormalities directly impact other systems such as negative affect and cognition. For example, basal ganglia circuits also contribute to functions included in the Cognitive Systems domain allowing for flexible modulation of internally generated/externally evoked behavioral responses to environmental cues (Aron et al., 2007; Middleton and Strick, 2000). Additionally, both cerebellum and basal ganglia are implicated in emotion processing and regulation (Turner et al., 2007), but whether dysfunctions in these areas underlie affective-emotional abnormalities observed in some patients with FMD, directly or via modulatory effects on prefrontal areas, is a largely unexplored areas of research. To this extent, comparing patients with FMD not only to healthy controls or patients with organic neurological disorders, but also with those with a psychiatric disorder exhibiting motor manifestations, may help elucidate the link between motor and behavioral symptoms in a more integrated way. The RDoC system, providing tools to look across systems in a mechanistically informed way, offers particular strengths in this regard.
3.4.2. Agency and ownership
There is a substantial body of clinical and neurophysiological evidence indicating that neural pathways implicated in volitional motor control are recruited during functional movements (Schwingenschuh et al., 2011) (Reuber et al., 2002; Avbersek and Sisodiya, 2010; Hallett, 2010; van der Salm et al., 2010; Schwingenschuh et al., 2016; Baizabal-Carvallo and Fekete, 2015). However, patients with FMD feel that the abnormal excessive movement (or limb weakness) is involuntary, which may imply there is no perceived self-agency. The sense of agency refers to the feeling that ‘one is initiating, executing, and in control of one’s volitional actions and their sensory consequences’, and this construct has been specifically linked to FMD in the RDoC matrix. Indeed, several fMRI studies have reported abnormal patterns of activity and connectivity in areas believed to be critically involved in the sense of agency (i. e., temporoparietal junction (TPJ), dlPFC, pre-SMA) (Voon et al., 2010b; Nahab et al., 2010; Nahab et al., 2017). For example, Voon and colleagues (Voon et al., 2010b) using a within-subject design found that functional tremor compared to voluntary mimicked tremor was associated with decreased TPJ activity and lower connectivity between this area and regions involved in sensory feedback (sensorimotor cortices and cerebellar vermis) and limbic regions (ventral anterior cingulate and ventral striatum). Decreased connectivity between the right TPJ and sensorimotor cortex, cerebellar vermis, bilateral SMA, and right insula was observed in a further group of FMD patients (Maurer et al., 2016a), thus confirming dysfunctions of the agency network characterized this disorder.
Consistent with this, Kranick and colleagues (Kranick et al., 2013) found that patients with FMD had decreased action-effect binding for normal voluntary movements compared with healthy volunteers, suggesting a reduced sense of agency. The action-effect binding paradigm has been used to quantify the sense of self-agency, because it requires subjects to judge the perceived time between an action (e.g. button press) and the subsequent sensory consequence of this movement (e.g., a sound played thereafter) (Moore and Obhi, 2012). Subjective sensation that our actions are associated with an effect only occurs if we feel being the agent responsible for the action, and this perception of volition appears reduced in patients with FMD. A further indication of impaired sense of agency came from a study reporting loss of sensory attenuation in FMD patients compared to healthy controls (Pareés et al., 2014). Sensory attenuation describes the normal reduction of intensity of sensation caused by movements that are self-generated compared with by others, a phenomenon believed important in labeling movements as self-generated (Hughes et al., 2013).
Future studies may help to further characterize the neural correlates of the sense of agency and how impairment in this construct may lead to alterations in attention and in predictive processing, which is a key process of many cognitive functions as well as motor control. In this regard, a further important construct to consider is interoception. While distinct from attention, perception and agency, interoception – i.e. receiving, processing, and integrating body-relevant signals with external stimuli to affect ongoing motivated behavior (Craig, 2002) – is an important overlapping construct, which may play a key role in the pathophysiology of FMD. Indeed, continuous interoceptive inputs are thought to be critical for conscious forms of agentic action control and interoceptive awareness has been implicated in the generation of subjective motor-related feelings states (Marshall et al., 2018). The investigation of interoceptive processing in FMD is still in its infancy, with one study showing that patients with FMD compared to controls exhibited reduced interoceptive awareness, as measured by the heartbeat detection task (Ricciardi et al., 2016). Future work is needed to examine whether dysfunctions in interoceptive processing are associated with FMD, and particularly with impaired sense of agency. To this extent, Table 2 we provide some examples of task-based and self-reported measures used to assess the different dimensions of interoception.
4. The RDoC matrix and the genetics of FMD
Currently, the genetic basis of FMD, as well as of other FND, are a largely uncharted frontier, in contrast with other neuropsychiatric disorders. In the last two decades, the field of psychiatric genetics has grown enormously, due to advances in genomic research, coupled with large-scale collaborative efforts like the Psychiatric Genomics Consortium (Sullivan et al., 2018). These efforts have identified hundreds of common and rare genetic variations that, in interaction with each other and in conjunction with environmental events, contribute to a range of neuropsychiatric disorders. Importantly, a substantial fraction of genetic influences on these disorders transcends clinical diagnostic boundaries (Lee et al., 2019). Furthermore, many of the genes and variants that have been associated to neuropsychiatric disorders are necessary for brain circuitry development and function and could be linked to distinct brain-behavior domains, instead of multifaceted clinical syndromes. For instance, genetic variants in the serotoninergic system have been associated to alterations in fronto-amygdala circuitry and amygdala activity, which in turn underlie several constructs of the Negative Valence Systems (Savage et al., 2017). These finding highlight the value of using the RDoC framework to guide research into the genetic basis of complex and heterogenous disorders, as FMD. Indeed, a recent study has applied a similar transdiagnostic approach to identify genes and gene x environment interactions implicated in FMD pathophysiology (Spagnolo et al., 2020). This approach may yield reliable endophenotypes and biomarkers of FMD, thus being of pragmatic utility in the evaluation of patterns of individual pathogenesis.
