Abstract
Background
Birthweight is a strong predictor of normal growth, healthy development, and survival. Several studies have found associations between temperature, fine particulate matter (PM2.5), and birth weight. However, the relevant timing of exposures varies between studies and is yet unclear. Therefore, we assessed the difference in term birthweight (TBW) associated with weekly exposure to temperature and PM2.5 throughout 37 weeks of gestation.
Methods
We included all singleton live term births in Massachusetts, U.S between 2004 and 2015 (n=712,438). Weekly PM2.5 and temperature predictions were estimated on a 1 km grid from satellite-based models. We utilized a distributed lag nonlinear model (DLNM) to estimate the difference in TBW associated with weekly exposures from the last menstrual period to 37 weeks of gestation.
Results
We found a nonlinear association with prenatal temperature exposure. Larger effects were observed in warmer temperatures, where higher temperatures were negatively associated with TBW. Temperature effects were larger in the first and final weeks of gestation. We observed a negative difference in TBW associated with PM2.5 exposure. Overall, a 1 μg/m3 increase in prenatal exposure was associated with 3.9g lower TBW (95% CI −5.0g; −2.9g). PM2.5 effects were larger in the final weeks of gestation.
Conclusion
We found heat and PM2.5 exposure to be related to lower TBW. Our findings suggest that women are more susceptible to both exposures towards the end of pregnancy. Susceptibility to heat was higher in the initial weeks of pregnancy as well. These critical windows of susceptibility can be communicated to pregnant women during routine prenatal visits to increase awareness and target interventions to reduce exposures.
Keywords: air pollution, temperature, Birth weight, PM2.5
1. Introduction
Birth weight is a marker for development in early life [1] with long-term health consequences in childhood and adulthood [2]. The World Health Organization has targeted the achievement of a 30% reduction in low birth weight deliveries as a sustainable developmental goal [3], emphasizing the importance of birth weight as a predictor of morbidity and mortality. Although birth weight is mostly determined by genetic factors and length of gestation [4], it is also affected by maternal health and exposures during pregnancy [5–11]. Several studies have found associations between temperature [12–16], particulate matter smaller than 2.5μm (PM2.5) [17–21], and birthweight. However, exposure assessment methods and the definition of the time windows of susceptibility vary across studies.
In most current studies, temperature and PM2.5 exposures are averaged over trimesters or the entire pregnancy [15, 21–25]. However, the true time windows of susceptibility are unlikely to follow the strict categorization of trimesters [12]. Another problem that can potentially arise from this approach is seasonality bias, as described by Wilson et al. Although this bias can be reduced by including all three trimester exposures in the model, it does not necessarily eliminate the bias [26].
Distributed lag models (DLMs) are a useful tool to address both limitations. DLMs can be used to identify critical windows of susceptibility in a flexible way [27]. They allow a joint assessment of multiple short intervals of exposure (i.e., the lag structure) throughout the gestation period [26]. This approach has been previously used to assess the effects of meteorological exposures or PM2.5 exposure during pregnancy on term birth weight (TBW) [12, 26].
As we know from previous studies, there is evidence of nonlinear associations between temperature exposure and birth outcomes [5, 25, 28]. In addition, we hypothesize that the lag structure of the associations throughout the pregnancy does not necessarily follow a linear trend, and exposures during some periods of gestation may be more critical to fetal growth than others. Distributed lag nonlinear models (DLNM) are an extension of DLMs, which allow us to flexibly model both the nonlinear exposure-effects and the lag structure of the associations [27]. Although this approach was used to assess temperature and humidity effects on the rate of preterm births [29, 30], or TBW [12] to our knowledge, it has not been used to assess the simultaneous effects of temperature and PM2.5 exposure on TBW.
In this study, we investigate the complex lag structures and nonlinear temperature and PM2.5 effects on TBW in a large population-based birth registry. We aim to identify critical windows of prenatal exposures using fine spatiotemporal models of temperature and PM2.5.
2. Materials and methods
2.1. Study population
We included all eligible singleton live births in the Massachusetts birth registry data (n=712,438 births) for years with available exposures data (2004–2015). The birth registry data included 834,886 records within the study years. This data includes the newborn’s information (date of birth, sex (male or female), birth weight in grams, gestational age at birth), maternal sociodemographic information (age at birth, race (White, Black, or other), parity (first delivery/ 2nd or more), whether the mother received government support for prenatal care (yes/no), smoking before or during pregnancy (yes/no), the highest level of education attained (no high school diploma, high school, some college, or college), chronic conditions (e.g. diabetes (yes/no) or hypertension (yes/no)), and geocoded residential address at birth. We excluded a total of 122,448 (14.6%) of the births according to the following exclusion criteria: preterm births (<37 weeks, n=57,557), records of gestational age >42 weeks (n=8,506), records of birthweight<400 grams (n=10) or over 6 kg (n=20), and records that had missing covariates, or residence information (n=93,527). Most of the missingness was driven by missing address information, and missing information on diabetes and hypertension. Since we only included term births, we a priori assumed linear relationship between gestational age and the outcomes. We observed stronger negative associations between maternal age and TBW among the youngest and eldest mothers and therefore used a penalized spline function to allow for a nonlinear association with maternal age at delivery.
This study was approved by the Massachusetts Department of Public Health and the human subjects committee at the Harvard T. H. Chan School of Public Health.
2.2. Exposures
PM2.5: Residential exposure to PM2.5 was estimated during pregnancy using a novel model [31]. We applied extreme gradient boosting (XGBoost) modeling to predict daily PM2.5 on a 1-km resolution for a 13-state region in the Northeastern USA. We used satellite-derived aerosol optical depth (AOD) and implemented a recursive feature selection among land use covariates to develop a robust yet parsimonious model. The model performance was excellent, with a root mean square error (RMSE) of 3.11 μg/m3 in our spatial cross-validation withholding nearby sites and a RMSE of 2.10 μg/m3 using a more conventional random ten-fold splitting of the dataset. For more in depth details on model methods and performance please see Just 2020 [31]. Daily estimates were averaged to calculate weekly exposures for each week of gestation in each woman.
Temperature: Ambient temperature at the residential address was estimated during pregnancy using a second novel model [32]. We applied XGBoost to predict hourly air temperature on a 1-km resolution for the same 13-state region in the Northeastern USA. We used land surface temperature (LST) from two NASA satellites (up to four overpasses daily) as well as physiographic and land use covariates and inverse-distance weighted temperature surfaces from ~4,000 weather stations of the Integrated Mesonet. A rigorous spatial cross-validation showed excellent performance with RMSEs around 1.6 K compared with standard deviations around 11.0 K. For comparison, the mean square error (MSE) was about 1/3 of the MSE of the widely used ~11 km by 14 km NASA NLDAS-2 forcing temperature. Hourly estimates were averaged to calculate weekly exposures for each week of gestation in each woman.
