Summary
A higher proportion of CD68-positive tumour associated macrophages (TAMs) has been associated with poorer outcomes in HIV-negative patients with Hodgkin lymphoma (HL), but whether this is true in HIV-positive patients with HL is not known. In this study, we investigated the number of CD68-positive TAMs and expression of programmed cell death-ligand 1 (PD-L1) in lymph node specimens from HL patients and correlated expression with clinical features (HIV status, disease severity and survival) and histopathological features (EBV latent positivity and subtype of HL).
We stained archived lymph node specimens from 77 patients diagnosed with HL for CD68 and PD-L1. Stains were graded as: CD68 low (≤25%), CD68 high (>25%), PD-L1 low (≤50%), and PD-L1 high (>50%). Expression levels were correlated with the clinical and histopathological features using bivariate and multivariate analyses. Survival was analysed by overall and progression-free survival.
Thirty-four of the 77 included patients (44%) were HIV-positive. EBV latency was detected in 97% of HIV-positive HL patients and in 14% of HIV-negative HL patients. A high CD68 score was associated with lower median haemoglobin levels (9.4 vs 11.4 g/dL; p=0.02), platelet numbers (262 vs 424 cells ×109/L; p=0.01), and lymphocyte numbers (0.99 vs 1.70 cells ×109/L, p=0.01) and a trend towards advanced disease (international prognostic score ≥4; hazard ratio 2.4; confidence interval 0.89–6.47; p=0.08). HIV status did not affect CD68 or PD-L1 expression. A higher proportion of CD68-positive TAMs was found in samples that were EBV-positive. HIV positivity and EBV negativity correlated with poorer survival. CD68 and PD-L1 expression were not predictive of survival.
High CD68 expression was associated with EBV positivity but not HIV positivity and did not predict adverse outcomes. PD-L1 expression was unaffected by HIV status or EBV positivity and did predict adverse outcomes.
Keywords: Hodgkin lymphoma, HIV, Epstein–Barr virus, South Africa, tumour associated macrophage, tumour microenvironment
INTRODUCTION
Classical Hodgkin lymphoma (HL) is a B-cell lymphoma. Due to immune system dysfunction, HIV-infected persons have a seven-fold increased risk of HL compared with age and gender matched persons from the general population, even in the era of antiretroviral therapy.1 Differences in tumour histology and biology have been described in HIV-positive and HIV-negative HL patients, but studies in well resourced settings have shown no difference in outcome between these two patient groups.2 However, in sub-Saharan Africa, survival outcomes are significantly poorer in HIV-positive HL patients.3,4 In HIV-negative patients, current treatment strategies cure around 80% of patients.5 In order to improve this, and particularly in the setting of relapsed/refractory disease, targeted biological therapies for HL have been approved. These therapies include the anti-CD30 antibody, brentuximab,6,7 and programmed cell death-1 ligand (PD-L1) inhibitors.8 The development and use of these novel agents have been guided by an understanding of the tumour biology and the tumour microenvironment (TME).
HL is unique in that it is characterised by a paucity of tumour cells. HL tumour cells are Hodgkin Reed–Sternberg (HRS) and Hodgkin mononuclear cells. These large cells arise from crippled B cells and occupy a small proportion (1–5%) of the overall tumour. They are enveloped by inflammatory cells (macrophages, CD4-positive and CD8-positive T cells, plasma cells, eosinophils, and other cells) comprising the TME.9 The TME has been associated with treatment outcomes in HIV-negative HL patients; a high proportion of tumour associated macrophages (TAMs) resulted in treatment failure and poorer outcome,10–14 although this was not supported by all studies.15 There are variations in the tumour biology and TME of HIV-positive and HIV-negative HL patients. These include differences in proportions of mixed cellularity HL,16 latent Epstein–Barr virus (EBV) infection in HRS cells,17,18 CD4-positive and CD8-postive T cells (inverted CD4:CD8 ratio), and the pattern of T cells surrounding HRS cells.19–21 One small study has shown similar proportions of CD68-positive TAMs in HIV-positive and HIV-negative HL patients,22 but this study did not look at survival outcomes or correlate CD68 expression with EBV positivity.
TAMs and HRS cells express PD-L1, and this is a key mechanism by which HRS cells achieve immune evasion. PD-L1 binds to the PD-1 receptor (CD279) on the surface of antigen-experienced T cells and induces immune tolerance by suppressing T-cell activation. PD-1 inhibitors work by interrupting this interaction, thereby enhancing tumour cell recognition by T cells.8,23 In HRS cells, increased PD-L1 expression is attributed to copy gains of chromosome 9p24.1, which includes the PD-L1, PD-L2, and JAK2 loci, and directly increases the level of PD-L1 and PD-L2 protein expression. An indirect increase in PD-L1 and PD-L2 protein expression is also achieved through augmented JAK-STAT signalling24,25 which is likely the predominant mechanism by which EBV-positive HL induces PD-L1 expression.26
In this study, using archived lymph node tissue from patients with HL, we correlate the expression of CD68-positive TAMs and PD-L1 expression with histopathological factors (EBV latency and HL subtype) and clinical factors (HIV status, HL stage, and survival).
METHODS
Patients
Formalin-fixed, paraffin-embedded lymph node tissue specimens from 77 patients diagnosed with HL between 2004 and 2018 were obtained from the archives of the National Health Laboratory Service at Groote Schuur Hospital (GSH), Cape Town. GSH is one of two major tertiary referral hospitals in Cape Town and South Africa. Patients were included if there was sufficient tissue available for further immunohistochemical staining. Demographic and baseline clinical characteristics including HIV status, modified Lugano staging,27 International Prognostic Score (IPS),28 treatment details, and treatment outcome were extracted by retrospective chart review. The study was approved by the University of Cape Town and hospital ethics review boards (HREC 610/2016). Patient consent was waived in view of the retrospective nature of the study.