5. RDoC-informed assessment of FMD
In addition to “mapping” the motor, behavioral and brain features of FMD into specific functional domains and neurocircuitry, the RDoC framework can also be used to implement a panel of instruments that researchers can use to perform a neuroscience-based, dimensional assessment of patients with FMD. In Table 2, we provide an example of such panel, which includes task-based, neurophysiological, self-report and behavioral measures, organized by domain. This battery integrates measures used in studies on FMD discussed above, a recently identified core of FMD clinical measures (Pick et al., 2020), and the domain-related instruments provided in the RDoC matrix (National Institute on Mental Health, 2020a), together with several assessment tools used in cognitive, affective and behavioral neuroscience.
Similar initiatives are underway worldwide for other neuropsychiatric disorders, such as the Addiction Neuroclinical Assessment (ANA) (Kwako et al., 2016) and the Addiction Neuroimaging Assessment (ANiA) (Voon et al., 2020), as well as the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) (Carter and Barch, 2007), with promising results.
The increasing recognition of FMD, which facilitates case diagnosis and ascertainment, and the growing number of collaborative research projects focused on these disorders, may promote the adoption of a common set of research tools and instruments assessing FMD phenotype across multiple domains. Furthermore, the collection of multidimensional information in patients with different overt symptoms or at different points in the clinical course of FMD may improve the depth, breadth, and specificity of characterization of the individual patient, leading, for example, to the identification of meaningful subtypes of the disorder.
6. Conclusions
In this review, we have provided a conceptual map of FMD symptoms and features into RDoC domains and constructs, with the goal of showing that research on these disorders may be conducted using an integrated approach focused on functional domains and their biological underpinnings. This approach offers the advantage to embrace experimental findings across multiple levels of analysis and to consider the multiplicity of factors (e.g., developmental, environmental, genetics) that shape brain-function-disorder relationships. By doing so, it may also facilitate the identification of endophenotypes and distinct clinical phenotypes, which can be used to better tailor treatment options.
However, we acknowledge that the use of a dimensional approach to FMD is not flawless as it does not completely avoid essentialism and reification and may overemphasize the overlap between different conditions. Furthermore, although we have highlighted the link between motor, behavioral and brain manifestations of FMD and multiple RDoC domains and constructs, not all individuals with these disorders show alterations in these domains. Indeed, the RDoC matrix may not include the totality of disturbances related to FMD but may represent a useful starting framework for further exploration. For example, several constructs in the Social Processes domain may be relevant to FMD, since they focus on the perception and interpretation of one’s self and others’ actions (National Institute on Mental Health, 2020a). These processes are critically linked to the ability to recognize an action as self-generated (agency), to acquire and integrate sensory data to understand and guide actions (perception) and, importantly, they also require simulating (‘mirroring’) observed actions to understand their goal, including their emotional value (Iacoboni and Dapretto, 2006; Iacoboni et al., 2005). Interestingly, alterations in mirror mechanisms have been suggested to play an important role in the definition of the motor phenotype exhibited by FMD patients (Pellicciari et al., 2014). Thus, future studies should investigate processes related to the Social Process domain in FMD patients. Furthermore, such studies should be integrated with the investigation of constructs related to perception and other cognitive processes, since this may yield to a better understanding of how functional movements are generated and maintained.
Certainly, there are many challenges that come with embracing and advancing a dimensional approach to FMD, however, we believe that this perspective may support the ongoing efforts to integrate psychiatry, neurology and neuroscience in the investigation of the underlying mechanisms of FMD, as well as may hold promises for optimizing clinical assessment and therapeutic management.
Acknowledgments
Funding
This work was supported by National Institute on Nuerological Disorders and Stroke (NINDS) Intramural Research Program, Bethesda, MD, USA.
