Abstract
Background and Objectives:
Prenatal exposure to environmental tobacco smoke (ETS) is associated with increased attention problems in children, however, the effects of such exposure on children’s brain structure and function have not been studied. Herein, we probed effects of prenatal ETS on children’s cognitive control circuitry and behavior.
Methods:
Forty-one children (7-9 years) recruited from a prospective longitudinal birth cohort of non-smoking mothers completed structural and task-functional magnetic resonance imaging to evaluate effects of maternal ETS exposure, measured by maternal prenatal urinary cotinine. Attention problems and externalizing behaviors were measured by parent report on the Child Behavior Checklist.
Results:
Compared to non-exposed children, exposed children had smaller left and right thalamic and inferior frontal gyrus (IFG) volumes, with large effect sizes (p-FDR <.05, Cohen’s D range from 0.79-1.07), and increased activation in IFG during the resolution of cognitive conflict measured with the Simon Spatial Incompatibility Task (38 voxels; peak t(25)=5.25, p-FWE=.005). Reduced thalamic volume was associated with increased IFG activation and attention problems, reflecting poor cognitive control. Mediation analyses showed a trend toward left thalamic volume mediating the association between exposure and attention problems (p=.05).
Conclusions:
Our findings suggest that maternal ETS exposure during pregnancy has deleterious effects on the structure and function of cognitive control circuitry which in turn affects attentional capacity in school-age children. These findings are consistent with prior findings documenting the effects of active maternal smoking on chidlren’s neurodevleoment, pointing to the neurotixicity of nicotine regardless of exposure pathway.
Keywords: Cotinine, Environmental Tobacco Smoke, Cognitive Control, Attention, Frontostriatal circuit
1. Introduction
Prospective birth cohort studies have begun to document associations between environmental (second-hand) tobacco smoke (ETS) exposure during the prenatal period in non-smoking women and children’s health outcomes, including adverse neurodevelopmental effects.1-5 Specifically, prenatal ETS exposure has been associated with increases in externalizing behaviors as well as hyperactivity.6,7 These findings are consistent with studies showing associations between active maternal smoking during pregnancy and risk for attention problems and Attention Deficit Hyperactivity Disorder (ADHD) among children,8-10 pointing to the neurotoxicity of transplacental nicotine11,12 and other compounds found in ETS, including heavy metals.13,14
Effects of prenatal ETS exposure on attention and cognitive control processes are likely driven in part by adverse effects of nicotine on fetal brain development.15,16 In animals, prenatal nicotine exposure acts on nicotinic acetylcholine receptors, thereby altering cholinergic signaling of cell replication and differentiation, apoptotic cell death, or decreasing cell size.17 Additionally, abnormal activation of nicotinic receptors interferes with the development of neurotransmitter systems (i.e., dopamine, norepinephrine, serotonin17) that innervate cognitive control circuitry and are implicated in ADHD.18 Last, animal models document effects of prenatal exposure to nicotine on attention and inhibitory control,19 suggesting that prenatal ETS exposure may adversely impact human brain development, and specifically cognitive control circuits.
In humans, prenatal exposure to nicotine through active maternal smoking during pregnancy is associated with regional thinning of cognitive control regions, including superior frontal, parietal, lateral occipital, and precentral cortices in 6-year-old children, as well as smaller total brain, gray, and white matter volumes.20 In adolescents and adults, active maternal smoking during pregnancy is associated with smaller right inferior frontal gyrus (IFG) volumes21 and reduced thickness in lateral orbitofrontal, middle frontal, and parahippocampal cortices.22 Functional magnetic resonance imaging (MRI) data also link prenatal exposure to nicotine and increased activity in IFG, inferior parietal lobule, thalamus, and basal ganglia during response inhibition in children and adolescents performing a Go/No-Go task.23,24 In contrast, adults with prenatal exposure showed decreased activity in anterior cingulate cortex (ACC), IFG, and supramarginal gyrus during the Go/No-Go task.21 Thus, prenatal exposure to nicotine through active maternal smoking affects both the structure and function of control regions with notable differences in these effects across development. However, the effects of prenatal exposure to ETS (rather than active maternal smoking) on the structure and function of circuits that support cognitive control processes remain unstudied.
Herein, we address this important gap in the literature. We assessed the effects of prenatal ETS exposure, measured by maternal prenatal urinary cotinine levels-a biomarker of nicotine exposure-on cognitive control circuits in 7-9-year-old children. Using structural and task-based fMRI, we probed effects of prenatal ETS exposure on the frontostriatal portions of cortico-striatal-thalamo-cortical (CSTC) circuits, which interconnect frontal, parietal, and temporal cortices with the basal ganglia and thalamus. These circuits are known to support cognitive control processes25 and are vulnerable to active maternal smoking.20,21,23,24 Consistent with the effects of active maternal smoking on brain structure and function,20,21,23,24 we hypothesized that relative to non-exposed children, those with prenatal ETS exposure would show altered frontostriatal morphometry and smaller total brain, gray, and white matter volumes as well as increased frontostriatal activity during the engagement of cognitive control on the Simon Spatial Incompatibility Task.26 This task requires the engagement of control to respond correctly on incongruent trials, when a task-irrelevant feature of a stimulus (the side of the screen on which an arrow appears) conflicts with the task-relevant feature (direction of the arrow). Both adults and children typically respond with slower reaction times (RT) on these incongruent trials.27,28 We hypothesized that, relative to non-exposed children, ETS-exposed children would show greater trial-wise associations between brain activity and RT and greater activation during incongruent versus congruent trials. Consistent with studies of clinical and behavioral outcomes,6,7 we explored associations of prenatal ETS exposure with greater attention problems and other behaviors reflecting poor cognitive control. Last, given that ETS exposure occurred prenatally, a period when brain structure and function are rapidly developing,29 we explored if effects of ETS on brain structure might influence brain function.
