Abstract
Dyslexia is a common learning disability that affects processing of written language despite adequate intelligence and educational background. If learning disabilities remain untreated, a child may experience long term social and emotional problems which influence future success in all aspects of their life. Dyslexia has a 60% heritability rate, and genetic studies have identified multiple dyslexia susceptibility genes (DSGs). DSGs, such as DCDC2, are consistently associated with the risk and severity of reading disability (RD). Altered neural connectivity within temporo-parietal regions of the brain are associated with specific variants of DSGs in individuals with RD. Genetically altering DSG expression in mice results in visual and auditory processing deficits as well as neurophysiological and neuroanatomical disruptions. Previously, we demonstrated that learning deficits associated with RD can be translated across species using virtual environments. In this two-year longitudinal study, we demonstrate that performance on a virtual Hebb-Williams maze in pre-readers, is able to predict future reading impairment, and the genetic risk strengthens, but is not dependent on, this relationship. Due to the lack of oral reporting and use of letters, this easy-to-use tool may be particularly valuable in a remote working environment, as well as working with vulnerable populations such as English language learners.
Keywords: READ1, reading disorder, virtual maze, translational research, early identification
Introduction
Nearly 2.4 million children, between 6-21 years of age, with specific learning disabilities received special education services under the Individuals with Disabilities Education Act in 2018 (US Department of Education). Reading disabilities, including dyslexia, accounts for approximately 80% of all learning disabilities. Dyslexia is a heritable, neurodevelopmental reading disorder characterized by an unexplained reading impairment, which significantly interferes with academic achievement or daily life. This multifaceted disorder has a 60% heritability rate and is associated with alterations in neural networks involved in auditory and visual processing associated with reading. Research examining the connection among genetic, cognitive and behavioral aspects of reading disorder offers promise for developing early identification and intervention for successfully addressing specific phenotypes of RD[1]. Early detection and intervention are necessary to prevent long term social and emotional problems that may occur if learning disabilities are untreated.
Genetic linkage and association studies, carried out across multiple countries (e.g. Finland, Germany, United Kingdom and the United States), have led to the identification of nine risk loci (DYX1-9). One of the most replicated DSGs is DCDC2 located within the DYX2 locus (for review see[2]). A naturally occurring 2,445 bp microdeletion within intron 2 of DCDC2 is associated with dyslexia and impaired processes associated with reading[3–5]. In rodents, deletion of exon 2 of Dcdc2 is associated with impaired auditory processing [6–8], which may underlie phonological processing deficits in humans. In addition, visual working memory, and visual-spatial and attentional processing have also been identified in this genetic model[9], and similar deficits were identified in children with reading impairment[10, 11]. Gruen and colleagues identified a regulatory element within this 2.4 kb region of intron 2 of DCDC2, called READ1 (regulatory element associated with dyslexia-1). READ1 has been shown to positively and negatively influence reading and language depending on the presence of specific READ1 alleles[12]. READ1 has six common and more than 34 rare alleles. Allele 5 is associated with severe reading disability, allele 6 with severe language impairment, however, both disorders are evident when two alleles are combined[13]. In contrast allele 3 is not associated with an increased risk of reading impairment, and even appears to protect against it when in the presence of another risk haplotype in KIAA0319, KIA-hap, located within the same DYX2 locus[14]. Altered expression of DCDC2 and KIAA0319 are associated with aberrant neural connectivity[15] and functional neural activity[16] within temporo-parietal regions of the brain which may underlie deficits associated with reading impairment[17]. In addition, DCDC2 microdeletion is associated with deficits in motion processing associated with the magnocellular-dorsal pathway in typical and atypical readers[18–20].
Word reading requires mapping of letters and sounds (i.e. decoding), which is influenced by orthographic and phonological processing, as well as executive functions, such as working memory, and cognitive flexibility[21]. Reading fluency requires visual processing of several graphemes, which may be influenced by visual attention and visual-spatial abilities[22, 23]. The fronto-parietal network and the magnocellular-dorsal pathway has been implicated in visual attention and motion sensing[24–27]. Visual motion perception deficits associated with reading impairment resulted in the magnocellular-dorsal pathway theory of dyslexia[28–33, 20]. Despite some negative findings for the role of the magnocellular pathway in visual motion perception[34–36], recent studies have found additional evidence to support the relationship between visual-spatial processing and reading ability[37–39, 1, 20]. The link between these visual deficits of the magnocellular pathway and reading impairment has also been observed in non-alphabetic, logographic languages[40, 41]. In addition, cingulo-opercular network (detection of behaviorally relevant information, maintaining task goals, and reward processing) are engaged during reading[42–46]. The cingulo-opercular network has been shown to be uniquely activated in multiple tasks when individuals search for behaviorally relevant information[47, 48, 45]. Reading intervention has been associated with greater functional connectivity in the cingulo-opercular network, particularly within reading impaired groups, demonstrating the importance of this network in reading[43].
In our own research we found evidence that animal models of reading impairment (knockdown of the Dcdc2 gene[9]) and children (readers and pre-readers) with reading impairment exhibit deficits performing the Hebb-Williams maze task[10], which requires the participant to process visual-spatial information (i.e. store, recall, and decode information) about their location in order to successfully navigate their environment. Successfully navigating a virtual maze environment requires proficient attentional shifting, working and reference memory, and cognitive flexibility. Recent evidence has demonstrated that both the fronto-parietal and the cingulo-opercular networks are important in reading, and are necessary for successful navigation of the virtual maze task[49]. The fronto-parietal network is crucial for the integration of environmental cues and the retention of a cognitive map[49], and the cingulo-opercular network is needed to identify salient features in the environment[47], and to monitor and adjust performance throughout a task[50–52]. Together these data suggest a neurocognitive link between maze performance and reading. These data support our findings demonstrating that genetic animal models of dyslexia exhibit impaired maze learning abilities, and the association between maze performance and reading ability in humans [10].
In this study we examined the link between impaired performance on a virtual version of the Hebb-Williams maze (vHW) with specific READ1 risk variants. In addition, we hypothesized that the impaired performance that we identified in children 8-12-years of age will also be evident in pre-readers (i.e. children enrolled in kindergarten). Lastly, we examined whether performance on the maze task is a strong predictor of future reading ability by tracking reading ability and maze performance in a cohort of participants over two years (i.e. kindergarten and second grade). These studies are essential to understand the link between genetic risk variants and cognitive processing deficits reported in individuals with dyslexia. In addition, these studies will provide powerful insight into our ability to use these tools as early detection methods for identifying children at risk for RD.
Materials and Methods
STUDY 1: Pre-readers
Participants
A total of 163 (75 female, 88 male) children participated in this study. Participants were recruited from two school districts in the Northwest (i.e. Idaho, 46.6% of participants), and two school districts in the Northeast (i.e. Pennsylvania, 53.4% of participants). We recruited children between the ages of 5-7 years (M = 6.14, SD = 0.46) to participate in this study between April, 2014 – March, 2020. Eighty-six participants reported their ethnicity with 53.5% (46/86) reporting White, 43.0% (37/86) Latino/a, 2.3% (2/86) Asian, and 1.2% (1/86) Black. No participants self-identified as American Indian/Alaska Native or Pacific Islander. Exclusionary criteria for all participants included: 1) speaking a language other than English as their first language, and 2) having an identified disability other than specific learning disability. These exclusionary criteria helped to rule out low reading scores due to factors other than RD, such as language background or IQ. Legal guardians provided written informed consent and all participants provided their assent prior to the start of the study. Institutional Review Boards at Lafayette College, Boise State University and Lehigh University approved all procedures.
Reading Measures
Participants completed Woodcock Reading Mastery Test (WRMT-III) Basic Reading subtests during the months between January to May in a given year. The WRMT-III is a standardized, norm-referenced, individually administered battery of nine tests designed to evaluate struggling readers, identify specific strengths and weaknesses in reading skills in order to plan targeted remediation, screen for reading readiness, and guide instructional decisions [53]. For this study, we administered three reading subtests to produce a Basic Reading cluster score: Phonological Awareness, Letter Identification, and Rapid Automatic Naming. Scores for all subtests and clusters are reported as standard scores on a mean of 100 with a standard deviation of 15. We identified participants with Basic Reading cluster score below 86 on the Woodcock-Johnson III as struggling readers (atypical) and all others as typically developing readers. Reliability coefficients of subtests range from .85 -.94; the average reliability for cluster scores is .95.
Virtual Hebb-Williams Maze (vHW)
The virtual maze environment and analysis software used to test participants, were created using the Unreal Development Kit and Java, respectively, and run on a Dell PC. Previous research from our laboratory suggests that type of computer and/or graphics card did not influence study outcomes [10]. Mazes were displayed at a resolution of 1600x900 in full screen mode. There are 6x6 cells that make up the interior of the maze, similar to the grid of the physical maze. Each cell consists of 256 units, with the goal and start boxes 300 units deep, and a viewing height (eye level) of 85 units from the ground. Participants navigated through the virtual environment at a constant velocity of 175 units/second and a maximum turn rate of 96 degrees/second using the arrow keys on the keyboard to navigate through the maze task. The backward arrow was disabled, requiring participants to face the direction they were moving, rather than being able to walk backwards in the maze. Participants navigated the maze using a joystick, or the arrow keys on the keyboard.
Participants were habituated to the virtual environment using the Maze 1 configuration (Fig. 1a). Participants were told how to navigate through the maze and were told to “find the red ball.” In Maze 1, unlike the other mazes, the red ball is immediately visible from the start box, and therefore serves as a practice for navigating a maze with the keys and to learn the general procedures for the task. Once the participant acquired the target (i.e. red ball), performance was reinforced with a smiling yellow cartoon star, and a sticker was placed on a sticker chart for the participant to take with them at the end of the study. Immediately after the yellow star appears the participant is placed back in the start box and the next trial begins. Participants completed each trial consecutively until all six trials were completed, with a 120 second maximum completion time per trial. The trial did not begin until the participant walked over the threshold of the start box into the maze. If the participant exceeded the maximum allowable time, floating yellow arrows appeared on the floor of the maze between the start and the goal box to guide the participant to the target. Participants were randomly oriented within the start box, at the beginning of each trial to simulate the physical version of the maze used with animal models of dyslexia[9, 10]. Following the habituation period, participants completed a training maze, Maze 5, which is used to train students to search for the red ball since children in this early age group needed to learn how to explore the maze (i.e. look around walls and in hallways) to find the red ball. The use of Maze 5 as a training maze is unlike previous research examining children between 8-12 and 18-22 years of age[10], however, children within this age group benefited from the additional training to understand the goal of the task. Following habituation and training, all participants completed vHW mazes 6, 8, 11, and 12. Mazes were completed in the succession, with a two-minute distractor task in between each maze. Unlike previous research[10], Maze 1 trained the students to use the keys to move throughout the maze and Maze 5 provided training on how to explore different areas of the maze to locate the red ball. Performance on Mazes 6, 8, 11, and 12 was examined across groups (i.e. typical and atypical readers). Statistical analysis revealed that the type of distractor task had no influence on maze performance on this task (e.g. puzzle, children’s movie, reading measure). The majority of participants completed all reading measures and maze tasks within a single 45-minute session, with reading measures conducted between maze tasks. In a few cases participants completed the tasks over two sessions <30 days apart, with the majority of sessions taking place within a 7-day period of time.
Fig. 1.
