Abstract
Introduction
Hemostasis depends on the delicate balance between coagulants and anti-coagulants. Higher levels of circulating coagulants have been associated with higher risk of cerebral infarctions and dementia. In contrast, higher levels of circulating protein C, an endogenous anticoagulant, have been associated with lower risk of cerebral infarctions, and the association between protein C levels and the risk of dementia is unknown. The goal of this study is to evaluate the association of circulating protein C levels in midlife and late life with incident dementia.
Methods
Circulating protein C levels were measured using blood samples collected at the midlife baseline (1987-89) and the late-life baseline (2011-13) among 14,462 and 3,614 participants, respectively, in the Atherosclerosis Risk in Communities study. Protein C levels were measured using ELISA at midlife and a modified aptamer-based assay at late life. Participants were followed up to 2013 from midlife and up to 2017 from late life. Incident dementia was ascertained during the follow-up periods using in-person cognitive and functional assessment, informant interviews, and International Classification of Diseases codes at hospitalization discharge and on death certificates. Cause-specific Cox regression models were used to evaluate the association between quintiles of circulating protein C and incident dementia.
Results
From midlife (mean age of 54), 1,389 incident dementia events were observed over a median follow-up of 23 years. From late life (mean age of 75), 353 incident dementia events were observed over a median follow-up of 4.9 years. At both midlife and late life, circulating protein C had an inverse association with incident dementia after adjusting for demographic, vascular and hemostatic risk factors, incident stroke as time-dependent covariate, and incorporating stabilized weights based on propensity scores (quintile 5 vs quintile 1 as the reference, midlife hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.66 to 0.96, p-value for trend 0.04; late life HR 0.84, 95% CI: 0.55 to 1.28, p-value for trend 0.04).
Discussion/Conclusion
Circulating protein C has an inverse association with incident dementia independent of established risk factors, including stroke. Our results suggest studying anticoagulants in addition to coagulants can increase our understanding on the relationship between hemostasis and dementia.
Keywords: protein C, dementia, hemostasis
Introduction
Hemostasis, which maintains blood fluidity and stops bleeding in the event of vascular injury, depends on the delicate balance between coagulants and anticoagulants (1). In population-based studies, higher levels of circulating coagulants have been associated with higher risk of cerebral infarctions, cognitive decline, and dementia (2-5). In contrast, higher levels of circulating protein C have been associated with lower risk of cerebral infarctions, a risk factor for dementia (4, 6). Among patients with atrial fibrillation, oral anticoagulant use has been associated with lower risk for dementia (7, 8). Little is known about the association between endogenous anticoagulant levels and the risk of dementia in the wider population of middle aged and older adults.
Protein C is an endogenous anticoagulant and, upon activation, deactivates two coagulants, factor V and factor VIII, whose levels have a positive association with cognitive decline and the risk of dementia (2, 9). Higher circulating protein C levels have been associated with lower risk of ischemic stroke, an established risk factor for dementia (10). However, many patients with dementia do not have stroke preceding dementia but have evidence of cerebral small vessel disease as revealed in magnetic resonance imaging (MRI) scans (11). Studying the association between circulating protein C levels and incident dementia may provide insight into the early role of the balance of hemostasis in dementia risk. We evaluated the potential etiological relationship of protein C levels in midlife and late life with incident dementia using cause-specific Cox regression models (12).
Materials and Methods
Overview of Study Design
The Atherosclerosis Risk in Communities (ARIC) study is a longitudinal cohort study of 15,972 adults from four communities (Washington County, Maryland; Forsyth County, North Carolina; northwestern suburbs of Minneapolis, Minnesota; and Jackson, Mississippi) with Jackson by design enrolling self-reported Black participants only. Since enrollment (visit 1, 1987-89), seven visits have been conducted.
Our primary analysis assessed the associations of circulating protein C levels in midlife and late life with incident dementia using two follow-up periods with non-overlapping sets of incident dementia cases (Figure 1). The midlife baseline was 1987-89 (visit 1, mean age: 54 years). The association analysis from midlife included 14,462 participants who were followed up to the end of visit 5 (December 31, 2013). The exclusions at midlife were: 103 participants with numbers in self-reported race and center combinations too small for analysis (self-reported race that was not White or Black, or self-reported as Black in Washington County or Minneapolis), 1 with missing incident dementia status, 275 with missing protein C measures, 587 with missing values for APOE ε4 genotype, 289 with missing values for other baseline covariates, and 75 with anticoagulant use (warfarin for all). Details are reported in Supplementary Figure 1. The late-life baseline was 2011-13 (visit 5, mean age: 75 years). The association from late life included 3,614 of the 6,538 participants who attended the late-life baseline visit, and participants were followed up to December 31, 2017, when incident stroke information was available for all participants from the ARIC cohort surveillance (13). The exclusions at late life were: 42 participants with self-reported race that was not White or Black, or self-reported as Black in Washington County or Minneapolis, 341 with prevalent dementia, 609 missing dementia status, 1081 without protein C measures, 185 with missing values for APOE ε4 genotype, 430 with missing values for other baseline covariates, and 236 with anticoagulant use (warfarin use: 218, other anticoagulants: 18). Details are reported in Supplementary Figure 2. Given that warfarin is known to suppress protein C activity (14), participants who reported the use of warfarin at the midlife and late-life baselines were excluded. Participants who reported the use of other anticoagulants were also excluded because the small number of users made it infeasible to include the use of other anticoagulants as a covariate.