Study patients were treated by institutional protocol with ABVD chemotherapy regimen (doxorubicin, bleomycine, vinblastine, dacarbazine). For early stage disease (I and II), ABVD was given for two cycles followed by involved-site radiation therapy (IFRT) or up to six cycles for patients either at risk of long-term complications for radiation therapy or with unfavourable early stage disease (as defined by the German Hodgkin Study Group).27 For advanced stage disease (III and IV), ABVD was given for six to eight cycles. Response to therapy was assessed after cycle two of ABVD, in most cases using computed tomography (CT), and where available positron emission tomography-CT (PET-CT). Salvage chemotherapy was given to patients with primary progressive or relapsed disease, followed by autologous transplantation in patients with responsive disease.
Morphology and immunohistochemical staining
Tissue sections of 3–4 mm thickness were cut from formalin-fixed, paraffin-embedded tissue blocks. Sections were stained with haematoxylin and eosin stain and visualised using light microscopy. The World Health Organization (WHO) 2017 Classification of Tumours of Haematopoietic and Lymphoid Tissues was used to classify the HL subtype.29 Tissue sections, original diagnostic reports, and immunohistochemical staining results were reviewed by an expert anatomical pathologist (DC).
CD30 and CD15 staining had been undertaken as part of the routine clinical work-up. For this study, we performed new immunohistochemical stains for CD68 and PD-L1. If EBV-encoded small RNA (EBER) in situ hybridisation (EBER-ISH) had not been done during the routine clinical work-up, we tested latent membrane protein-1 (LMP1) expression to assess EBV positivity (because LMP-1 is cheaper than EBER-ISH and detects latent EBV with high sensitivity in HL.30) Three-micron sections were cut from the tissue blocks, placed onto silanised slides and heat fixed on a hotplate at 75°C for 30 min. Tissue sections were then dewaxed in xylene, cleared in ethanol, and rehydrated in water. All immunohistochemistry stains were performed with the Envision Detection System on a Dako Autostainer (Universal Staining System; Dako, Denmark) using routine staining protocols and the antibodies listed in Table 1. EBER-ISH was performed using the Ventana ISH iVIEW Blue Plus Detection Kit (Ventana Medical Systems, USA) on the BenchMark ULTRA IHC/ISH System (automated slide stainer).
Table 1.
Antibodies used
| Antibody | Clone | Dilution (PBS) | Antigen retrieval | Control | Supplier |
|---|---|---|---|---|---|
| CD30 | Ber-H2 | 1:400 | Tris-EDTA | HL lymph node | Dako, Denmark |
| CD15 | MMA | Ready to use | Tris-EDTA | HL lymph node | Roche, Switzerland |
| LMP-1 | Cs.1-4 | 1:300 | Tris-EDTA | Nasopharyngeal carcinoma | Dako, Denmark |
| CD68 | PG-M1 | 1:50 | Protease | Lymph node | Abcam, USA |
| PD-L1 | B7–H1/CD274 | 1:300 | Citric acid | Placenta | Sino Biological, USA |
EDTA, ethylenediamine tetraacetic acid; HL, Hodgkin lymphoma.
CD68 and PD-L1 stains were graded only in areas containing tumour cells; areas with fibrosis, medium or large blood vessels, residual reactive lymph nodes, and necrosis or crush artefacts were excluded. The CD68 stain was graded as one (<5% of the TME is positive), two (5–25% of the TME is positive) and three (>25% of the TME is positive) as per Tan et al.13 For regression modelling, categories one and two were combined into a single category (‘CD68 low’) and compared with category three (‘CD68 high’). The PD-L1 stain was graded as low (<50%) or high (≥50%); there was no established method of quantification from the literature we identified. On original analysis the PD-L1 groups groups were: <5%, 5–10%, 11–25%, 25–50%, and >50%; however, there was no association between any of these categories and the variables analysed and the group was simplified with a 50% cut-off for grading the stain.
Statistical analysis
Categorical and continuous variables were summarised as frequencies and percentages or medians and interquartile ranges (IQRs), respectively. Univariate comparisons between categorical variables were made with the chi-squared test. Medians for non-parametric data were compared using the Wilcoxon rank-sum test, or the Kruskal–Wallis test for categorical variables with more than two groups.
For each patient, overall survival (OS) was calculated as the time between the date of diagnosis and the date of death or last follow-up for censored cases. Progression-free survival (PFS) was calculated as the time from the date of diagnosis until date of relapse, progression, or death from any cause. A multivariable Cox proportional hazards model was developed to assess the impact of variables on OS and PFS. Covariates in the Cox proportional hazards model were: HIV status, age, IPS, HL stage, EBV positivity, CD68 expression, and PD-L1 expression. The Kaplan–Meier method was used to estimate survival curves, and differences between survival distributions were determined using the log-rank test. Two-sided p values less than 0.05 indicated statistical significance.
STATA v14 (StataCorp)31 was used for all descriptive and quantitative analyses.
RESULTS
Clinical features
Of the 77 included patients, 44% were HIV-positive (n=34). HIV-positive participants were older than HIV-negative participants (median age 36 vs 27 years; p=0.04). In HIV-positive patients, the median number of CD4-positive cells was 194 cells/mm3 (IQR 123–275). Twenty-seven HIV-positive patients (79%) were on antiretroviral therapy at the time of diagnosis, and 19 (56%) had a lower than detectable viral load. A high proportion of patients (56%) had stage IV disease, and 34% had advanced disease indicated by an IPS ≥4 (Table 2).