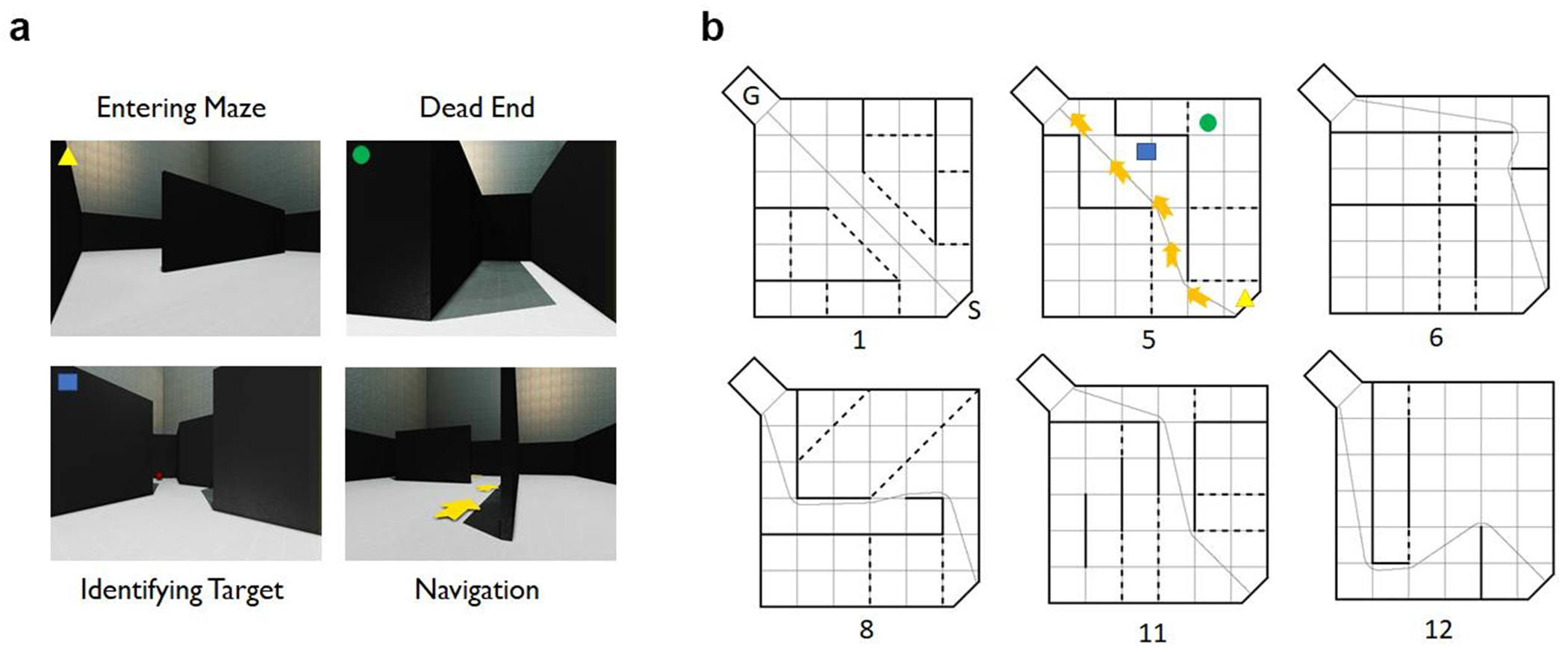
Hebb-Williams maze configurations and virtual platform. (a) Scenes from maze 5 on the virtual platform, including entering the maze, reaching a dead end, visualizing the target, and the appearance of arrows which occurs if the target is not reached in 120 seconds. (b) Line drawings of the mazes used for training (Mazes 1 and 5) and testing (Mazes 6, 8, 11, and 12). The goal box containing the target (i.e. red ball) is identified by the letter “G” and the start position is identified by the letter “S”. The number listed below the drawing indicates the Hebb-Williams maze configuration. The solid black lines represent walls in the maze, the black dotted lines represent error zones, which are not visible to participants, and the grey trace is the true path between the start and the goal box. The specific position of the avatar in the scenes from maze 5 (left) are represented by specific points in the schematic of maze 5 (triangle, entering the maze; circle, dead end; square, identifying the target; arrows, true path revealed once 120 s has elapsed).
Adult Reading History Questionnaire (ARHQ)
Biological parents of the participants completed the ARHQ. The questionnaire consists of 23 self-report items on a 5-point Likert scale ranging from 0 to 4[54]. The total score was divided by the maximum possible score (i.e. 92) to generate a ratio ranging from 0 to 1. Higher scores are associated with greater reading difficulty. Familial risk is defined by ARHQ scores of 0.40 or greater, which has a ~80% sensitivity, specificity, and overall correct classification rate[54].
Genetic Screening
In this study we examined READ1 within the DCDC2 gene, a dyslexia susceptibility gene (DSG) previously shown to associate with several reading phenotypes [13]. READ1 is a highly polymorphic, purine-rich, compound short tandem repeat (STR) located in intron 2 of DCDC2 [3]. Gruen and colleagues showed that READ1 binds a human brain-expressed transcription factor called ETV6 with very high specificity and is capable of modulating reporter-gene expression from the DCDC2 promoter in an allele-specific manner [55].
READ1 Genotyping:
READ1 was genotyped by PCR amplification, purification of PCR products with ExoSAP-IT enzyme mix, and Sanger sequencing as previously described [13]. Sanger sequencing was performed at the Yale W.M. Keck DNA Sequencing Facility as per their standard sequencing protocol. Alleles were called by an in-house C language program developed for this purpose [13].
READ1 Microdeletion Genotyping:
The 2,445bp DCDC2 microdeletion encompasses the entire READ1 STR within its breakpoints, therefore it was genotyped in addition to READ1 to ensure an accurate genotype was achieved for apparent READ1 homozygotes (methods described in [13]). The microdeletion was genotyped by allele-specific PCR resolved on agarose-gel electrophoresis with the use of a three-primer reaction that generates a ~600bp amplicon from intact chromosomes and a ~200bp amplicon from chromosomes with the deletion, allowing heterozygotes and both homozygotes to be readily distinguishable from one another. PCR products were electrophoresed on 1% agarose gels with the use of standard 1X TBE buffer and ethidium bromide (0.2 mg/ ml) via standard methods at 100–150 V, depending on gel size. Gels were imaged on a UV transilluminator and documented with a Bio-Rad Gel Doc XR imaging system. Genotypes were called from the gels manually. Markers that deviated substantially from Hardy-Weinberg equilibrium or that had an overall call rate <85% were excluded from genetic analyses. Single marker SNP analyses of case-control status and quantitative traits were performed with SNP and Variation Suite (SVS) v7.6.4 (Bozeman, Mt). Haplotype-based association tests were performed with KIAA-Hap haplotypes using PLINK v1.07 [56, 57].
Statistical Analyses
Statistical tests were performed using IBM SPSS Statistics 26 software (IBM Corporation, Somers, NY, USA) or Microsoft Excel. A 2 (typical vs. atypical populations) x 2 (unknown genetic risk vs. known risk) x 4 (Mazes 6, 8, 11, 12) x 6 (trials 1-6) mixed factorial repeated measures ANOVA was performed with maze and trial as the within-subjects’ factors. Genetic risk was defined as an individual with the DCDC2 microdeletion, READ1 risk allele 5, and/or an ARHQ ratio greater than 0.4. Gender was used as a covariate due to reported gender differences in reading[58] and spatial ability[59–61], including wayfinding tasks in virtual environments[62], in children. Errors and trial duration data were collected and used to calculate performance efficiency. Performance efficiency was based on a calculation described by Shore et al. (2001), which calculates the average of the standardized duration and error scores to provide a compound measure of performance on the Hebb-Williams maze[63]. More specifically, performance efficiency was measured by computing the z-score (using the overall grand means and standard deviations from all subjects) for both error and time to complete the task. These two z-scores were averaged and both time and error were weighted equally. This provided a composite measure where large positive numbers reflected a relatively poor performance (i.e., longer duration and more errors than average). The dependent variables of errors, time to complete the task and performance efficiency on the vHW task were analyzed.
In addition, performance efficiency scores were used to calculate slope and learning rate. Slope was calculated from performance efficiency z scores across trials 1-6. Briefly, z scores were converted to T scores (T=[z*10]+50), the natural log of the T scores were taken and the slope was calculated (m= [y2−y1]/[x2−x1]). Learning rate across groups was calculated based on performance efficiency (PE) scores across trials 1-6 (T1-T6), as described in Boutet et al. (2018). The learning rate represented the degree efficiency changed across trials[64] using performance efficiency (PE) scores: [(PET1−PET2) + (PET2−PET3) + (PET3−PET4) + (PET4−PET5) + (PET5−PET6)]/5[64]. Positive learning rate values indicated increased performance efficiency across trials. A 2 (typical vs. atypical) x 2 [genetic risk] x 4 (mazes 6, 8, 11, 12) mixed factorial ANOVA was performed to examine the interaction of these factors on slope and learning rate. For assumption was not met, a Huynh-Feldt correction was applied.
Odds ratio and Risk Assessment
An odds ratio was calculated to examine the relative risk of an individual having a reading impairment based on their performance on the vHW task (Mazes 6, 8, 11, and 12) during trial 3, or genetic risk (ARHQ ratio >0.4, allele 5, or microdeletion). The odds ratio is reported along with the 95% confidence interval (95% CI), and frequency of occurrence for typical and struggling readers (atypical) in kindergarten.
STUDY 2: Longitudinal Study
Participants
A total of 42 (21 female) children (M=8.30 ± 0.61 years of age) participated in the two-year follow-up of this study between Feb, 2016 – March 2020. Thirty-three participants reported their ethnicity 60.6% White, 33.3% Latino/a, 3.0% Asian, and 3.0% Black. Exclusionary criteria, consent, and assent processes were the same as in Study 1. Written informed consent was provided by the legal guardians and all participants provided their assent prior to the start of the study. Institutional Review Boards at Lafayette College, Boise State University and Lehigh University approved all procedures.
Reading Measures
The Woodcock-Johnson IV Tests of Achievement (WJ-IV) is a standardized, norm-referenced, individually administered battery of 22 tests designed to assess academic skill levels in several curricular areas [65]. For this study, four reading subtests, Letter-Word Identification (Test 1), Passage Comprehension (Test 4), Word Attack (Test 7) and Reading Recall (Test 12) were administered. These four reading subtests are combined to create three reading cluster scores (i.e. Reading, Basic Reading, and Reading Comprehension). The reading cluster included performance on Letter-Word Identification, in which a child names letters and reads words aloud from a list, and Passage Comprehension, in which students orally supply a missing word from a passage they read silently. Word Attack, in which a child reads nonsense words aloud to test their phonetic knowledge, and Letter-Word Identification provide a Basic Reading Skills cluster score. Reading Comprehension is based on the Reading Recall subtest, which asks a child to recall as many components of a story they read to themselves, and Word Attack performance. Because this study was completed over several years, students who participated in the study prior to the publication of the WJ-IV (i.e. January 2016) completed the WJ-III. The WJ-III the Basic Reading cluster was the same as in WJ-IV, however the Reading cluster (Broad Reading) included Letter-Word Identification, Reading Fluency, which examines the time it takes to read sentences and answer yes or no questions, as well as Passage Comprehension. The Reading Comprehension cluster in the WJ-III includes the subtests Passage Comprehension and Reading Vocabulary, in which students orally state synonyms and antonyms to given words. The three reading cluster scores are used to help determine areas of strength and weakness as related to the reading task. Scores for all subtests and clusters are reported as standard scores on a mean of 100 with a standard deviation of 15. Reliability coefficients of these five subtests range from 0.88 - 0.94. Because cluster scores consist of two or more subtests, their reliability is higher, ranging from 0.93 - 0.95.
For students who completed the WJ-IV Basic Reading, Reading, and Reading Comprehension clusters, which did not include a reading fluency measure, reading fluency was determined based on performance of the DIBELS Oral Reading Fluency (DORF) test. A child’s performance is measured by the number of errors made (i.e. words omitted, substituted, and hesitations lasting more than three seconds) and the number of words read out loud from a passage within one minute. The passages are calibrated for the goal level of reading for each grade level. The number of correct words per minute from the passage is the oral reading fluency rate. Test-retest reliabilities for elementary students ranged from .92 to .97[66]. Criterion-related validity studied in eight separate studies in the 1980s reported coefficients ranging from .52 - .91 [67]. For this study, we identified children as atypical readers if they had one cluster score (Basic Reading and/or Reading) that was at or below a standard score of 85. A participant was not classified as an atypical reader if Reading Comprehension was the only cluster score below 85.
Virtual Hebb-Williams Maze
As described in Study 1, participants were habituated to the virtual environment using Maze 1 configuration (Fig. 1a & Fig. 6) and the participants were told to “find the red ball”. For Study 2, we measured performance on Mazes 6, 8, and 9 (Fig. 6). By examining performance on two of the same mazes tested in Study 1 (i.e. Mazes 6 and 8 were performed at both time points) we were able to determine whether there was consistency in performance over time. In addition, we acquired additional data on a maze configuration (Maze 9) that has been described showing similar performance efficiencies across species[63], but had not been previously presented to the participants in this longitudinal study. As previously described, participants completed six consecutive trials with a 120 second maximum completion time per trial. If the participant exceeded the maximum allowable time floating, yellow arrows appeared on the floor of the maze guiding the participant to the goal box; data was not collected once the time allotment had been reached. Participants completed the selected mazes in a single day of testing and were given a two- minute distracter task (i.e., video clip from an age-appropriate, popular children’s movie, or reading measure) in between each maze. Participants completed all mazes and reading measures within 45 minutes, when reading measures were completed between maze tasks. If an alternative distractor task was used, reading measures were completed during a separate testing day ≤30 days. All procedures were approved by the Institutional Review Board.
Fig. 6.