2. Methods
2.1. Participants
We recruited all 53 children in the Sibling-Hermanos birth cohort30 age 7-years or older (Supplement). Mothers were non-smokers.31 Five children opted not to participate leaving 48 children who came for a visit and attempted a scan. Of these 48, five children were unable to complete a structural scan and two children were excluded during quality control procedures (Supplement) for excessive head motion during the structural sequence, leaving 41 with useable structural data. Of 39 children who completed the Simon task, six were excluded for poor task accuracy (<50% correct on each run), two for excessive head motion (>20% of frames were outliers with >1mm frame-wise displacement on each run32,33), and 1 for a technical error, leaving 30 with useable task data. The institutional review boards at Columbia University and New York State Psychiatric Institute approved the study; children and guardians provided written informed assent and consent.
2.2. Cotinine Measurement
Cotinine was measured in prenatal maternal and postnatal child urine: individuals with values between 0.05-0.99ng/ml were classified as non-exposed, and those with 1.00–16.00ng/ml as ETS-exposed (Supplement).34-36 Among participants who attempted a scan, 28 children were non-exposed and 20 (41.7%) exposed, mirroring US population estimates that 40% of children are ETS exposed.37 Of the 41 children who successfully completed the structural scan, 24 were exposed, and of the 30 children who successfully completed the Simon task, 10 were exposed.
2.3. Behavioral Measures
Maternal report on the Child Behavior Checklist (CBCL), a clinically validated questionnaire, was used to measure behavioral features of ADHD. Parent-report is the most commonly used clinical tool for assessing these behaviors.38 We examined three CBCL subscales: the empirically-based Attention Problems score measuring symptoms associated with ADHD, the DSM-oriented ADHD scale reflecting likelihood of an ADHD diagnosis, and the Externalizing behavior scale. Children completed the Wechsler Abbreviated Scale of Intelligence-2nd edition.39
2.4. MRI Acquisition
Data were acquired on a 3T GE 750 scanner with a 32-channel head coil. Two structural T1 images were collected for each participant using a 3D-FSPGR sequence (flip angle=11, TE=2.588ms, TR=6.412ms, 180 slices, 1mm isotropic resolution). Three runs of Simon task data were acquired with an echo planar imaging sequence (flip angle=77, TE=25ms, TR=2000ms, 44 slices, 3.0mm isotropic resolution, 160 acquisition frames, 5 minutes and 32 seconds long). Structural data were processed using standard FreeSurfer v6.0 pipeline (recon-all)40,41 and functional data using Statistical Parametric Mapping (SPM)12, AFNI, and FSL (Supplement).
2.5. Simon Task
During the Simon task,26 individuals press a button to indicate the direction an arrow is facing. Congruent (C) trials occur when a right-facing arrow appears on the right side of the screen, and incongruent (I) trials when a left-facing arrow appears on the right side of the screen or vice versa. The task thus requires engagement of control processes (inhibit the prepotent response to the arrow location and instead indicate the direction). Typically developing children respond faster to congruent than incongruent trials, activating bilateral IFG, ACC, and parietal regions during the resolution of conflict.28 Prior studies suggest that reaction time (RT), indexing trial-wise difficulty resolving cognitive conflict, similarly associates with activation in the ACC, IFG, and thalamus.42,43 Thus, we were interested in trial-wise associations between brain activity and RT as well as associations between brain activation and event-related contrasts.
First-level general linear models were generated in two ways (SPM12; Supplement). First, models include a regressor of interest for correct trials parametrically modulated by trial-wise RT and one for error trials with duration as RT. Second, models included 5 task regressors (duration as trial-wise RT) to examine second-level Incongruent-Congruent (I-C) contrast: post-congruent congruent, post-incongruent congruent, post-congruent incongruent, post-incongruent incongruent, and errors. All analyses included 24 head motion regressors (translation/rotation, their derivatives, and squares) and regressed out frames with framewise displacement >1mm, as in pediatric task-based studies with similar TR.44
2.6. Statistical Analyses
Distributions of continuous variables (quantile-quantile plots in Figure S1) were transformed as needed to fit a normal distribution. Outliers (z-score>3) were winsorized. Analyses excluded cases with missing data. All tests were conducted using R, with two-tailed tests, alpha p<.05.