Fig. 1.
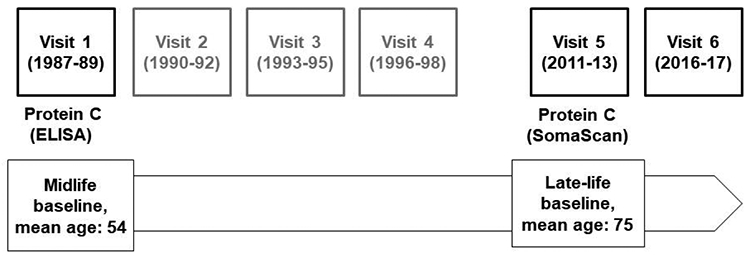
Overview of the study design. The associations between protein C levels and incident dementia were assessed using two follow-up periods with non-overlapping sets of cases. The midlife baseline was 1987-89 (visit 1) with follow-up period up to the end of 2013. The late-life baseline was 2011-13 (visit 5) with follow-up period up to the end of 2017. Abbreviation. ELISA, enzyme-linked immunosorbent assay.
Standard Protocol Approvals, Registrations, and Patient Consents.
This study was approved by the Institutional Review Board of each ARIC participating site: University of North Carolina at Chapel Hill, Chapel Hill, NC; Wake Forest University, Winston-Salem, NC; Johns Hopkins University, Baltimore, MD; University of Minnesota, Minneapolis, MN; and University of Mississippi Medical Center, Jackson, MS. All participants provided written informed consent at each study visit, and proxies provided consent for participants who were determined to lack capacity.
Measurement of circulating protein C levels
The blood samples used for the assay of protein C were drawn from the antecubital vein after an 8-hour fast using a citrated plasma tube in midlife and an EDTA plasma tube in late life. Samples were processed according to a standardized protocol and shipped on dry ice to the ARIC central laboratory for storage at −70°C or −80°C. At the midlife baseline, circulating protein C antigen levels were measured within a few weeks of blood draw using ELISA (15-18). The coefficient of variation (CV) from blind duplicates was 4% to 5% (17). The short-term reliability coefficients obtained from repeated testing of individuals over several weeks were 0.56 for protein C, 0.72 for fibrinogen, and 0.68 for von Willebrand factor (19). At the late-life baseline, the relative concentration of protein C in relative fluorescence units (RFU) was measured after 5 to 7 years of storage using SomaScan version 4, a modified aptamer-based assay (20, 21). Based on binding specificity tests performed by the manufacturer, the modified aptamers that targeted protein C did not bind to related proteins (factor VII, factor IX or factor X). The specificity of the modified aptamer targeting protein C was also confirmed by data dependent analysis (DDA) mass spectrometry (22). The protein C measures were normalized at the modified aptamer, plate, and sample levels based on the manufacturer standards and finally adjusted using the protein measures of a pool of healthy controls (23). Based on blind duplicates (n=197 pairs), the CV of protein C at the late life baseline was 4.6% (24). The Spearman correlation between the midlife and late life protein C measures was 0.18.
Incident dementia ascertainment
We followed participants from enrollment (1987-89, visit 1) up to the end of visit 6 (December 31, 2017). Dementia status was determined by an expert committee that included physicians and neuropsychologists. The methods for dementia ascertainment in the ARIC study have been reported previously (25). Briefly, the ascertainment was based on detailed cognitive and functional assessment (Supplementary Table 1) at ARIC visits 5 (2011–2013) and 6 (2016–17), cognitive tests conducted at visits 2 (1990–92) and 4 (1996–98), and informant interviews. Participants who did not attend visit 5 were contacted by phone and administered the modified Telephone Interview for Cognitive Status (TICS) (26). TICS scores were education-adjusted (25). Informant interviews were sought for participants who could not be contacted for visits 5 or 6. During visit 5, informant interviews used the Clinical Dementia Rating (CDR) and the Functional Activities Questionnaire (FAQ). After visit 5, telephone interviews were sought for all participants semi-annually between study visits using the Six Item Screener (SIS) scores and for informant interviews using the Alzheimer’s Dementia 8-Item (AD8) Informant Questionnaire (27). Finally, dementia cases were also ascertained using ICD-9 dementia codes at hospitalization discharge and on death certificates obtained by ARIC cohort surveillance.
Dementia date was first set as the earliest of either the hospitalization date with an ICD-9 code for dementia, death date if a dementia code was listed on the death certificate, date of telephone communication with the participant or proxy with indication of dementia, or date of the first visit when dementia was indicated. Dementia onset date ascertained from informant interviews, hospitalization and death certificates was reassigned as six months earlier to account for the expected lag in the reporting of the event. Participants who were classified as not having dementia were censored at their last study contact date or the date of death collected by ARIC cohort surveillance.