Footnotes
Declaration of Competing Interest
The authors have nothing to disclose
References
- Alexander GE, Crutcher MD, DeLong MR, 1990. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res 85, 119–146 [published Online First: 1990/01/01]. [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C. [Google Scholar]
- Anna Kaplan R.O., 2004. Being Bertha Pappenheim–historiography and biography. Australas. Psychiatry 12 (1), 62–68. 10.1046/j.1039-8562.2003.02062.x [published Online First: 2005/02/18]. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, et al. , 2007. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J. Neurosci 27 (44), 11860–11864. 10.1523/jneurosci.3644-07.2007 [published Online First: 2007/11/06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avbersek A, Sisodiya S, 2010. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J. Neurol. Neurosurg. Psychiatr 81 (7), 719–725. 10.1136/jnnp.2009.197996 [published Online First: 2010/06/29]. [DOI] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, et al. , 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry 76 (3), 258–266. 10.1016/j.biopsych.2013.11.027 [published Online First: 2014/01/07]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybek S, Nicholson TR, O’Daly O, et al. , 2015. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One 10 (4), e0123273. 10.1371/journal.pone.0123273 [published Online First: 2015/04/11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybek S, Nicholson TR, Zelaya F, et al. , 2014. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 71 (1), 52–60. 10.1001/jamapsychiatry.2013.2842 [published Online First: 2013/11/22]. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ, 1994. The twenty-item Toronto Alexithymia Scale–I. Item selection and cross-validation of the factor structure. J. Psychosom. Res 38 (1), 23–32. 10.1016/0022-3999(94)90005-1 [published Online First: 1994/01/01]. [DOI] [PubMed] [Google Scholar]
- Baizabal-Carvallo JF, Fekete R, 2015. Recognizing uncommon presentations of psychogenic (functional) movement disorders. Tremor Other Hyperkinet. Mov. N. Y. (N Y) 5, 279. 10.7916/d8vm4b13 [published Online First: 2015/02/11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakvis P, Spinhoven P, Roelofs K, 2009. Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav.: E&B 16, 558–560. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1988a. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin. Psychol. Rev 8 (1), 77–100. [Google Scholar]
- Beck AT, Epstein N, Brown G, et al. , 1988b. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol 56 (6), 893–897. 10.1037//0022-006x.56.6.893 [published Online First: 1988/12/01]. [DOI] [PubMed] [Google Scholar]
- Bègue I, Adams C, Stone J, et al. , 2019. Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? Neuroimage Clin. 22, 101798. 10.1016/j.nicl.2019.101798 [published Online First: 2019/05/31]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA, 2016. Differential motor and prefrontal cerebello-cortical network development: evidence from multimodal neuroimaging. NeuroImage 124 (Pt A), 591–601. 10.1016/j.neuroimage.2015.09.022 [published Online First: 2015/09/24]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, et al. , 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 27 (2), 169–190. 10.1016/s0145-2134(02)00541-0 [published Online First: 2003/03/05]. [DOI] [PubMed] [Google Scholar]
- Blakemore RL, Sinanaj I, Galli S, et al. , 2016. Aversive stimuli exacerbate defensive motor behaviour in motor conversion disorder. Neuropsychologia 93 (Pt A), 229–241. 10.1016/j.neuropsychologia.2016.11.005 [published Online First: 2016/11/15]. [DOI] [PubMed] [Google Scholar]
- Boy F, Husain M, Singh KD, et al. , 2010. Supplementary motor area activations in unconscious inhibition of voluntary action. Exp. Brain Res 206 (4), 441–448. 10.1007/s00221-010-2417-x [published Online First: 2010/09/28]. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. , 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28 (2), 193–213. 10.1016/0165-1781(89)90047-4 [published Online First: 1989/05/01]. [DOI] [PubMed] [Google Scholar]
- Carson A, Epidemiology, Lehn A, 2016. Handbook of Clinical Neurology, 139, pp. 47–60. 10.1016/b978-0-12-801772-2.00005-9 [published Online First: 2016/10/11]. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM, 2007. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr. Bull 33 (5), 1131–1137. 10.1093/schbul/sbm081 [published Online First: 2007/07/17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ, et al. , 2014. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatry 5, 80. 10.3389/fpsyt.2014.00080 [published Online First: 2014/07/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojan Y, Waber L, Carruzzo A, et al. , 2009. Motor inhibition in hysterical conversion paralysis. NeuroImage 47 (3), 1026–1037. 10.1016/j.neuroimage.2009.05.023 [published Online First: 2009/05/20]. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3 (8), 655–666. 10.1038/nrn894 [published Online First: 2002/08/03]. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, 2014. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology 51 (12), 1205–1206. 10.1111/psyp.12342 [published Online First: 2014/11/13]. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126. 10.1186/1741-7015-11-126 [published Online First: 2013/05/16]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 2006. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol 61 (8), 741–756. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Roelofs K, Toni I, 2007. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia 45 (9), 2051–2058. 10.1016/j.neuropsychologia.2007.02.002 [published Online First: 2007/03/21]. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T, 2007. Circuits and circuit disorders of the basal ganglia. Arch. Neurol 64 (1), 20–24. 10.1001/archneur.64.1.20 [published Online First: 2007/01/11]. [DOI] [PubMed] [Google Scholar]
- Demartini B, D’Agostino A, Gambini O, 2016. From conversion disorder (DSM-IV-TR) to functional neurological symptom disorder (DSM-5): when a label changes the perspective for the neurologist, the psychiatrist and the patient. J. Neurol. Sci 360, 55–56. 10.1016/j.jns.2015.11.026 [published Online First: 2016/01/03]. [DOI] [PubMed] [Google Scholar]