2.3. Statistical analysis
We assessed the association between weekly mean temperature and PM2.5 exposures during gestation and TBW using DLNMs [27]. We utilized a DLNM generalized additive model to estimate the difference in TBW associated with weekly exposures from the last menstrual period to 37 weeks of gestation. Both exposures were included in simultaneously in the multivariate model. The advantage of the DLNM framework is that it allows nonlinear exposure-response and time-response functions [27]. Associations between temperature exposure and birth outcomes are often nonlinear [5, 25, 28]. Therefore, we modeled the association between weekly mean temperature and TBW using a natural cubic spline with two degrees of freedom and modeled the lag function using a penalized spline. For PM2.5 exposure, we assumed a linear association with TBW and modeled the lag function using a natural cubic spline with three degrees of freedom. Both for the association with temperature and for the lag function of PM2.5, we selected the number of degrees of freedom that minimizes the Akaike Information Criterion (AIC). We estimated the overall cumulative exposure-response curve of temperature and PM2.5 exposure effects on TBW. We additionally assessed the difference in TBW associated with increases in PM2.5 and low and high temperatures.
We adjusted the model for the variables available in the birth records shown to be potential confounders of the associations between temperature, PM2.5, and birthweight: season and year of birth, a cyclic spline of day of the year, government support for prenatal care, race, age of mother at birth, parity, maternal smoking before or during pregnancy, the maternal highest level of education attained, chronic diabetes, chronic hypertension, and gestational age at birth [6, 10, 33]. We additionally adjusted the model for census block-group socioeconomic characteristics (i.e., population density and median household income).
We present the results of the cumulative exposure-response curves, defined as the net effect of PM2.5 or temperature across the entire lag period [27]. We additionally present the difference in birthweight, associated with 1 μg/m3 increase in PM2.5, and 1 °C increase in temperature in different weeks of gestation. Since the cumulative exposure-response curve suggested a nonlinear association with temperature exposure, we present the effect estimates associated with a rise of 1 °C in the 5th (−4 °C) and the 95th (23 °C) percentiles of the temperature distribution.
2.3.1. Secondary analyses
To identify a potential modification of the associations by maternal race and income, and by newborn sex, we repeated our model separately among White versus non-White mothers, among mothers who receive governmental support for prenatal care versus mothers who do not receive governmental support, and among male versus female newborns. We treat eligibility to governmental support as a proxy for socioeconomic status.
Additionally, to capture the effects of the studied exposures on extreme abnormal fetal growth, we have assessed the effects of temperature and PM2.5 exposures in pregnancy on newborns small for gestational age (SGA). We calculate the newborn size for gestational age (SGA, appropriate for gestational age (AGA) or large for gestational age (LGA)) using the Fenton sex-specific reference growth curves [34].
2.3.2. Sensitivity analysis
The DLNM requires identical length exposure periods across the study population. This poses a challenge in defining the exposure period when studying pregnancy outcomes where the intrauterine exposures differ in length and depend on the gestational age at birth. To overcome this limitation, we defined the exposure period in our main analysis as the first 37 weeks of gestation, starting at date of last menstrual period (LMP), and limited our data to term births. For women who gave birth after the start of 37 weeks of gestation, exposures following the 37th week were unaccounted for in our main model. We, therefore, added a sensitivity analysis aimed to examine the robustness of our findings. We created a subcohort of women who delivered at 39 weeks of gestation. Among this population, we compared the results obtained when assessing the weekly effects of temperature and PM2.5 exposure at the first 37 weeks of gestation versus the entire pregnancy (39 weeks of gestation).
3. Theory
Multiple factors determine birthweight; some are not fully understood. It is, therefore, hard to identify a single etiology of lower birth weight. Maternal exposure to high temperatures during pregnancy can affect birthweight through different mechanisms. The first trimester of pregnancy is crucial for the correct implantation of the placenta [35]. Maternal exposure to heat in the early stages of pregnancy can reduce placental weight and umbilical cord flow [5]. The final stages of pregnancy are characterized by the fastest somatic growth of the fetus [35]. Therefore, high-temperature exposures in late gestation can also affect birth weight. Higher heat production during pregnancy, alongside weight gain and the burden of the fetus, limit the maternal ability to tolerate heat stress [13]. Heat stress and dehydration during pregnancy can cause uterine constriction and impair the uterine blood flow [36].
The PM2.5 effect on intrauterine growth can be attributed to the limited placental ability to filter environmental chemicals [37]. Maternal exposure to air pollution during pregnancy may cause oxidative stress and endocrine disruption in the placenta, impair the transport of oxygen and nutrients to the fetus, and adversely affect the intrauterine growth [35, 38]. PM2.5 has also been shown to impair vascular development in the placenta [39], decrease placental methylation in the leptin gene promotor [40], and decrease mitochondrial DNA content and increase mitochondrial DNA methylation in the placenta [41].
4. Results
We included 712,438 births; 71.9% were of White mothers. The mean maternal age at delivery was 30 years, 6.8% reported smoking during pregnancy, 0.9% had diabetes mellitus, and 1.3% had chronic hypertension. Half of the newborns were males, the mean gestational age at birth was 39.3 weeks, and the mean TBW was 3.4 kg (Table 1). The median household income at the maternal block group of residences was $51,700 on average.
Table 1.
Population characteristics.
| Population characteristics | N=712,438 |
|---|---|
| Maternal age, Mean (SD) | 30.08 (5.92) |
| Maternal race, n (%) | |
| White | 512,355 (71.9) |
| Black | 67,435 (9.5) |
| Other | 132,648 (18.6) |
| Maternal education, n (%) | |
| Less than high school | 75,511 (10.6) |
| High school | 158,733 (22.3) |
| Some college | 162,002 (22.7) |
| College or more | 316,192 (44.4) |
| Governmental support for prenatal care, n (%) | 251,633 (35.3) |
| Chronic hypertension, n (%) | 9,073 (1.3) |
| Diabetes mellitus, n (%) | 6,280 (0.9) |
| Smoking in pregnancy, n (%) | 48,405 (6.8) |
| Gestational age in weeks, Mean (SD) | 39.31 (1.18) |
| Parity>1, n (%) | 387,393 (54.4) |
| Newborn sex, female, n (%) | 349,086 (49.0) |
| Birthweight in g, Mean (SD)) | 3428.90 (464.14) |
| Birthweight for gestational age, n (%) | |
| AGA | 590,715 (82.9) |
| LGA | 81,300 (11.4) |
| SGA | 40,423 (5.7) |
| Term low birthweight (<2500 g), n (%) | 15,510 (2.1) |
| Median household income, Mean (SD) | 51,700 (21,099) |
SD=standard deviation; AGA= appropriate for gestational age; LGA=large for gestational age; SGA= small for gestational age.
Newborn size for gestational age was calculated using the Fenton sex-specific reference growth curves.
During the study period, the mean temperature exposure was 10.2 °C, and mean PM2.5 exposure was 8.9 μg/m3 (Figure 1 and Table 2). Weekly temperature exposures ranged from −16.7 °C to 30.2 °C with a standard deviation of 9.2 °C. The births were distributed evenly throughout the year, with slightly more births in the summer months (Table 2). The correlation between weekly temperature and PM2.5 exposures was low (r=0.15, p<0.001).