Measurement of other variables
Race, education levels (< high school, high school graduate or vacation school, and at least some college, graduate or professional school), and current smoking status were self-reported. Body mass index (BMI) was calculated using height and weight measured at study visit. Prevalent diabetes mellitus was defined as having a fasting glucose level ≥ 126 mg/dl, non-fasting glucose level ≥ 200 mg/dl, self-reported diabetes medication use, or self-reported physician diagnosis of diabetes. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or self-reported use of anti-hypertension medications. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (28) with calibrated and standardized serum creatinine (29). Anticoagulant use was determined based on the medication bottles that the participants provided at study visit. Platelet count was measured by Coulter counters. At the midlife baseline, factor VIII activity (factor VIIIc) was measured by the coagulation test; fibrinogen by the thrombin-time titration method; and von Willebrand factor antigen by ELISA. At the late-life baseline, factor VIII, fibrinogen, and von Willebrand factor were measured by the SomaScan assay. Global cognition Z scores were calculated by averaging the Z scores of three tests: the Delayed Word Recall Test, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Word Fluency Test (30, 31). Prevalent stroke at the midlife baseline was based on self-report. Subsequent stroke events were based on hospital records from local hospital surveillance and annual telephone interview with ARIC participants. Stroke events were classified by a combination of computer algorithm and physician review (32). Atrial fibrillation events were ascertained based on electrocardiogram (ECG) during study visits, ICD codes for hospitalization discharge and on death certificate (33). Genotyping of the two single nucleotide polymorphisms (rs429358, rs7412) that define the APOE ε4 genotypes was performed using the TaqMan assay (Thermo Fisher Scientific, Waltham MA).
Statistical analysis
Baseline characteristics were compared across quintiles of circulating protein C levels using the chi-squared test for categorical variables with ≥ 5 observations in all cells and the Fisher exact test otherwise, one-way ANOVA for non-skewed continuous variables, and Kruskal-Wallis test for skewed continuous variables.
We performed cause-specific analysis using Cox regression models, which are appropriate for etiological investigation (12) to evaluate the association of circulating protein C levels at midlife and late life with incident dementia. Protein C levels at both midlife and late-life baselines were categorized in quintiles. Covariates were established demographic, genetic, or vascular risk factors for dementia (34, 35) or novel risk factors that may be confounders in the relationship between protein C levels and dementia: eGFR (36), three coagulation factors (factor VIII, fibrinogen, and von Willebrand factor) (3), and platelet count (37, 38).
Our primary analysis consists of five cause-specific Cox regression models. At the midlife baseline, the covariates of Model 1 included demographics (age, race-center, sex, education levels) and APOE ε4 carrier status. Model 2 added vascular risk factors (diabetes, hypertension, BMI, current smoking, and prevalent stroke). Model 3 added coagulants (factor VIIIc, fibrinogen, and von Willebrand factor) and platelet count. Model 4 added incident stroke as a time-varying covariate. Model 5 added the inverse of propensity scores as weights to further control for potential confounding. The propensity scores represent the conditional probabilities of belonging to one of the quintiles of protein C given the covariates (39, 40). Stabilized weights were calculated as a ratio with the numerator being the density of the quintiles of protein C and the denominator being the propensity scores generated using all covariates in Model 4. To assess whether these stabilized weights indeed balanced the covariates across quintiles of protein C, we conducted weighted regression analysis using each covariate as the outcome and quintiles of protein C as predictors incorporating the stabilized weights. The results showed that the covariates were largely independent of protein C after incorporating the stabilized weights. (Supplementary Table 2).
At the late-life baseline, the five models were essentially the same as those defined for the midlife baseline except as noted here. First, the values of BMI, current smoking status, prevalent stroke, diabetes and hypertension status, eGFR, factor VIII, von Willebrand factor, and platelet count were obtained at the late life baseline. Second, Models 2 to 5 included global cognition Z score, which was not available at the midlife baseline. Lastly, Models 3 to 5 did not include fibrinogen because the modified aptamer targeted fibrinogen did not pass quality control for binding specificity. Similar to the results of the midlife baseline, the covariates were largely independent of protein C after incorporating the stabilized weights (Supplementary Table 3).
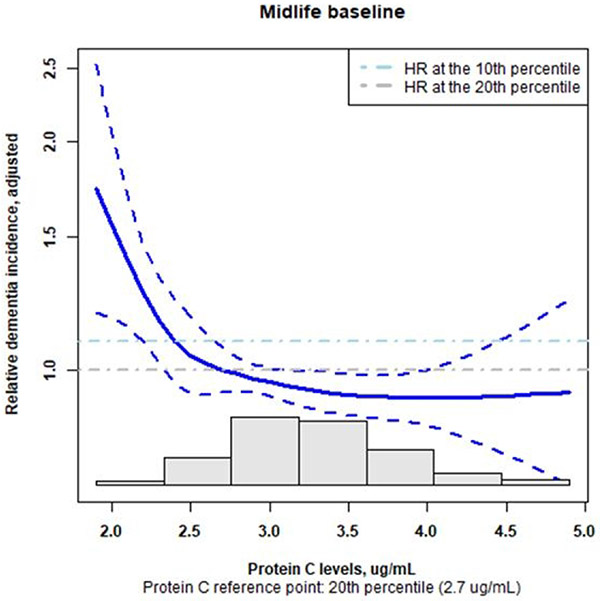
We conducted two secondary analyses. Given that protein C levels might be more related to vascular risk factors of dementia, and the APOE ε4 genotype is more specific for Alzheimer’s disease, the first secondary analysis assessed the association between protein C levels and incident dementia excluding participants who were APOE ε4 homozygotes (Model 6) and APOE ε4 carriers (Model 7). Model 6 had the same covariates as Model 5, and Model 7 removed APOE ε4 carrier status as a covariate. Both Models 6 and 7 were evaluated incorporating stabilized weights based on propensity scores as used in Model 5. The other secondary analysis was conducted at the midlife baseline to explore whether only very low levels of protein C within quintile 1 were associated with higher risk of dementia given that compared with quintile 1, all higher quintiles (from 2 to 5) had similar hazard ratios. This analysis modeled protein C levels using cubic spline with knots at the 10th and 20th percentiles and included the same covariates and propensity score-based weights as in Model 5. This analysis was not performed for the late-life baseline given that compared to quintile 1, quintile 2 had slightly higher risk estimate and quintiles 3 to 5 had lower risk estimates for dementia..