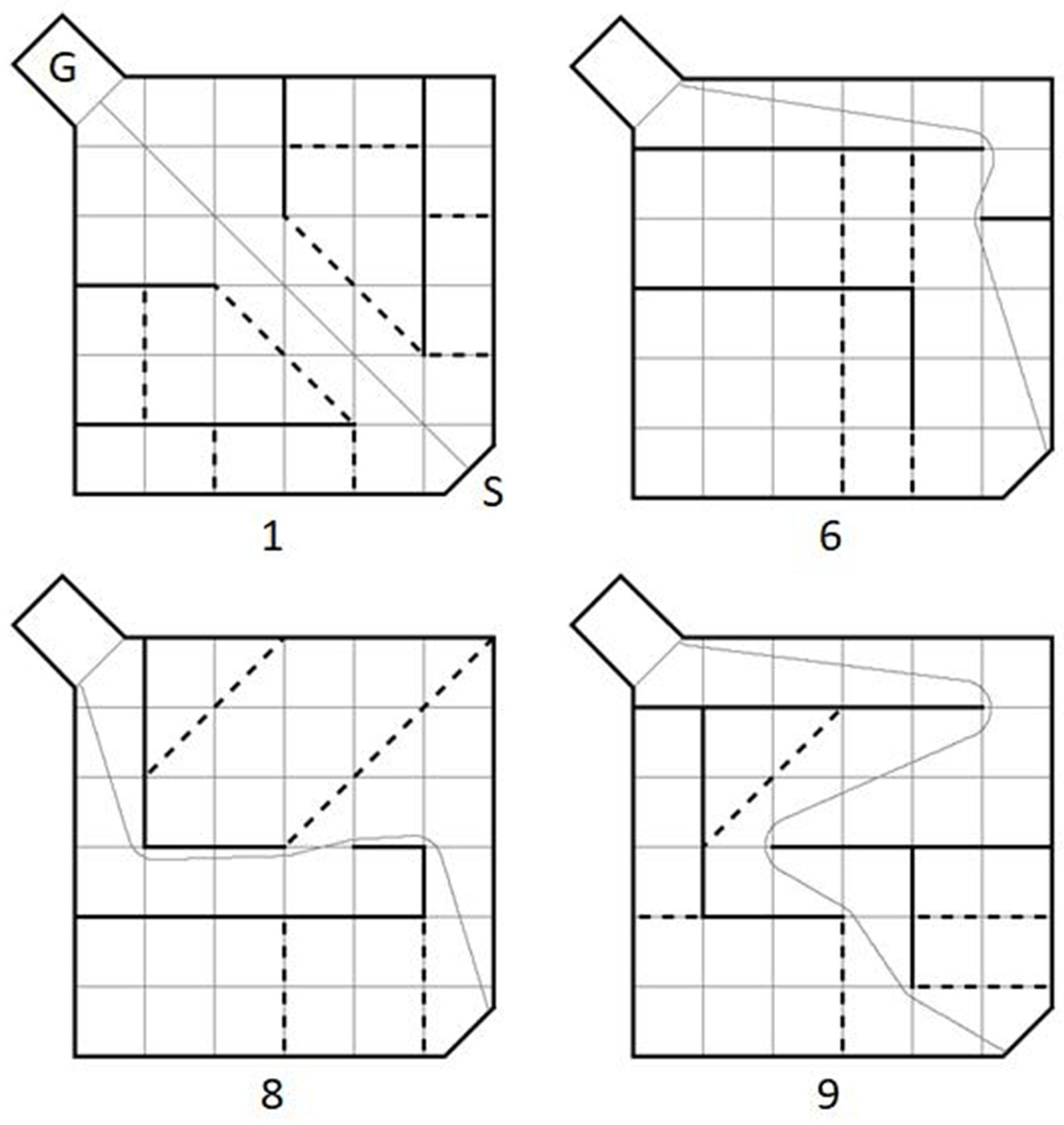
Schematic of Hebb-Williams mazes 1, 6, 8 and 9. The goal box containing the target (i.e. red ball) is identified by the letter “G” and the start position is identified by the letter “S”. The number listed below the drawing indicates the Hebb-Williams maze configuration. The solid black lines represent walls in the maze, the black dotted lines represent error zones, which are not visible to participants, and the grey trace is the true path between the start and the goal box.
Statistical Analyses
Statistical tests were performed using IBM SPSS Statistics 26 software (IBM Corporation, Somers, NY, USA) or Microsoft Excel. A 2 (typical vs. atypical) x 2 (unknown genetic risk vs. genetic risk) x 2 (mazes 6, 8) x 6 (trials 1-6) mixed factorial MANOVA was performed with maze and trial as the within-subjects’ factors. Errors committed during the completion of the maze task (errors), the amount of time it took for the participants to complete the task (duration), and performance efficiency on the vHW task were analyzed. Performance efficiency is based on a calculation described by Shore et al. (2001), which calculates the average of the standardized duration and error scores to provide a compound measure of performance on the Hebb-Williams maze[63]. More specifically, performance efficiency is measured by computing the z score (using the overall grand means and standard deviations from all subjects) for both error and time to complete the task. The average z score is calculated for each participant, with error and time weighted equally. This provides a composite measure where large positive numbers reflect a relatively poor performance.
Odds Ratio and Risk Assessment
An odds ratio was calculated to examine the relative risk of an individual having a reading impairment based on their performance on the vHW task (Mazes 6, 8, and 9) during trial one, or genetic risk (ARHQ ratio >0.4, allele 5, or microdeletion). Trial one was examined based on previously published data which demonstrated that participants do not have performance efficiency scores greater than 1 SD above the mean in both typical and atypical populations on Mazes 6 & 8. However, there is still considerable variability in performance during Trial 1 in this age group[10]. In contrast, performance Maze 9, which has not been examined before in this population, is still highly varied during later trials (i.e. Trial 3). The odds ratio is reported along with the 95% confidence interval (95% CI), and frequency of occurrence for typical and atypical readers in second grade.
Results
Study 1: Pre-readers
Children with RD show impaired performance on maze learning task
A total of 163 participants (54.0% male, 88/163), 6.14 ± 0.46 years of age were tested on reading measures. A total of 27 participants (16.6%, 13 females and 14 males) scored one standard deviation, or more, below the mean on cluster scores for Basic Reading skills on the WRMT-III, and are referred to as atypical readers (i.e. a typical). Of the 27 atypical readers, twenty reported ethnicities: 90% reporting Latino/a, 5% Black, and 5% White. Typical readers (i.e. typical) scored ≤1 standard deviation below the mean. Of the 136 typical readers, 66 reported ethnicities: 68.2% White, 28.8% Latino/a, and 3.0% Asian. Participant performance was examined on four separate vHW maze configurations (i.e. Mazes 6, 8, 11, and 12; with Mazes 1 and 5 serving as training mazes), across six trials per maze. It is important to note that all participants understood the objective of the task, and successfully learned how to control the avatar and complete the training mazes. Descriptive statistics (minimum and maximum values; mean ± standard deviation) are provided for the individual test scores and composite scores for both the typical and atypical populations in Table 1 and Table 2. Latency to complete the maze and errors committed were measured and standardized to calculate a performance efficiency score. Slope was also calculated from T-scores of performance efficiency across trials 1-4. Representative paths taken to complete the maze for typical and atypical readers for each maze are shown in Figure 2.
Table 1.
WRMT-III Subtest Reading Scores, Pre-readers.
| Group (N) | Letter-Word Identification | Phonological Processing | Rapid Automatic Naming | |||
|---|---|---|---|---|---|---|
| Typical (135) | 103.3 ± 6.7 | 110.3 ± 13.0 | 106.7 ± 11.8 | |||
| 78 | 116 | 75 | 138 | 73 | 138 | |
| Reading Impaired (27) | 79.5 ± 15.9 | 82.0 ± 10.6 | 80.9 ± 11.6 | |||
| 55 | 106 | 63 | 101 | 56 | 111 | |
Values are presented as mean ± standard deviation with the minimum and maximum values for each measure listed below. An independent samples t-test shows a statistically significant different on all measures between groups (p<0.001)
Table 2.
WRMT-III Reading Cluster Scores, Pre-readers.
| Group (N) | Basic Reading Skills | |
|---|---|---|
| Typical (135) | 108.3 ± 11.3 | |
| 86 | 133 | |
| Reading Impaired (27) | 72.2 ± 8.7 | |
| 56 | 85 | |
Values are presented as mean ± standard deviation with the minimum and maximum values for each measure listed below. An independent samples t-test shows a statistically significant different between groups (p<0.001)
Fig. 2.
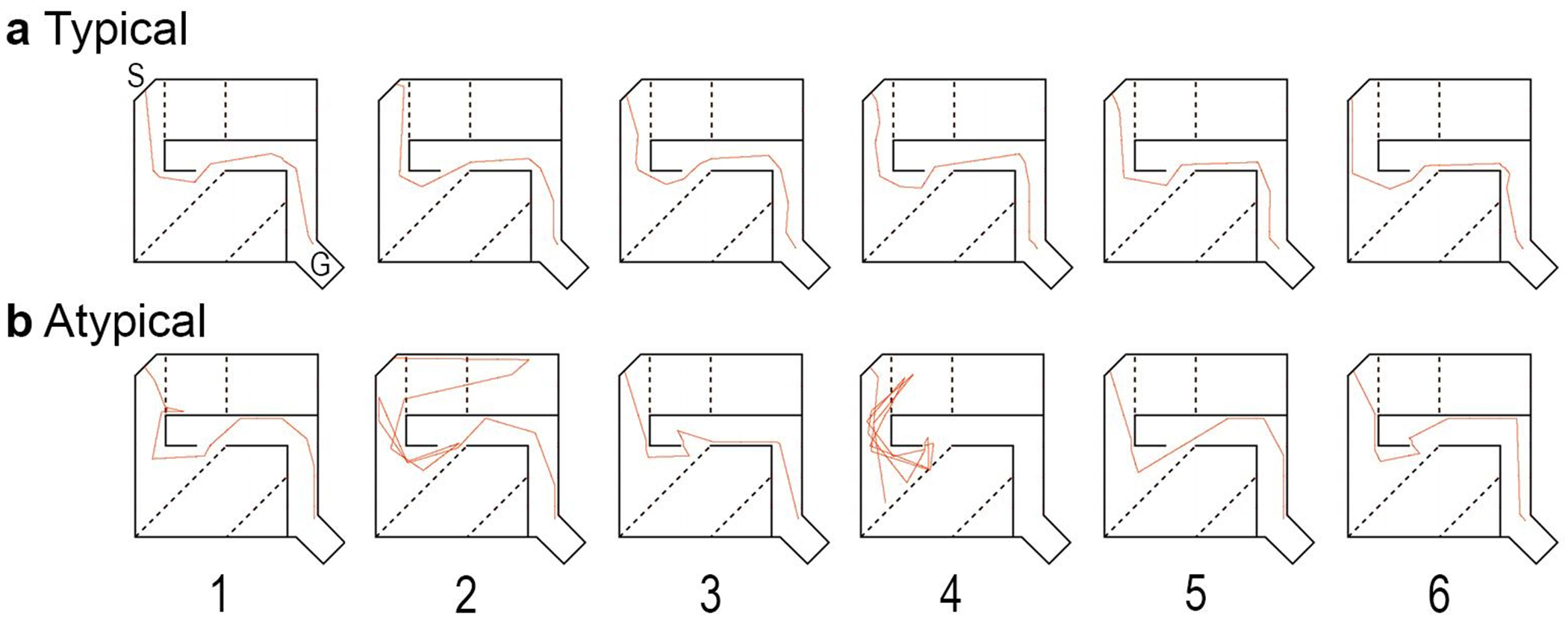
Children with reading impairment show difficulty maintaining a true path to solve the maze task. Representative traces of paths (shown in red) taken by (a) typical and (b) atypical readers while solving Maze 8. The goal box (G) and the starting position (S) are indicated on the schematic. The solid black lines represent walls in the maze, the black dotted lines represent error zones, which are not visible to participants Children with reading impairment (atypical) experienced difficulty identifying and maintaining the correct path to the target within the goal box of the maze. For example, in trial 4 you can see that the participant was unable to find the goal box within 120 s despite having successfully found the target in the previous trials. This is in contrast to a typical reader who is able to identify the true (i.e. correct path) in the first trial, and to repeat the behavior on subsequent trials.