All models testing group differences (exposed versus non-exposed) in brain structure or function controlled for age and sex given known associations with brain development;45 models testing regional brain volume controlled for intracranial volume (ICV), and those measuring brain function controlled for mean motion during fMRI acquisition. Although brain structure and function associate with socioeconomic status and FSIQ,46,47 groups did not differ on these variables, and thus they were not included as covariates. To test the effects of postnatal exposure (e.g., specificity of the timing of exposure), post-hoc analyses evaluated the effects of prenatal exposure (exposed versus non-exposed) controlling for postnatal exposure (exposed versus non-exposed) measured with cotinine at child age 3, given their high intercorrelation (prenatal continuous cotinine and postnatal continuous cotinine, r=.45). In addition, postnatal exposure via parent report of a smoker in the house at child age 7 was tested (Supplement). For all significant findings, we explored the dose-response relationship using prenatal cotinine as a continuous variable.
Linear regressions evaluated group differences in volumes of frontostriatal regions (IFG, ACC, putamen48) and thalamus, given our interest in CSTC circuitry as well as global brain measures (total brain, cerebral gray, white matter volumes). Cortical thickness (CT) and surface area (SA) were explored for frontostriatal regions. Cohen’s D, based on the regression coefficient of the ETS term, estimated effect sizes.49 For completeness, vertex-wise analyses evaluated effects of exposure on CT and SA across the entire cortex. All models were false discovery rate (FDR) corrected for multiple comparisons.
T-tests were used to analyze Simon task performance and demographic differences between children included in analyses (n=30) versus excluded (n=18), as well as differences in task performance and head motion between exposure groups. Second-level regressions were used to examine group differences in brain activation. First, we identified clusters that showed significant trial-wise association between activity and RT across all correct trials; we used this parametric modulation to understand trial-level differences in activation associated with engagement of cognitive control (i.e., longer RTs should characterize trials with more cognitive conflict). Second, we identified clusters significantly associated with brain activation during incongruent vs. congruent trials, which does not account for trial-level differences in performance, but rather evaluates differences in average activity between trial types (I-C). Analyses were thresholded at voxel-level significance p<.001, cluster size threshold p-FWE<.05. Consistent with our previous papers using the Simon task in other samples of children, adolescents, and adults,28,48,50,51 group differences (exposed vs. non-exposed) were assessed within our predefined frontostriatal mask,48 that included the same regions investigated in structural analyses (IFG, ACC, putamen, thalamus), defined by the Anatomical Automatic Labeling (AAL) atlas.
Linear regression was used to explore if any observed ETS-related structural and functional alterations were associated with one another. We also explored whether ETS-related brain effects that passed FDR correction were associated with attention problems or externalizing behaviors, and if so, whether these effects mediated associations between exposure and behavior (Supplement).
3. Results
3.1. Participants
Black children and Hispanic children age 7-9 years participated in this study. Exposed and non-exposed children did not differ in age, sex, FSIQ, maternal education, or maternal ADHD symptoms. Black were more likely than Hispanic children to be exposed (Table 1).
Table 1.
Demographic data.
| All Participants (n=41) | Exposed (n=17) | Non-exposed (n=24) | Group Comparison |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | χ2 | P | |
| Sex (female) | 24 | 59 | 9 | 53 | 15 | 63 | 0.37 | .54 |
| Black | 19 | 46 | 11 | 52 | 8 | 33 | 3.94 | .05 |
| Hispanic | 22 | 53 | 6 | 35 | 16 | 67 | ||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | t | P | |
| Age (months) | 103.8 (9.0) | 84-118 | 102 (10.3) | 84-115 | 105 (7.8) | 84-118 | 1.01 | .32 |
| FSIQ | 89.8 (13.3) | 70-119 | 90.1 (16.1) | 70-119 | 89.6 (11.3) | 72-117 | −0.10 | .92 |
| Mat. Ed.(years) | 12.3 (1.7) | 10-16 | 12.0 (1.4) | 10-14 | 12.6 (1.8) | 10-16 | 0.34 | .26 |
| Mat ADHD | 35.9 (5.5) | 31-57 | 36.1 (4.6) | 31-47 | 35.8 (6.1) | 31-57 | −0.18 | .86 |
| Cotinine | 2.09 (2.9) | 0.10-14.2 | 4.2 (3.5) | 1.3-14.2 | 0.6 (0.26) | 0.1-1.0 | −4.23 | .001 |
Note: Mat. Ed. = maternal education. Maternal ADHD symptoms were measured with the Connors Adult ADHD Rating Scale. FSIQ = Full Scale Intelligence Quotient.