- Diez I, Larson AG, Nakhate V, et al. , 2020. Early-life trauma endophenotypes and brain circuit-gene expression relationships in functional neurological (conversion) disorder. Mol. Psychiatry 10.1038/s41380-020-0665-0 [published Online First: 2020/02/14]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Fani N, Hallett M, et al. , 2020. A framework for understanding the pathophysiology of functional neurological disorder. CNS Spectr. 1–7. 10.1017/s1092852920001789 [published Online First: 2020/09/05]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Adams RA, Brown H, et al. , 2012. A Bayesian account of ‘hysteria’. Brain 135 (Pt 11), 3495–3512. 10.1093/brain/aws129 [published Online First: 2012/05/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Fotopoulou A, Pareés I, 2013. Neurobiology of functional (psychogenic) movement disorders. Curr. Opin. Neurol 26 (4), 442–447. 10.1097/WCO.0b013e3283633953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejareh Dar M, Kanaan RA, 2016. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr. Dis. Treat 12, 143–153. 10.2147/ndt.S65880 [published Online First: 2016/02/03]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayake V, Kranick S, LaFaver K, et al. , 2017. Personality traits in psychogenic nonepileptic seizures (PNES) and psychogenic movement disorder (PMD): neuroticism and perfectionism. J. Psychosom. Res 97, 23–29. 10.1016/j.jpsychores.2017.03.018 [published Online First: 2017/06/14]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erro R, Brigo F, Trinka E, et al. , 2016. Psychogenic nonepileptic seizures and movement disorders. A comparative review. Neurol. Clin. Pract 6 (2), 138–149. 10.1212/cpj.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Aybek S, Carson A, et al. , 2018a. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol. 75 (9), 1132–1141. 10.1001/jamaneurol.2018.1264 [published Online First: 2018/06/06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Maloney T, Vannest J, et al. , 2018b. Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov. Disord 33 (1), 136–145. 10.1002/mds.27217 [published Online First: 2017/11/11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Maloney T, Vannest J, et al. , 2018c. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin. 17, 179–187. 10.1016/j.nicl.2017.10.020 [published Online First: 2017/11/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul L, Knight LK, Espay AJ, et al. , 2020. Neural activity in functional movement disorders after inpatient rehabilitation. Psychiatry Res. Neuroimaging 303, 111125. 10.1016/j.pscychresns.2020.111125 [published Online First: 2020/06/26]. [DOI] [PubMed] [Google Scholar]
- Feinstein A, Stergiopoulos V, Fine J, et al. , 2001. Psychiatric outcome in patients with a psychogenic movement disorder: a prospective study. Neuropsychiatry Neuropsychol. Behav. Neurol 14 (3), 169–176. [PubMed] [Google Scholar]
- Frith U, Frith CD, 2003. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond., B, Biol. Sci 358 (1431), 459–473. 10.1098/rstb.2002.1218 [published Online First: 2003/04/12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Cuthbert BN, 2017. Developing a motor systems domain for the NIMH RDoC program. Schizophr. Bull 43 (5), 935–936. 10.1093/schbul/sbx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, 2003. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin. Neurophysiol 114, 1557–1579. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB, 1997. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J. Psychopharmacol. (Oxf. Engl.) 11 (2), 99–106. 10.1177/026988119701100202 [published Online First: 1997/01/01]. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB, 2013. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci 14 (7), 488–501. 10.1038/nrn3524 [published Online First: 2013/06/21]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, 2010. Physiology of psychogenic movement disorders. J. Clin. Neurosci 17 (8), 959–965. 10.1016/j.jocn.2009.11.021 [published Online First: 2010/05/25]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, et al. , 2002. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage 17 (1), 317–323. 10.1006/nimg.2002.1179 [published Online First: 2002/12/17]. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Schmidt FM, Sander C, et al. , 2018. Heart rate variability as Indicator of clinical state in depression. Front. Psychiatry 9, 735. 10.3389/fpsyt.2018.00735 [published Online First: 2019/02/02]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa T, Sebastian A, Liepert J, et al. , 2017. Symptom-specific amygdala hyperactivity modulates motor control network in conversion disorder. Neuroimage Clin. 15, 143–150. 10.1016/j.nicl.2017.04.004 [published Online First: 2017/05/23]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, et al. , 2016. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol 6 (2), 603–621. 10.1002/cphy.c150015 [published Online First: 2016/04/12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilti CC, Hilti LM, Heinemann D, et al. , 2010. Impaired performance on the Rapid Visual Information Processing task (RVIP) could be an endophenotype of schizophrenia. Psychiatry Res. 177 (1–2), 60–64. 10.1016/j.psychres.2009.12.012 [published Online First: 2010/01/30]. [DOI] [PubMed] [Google Scholar]
- Horvath T, Friedman J, Meares R, 1980. Attention in hysteria: a study of Janet’s hypothesis by means of habituation and arousal measures. Am. J. Psychiatry 137 (2), 217–220. 10.1176/ajp.137.2.217 [published Online First: 1980/02/01]. [DOI] [PubMed] [Google Scholar]
- Hughes G, Desantis A, Waszak F, 2013. Mechanisms of intentional binding and sensory attenuation: the role of temporal prediction, temporal control, identity prediction, and motor prediction. Psychol. Bull 139 (1), 133–151. 10.1037/a0028566 [published Online First: 2012/05/23]. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M, 2006. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci 7 (12), 942–951. 10.1038/nrn2024 [published Online First: 2006/11/23]. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, et al. , 2005. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 3 (3), e79. 10.1371/journal.pbio.0030079 [published Online First: 2005/03/02]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. , 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167 (7), 748–751. 10.1176/appi.ajp.2010.09091379 [published Online First: 2010/07/03]. [DOI] [PubMed] [Google Scholar]
- Keynejad RC, Frodl T, Kanaan R, et al. , 2019. Stress and functional neurological disorders: mechanistic insights. J. Neurol. Neurosurg. Psychiatr 90 (7), 813–821. 10.1136/jnnp-2018-318297 [published Online First: 2018/11/10]. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, et al. , 1995. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med 57 (1), 23–31. 10.1097/00006842-199501000-00004 [published Online First: 1995/01/01]. [DOI] [PubMed] [Google Scholar]
- Kozlowska K, Brown K, Palmer D, et al. , 2021. Specific biases for identifying facial expression of emotion in children and adolescents with conversion disorders. Psychosom. Med 75. [DOI] [PubMed] [Google Scholar]
- Kranick SM, Moore JW, Yusuf N, et al. , 2013. Action-effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov. Disord 28 (8), 1110–1116. 10.1002/mds.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranick SM, Gorrindo T, Hallett M, 2011a. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics 52 (2), 109–116. 10.1016/j.psym.2010.12.017 [published Online First: 2011/03/15]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranick S, Ekanayake V, Martinez V, et al. , 2011b. Psychopathology and psychogenic movement disorders. Mov. Disord 26 (10), 1844–1850. 10.1002/mds.23830 [published Online First: 2011/06/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Begeman M, Tolosa E, et al. , 2007. Dual task interference in psychogenic tremor. Mov. Disord 22 (14), 2077–2082. 10.1002/mds.21670 [published Online First: 2007/08/04]. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, et al. , 2016. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol. Psychiatry 80 (3), 179–189. 10.1016/j.biopsych.2015.10.024 [published Online First: 2016/01/17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader M, Sartorius N, 1968. Anxiety in patients with hysterical conversion symptoms. J. Neurol. Neurosurg. Psychiatr 31 (5), 490–495. 10.1136/jnnp.31.5.490 [published Online First: 1968/10/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFaver K, 2020. Treatment of functional movement disorders. Neurol. Clin 38 (2), 469–480. 10.1016/j.ncl.2020.01.011 [published Online First: 2020/04/14]. [DOI] [PubMed] [Google Scholar]