Figure 1. The distribution of mean temperature and PM2.5 exposures over gestation.
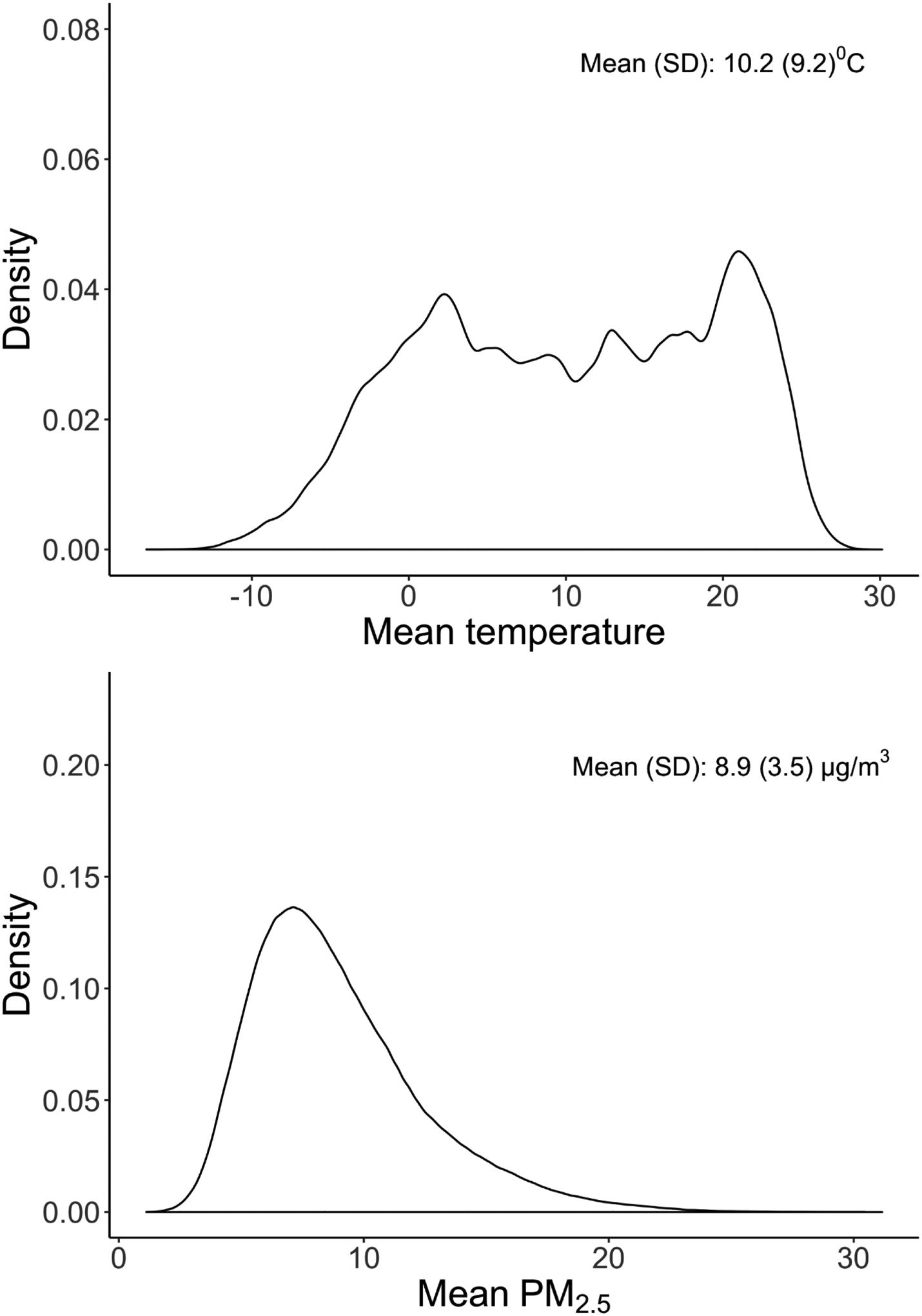
Figure 1 shows the distribution of weekly temperature and PM2.5 exposures. Each woman contributed 37 weeks of weekly averaged exposures.
Table 2.
Summary statistics of exposures.
| Exposure metrics | Summary statistics |
|---|---|
| mean temperature over gestation, °C | |
| Mean (SD) | 10.2 (9.2) |
| 25th percentile | 2.3 |
| 50th percentile | 10.4 |
| 75th percentile | 18.5 |
| mean PM2.5 over gestation, μg/m3 | |
| Mean (SD) | 8.9 (3.5) |
| 25th percentile | 6.4 |
| 50th percentile | 8.2 |
| 75th percentile | 10.8 |
| Season of birth, n (%) | |
| Winter | 167,435 (23.5) |
| Spring | 180,110 (25.3) |
| Summer | 188,097 (26.4) |
| Fall | 176,796 (24.8) |
SD=standard deviation, PM2.5=fine particulate matter.
We observed significant associations between temperature and TBW, independent of PM2.5 exposure. The cumulative temperature exposure-response curves over 37 weeks of gestation are presented in Figure 2. In temperatures below zero, we observed a positive difference in TBW associated with higher temperatures. The associations in colder temperatures were mostly non-significant. In warmer temperatures, we observed a negative difference in TBW associated with higher temperatures. For example, we observed a positive non-significant difference in TBW for a rise in 1 °C in temperature exposure from −4 °C to −3 °C (2.4g, 95% CI −0.1g; 4.9g). However, a rise in 1 °C in temperature exposure from 23 °C to 24 °C was associated with 7.9g lower TBW (95% CI −11.2g; −4.6g) (Figure 2.a). We observed a negative cumulative difference in TBW associated with PM2.5 exposure, independent of temperature exposure. A 1 μg/m3 increase was associated with 3.9g lower TBW (95% CI −5.0g; −2.9g) (Figure 2.b).
Figure 2. The cumulative exposure-response curves for differences in birth weight, associated with weekly (a) temperature and (b) PM2.5 exposures in weeks 0–37 of pregnancy.
