Abstract
Background/objectives:
Pregnant women are ubiquitously exposed to phthalates from food packaging materials and personal care products. Phthalates alter estrogen and testosterone concentrations in experimental models, but their ability to impact these hormones in human pregnancy is not well characterized.
Methods:
We recruited women ages 18 to 40 into the Illinois Kids Development Study (I-KIDS) in early pregnancy. Participants provided up to 5 first-morning urine samples across pregnancy (8–40 weeks gestation) that we pooled for quantification of 19 phthalate or phthalate alternative metabolites. Either individual (ng/mL) or molar sums (nmol/mL) of metabolites were used as exposure biomarkers. We summed urinary concentrations (ng/mL) of eight major estrogen (SumEstrogens) and two major testosterone (SumTestosterones) metabolites measured at median 13, 28, and 34 weeks gestation. We also estimated the ratio of estrogens-to-androgens. Linear mixed-effects models assessed relationships of phthalates/alternatives as continuous measures or as concentration quartiles with SumEstrogens, SumTestosterones, and the Estrogen/Androgen ratio in 434 women. In our models we controlled for age, race, education, parity, smoking in first trimester, pre-pregnancy body mass index, diet quality, conception season, fetal sex, and gestational age at hormone assessment. We also explored whether gestational age at hormone assessment or fetal sex modified these associations. All biomarkers and outcomes were specific gravity-adjusted, and continuous exposures and outcomes were also natural log-transformed.
Results:
Most participants were non-Hispanic white (80.9%), college educated (82.2%), and had urinary phthalate/alternative metabolite concentrations similar to those of reproductive-aged U.S. women. Overall, select phthalate metabolites were positively associated with SumEstrogens and SumTestosterones, but negatively associated with the Estrogen/Androgen ratio. For example, SumEstrogens was 5.1% (95%CI: 1.8, 8.5) higher with every 2-fold increase in sum of di(2-ethylhexyl) phthalate metabolites, while SumTestosterones was 7.9% (95%CI: 1.0, 15.3) higher and Estrogen/Androgen ratio was −7.7% (95%CI: −13.6, −1.4) lower with every 2-fold increase in monoethyl phthalate. However, phthalate alternatives were only positively associated with SumEstrogens, which was 2.4% (95%CI: 0.4, 4.5) and 3.2% (95%CI: 0.7, 5.8) higher with every 2-fold increase in sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites and sum of di(2-ethylhexyl) terephthalate metabolites, respectively. Gestational age- and fetal sex-specific associations were only consistently observed for associations of phthalates/alternatives with SumEstrogens, where associations were strongest in mid-to-late pregnancy in women carrying females.
Conclusion:
Phthalates/alternatives may impact gestational hormones, with potential for gestational age- and fetal sex-specific associations. Whether maternal urinary estrogens and testosterones mediate associations of phthalates/alternatives with pregnancy and fetal outcomes merits further investigation.
Keywords: Pregnancy, phthalates, phthalate replacements, urinary estrogen metabolites, urinary testosterone metabolites
1. INTRODUCTION
Estrogens and androgens (e.g. testosterone) are sex-steroid hormones that support pregnancy progression and fetal development (Noyola-Martinez et al. 2019). While maternal ovaries and adrenal glands contribute to maternal circulating sex-steroid hormones in early pregnancy, by the second trimester, the maternal-fetal-placental unit is almost solely responsible for synthesizing sex-steroid hormones from maternal and fetal cholesterol (Tal et al. 2000; Xu et al. 2005). Longitudinal studies demonstrate that estrogens and testosterone increase across gestation to support numerous pregnancy-related events (O’Leary et al. 1991; Schock et al. 2016). For example, substantial increases in major circulating estrogens (i.e. estrone, estradiol, estriol) are responsible for supporting implantation, placental angiogenesis, maternal metabolism, and parturition (Schock et al. 2016). Although less understood, minor increases in gestational testosterone support cervical remodeling and myometrial function responsible for parturition (Makieva et al. 2014). As a result, deviations from normal gestational estrogen and testosterone patterns may result in adverse maternal and fetal outcomes. To that end, observational studies suggest that disrupted estrogens and androgens are associated with increased risk of pre-eclampsia, gestational diabetes, and pre-term birth (Makieva et al. 2014; Morisset et al. 2013; Salamalekis et al. 2006). To protect maternal and child health, it is critical to understand factors that could disrupt the patterns of estrogens and testosterone in pregnancy.
Pregnant women are widely exposed to phthalates, which are endocrine disrupting chemicals that modify the synthesis and function of sex-steroid hormones. In the 2015–16 National Health and Nutrition Examination Survey (NHANES), >90% of reproductive-aged 18–40 year old U.S. women had detectable concentrations of at least one phthalate metabolite in their urine, which may be related to the use of phthalates in common consumer products, including food packaging materials and personal care products ((CDC), 2015–2016). Prenatal phthalate exposure has been associated with poor pregnancy and birth outcomes, including early pregnancy loss and pre-term birth (Ferguson et al. 2014; Toft et al. 2012), as well as adverse child outcomes, including cognitive and metabolic problems (Harley et al. 2017; Ipapo et al. 2017). Evidence for the endocrine disrupting potential of phthalates originates from in vitro and in vivo studies showing that certain phthalates can bind to estrogen and androgen receptors and may have weak estrogenic or anti-androgenic properties (Harris et al. 1997; Howdeshell et al. 2007; Jobling et al. 1995; Parks et al. 2000). Phthalates may also indirectly impact estrogen and testosterone synthesis and function by altering follicle stimulating hormone or by interacting with peroxisome proliferator-activated receptors or thyroid receptors, which are part of estrogen and androgen regulatory pathways (Benjamin et al. 2017). Consistently, studies in animals and humans have also demonstrated adverse associations of prenatal phthalate exposure with reproductive capacity or sex hormone-mediated outcomes (Marsee et al. 2006; Wang et al. 2012). However, few epidemiological studies have directly evaluated the impact of prenatal phthalate exposure on maternal hormone levels during pregnancy (Cathey et al. 2019; Johns et al. 2015; Sathyanarayana et al. 2014; Sathyanarayana et al. 2017). Additionally, while policies have led to reduced use of phthalates in certain consumer products (Commission 2017; Zota et al. 2014), phthalate alternatives have been introduced to replace phthalates in some plastic materials, with limited data related to the consequences of exposure to these replacements on human health.
To our knowledge, five prospective cohort studies have evaluated associations of maternal phthalate exposure with serum or plasma sex-steroid hormones in pregnant women (Banker et al. 2020; Cathey et al. 2019; Johns et al. 2015; Sathyanarayana et al. 2014; Sathyanarayana et al. 2017), and only one evaluated relationships of phthalate alternatives with these hormones (Cathey et al. 2019). Furthermore, only two studies evaluated fetal sex-specific associations, which may be important given that maternal phthalate exposures are sex-specifically associated with fetal and child health outcomes (Banker et al. 2020; Sathyanarayana et al. 2014). Additionally, previous studies related to phthalates and maternal hormones only evaluated associations of phthalates with sex-steroid hormones during a single trimester – either in the first trimester (Sathyanarayana et al. 2017) or the second/third trimesters (Cathey et al. 2019; Johns et al. 2015; Sathyanarayana et al. 2014), although a recent study evaluated maternal sex-steroid hormones in the first trimester and at term upon arrival to the hospital for delivery (Banker et al. 2020). Given the dynamic cross-pregnancy changes in estrogen and, to a lesser extent, testosterone levels, and the fact that previous studies collectively suggest that associations of phthalates/alternatives with hormones differ across pregnancy (Banker et al. 2020; Cathey et al. 2019; Johns et al. 2015; Sathyanarayana et al. 2014; Sathyanarayana et al. 2017), additional studies need to evaluate these associations at multiple timepoints across gestation.
Repeated blood sampling across gestation may not be feasible in large cohorts of pregnant women, but other analytical methods can detect sex-steroid hormone concentrations in urine (Xu et al. 2005), which in some cases may be easier to obtain than blood. This approach was developed in non-pregnant women as a proxy biomarker of circulating sex-steroid hormones (Coburn et al. 2019; Xu et al. 2005). More importantly, there is some evidence that urinary hormones may recapitulate observed associations of plasma/serum hormones with certain reproductive and lifestyle factors (Fortner et al. 2012; Gu et al. 2013). For example, urinary estrogen metabolites correlate with age at first birth and smoking status analogously to correlates of serum estrogen (Fortner et al. 2012; Gu et al. 2013), and urinary testosterone metabolite concentrations decrease with women’s age similar to what is observed with serum testosterone (Hall Moran et al. 2001). Conversely, other studies suggest that urinary hormones or their metabolites may not reflect circulating hormone concentrations, but rather represent hormone metabolism (Cantonwine et al. 2019; Eliassen et al. 2012). To circumvent these discrepancies with assessing hormones in blood versus urine, previous studies created sums of multiple urinary estrogen and testosterone metabolites to provide an estimate of total circulating estrogen or testosterone concentrations (Cantonwine et al. 2019; Eliassen et al. 2012). While this approach does not allow for evaluating associations between phthalates/alternatives and individual hormones, it does make it easier to conduct cross-pregnancy assessment of hormone status in large pregnancy cohorts.