In all analyses, to reduce skewedness and potential influence of outliers, a log2 transformation was applied to factor VIII, fibrinogen, von Willebrand factor, and platelet count followed by winsorization at the 1st and 99th percentile. The proportional hazards assumption was assessed by inspecting the Schoenfeld residual plot and testing whether the slope of the time-dependent coefficient across time was zero (41). The p-value for trend was obtained by using the five quintiles of protein C as a continuous variable with values from 1 to 5. The Wald test with a degree of freedom of 4 was used to assess the joint effects of all five quintiles. All analyses were conducted using R 4.0.2. The stabilized weights based on propensity scores were generated using the R ipw package (40).
Data Availability Statement
ARIC data from visit 1 to visit 5 are available through the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Data that are not yet available through BioLINCC are available upon request through the ARIC Coordinating Center at the University of North Carolina.
Results
At the midlife baseline (mean age: 54 years, 45% men, 26% Black overall), the median protein C levels was 3.1 ug/mL (25th, 75th percentile: 2.7, 3.5). Higher quintiles of circulating protein C levels had lower proportions of men, current smokers, Black participants, as well as higher BMI, and proportions of prevalent diabetes and hypertension. The proportions of participants with prevalent atrial fibrillation and APOE ε4 allele were similar across the quintiles (Table 1).
Table 1.
Population characteristics at the midlife baseline (visit 1, n=14,462)
| Overall | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-value | |
|---|---|---|---|---|---|---|---|
| Range of circulating protein C levels, ug/mL | 0.7, 9.6 | 0.7, 2.6 | 2.7, 2.9 | 3.0, 3.2 | 3.3, 3.6 | 3.7, 9.6 | |
| Variable | |||||||
| N | 14,462 | 2809 | 2738 | 3011 | 2963 | 2941 | |
| Age, year, mean (SD) | 54.2 (5.8) | 53.8 (5.9) | 53.9 (5.9) | 54.1 (5.8) | 54.5 (5.7) | 54.6 (5.5) | <0.001 |
| Male, n (%) | 6486 (44.9) | 1594 (56.7) | 1397 (51) | 1368 (45.4) | 1208 (40.8) | 919 (31.2) | <0.001 |
| Self-reported black, n (%) | 3784 (26.2) | 846 (30.1) | 680 (24.8) | 757 (25.1) | 731 (24.7) | 770 (26.2) | <0.001 |
| APOE ε4 1 or 2 copies, n (%) | 4452 (30.8) | 913 (32.5) | 836 (30.5) | 885 (29.4) | 911 (30.7) | 907 (30.8) | 0.15 |
| Education, year, n (%) | <0.001 | ||||||
| < High school | 3399 (23.5) | 684 (24.4) | 629 (23) | 698 (23.2) | 658 (22.2) | 730 (24.8) | |
| High school graduate or vocational school | 5921 (40.9) | 1092 (38.9) | 1069 (39) | 1218 (40.5) | 1278 (43.1) | 1264 (43) | |
| College, graduate or professional school | 5142 (35.6) | 1033 (36.8) | 1040 (38) | 1095 (36.4) | 1027 (34.7) | 947 (32.2) | |
| BMI, kg/m2, mean (SD) | 27.7 (5.3) | 26.9 (5.2) | 27.6 (5.3) | 27.7 (5.3) | 27.9 (5.5) | 28.3 (5.3) | <0.001 |
| Current smoker, n (%) | 3775 (26.1) | 918 (32.7) | 745 (27.2) | 802 (26.6) | 687 (23.2) | 623 (21.2) | <0.001 |
| Prevalent diabetes, n (%) | 1701 (11.8) | 277 (9.9) | 288 (10.5) | 287 (9.5) | 344 (11.6) | 505 (17.2) | <0.001 |
| Prevalent hypertension, n (%) | 4995 (34.5) | 844 (30) | 877 (32) | 1003 (33.3) | 1042 (35.2) | 1229 (41.8) | <0.001 |
| Prevalent stroke, n (%) | 236 (1.6) | 51 (1.8) | 34 (1.2) | 45 (1.5) | 42 (1.4) | 64 (2.2) | 0.04 |
| Prevalent atrial fibrillation, n (%) | 25 (0.18) | 9 (0.3) | 4 (0.1) | 5 (0.2) | 5 (0.2) | 2 (0.1) | 0.27 |
| eGFR, mL/min/1.73m2, mean (SD) | 102.4 (15.8) | 103.8 (15.8) | 101.9 (15.3) | 102 (15.9) | 102.1 (15.1) | 102.4 (16.5) | <0.001 |
| Platelet count, K/uL, median (25th, 75th percentile) | 251 (214, 295) | 238 (200, 281) | 243 (208.25, 285) | 252 (216, 293) | 256 (219, 300) | 267 (228, 309) | <0.001 |
| Factor VIIIc, % | 126 (105, 151) | 122 (101, 147) | 123 (104, 148) | 126 (105, 150) | 128 (107, 151) | 132 (109, 158) | <0.001 |
| Fibrinogen, mg/dL | 295 (260, 337) | 290 (254, 331) | 290 (256, 332) | 295 (260.5, 337) | 297 (262, 341) | 302 (267, 345) | <0.001 |
| von Willebrand factor, % | 110 (84, 142) | 106 (80, 138) | 107 (82, 138) | 109 (83, 140) | 111 (86, 145) | 115 (89, 151) | <0.001 |