3.2. Prenatal Exposure to ETS and Brain Structure
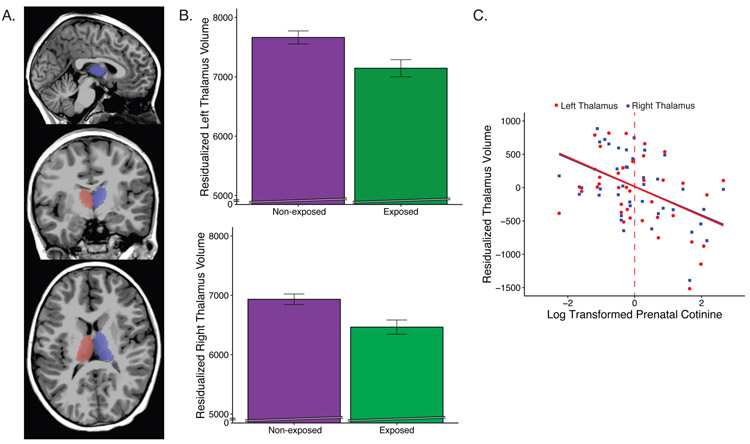
Relative to non-exposed children, exposed children had smaller left and right thalamic and IFG volumes, with large effect sizes (Figure 1; Table 2). Group differences with medium effect sizes that did not pass FDR correction were detected in total brain and white matter volumes (Table 2). Exploratory analyses also revealed medium effects sizes in right IFG surface area and left ACC thickness (Table S1). All brain measures showing group differences also showed significant dose-response relationships using continuous prenatal cotinine (Figures 1c, S2; Table S2). Given group differences in exposure, ethnicity was tested as a covariate in supplementary analyses with no effect on results (Table S3). Postnatal exposure (at age 3 or 7 years) did not associate with brain measures when included in the model with prenatal exposure (p’s>0.3; Table S4, S5). Using birthweight rather than ICV did not change these results (left thalamus: b=−513.69, p<.014; right thalamus: b=−452.2, p<.007). In vertex-wise analyses, no significant group differences were detected.
Figure 1.
Children with prenatal exposure to tobacco smoke have reduced thalamic volumes. A) Thalamus segmentation. B) Group differences in thalamic volumes adjusted for age, sex, and intracranial volume. C) Association between continuous prenatal cotinine and right/left thalamic volumes. Dashed vertical line denotes threshold for classification as prenatally exposed.
Table 2.
Associations between group (exposed vs non-exposed) and brain volume.
| Outcomes | % difference | b | P | p-FDR | Cohen’s D |
|---|---|---|---|---|---|
| Total Brain | 5.62 | −64868 | .039* | .059 | 0.72 |
| Cerebral Gray Matter | 5.05 | −25648 | .079 | .079 | 0.61 |
| Cerebral White Matter | 7.69 | −27909 | .026* | .059 | 0.79 |
| Left Thalamus | 7.82 | −538.7 | .007* | .028** | 0.97 |
| Right Thalamus | 7.48 | −487.3 | .003* | .026** | 1.07 |
| Left Putamen | 1.70 | 91.0 | .635 | .754 | 0.16 |
| Right Putamen | 2.82 | −133.8 | .442 | .708 | 0.26 |
| Left IFG | 8.63 | −734.1 | .026* | .052** | 0.79 |
| Right IFG | 10.91 | −987.8 | .025* | .052** | 0.79 |
| Left ACC | 2.70 | −91.5 | .662 | .754 | 0.15 |
| Right ACC | 2.04 | −71.9 | .754 | .754 | 0.11 |
Note: Regression models include group, age, sex, and intracranial volume; total brain volume model excludes intracranial volume.
p≤.05 corrected
p≤.05 uncorrected. Models were FDR corrected separately for 3 global and 8 regional brain measures. % difference = percent difference between groups in volume. b = unstandardized beta. ACC = anterior cingulate cortex. IFG = inferior frontal gyrus.
3.3. Prenatal Exposure to ETS and Brain Function
Children who completed the Simon task were significantly older than those who did not (t(46)=−2.91, p=.007; Table S6), but did not differ in prenatal cotinine, FSIQ, or maternal education (Table S6). Children included in the task analysis showed slower mean RT for incongruent (M=960ms) than congruent trials (M=890ms; t(29)=3.83, p=.001). No group differences (exposed, non-exposed) were detected in mean RTs for incongruent or congruent trials, I-C RT, or mean head motion (p’s>.24).