Of the 163 students who participated in the study, 148 participants (124 typical; 24 atypical) completed all mazes (mazes 1,5,6,8,11,12), with 158 readers (132 typical; 26 atypical) participants completing mazes 1, 5, 6, 8, 11 only due to time constraints. Analyzing data for maze 12 separately, and applying a conservative Bonferroni correction for the number of comparisons, did not change the outcome of the study. Of 120 participants who provided information regarding biological risk, 59 participants provided Adult Reading History Questionnaire (ARHQ) data from biological parents, and we were able to analyze biological samples from 88 participants for READ1 microdeletion genotyping. Some participants provided both ARHQ data, and a biological sample. Though additional biological samples were collected, not all samples were able to be processed by the time of publication, and are not included in this study. Genetic risk was determined based on the presence of DCDC2 allele 5, the microdeletion, and/or an ARHQ ratio of 0.4 or greater[54]. Individuals who did not meet these criteria were identified as having an unknown genetic risk. Because several dyslexia susceptibility genes (DSGs) have been identified, we cannot rule out the possibility of other genetic risk factors that we did not examine in this study.
Examination of the interactions between reading ability (typical vs. atypical), mazes performed, and trials, with gender as a covariate, demonstrated that children with reading impairment exhibit impaired performance on this task. We calculated a significant three-way interaction between maze, trial, and reading impairment for performance efficiency (F(11.8,1226.5) = 2.2, p< 0.01, ηp2=.021, Fig. 3a), and errors committed (F(7.1,739.0)= 2.9, p<0.01, ηp2=.027, Fig. 3b), but not for time to complete the maze (F(9.4,981.4) = 1.6, n.s., Fig. 3c). There was a not a statistically significant interaction between maze and reading impairment for performance efficiency (F(2.9,304.4) <1, n.s.,), time to complete the maze (F(2.8,291.4) <1, n.s.), and errors committed (F(1.8, 189.4)<1, n.s.), However, there was a statistically significant interaction between maze and reading impairment for slope (F(2, 312)= 3.8, p = 0.02, ηp2=.023, Fig. 4a) and learning rate (F(2,312)= 3.0, p = 0.05, ηp2=.019 Fig. 4b, indicating differences in learning curves.
Fig. 3.
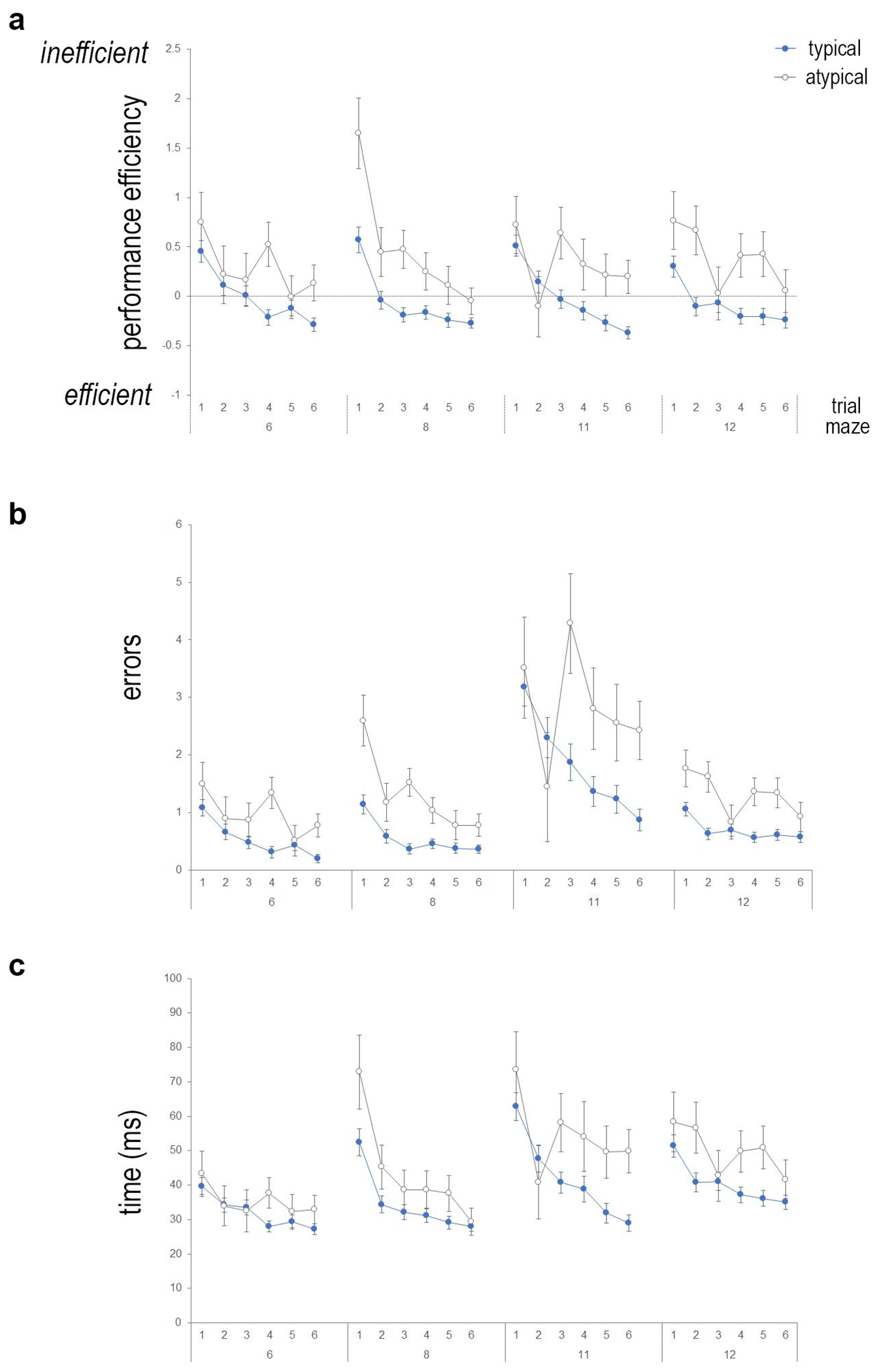
Young children with reading impairment exhibit impairment performance on the virtual maze learning task. (a) Performance efficiency is plotted for all mazes completed (Mazes 6, 8, 11, 12) during testing, across six consecutive trials for typical (blue closed circles) and atypical (grey open circles) readers. Positive performance efficiency values indicate inefficient performance, whereas negative values show greater efficiency compared to the mean performance (Mean performance = 0). Young children who struggle with reading (atypical) exhibit impaired performance across trials. Typical readers exhibit linear learning curves and improved performance across trials, whereas struggling readers exhibit a greater degree of variability across the consecutive trials for each maze task. Performance efficiency is calculated by equally weighting standardized values (z-scores) for (b) errors committed during the completion of the maze task and (c) time to complete the maze.
Fig. 4.
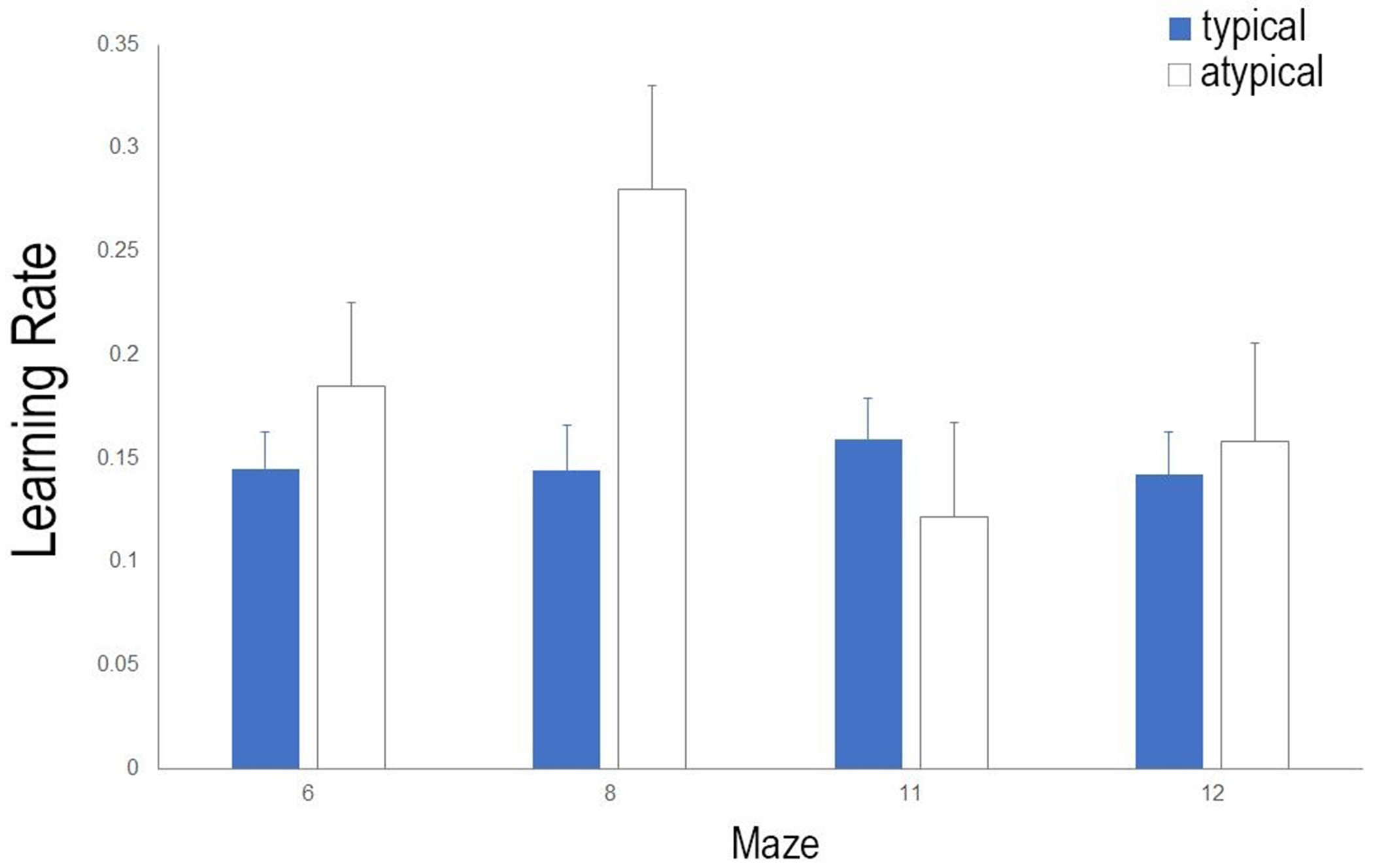
Learning rate among children with reading impairment show a greater degree of variability across mazes. Mean learning rate and standard error for typical and atypical readers is plotted against mazes 6, 8, 11, and 12. Learning rate is calculated by taking the average difference in performance efficiency scores across trials. A larger value indicates a greater rate of learning (i.e. steeper learning curve) across trials. Typical readers show a consistent learning rate across mazes, whereas the learning rate is variable across mazes for children with reading impairment (atypical).
The interaction between trial and reading performance was not statistically significant for performance efficiency (F(4.1,430.9) <1, n.s.), errors (F(4.3,447.7) = 2.0, p=0.09.) and latency (F(3.8,394.2) <1, n.s.). There was a statistically significant main effect of reading level for performance efficiency (F(1,104) = 11.2, p<0.01, ηp2=.097), errors committed (F(1,104) = 10.8, p<0.01, ηp2=.094), and time to complete the maze (F(1,104) = 4.4, p < 0.05, ηp2=.041), but not for slope (F(1,152)<1, n.s.) or learning rate (F(1,152) = 1.6, n.s).
As expected there was a main effect of trial identified for performance efficiency (F(4.1,430.9)= 5.7, p< 0.001, ηp=.052), time to complete the maze (F(3.8,394.2)=4.0, p< 0.01, ηp2=.037), and errors committed (F(4.3,447.7)= 2.7, p = 0.02, ηp2=.026). However, there was no main effect of maze for performance efficiency (F(2.9,304.4) = 1.4, n.s.), errors committed (F(1.8,189.4) <1, n.s.), time to complete the maze task (F(2.8,291.4) =2.5, p = 0.07), slope (F(2,312) <1, n.s), or learning rate (F(2,312) = 2.1, n.s).
There was no significant interaction between gender, maze, and trial, or gender and trial for any measure. However, there was a significant interaction between maze and gender on the number of errors committed (F(1.8,189.4) = 8.9, p<0.001, ηp2=.078,) and time to complete the maze (F(2.8,291.4) = 4.0, p<0.01, ηp2=.037), but not for performance efficiency (F(2.9,304.4) =2.0, n.s.), slope (F(2,312) <1, n.s.), or learning rate (F(2,312) <1, n.s.). There was a significant main effect of gender for errors (F(1, 104) = 10.2, p<0.01, ηp2=.09), latency, F(1, 104) = 13.8, p<0.001, ηp2=.117) and performance efficiency (F(1, 104) = 13.9, p<0.001, ηp2=.118), but not for slope (F(1,156) <1, n.s.) or learning rate (F(1,156) <1, n.s.). These data provide evidence that pre-readers that display an impaired reading ability based on early reading measures, exhibit a similar impairment on the maze learning task to children with reading impairment[10] and the dcdc2 knockout mouse[9], a genetic model of developmental dyslexia.