Abbreviation. BMI, body mass index; eGFR, estimated glomerular filtration rate; factor VIIIc, factor VIII activity P-values were obtained by comparing the values among the quintiles of protein C: Chi-squared test was used for categorical variables with at least 5 observations in all cells, otherwise Fisher-exact test was used; one-way ANOVA was used for non-skewed variables and Kruskal-Wallis test was used for skewed variables
After a median follow-up of 23 years, we observed 1,389 incident dementia events. The primary data sources for dementia diagnoses were in-person cognition and functional assessment (22.1%), CDR and/or FAQ (38.7%), ICD codes at hospitalization discharge (26.9%), TICS (6.7%), and ICD codes on death certificates (5.5%, Supplementary Table 4). The curves of the crude cumulative incidence by quintiles of protein C levels from the midlife baseline were close together (Supplementary Figure 3). After adjusting for demographic factors (age, sex, race-center, education levels) and APOE ε4 carrier status, there was a weak inverse association between protein C levels and incident dementia (Model 1, quintile 1 as reference, quintile 5 adjusted hazard ratio [HR], 0.82, 95% confidence interval [Cl]: 0.69 to 0.97, p-value for trend 0.14, Wald test p-value for the joint effects of all quintiles: 0.05, Table 2). After adjusting for vascular and hemostatic risk factors, the inverse association between protein C levels and incident dementia became stronger (Model 3, quintile 5 adjusted HR, 0.74, 95% CI: 0.62 to 0.88, p-value for trend 0.01, Wald test p-value: 0.004, Table 2). This stronger inverse association might be partly due to the inclusion of some vascular risk factors of dementia that had positive univariate relationship with protein C levels, such as BMI and diabetes reported above. After adjusting for additional covariates and incident stroke as a time-varying covariate (Model 4), higher levels of protein C were associated with lower risk for dementia (quintile 5 adjusted HR, 0.77, 95% CI: 0.64 to 0.92, p-value for trend 0.04, Wald test p-value 0.01 Table 2). Results were similar in Model 5 incorporating the stabilized weights based on the propensity scores (quintile 5 adjusted HR, 0.80, 95% CI: 0.66 to 0.96, p-value for trend 0.04, Wald’s test p-value 0.04). The hazard ratios for quintiles 2 to 5 were similar. In the secondary analysis excluding APOE ε4 homozygotes and heterozygotes (Models 6 and 7), the hazard ratio estimates were similarly to those from Model 5 (Supplementary Table 5). We did not observe a monotonic dose-response pattern. The secondary analysis exploring the shape of association within quintile 1 using a cubic spline with knots at the 10th and 20th percentiles revealed an inverse dose-response pattern within quintile 1 (cubic spline term overall p-value 0.004), i.e. the risk for dementia were higher as the levels of protein C became lower (Figure 2).
Table 2.
Association between circulating protein C levels in midlife and incident dementia (n=14,462)
| Model | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-value for trend |
Wald test p- value |
|---|---|---|---|---|---|---|---|
| Model 1 | Reference | 0.76 (0.64, 0.91) | 0.87 (0.73, 1.02) | 0.84 (0.71, 1.00) | 0.82 (0.69, 0.97) | 0.14 | 0.05 |
| Model 2 | Reference | 0.75 (0.63, 0.90) | 0.87 (0.73, 1.03) | 0.82 (0.69, 0.98) | 0.77 (0.64, 0.91) | 0.03 | 0.01 |
| Model 3 | Reference | 0.74 (0.62, 0.89) | 0.84 (0.71, 1.00) | 0.80 (0.67, 0.95) | 0.74 (0.62, 0.88) | 0.01 | 0.004 |
| Model 4 | Reference | 0.73 (0.61, 0.88) | 0.85 (0.71, 1.00) | 0.81 (0.68, 0.96) | 0.77 (0.64, 0.92) | 0.04 | 0.01 |
| Model 5 | Reference | 0.75 (0.61, 0.91) | 0.88 (0.73, 1.06) | 0.83 (0.69, 0.99) | 0.80 (0.66, 0.96) | 0.04 | 0.04 |
Covariates for each model
Model 1: age, sex, race-center, education levels, APOE ε4 carrier status
Model 2: Model 1 + vascular factors (body mass index, current smoking status, prevalent stroke, diabetes and hypertension status, estimated glomerular filtration rate)
Model 3: Model 2 + coagulants (factor VIII activity, fibrinogen, von Willebrand factor) + platelet count
Model 4: Model 3 + incident stoke as time-varying covariate
Model 5: Model 4 incorporating stabilized weights based on the inverse of propensity scores
P-value for trend was obtained by using the five quintiles of protein C as a continuous variable with values from 1 to 5 Wald test p-values were obtained by testing the joint effects of all five quartiles
P-value for proportional hazards assumption for all protein C quintile variables > 0.10 for all models
Fig. 2.