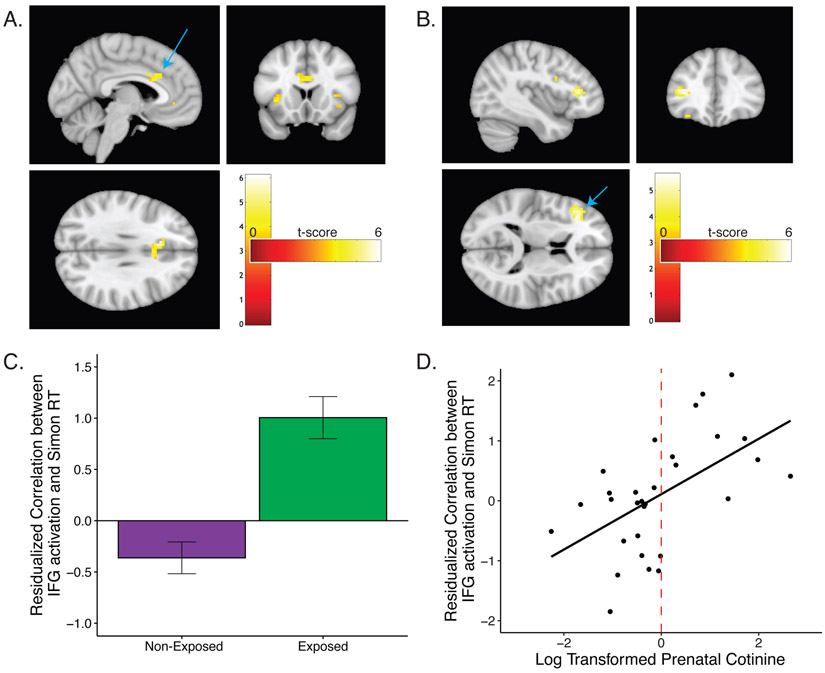
We detected significant positive trial-wise associations between RT and activation in ACC (Montreal Neurological Institute (MNI) coordinates: x=−9,y=21,z=30; 139 voxels; peak t=6.10, p-FWE=.0003, Figure 2A) and significant group differences (exposed vs non-exposed) in the trial-wise association between RT and activation in left IFG (MNI coordinates: x=−36,y=30,z=9; 38 voxels; peak t(25)=5.25, p-FWE=.005, Figure 2B). Non-exposed children showed an inverse association between activation in IFG and RT (less IFG activation on longer RT trials), whereas exposed children showed a positive association (more IFG activation on longer RT trials; Figure 2C). The trial-wise association between RT and left IFG activation was similarly associated with continuous prenatal cotinine (b=0.497, p=.002, Figure 2D). Group differences in prenatal exposure remained the strongest predictor of the trial-wise association between RT and left IFG activation when postnatal exposure was included in the model (prenatal: b=1.363, p<.001; postnatal: b=0.518; p=.076). Including ethnicity did not affect results (Table S3). A cluster in the ACC was associated with the resolution of post-congruent conflict across all children (p-uncorrected=.02, 29 voxels, MNI coordinates: x=−6,y=−12,z=33), but no clusters were associated with group differences in conflict or post-congruent conflict.
Figure 2.
Simon task. A) Significant associations between RT and ACC activation across all participants. B,C) Associations between RT and left IFG activation is higher in children with prenatal environmental tobacco exposure (group differences), and D) with prenatal cotinine modeled continuously. Dashed vertical line denotes threshold for classification as prenatally exposed. The frontostriatal mask was defined by the Anatomical Automatic Labeling atlas.
3.4. Associations between ETS-related Structural and Functional Alterations
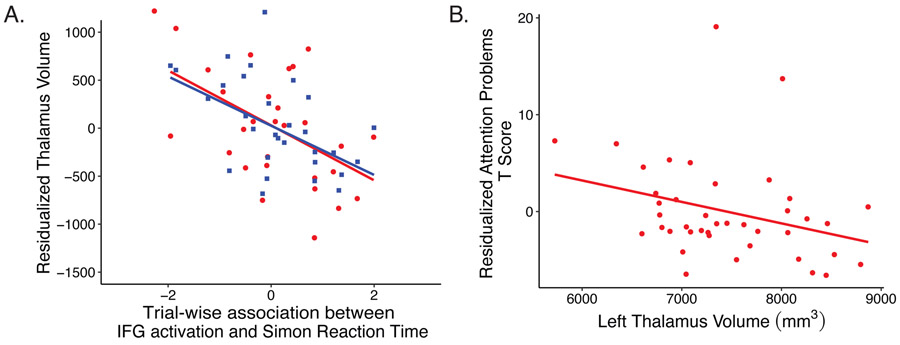
Exploratory analyses revealed that left and right thalamic volumes were inversely associated with the trial-wise association between left IFG activation and RT (left: b=−0.001, t(23)=−2.455, p=02; right: b=−0.001, t(23)=−3.12, p=005; Figure 3A).
Figure 3.
Structure-function associations. A) Inverse associations between thalamic volume (red circle=left; blue square=right) and RT-modulated IFG activation during the Simon Task, controlling for age, sex, intracranial volume, and motion. B) Associations between thalamic volume and parent reported attention problems, controlling for age, sex and intracranial volume. Mm3 = millimeters cubed.
3.5. Exploratory Brain-Behavior Analyses
Of the regions significantly associated with prenatal environmental tobacco smoke after multiple comparisons correction, left thalamus volume was associated with CBCL Attention Problems, and left IFG volume was associated with CBCL Attention Problems, ADHD Scale, and Externalizing Scale (ps<.05, Table S6, Figure 3B). Right thalamus, right IFG, and trial-wise association between left IFG and RT were not associated with behavior. Four mediation analyses were thus conducted. Left thalamus volume mediated the association between prenatal ETS exposure and attention problems (average causal mediation effect=1.42, p=.05),52 whereas left IFG did not mediate any ETS-behavior associations. For completeness, associations between behavioral measures and brain volumes that showed uncorrected associations with ETS are reported (Table S6).