Table 2.
Demographics and clinical data
| Demographics | Total (n=77) | HIV-negative (n=43) | HIV-positive (n=34) | p |
|---|---|---|---|---|
| No. (%) | No. (% of HIV-negative) | No. (% of HIV-positive) | ||
| Male sex | 38 (49) | 21 (49) | 17 (50) | 0.92 |
| Age, years, median (IQR) | 31 (25–43) | 27 (24–43) | 36 (30–43) | 0.03 |
| <30 | 32 (42) | 24 (56) | 8 (24) | 0.013 |
| 30–50 | 37 (48) | 15 (35) | 22 (65) | |
| >50 | 8 (10) | 4 (9) | 4 (12) | |
| Stage | ||||
| 1 | 5 (6) | 1 (2) | 4 (12) | 0.089 |
| 2 | 19 (25) | 14 (33) | 5 (15) | |
| 3 | 7 (9) | 5 (12) | 2 (6) | |
| 4 | 46 (60) | 22 (52) | 24 (71) | |
| Blood results, median (IQR) | ||||
| Haemoglobin, g/dL, n=77 | 10.8 (8.9–12.4) | 10.8 (8.9–12.6) | 10.6 (8.9–12.3) | 0.45 |
| Platelet, cells×109/L, n=73 | 398 (215–494) | 460 (314–568) | 270 (199–424) | 0.005 |
| Total white cell count, cells×109/L, n=77 | 9.9 (6.4–14.7) | 13.7 (9.3–16.6) | 6.5 (4.3–9.0) | <0.01 |
| Lymphocyte count, cells×109/L, n=65 | 1.43 (0.8–2.2) | 1.69 (1.4–2.6) | 1.01 (0.7–1.9) | 0.018 |
| Lactate dehydrogenase, IU/L, n=72 | 470 (352–609) | 477 (365–631) | 437 (352–585) | 0.5 |
| ESR, mm/h, n=55 | 78 (39–122) | 78 (32–107) | 83 (46–127) | 0.362 |
| Albumin, g/L, n=74 | 37 (27–41) | 37 (32–42) | 36 (24–39) | 0.1 |
| IPS ≥4 (high risk) | 26 (34) | 14 (33) | 12 (35) | 0.8 |
| EBV positive | 39 (51) | 6 (14) | 33 (97) | <0.01 |
| HL subtype | ||||
| Nodular sclerosing | 56 (73) | 38 (88) | 18 (53) | 0.003 |
| Mixed cellularity | 10 (13) | 2 (5) | 8 (24) | |
| Lymphocyte rich | 0 (0) | 0 | 0 (0) | |
| Lymphocyte depleted | 2 (3) | 1 (2) | 1 (3) | |
| HL unspecified | 9 (12) | 2 (5) | 7 (21) | |
| Treatment | ||||
| Primary treatment | ||||
| ABVD chemotherapy +/− radiation | 75 (97) | 43 (57) | 32 (43) | 0.32 |
| Died before chemo | 2 (3) | 0 (0) | 2 (100) | 0.46 |
| Secondary treatment | ||||
| Other therapy with curative intent (i.e., DHAP, IGEV or ICE) | 10 (16) | 9 (29) | 1 (3) | 0.04 |
| Autologous stem-cell transplantation | 5 (8) | 5 (16) | 0 | 0.08 |
Data are median (interquartile range) or n/N(%).
DHAP, dexamethasone, high dose ara-C, cisplatin; EBV, Epstein–Barr virus; ESR, erythrocyte sedimentation rate; HL, Hodgkin lymphoma; ICE, ifosfamide, carboplatin, etoposide; IGEV, ifosfamide, gemcitabine, vinorelbine; IPS, International Prognostic Score; IQR, interquartile range.
First-line treatment in all cases was ABVD chemotherapy with or without radiation according to the institutional protocol. Fifteen patients had relapsed or refractory disease of whom only three were HIV-positive. Ten of these 15 patients (nine HIV-negative, one HIV-positive patient) were fit enough to receive salvage chemotherapy with curative intent. Five of these 10 patients went on to have an autologous stem-cell transplant and they were HIV-negative (Table 2).
Histological features
The most frequent histological HL subtype was nodular sclerosing HL in HIV-positive and HIV-negative HL patients. However, the proportion of mixed cellularity HL was higher in HIV-positive HL patients (24% vs 4%, p<0.01). Positivity for EBV latent infection was found in 14% (6/43) of HIV-negative cases and in 97% (33/34) of HIV-positive cases (p<0.01).
The percentage of CD68-positive macrophages was <5% in 17% of patients (13/77), 5–25% in 47% of patients (36/77), and >25% in 28% of patients (36/77). The association of these categories with histological and clinical variables is shown in Table 3. When the groups were categorised as low (<25%) or high (>25%) in univariate analysis, high CD68 expression was more likely to be associated with EBV latent infection [hazard ratio (HR) 6.9; confidence interval (CI) 2.3–20.2; p<0.01], and HIV infection (HR 2.9; CI 1.1–7.5; p=0.03). There was a non-statistically significant association of high CD68 expression with the mixed cellularity HL subtype (HR 3.1; CI 0.8–12.5; p=0.1). In the multivariate model, CD68 expression was still associated with EBV positivity (HR 25; CI, 2.6–256; p=0.06), but the association with HIV infection and mixed cellularity was no longer observed (HR 0.19; CI 0.13–2.68; p=0.15; and HR 1.7; CI 0.37–7.4; p=0.51, respectively). This signifies that HIV infection and the mixed cellularity HL subtype are not independently associated with increased CD68 expression, but rather are a consequence of EBV positivity. High CD68 expression was not associated with high PD-L1 expression (HR 1.38; CI 0.5–3.5; p=0.49).