At the midlife baseline, within quintile 1, an inverse dose-response relation between protein C levels and dementia risk was apparent when protein C levels were modeled as a cubic spline with knots at the 10th and 20th percentiles using the same covariates and stabilized weights based on propensity score as in Model 5 (p-value of the cubic spline 0.004). The grey bars in the lower part of the plot is the histogram of protein C levels. Abbreviation. HR, hazard ratio.
At the late-life baseline (mean age: 75 years, 41.3% men, 17.5% Black overall), similar to the midlife baseline, higher quintiles of circulating protein C levels had lower proportions of men and Black participants. The proportions of participants with prevalent atrial fibrillation and APOE ε4 allele were also similar across the quintiles. In contrast to the midlife baseline, higher quintiles of circulating protein C levels had lower BMI and proportions of prevalent diabetes and hypertension (Supplementary Table 6). After a median follow-up of 4.9 years, we observed 353 incident dementia events. The primary data sources for dementia diagnoses were in-person cognition and functional assessments (37.4%), AD8 (35.4%), SIS (6.8%), and ICD codes at hospitalization discharge (19.3%) and on death certificates (1.1%, Supplementary Table 7). In contrast to the midlife baseline, the curves of the crude cumulative incidence by quintiles of protein C levels at late-life showed a separation pattern reflecting the inverse association between protein C levels and incident dementia (Supplementary Figure 4). The results from the five multivariate models did not differ appreciably due to covariate adjustment. In all model tested, the overall associations between quintiles of protein C and incident dementia were significant (Wald test p-value for the joint effect of all quintiles ≤ 0.02). The hazard ratios of the quintiles had wide confidence interval, and quintile 3 to 5 had lower hazard ratio estimates compared with quintile 1. For example, in Model 5 when the stabilized weights based on propensity scores were incorporated: quintile 1 as reference, quintile 3 adjusted HR, 0.78, 95% CI: 0.56 to 1.10; quintile 4 adjusted HR, 0.73, 95% CI: 0.50 to 1.07; quintile 5 adjusted HR, 0.84, 95% CI: 0.55 to 1.28; p-value for trend 0.04). In the secondary analysis excluding APOE ε4 homozygotes and heterozygotes, the inverse association between protein C levels and incident dementia seemed to be slightly stronger in Model 7 (quintile 1 as reference, quintile 5 adjusted HR 0.62, 95% CI: 0.37, 1.04, p-trend: 0.01, Supplementary Table 5). Similar to the association in midlife, we did not observe a monotonic dose response pattern.
Discussion/Conclusion
In this study of a community-based cohort with measures of circulating protein C levels in midlife and late life, cause-specific Cox regression models showed that protein C levels and incident dementia had an inverse association independent of stroke and coagulants, including factor VIII, fibrinogen, and von Willebrand factor. These results suggest more research on the balance between coagulants and anticoagulants in hemostasis may yield insight on the pathophysiology of dementia.
In the coagulation cascade, protein C, upon activation, can inactivate factor VIII resulting in lower production of fibrin downstream (42). In addition, activated protein C reduces the activity of plasminogen activator inhibitor. This function enhances the production of plasmin, which degrades fibrin clots (43). Therefore, adequate levels of protein C are critical for avoiding stasis and maintaining blood fluidity. Protein C levels have had an inverse association with incident stroke, an established risk factor for dementia (10, 44). In this study, the association between protein C and dementia was independent of stroke. Subclinical vascular injuries, such as brain infarcts, may be among the links underlying this independent inverse association between protein C and dementia risk. Among participants in the ARIC Brain MRI study, which were stroke-free at the time of the MRI examination, higher levels of circulating protein C and lower levels of fibrinogen at the midlife baseline were associated with lower odds for subclinical brain infarcts six years later (4). Subclinical brain infarcts have been shown to be detectable in about 20% of older adults without stroke and have been associated with higher risk for dementia in population-based studies (6, 45, 46). These results suggest the inverse association between protein C levels and incident dementia reported in the present study may not be mediated by stroke, and the balance between anticoagulants and coagulants in hemostasis may be important in the maintenance of vascular health in the brain for the prevention of dementia.
The inverse association between protein C and incident dementia is consistent with the protective association between anticoagulant treatment and incident dementia among patients with atrial fibrillation (7, 8, 47). While atrial fibrillation is an established risk factor for dementia, the underlying mechanism is still unclear (48). Dysrhythmia in atrial fibrillation is considered a cause of thromboembolism, which could lead to ischemic stroke or other vascular defects in the brain and thus increases the risk of dementia (49). In addition, atrial fibrillation could also be a marker of the Virchow’s triad for thromboembolism: a hypercoagulable state, stasis, and endothelial injury, which are common among older adults (48, 49). The results of the present study suggest that levels of endogenous anti-coagulant levels may be relevant for the study of dementia pathophysiology in a wider population.