Given the importance of estrogens and testosterone in pregnancy, our first objective was to evaluate associations of gestational urinary biomarkers of phthalates/alternatives exposures with urinary estrogens, testosterones, and the estrogen/androgen ratio. Unlike previous studies, we evaluated hormones across gestation in urine and explored whether these relationships may be dose-dependent. Because estrogens/androgens change across pregnancy and some differ by fetal sex, our second objective was to evaluate whether associations of gestational phthalate/alternative metabolite concentrations with urinary estrogens/testosterones differ across three gestational timepoints, or if they differ depending on the sex of the fetus. Results from our analyses provide additional insights into the endocrine disrupting potential of phthalates and phthalate alternatives across pregnancy, which may have long-term implications for maternal and child health.
2. MATERIALS AND METHODS
2.1. Illinois Kids Development Study recruitment and enrollment
This study includes pregnant participants in the Illinois Kids Development Study (I-KIDS), an ongoing prospective pregnancy cohort with the overarching goal of evaluating the impacts of prenatal environmental chemical exposures on infant neurodevelopment. We recruited pregnant women from two local obstetric clinics in Champaign-Urbana, IL at their first prenatal care appointment. Women who expressed interest in the study were contacted by I-KIDS staff and were eligible to participate if they were ≥10 but <15 weeks pregnant, 18–40 years old, fluent in English, not in a high-risk pregnancy or carrying multiple fetuses, living within a 30-minute drive of the University of Illinois campus, and not planning to move out of the area before their child’s first birthday. Enrolled women provided written informed consent according to the Institutional Review Board at the University of Illinois. The current study includes the first 439 women who enrolled in I-KIDS between December 2013 and July 2018. For the current study (and all chemical/hormone analyses) women must have remained in the study through the birth of their infant. The analysis of de-identified specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
2.2. Collection of maternal sociodemographic and lifestyle information at enrollment
Immediately after enrollment, we visited each pregnant I-KIDS participant at home to obtain information about their demographics, lifestyle, and health. We interviewed participants approximately monthly after this initial visit to ascertain important pregnancy-related updates, but only used information collected at the initial visit in the current analysis. Specifically, the following relevant demographic and lifestyle variables were self-reported by women at baseline: maternal age, race/ethnicity, highest educational level attained, smoking status since conception, and parity. Women also reported pre-pregnancy weight in pounds and height in feet and inches, which we used to calculate pre-pregnancy body mass index (BMI in kg/m2). Self-reported pre-pregnancy BMI has been shown to be highly correlated with measured first trimester BMI (Bannon et al. 2017; Holland et al. 2013; Natamba et al. 2016), which we also confirmed in a subset of women from our study (r = 0.93; data not shown). Estimated due date based on the last menstrual period was collected at baseline and confirmed after the first trimester ultrasound, while information about fetal sex was collected at birth. At 8–15 and 32–40 weeks gestation, women filled out a semi-quantitative food frequency questionnaire (FFQ) adapted from the full-length Block-98 FFQ (NutritionQuest, Berkeley, CA). The FFQ asked about maternal diet during the previous three months (Boucher et al. 2006) and was used to calculate Alternative Healthy Eating Index 2010 (AHEI-2010) in early and late gestation. AHEI-2010 is an 11 component diet quality measure (scored out of 110) based on food/nutrients predictive of chronic disease risk, and a higher score is reflective of better overall diet quality (McCullough et al. 2002). Because overall diet quality was relatively stable from early to late pregnancy in the I-KIDS population (data not shown), we used the mean of AHEI-2010 scores across the two timepoints to estimate maternal diet quality across gestation.
2.3. Collection and processing of urine samples for chemical and hormone analyses
Pregnant women provided first-morning urine samples at 8–15, 14–22, 19–28, 25–33, and 32–40 weeks gestation (median 13, 17, 23, 28, and 34 weeks, respectively). Urine samples were collected in polypropylene urine cups. All samples were refrigerated immediately after collection and transported on ice to the I-KIDS laboratory. Within 24 hours of collection, urine samples were warmed for 30 minutes to room temperature, vortexed, and assessed for specific gravity using a handheld refractometer (TS400; Reichert Technologies, Depew, NY). Each urine sample was aliquoted into polypropylene cryovial tubes (Nalgene, Rochester, NY) using disposable polyethylene bulb transfer pipettes (Fisher Scientific, Ann Arbor, MI). Duplicates and purified water blanks were collected and analyzed for every 10 samples. In addition to creating individual aliquots of urines at each timepoint, we also pooled all five urines for each participant by adding 900 μL of fresh urine from each timepoint to a 5 mL cryovial tube containing frozen urine from previous gestational timepoints. Specific gravity of pooled samples was measured at the end of pregnancy, when each pooled sample was thawed, vortexed, and aliquoted as described above. All urine was stored at −80 °C.
2.4. Quantification of urinary phthalate/alternative metabolites
Because phthalates/phthalate alternatives have short half-lives and high within-individual exposure variability (Shin et al. 2019), the current study assessed phthalate and phthalate alternative metabolites in pooled samples of up to five first morning urines to approximate maternal phthalate/alternative exposure across pregnancy. We shipped frozen pooled urine samples to the CDC on dry ice in three batches in the order of participant enrollment (batch one enrolled December 2013–February 2015, batch two enrolled February 2015–July 2016, and batch three enrolled July 2016–February 2018). Urinary phthalate and phthalate metabolite concentrations were quantified at the CDC using on-line solid phase extraction coupled with isotope dilution-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (Silva et al. 2007). The following phthalates and phthalate alternative metabolites were quantified in all batches: mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-isononyl phthalate (MiNP), monocarboxyoctyl phthalate (MCOP), monocarboxynonyl phthalate (MCNP), mono(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), mono-hydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-hydroxy-isobutyl phthalate (MiHBP), cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester (MCOCH). Three additional phthalate and phthalate alternative metabolites were added to the CDC analytical panel for women in batches two and three (monooxononyl phthalate (MONP), mono(2-ethyl-5-hydroxyhexyl) terephthalate (MEHHTP), and mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP)).
2.5. Measurement of urinary estrogen and testosterone metabolites
We measured eight major estrogens (estrone, estradiol, estriol, 16α-hydroxyestrone, 2-hydroxyestrone, 2-methoxyestrone, 4-hydroxyestrone, and 4-methoxyestrone) and two major testosterones (testosterone and 5α-dihydrotestosterone) in three individual first-morning urine samples collected at 8–15, 25–33, and 32–40 weeks gestation (median 13, 28, and 34 weeks, respectively) corresponding to early, middle, and late gestational plasma estrogen and testosterone concentrations (bolded in Figure 1, adapted from: (Tal et al. 2000; Xu et al. 2005)). All samples were analyzed in one analytical batch using methods adapted from Xu et al. (Xu et al. 2005). Urine samples for hormone analyses were prepared as follows: 500 μL of urine was mixed with 100 μL 1 M acetate buffer (pH 4.0), 20 μL of 0.5 μg/mL D3-testosterone (Sigma Aldrich Co. St. Louis, MO), and 20 μL beta-glucuronidase (Roche through Sigma Aldrich). The mixture was then vortexed, incubated in a heat block at 63° Celsius for 30 minutes, and centrifuged for 5 minutes at 8,000 rpm. Standards for all hormones were purchased from Steraloids Inc, Newport, RI. Solid-phase extraction (SPE) cleanup was performed using polymeric reverse phase cartridges (StrataTM-X, Phenomenex, Torrence, CA). Prepared and cleaned samples were analyzed with a 5500 QTRAP LC/MS/MS system (AB Sciex, Framingham, MA) with 1200 series HPLC system (Agilent Technologies) in the Roy J. Carver Biotechnology Center Metabolomics Lab at the University of Illinois at Urbana-Champaign.
Figure 1. Estrogen and testosterone metabolism pathway.