4. Discussion
Our study is the first to report effects of prenatal ETS exposure in non-smoking mothers on children’s brain structure and function. Children with prenatal exposure exhibited significantly smaller volumes of cortical and subcortical regions of frontostriatal circuits. Additionally, we detected alterations in frontostriatal activation during the resolution of cognitive conflict in ETS exposed children that were not present in non-exposed children. Significant dose-response relationships examining continuous prenatal cotinine were observed with all brain measures showing group differences. In exploratory analyses, the trial-wise association between RT and left IFG activation was associated with smaller thalamus volume, and trend-level findings suggest that smaller left thalamic volume mediated the pathway from ETS exposure to children’s attention problems. These ETS-related effects on neurodevelopment closely parallel those of active maternal smoking,8,9 suggesting similar neurotoxic effects arising from active and environmental exposure.
Compared to non-exposed children, those with prenatal ETS exposure had significantly smaller IFG and thalamus volumes, key areas of frontostriatal portions of CSTC circuits. Exploratory analyses also revealed ETS-related effects on right IFG surface area and left ACC thickness. These findings were also significant using cotinine as a continuous measure of exposure, indicating a significant dose-response effect. We additionally detected significant associations of maternal report of children’s attention problems and externalizing behaviors with smaller left thalamic and IFG volumes. Last, trend-level findings suggest that ETS-related reductions in thalamic volumes mediated the pathway from ETS exposure to increased attention problems. Given that these frontostriatal control circuits are disrupted in ADHD,53 our findings suggest that control circuit alterations are a plausible mechanism through which prenatal ETS increases risk for attention problems.
Further evidence supporting our hypothesis that prenatal ETS disrupts frontostriatal control circuits comes from our task fMRI data. We detected ETS-related effects on the function of frontostriatal control circuits during the resolution of cognitive conflict. Exposed children showed positive associations between IFG activation and trial-wise RT, whereas non-exposed children showed no association between activation in this region and RT; a significant dose-response relationship was detected using cotinine as a continuous measure of exposure. Such findings potentially point to a compensatory recruitment of the IFG on long RT trials during the Simon task in exposed children. Our result is consistent with prior findings that active maternal smoking is associated with greater IFG activation during Go/No-Go tasks in nicotine exposed youth.23,24 In contrast, adults with prenatal exposure to active maternal smoking show decreased activity in IFG during the Go/No Go task.21 Thus ETS-related functional effects may change over development, pointing to the need for longitudinal study of this developmental process. Furthermore, ETS-related differences in thalamic volumes were inversely associated with increased activity in IFG, further suggesting that ETS has distributed effects on frontostriatal circuit architecture. The frontostriatal portion of CSTC circuits is known to underlie cognitive control processes.25 Thalamocortical processing is hypothesized to be involved in efference copies,54 which send information from motor cortex back to the sensory processing stream in order to anticipate impending self-generated behavior.55 ETS-associated effects on thalamic structure and IFG activation could work in concert through this mechanism to underlie difficulty anticipating and, thus, regulating self-generated behavior. ETS exposure may act on thalamic volumes, contributing to compensatory IFG engagement, or on frontostriatal function, contributing to altered neuroplasticity and thus reductions in thalamic volumes, underscoring the need for longitudinal study of the effects of prenatal ETS on children’s neurodevelopment.
Our study has a relatively small sample size and, thus, was powered to detect the large effect sizes reported. Future studies, however, with larger samples are needed to replicate these findings and to detect other brain regions that may show smaller prenatal ETS effect sizes. In addition, future studies should examine effects of ETS on other brain regions important for other cognitive control subprocesses. Furthermore, results remained consistent when cotinine was modeled as a continuous variable and show significant dose-response relationships, as well as when postnatal exposure at age 3 or age 7 was included in models. Given the correlation between prenatal and postnatal exposure, we cannot exclude the possibility that the more proximal exposure window to outcome assessment may also be important. Future research is needed to disentangle effects of prenatal from postnatal exposure to ETS on the structure and function of control circuits in children. In our study, maternal ADHD symptoms did not vary between exposed and non-exposed children, suggesting that maternal genetic risk was not a confound. Future prospective studies that incorporate biomarkers of exposure, as this study did, should consider the role of paternal genetics as well as timing of exposure during pregnancy. Although we detected effects of prenatal exposure on conflict-related activation when RT was parametrically modulated, group differences in conflict or post-congruent conflict condition contrasts were not significant. However, RT was associated with activity in the ACC across all children and a cluster in the ACC was associated with post-congruent conflict contrast across all children, suggesting convergence across methods. Future studies with larger samples are needed to better understand the differences between contrast analyses, which average activity across trials within individuals, and RT modulation, which reflects trial-level individual differences that can stem from various factors beyond condition, like prior trial/sequence effects.
Although anti-smoking campaigns have reduced cigarette use, 14% of Americans still smoke,56,57 and another 14.9% have used e-cigarettes,57 and twelve states have no public smoking laws,58 greatly increasing pregnant women’s risk of exposure.59 Environmental injustice places Black children at greatest risk of exposure: ~40% of children in general, versus ~70% of Black children are ETS exposed.60 The CDC does not list cognitive outcomes as a risk of ETS exposure.61 Public health programming aimed at increased awareness of the risk of ETS exposure for pregnant women may reduce the incidence of attention problems, particularly among Black children.