This study has several strengths. We demonstrated consistent inverse associations of circulating protein C levels with incident dementia in midlife and late life using two follow-up periods with non-overlapping cases in a large community-based cohort including White and Black participants. The dementia assessment was rigorous combining in-person and informant interviews with cohort surveillance. Some limitations warrant mentioning. At the midlife baseline, dementia assessment was not available. We assumed all participants were free of dementia at this baseline given that the age range at this baseline was 45 to 64. Specific forms of dementia diagnosis, such as vascular dementia and Alzheimer’s disease, were not available in the ARIC study. Therefore, we could not evaluate the association between protein C levels and specific forms of dementia. Measures of protein S, a cofactor of protein C, were not available to inform whether the effects of protein C might have been affected by this cofactor. Protein C measured at midlife had higher within-person variability than other hemostatic factors (19). We could have underestimated the true association due to this measurement variability. Given that the protein C levels at the midlife baseline were absolute measures and at the late-life baseline were relative measures, we could not directly compare the range of the protein C values or the changes between these two baselines. While the inverse associations between protein C levels and the risk for dementia were consistent between the midlife and the late-life baselines, the sample size was smaller at the late-life baseline resulting in larger confidence intervals in the comparisons between quintiles. Finally, given that this is an observational study with relative quantification of protein C levels at the late-life baseline, our data could not provide precise estimates of protein C levels that could be protective for dementia risk.
In summary, we have showed that circulating protein C levels have an inverse association with incident dementia. Our results suggest investigating anticoagulants in conjunction with coagulants in hemostasis may yield insight into the pathophysiology of dementia, potentially leading to its prevention.
Supplementary Material
Table 3.
Association between circulating protein C levels in late life and incident dementia (n=3,614)
| Model | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-value for trend |
Wald test p-value |
|---|---|---|---|---|---|---|---|
| Model 1 | Reference | 1.07 (0.80, 1.44) | 0.69 (0.50, 0.96) | 0.66 (0.46, 0.94) | 0.79 (0.55, 1.13) | 0.01 | 0.01 |
| Model 2 | Reference | 1.21 (0.89, 1.64) | 0.78 (0.56, 1.08) | 0.71 (0.49, 1.03) | 0.92 (0.63, 1.34) | 0.07 | 0.02 |
| Model 3 | Reference | 1.21 (0.89, 1.63) | 0.77 (0.55, 1.07) | 0.70 (0.49, 1.02) | 0.92 (0.63, 1.35) | 0.07 | 0.02 |
| Model 4 | Reference | 1.20 (0.89, 1.62) | 0.78 (0.56, 1.08) | 0.71 (0.49, 1.02) | 0.93 (0.64, 1.36) | 0.08 | 0.02 |
| Model 5 | Reference | 1.26 (0.92, 1.72) | 0.78 (0.56, 1.10) | 0.73 (0.50, 1.07) | 0.84 (0.55, 1.28) | 0.04 | 0.02 |
Covariates for each model
Model 1: age, sex, race-center, education levels, APOE ε4 carrier status
Model 2: Model 1 + vascular factors (body mass index, current smoking status, prevalent stroke, diabetes and hypertension status, estimated glomerular filtration rate)
Model 3: Model 2 + coagulants (factor VIII, von Willebrand factor) + platelet count
Model 4: Model 3 + incident stoke as time-varying covariate
Model 5: Model 4 incorporating stabilized weights based on the inverse of propensity scores
P-value for trend was obtained by using the five quintiles of protein C as a continuous variable with values from 1 to 5 Wald test p-values were obtained by testing the joint effects of all five quartiles
P-value for proportional hazards assumption for each quintile variable > 0.1 for all
Acknowledgement
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Statement of Ethics
Study participants have given their written informed consent and that the study protocol was approved by the institute’s committee on human research.
References
- 1.Fisher MJ. Brain regulation of thrombosis and hemostasis: from theory to practice. Stroke. 2013;44(11):3275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander KS, Zakai NA, Gillett S, McClure LA, Wadley V, Unverzagt F, et al. ABO blood type, factor VIII, and incident cognitive impairment in the REGARDS cohort. Neurology. 2014;83(14):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH, et al. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology. 2019;92(11):e1256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knuiman MW, Folsom AR, Chambless LE, Liao D, Wu KK. Association of hemostatic variables with MRI-detected cerebral abnormalities: the atherosclerosis risk in communities study. Neuroepidemiology. 2001;20(2):96–104. [DOI] [PubMed] [Google Scholar]
- 5.Quinn TJ, Gallacher J, Deary IJ, Lowe GDO, Fenton C, Stott DJ. Association between circulating hemostatic measures and dementia or cognitive impairment: systematic review and meta-analyzes. Journal of Thrombosis and Haemostasis. 2011;9(8):1475–82. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdsson S, Aspelund T, Kjartansson O, Gudmundsson Elias F, Jonsdottir Maria K, Eiriksdottir G, et al. Incidence of Brain Infarcts, Cognitive Change, and Risk of Dementia in the General Population. Stroke. 2017;48(9):2353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhavan M, Hu TY, Gersh BJ, Roger VL, Killian J, Weston SA, et al. Efficacy of Warfarin Anticoagulation and Incident Dementia in a Community-Based Cohort of Atrial Fibrillation. Mayo Clin Proc. 2018;93(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Lutsey Pamela L, MacLehose Richard F, Claxton J'Neka S, Norby Faye L, Chamberlain Alanna M, et al. Association of Oral Anticoagulant Type With Risk of Dementia Among Patients With Nonvalvular Atrial Fibrillation. Journal of the American Heart Association. 2018;7(21):e009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallacher J, Bayer A, Lowe G, Fish M, Pickering J, Pedro S, et al. Is sticky blood bad for the brain?: Hemostatic and inflammatory systems and dementia in the Caerphilly Prospective Study. Arterioscler Thromb Vasc Biol. 2010;30(3):599–604. [DOI] [PubMed] [Google Scholar]