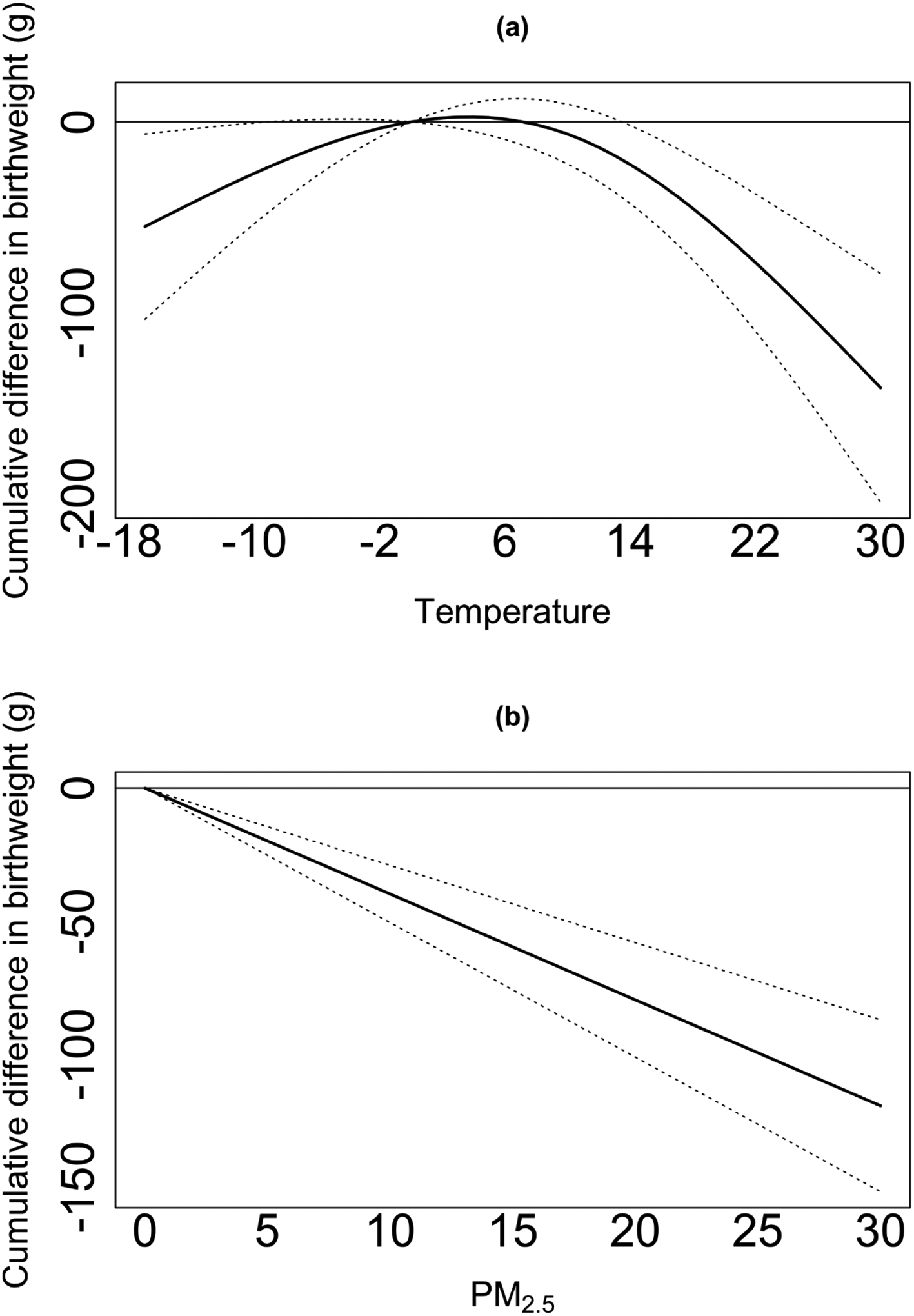
Figure 2 presents the cumulative exposure-response curves for the association between temperature, PM2.5, and term birth weight, defined as the net effects of the exposures across the entire lag period (weeks 0–37 of pregnancy). The model is adjusted for: season and year of birth, a cyclic spline of day of the year, government support for prenatal care, race, age of mother at birth, parity, maternal smoking before or during pregnancy, the maternal highest level of education attained, chronic diabetes, chronic hypertension, gestational age at birth, and census block-group socioeconomic characteristics (i.e., population density and median household income).
Figure 3 shows the difference in TBW associated with a unit increase in exposures across the 37 lags of exposures. In the 5th percentile of temperature (−4 °C), we observed positive associations with TBW, that were similar across different weeks of gestation (Figure 3.a). In the 95th percentile of temperature (23 °C), we observed larger TBW reductions associated with temperature exposure at the first and final weeks of gestation. We found smaller TBW reductions associated with weekly temperature exposure around the second trimester of pregnancy (Figure 3.b). Finally, we observed larger TBW reductions associated with PM2.5 exposure in the final weeks of gestation (Figure 3.c). Figure 4 shows the odds ratio of SGA associated with a unit increase in exposures across the 37 lags of exposures. We did not find a statically significant association between temperature exposure and SGA. A 1 μg/m3 increase in cumulative PM2.5 exposure across the pregnancy was associated with a 3% (odds ratio 95%CI 1.02; 1.04) higher odds of SGA. The effects were similar across the different weeks of gestation. For example, the odds of SGA were 0.008% higher for each 1 μg/m3 increase in PM2.5 exposure in the 37th week of gestation, and 0.01% higher for each 1 μg/m3 increase in PM2.5 exposure in the first week of gestation.
Figure 3. The difference in birth weight for temperature at the 5th percentile (−4 °C) (a), temperature at the 95th percentile (23 °C) (b), and PM2.5 exposure (c) across the different weeks of gestation.
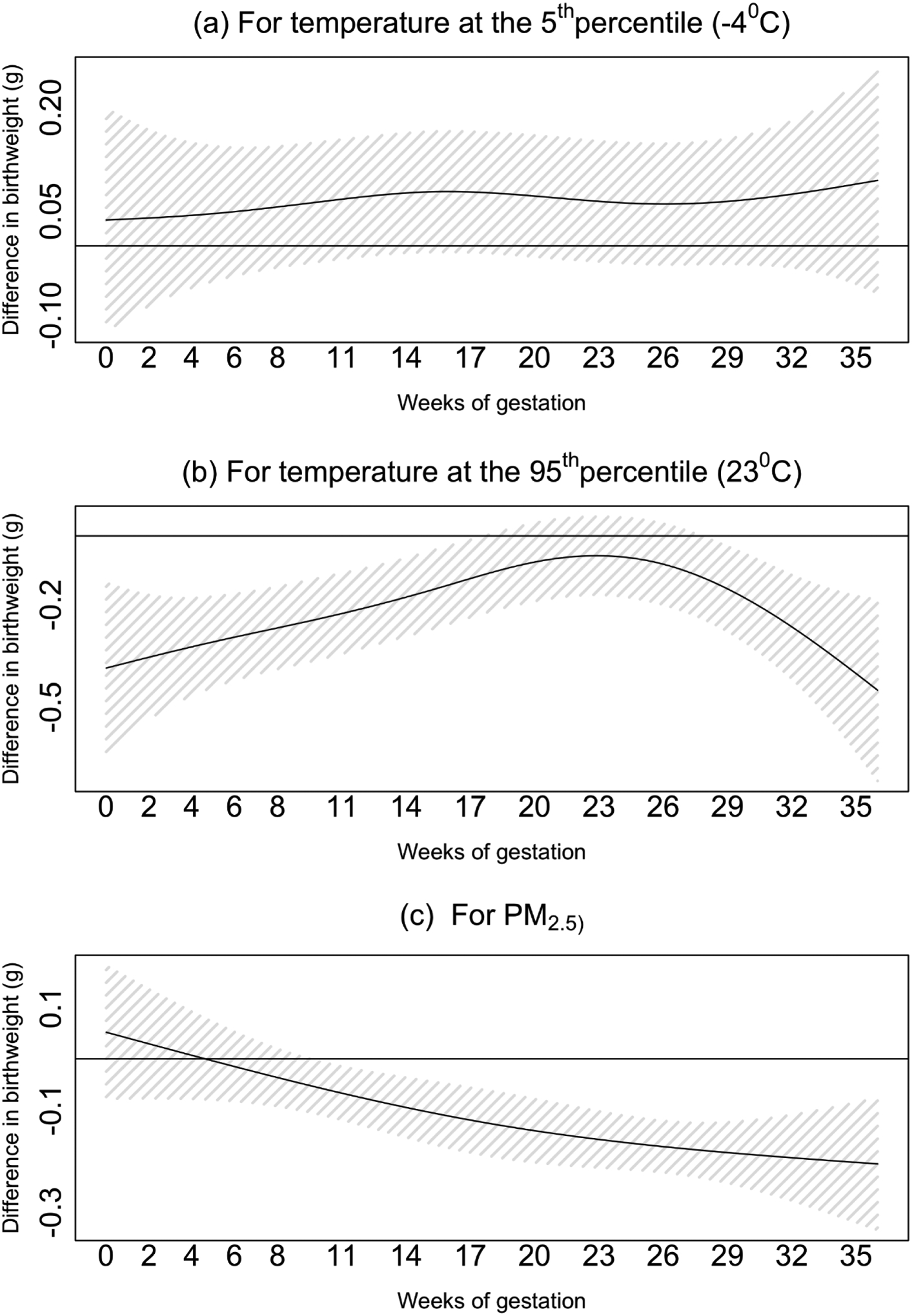
The model is adjusted for: season and year of birth, a cyclic spline of day of the year, government support for prenatal care, race, age of mother at birth, parity, maternal smoking before or during pregnancy, the maternal highest level of education attained, chronic diabetes, chronic hypertension, gestational age at birth, and census block-group socioeconomic characteristics (i.e., population density and median household income).