Table 3.
Clinical and histopathological correlations with CD68 and PD-L1 expression
| Total (%) | CD68 <5% | CD68 5–25% | CD68 >25% | p | PD-L1 low | PD-L1 high | p | |
|---|---|---|---|---|---|---|---|---|
| HIV status | ||||||||
| Positive | 34 (44) | 2 | 15 | 17 | 0.02 | 15 | 19 | 0.54 |
| Negative | 43 (56) | 11 | 21 | 11 | 22 | 21 | ||
| EBV latent status (LMP-1/EBER-ish) | ||||||||
| Negative | 38 (49) | 2 | 15 | 22 | <0.00 | 21 | 17 | 0.21 |
| Positive | 39 (51) | 11 | 21 | 6 | 16 | 23 | ||
| CD15 | ||||||||
| CD15− | 5 (6) | 13 | 33 | 26 | 0.57 | 2 | 3 | 0.71 |
| CD15+ | 72 (94) | 0 | 3 | 2 | 35 | 37 | ||
| PD-L1 | ||||||||
| Low | 37 (12) | 8 | 17 | 12 | 0.53 | |||
| High | 40 (52) | 5 | 19 | 16 | ||||
| HL subtype | ||||||||
| Nodular sclerosing | 56 (72) | 10 | 29 | 17 | 0.2 | 26 | 30 | 0.88 |
| Mixed cellularity | 10 (13) | 0 | 4 | 6 | 6 | 4 | ||
| Other | 11 (14) | 3 | 3 | 5 | 5 | 6 | ||
| IPS ≥4 | 26 (34) | 2 | 10 | 14 | 0.05 | 10 | 16 | 0.23 |
| Stage IV disease | 45 (58) | 7 | 19 | 20 | 0.19 | 18 | 27 | 0.09 |
| Peripheral blood counts | ||||||||
| Haemoglobin, g/dL | 10.8 (8.9–12.4) | 10.8 (9.1–12.4) | 11.7 (9.5–12.9) | 9.4 (8.7–10.9) | 0.057 | 10.8 (8.9–12.6) | 10.5 (8.5–12.2) | 0.39 |
| Platelets, cells×109/L | 398 (215–494) | 425 (301–603) | 424 (290–517) | 262 (168–439) | 0.032 | 424 (268–520) | 315 (192–486) | 0.13 |
| Total white cell count, cells×109/L | 9.8 (6.4–14.7 | 12.9 (8.2–15.7) | 10.2 (7.1–15.4) | 7.25 (4.23–11.4) | 0.01 | 11.2 (7.0–14.9) | 8.2 (5.3–13.8) | 0.21 |
| Lymphocytes, cells×109/L | 1.4 (0.8–2.2) | 1.7 (1.4–2.6) | 1.4 (1.0–2.3) | 1 (0.5–1.6) | 0.020 | 1.8 (1.2–3.0) | 1.4 (0.7–1.7) | 0.08 |
Data are median (interquartile range) or n/N (%).
EBV, Epstein–Barr virus; HL, Hodgkin lymphoma; IPS, International Prognostic Score.
Patients with a high CD68 score were more likely to show features of bone marrow suppression with a lower median haemoglobin (9.4 vs 11.4 g/dL; p=0.02), lower platelet count (262 vs 424 cells ×109/L, p=0.01), and lower lymphocyte count (0.99 vs 1.70 cells ×109/L, p=0.01). Patients with a high CD68 score also showed a trend towards more advanced disease (IPS ≥4), but this trend was not statistically significant (HR 2.4; CI 0.89–6.47; p=0.08).
PD-L1 expression was low in 48% (37/77) and high in 52% (40/77) of patients. High PD-L1 expression was not associated with HIV status (HR 1.32; CI 0.5–3.3; p=0.54), disease severity nor survival. In addition, high PD-L1 expression was not associated with EBV status (HR 1.77; CI 0.71–4.3; p=1.25), nor the mixed cellularity HL subtype (HR 1.74; CI 0.45–6.74; p=0.8).
Treatment and survival outcomes
After a median follow-up of 51 months (range 0.65–149 months), the OS at 2 and 5 years was 89% (95% CI 79–94) and 67.8% (95% CI 59–78), respectively; and the PFS at 2 and 5 years was 75% (95% CI 63–84) and 66% (95% CI 53–76), respectively. Univariate analysis showed that the following factors were not predictive for OS nor PFS: HIV status, age >45 years, stage four disease, EBV positivity, CD68 expression, and PD-L1 expression. An IPS ≥4 trended towards a poorer OS (HR 2.2; 95% CI 0.98–4.97; p=0.06) but this trend was not statistically significant. EBV-negative patients had a higher risk of relapse, with a lower PFS (HR 3.3; 95% CI 0.5–3.22; p=0.01). Kaplan–Meier curves show the effect of HIV status, EBV status, CD68 expression, and PD-L1 expression on OS (Fig. 1) and PFS (Fig. 2).
Fig. 1.