Genetic risk associated with greater impairment on maze learning task
Genetic risk was determined based on the presence of the DCDC2 READ1 risk allele 5, a 2,445 bp microdeletion in intron 2 of DCDC2, and/or a biological parent with reported ARHQ ratio of ≥ 0.4 [54]. Of the 163 participants in this study, 116 participants (101 typical and 15 atypical) provided a saliva sample for analysis, and/or provided a completed ARHQ from one or both biological parents. Forty-one participants (34.5% of 116) were identified as having a known genetic risk for reading impairment. Of the 101 typical readers, thirty-six (35.6%) were identified as having a genetic risk for dyslexia, compared to five out of fifteen (33.3%) of atypical readers.
We found a significant four-way interaction between reading ability, genetic risk, maze, and trial for performance efficiency (F(11.8, 1226.5) = 2.17, p= 0.01, ηp2=.020, Fig. 5a), and errors committed (F(7.1, 739.0)= 2.8, p<0.01,, ηp2=.026, Fig. 5b), but not time to complete the maze (F(9.4,981.4) = 1.4, n.s., Fig. 5c). There was a significant interaction between trial, reading ability and genetic risk for errors committed (F(4.3, 447.7)= 2.6, p=0.03, ηp2=.024), but not for performance efficiency (F(4.1, 430.9) = 2.1, p=0.08 or time to complete the maze [F(3.8,394.2) = 1.4, n.s.). Conversely, there was a significant three-way interaction for maze, reading ability, and genetic risk for all three measures (performance efficiency [F(2.9,304.4)= 3.4, p=0.02, ηp2=.031], time to complete the maze [F(2.8,291.4)= 4.8, p0.01, ηp2=.044), and errors committed (F(1.8, 189.4)= 5.3, p0.01, ηp2=.049), as well as for slope (F(2,224) = 4.7, p = 0.02, ηp2=.037), but not learning rate (F(2,224) = 2.5, p = 0.08.). However, there was not a statistically significant interaction between maze, trial, and genetic risk for these measures (performance efficiency [F(11.8, 1226.5) =1.3, n.s.), time to complete the maze [F(9.4,981.4) <1, n.s.], and errors committed [F(7.1, 739.0) = 1.5, n.s.]). There was no significant interaction found for trial and genetic risk, nor maze and genetic risk for performance efficiency, time to complete the maze, or errors committed. There was no significant interaction between maze and genetic risk, or reading ability and genetic risk, for slope or learning rate. These data suggest that struggling readers exhibit impaired performance on this maze learning task, and that this impairment is exacerbated by genetic risk for dyslexia.
Fig. 5.
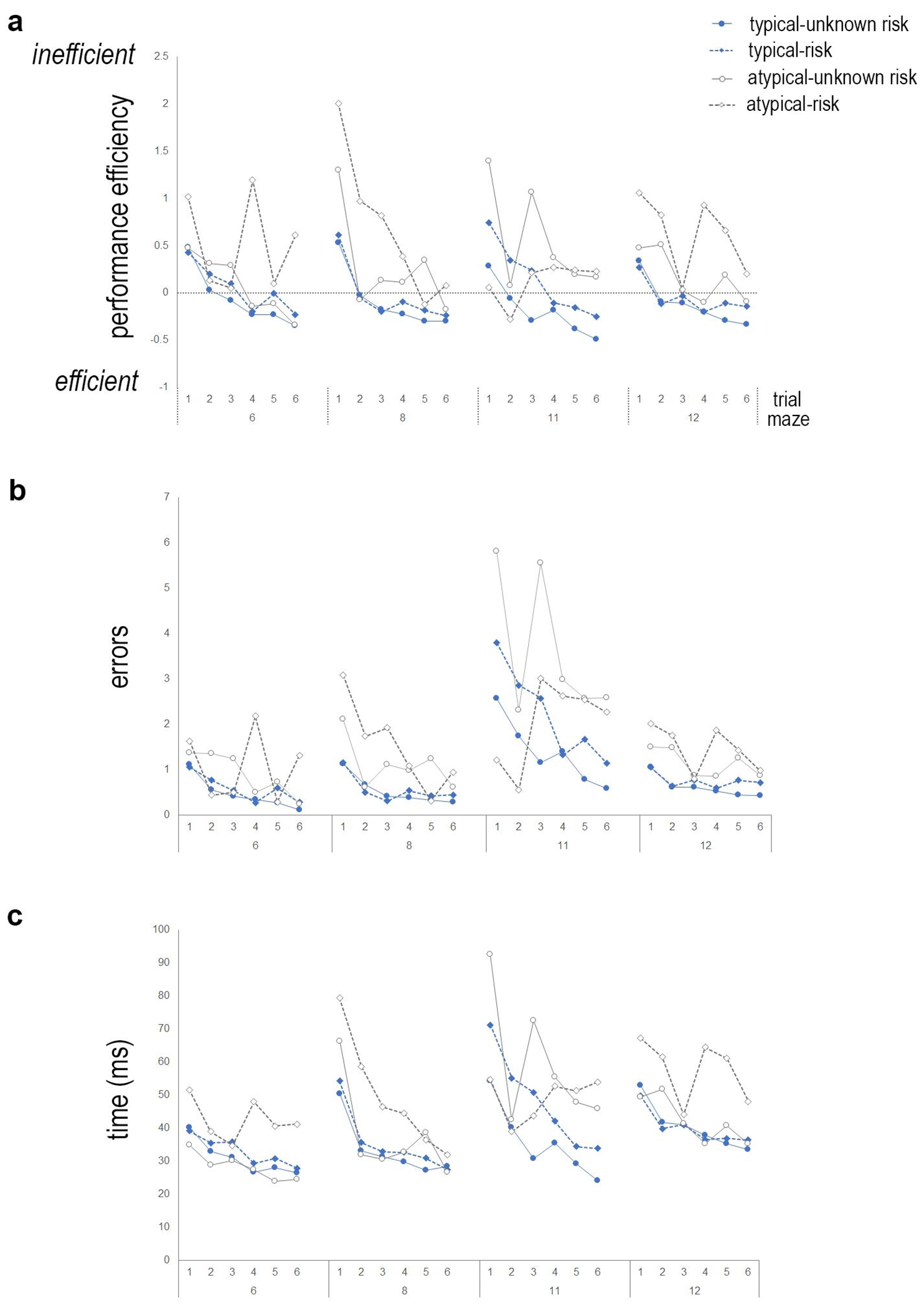
Genetic risk for dyslexia is associated with greater impairment on the maze task. (a) Performance efficiency is plotted for all mazes completed (Mazes 6, 8, 11, 12) during testing, across six consecutive trials for typical (blue) and atypical (grey) readers based on genetic risk (unknown risk [solid lines], and genetic risk [dotted lines]). Children with reading impairment (atypical) exhibit impaired performance across trials, which is exacerbated by known genetic risk. Genetic risk is defined as 2.4 kb microdeletion in intron 2 of DCDC2, READ1 risk allele 5, and or biological risk based on the adult reading history questionnaire from one, or both, biological parents. Positive performance efficiency values indicate inefficient performance, whereas negative values show greater efficiency compared to the mean performance (Mean performance = 0). Performance efficiency is calculated by equally weighting standardized values (z-scores) for (b) errors committed during the completion of the maze task and (c) time to complete the maze.
Risk assessment demonstrates ability of performance on maze task to predict reading impairment
We examined risk of reading impairment based on maze performance greater than 1.5 SD above the mean (i.e. poor performance efficiency) on trial three, as well as genetic risk associated with an ARHQ ratio greater than 0.4, presence of the microdeletion in intron 2 of the DCDC2 gene, or the presence of DCDC2 READ1 allele 5. The odds of being a struggling reader are 2.1 times greater (95% CI: 0.4-11.4; 2/27 atypical, 5/136) for individuals who score greater than 1.5 standard deviations above the mean on trial 3 of Maze 6, 3.5 times greater (95% CI: 0.6-22.2; 2/27 atypical, 3/135 typical) on Maze 8, 3.7 times greater (95% CI: 1.2-11.4; 6/26 atypical, 10/134 typical) on Maze 11, and 1.2 times greater (95% CI: 0.3-4.5; 3/25 atypical, 13/126 typical) on Maze 12. The odds ratio for genetic risk 0.9 (95% CI: 0.4-1.9; 5/16 atypical, 37/104 typical) suggests that the maze task is a better predictor of reading ability than genetic risk as defined in this study.
Study 2: Longitudinal
Performance on the maze is stable over time among children with reading impairment.
A total of 42 participants (21, 50% male), were tested at two timepoints, kindergarten and second grade. Ten participants (25% of the sample, 4 females and 6 males) scored one standard deviation or more, below the mean on the Basic Reading or Reading cluster on the WRMT-III or WJ-IV, and are referred to as atypical readers (i.e. atypical). One participant identified as a typical reader had a Reading Comprehension cluster score below 85. The reading ability designation (i.e. typical or atypical) was changed in 16.7% of the participants between time one and time two (7/42). Approximately 71% (5/7) were identified as typical readers at time one, and were later identified as atypical readers at time two. Participant performance was examined on four separate Hebb-Williams maze configurations (i.e. Mazes 6, 8, and 9; with Maze 1 serving as a training maze, see Fig. 6), across six trials per maze. Descriptive statistics (minimum and maximum values; mean ± standard deviation) are provided for cluster scores of Basic Reading and Reading for both the typical and atypical populations in Table 3. As described above, latency to complete the maze and errors committed were measured and standardized to calculate a performance efficiency score. Representative paths taken to complete the maze for typical and atypical readers for each maze are shown in Fig. 7.
Table 3.
Reading Cluster Scores, Readers.
| Group (N) | Basic Reading | Reading | ||
| Typical (30) | 106.3 ± 11.4 | 104.4 ± 11.5 | ||
| 86 | 130 | 89 | 131 | |
| Reading Impaired (10) | 76.0 ± 9.5 | 74.1 ± 11.3 | ||
| 61 | 86 | 55 | 90 | |
Values are presented as mean ± standard deviation with the minimum and maximum values for each measure listed below. An independent samples t-test shows a statistically significant different between groups (p<0.001)
Fig. 7.