Figure 4. The cumulative exposure-response curves for the odds ratio of SGA, associated with weekly (a) temperature and (b) PM2.5 exposures in weeks 0–37 of pregnancy.
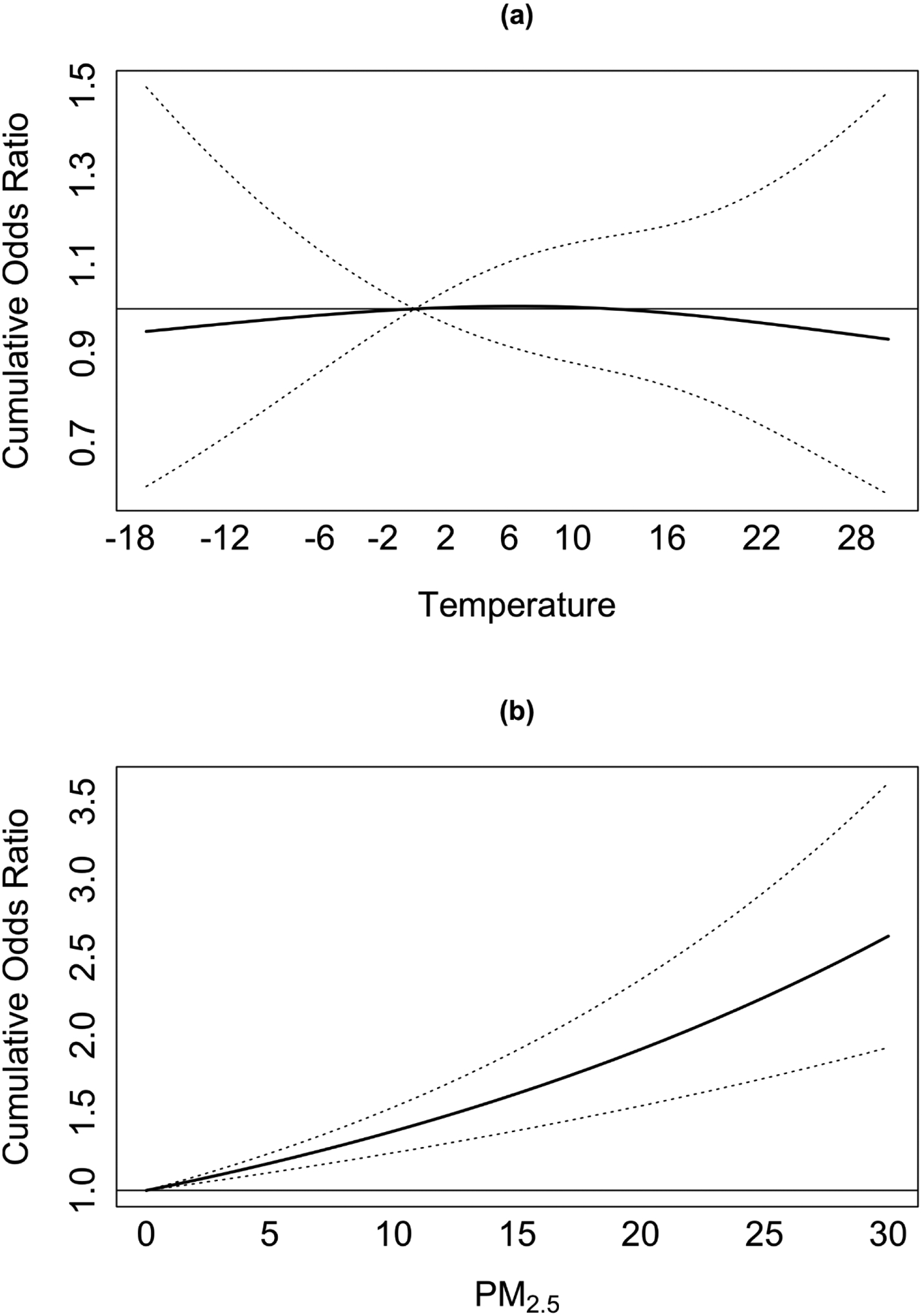
Figure 2 presents the cumulative exposure-response curves for the association between temperature, PM2.5, and newborns small for gestational age (SGA), defined as the net effects of the exposures across the entire lag period (weeks 0–37 of pregnancy). The model is adjusted for: season and year of birth, a cyclic spline of day of the year, government support for prenatal care, race, age of mother at birth, parity, maternal smoking before or during pregnancy, the maternal highest level of education attained, chronic diabetes, chronic hypertension, gestational age at birth, and census block-group socioeconomic characteristics (i.e., population density and median household income).
Thirty-one percent of the women in our analytic dataset gave birth at 39 weeks of gestation. We repeated our model among this subcohort and compared the results for 37 weeks of exposure and complete gestational exposure (39 weeks) in this population. The cumulative effects of temperature and PM2.5 were similar, regardless of the exposure period (Supplementary Figure 1).