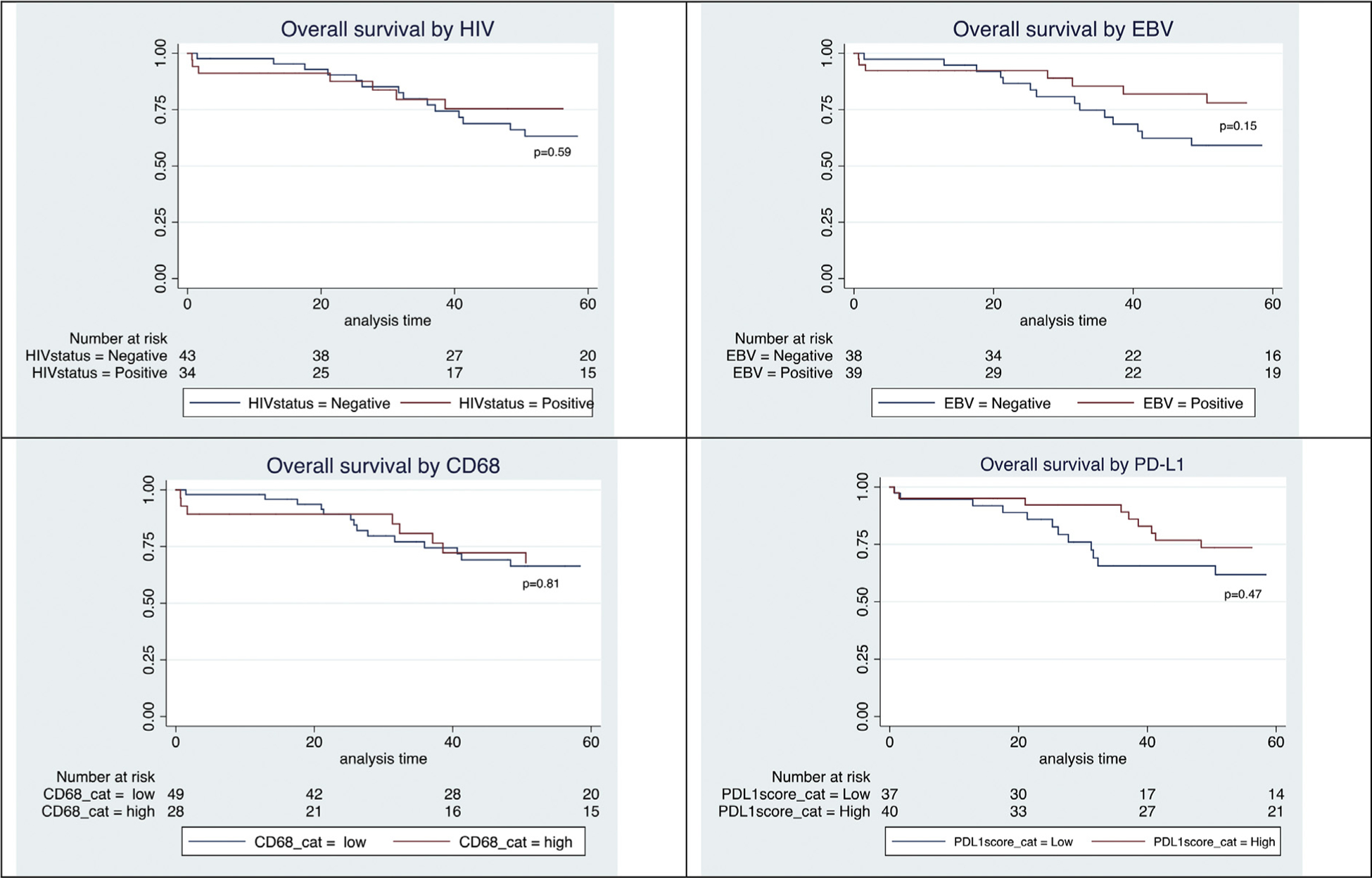
(A–D) Kaplan–Meier curves for overall survival.
Fig. 2.
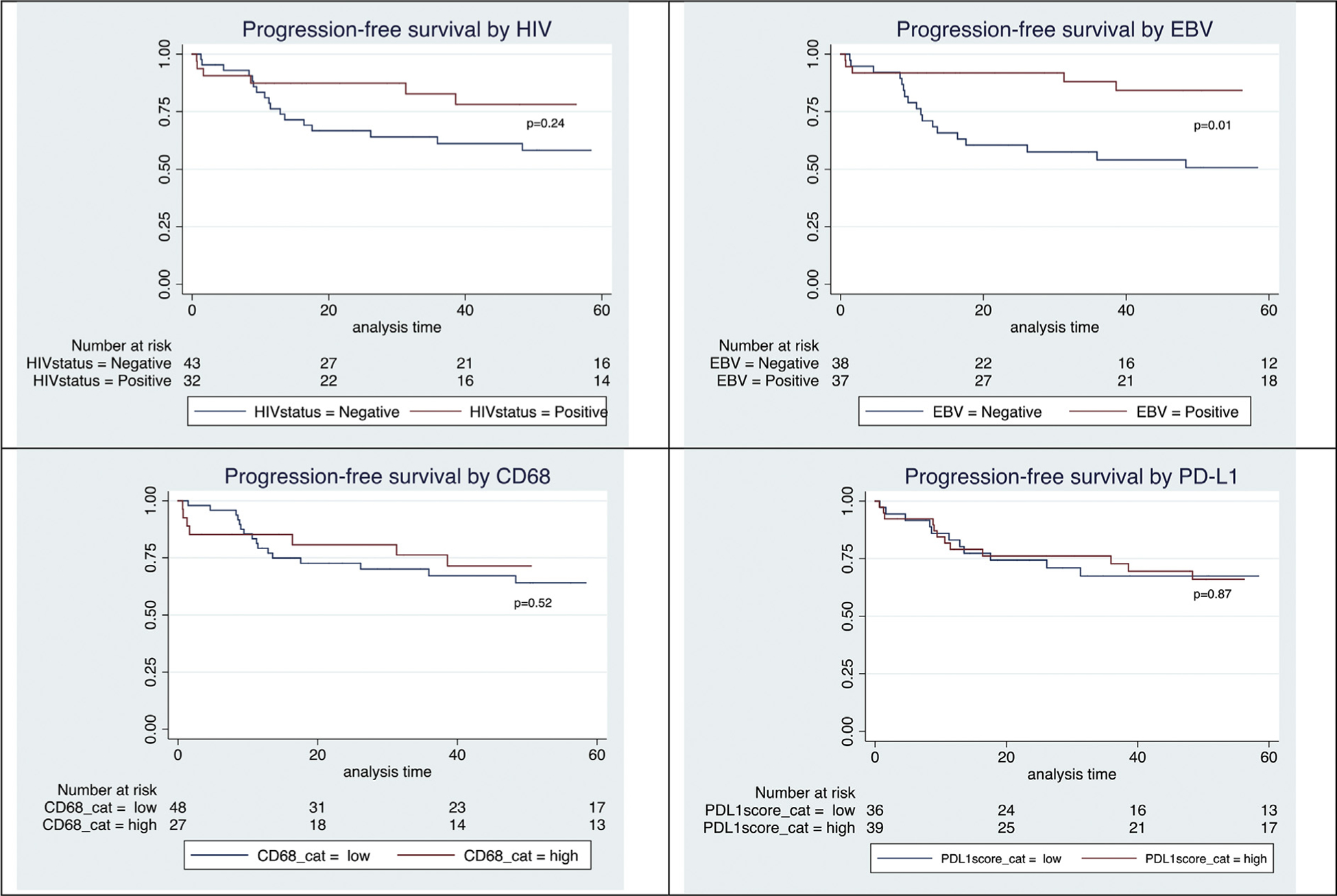
(A–D) Kaplan–Meier curves for progression-free survival.
A multivariate model for OS and PFS is shown in Table 4. In the multivariate model, HIV positivity predicted statistically poorer OS and PFS (HR 7.5; CI 1.05–53.7; p=0.05; and HR 8.8; CI 1.26–61.67; p=0.03). EBV negativity also predicted lower OS and PFS (HR 12.5; CI 1.61–100; p=0.02; and HR 33.3; CI 3.8–100; p≤0.01). The remaining variables were not statistically significant, although the IPS again showed a trend towards poorer OS.
Table 4.
Multivariate Cox model for OS and PFS
| Variable | HR for OS | p | 95% CI | HR for PFS | p | 95% CI |
|---|---|---|---|---|---|---|
| HIV-positive (vs negative) | 7.50 | 0.05 | 1.05–53.70 | 8.80 | 0.03 | 1.26–61.67 |
| Age >45 (vs ≤45) | 1.81 | 0.24 | 0.67–4.83 | 0.76 | 0.68 | 0.21–2.78 |
| IPS >4 (vs ≤4) | 2.57 | 0.06 | 0.97–6.80 | 2.22 | 0.11 | 0.82–5.99 |
| Stage 4 (vs stages 1–3) | 1.20 | 0.73 | 0.44–3.27 | 1.59 | 0.39 | 0.56–4.50 |
| EBV-negative (vs positive) | 12.5 | 0.02 | 1.61–100.0 | 33.3 | 0.00 | 3.84–100.0 |
| CD68 high (vs low) | 1.40 | 0.45 | 0.58–3.39 | 1.29 | 0.62 | 0.48–3.45 |
| PD-L1 high (vs low) | 0.68 | 0.39 | 0.28–1.66 | 0.97 | 0.94 | 0.40–2.32 |
CI, confidence interval; EBV, Epstein–Barr virus; HR, hazard ratio; IPS, International Prognostic Score; OS, overall survival; PFS, progression-free survival.
DISCUSSION