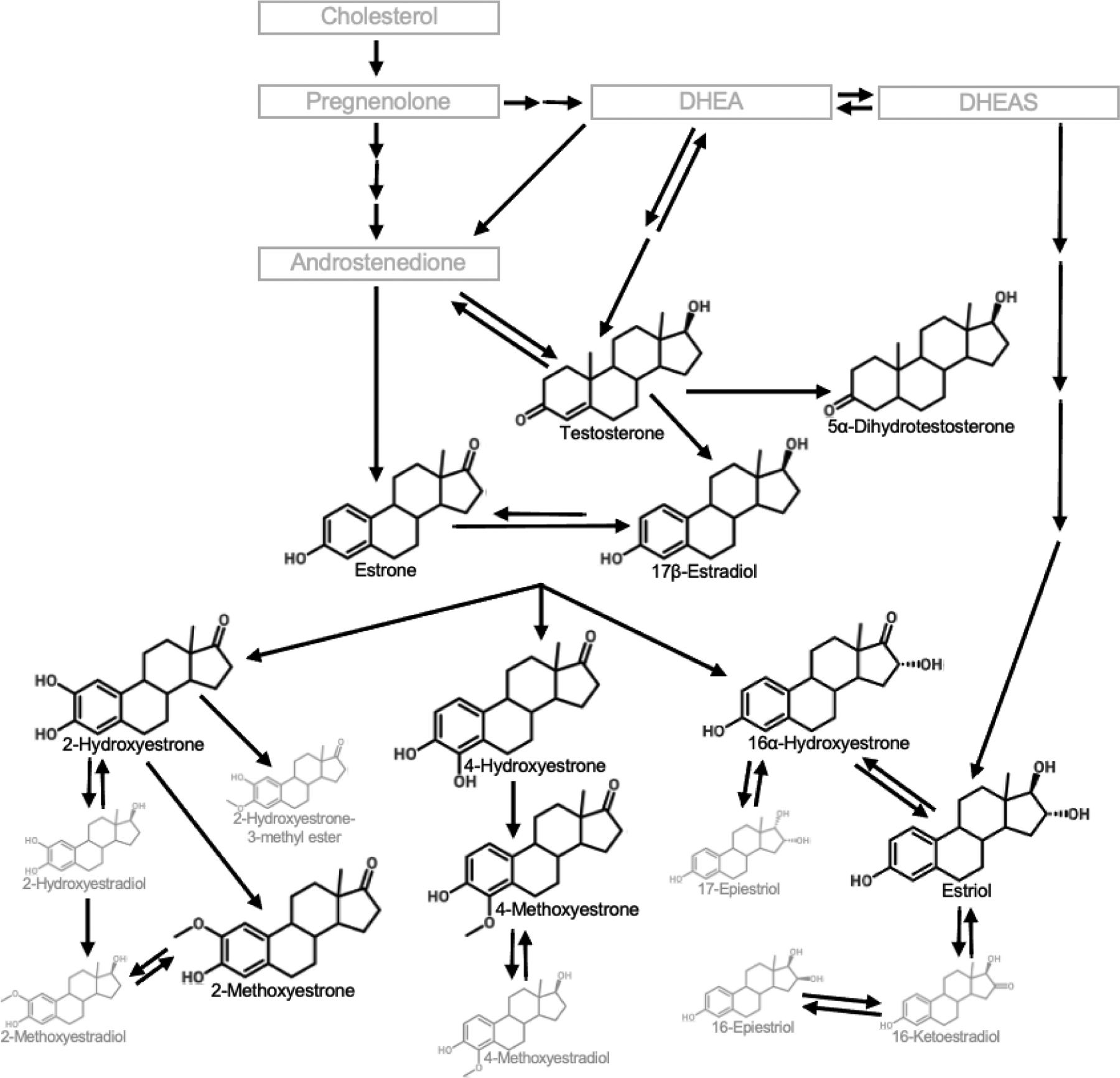
Maternal estrogens and testosterones are cholesterol-derived hormones that are primarily synthesized from maternal cholesterol in the ovaries, adrenal glands, and adipose tissue (to a lesser extent) in the first trimester. The maternal-fetal-placental unit become the primary producer of hormones after the first trimester. Estrogens and testosterones are metabolized in the maternal liver, and the parent compounds as well as resulting metabolites are excreted in urine. The bolded compounds are the eight major urinary estrogen and two major urinary testosterone parent compounds or metabolites measured in this study. DHEA, dihydroepiandrostenediene; DHEAS, dihydroepiandrostenediene sulfate.
2.6. Statistical analysis
2.6.1. Final sample size and covariate selection
Data from 439 women available for statistical analyses included all women who remained in the study through the birth of their infant (by July 2018) and therefore had information about prenatal phthalates/phthalate alternatives and hormones. In all statistical models, we included the following covariates selected a priori using previous literature: maternal age, race/ethnicity, education, parity, smoking since conception, pre-pregnancy BMI, AHEI-2010, season of conception, fetal sex, and timepoint of hormone assessment. We evaluated correlations between all potential confounders to test for multicollinearity. Three women had missing information about pre-pregnancy BMI, and two others had missing information about race/ethnicity or AHEI-2010 (Table 1). Therefore, 434 pregnant women were included in final statistical models. For hormone analysis, a total of 433, 424, and 426 women contributed urine samples at median 13, 28, and/or 34 weeks gestation, respectively, with 414 contributing urine samples at all three timepoints and 20 contributing urine samples at only two timepoints. Maternal age, AHEI-2010, and pre-pregnancy BMI were included as continuous variables, while the remaining covariates were categorical (Table 1).
Table 1.
Baseline demographic and lifestyle characteristics of I-KIDS pregnant women.
| Demographic or lifestyle characteristic | I-KIDS women (n=439)1 |
|---|---|
| median (range) or n (%) | |
| Age | 30.0 (18.0 – 40.0) |
| Alternative Healthy Eating Index-2010 (1 missing) | 55.8 (28.1 – 82.8) |
| Race/ethnicity (1 missing) | |
| Non-Hispanic White | 355 (80.9) |
| Others2 | 83 (18.9) |
| Education | |
| Some college of less | 78 (17.8) |
| College graduate or higher | 361 (82.2) |
| Parity | |
| Nulliparous | 228 (51.9) |
| Primiparous | 139 (31.7) |
| Multiparous | 72 (16.4) |
| Smoking during 1st trimester | |
| No | 385 (87.7) |
| Yes | 21 (4.8) |
| Unknown | 33 (7.5) |
| Pre-pregnancy BMI (3 missing) | |
| Underweight (<18.5 kg/m2) | 10 (2.3) |
| Normal weight (18.5–24.9 kg/m2) | 227 (51.7) |
| Overweight (25–29.9 kg/m2) | 103 (23.5) |
| Obese (≥30 kg/m2) | 96 (21.9) |
| Season of conception | |
| Winter | 108 (24.6) |
| Spring | 114 (25.9) |
| Summer | 100 (22.8) |
| Fall | 117 (26.7) |
| Fetal sex | |
| Female | 229 (52.2) |
| Male | 210 (47.8) |
Women who enrolled in the study through February 2018 and remained in the study through the birth of their infant.
Includes Hispanic Whites, Native American or Alaska Natives, Asians, Blacks or African Americans, Native Hawaiians or other Pacific Islanders, and others. Percentages may not add up to 100% due to missing. BMI, body mass index; I-KIDS, Illinois Kids Development Study
2.6.2. Exposure and outcome variables
To avoid bias associated with imputing values below the limit of detection (LOD) (Succop et al. 2004), we used instrument-read values for all samples rather than imputing phthalate/alternative and hormone metabolite concentrations below the LOD. Across the chemical and hormone analyses, only 5 values were zero (n=1 for SumDiNCH; n=3 and n=1 for SumTestosterones at median 13 and 28 weeks gestation, respectively), so in final statistical models we added a constant 0.0001 to these zero values before natural log-transformation (ln-transformation) to avoid undefined estimates (Weiss et al. 2015). To account for urine dilution, we used the following formula to adjust all urinary chemical and hormone metabolite concentrations: Pc = P[(SG − 1)/(SGi − 1)], where Pc is the specific gravity adjusted chemical or hormone metabolite concentration, P is the measured chemical metabolite concentration or hormone metabolite concentration (ng/mL), SG is the median specific gravity of the pooled samples used for chemical analysis (1.016) and three urine samples used for hormone analysis (1.015), and SGi is the specific gravity of each individual urine sample (Meeker et al. 2009).
We approximated exposure to phthalate and phthalate alternative parent compounds that are metabolized and excreted as multiple urinary metabolites using the following molar-sum (in nmol/mL) equations: sum of di(2-ethylhexyl) phthalate metabolites (SumDEHP) = (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308), sum of di-isononyl phthalate metabolites (SumDiNP) = (MiNP/292) + (MCOP/322), sum of di-n-butyl phthalate metabolites (SumDBP) = (MBP/222) + (MHBP/238), sum of di-iso-butyl phthalate metabolites (SumDiBP) = (MiBP/222) + (MHiBP/238), sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites (SumDiNCH) = (MHiNCH/314) + (MCOCH/328), and sum of di(2-ethylhexyl) terephthalate metabolites (SumDEHTP) = (MEHHTP/294) + (MECPTP/308). We excluded MONP from SumDiNP because this metabolite was only measured in batches 2 and 3, and we did not observe marked differences in associations between SumDiNP and hormones when MONP was included in the sum (data not shown). We approximated exposure to di-isodecyl phthalate, di-n-octyl phthalate, benzylbutyl phthalate, and diethyl phthalate using the concentrations (in ng/mL) of their non-molar converted major urinary metabolites MCNP, MCPP, MBzP, and MEP, respectively. Additionally, we molar-summed (nmol/mL) some phthalate metabolites based on common exposure sources by calculating the sum of phthalate metabolites of parent compounds found in plastics (SumPlastics) and personal care products (SumPCP) as follows (Wesselink et al. 2020): SumPlastics = (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308) + (MiNP/292) + (MCOP/322) + (MCNP/336) + (MCPP/252) + (MBzP/256) and SumPCP = (MEP/194) + (MBP/222) + (MHBP/238) + (MiBP/222) + (MHiBP/238). SumPlastics and SumPCP are reflective of high and low molecular weight phthalate metabolites, respectively.
Urine and blood likely capture different hormone forms, such that unconjugated (biologically active) hormones are measured in blood, whereas conjugated (biologically inactive) hormones are generally measured in urine (Eliassen et al. 2012). Therefore, individual urinary hormones, especially the downstream metabolites of the parent hormones, may not be representative of hormones in circulation, but rather provide insights into steroid hormone metabolism. This is particularly evident when comparing studies that quantified maternal parent estrogen concentrations (estrone, estradiol, and estriol) across pregnancy in blood versus urine, where estradiol and estriol tend to have the highest concentrations in blood and urine, respectively, relative to the other parent estrogens (Mistry et al. 2015; Tal et al. 2000). To circumvent this, we created hormone sums at each of the three gestational timepoints to represent total estrogens (SumEstrogens of eight major urinary estrogen metabolites) and testosterones (SumTestosterones of two major testosterone metabolites) in early, mid, and late gestation – an approach that has been previously used to characterize hormone status using hormones measured in urine (Cantonwine et al. 2019; Eliassen et al. 2012). Because the estrogen-to-androgen ratio may be an important indicator of pregnancy health (Villarroel et al. 2017), we additionally created an Estrogen/Androgen ratio by dividing SumEstrogens by SumTestosterones at each gestational timepoint. The Estrogen/Androgen ratio was only created for women who had non-zero values for both SumEstrogens and SumTestosterones.