- Lee Phil, Anttila Verneri, Won Hyejung, Feng Yen-Chen, Rosenthal Jacob, Zhu Zhaozhong, Tucker-Drob Elliot, Nivard Michel, Grotzinger Andrew, Posthuma Danielle, Wang Meg, Yu Dongmei, Rujescu Dan, Tooney Paul, Grünblatt Edna, Falkai Peter, Faraone Stephen, Gelernter Joel, Mathews Carol, Nærland Terje P.Y., 2019. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 179 (7), 1469–1482. 10.1016/j.cell.2019.11.020e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Hassa T, Tüscher O, et al. , 2008. Electrophysiological correlates of motor conversion disorder. Mov. Disord 23 (15), 2171–2176. 10.1002/mds.21994 [published Online First: 2008/09/12]. [DOI] [PubMed] [Google Scholar]
- Liu D, Li W, Ma C, et al. , 2020. A common hub for sleep and motor control in the substantia nigra. Science 367 (6476), 440–445. 10.1126/science.aaz0956. [DOI] [PubMed] [Google Scholar]
- Løvstad M, Sigurdardottir S, Andersson S, et al. , 2016. Behavior rating inventory of executive function adult version in patients with neurological and neuropsychiatric conditions: symptom levels and relationship to emotional distress. J. Int. Neuropsychol. Soc. JINS 22 (6), 682–694. 10.1017/s135561771600031x [published Online First: 2016/04/30]. [DOI] [PubMed] [Google Scholar]
- Loh S, Lamond N, Dorrian J, et al. , 2004. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav. Res. Methods Instrum. Comp 36 (2), 339–346. 10.3758/bf03195580 [published Online First: 2004/09/10]. [DOI] [PubMed] [Google Scholar]
- Lovallo W, 1975. The cold pressor test and autonomic function: a review and integration. Psychophysiology 12 (3), 268–282. 10.1111/j.1469-8986.1975.tb01289.x [published Online First: 1975/05/01]. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N, 2013. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77 (3), 406–424. 10.1016/j.neuron.2013.01.020 [published Online First: 2013/02/12]. [DOI] [PubMed] [Google Scholar]
- Ludwig L, Pasman JA, Nicholson T, et al. , 2018. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 5 (4), 307–320. 10.1016/s2215-0366(18)30051-8 [published Online First: 2018/03/13]. [DOI] [PubMed] [Google Scholar]
- Mahon A, Bendžiūte S, Hesse C, et al. , 2020. Shared attention for action selection and ˙ action monitoring in goal-directed reaching. Psychol. Res 84 (2), 313–326. 10.1007/s00426-018-1064-x [published Online First: 2018/08/12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JC, Halligan PW, Fink GR, et al. , 1997. The functional anatomy of a hysterical paralysis. Cognition 64 (1), B1–8. 10.1016/s0010-0277(97)00020-6 [published Online First: 1997/07/01]. [DOI] [PubMed] [Google Scholar]
- Marshall AC, Gentsch A, Schütz-Bosbach S, 2018. The interaction between Interoceptive and action states within a framework of predictive coding. Front. Psychol 9, 180. 10.3389/fpsyg.2018.00180 [published Online First: 2018/03/09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti S, Sigman M, Dehaene S, 2012. A shared cortical bottleneck underlying attentional blink and psychological refractory period. NeuroImage 59 (3), 2883–2898. 10.1016/j.neuroimage.2011.09.063 [published Online First: 2011/10/13]. [DOI] [PubMed] [Google Scholar]
- Matt E, Amini A, Aslan T, et al. , 2019. Primary motor cortex deactivation as a new mechanism of motor inhibition in conversion paralysis. Mov. Disord 34 (1), 148–149. 10.1002/mds.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, LaFaver K, Limachia GS, et al. , 2018. Gray matter differences in patients with functional movement disorders. Neurology 91 (20), e1870–e1879. 10.1212/wnl.0000000000006514 [published Online First: 2018/10/12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, LaFaver K, Ameli R, et al. , 2015. A biological measure of stress levels in patients with functional movement disorders. Parkinsonism Relat. Disord 21 (9), 1072–1075. 10.1016/j.parkreldis.2015.06.017 [published Online First: 2015/06/29]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, LaFaver K, Ameli R, et al. , 2016a. Impaired self-agency in functional movement disorders: a resting-state fMRI study. Neurology 87 (6), 564–570. 10.1212/wnl.0000000000002940 [published Online First: 2016/07/08]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, Liu VD, LaFaver K, et al. , 2016b. Impaired resting vagal tone in patients with functional movement disorders. Parkinson. Relat. Disord 30, 18–22. 10.1016/j.parkreldis.2016.06.009 [published Online First: 2016/06/24]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, et al. , 2020. Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol. Psychiatry 25 (5), 993–1005. 10.1038/s41380-018-0215-1 [published Online First: 2018/08/19]. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L, 1971. Manual for the Profi le of Mood States. Educational and Industrial Testing Service, San Diego. [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, et al. , 2012. The multidimensional assessment of interoceptive awareness (MAIA). PLoS One 7 (11), e48230. 10.1371/journal.pone.0048230 [published Online First: 2012/11/08]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL, 2000. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 42 (2), 183–200. 10.1006/brcg.1999.1099 [published Online First: 2000/04/04]. [DOI] [PubMed] [Google Scholar]
- Moore JW, Obhi SS, 2012. Intentional binding and the sense of agency: a review. Conscious. Cogn 21 (1), 546–561. 10.1016/j.concog.2011.12.002 [published Online First: 2012/01/14]. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, et al. , 2016. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41 (1), 80–102. 10.1038/npp.2015.166 [published Online First: 2015/06/13]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, et al. , 2007. The role of the pre-supplementary motor area in the control of action. NeuroImage 36 (Suppl 2(3–3)), T155–63. 10.1016/j.neuroimage.2007.03.034 [published Online First: 2007/05/15]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahab FB, Kundu P, Gallea C, et al. , 2010. The neural processes underlying self-agency. Cereb. Cortex 21 (1), 48–55. 10.1093/cercor/bhq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahab FB, Kundu P, Maurer C, et al. , 2017. Impaired sense of agency in functional movement disorders: an fMRI study. PLoS One 12 (4), e0172502. 10.1371/journal.pone.0172502 [published Online First: 2017/04/28]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Mental Health, 2020a. RDoC Matrix 2016 [Available from: https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/constructs/rdoc-matrix.shtml Accessed September 9th, 2020.