In this study, we investigated the expression of CD68 and PD-L1 in lymph node tissue of HL patients, and evaluated correlation of this with clinical and histopathological features. We revealed higher CD68 expression in EBV-positive HL. Neither CD68 nor PD-L1 expression impacted on survival. An interesting finding from the study was that EBV-positive patients had a better overall survival, and given the high rates of EBV positivity in HIV-positive HL this effect might mask the negative impact of HIV on survival.
Almost 100% of HIV-positive HL patients are EBV-positive, whereas approximately 40% of HIV-negative patients are EBV-positive. Regional variation and dissimilarity has also been reported among race groups.30,32,33 PD-L1 is produced by HRS cells and macrophages. In HIV-negative HL patients, chromosome 9p24 amplification in HRS cells increases PD-L1 and PD-L2 expression.24,25 In EBV-positive HL patients, PD-L1 expression is increased by direct effects of EBV. LMP-1 is an EBV protein that activates the NF-kB, JAK/STAT, and P12–K pathways, all of which recruit macrophages and increase PD-L1 expression, thereby evading T-cell immunity. Differences in the mutational status of chromosome 9 between EBV-positive and negative cases may partly explain why EBV-positive HL had improved survival, this needs further investigation.
PD-L1 is expressed predominately by TAMs and to a lesser degree by HRS.34 In this study, PD-L1 expression did not correlate with the number of CD68-positive TAMs in the tumour samples, suggesting that CD68-positive TAMs are not the main producers of PD-L1 in EBV-positive HL. Further study of the PD-L1/PD-1 axis in EBV-positive HL is required, using immunohistochemical techniques to elucidate the topography of PD-L1 in relation to the surrounding cells.