2.6.3. Linear mixed model approach
We used linear mixed models to accommodate our longitudinal prospective design with hormone outcomes at three timepoints in gestation. SumEstrogens, SumTestosterones, and Estrogen/Androgen ratio were analyzed separately. An unstructured covariance 3×3 matrix was specified for the model’s residuals. All statistical analyses were conducted in SAS Software, version 9.4 (SAS Institute Inc, Cary, NC). Associations were considered significant at P < 0.05, and we did not adjust for multiple comparisons (Rothman 1990).
The first part of our first objective was to evaluate associations of continuous phthalate/alternative biomarkers with maternal sums of urinary hormone metabolites across pregnancy. We ln-transformed our exposures and outcomes to fit normality assumptions and added 0.0001 to phthalate/alternative or hormones that had zero as a minimum value (see section 2.6.2 for specific details). The second part of our first objective was to assess potential dose-response relationships between phthalates/alternatives and hormones. Phthalates were categorized based on quartiles of urinary phthalate/alternative biomarkers concentrations, while hormones were ln-transformed as described previously. For our second objective, because previous studies suggest that associations of phthalate and phthalate alternative biomarkers with hormones may be gestational age- or fetal sex-specific (Banker et al. 2020; Cathey et al. 2019; Johns et al. 2015; Sathyanarayana et al. 2014), we also evaluated gestational age- and fetal sex-specific relationships between phthalate/alternative biomarkers and hormones by including a three-way interaction (and all relevant two-way interactions) between exposure, gestational age at hormone sampling, and fetal sex. We only evaluated gestational age- and fetal sex-specific associations of continuous phthalate/alternative biomarkers with hormones.
2.6.4. Reporting of results
All gestational age- and fetal sex-specific results were reported regardless of the significance of the three-way interaction term. All β-estimates and 95% confidence intervals (CIs) were back transformed and presented as a percent change in hormones in tables and figures. In models with both ln-transformed phthalate/alternative biomarkers and hormones (objectives 1 and 2), β-estimates and 95% CIs were back transformed using the equation [((2.00)β − 1)*100] to represent a percent change in hormones for each 2-fold increase in phthalate/alternative (Table 2). For models with ln-transformed hormones and phthalate/alternatives biomarkers in quartiles (objective 1), β-estimates and 95% CIs were back transformed using the equation [(eβ − 1)*100] to represent the percent change in hormones among women in quartiles two (Q2), three (Q3), and four (Q4) of urinary phthalate/alternative biomarkers concentrations, which were each compared to quartile one (Q1) (Table 2, Figures 4–6).
Table 2.
Overall associations of phthalate and phthalate alternative biomarkers with SumEstrogens, SumTestosterones, and Estrogen/Androgen ratio.
| Biomarker | % Change (95%CI) in SumEstrogens | P | % Change (95%CI) in SumTestosterones | P | % Change (95%CI) in Estrogen/Androgen ratio | P |
|---|---|---|---|---|---|---|
| SumDEHP | ||||||
| Linear association | 5.1 (1.8, 8.5) | 0.002 | −4.2 (−12.6, 4.9) | 0.35 | 8.2 (−1.2, 18.5) | 0.09 |
| Q2 (ref=Q1) | −3.1 (−11.0, 5.4) | 0.46 | 10.9 (−13.2, 41.8) | 0.41 | −6.2 (−26.5, 19.8) | 0.61 |
| Q3 (ref=Q1) | 1.8 (−6.4, 10.9) | 0.67 | 34.3 (5.0, 71.7) | 0.02 | −21.5 (−38.6, 0.3) | 0.05 |
| Q4 (ref=Q1) | 12.7 (3.3, 23.0) | 0.01 | 7.2 (−16.7, 38.0) | 0.59 | 9.8 (−14.6, 41.3) | 0.46 |
| SumDiNP | ||||||
| Linear association | 0.1 (−3.2, 3.4) | 0.97 | 0.4 (−9.0, 10.9) | 0.93 | −3.3 (−12.3, 6.6) | 0.49 |
| Q2 (ref=Q1) | 2.3 (−6.1, 11.3) | 0.61 | −7.2 (−27.4, 18.7) | 0.55 | 7.8 (−15.7, 37.8) | 0.55 |
| Q3 (ref=Q1) | −3.2 (−11.3, 5.7) | 0.47 | −0.6 (−22.9, 28) | 0.96 | 6.3 (−17.5, 36.9) | 0.63 |
| Q4 (ref=Q1) | 2.4 (−6.5, 12) | 0.61 | 0.9 (−22.2, 30.9) | 0.94 | 5.3 (−18.8, 36.5) | 0.70 |
| MCNP | ||||||
| Linear association | 2.2 (−0.7, 5.1) | 0.14 | 4.8 (−3.3, 13.7) | 0.25 | −0.8 (−8.5, 7.6) | 0.85 |
| Q2 (ref=Q1) | −2.2 (−10.4, 6.7) | 0.61 | 14.6 (−10.8, 47.3) | 0.29 | −19.3 (−37.2, 3.7) | 0.09 |
| Q3 (ref=Q1) | 0.5 (−7.8, 9.6) | 0.91 | 13.0 (−11.9, 45.1) | 0.34 | −15.4 (−34.0, 8.5) | 0.19 |
| Q4 (ref=Q1) | 2.2 (−6.5, 11.7) | 0.63 | 17.0 (−9.4, 51.0) | 0.23 | −10.9 (−30.9, 14.9) | 0.37 |
| MCPP | ||||||
| Linear association | 2.6 (−0.02, 5.4) | 0.05 | 4.6 (−3.0, 12.9) | 0.25 | −1.1 (−8.3, 6.7) | 0.78 |
| Q2 (ref=Q1) | 7.3 (−1.5, 16.9) | 0.11 | 22.0 (−4.6, 56.1) | 0.11 | −10.5 (−30.0, 14.6) | 0.38 |
| Q3 (ref=Q1) | 6.7 (−2.1, 16.3) | 0.14 | 35.8 (6.1, 73.9) | 0.02 | −21.6 (−38.8, 0.3) | 0.05 |
| Q4 (ref=Q1) | 9.3 (0.3, 19.1) | 0.04 | 23.1 (−3.9, 57.6) | 0.10 | −3.8 (−24.9, 23.2) | 0.76 |
| MBzP | ||||||
| Linear association | 1.2 (−0.8, 3.4) | 0.24 | 6.3 (0.1, 12.8) | 0.05 | −5.0 (−10.5, 0.9) | 0.09 |