- National Institute on Mental Health, 2020b. RDoC Changes to the Matrix (CMAT) Workgroup Update: Addition of the Sensorimotor Domain 2018 [Available from: https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/rdoc-changes-to-the-matrix-cmat-workgroup-update-addition-of-the-sensorimotor-domain.shtml Accessed Sepptember 9th, 2020.
- Nicholson TR, Aybek S, Craig T, et al. , 2016. Life events and escape in conversion disorder. Psychol. Med 46 (12), 2617–2626. 10.1017/s0033291716000714 [published Online First: 2016/07/06]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, 2002. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav. Brain Sci 25 (5), 555–577 discussion 78–604. doi: [published Online First: 2003/09/10]. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Fink GR, 2009. Psychogenic movement disorders: aetiology, phenomenology, neuroanatomical correlates and therapeutic approaches. NeuroImage 47 (3), 1015–1025. 10.1016/j.neuroimage.2009.04.082 [published Online First: 2009/05/12]. [DOI] [PubMed] [Google Scholar]
- Pareés I, Brown H, Nuruki A, et al. , 2014. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain 137 (11), 2916–2921. 10.1093/brain/awu237. [DOI] [PubMed] [Google Scholar]
- Pareés I, Saifee TA, Kassavetis P, et al. , 2012a. Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain 135 (Pt 1), 117–123. 10.1093/brain/awr292 [published Online First: 2011/11/15]. [DOI] [PubMed] [Google Scholar]
- Pareés I, Kassavetis P, Saifee TA, et al. , 2012b. ‘Jumping to conclusions’ bias in functional movement disorders. J. Neurol. Neurosurg. Psychiatr 83 (4), 460–463. 10.1136/jnnp-2011-300982 [published Online First: 2012/02/18]. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Superbo M, Gigante AF, et al. , 2014. Disease modeling in functional movement disorders. Parkinsonism Relat. Disord 20 (11), 1287–1289. 10.1016/j.parkreldis.2014.09.017 [published Online First: 2014/09/30]. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ, 2017. Motor abnormalities: from neurodevelopmental to neurodegenerative through “Functional” (Neuro) psychiatric disorders. Schizophr. Bull 43 (5), 956–971. 10.1093/schbul/sbx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, 2020. The CODES trial for dissociative seizures: a landmark study and inflection point. Lancet Psychiatry 7 (6), 464–465. 10.1016/s2215-0366(20)30143-7 [published Online First: 2020/05/24]. [DOI] [PubMed] [Google Scholar]
- Perez DL, Dworetzky BA, Dickerson BC, et al. , 2015. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin. EEG Neurosci 46 (1), 4–15. doi. 10.1177/1550059414555905 [published Online First: 2014/11/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Matin N, Barsky A, et al. , 2017. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J. Neurol. Neurosurg. Psychiatr 88 (6), 491–497 doi. 10.1136/jnnp-2016-314998 [published Online First: 2017/04/19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick S, Anderson DG, Asadi-Pooya AA, et al. , 2020. Outcome measurement in functional neurological disorder: a systematic review and recommendations. J. Neurol. Neurosurg. Psychiatr 91 (6), 638–649. 10.1136/jnnp-2019-322180 [published Online First: 2020/03/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree A, Morrison I, Olausson H, et al. , 2019. Using facial electromyography to assess facial muscle reactions to experienced and observed affective touch in humans. J. Visual. Exper.: JoVE (145) doi: 10.3791/59228 [published Online First: 2019/04/02]. [DOI] [PubMed] [Google Scholar]
- Reuber M, Fernández G, Bauer J, et al. , 2002. Interictal EEG abnormalities in patients with psychogenic nonepileptic seizures. Epilepsia 43 (9), 1013–1020. 10.1046/j.1528-1157.2002.52301.x [published Online First: 2002/08/30]. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Demartini B, Crucianelli L, et al. , 2016. Interoceptive awareness in patients with functional neurological symptoms. Biol. Psychol 113, 68–74. 10.1016/j.biopsycho.2015.10.009 [published Online First: 2015/11/04]. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, et al. , 2002. The continuous performance test: a window on the neural substrates for attention? Arch. Clin. Neuropsychol 17 (3), 235–272 [published Online First: 2003/11/01]. [PubMed] [Google Scholar]
- Rinck M, Becker ES, 2007. Approach and avoidance in fear of spiders. J. Behav. Ther. Exp. Psychiatry 38 (2), 105–120. 10.1016/j.jbtep.2006.10.001 [published Online First: 2006/11/28]. [DOI] [PubMed] [Google Scholar]
- Roelofs JJ, Teodoro T, Edwards MJ, 2019. Neuroimaging in functional movement disorders. Curr. Neurol. Neurosci. Rep 19 (3), 12. 10.1007/s11910-019-0926-y [published Online First: 2019/02/13]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, Keijsers GP, Hoogduin KA, et al. , 2002. Childhood abuse in patients with conversion disorder. Am. J. Psychiatry 159 (11), 1908–1913. 10.1176/appi.ajp.159.11.1908 [published Online First: 2002/11/02]. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Spinhoven P, Sandijck P, et al. , 2005. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J. Nerv. Ment. Dis 193 (8), 508–514. 10.1097/01.nmd.0000172472.60197.4d [published Online First: 2005/08/06]. [DOI] [PubMed] [Google Scholar]