Supplementary Figures 2–4 shows the cumulative differences in TBW by race, maternal governmental support for prenatal care visits, and newborn sex. The differences in TBW associated with temperature at the 5th and 95th percentiles and PM2.5 in each sub cohort are presented in Supplementary Table 1. We observed stronger temperature effects among mothers who received governmental support, and among female newborns. Additionally, we observed stronger effects of hotter temperatures among White mothers. The effects of colder temperatures did not differ by race. The effects of PM2.5 exposure were similar in all sub cohorts.
5. Discussion
5.1. Main findings
Our findings show that the first and final weeks of gestation are the critical windows of exposure to heat. We observed a nonlinear temperature effect, with higher temperatures associated with lower TBW in warmer temperatures. We found a significant decrease in TBW and a significant increase in the odds of SGA associated with PM2.5 exposure. The negative association of PM2.5 exposure with TBW was larger towards the end of pregnancy.
5.2. Critical windows of exposure
This study confirms evidence from previous studies that found decreases in TBW associated with maternal exposure to heat during pregnancy. A recent review investigating the effects of prenatal exposure to air pollution and heat on birth outcomes in the U.S. has found three studies that evaluated the association between maternal exposure to heat and low birth weight [42]. In each of these studies, temperature exposure was associated with low birth weight [15, 43, 44]. Basu et al. have concluded that the observed effects of apparent temperature on low birth weight over the entire pregnancy are driven largely by exposure in the third trimester of pregnancy [43]. Similarly, Ha et al. [15] and Kloog et al. [45] have found an increased risk for low birth weight associated with heat exposure during the whole pregnancy, and the third trimester specifically. To date, there is no consensus regarding the critical windows of exposure to heat during pregnancy [46]. As pointed in the examples above, most studies identify the third trimester as the important window of susceptibility. However, our findings show that the first trimester of pregnancy is critical as well. We found that newborns are more susceptible to maternal heat exposure in the initial and final weeks of pregnancy, adjusting for the exposure across all other weeks of gestation. With the progression of pregnancy towards the 19th week of gestation, the observed effects gradually became smaller. After remaining constant in mid-pregnancy, following the 25th week of gestation, the effects gradually became larger. The early pregnancy exposure-effect might not be captured in studies averaging the exposures over trimesters or the whole pregnancy. The added value of our study is the finer isolation of the critical windows of susceptibility.
Unlike temperature exposure, the lag-structure of the PM2.5 effect on TBW followed a linear trend with larger effects in later weeks of gestation. The increased odds of SGA, associated with PM2.5 exposures were very similar across the different weeks of gestation. Similar to our analysis, numerous studies have found significant associations between PM2.5 and low birth weight, TBW [42] or SGA [20]. However, the critical windows of susceptibility for PM2.5 are inconsistent across studies. For example, Kumar et al. [47] have identified the highest PM2.5 effect on birth weight in the first trimester of pregnancy. Ha et al. [15] have found associations with PM2.5 exposure in the second trimester, and Savitz et al. [48] have found significant effects of PM2.5 exposures in all three trimesters of pregnancy. In a previous analysis done by our group, we have found significant effects of PM2.5 exposures in all three trimesters of pregnancy. These effects were attenuated when we included exposures to greenspace, noise, and walkability in the model [49]. Studies that investigated the effects of extreme pollution events on obstetric outcomes have found exposure to PM2.5 originating in wildfire smoke in first [50, 51], second and third trimesters [51] of pregnancy to be associated with lower birthweight.
5.3. Nonlinear association with temperature exposure
Another important finding of this study is the nonlinear relationship between temperature and TBW. The same exposure response curve was reported in animal studies which found extremely high and low temperatures to be associated with maternal stress, lower birthweight, placental weight and diameter. We observed an inverted U-shaped exposure-response curve, which suggested that both temperature extremes were associated with lower TBW. The effects were stronger in warmer temperatures, where higher exposure was associated with lower TBW. These findings are in line with current evidence, suggesting that the adverse neonatal effects of temperature exposure are stronger for heat than for cold [52].
5.4. Susceptible groups
The results of our sub cohort analyses varied by exposure. We found similar associations for PM2.5 regardless of sex, socioeconomic and racial groups. Similarly, Bell et al also found similar air pollution effects on birthweight among male and female newborns [53]. Unlike our findings, other studies have found racial minorities and lower socioeconomic groups to be more susceptible to air pollution effects [54, 55]. This may be related to higher exposure concentrations, higher frequency of psychological stressors, or limited access to health care [56]. In accordance with the literature, we did find mothers of lower socioeconomic status to be more susceptible to both colder and hotter temperatures [57]. This may be related to limited access to air conditioning, and residence in densely populated neighborhoods. Unexpectedly, White mothers were more susceptible to hotter temperatures compared to non-White mothers. With 80% White population in Massachusetts, it is possible that our findings were not robust due to a small sample size among minority groups.
Finally, we found larger TBW reductions associated with temperature among females, especially in hotter temperatures. Boys and girls are different in terms of fetal growth patterns, placental efficiency, and susceptibility to various prenatal exposures [12]. The underlying mechanisms of susceptibility are unclear and only a few studies have investigated potential modification by sex. Jakpor et al have found higher temperature variability to be associated with lower birthweight. Unlike our findings, they have found males to be more vulnerable to temperature variability [12]. Additional studies are required to investigate the differential vulnerability to prenatal temperature exposure among males and females.
5.5. Clinical implications
The current guidelines of the American College of Obstetricians and Gynecologists for prenatal care cover a wide range of maternal risk factors during pregnancy. Health care providers advise pregnant women on topics related to immunizations, infections, nutrition, exposure to violence, food, and medication consumption during pregnancy, and more. Although the guidelines address teratogenic environmental exposures, they do not include specific recommendations regarding heat and air pollution exposure during pregnancy.
Health care providers can communicate these critical windows of susceptibility to prenatal temperature and air pollution exposure to pregnant women during routine prenatal visits. This information will increase awareness and hopefully motivate the mothers to reduce exposure as much as possible, especially during the critical gestation weeks. For example, women can be advised to limit their exposure to traffic (e.g., avoid exercising near main roads, maintain proper ventilation indoors) – especially during the final weeks of pregnancy.
5.6. Strengths and limitations
This is a statewide analysis incorporating time-varying exposures to PM2.5 and temperature throughout the pregnancy. The large sample size and the use of fine spatiotemporally resolved exposure models are the major strengths of this analysis. Another strength is the use of DLNM, which allowed us to flexibly investigate exposure-effects in different stages of gestation. Our study had several limitations. Since we did not collect the data for this analysis but used routinely collected data, 12% of the records had missing covariates data. This problem is common in retrospective studies. Second, since we assigned exposure based on maternal place of residence at birth, we might have had misclassified exposure for women who changed addresses during pregnancy. However, since the new residence choice is unlikely to be based on air pollution or temperature exposure and unlikely to differ greatly in terms of the socioeconomic environment, we expect the exposure measurement error to be non-differential [58, 59]. Moreover, since we assign ambient exposures, and do not have information on indoor exposures or air conditioning use, we might have misclassified the exposure. If the true exposure of women during heat events tends to be much lower than the ambient temperature exposure due to use of air conditioning, this may have attenuated our results. If the misclassification was non differential, the results may be biased either toward or away from the null. Third, since we could not control for all potential confounders (such as maternal weight), residual confounding may still be present although the specific temporal pattern of associations decreases the likelihood to non-time varying confounding factors. In addition, since our study population is restricted to live births, we do not have information on spontaneous abortions. Therefore, our findings do not reflect the exposure effects on pregnancies which ended in an early stage. Finally, since most of our study population were White mothers, we might have not had enough representation of minority groups to allow the investigation of modification by racial group.