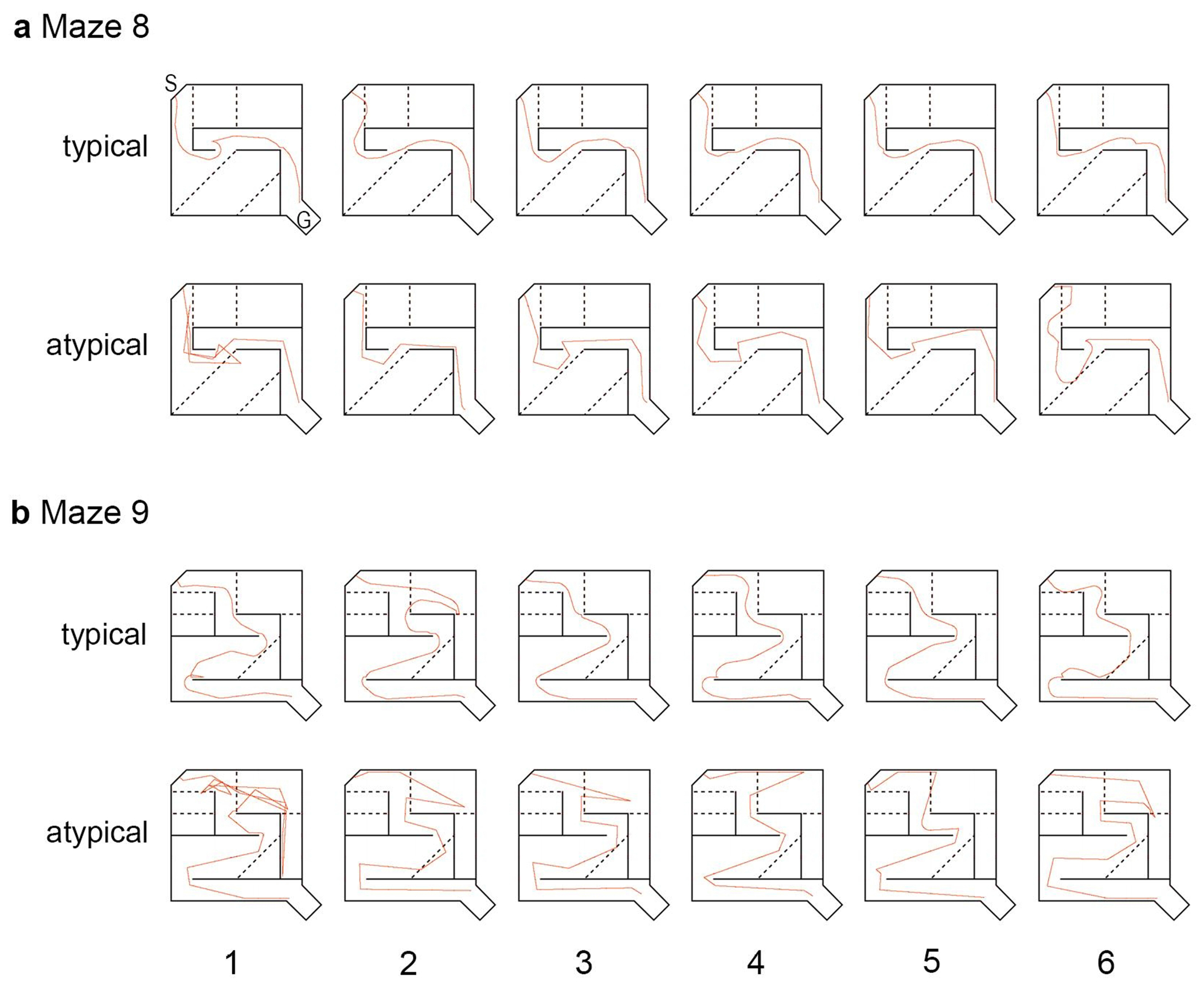
Children with reading impairment show difficulty maintaining a true path to solve the maze task. (a) Representative traces of paths (shown in red) taken by typical (top) and atypical (bottom) readers while solving Maze 8, a task they were introduced to two years prior. The goal box (G) and the starting position (S) are indicated on the schematic. The solid black lines represent walls in the maze, the black dotted lines represent error zones, which are not visible to participants Children with reading impairment (atypical) experienced difficulty identifying and maintaining the correct path to the target within the goal box of the maze. (b) Representative traces of paths taken by typical (top) and atypical (bottom) readers while solving Maze 9, a task they had no prior experience solving. Children with reading impairment exhibited a greater degree of difficulty identifying and maintaining the correct path to the target within the goal box of the maze. It is important to note that the examples in this figure are from the same participant whose traces are represented in Figure 2. A direct comparison in performance on Maze 8 can be seen between time one (Kindergarten) and time two (second grade).
Examination of the reading ability (typical vs. atypical), mazes performed (maze 6 and maze 8), and trials (1-6); with gender as a covariate, demonstrated that children with reading impairment continue to exhibit impaired performance on this task, despite having previously performed the task. There was not a significant three-way interaction between maze, trial and reading impairment (Wilks’ Lambda = .91, F(15, 505.6) = 1.18, n.s.). However, there was a statistically significant interaction between maze and reading ability (Wilks’ Lambda = .79, F(3,35) = 3.0, p=0.04, ηp=.206, Fig. 8), but not trial and reading (Wilks’ Lambda = .94, F(15,505.6) <1, n.s.). There was a significant interaction between maze, trial, and gender (Wilks’ Lambda = .87, F(15,505.6) = 1.79, p=0.03, ηp2=.047); maze and trial (Wilks’ Lambda = .87, F(15,505.6) = 1.78, p=0.04, ηp2=.046), and trial and gender (Wilks’ Lambda = .87, F(15,505.6) = 1.71, p0.05, ηp2=.044), but not maze and gender (Wilks’ Lambda = .90, F(3,35)=1.28, n.s.); maze (Wilks’ Lambda = .97, F(3,35)<1, n.s.), or trial (Wilks’ Lambda = .90, F(15,505.6) =1.38, n.s.).
Fig. 8.
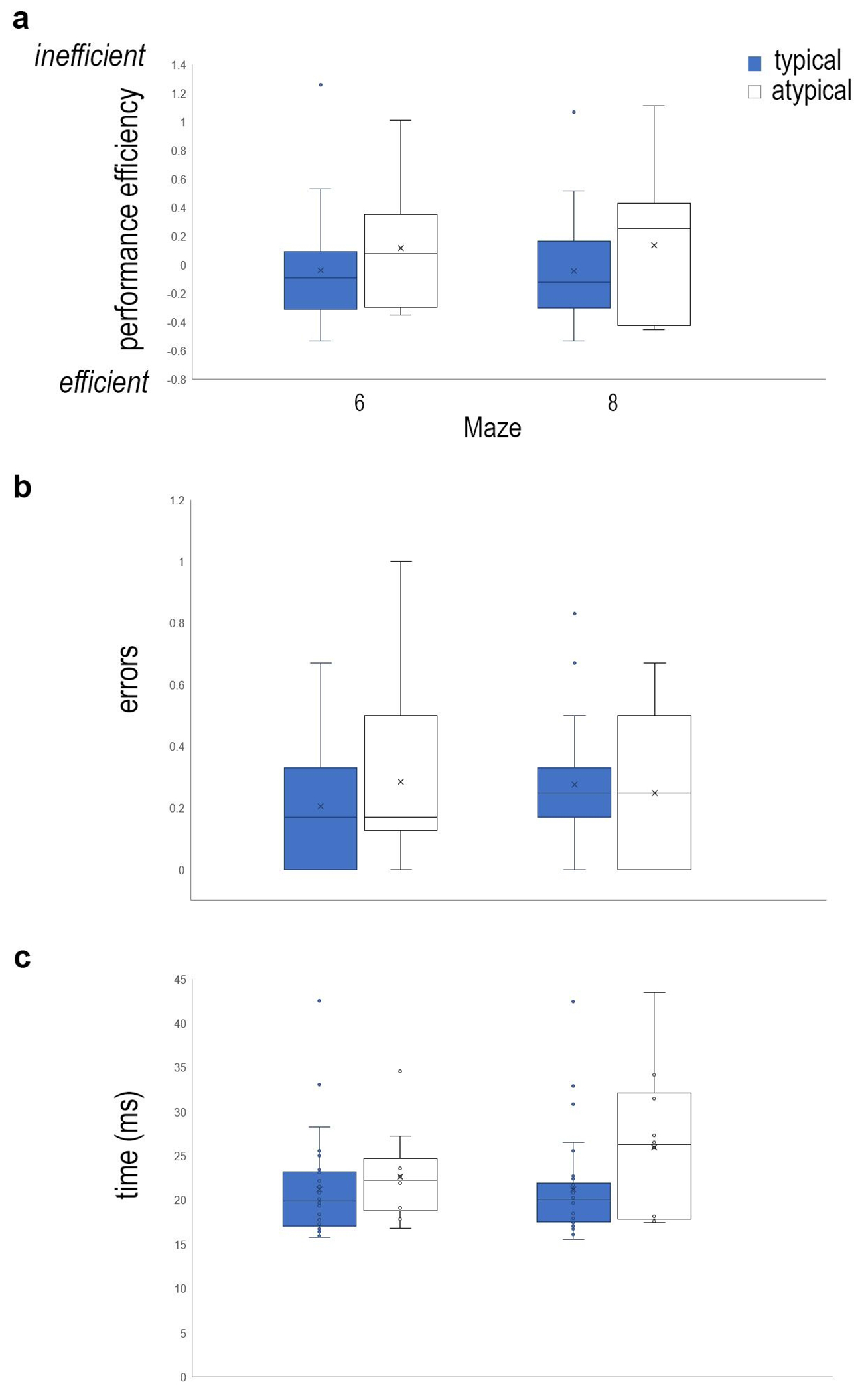
Performance on the virtual maze task is stable over time. (a) Box plots of performance efficiency is shown for mazes 6 and 8 for typical and atypical readers two years after initial testing. Positive values indicate inefficient performance, whereas negative values show greater efficiency compared to the mean performance (Mean performance = 0). Children with reading impairment, continue to show inefficient performance on these maze tasks. Performance efficiency is calculated by equally weighting standardized values (z-scores) for (b) errors committed during the completion of the maze task and (c) time to complete the maze.
Odds ratio and Risk Assessment
We conducted a risk assessment for reading impairment based on performance on mazes 6 and 8, which were assessed at both timepoints (kindergarten and second grade). Unlike the performance of 5-6-year-old children, 8-9-year-old children do not exhibit inefficient performance greater than 1 SD above the mean in later trials of the task (i.e. trial 3) in this sample. However, assessment of risk following trial 1 demonstrates that the odds of being an atypical reader are 1.3 times greater (95% CI: 0.3-5.5; 5/10 atypical, 13/30 typical) for individuals who score greater than one standard deviation above the mean on trial 1 of Maze 6, as well as Maze 8 (1.3 times greater [95% CI: 0.3-5.8; 4/10 atypical, 10/30 typical]). Examination of previously published data [10], which did not report this assessment, demonstrates that only 11% of atypical readers performed greater than 1 standard deviation above the mean (2/18), and 5.5% of typical readers (4/73). With a small sample size of individuals who participated in this study over a two-year time period, it is difficult to know whether the sample was too small to detect a similar variability in performance (only 1/10 atypical readers, and 2/30 typical readers, would have been expected to perform greater than 1 SD above the mean during trial 3), or if we are detecting an effect of re-testing participants on the same maze task. Based on the previously published data, the odds of being an atypical reader were 2.9 times greater for individuals who score greater than one standard deviation above the mean on trial 1 of Maze 6, and 1.6 times greater on Maze 8, which is different than what is currently reported in this study. Interestingly, the odds of being an atypical reader are 17.3 times greater (95% CI: 1.9-253.2; 2/5 atypical, 1/27 typical) for individuals who score greater than one standard deviation above the mean on trial 3 of Maze 9. Participants were not previously exposed to Maze 9 in kindergarten, whereas participants completed Mazes 6 & 8 at both timepoints. Future studies will need to examine performance on maze 9 with a larger sample of this population to determine if the risk of reading impairment can be predicted to such a degree.
Discussion/Conclusions
The current data demonstrate that young children (i.e. kindergarteners) who are struggling readers perform poorly on the virtual maze learning task, in a similar fashion as struggling readers ages 8-12[10], and genetic animal models of dyslexia (dcdc2 knockdown in mice[9]. In struggling readers, performance is marked by a lack of a typical learning curve, and often characterized by inconsistent performance between trials (Fig. 3). Representative traces of paths taken to complete the maze task demonstrate that typical readers will continue to follow the true path to the goal, consistently across trials, once the target has been successfully identified (Fig. 2 typical). However, struggling readers often exhibit inconsistent path finding behaviors even after successfully identifying the true path to the target (Fig. 2 atypical). These data may suggest a general impairment in the ability to acquire, store, recall, and decode visual information effectively, even over a relatively short period of time (i.e. the trials are run consecutively without delay). This is further highlighted by the statistically significant differences in the learning rates calculated from the performance efficiency scores across trials. Positive learning rates indicate that the performance efficiency increased across trials. Learning rates for typical readers remained stable across mazes, however learning rates for struggling readers were variable (Fig. 7). For example, struggling readers showed significantly inefficient performance on the first completed trial compared to typical readers, but their learning rate was also significantly higher; this suggested that although they initially struggled they were able to learn the task and improve performance. However, on Maze 11, the learning rate among struggling readers was lower on average than typical readers, and the learning curve was considerably more flat, similar to learning curves for dcdc2 knockout mice[9].
Genetic risk for reading impairment, defined by READ1 allele 5, the 2,445 bp microdeletion, and/or biological risk assessed by the ARHQ, is associated with a greater degree of inefficiency on this task (Fig. 5). Individuals with a genetic risk factor showed more positive performance efficiency scores on this task in comparison to individuals without. Typical readers exhibited a linear learning curve in comparison to struggling (i.e. atypical) readers, however, even typical readers with a genetic risk for dyslexia exhibited greater performance inefficiency compared to typical readers without. Atypical readers with a genetic risk for dyslexia showed the most inefficient performance on this task. However, the natural grouping of the participants resulted in the lowest sample size in this condition, with only five of the 116 participants exhibiting both atypical reading ability and a genetic risk for dyslexia. Although a Huynh-Feldt correction can control for Type I error rate in even smaller samples[68], statistical power is influenced by the smallest sample size. Therefore, additional studies need to be performed to further examine the interaction between genetic risk and reading ability on maze performance to confirm these findings. In addition to DCDC2 there are eight other dyslexia susceptibility genes (DSG), DYX1C1, KIAA0319, C2orf3, MPRL19, ROBO1, GRIN2B, FOXP2, and CNTNAP2 which have been replicated in at least one study (for review see [17]). Future studies should be aimed at determining if the link between genetic risk and performance on this task are specific to a DSG or subsets of DSGs.