| Q2 (ref=Q1) | 4.2 (−4.2, 13.4) | 0.34 | 10.5 (−13.3, 40.8) | 0.42 | −6.1 (−26.3, 19.7) | 0.61 |
| Q3 (ref=Q1) | 5.3 (−3.5, 14.9) | 0.25 | 9.4 (−14.9, 40.7) | 0.48 | −10.2 (−30.2, 15.5) | 0.40 |
| Q4 (ref=Q1) | 6.3 (−2.5, 15.9) | 0.16 | 26.3 (−1.5, 61.9) | 0.07 | −15.6 (−34.2, 8.3) | 0.18 |
| SumPlastics | ||||||
| Linear association | 3.7 (0.5, 6.9) | 0.02 | 0.9 (−7.7, 10.3) | 0.84 | 3.2 (−5.6, 12.8) | 0.48 |
| Q2 (ref=Q1) | 2.9 (−5.7, 12.1) | 0.52 | −0.1 (−22.1, 28.1) | 0.99 | 9.2 (−14.9, 40.2) | 0.49 |
| Q3 (ref=Q1) | 10.4 (1.2, 20.4) | 0.03 | 28.2 (−0.3, 64.8) | 0.05 | −3.5 (−25.0, 24.2) | 0.78 |
| Q4 (ref=Q1) | 8.8 (−0.4, 18.9) | 0.06 | 6.7 (−17.4, 37.8) | 0.62 | 10.0 (−14.9, 42.3) | 0.47 |
| SumDiNCH | ||||||
| Linear association | 2.4 (0.4, 4.5) | 0.02 | 0.3 (−5.4, 6.3) | 0.92 | −1.1 (−6.7, 4.8) | 0.71 |
| Q2 (ref=Q1) | −1.7 (−9.9, 7.2) | 0.69 | −10.7 (−30.5, 14.8) | 0.38 | 2.9 (−19.9, 32.2) | 0.82 |
| Q3 (ref=Q1) | 6.3 (−2.7, 16.1) | 0.18 | −20.6 (−38.5, 2.6) | 0.08 | 23.4 (−4.4, 59.3) | 0.11 |
| Q4 (ref=Q1) | 8.7 (−0.4, 18.7) | 0.06 | −5.7 (−26.8, 21.4) | 0.65 | −0.9 (−23.0, 27.6) | 0.94 |
| SumDEHTP | ||||||
| Linear association | 3.2 (0.7, 5.8) | 0.01 | 3.0 (−4.5, 11.0) | 0.44 | −1.9 (−8.9, 5.7) | 0.62 |
| Q2 (ref=Q1) | −1.5 (−12.0, 10.3) | 0.80 | −11.3 (−36.9, 24.7) | 0.49 | 13.9 (−18.6, 59.3) | 0.45 |
| Q3 (ref=Q1) | 5.8 (−5.5, 18.4) | 0.32 | 23.2 (−12.4, 73.2) | 0.23 | −18.2 (−41.5, 14.5) | 0.24 |
| Q4 (ref=Q1) | 7.3 (−4.4, 20.3) | 0.23 | 0.3 (−29.1, 41.9) | 0.98 | 0.7 (−28.4, 41.8) | 0.97 |
| MEP | ||||||
| Linear association | 1.4 (−0.9, 3.8) | 0.23 | 7.9 (1.0, 15.3) | 0.02 | −7.7 (−13.6, −1.4) | 0.02 |
| Q2 (ref=Q1) | 2.5 (−5.9, 11.6) | 0.57 | 22.1 (−4.4, 56.1) | 0.11 | −16.8 (−34.9, 6.2) | 0.14 |
| Q3 (ref=Q1) | 7.6 (−1.3, 17.3) | 0.10 | 34.1 (4.7, 71.8) | 0.02 | −28.7 (−44.3, −8.7) | 0.01 |
| Q4 (ref=Q1) | 0.8 (−7.7, 10.1) | 0.85 | 29.2 (0.4, 66.4) | 0.05 | −25.2 (−41.9, −3.8) | 0.02 |
| SumDBP | ||||||
| Linear association | 2.8 (−0.5, 6.3) | 0.10 | −2.9 (−11.9, 6.9) | 0.54 | −0.2 (−9.0, 10.3) | 0.97 |
| Q2 (ref=Q1) | 9.3 (0.4, 19.0) | 0.04 | 2.2 (−20.2, 30.8) | 0.86 | −4.9 (−25.7, 21.7) | 0.69 |
| Q3 (ref=Q1) | 7.1 (−1.6, 16.6) | 0.11 | 15.6 (−9.5, 47.7) | 0.24 | −15.7 (−34.0, 7.7) | 0.17 |
| Q4 (ref=Q1) | 9.0 (−0.04, 18.9) | 0.05 | 5.3 (−18.0, 35.3) | 0.68 | −3.1 (−24.5, 24.4) | 0.81 |
| SumDiBP | ||||||
| Linear association | 0.9 (−1.9, 3.8) | 0.52 | −2.6 (−10.1, 5.6) | 0.53 | −3.0 (−10.5, 5.2) | 0.46 |
| Q2 (ref=Q1) | 11.2 (2.2, 20.9) | 0.01 | 19.8 (−6.1, 52.9) | 0.15 | −8.1 (−28.0, 17.4) | 0.50 |
| Q3 (ref=Q1) | 3.7 (−4.8, 13.0) | 0.41 | −3.8 (−25.0, 23.3) | 0.76 | −8.7 (−28.8, 17.1) | 0.47 |
| Q4 (ref=Q1) | 6.5 (−2.2, 16.0) | 0.15 | −2.7 (−24.0, 24.5) | 0.83 | −4.4 (−25.4, 22.5) | 0.72 |
| SumPCP | ||||||
| Linear association | 1.7 (−1.4, 4.9) | 0.29 | 5.0 (−4.1, 14.9) | 0.29 | −8.6 (−16.4, −0.0) | 0.05 |
| Q2 (ref=Q1) | 4.4 (−4.3, 13.8) | 0.33 | 14.0 (−11.1, 46.2) | 0.30 | −17.4 (−35.4, 5.8) | 0.13 |
| Q3 (ref=Q1) | 3.2 (−5.4, 12.5) | 0.48 | 14.1 (−11, 46.3) | 0.30 | −14.3 (−33.0, 9.7) | 0.22 |
| Q4 (ref=Q1) | 2.3 (−6.4, 11.7) | 0.61 | 19.4 (−7.4, 53.9) | 0.17 | −28.5 (−44.5, −8.0) | 0.01 |
Linear mixed models were used to evaluate overall associations of phthalates/phthalate alternatives with ln-transformed hormones controlling for age, race/ethnicity, education, parity, smoking during 1st trimester, pre-pregnancy body mass index, diet quality, season of conception, fetal sex, and gestational age at hormone assessment. Phthalates/phthalate alternatives are either included as ln-transformed continuous variables or variables categorized into quartiles of exposure with Q1 as the reference group. β-estimates and 95%CIs for associations of continuous phthalates/phthalate alternatives with hormones were back-transformed to represent a % change in hormones for every 2-fold increase in phthalate/alternative. CI, confidence interval; Q1–4, quartiles 1–4; Ref, reference. Phthalate/alternative concentrations in ng/mL or nmol/mL and hormone concentrations in ng/mL.
Figure 4. Gestational age- and fetal sex-specific associations of phthalate and phthalate alternative molar sums and metabolites with urinary SumEstrogens.
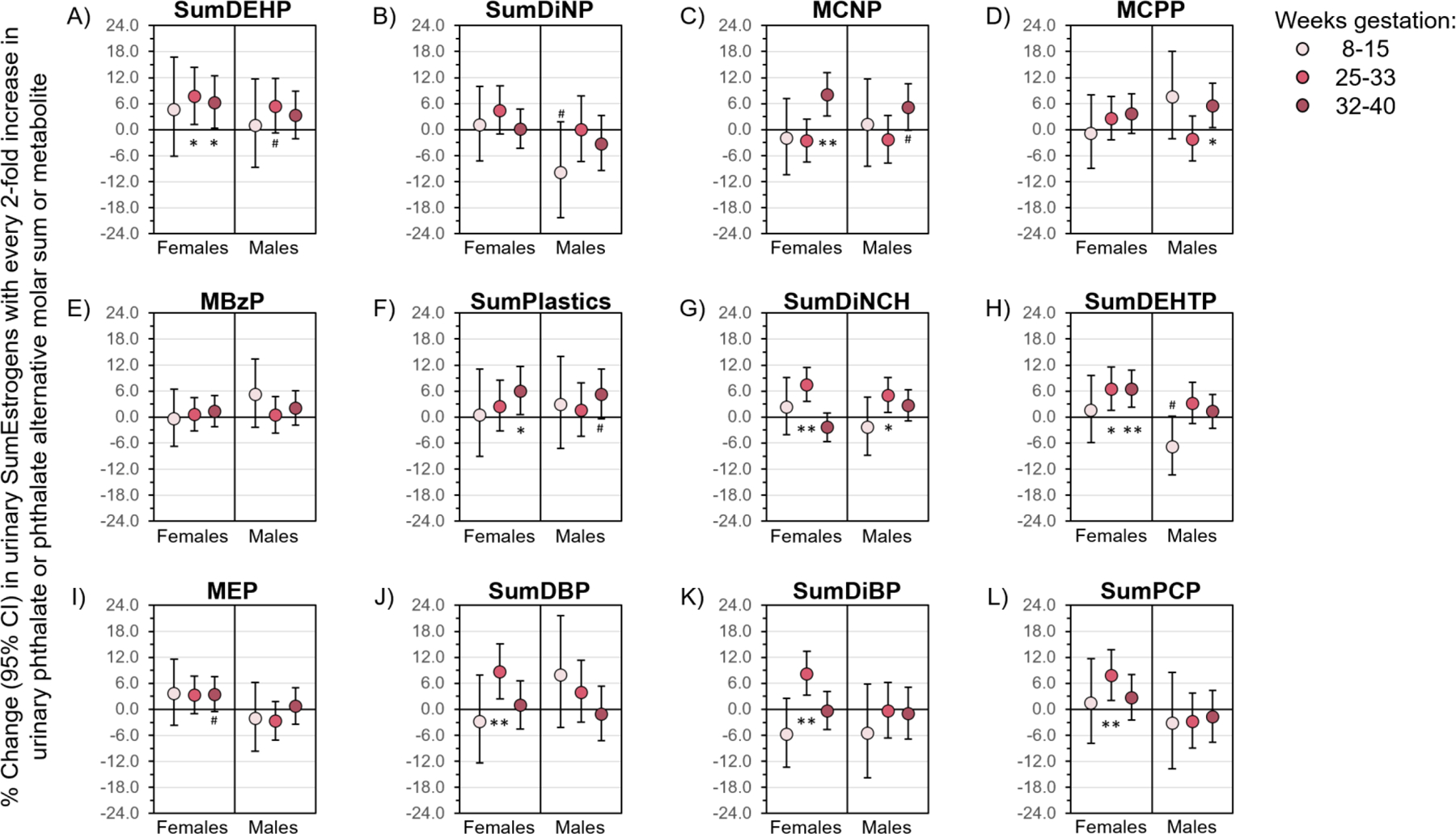
Linear mixed models controlled for age, race/ethnicity, education, parity, smoking during 1st trimester, pre-pregnancy body mass index, diet quality, season of conception, fetal sex, and gestational age at hormone assessment. Data are presented as the percent change (filled circle) and 95% CI (solid lines) in urinary SumEstrogens with every 2-fold increase in urinary phthalate or phthalate alternative biomarker. CIs that do not cross the null are significant at #P<0.1, *P<0.05, and **P<0.01. CI, confidence interval.
Figure 6. Gestational age- and fetal sex-specific associations of phthalate and phthalate alternative molar sums and metabolites with urinary Estrogen/Androgen ratio.