- Roelofs K, de Bruijn ER, Van Galen GP, 2006. Hyperactive action monitoring during motor-initiation in conversion paralysis: an event-related potential study. Biol. Psychol 71 (3), 316–325. 10.1016/j.biopsycho.2005.07.002 [published Online First: 2005/08/23]. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Daum C, Meppelink AM, et al. , 2020. Reduced drift rate: a biomarker of impaired information processing in functional movement disorders. Brain 143 (2), 674–683. 10.1093/brain/awz387 [published Online First: 2019/12/23]. [DOI] [PubMed] [Google Scholar]
- Savage JE, Sawyers C, Roberson-Nay R, et al. , 2017. The genetics of anxiety-related negative valence system traits. Am. J. Med. Genet. Part B, Neuropsychiatr. Genet 174 (2), 156–177. 10.1002/ajmg.b.32459 [published Online First: 2016/05/20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, et al. , 2008. Evidence for recovery of fear following immediate extinction in rats and humans. Learn. Mem 15 (6), 394–402. 10.1101/lm.909208 [published Online First: 2008/05/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag AE, Mehta AR, Bhatia KP, et al. , 2013. The functional neuroimaging correlates of psychogenic versus organic dystonia. Brain 136 (Pt 3), 770–781. 10.1093/brain/awt008 [published Online First: 2013/02/26]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingenschuh P, Katschnig P, Seiler S, et al. , 2011. Moving toward “laboratory-supported” criteria for psychogenic tremor. Mov. Disord 26 (14), 2509–2515. 10.1002/mds.23922 [published Online First: 2011/10/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingenschuh P, Saifee TA, Katschnig-Winter P, et al. , 2016. Validation of “laboratory-supported” criteria for functional (psychogenic) tremor. Mov. Disord 31 (4), 555–562. 10.1002/mds.26525. [DOI] [PubMed] [Google Scholar]
- Seignourel PJ, Miller K, Kellison I, et al. , 2007. Abnormal affective startle modulation in individuals with psychogenical movement disorder. Mov. Disord 22 (9), 1265–1271. 10.1002/mds.21451. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, et al. , 2013. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol. Psychiatry 73 (11), 1103–1110. 10.1016/j.biopsych.2013.01.019 [published Online First: 2013/02/26]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Cornelisse S, Quaedflieg CW, et al. , 2012. Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 37 (12), 1998–2008. 10.1016/j.psyneuen.2012.04.012 [published Online First: 2012/05/23]. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Norato G, Maurer CW, et al. , 2020. Effects of TPH2 gene variation and childhood trauma on the clinical and circuit-level phenotype of functional movement disorders. J. Neurol. Neurosurg. Psychiatr 91 (8), 814–821. 10.1136/jnnp-2019-322636 [published Online First: 2020/06/25]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Crimlisk HL, Cope H, et al. , 2000. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet 355 (9211), 1243–1244. [DOI] [PubMed] [Google Scholar]
- Stone J, Carson A, Duncan R, et al. , 2010. Who is referred to neurology clinics?–The diagnoses made in 3781 new patients. Clin. Neurol. Neurosurg 112 (9), 747–751. 10.1016/j.clineuro.2010.05.011 [published Online First: 2010/07/22]. [DOI] [PubMed] [Google Scholar]
- Stone J, Zeman A, Simonotto E, et al. , 2007. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom. Med 69 (9), 961–969. [DOI] [PubMed] [Google Scholar]
- Stone J, Sharpe M, Binzer M, 2004. Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics 45 (6), 492–499. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, et al. , 2018. Psychiatric genomics: an update and an agenda. Am. J. Psychiatry 175 (1), 15–27. 10.1176/appi.ajp.2017.17030283 [published Online First: 2017/10/04]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan T, Tesar GE, Jimenez XF, 2016. Biomarkers in the diagnosis and study of psychogenic nonepileptic seizures: a systematic review. Seizure 35, 11–22. 10.1016/j.seizure.2015.12.011 [published Online First: 2016/01/18]. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Thier P, Leube DT, et al. , 2010. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain 133 (Pt 1), 262–271. 10.1093/brain/awp291 [published Online First: 2009/12/10]. [DOI] [PubMed] [Google Scholar]
- Tapal A, Oren E, Dar R, et al. , 2017. The sense of agency scale: a measure of consciously perceived control over one’s mind, body, and the immediate environment. Front. Psychol 8 (1552) 10.3389/fpsyg.2017.01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca C, Rapetti M, Carta MG, et al. , 2012. Women and hysteria in the history of mental health. Clin. Pract. Epidemiol. Mental Health: CP & EMH 8, 110–119. 10.2174/1745017901208010110 [published Online First: 2012/11/02]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro T, Koreki A, Meppelink AM, et al. , 2020. Contingent negative variation: a biomarker of abnormal attention in functional movement disorders. Eur. J. Neurol 27 (6), 985–994. 10.1111/ene.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble M, Reynolds EH, 2016. A brief history of hysteria: from the ancient to the modern. Handb. Clin. Neurol 139, 3–10. 10.1016/b978-0-12-801772-2.00001-1 [published Online First: 2016/10/11]. [DOI] [PubMed] [Google Scholar]