In addition, it is unclear from the data whether other READ1 risk alleles would be associated with poor performance on this task. For example, three functional groups of READ1 alleles have been reported[69], RU1-1,RU2-Long, and RU2-Short. RU1-1 alleles only have one copy of Repeat Unit 1 (RU1), and has been associated with a moderate protective effect on reading performance in the Avon Longitudinal Study of Parents and Children (ALSPaC), a longitudinal cohort of European descent[12]. RU2-Long alleles have two copies of RU1 and greater than seven copies of Repeat Unit 2 (RU2). Allele 5 belongs in the RU2-long functional group, along with alleles 6, 13, 14, 19, 20, 22 and 23, and is associated with increased risk for reading impairment[12]. RU2-Short is characterized by alleles that have fewer than six copies of RU2, and has recently been shown to be associated with poor reading comprehension in the Genes Reading and Dyslexia (GRaD) study[70]. The microdeletion was identified in 10 participants in this study, 10 participants had READ1 alleles within the RU1_1 functional group, 16 participants with READ1 alleles within the RU2_Long, 15 within RU2-Short, and 37 participants had READ1 alleles not associate with these functional groupings (e.g.1_1). Of the 16 participants with READ1 alleles within the RU2-Long functional group, 4 participants had one copy of allele 5, one of those participants had a combined 5_6 allele; of the 10 participants with a microdeletion two had one copy of allele 5 (D_5) and one had two copies of the microdeletion (D_D). Additional studies should be performed in order to determine if other READ1 risk alleles, and/or dyslexia susceptibility genes (DSGs), are also associated with poor performance on this task.
The exact link between performance on the vHW task and reading ability has yet to be determined. Research indicates that children and adults with reading difficulties exhibit impaired phonological processing, visual spatial attention, working memory, and unsuccessful use of visual cues[71–76, 27]. In our initial studies using the vHW task, we examined participants’ ability to successfully solve a series of maze configurations. The completion of the task requires appropriate use of visual cues (i.e. based on the unique configuration of walls and alleys in each maze) and working and reference abilities as the individual completes successive trials of each maze. Working and reference abilities are dependent on visual processing and attention (i.e. selecting the most relevant information) while navigating through the maze[77–80]. These processes parallel those involved in word reading, which may explain why both animal models of reading disorder, and children with impaired reading ability exhibit reduced performance on this task[10]. In addition, it has been demonstrated that the fronto-parietal and cingulo-opercular networks are activated in response to distinct, but complementary processes underlying reading. These networks are augmented by reading intervention, and also underlie successful learned maze navigation[42, 43, 49, 44–46].
Maze tasks, like the vHW maze, result in activation of multiple networks to successfully find the solutions (correct path), including the left superior and inferior parietal cortex, inferior frontal gyrus, and insula[81]. In addition, the activation of these regions was greater during the maze task compared to a pseudo-maze which removed the incorrect paths (i.e. dead ends) but required the same trajectory to get from the start to the goal box[81]. These data suggest that these regions are activated in response to specific mental processes needed to solve the maze task, as opposed to simply following the path to the goal. During tasks associated with reading, the superior parietal and inferior prefrontal cortex is activated in response to letter identification tasks[82] and the superior parietal cortex is involved in visual attention during a task involving letter and non-alphabetic character strings[83]. In individuals with reading impairment, the left inferior parietal cortex and superior parietal cortex show hypoactivation in response to orthographic mapping tasks[84–86]. The fronto-parietal network has been shown to be involved in phasic aspects of attention, which was suggested to occur when adapting to error across a wide range of tasks[87], as well as the development and retention of a cognitive map[49]. This is in contrast to the cingulo-opercular network which is involved in sustained engagement in a task[47, 88] requiring stable goal-directed behavior[87]. The virtual Hebb-Williams maze task likely involves activation of these same networks during the process of successful navigation to the target. These networks are also involved in multiple aspects of reading. Moreover, children with reading impairment exhibit differential activation patterns within these networks, in comparison to typical readers, during reading tasks[43]. Therefore, it is not surprising that this task would be able to show differences between these groups. Future studies should examine the neural networks underlying successful navigation of the maze task to determine if there is a direct link between performance on this task and the fronto-parietal and cingulo-opercular networks in children with and without reading impairment.
Translational research involves the application of research findings using animal models in the laboratory to human studies. The purpose is to develop and enhance prevention and treatment options for disorders, and improve quality of life. In this study we created a virtual version of the Hebb-Williams maze paradigm that directly models the experiments conducted in animal models of dyslexia (i.e. genetic models and models of focal cortical dysplasia [89, 9, 10]. Accurate rendering of virtual environments allows for cross-species comparisons between animal models of dyslexia and humans with the disorder, in order to gain a better understanding of the biological basis of reading impairment. In this study, we provide new evidence that young children (pre-readers) who are struggling readers are impaired on the virtual maze task, similarly to older children (8-13 years old) with reading impairment [10], and which parallels the deficits identified in a genetic model of the disorder [9]. In addition, we provide evidence that a genetic risk is associated with greater impairment on this task when compared to individuals with an unknown genetic risk for dyslexia. This task may provide an efficient, cost-effective mechanism, an important part of translational science, for identifying young children who are likely to have reading impairment, prior to learning how to read. Early identification of dyslexia is the key factor in positive outcomes for children with specific reading impairment. However, current early assessments still suffer from high rates of over- and under-identification of children at risk for reading impairment[90–92]. In addition, current measures of cognitive processing require a certified professional to administer and assess outcomes which may lead to a bottleneck for students to receive necessary interventions. The virtual maze task taps into various processes which underlie reading, is not influenced by potential differences in reading experience due to the lack of text or oral reporting[93], and as a fully automated system, does not require specialized training to administer. Taken together, these data suggest that the vHW maze task may be a practicable screening tool for the early identification of reading impairment.
Acknowledgements
We would like to thank current and former Lafayette College undergraduates Nick Escalona and Monica Manglani for their original work on the virtual maze environment, and Margaret DenBleyker, Will Duncan, Milena Berestko, Margaret Schiazza, Olivia Grigaux, Alina Sosa, Amanda Literati, Jessica Cysner, and Alexandria Battison for help with data collection. We would also like to thank Lehigh University students Mariangela Perrella and Michaela Ott for help with data collection. In addition, we would like to thank the administrators and teachers at the school districts we worked with for their help and support of this research program.
Funding sources
This work was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health Award Number R15HD087937 to LAG (PI), EJ, ERL, and JRG, Alan & Wendy Pesky Foundation Research Grant to LAG, and Humboldt Research Fellowship to LAG.
Footnotes
Statement of Ethics
Legal guardians provided written informed consent and all participants provided their assent prior to the start of the study. The Lafayette College Institutional Review Board [IRB] (protocol # AY2021-32), Boise State University Social & Behavioral IRB (protocol #: 112-SB17-163), and Lehigh University IRB (protocol # 1506860-2) approved all procedures. The Yale University IRB (#2000021986) determined that the Yale University investigator contributions to this manuscript (i.e. genetic screening of saliva samples) do not constitute research involving human subjects. As such, no consent documentation was required at the Yale site.
Conflict of Interest Statement
Dr. Lisa A. Gabel has a US patent pending (US patent Apl. No. 15/934,567) for the use of the virtual Hebb-Williams maze as a tool for the early identification of dyslexia.
References
- 1.Gori S, Mascheretti S, Giora E, Ronconi L, Ruffino M, Quadrelli E, et al. The DCDC2 intron 2 deletion impairs illusory motion perception unveiling the selective role of magnocellular-dorsal stream in reading (dis)ability. Cereb Cortex. 2015. June;25(6):1685–95. [DOI] [PubMed] [Google Scholar]
- 2.Gabel LA, Gibson CJ, Gruen JR, LoTurco JJ. Progress towards a cellular neurobiology of reading disability. Neurobiol Dis. 2010. May;38(2):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng H, Smith S, Hager K, Held M, Liu J, Olson R, et al. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci U S A. 2005. November;102(47):17053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig K, Schumacher J, Schulte-Körne G, König I, Warnke A, Plume E, et al. Investigation of the DCDC2 intron 2 deletion/compound short tandem repeat polymorphism in a large German dyslexia sample. Psychiatr Genet. 2008. December;18(6):310–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marino C, Scifo P, Della Rosa PA, Mascheretti S, Facoetti A, Lorusso ML, et al. The DCDC2/intron 2 deletion and white matter disorganization: focus on developmental dyslexia. Cortex. 2014. August;57:227–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centanni TM, Chen F, Booker AM, Engineer CT, Sloan AM, Rennaker RL, et al. Speech sound processing deficits and training-induced neural plasticity in rats with dyslexia gene knockdown. PLoS One. 2014;9(5):e98439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong DT, Che A, Rendall AR, Szalkowski CE, LoTurco JJ, Galaburda AM, et al. Mutation of Dcdc2 in mice leads to impairments in auditory processing and memory ability. Genes Brain Behav. 2014. November;13(8):802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centanni TM, Booker AB, Chen F, Sloan AM, Carraway RS, Rennaker RL, et al. Knockdown of Dyslexia-Gene Dcdc2 Interferes with Speech Sound Discrimination in Continuous Streams. J Neurosci. 2016. 04;36(17):4895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabel LA, Marin I, LoTurco JJ, Che A, Murphy C, Manglani M, et al. Mutation of the dyslexia-associated gene Dcdc2 impairs LTM and visuo-spatial performance in mice. Genes Brain Behav. 2011. November;10(8):868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabel LA, Manglani M, Escalona N, Cysner J, Hamilton R, Pfaffmann J, et al. Translating dyslexia across species. Annals of Dyslexia. 2016;66(3):319–36. [DOI] [PubMed] [Google Scholar]
- 11.Centanni TM, Norton ES, Ozernov-Palchik O, Park A, Beach SD, Halverson K, et al. Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. Neuroimage Clin. 2019;22:101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers NR, Eicher JD, Miller LL, Kong Y, Smith SD, Pennington BF, et al. The regulatory element READ1 epistatically influences reading and language, with both deleterious and protective alleles. J Med Genet. 2016. December;53(3):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers NR, Eicher JD, Butter F, Kong Y, Miller LL, Ring SM, et al. Alleles of a polymorphic ETV6 binding site in DCDC2 confer risk of reading and language impairment. Am J Hum Genet. 2013. July;93(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eicher JD, Powers NR, Miller LL, Mueller KL, Mascheretti S, Marino C, et al. Characterization of the DYX2 locus on chromosome 6p22 with reading disability, language impairment, and IQ. Hum Genet. 2014. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darki F, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biol Psychiatry. 2012. October;72(8):671–6. [DOI] [PubMed] [Google Scholar]
- 16.Cope N, Eicher JD, Meng H, Gibson CJ, Hager K, Lacadie C, et al. Variants in the DYX2 locus are associated with altered brain activation in reading-related brain regions in subjects with reading disability. Neuroimage. 2012. October;63(1):148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eicher JD, Gruen JR. Imaging-genetics in dyslexia: connecting risk genetic variants to brain neuroimaging and ultimately to reading impairments. Mol Genet Metab. 2013. November;110(3):201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gori S, Cecchini P, Bigoni A, Molteni M, Facoetti A. Magnocellular-dorsal pathway and sub-lexical route in developmental dyslexia. Front Hum Neurosci. 2014;8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicchini GM, Marino C, Mascheretti S, Perani D, Morrone MC. Strong Motion Deficits in Dyslexia Associated with DCDC2 Gene Alteration. J Neurosci. 2015. May;35(21):8059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gori S, Seitz AR, Ronconi L, Franceschini S, Facoetti A. Multiple Causal Links Between Magnocellular-Dorsal Pathway Deficit and Developmental Dyslexia. Cereb Cortex. 2015. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: a multiparametric approach. Cereb Cortex. 2010. August;20(8):1799–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobier M, Dubois M, Valdois S. The role of visual processing speed in reading speed development. PLoS One. 2013;8(4):e58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobier M, Valdois S. Visual attention deficits in developmental dyslexia cannot be ascribed solely to poor reading experience. Nat Rev Neurosci. 2015. April;16(4):225. [DOI] [PubMed] [Google Scholar]