6. Conclusions
We found heat and PM2.5 exposure to be related to lower birth weight among term singleton births. Our findings suggest that women are more susceptible to both exposures towards the end of pregnancy. Susceptibility to heat was higher in the initial weeks of pregnancy as well. These critical windows of susceptibility can be communicated to pregnant women during routine prenatal visits to increase awareness and motivate them to reduce exposure.
Supplementary Material
Heat and PM2.5 exposure were independently associated with lower TBW.
Women are more susceptible to both exposures towards the end of pregnancy.
Susceptibility to heat was higher in the initial weeks of pregnancy as well.
7. Acknowledgments
This research was funded by grant 2017277 from the Binational Science Foundation (BSF) and NIH grants UH3 OD023337 and P30 ES023515.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Goldenberg RL, Culhane JF: Low birth weight in the United States. American Journal of Clinical Nutrition 2007, 85(2):584S–590S. [DOI] [PubMed] [Google Scholar]
- 2.Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I: Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. Bmc Medicine 2016, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH: Health in 2015: from MDGs to SDGs. In.: World Health Organization; 2015: 90–91. [Google Scholar]
- 4.Mann N: Birth weight symposium. Archives of Disease in Childhood 2002, 86(1):F2–F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells JCK: Thermal environment and human birth weight. Journal of Theoretical Biology 2002, 214(3):413–425. [DOI] [PubMed] [Google Scholar]
- 6.Ebisu K, Holford TR, Bell ML: Association between greenness, urbanicity, and birth weight. Science of the Total Environment 2016, 542:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RB, Fecht D, Gulliver J, Beevers SD, Dajnak D, Blangiardo M, Ghosh RE, Hansell AL, Kelly FJ, Anderson HR et al. : Impact of London’s road traffic air and noise pollution on birth weight: retrospective population based cohort study. Bmj-British Medical Journal 2017, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieuwenhuijsen MJ, Ristovska G, Dadvand P: WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Adverse Birth Outcomes. International Journal of Environmental Research and Public Health 2017, 14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz J, Arroyo V, Ortiz C, Carmona RO, Linares C: Effect of Environmental Factors on Low Weight in Non-Premature Births: A Time Series Analysis. PLoS One 2016, 11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong KC, Kloog I, Coull BA, Koutrakis P, Laden F, Schwartz JD, James P: Residential Greenness and Birthweight in the State of Massachusetts, USA. International Journal of Environmental Research and Public Health 2018, 15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yitshak-Sade M, Novack L, Landau D, Kloog I, Sarov B, Hershkovitz R, Karakis I: Relationship of ambient air pollutants and hazardous household factors with birth weight among Bedouin-Arabs. Chemosphere 2016, 160:314–322. [DOI] [PubMed] [Google Scholar]
- 12.Jakpor O, Chevrier C, Kloog I, Benmerad M, Giorgis-Allemand L, Cordier S, Seyve E, Vicedo-Cabrera AM, Slama R, Heude B et al. : Term birthweight and critical windows of prenatal exposure to average meteorological conditions and meteorological variability. Environment international 2020, 142:105847. [DOI] [PubMed] [Google Scholar]
- 13.Beltran AJ, Wu J, Laurent O: Associations of meteorology with adverse pregnancy outcomes: a systematic review of preeclampsia, preterm birth and birth weight. International journal of environmental research and public health 2014, 11(1):91–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chodick G, Flash S, Deoitch Y, Shalev V: Seasonality in Birth Weight: Review of Global Patterns and Potential Causes. Human Biology 2009, 81(4):463–477. [DOI] [PubMed] [Google Scholar]
- 15.Ha S, Zhu Y, Liu D, Sherman S, Mendola P: Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environmental research 2017, 155:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Wang J, Xu Z, Wang X, Xu G, Zhang J, Shen X, Tong S: Exploring associations of maternal exposure to ambient temperature with duration of gestation and birth weight: a prospective study. BMC pregnancy and childbirth 2018, 18(1):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischer NL, Merialdi M, van Donkelaar A, Vadillo-Ortega F, Martin RV, Betran AP, Souza JP, O’Neill MS: Outdoor Air Pollution, Preterm Birth, and Low Birth Weight: Analysis of the World Health Organization Global Survey on Maternal and Perinatal Health. Environmental Health Perspectives 2014, 122(4):425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML: PM2.5 Exposure and Birth Outcomes Use of Satellite- and Monitor-Based Data. Epidemiology 2014, 25(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dadvand P, Ostro B, Figueras F, Foraster M, Basagana X, Valentin A, Martinez D, Beelen R, Cirach M, Hoek G et al. : Residential Proximity to Major Roads and Term Low Birth Weight The Roles of Air Pollution, Heat, Noise, and Road-Adjacent Trees. Epidemiology 2014, 25(4):518–525. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XX, Liu Y, Chen YY, Yao CJ, Che Z, Cao JY: Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environmental Science and Pollution Research 2015, 22(5):3383–3396. [DOI] [PubMed] [Google Scholar]
- 21.Li XY, Huang SQ, Jiao AQ, Yang XH, Yun JF, Wang YX, Xue XW, Chu YY, Liu FF, Liu YS et al. : Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environmental Pollution 2017, 227:596–605. [DOI] [PubMed] [Google Scholar]
- 22.Dugandzic R, Dodds L, Stieb D, Smith-Doiron M: The association between low level exposures to ambient air pollution and term low birth weight: a retrospective cohort study. Environmental health : a global access science source 2006, 5:3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elter K, Ay E, Uyar E, Kavak ZN: Exposure to low outdoor temperature in the midtrimester is associated with low birth weight. Australian & New Zealand Journal of Obstetrics & Gynaecology 2004, 44(6):553–557. [DOI] [PubMed] [Google Scholar]
- 24.Geer LA, Weedon J, Bell ML: Ambient air pollution and term birth weight in Texas from 1998 to 2004. Journal of the Air & Waste Management Association (1995) 2012, 62(11):1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloog I, Novack L, Erez O, Just AC, Raz R: Associations between ambient air temperature, low birth weight and small for gestational age in term neonates in southern Israel. Environmental Health 2018, 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson A, Chiu Y-HM, Hsu H-HL, Wright RO, Wright RJ, Coull BA: Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. American journal of epidemiology 2017, 186(11):1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasparrini A: Modeling exposure-lag-response associations with distributed lag non-linear models. Statistics in Medicine 2014, 33(5):881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloog I: Air pollution, ambient temperature, green space and preterm birth. Current Opinion in Pediatrics 2019, 31(2):237–243. [DOI] [PubMed] [Google Scholar]