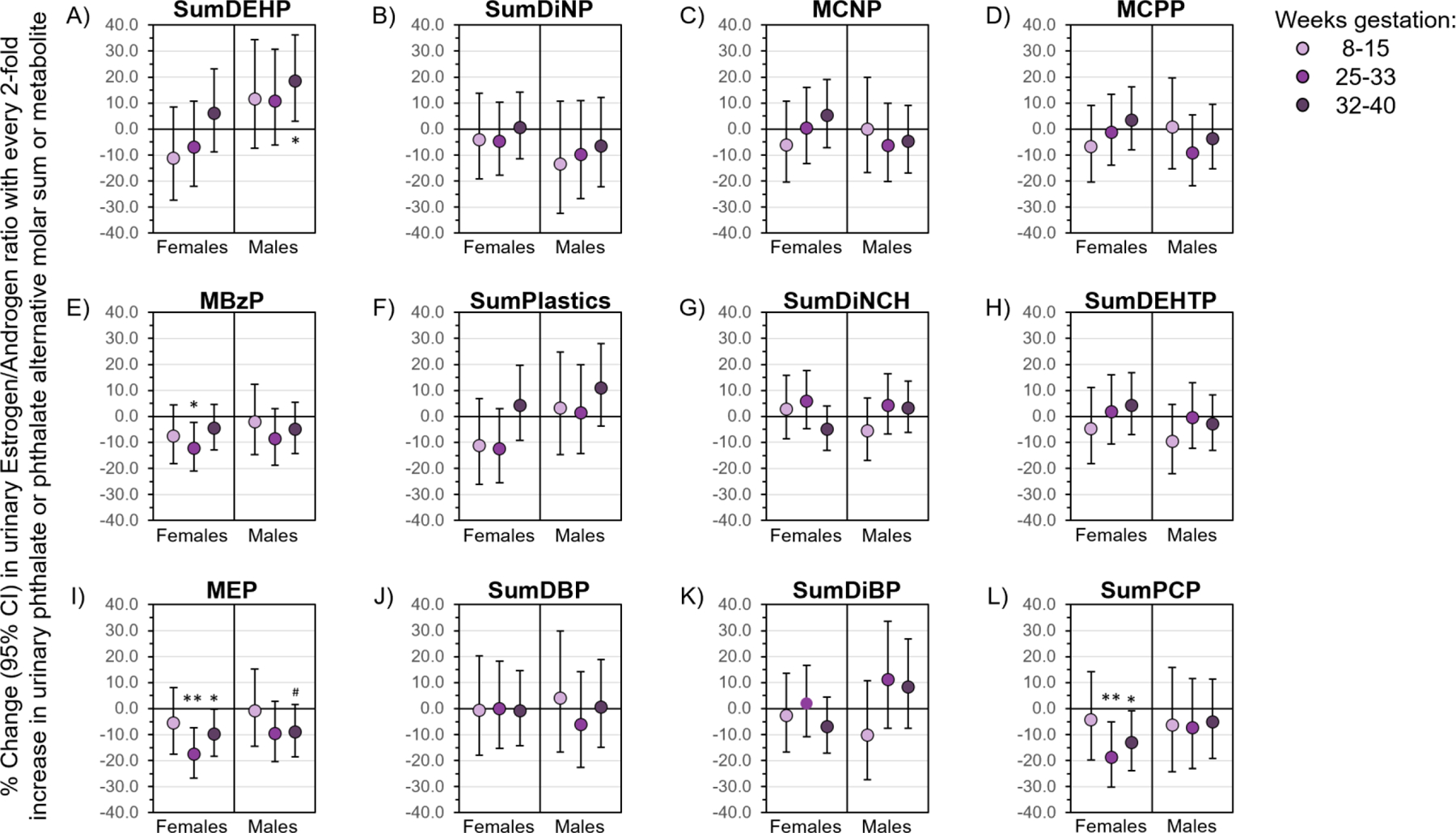
Linear mixed models controlled for age, race/ethnicity, education, parity, smoking during 1st trimester, pre-pregnancy body mass index, diet quality, season of conception, fetal sex, and gestational age at hormone assessment. Data are presented as the percent change (filled circle) and 95% CI (solid lines) in urinary Estrogen/Androgen ratio with every 2-fold increase in urinary phthalate or phthalate alternative biomarker. CIs that do not cross the null are significant at #P<0.1, *P<0.05, and **P<0.01. CI, confidence interval.
3. RESULTS
3.1. Demographic and lifestyle characteristics of the I-KIDS population
Baseline characteristics of 439 pregnant I-KIDS women are reported in Table 1. Participants had a median (range) age of 30 years (18–40), and the majority were non-Hispanic white (81%) and college educated (82%). Approximately 52% of women were nulliparous, while 48% were primiparous or multiparous. The majority of women did not smoke since conception (88%), 52% were normal weight and 45% were overweight or had obesity before pregnancy, and median (range) diet quality score measured by the AHEI-2010 was 55.8 (28.1–82.8) out of 110. Season of conception was equally distributed across all seasons, and fetal sex was also relatively evenly distributed, with 52% and 48% of women carrying a female or male fetus, respectively. Median (range) gestational ages at hormone assessment were as follows: 13.4 weeks (8.9–15.3), 28.7 weeks (25.7–33.6), and 34.9 weeks (32.7–40.0).
3.2. Urinary phthalate and phthalate alternative metabolite concentrations
The concentrations of most phthalate and phthalate alternative metabolites were detectable in 100% of women, except for the following metabolites (% of women with metabolite concentrations >LOD): MEHP (74%), MiNP (42%), MCPP (97%), MBzP (99%), MHBP (90%), MiBP (99%), MHiBP (99%), MHiNCH (77%), and MCOCH (49%) (data not shown). Urinary phthalate/alternative metabolite concentrations in I-KIDS women were generally comparable to those in 18–40-year-old women from NHANES cycles 2013–14 and 2015–16 (Figure 2).
Figure 2. Urinary phthalate and phthalate alternative metabolite concentrations in pregnant women from I-KIDS 2013–19 compared to women from the NHANES 2013–16.

Urinary phthalate (A-J, O-S) and phthalate alternative (K-N) metabolite concentrations were assessed from pooled sample of five urine samples per participant (n=439). Results are presented as 1.5 times the interquartile range below and above the 25th and 75th percentiles (lower and upper endpoints of whisker), the 25th and 75th percentiles (lower and upper edges of box), median (line inside box), and mean (diamond). Urinary phthalate and phthalate alternative metabolite concentrations assessed from a spot urine sample per participant were obtained for 18–40-year-old non-pregnant women from NHANES survey years 2013–14 (n=394 or 392) and 2015–16 (n=348). I-KIDS, Illinois Kids Development Study; NHANES, National Health and Nutrition Examination Survey.
3.3. Urinary sex-steroid hormone concentrations
As expected, urinary SumEstrogens, SumTestosterones, and the Estrogen/Androgen ratio increased across gestation (Figure 3; Ptime<0.0001). Median (range) urinary specific gravity-adjusted hormone concentrations were as follows at 8–15, 25–33, and 32–40 weeks gestation: SumEstrogens: 3064.4 ng/mL (313.5–57,879.9), 11,168.9 ng/mL (2,845.3–40,420.4), and 14350.1 ng/mL (4,063.1–41,561.0), respectively (Figure 3A); SumTestosterones: 3.6 ng/mL (0.0–61.9), 3.4 ng/mL (0.0–84.6), and 4.3 ng/mL (0.1–73.2), respectively (Figure 3B); and Estrogen/Androgen ratio: 848.3 (75.5–236,684.0), 3,720.2 (43.3–332,635.5), and 3,659.1 (176.7–106,960.2), respectively (Figure 3C).
Figure 3. Distribution of urinary specific gravity-adjusted SumEstrogen, SumTestosterone, and Estrogen/Androgen ratio across gestation (n=439).

Distributions of urinary A) SumEstrogens (ng/mL), B) SumTestosterones (ng/mL), and C) Estrogen/Androgen ratio at 8–15, 25–33, and 32–40 weeks gestation. Results are presented as 1.5 times the interquartile range below and above the 25th and 75th percentiles (lower and upper endpoints of whisker), the 25th and 75th percentiles (lower and upper edges of box), median (line inside box), and mean (diamond). Linear mixed models were used to assess whether hormone concentrations differed across the three gestational timepoints (Ptime).
3.4. Overall associations of phthalate/alternative biomarkers with hormones
In linear regression models, SumDEHP, MCPP, SumPlastics, SumDiNCH, and SumDEHTP were positively associated with SumEstrogens (Table 2). For example, each 2-fold increase in SumDEHP and SumDEHTP was associated with 5.1% (95%CI: 1.8, 8.5) and 3.2% (95%CI: 0.7, 5.8) increase in SumEstrogens, respectively. Certain analyses where phthalate/alternative biomarkers were modeled in quartiles generally supported the linear models. SumEstrogens were higher in women at the upper quartiles of SumDEHP (Q4), MCPP (Q4), SumPlastics (Q3 and Q4), and SumDiNCH (Q4) compared to those in Q1. For example, compared to Q1, SumEstrogens was 12.7% (95%CI: 3.3, 23.0) higher in SumDEHP Q4. However, associations with SumDBP and SumDiBP did not appear to follow a linear trend, such that compared to Q1, SumEstrogens were 9.2% (95%CI: 0.4, 19.0) and 9.0% (95%CI: −0.04, 18.9) higher in SumDBP Q2 and Q4, respectively, and 11.2% (95%CI: 2.2, 20.9) higher only in SumDiBP Q2.