- 24.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–49. [DOI] [PubMed] [Google Scholar]
- 25.Franceschini S, Gori S, Ruffino M, Pedrolli K, Facoetti A. A causal link between visual spatial attention and reading acquisition. Curr Biol. 2012. May;22(9):814–9. [DOI] [PubMed] [Google Scholar]
- 26.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gori S, Facoetti A. How the visual aspects can be crucial in reading acquisition? The intriguing case of crowding and developmental dyslexia. J Vis. 2015;15(1):15.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc Natl Acad Sci U S A. 1991. September;88(18):7943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galaburda A, Livingstone M. Evidence for a magnocellular defect in developmental dyslexia. Ann N Y Acad Sci. 1993. June;682:70–82. [DOI] [PubMed] [Google Scholar]
- 30.Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996. July;382(6586):66–9. [DOI] [PubMed] [Google Scholar]
- 31.Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997. April;20(4):147–52. [DOI] [PubMed] [Google Scholar]
- 32.Facoetti A, Paganoni P, Lorusso ML. The spatial distribution of visual attention in developmental dyslexia. Exp Brain Res. 2000. June;132(4):531–8. [DOI] [PubMed] [Google Scholar]
- 33.Vidyasagar TR, Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn Sci. 2010. February;14(2):57–63. [DOI] [PubMed] [Google Scholar]
- 34.Vanni S, Uusitalo MA, Kiesilä P, Hari R. Visual motion activates V5 in dyslexics. Neuroreport. 1997. May;8(8):1939–42. [DOI] [PubMed] [Google Scholar]
- 35.Kronbichler M, Hutzler F, Wimmer H. Dyslexia: verbal impairments in the absence of magnocellular impairments. Neuroreport. 2002. April;13(5):617–20. [DOI] [PubMed] [Google Scholar]
- 36.Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. Deficits in perceptual noise exclusion in developmental dyslexia. Nat Neurosci. 2005. July;8(7):862–3. [DOI] [PubMed] [Google Scholar]
- 37.Facoetti A, Corradi N, Ruffino M, Gori S, Zorzi M. Visual spatial attention and speech segmentation are both impaired in preschoolers at familial risk for developmental dyslexia. Dyslexia. 2010. August;16(3):226–39. [DOI] [PubMed] [Google Scholar]
- 38.Boets B, De Smedt B, Ghesquière P. Coherent motion sensitivity predicts individual differences in subtraction. Res Dev Disabil. 2011 2011. May-Jun;32(3):1075–80. [DOI] [PubMed] [Google Scholar]
- 39.Franceschini S, Gori S, Ruffino M, Viola S, Molteni M, Facoetti A. Action video games make dyslexic children read better. Curr Biol. 2013. March;23(6):462–6. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Qian Y, Bi HY, Coltheart M. The visual magnocellular-dorsal dysfunction in Chinese children with developmental dyslexia impedes Chinese character recognition. Sci Rep. 2014. November;4:7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Y, Zhao J, He T, Tan Y, Zheng L, Wang Z. Selective Impairments in Covert Shifts of Attention in Chinese Dyslexic Children. Dyslexia. 2016. November;22(4):362–78. [DOI] [PubMed] [Google Scholar]
- 42.Vaden KI, Kuchinsky SE, Cute SL, Ahlstrom JB, Dubno JR, Eckert MA. The cingulo-opercular network provides word-recognition benefit. J Neurosci. 2013. November;33(48):18979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horowitz-Kraus T, Toro-Serey C, DiFrancesco M. Increased Resting-State Functional Connectivity in the Cingulo-Opercular Cognitive-Control Network after Intervention in Children with Reading Difficulties. PLoS One. 2015;10(7):e0133762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallis G, Stokes M, Cousijn H, Woolrich M, Nobre AC. Frontoparietal and Cingulo-opercular Networks Play Dissociable Roles in Control of Working Memory. J Cogn Neurosci. 2015. October;27(10):2019–34. [DOI] [PubMed] [Google Scholar]
- 45.Crittenden BM, Mitchell DJ, Duncan J. Task Encoding across the Multiple Demand Cortex Is Consistent with a Frontoparietal and Cingulo-Opercular Dual Networks Distinction. J Neurosci. 2016. 06;36(23):6147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, et al. Evidence for Accelerated Decline of Functional Brain Network Efficiency in Schizophrenia. Schizophr Bull. 2016. May;42(3):753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007. February;27(9):2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011. March;31(12):4407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shikauchi Y, Ishii S. Decoding the view expectation during learned maze navigation from human fronto-parietal network. Sci Rep. 2015. December;5:17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004. February;303(5660):1023–6. [DOI] [PubMed] [Google Scholar]
- 51.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006. July;9(7):971–8. [DOI] [PubMed] [Google Scholar]
- 52.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008. March;12(3):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodcock RW. Woodcock reading mastery tests: WRMT-III. 3 ed. Pearson; 2011. [Google Scholar]
- 54.Lefly DL, Pennington BF. Reliability and validity of the adult reading history questionnaire. J Learn Disabil. 2000. 2000 May-Jun;33(3):286–96. [DOI] [PubMed] [Google Scholar]
- 55.Meng H, Powers NR, Tang L, Cope NA, Zhang PX, Fuleihan R, et al. A dyslexia-associated variant in DCDC2 changes gene expression. Behav Genet. 2011. January;41(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005. January;21(2):263–5. [DOI] [PubMed] [Google Scholar]
- 57.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007. September;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reilly D, Neumann DL, Andrews G. Gender differences in reading and writing achievement: Evidence from the National Assessment of Educational Progress (NAEP). Am Psychol. 2019. 2019 May-Jun;74(4):445–58. [DOI] [PubMed] [Google Scholar]
- 59.Levine SC, Huttenlocher J, Taylor A, Langrock A. Early sex differences in spatial skill. Dev Psychol. 1999. July;35(4):940–9. [DOI] [PubMed] [Google Scholar]
- 60.Levine SC, Vasilyeva M, Lourenco SF, Newcombe NS, Huttenlocher J. Socioeconomic status modifies the sex difference in spatial skill. Psychol Sci. 2005. November;16(11):841–5. [DOI] [PubMed] [Google Scholar]
- 61.Tzuriel D, Egozi G. Gender differences in spatial ability of young children: the effects of training and processing strategies. Child Dev. 2010. 2010 Sep-Oct;81(5):1417–30. [DOI] [PubMed] [Google Scholar]
- 62.Merrill EC, Yang Y, Roskos B, Steele S. Sex Differences in Using Spatial and Verbal Abilities Influence Route Learning Performance in a Virtual Environment: A Comparison of 6- to 12-Year Old Boys and Girls. Front Psychol. 2016;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shore DI, Stanford L, MacInnes WJ, Klein RM, Brown RE. Of mice and men: virtual Hebb-Williams mazes permit comparison of spatial learning across species. Cogn Affect Behav Neurosci. 2001. March;1(1):83–9. [DOI] [PubMed] [Google Scholar]
- 64.Boutet I, Collin CA, MacLeod LS, Messier C, Holahan MR, Berry-Kravis E, et al. Utility of the Hebb-Williams Maze Paradigm for Translational Research in Fragile X Syndrome: A Direct Comparison of Mice and Humans. Front Mol Neurosci. 2018;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Riverside, IL: Riverside Publishing; 2001. [Google Scholar]
- 66.Christ TJ, Ardoin SP. Measurement of oral reading: Passage equivalence and probe-set development. Journal of School Psychology. 2009;47(1):55–75. [Google Scholar]
- 67.Good RH, Jefferson G. Contemporary perspectives on curriculum-based measurement validity. In: Shinn MR, editor. Advanced applications of curriculum based measurement. New York: Guilford Publications.; 1998. p. 61–88. [Google Scholar]
- 68.Oberfeld D, Franke T. Evaluating the robustness of repeated measures analyses: the case of small sample sizes and nonnormal data. Behav Res Methods. 2013. September;45(3):792–812. [DOI] [PubMed] [Google Scholar]
- 69.Li M, Truong DT, DeMille M, Malins JG, Lovett MW, Bosson-Heenan J, et al. Effect of READ1 on latent profiles of reading disorder and comorbid attention and language impairment subtypes. Child Neuropsychol. 2020. 02;26(2):145–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M, Malins JG, DeMille MMC, Lovett MW, Truong DT, Epstein K, et al. A molecular-genetic and imaging-genetic approach to specific comprehension difficulties in children. NPJ Sci Learn. 2018;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Facoetti A. Facilitation and inhibition mechanisms of human visuospatial attention in a non-search task. Neurosci Lett. 2001. January;298(1):45–8. [DOI] [PubMed] [Google Scholar]
- 72.Sireteanu R, Goertz R, Bachert I, Wandert T. Children with developmental dyslexia show a left visual “minineglect”. Vision Res. 2005. November;45(25-26):3075–82. [DOI] [PubMed] [Google Scholar]
- 73.Sireteanu R, Goebel C, Goertz R, Werner I, Nalewajko M, Thiel A. Impaired serial visual search in children with developmental dyslexia. Ann N Y Acad Sci. 2008. December;1145:199–211. [DOI] [PubMed] [Google Scholar]
- 74.Frijters JC, Lovett MW, Steinbach KA, Wolf M, Sevcik RA, Morris RD. Neurocognitive predictors of reading outcomes for children with reading disabilities. J Learn Disabil. 2011. 2011 Mar-Apr;44(2):150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menghini D, Finzi A, Carlesimo GA, Vicari S. Working Memory Impairment in Children With Developmental Dyslexia: Is it Just a Phonological Deficity? Dev Neuropsychol. 2011. February;36(2):199–213. [DOI] [PubMed] [Google Scholar]
- 76.Moores E, Cassim R, Talcott JB. Adults with dyslexia exhibit large effects of crowding, increased dependence on cues, and detrimental effects of distractors in visual search tasks. Neuropsychologia. 2011. December;49(14):3881–90. [DOI] [PubMed] [Google Scholar]
- 77.McConnell J, Quinn JG. Interference in visual working memory. Q J Exp Psychol A. 2000. February;53(1):53–67. [DOI] [PubMed] [Google Scholar]
- 78.Klauer KC, Zhao Z. Double dissociations in visual and spatial short-term memory. J Exp Psychol Gen. 2004. September;133(3):355–81. [DOI] [PubMed] [Google Scholar]
- 79.Baumann O, Skilleter AJ, Mattingley JB. Short-term memory maintenance of object locations during active navigation: which working memory subsystem is essential? PLoS One. 2011;6(5):e19707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ploran EJ, Bevitt J, Oshiro J, Parasuraman R, Thompson JC. Self-motivated visual scanning predicts flexible navigation in a virtual environment. Front Hum Neurosci. 2014;7:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirsch P, Lis S, Esslinger C, Gruppe H, Danos P, Broll J, et al. Brain activation during mental maze solving. Neuropsychobiology. 2006;54(1):51–8. [DOI] [PubMed] [Google Scholar]
- 82.Carreiras M, Monahan PJ, Lizarazu M, Duñabeitia JA, Molinaro N. Numbers are not like words: Different pathways for literacy and numeracy. Neuroimage. 2015. September;118:79–89. [DOI] [PubMed] [Google Scholar]
- 83.Lobier M, Peyrin C, Le Bas JF, Valdois S. Pre-orthographic character string processing and parietal cortex: a role for visual attention in reading? Neuropsychologia. 2012. July;50(9):2195–204. [DOI] [PubMed] [Google Scholar]
- 84.Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 2006. October;47(10):1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bitan T, Burman DD, Chou TL, Lu D, Cone NE, Cao F, et al. The interaction between orthographic and phonological information in children: an fMRI study. Hum Brain Mapp. 2007. September;28(9):880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paz-Alonso PM, Oliver M, Lerma-Usabiaga G, Caballero-Gaudes C, Quiñones I, Suárez-Coalla P, et al. Neural correlates of phonological, orthographic and semantic reading processing in dyslexia. Neuroimage Clin. 2018;20:433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007. June;104(26):11073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sadaghiani S, D’Esposito M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cereb Cortex. 2015. September;25(9):2763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoplight BJ, Sherman GF, Hyde LA, Denenberg VH. Effects of neocortical ectopias and environmental enrichment on Hebb-Williams maze learning in BXSB mice. Neurobiology of learning and memory. 2001. Jul 5/6/2009;76(1):33. [DOI] [PubMed] [Google Scholar]
- 90.Scarborough HS. Early identification of children at risk for reading disabilities: phonological awareness and some other promising predictors. In: Shapiro BK, Accardo PJ, Capute AJ, editors. Specific Reading Disability: A View of the Spectrum. Timonium, Maryland: york Press, Inc.; 1998. p. 75–107. [Google Scholar]
- 91.Jenkins JR, Hudson RF, Johnson ES. Screening for at-risk readers in a response to intervention framework. School Psychology Review. 2007;36(4):582–600. [Google Scholar]
- 92.Thomson JM, Hogan TP. Introduction: Advances in early detection of reading risk. J Learn Disabil. 2010. 2010 Jul-Aug;43(4):291–3. [DOI] [PubMed] [Google Scholar]
- 93.Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nat Rev Neurosci. 2015. January;16(1):43–54. [DOI] [PubMed] [Google Scholar]