- 29.Song J, Lu J, Wang E, Lu M, An Z, Liu Y, Zeng X, Li W, Li H, Xu D et al. : Short-term effects of ambient temperature on the risk of premature rupture of membranes in Xinxiang, China: A time-series analysis. Sci Total Environ 2019, 689:1329–1335. [DOI] [PubMed] [Google Scholar]
- 30.Cheng P, Peng L, Hao J, Li S, Zhang C, Dou L, Fu W, Yang F, Hao J: Short-term effects of ambient temperature on preterm birth: a time-series analysis in Xuzhou, China. Environ Sci Pollut Res Int 2020. [DOI] [PubMed] [Google Scholar]
- 31.Just AC, Arfer KB, Rush J, Dorman M, Shtein A, Lyapustin A, Kloog I: Advancing methodologies for applying machine learning and evaluating spatiotemporal models of fine particulate matter (PM(2.5)) using satellite data over large regions. Atmos Environ (1994) 2020, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrión D, Arfer K, Rush J, Dorman M, Rowland S, Kioumourtzoglou M-A, Kloog I, Just A: Development and application of a 1-km hourly air-temperature model for the Northeastern and Mid-Atlantic United States using remotely sensed and ground-based measurements. 2020. [DOI] [PMC free article] [PubMed]
- 33.Casey JA, James P, Rudolph KE, Wu CD, Schwartz BS: Greenness and Birth Outcomes in a Range of Pennsylvania Communities. International Journal of Environmental Research and Public Health 2016, 13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenton TR, Kim JH: A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013, 13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proietti E, Roosli M, Frey U, Latzin P: Air Pollution During Pregnancy and Neonatal Outcome: A Review. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2013, 26(1):9–23. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg RL, Culhane JF, Iams JD, Romero R: Epidemiology and causes of preterm birth. Lancet (London, England) 2008, 371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Tang Y, Song X, Lazar L, Li Z, Zhao J: Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicology and environmental safety 2019, 169:248–254. [DOI] [PubMed] [Google Scholar]
- 38.Schlesinger RB, Kunzli N, Hidy GM, Gotschi T, Jerrett M: The health relevance of ambient particulate matter characteristics: coherence of toxicological and epidemiological inferences. Inhalation toxicology 2006, 18(2):95–125. [DOI] [PubMed] [Google Scholar]
- 39.Yue H, Ji X, Zhang Y, Li G, Sang N: Gestational exposure to PM(2.5) impairs vascularization of the placenta. Sci Total Environ 2019, 665:153–161. [DOI] [PubMed] [Google Scholar]
- 40.Saenen ND, Vrijens K, Janssen BG, Roels HA, Neven KY, Vanden Berghe W, Gyselaers W, Vanpoucke C, Lefebvre W, De Boever P et al. : Lower Placental Leptin Promoter Methylation in Association with Fine Particulate Matter Air Pollution during Pregnancy and Placental Nitrosative Stress at Birth in the ENVIRONAGE Cohort. Environ Health Perspect 2017, 125(2):262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen BG, Byun HM, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS: Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics 2015, 10(6):536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekkar B, Pacheco S, Basu R, DeNicola N: Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA network open 2020, 3(6):e208243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu R, Rau R, Pearson D, Malig B: Temperature and Term Low Birth Weight in California. American journal of epidemiology 2018, 187(11):2306–2314. [DOI] [PubMed] [Google Scholar]
- 44.Kloog I, Melly SJ, Coull BA, Nordio F, Schwartz JD: Using Satellite-Based Spatiotemporal Resolved Air Temperature Exposure to Study the Association between Ambient Air Temperature and Birth Outcomes in Massachusetts. Environment Health Prospectives 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J: Using new satellite based exposure methods to study the association between pregnancy pm(2.5) exposure, premature birth and birth weight in Massachusetts. Environmental Health 2012, 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuehn L, McCormick S: Heat Exposure and Maternal Health in the Face of Climate Change. International journal of environmental research and public health 2017, 14(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar N: The Exposure Uncertainty Analysis: The Association between Birth Weight and Trimester Specific Exposure to Particulate Matter (PM2.5 vs. PM10). Int J Environ Res Public Health 2016, 13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savitz DA, Bobb JF, Carr JL, Clougherty JE, Dominici F, Elston B, Ito K, Ross Z, Yee M, Matte TD: Ambient fine particulate matter, nitrogen dioxide, and term birth weight in New York, New York. Am J Epidemiol 2014, 179(4):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yitshak-Sade M, Fabian MP, Lane KJ, Hart JE, Schwartz JD, Laden F, James P, Fong KC, Kloog I, Zanobetti A: Estimating the Combined Effects of Natural and Built Environmental Exposures on Birthweight among Urban Residents in Massachusetts. Int J Environ Res Public Health 2020, 17(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdo M, Ward I, O’Dell K, Ford B, Pierce JR, Fischer EV, Crooks JL: Impact of Wildfire Smoke on Adverse Pregnancy Outcomes in Colorado, 2007–2015. Int J Environ Res Public Health 2019, 16(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holstius DM, Reid CE, Jesdale BM, Morello-Frosch R: Birth weight following pregnancy during the 2003 Southern California wildfires. Environ Health Perspect 2012, 120(9):1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Yu C, Wang L: Temperature exposure during pregnancy and birth outcomes: An updated systematic review of epidemiological evidence. Environ Pollut 2017, 225:700–712. [DOI] [PubMed] [Google Scholar]
- 53.Bell ML, Ebisu K, Belanger K: The relationship between air pollution and low birth weight: effects by mother’s age, infant sex, co-pollutants, and pre-term births. Environ Res Lett 2008, 3(4):44003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westergaard N, Gehring U, Slama R, Pedersen M: Ambient air pollution and low birth weight - are some women more vulnerable than others? Environ Int 2017, 104:146–154. [DOI] [PubMed] [Google Scholar]
- 55.Thayamballi N, Habiba S, Laribi O, Ebisu K: Impact of Maternal Demographic and Socioeconomic Factors on the Association Between Particulate Matter and Adverse Birth Outcomes: a Systematic Review and Meta-analysis. J Racial Ethn Health Disparities 2020. [DOI] [PubMed] [Google Scholar]
- 56.Yitshak-Sade M, Lane KJ, Fabian MP, Kloog I, Hart JE, Davis B, Fong KC, Schwartz JD, Laden F, Zanobetti A: Race or racial segregation? Modification of the PM2. 5 and cardiovascular mortality association. PloS one 2020, 15(7):e0236479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, Wernecke B, Robinson M, Hetem R, Boeckmann M et al. : Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. Bmj 2020, 371:m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Bell EM, Caton AR, Druschel CM, Lin S: Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res 2010, 110(2):162–168. [DOI] [PubMed] [Google Scholar]
- 59.Warren JL, Son JY, Pereira G, Leaderer BP, Bell ML: Investigating the Impact of Maternal Residential Mobility on Identifying Critical Windows of Susceptibility to Ambient Air Pollution During Pregnancy. Am J Epidemiol 2018, 187(5):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