In summary, our findings point to the effects of prenatal ETS exposure on the structure and function of frontostriatal circuits in children, alterations that contribute to their attention problems. Such effects are similar to the effects of exposure to active maternal smoking during pregnancy. Moreover, effects on frontostriatal circuits are consistent with neurological markers of attention problems as detected in ADHD62. Attention problems and ADHD have high heritability rates of approximately 70 percent63, leaving the origins of up to 30 percent of idiopathic cases unexplained. Thus, the effects of environmental exposure to prenatal ETS may represent a novel phenotype of attention problems.
Supplementary Material
Highlights.
Prenatal environmental tobacco smoke (ETS) increases risk for attention problems.
The neurobiological effects of prenatal ETS are unknown.
Prenatal ETS alters the structure and function of frontostriatal control circuitry.
Smaller thalamic volume mediated effects of exposure on attention problems.
ETS and smoking in pregnancy confer similar risk on brain structure and behavior.
Acknowledgments
Funding/Support: All phases of this study were supported by NIEHS K23 - ES026239 and R01 ES030950, and The NVLD Project.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Sharing Statement: Deidentified individual participant data will be made available upon reasonable request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polanska K, Krol A, Merecz-Kot D, et al. Environmental Tobacco Smoke Exposure during Pregnancy and Child Neurodevelopment. Int J Environ Res Public Health 2017;14:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B, Hong YC, Park HK, et al. Prenatal Exposure to ETS and Infantile Neurodevelopment. Epidemiology 2009;20:S194 [Google Scholar]
- 3.Cho K, Frijters JC, Zhang H, Miller LL, Gruen JR. Prenatal exposure to nicotine and impaired reading performance. J Pediatr 2013;162:713–8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect 2005;113:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Luo R, Wang T, Gao J, Liu C. Prenatal Exposure to Environmental Tobacco Smoke and Early Development of Children in Rural Guizhou Province, China. Int J Environ Res Public Health 2018;15:2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Q, Hou XY, Yin XN, et al. Prenatal Exposure to Environmental Tobacco Smoke and Hyperactivity Behavior in Chinese Young Children. Int J Environ Res Public Health 2017;14:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Leung PW, McCauley L, Ai Y, Pinto-Martin J. Mother's environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children. Neurotoxicology 2013;34:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekblad M, Lehtonen L, Korkeila J, Gissler M. Maternal Smoking During Pregnancy and the Risk of Psychiatric Morbidity in Singleton Sibling Pairs. Nicotine Tob Res 2017;19:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sourander A, Sucksdorff M, Chudal R, et al. Prenatal Cotinine Levels and ADHD Among Offspring. Pediatrics 2019;143:e20183144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovess V, Keyes KM, Hamilton A, et al. Maternal smoking and offspring inattention and hyperactivity: results from a cross-national European survey. Eur Child Adolesc Psychiatry 2015;24:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 1985;8:384–95. [DOI] [PubMed] [Google Scholar]
- 12.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther 1998;285:931–45. [PubMed] [Google Scholar]
- 13.Li L, Guo L, Chen X, et al. Secondhand smoke is associated with heavy metal concentrations in children. Eur J Pediatr 2018. 177:257–64. [DOI] [PubMed] [Google Scholar]
- 14.Willers S, Gerhardsson L, Lundh T. Environmental tobacco smoke (ETS) exposure in children with asthma—relation between lead and cadmium, and cotinine concentrations in urine. Respir Med 2005;99:1521–7. [DOI] [PubMed] [Google Scholar]
- 15.Guillette EA. Examining childhood development in contaminated urban settings. Environ Health Perspect 2000;108 Suppl 3:389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss B Vulnerability of children and the developing brain to neurotoxic hazards. Environ Health Perspect 2000;108 Suppl 3:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AM, Dwoskin LP, Pauly JR. Early exposure to nicotine during critical periods of brain development: Mechanisms and consequences. J Pediatr Biochem 2010;1:125–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraone SV. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev 2018;87:255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryden DW, Burton AC, Barnett BR, et al. Prenatal Nicotine Exposure Impairs Executive Control Signals in Medial Prefrontal Cortex. Neuropsychopharmacology 2016;41:716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Marroun H, Schmidt MN, Franken IHA, et al. Prenatal Tobacco Exposure and Brain Morphology: A Prospective Study in Young Children. Neuropsychopharmacology 2014;39:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holz NE, Boecker R, Baumeister S, et al. Effect of prenatal exposure to tobacco smoke on inhibitory control: neuroimaging results from a 25-year prospective study. JAMA Psychiatry 2014;71:786–96. [DOI] [PubMed] [Google Scholar]
- 22.Toro R, Leonard G, Lerner JV, et al. Prenatal Exposure to Maternal Cigarette Smoking and the Adolescent Cerebral Cortex. Neuropsychopharmacology 2008;33:1019–27. [DOI] [PubMed] [Google Scholar]
- 23.Bennett DS, Mohamed FB, Carmody DP, et al. Response inhibition among early adolescents prenatally exposed to tobacco: an fMRI study. Neurotoxicol Teratol 2009;31:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longo CA, Fried PA, Cameron I, Smith AM. The long-term effects of prenatal nicotine exposure on response inhibition: An fMRI study of young adults. Neurotoxicol Teratol 2013;39:9–18. [DOI] [PubMed] [Google Scholar]