Several phthalate and phthalate alternative biomarkers were also associated with urinary SumTestosterone concentrations across pregnancy (Table 2). In linear models, every 2-fold increase in MBzP and MEP was associated with 6.3% (95%CI: 0.1, 12.8) and 7.9% (95%CI: 1.0, 15.3) increase in SumTestosterones, respectively. In models where phthalate/alternative biomarkers were modeled as quartiles, compared those to the lowest quartile, SumTestosterones were higher at higher quartiles of SumDEHP (Q3), MCPP (Q3), MBzP (Q4), SumPlastics (Q3), and MEP (Q3 and Q4). For example, SumTestosterones were 34.1% (95%CI: 4.7, 71.8) and 29.2% (95%CI: 0.4, 66.4) higher in MEP Q3 and Q4, respectively, compared to Q1.
Some phthalate, but not phthalate alternative, biomarkers were associated with the Estrogen/Androgen ratio (Table 2). Specifically, in linear models, the ratio tended to be negatively associated with SumDEHP and MBzP, and was negatively associated with MEP and SumPCP, such that each 2-fold increase in MEP and SumPCP was associated with −7.7% (95%CI: −13.6, −1.4) and −8.6% (95%CI: −16.4, 0.0) lower Estrogen/Androgen ratio. In quartile analyses, compared to those in Q1, the Estrogen/Androgen ratio tended to be lower for women in SumDEHP Q3, MCNP Q2, and MCPP Q3, and was lower in women at higher quartiles of MEP (Q3 and Q4) and SumPCP (Q4). For example, the Estrogen/Androgen ratio was −28.7% (95%CI: −44.3, −8.7) and −25.2% (95%CI: −41.9, −3.8) lower in MEP Q3 and Q4, respectively, compared to Q1.
3.5. Gestational age- and fetal sex-specific associations of phthalate/alternative biomarkers with hormones
Some associations between phthalate/alternative biomarkers and urinary SumEstrogen concentrations did differ by gestational age at hormone assessment and/or fetal sex (Figure 4). Values for all gestational age- and fetal sex-specific results are reported in Supplementary Tables 1–3. Positive associations of SumDEHP, MCNP, MCPP, SumPlastics, and SumDiNCH with SumEstrogens were observed in both sexes, but were strongest at 25–33 or 32–40 weeks gestation (Figures 4A, C, D, F, G). Associations of SumDEHTP with SumEstrogens were positive in women carrying female fetuses and strongest at 25–33 and 32–40 weeks gestation, but there was a trend for a negative association in women carrying male fetuses at 8–15 weeks gestation (Figure 4H). Positive associations of MEP, SumDBP, SumDiBP, and SumPCP with SumEstrogens were only observed in women carrying female fetuses and were strongest at 25–33 or 32–40 weeks gestation (Figures 4I, J, K, L). Interestingly, a trend towards a negative association between SumDiNP and SumEstrogens was observed in women carrying male fetuses and strongest at 8–15 weeks gestation (Figure 4B). In these stratified analyses, urinary concentrations of MBzP were not associated with SumEstrogens at any gestational timepoint or by fetal sex (Figure 4E).
Some associations of phthalate (but not phthalate alternative) biomarkers with urinary SumTestosterones also differed by gestational age and fetal sex (Figure 5). Positive associations of MBzP, MEP, and SumPCP with SumTestosterones were strongest in women carrying female fetuses at 25–33 or 32–40 weeks gestation (Figure 5E, I, L). However, SumDEHP and SumDiBP were negatively associated with SumTestosterones in women carrying male fetuses, with strongest associations observed at 8–15 or 25–33 weeks gestation, respectively (Figure 5A, K). Maternal SumDiNP, MCNP, MCPP, SumPlastics, SumDiNCH, SumDEHTP, and SumDBP were not associated with SumTestosterones, regardless of gestational timepoint of hormone assessment or fetal sex (Figures 5B, C, D, F, G, H, J).
Figure 5. Gestational age- and fetal sex-specific associations of phthalate and phthalate alternative molar sums and metabolites with urinary SumTestosterones.

Linear mixed models controlled for age, race/ethnicity, education, parity, smoking during 1st trimester, pre-pregnancy body mass index, diet quality, season of conception, fetal sex, and gestational age at hormone assessment. Data are presented as the percent change (filled circle) and 95% CI (solid lines) in urinary SumTestosterones with every 2-fold increase in urinary phthalate or phthalate alternative biomarker. CIs that do not cross the null are significant at #P<0.1, *P<0.05, and **P<0.01. CI, confidence interval.
We also observed that some associations of phthalate (but not phthalate alternative) biomarkers with the Estrogen/Androgen ratio differed by gestational age and/or fetal sex (Figure 6). Negative associations of MBzP, MEP, and SumPCP with the Estrogen/Androgen ratio were strongest in women carrying female fetuses at 25–33 or 32–40 weeks gestation (Figure 6E, I, L). However, associations of SumDEHP with Estrogen/Androgen ratio were positive in women carrying male fetuses and strongest at 32–40 weeks gestation (Figure 6A). In stratified analyses, associations of SumDiNP, MCNP, MCPP, SumPlastics, SumDiNCH, SumDEHTP, SumDBP, and SumDiBP with Estrogen/Androgen ratio did not differ by gestational age or fetal sex (Figures 6B, C, D, F, G, H, J, K).
4. DISCUSSION
4.1. Summary of major findings
Our study suggests that exposure to select phthalates in pregnancy is associated with higher maternal urinary SumEstrogens and SumTestosterones, and a lower Estrogen/Androgen ratio. Additionally, we found that two biomarkers of phthalate alternatives (SumDiNCH and SumDEHTP) were positively associated with SumEstrogens, but not with SumTestosterones or the Estrogen/Androgen ratio. Some associations of phthalate/alternative biomarkers with urinary hormones tended to be linear, with the strongest relationships observed at higher quartiles of phthalate or phthalate alternative biomarker concentrations. Importantly, many associations of phthalate and phthalate alternative biomarkers with SumEstrogens tended to be strongest in mid-to-late gestation and in women carrying females, while gestational age- and fetal sex-specific associations of phthalate/alternative biomarkers with SumTestosterones and Estrogen/Androgen ratio were less consistent. These findings further confirm that phthalates may have endocrine disrupting properties in pregnant women, which may have important public health implications for maternal and child life-long health. Our findings also support the need for additional studies evaluating the potential endocrine disrupting capacity of newer phthalate alternatives.
4.2. Assessment of gestational estrogens and testosterones in urine
Our study is one of few that measured gestational hormone metabolite concentrations in urine. Validation studies in pre-menopausal women found that urinary estrogen metabolite concentrations have high within-person reproducibility (Ziegler et al. 2010). Other studies in pre-menopausal women also suggest that associations of certain reproductive and lifestyle factors, including age at first birth and smoking status, with urinary estrogens are consistent with those assessing plasma or serum estrogens (Fortner et al. 2012; Gu et al. 2013). However, findings from other studies suggest that evaluating urinary hormones may require a different interpretation compared to those evaluating plasma or serum hormone concentrations. For example, in pre-menopausal women, breast cancer risk was not associated with luteal plasma estrogens, was positively associated with follicular plasma estrogens, but was negatively associated with urinary estrogens (Eliassen et al. 2012). Additionally, a nested case-control study of pregnant women found that women with pre-eclampsia had higher urinary estradiol concentrations than controls (Cantonwine et al. 2019), which is inconsistent with studies evaluating estradiol in serum. Authors hypothesized that urinary estrogens may be markers of hormone metabolism rather than direct measurements of circulating concentrations (Cantonwine et al. 2019), which further supports the use of summative measures of urinary estrogens rather than individual urinary metabolites. This is evident in studies assessing concentrations of estrone, estradiol, and estriol in blood or urine showing that estradiol and estriol are the parent estrogens with the highest concentrations in blood and urine, respectively (Mistry et al. 2015; Tal et al. 2000). Urine and blood likely capture different hormone forms where unconjugated hormones are measured in plasma or serum, while conjugated hormones are generally measured in urine (Eliassen et al. 2012). Whether this is also the case in pregnant women (where the placenta is the major source of steroid hormones) will need to be confirmed in additional studies. However, urine may allow researchers to measure different types of hormone metabolites that cannot be measured in plasma or serum. Compared to blood sampling, urine may also provide opportunities for more extensive cross-pregnancy assessment of hormonal disruption in response to environmental exposures. Despite this, while our study findings suggest that phthalates may disrupt maternal hormones, some caution may be warranted when directly comparing the directionality/magnitude of our findings to studies where hormones were measured in maternal circulation.