The effect of EBV positivity on survival in HL patients is controversial, with some studies showing improved survival,35–38 some showing poorer survival,39–42 and others showing no difference.43–47 EBV-positive HL is associated with extreme ages (<15 and >45 years), the mixed cellularity HL subtype, male sex, and HIV infection, which makes is difficult to accurately quantify the effect of EBV positivity on survival. Almost all HIV-positive HL patients are also EBV-positive, which means the CI in our multivariate model was wide (only a few included patients were HIV-positive and EBV-negative). Despite these limitations, the effect of EBV positivity on improved survival is interesting, and considering the high proportion of EBV latency in HIV-positive HL one does wonder if EBV positivity may have masked the negative impact on OS in other studies. In our study, the 17 HIV-negative patients with disease progression, relapse, or death were all EBV-negative.
Our study has several limitations. Firstly, it would have been preferable to use PET-CT to evaluate the response to therapy, but PET-CT was not consistently available. Secondly, it might have been more accurate to quantify the proportion of CD68-positive cells as a percentage initially before looking for correlations. Furthermore, quantification of immunohistochemical stains may have been improved by having more than one reviewing pathologist, and by using a computer assisted stereology system. Thirdly, we only included patients with sufficient lymph node tissue, which may have introduced selection bias because all patients with a primary diagnosis on other tissues (which may have represented a more aggressive type of HL) were excluded. For example, up to 39% of all HIV-positive patients in our local setting are diagnosed with HL based on analysis of bone marrow samples.4
CONCLUSION
In summary, a higher proportion of CD68-positive TAMs was seen in EBV-positive HL and correlated with disease severity but did not affect survival. PD-L1 expression was not affected by HIV and EBV status or by HL subtypes. Further research is needed to elucidate the relationship of different cells with the TME and to identify which cells in EBV-positive and HIV-positive HL predominantly produce PD-L1. The expression of PD-L1 in HIV-positive HL patients supports the use of PD-1 antibodies, but further clinical research is needed. Improved OS in EBV-positive patients with HL may help to explain why HIV-positive HL patients do not have a poorer outcome than HIV-negative counter-parts in first world settings and from this research we advise that EBV latency is accounted for within a multivariate model when analysing outcomes in HIV-positive HL. However, the existing literature remains inconclusive and further study is warranted.
Conflicts of interest and sources of funding:
The research reported in this publication was supported by the Fogarty International Center of the US National Institutes of Health (grant numbers: D43-TW010345 and D43-TW010543), the Discovery Foundation, and the Peter Jacobs Bursary Trust. EV was supported in part by the Thuthuka grant (TTK14052268878). The authors state that there are no conflicts of interest to disclose. The funders had no role in the design of the study; the collection, analysis, and interpretation of data; or writing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med 2018; 378: 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol 2012; 30: 4111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antel K, Levetan C, Mohamed Z, et al. The determinants and impact of diagnostic delay in lymphoma in a TB and HIV endemic setting. BMC Cancer 2019; 19: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swart L, Novitzky N, Mohamed Z, Opie J. Hodgkin lymphoma at Groote Schuur Hospital, South Africa: the effect of HIV and bone marrow infiltration. Ann Hematol 2019; 98: 381–9. [DOI] [PubMed] [Google Scholar]
- 5.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013; 31: 684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez-Espiridion B, Martin-Moreno AM, Montalban C, et al. Immunohistochemical markers for tumor associated macrophages and survival in advanced classical Hodgkin’s lymphoma. Haematologica 2012; 97: 1080–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. Soc Hematol 2012; 30: 2183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin’s lymphoma microenvironment and its role. J Pathol 2010; 221: 248–63. [DOI] [PubMed] [Google Scholar]
- 10.Gotti M, Nicola M, Lucioni M, et al. Independent prognostic impact of tumour-infiltrating macrophages in early-stage Hodgkin’s lymphoma. Hematol Oncol 2017; 35: 296–302. [DOI] [PubMed] [Google Scholar]
- 11.Greaves P, Clear A, Coutinho R, et al. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical hodgkin lymphoma is predictive of outcome. J Clin Oncol 2013; 31: 256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamper P, Bendix K, Hamilton-Dutoit S, Honoré B, Nyengaard JR, Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011; 96: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood 2012; 120: 3280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steidl C, Shah SP, Farinha P, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 2010; 362: 875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayal S, Mathur S, Karak AK, et al. CD68 tumor-associated macrophage marker is not prognostic of clinical outcome in classical Hodgkin lymphoma. Leuk Lymphoma 2014; 55: 1031–7. [DOI] [PubMed] [Google Scholar]
- 16.Lévy R, Colonna P, Tourani JM, et al. Human immunodeficiency virus associated hodgkin’s disease: report of 45 cases from the French registry of HIV-associated tumors. Leuk Lymphoma 1995; 16: 451–6. [DOI] [PubMed] [Google Scholar]