- 25.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci 2016;18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft JL, Simon JR. Processing symbolic information from a visual display: interference from an irrelevant directional cue. J Exp Psychol 1970;83:415–20. [DOI] [PubMed] [Google Scholar]
- 27.Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson BS. Adaptation to Conflict via Context-Driven Anticipatory Signals in the Dorsomedial Prefrontal Cortex. The Journal of Neuroscience 2011;31:16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis AE, Davis KS, Pao LS, et al. Verbal–spatial IQ discrepancies impact brain activation associated with the resolution of cognitive conflict in children and adolescents. Developmental science 2018;21:e12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andescavage NN, du Plessis A, McCarter R, et al. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb Cortex 2016;27:5274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowell WJ, Stapleton HM, Holmes D, et al. Prevalence of historical and replacement brominated flame retardant chemicals in New York City homes. Emerging Contaminants 2017;3:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel JS, Power Jd Fau, Dubis JW, Dubis Jw Fau, Vogel AC, et al. , 2014. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp 35, 1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centanni TM, Norton ES, Park A, et al. Early development of letter specialization in left fusiform is associated with better word reading and smaller fusiform face area. Developmental science 2018;21:e12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P 3rd. Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol 2009;170:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit 2009;31:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PN. Relation of urinary cotinine concentrations to cigarette smoking and to exposure to other people's smoke. Thorax 1991;46:274-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 38.Bied A, Biederman J, Faraone S. Parent-based diagnosis of ADHD is as accurate as a teacher-based diagnosis of ADHD. Postgrad Med 2017;129:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wechsler D Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 40.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- 41.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23 Suppl 1:S69–84. [DOI] [PubMed] [Google Scholar]
- 42.Weissman DH, Carp J. The congruency effect in the posterior medial frontal cortex is more consistent with time on task than with response conflict. PLoS One 2013;8:e62405–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage 2011;57:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Hulst BM, de Zeeuw P, Bos DJ, Rijks Y, Neggers SFW, Durston S. Children with ADHD symptoms show decreased activity in ventral striatum during the anticipation of reward, irrespective of ADHD diagnosis. Journal of Child Psychology and Psychiatry 2017;58:206–14. [DOI] [PubMed] [Google Scholar]
- 45.Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nature reviews Neuroscience 2018;19:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences 2009;13:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokota S, Takeuchi H, Hashimoto T, et al. Individual differences in cognitive performance and brain structure in typically developing children. Dev Cogn Neurosci 2015;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cyr M, Yang X, Horga G, Marsh R. Abnormal fronto-striatal activation as a marker of threshold and subthreshold Bulimia Nervosa. Hum Brain Mapp 2018;39 1796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews 2007;82:591–605. [DOI] [PubMed] [Google Scholar]
- 50.Margolis AE, Pagliaccio D, Davis KS, et al. Neural correlates of cognitive control deficits in children with reading disorder. Brain Imaging Behav 2020;14:1531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsh R, Horga G, Wang Z, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. The American journal of psychiatry 2011;168:1210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther 2017;98:39–57. [DOI] [PubMed] [Google Scholar]
- 53.Cai W, Griffiths K, Korgaonkar MS, Williams LM, Menon V. Inhibition-related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 2016;19:533–41. [DOI] [PubMed] [Google Scholar]
- 55.Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 2008;31:317–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, 2014. at https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm.) [Google Scholar]
- 57.Current Cigarette Smoking Among Adults in the United States. National Center for Chronic Disease Prevention and Health Promotion. at https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.) [Google Scholar]
- 58.Map of Smokefree Indoor Air - Private Worksites, Restaurants, and Bars. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. at https://www.cdc.gov/statesystem/factsheets/sfia/SmokeFreeIndoorAir.html.) [Google Scholar]
- 59.Sims M, Mindell Js Fau, Jarvis MJ, Jarvis Mj Fau, Feyerabend C, Feyerabend C, Fau-Wardle H, Wardle H, Fau-Gilmore A, Gilmore A, 2012. Did smokefree legislation in England reduce exposure to secondhand smoke among nonsmoking adults? Cotinine analysis from the Health Survey for England. Environ. Health Perspect 120, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vital Signs: Disparities in Nonsmokers' Exposure to Secondhand Smoke — United States, 1999–2012. Centers for Disease Control and Prevention, 2015. at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6404a7.htm?s_cid=mm6404a7_w.) [PMC free article] [PubMed] [Google Scholar]
- 61.Health Effects of Secondhand Smoke. at http://www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/health_effects/.)
- 62.Rubia K Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front Hum Neurosci 2018;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry 2019;24:562–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.