- Turner BM, Paradiso S, Marvel CL, et al. , 2007. The cerebellum and emotional experience. Neuropsychologia 45 (6), 1331–1341. 10.1016/j.neuropsychologia.2006.09.023 [published Online First: 2006/11/25]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegom M, Homberg JR, MJAG, Henckens, 2017. Modulation of the hypothalamic-pituitary-Adrenal Axis by early life stress exposure. Front. Cell. Neurosci 11 (87) 10.3389/fncel.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Feltz-Cornelis CM, Allen SF, 2020. Van Eck van der Sluijs JF. Childhood sexual abuse predicts treatment outcome in conversion disorder/functional neurological disorder. An observational longitudinal study. Brain Behav. 10 (3) 10.1002/brb3.1558e01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Salm SM, Koelman JH, Henneke S, et al. , 2010. Axial jerks: a clinical spectrum ranging from propriospinal to psychogenic myoclonus. J. Neurol 257 (8), 1349–1355. 10.1007/s00415-010-5531-6 [published Online First: 2010/03/31]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poppelen D, Saifee TA, Schwingenschuh P, et al. , 2011. Attention to self in psychogenic tremor. Mov. Disord 26 (14), 2575–2576. 10.1002/mds.23911 [published Online First: 2011/10/26]. [DOI] [PubMed] [Google Scholar]
- van Wouwe NC, Mohanty D, Lingaiah A, et al. , 2020. Impaired action control in patients with functional movement disorders. J. Neuropsychiatry Clin. Neurosci 32 (1), 73–78. 10.1176/appi.neuropsych.19030076 [published Online First: 2019/10/08]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, 2008. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci 12 (11), 418–424. 10.1016/j.tics.2008.07.005 [published Online First: 2008/09/19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, 2014. Functional neurological disorders: imaging. Neurophysiol. Clin 44 (4), 339–342. 10.1016/j.neucli.2014.07.003 [published Online First: 2014/10/13]. [DOI] [PubMed] [Google Scholar]
- Voon V, Cavanna AE, Coburn K, et al. , 2016. Functional neuroanatomy and neurophysiology of functional neurological disorders (Conversion disorder). J. Neuropsychiatry Clin. Neurosci 28 (3), 168–190. [DOI] [PubMed] [Google Scholar]
- Voon V, Ekanayake V, Wiggs E, et al. , 2013. Response inhibition in motor conversion disorder. Mov. Disord 28 (5), 612–618. 10.1002/mds.25435 [published Online First: 2013/04/05]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, et al. , 2011. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov. Disord 26 (13), 2396–2403. 10.1002/mds.23890 [published Online First: 2011/09/22]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Grodin E, Mandali A, et al. , 2020. Addictions NeuroImaging Assessment (ANIA): towards an integrative framework for alcohol use disorder. Neurosci. Biobehav. Rev 113, 492–506. 10.1016/j.neubiorev.2020.04.004 [published Online First: 2020/04/17]. [DOI] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, et al. , 2010a. Emotional stimuli and motor conversion disorder. Brain 133 (Pt 5), 1526–1536. 10.1093/brain/awq054 [published Online First: 2010/04/08]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Gallea C, Hattori N, et al. , 2010b. The involuntary nature of conversion disorder. Neurology 74 (3), 223–228. 10.1212/WNL.0b013e3181ca00e9 [published Online First: 2010/01/20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, 2005. Hysterical conversion and brain function. Prog. Brain Res 150, 309–329. 10.1016/s0079-6123(05)50023-2 [published Online First: 2005/09/28]. [DOI] [PubMed] [Google Scholar]
- Williams B, Ospina JP, Jalilianhasanpour R, et al. , 2019. Fearful attachment linked to childhood abuse, Alexithymia, and depression in motor functional neurological disorders. J. Neuropsychiatry Clin. Neurosci 31 (1), 65–69. 10.1176/appi.neuropsych.18040095 [published Online First: 2018/11/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissel BD, Dwivedi AK, Merola A, et al. , 2018. Functional neurological disorders in Parkinson disease. J. Neurol. Neurosurg. Psychiatr 89 (6), 566–571. 10.1136/jnnp-2017-317378 [published Online First: 2018/03/20]. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lu Y-C, Jacobs M, et al. , 2020. Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Network Open 3 (1). 10.1001/jamanetworkopen.2019.19940 e1919940-e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv I, Djaldetti R, Zoldan Y, et al. , 1998. Diagnosis of “non-organic” limb paresis by a novel objective motor assessment: the quantitative Hoover’s test. J. Neurol 245 (12), 797–802. 10.1007/s004150050289 [published Online First: 1998/12/05]. [DOI] [PubMed] [Google Scholar]