- 17.Carbone A, Gloghini A, Larocca LM, et al. Human immunodeficiency virus-associated Hodgkin’s disease derives from post-germinal center B cells. Blood 1999; 93: 2319–26. [PubMed] [Google Scholar]
- 18.Herndier BG, Sanchez HC, Chang KL, Chen YY, Weiss LM. High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of HIV-associated Hodgkin’s disease. Am J Pathol 1993; 142: 1073–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann S, Jakobus C, Rengstl B, et al. Spindle-shaped CD163+ rosetting macrophages replace CD4+ T-cells in HIV-related classical Hodgkin lymphoma. Mod Pathol 2013; 26: 648–57. [DOI] [PubMed] [Google Scholar]
- 20.Bosch Príncep R, Lejeune M, Salvadó Usach MT, Jaén Martínez J, Pons Ferré LE, Álvaro Naranjo T. Decreased number of granzyme B+ activated CD8+ cytotoxic T lymphocytes in the inflammatory background of HIV-associated Hodgkin’s lymphoma. Ann Hematol 2005; 84: 661–6. [DOI] [PubMed] [Google Scholar]
- 21.Kiyasu J, Aoki R, Tanaka PY, et al. FOXP3 (+) regulatory and TIA-1 (+) cytotoxic T lymphocytes in HIV-associated Hodgkin lymphoma. Pathol Int 2012; 62: 77–83. [DOI] [PubMed] [Google Scholar]
- 22.Koulis A, Trivedi P, Ibrahim H, Bower M, Naresh KN. The role of the microenvironment in human immunodeficiency virus-associated classical Hodgkin lymphoma. Histopathology 2014; 65: 749–56. [DOI] [PubMed] [Google Scholar]
- 23.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016; 17: 1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116: 3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome. J Clin Oncol 2016; 34: 2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 2012; 18: 1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano Classification. J Clin Oncol 2014; 32: 3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. N Engl J Med 1998; 339: 1506–14. [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization (WHO) classification of lymphoid neo-plasms. Blood 2016; 127: 1375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaser SL, Lin RJ, Stewart SL, et al. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer 1997; 70: 375–82. [DOI] [PubMed] [Google Scholar]
- 31.STATA V14.2 StataCorp LP. STATA. Version 10.1 College Station: StataCorp LP, 2008. [Google Scholar]
- 32.Audouin J, Diebold J, Pallesen G. Frequent expression of Epstein-Barr virus latent membrane protein-1 in tumour cells of Hodgkin’s disease in HIV-positive patients. J Pathol 1992; 167: 381–4. [DOI] [PubMed] [Google Scholar]
- 33.Hashmi AA, Hussain ZF, Hashmi KA, et al. Latent membrane protein 1 (LMP1) expression in Hodgkin lymphoma and its correlation with clinical and histologic parameters. World J Surg Oncol 2017; 15: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017; 130: 2420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray PG, Billingham LJ, Hassan HT, et al. Effect of Epstein-Barr virus infection on response to chemotherapy and survival in Hodgkin’s disease. Blood 1999; 94: 442–7. [PubMed] [Google Scholar]
- 36.Glavina-Durdov M, Jakic-Razumovic J, Capkun V, Murray P. Assessment of the prognostic impact of the Epstein-Barr virus-encoded latent membrane protein-1 expression in Hodgkin’s disease. Br J Cancer 2001; 84: 1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montalban C, Abraira V, Morente M, et al. Epstein-Barr virus-latent membrane protein 1 expression has a favorable influence in the outcome of patients with Hodgkin’s disease treated with chemotherapy. Leuk Lymphoma 2000; 39: 563–72. [DOI] [PubMed] [Google Scholar]
- 38.Krugmann J, Tzankov A, Gschwendtner A, et al. Longer failure-free survival interval of Epstein-Barr virus-associated classical Hodgkin’s lymphoma: a single-institution study. Mod Pathol 2003; 16: 566–73. [DOI] [PubMed] [Google Scholar]
- 39.Clarke CA, Glaser SL, Dorfman RF, et al. Epstein-Barr virus and survival after Hodgkin disease in a population-based series of women. Cancer 2001; 91: 1579–87. [DOI] [PubMed] [Google Scholar]
- 40.Diepstra A, van Imhoff GW, Schaapveld M, et al. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin’s lymphoma predicts adverse outcome in older adult patients. J Clin Oncol 2009; 27: 3815–21. [DOI] [PubMed] [Google Scholar]
- 41.Oudejans JJ, Jiwa NM, Meijer CJLM. Epstein-barr virus in Hodgkin’s disease: more than just an innocent bystander. J Pathol 1997; 181: 353–6. [DOI] [PubMed] [Google Scholar]
- 42.Stark GL, Wood KM, Jack F, Angus B, Proctor SJ, Taylor PR. Hodgkin’s disease in the elderly: a population-based study. Br J Haematol 2002; 119: 432–40. [DOI] [PubMed] [Google Scholar]
- 43.Herling M, Rassidakis GZ, Medeiros LJ, et al. Expression of Epstein-Barr virus latent membrane protein-1 in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma: associations with presenting features, serum interleukin 10 levels, and clinical outcome. Clin Cancer Res 2003; 9: 2114–20. [PubMed] [Google Scholar]
- 44.Fellbaum C, Hansmann ML, Niedermeyer H, et al. Influence of Epstein-Barr virus genomes on patient survival in Hodgkin’s disease. Am J Clin Pathol 1992; 98: 319–23. [DOI] [PubMed] [Google Scholar]
- 45.Enblad G, Sandvej K, Sundström C, Pallesen G, Glimelius B. Epstein-Barr virus distribution in Hodgkin’s disease in an unselected Swedish population. Acta Oncol 1999; 38: 425–9. [DOI] [PubMed] [Google Scholar]
- 46.Vestlev PM, Pallesen G, Sandvej K, Hamilton-Duroit SJ, Bendtzen SM. Prognosis of Hodgkin’s disease in not influenced by Epstein-Barr virus latent membrane protein. Int J Cancer 1992; 50: 670–1. [DOI] [PubMed] [Google Scholar]
- 47.Myriam BD, Sonia Z, Hanene S, Teheni L, Mounir T. Prognostic significance of Epstein–Barr virus (EBV) infection in Hodgkin lymphoma patients. J Infect Chemother 2017; 23: 121–30. [DOI] [PubMed] [Google Scholar]