- 10.Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018;14(11):1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez OL, Kuller LH, Becker JT, Jagust WJ, DeKosky ST, Fitzpatrick A, et al. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology. 2005;64(9):1539–47. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–33. [DOI] [PubMed] [Google Scholar]
- 14.Esmon CT, Vigano-D'Angelo S, D'Angelo A, Comp PC. Anticoagulation proteins C and S. Adv Exp Med Biol. 1987;214:47–54. [DOI] [PubMed] [Google Scholar]
- 15.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61(1):15–9. [PubMed] [Google Scholar]
- 16.Wu KK, Papp AC, Patsch W, Rock R, Eckfeldt J, Sharrett R. ARIC hemostasis study--II. Organizational plan and feasibility study. Thromb Haemost. 1990;64(4):521–5. [PubMed] [Google Scholar]
- 17.Chambless LE, McMahon R, Finch A, Sorlie P, Heiss G, Lyles R, et al. ARIC hemostasis study--III. Quality control. Atherosclerosis Risk in Communities. Thromb Haemost. 1993;70(4):588–94. [PubMed] [Google Scholar]
- 18.Nguyen ND, Ghaddar H, Stinson V, Chambless LE, Wu KK. ARIC hemostasis study--IV. Intraindividual variability and reliability of hemostatic factors. The Atherosclerosis Risk in Communities (ARIC). Thromb Haemost. 1995;73(2):256–60. [PubMed] [Google Scholar]
- 19.Chambless LE, McMahon R, Wu K, Folsom A, Finch A, Shen YL. Short-term intraindividual variability in hemostasis factors. The ARIC Study. Atherosclerosis Risk in Communities Intraindividual Variability Study. Ann Epidemiol. 1992;2(5):723–33. [DOI] [PubMed] [Google Scholar]
- 20.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5(12):e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostroff R, Foreman T, Keeney TR, Stratford S, Walker JJ, Zichi D. The stability of the circulating human proteome to variations in sample collection and handling procedures measured with an aptamer-based proteomics array. J Proteomics. 2010;73(3):649–66. [DOI] [PubMed] [Google Scholar]
- 22.Emilsson V, Ilkov M, Lamb JR, Finkel N, Gudmundsson EF, Pitts R, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018:eaaq1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candia J, Cheung F, Kotliarov Y, Fantoni G, Sellers B, Griesman T, et al. Assessment of Variability in the SOMAscan Assay. Sci Rep. 2017;7(1):14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313(7049):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter CR, DesPain B, Keeling TN, Shah M, Rothenberger M. The Six-Item Screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Annals of emergency medicine. 2011;57(6):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918–26. [DOI] [PubMed] [Google Scholar]
- 30.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46(2):141–5. [DOI] [PubMed] [Google Scholar]
- 31.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161(11):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–43. [DOI] [PubMed] [Google Scholar]
- 33.Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, et al. Association of Atrial Fibrillation With Cognitive Decline and Dementia Over 20 Years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc. 2018;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11(6):718–26. [DOI] [PubMed] [Google Scholar]
- 35.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 36.Christensson A, Ash JA, DeLisle RK, Gaspar FW, Ostroff R, Grubb A, et al. The Impact of the Glomerular Filtration Rate on the Human Plasma Proteome. Proteomics Clin Appl. 2017. [DOI] [PubMed] [Google Scholar]
- 37.White TC, Berny MA, Tucker EI, Urbanus RT, de Groot PG, Fernández JA, et al. Protein C supports platelet binding and activation under flow: role of glycoprotein Ib and apolipoprotein E receptor 2. J Thromb Haemost. 2008;6(6):995–1002. [DOI] [PubMed] [Google Scholar]
- 38.Catricala S, Torti M, Ricevuti G. Alzheimer disease and platelets: how's that relevant. Immunity & ageing : I & A. 2012;9(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Wal WM, Prins M, Lumbreras B, Geskus RB. A simple G-computation algorithm to quantify the causal effect of a secondary illness on the progression of a chronic disease. Statistics in Medicine. 2009;28(18):2325–37. [DOI] [PubMed] [Google Scholar]
- 40.van der Wal WM, Geskus RB. ipw: An R Package for Inverse Probability Weighting. Journal of Statistical Software; Vol 1, Issue 13 (2011). 2011. [Google Scholar]
- 41.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 42.Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–65. [PubMed] [Google Scholar]
- 43.van Hinsbergh VW, Bertina RM, van Wijngaarden A, van Tilburg NH, Emeis JJ, Haverkate F. Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood. 1985;65(2):444–51. [PubMed] [Google Scholar]
- 44.Folsom AR, Ohira T, Yamagishi K, Cushman M. Low protein C and incidence of ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost. 2009;7(11):1774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–22. [DOI] [PubMed] [Google Scholar]
- 46.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70(6):425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Field TS, Weijs B, Curcio A, Giustozzi M, Sudikas S, Katholing A, et al. Incident Atrial Fibrillation, Dementia and the Role of Anticoagulation: A Population-Based Cohort Study. Thromb Haemost. 2019;119(6):981–91. [DOI] [PubMed] [Google Scholar]
- 48.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke. 2016;47(3):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373(9658):155–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ARIC data from visit 1 to visit 5 are available through the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Data that are not yet available through BioLINCC are available upon request through the ARIC Coordinating Center at the University of North Carolina.