4.3. Phthalate/alternatives are endocrine disruptors that alter gestational hormone levels
Our findings related to urinary SumEstrogens are consistent with in vitro studies showing that phthalates are weakly estrogenic (Harris et al. 1997; Jobling et al. 1995), which is concerning since higher maternal third trimester circulating estrone and estradiol concentrations have been associated with higher risk of breast cancer in mothers years after pregnancy (Cohn et al. 2017). Our findings that some phthalates are associated with higher urinary SumTestosterones may be concerning given that elevated second and third trimester maternal testosterone levels may be associated with higher risk of pre-eclampsia and gestational diabetes (Morisset et al. 2013; Salamalekis et al. 2006). However, these findings in pregnant women are inconsistent with in vivo studies reporting anti-androgenic effects of maternal DEHP and DBP exposure in male offspring (Howdeshell et al. 2007; Parks et al. 2000) or reduced late pregnancy blood testosterone levels in pregnant dams with DEHP exposure (Saadeldin et al. 2018). These inconsistencies may be because these studies evaluated testosterone levels in male offspring rather than mothers or because they exposed pregnant dams to phthalates at doses irrelevant to humans (i.e. 100 mg/kg/day). It is also possible that urinary testosterone levels are not directly comparable to plasma testosterone concentrations, thus our findings should be corroborated using repeated plasma sampling. The Estrogen/Androgen ratio may be a relevant indicator of placental P450 aromatase activity, which is required to convert testosterone to estradiol (Kragie 2002), and our findings evaluating this ratio in urine may align with experimental models demonstrating that some phthalates reduce aromatase activity during gestation (Perez-Albaladejo et al. 2017). Most importantly, our study is one of the first to show that biomarkers of phthalate alternatives (SumDiNCH and SumDEHTP) may exert similar endocrine disrupting effects on gestational hormones as those of the phthalate parent compounds they replace (e.g. DEHP), which has not been shown in previous studies (Cathey et al. 2019; Engel et al. 2018). Given the importance of estrogens and testosterone for pregnancy outcomes, substantially more needs to be understood about the impacts of phthalates and their replacements on these hormones in pregnant women and the consequences of these disruptions for maternal and child health.
4.4. Associations of phthalate/alternative biomarkers with maternal hormones differed by gestational age
The current study appears to be the first to evaluate associations of phthalate/alternative biomarkers with urinary markers of early, middle, and late gestation estrogen and testosterone concentrations. Associations of phthalate biomarkers with urinary estrogens in mid-to-late gestation suggest that these exposures may be targeting placental hormonal pathways (Tal et al. 2000), which is consistent with experimental studies showing that phthalates modulate placental estrogen receptor activity and gene expression (Strakovsky and Schantz 2018). However, our results are somewhat inconsistent with those from other prospective pregnancy cohort studies (Banker et al. 2020; Cathey et al. 2019; Johns et al. 2015; Sathyanarayana et al. 2014; Sathyanarayana et al. 2017), which may be due to differences in assessing sex steroid concentrations in urine versus blood. One study of U.S. pregnant women found that urinary phthalate metabolites were positively associated with early pregnancy serum estrone or estradiol (consistent with our findings), but negatively associated with early pregnancy serum free testosterone (inconsistent with our findings) (Sathyanarayana et al. 2017). However, a recent study in Michigan pregnant women found that urinary phthalate metabolites were not associated with first trimester plasma estrone, estradiol, estriol, or testosterone, but observed a negative association between MBP and maternal plasma estrone measured before delivery (Banker et al. 2020). Additionally, in a cohort of Puerto Rican pregnant women, phthalate/alternative biomarkers were not associated with maternal second trimester serum estradiol or estriol, while MHBP and MEP were positively and negatively, respectively, associated with mid-pregnancy testosterone (Cathey et al. 2019; Johns et al. 2015). This same study also found that some associations of phthalate/alternative biomarkers with pregnancy serum estriol and testosterone differed by gestational age, such that positive and negative associations were observed in early and late second trimester, respectively (Cathey et al. 2019). However, given that estrogens and testosterones have specific patterns of increases across all pregnancy trimesters, our study design may better represent hormone disruption at three key gestational timepoints. Additionally, unlike the previous studies described here, we assessed phthalate metabolites in a pooled urine sample across pregnancy, which may more accurately represent average gestational exposure to these chemicals. Nevertheless, to corroborate our findings, additional studies are needed that simultaneously assess (and compare) urinary and blood hormone concentrations at multiple key timepoints in pregnancy that correspond to important developmental windows.
4.5. Associations of phthalate/alternative biomarkers with maternal hormones differed by fetal sex
Associations of prenatal phthalate metabolite concentrations with hormonally-driven pregnancy and birth outcomes, including pre-eclampsia, gestational diabetes, birth weight, and pre-term birth, often differ by fetal sex (Qian et al. 2020). However, prior to our study, only two studies of U.S. pregnant women evaluated fetal sex-specific associations of phthalates with gestational hormones, and their findings were inconsistent with ours (Banker et al. 2020; Sathyanarayana et al. 2014). In the multi-center cohort of U.S. women, SumDEHP was negatively associated with mid-to-late estradiol in women carrying females (Sathyanarayana et al. 2014). Additionally, this study found that SumDEHP, MBzP, and MBP were negatively associated with mid-to-late free/total testosterone in women carrying females, but MEP was positively associated with free/total testosterone in women carrying males (Sathyanarayana et al. 2014). However, a study of Michigan pregnant women found that associations of urinary phthalate metabolite concentrations with plasma estrone, estradiol, estriol, or testosterone measured during the first trimester or before delivery were not fetal sex-specific (Banker et al. 2020). In addition to differences in the biological medium used for hormone assessment, these inconsistencies may be related to exposure measurement since these studies quantified phthalate metabolites from individual spot urine samples collected during pregnancy. Additionally, these other studies may have been underpowered (n=180 for the multicenter U.S. cohort and n=121 for the Michigan cohort) to detect sex-specific associations of various phthalates biomarkers with hormones (Banker et al. 2020; Sathyanarayana et al. 2014). Consequently, future studies are needed to corroborate fetal sex-specific findings from both studies as well as from the current study.
4.6. Strengths and limitations
First, using a pooled sample to assess pregnancy exposure to phthalates/alternatives means that there is uncertainty regarding the directionality of associations as some hormone measures were obtained priori to some exposure measures. However, using a pooled sample of five first morning urines for quantification of nonpersistent chemicals reduces exposure measurement error, provides a more stable measure of mean gestational exposure, and may, in fact, be a better reflection of exposure at any given timepoint during pregnancy (Shin et al. 2019; Vernet et al. 2019). Second, while we did not validate urinary gestational sex-steroid hormones with those measured in serum or plasma, other studies in non-pregnant populations have established urine as a reliable biomarker for sex-steroid hormone assessment (Coburn et al. 2019). Third, although urinary hormones and their metabolites may not directly reflect hormone levels in circulation, we only evaluated associations of phthalate/alternative biomarkers with the sum of estrogen and testosterone metabolites to characterize maternal hormonal disruption (Eliassen et al. 2012). Fourth, our findings may not be generalizable to more diverse population since our midwestern U.S. population of pregnant women is predominately white and highly educated. However, this rather homogenous population allows us to easily evaluate and propose biological pathways that can later be confirmed in more diverse populations and in appropriate experimental models. Fifth, while we accounted for urine dilution (i.e. hydration status) by specific gravity adjusting urinary analyte concentrations, specific gravity can vary by physiologic factors such body composition (MacPherson et al. 2018). However, specific gravity has low within-person variability, which makes it the more favorable marker of hydration status in pregnant populations relative to creatinine or osmolality (MacPherson et al. 2018). Sixth, while there may be residual or unmeasured confounding unaccounted for in our statistical models, we used a priori consideration and previous literature to make informed decisions about covariate selection. Seventh, there may be increased type I error because we did not adjust for multiple comparisons. However, our focus was on a qualitative interpretation of the findings, especially the stratified results, to identify trends in associations of phthalates/alternatives with hormones by gestational age at hormone assessment and fetal sex that will guide future research (Rothman 1990). Lastly, our study was limited to estrogens and testosterones based on previous experimental studies, but studies in pregnant women suggest that phthalates can also alter maternal thyroid hormone, progesterone, and corticotropin-releasing hormone levels (Cathey et al. 2019; Johns et al. 2015). Given that phthalates may impact multiple hormonal pathways, future studies may be needed that consider a larger array of gestational hormones.
5. CONCLUSIONS
To our knowledge, this is the first study to evaluate associations of phthalate and phthalate alternative biomarkers with cross-pregnancy gestational sex-steroid hormones measured in urine. Our study suggests that phthalates and their replacements may have endocrine disrupting capacity during pregnancy, and that some of these associations differ by gestational age and fetal sex. Interestingly, our findings combined with results from previously published experimental and observational studies suggest that the direction of these associations remains inconsistent, likely because of numerous factors related to study design and data analysis. Given that pregnant women are exposed to multiple phthalates/alternatives, future studies should evaluate the combined or interactive effects of multiple phthalates/alternatives on gestational hormone concentrations. Most importantly, because altered gestational sex-steroid hormone levels are associated with numerous adverse pregnancy and fetal outcomes, pregnant women may benefit from limiting their use of phthalate and phthalate replacement-containing products during pregnancy.
Supplementary Material
Highlights.
Cross-pregnancy urinary sum estrogens & testosterones (E&T) mirror blood patterns.
Plasticizer phthalates/replacements associated with increased sum E.
Associations with sum E strongest in later gestation in women carrying females.
Personal care phthalates associated with higher sum T and lower E/T ratio.
Associations with sum T and E/T ratio are not gestational age- or sex-specific.
Funding sources:
This publication was made possible by the National Institute for Environmental Health Sciences (NIH/NIEHS) grant ES024795, ES032227, ES022848, the U.S. Environmental Protection Agency grant RD83543401, and National Institute of Health Office of the Director grant OD023272. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or NIH. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. This project was also supported by the USDA National Institute of Food and Agriculture and Michigan AgBioResearch.
Abbreviations:
- AHEI-2010
Alternative Healthy Eating Index 2010
- BMI
body mass index
- CI
confidence interval
- CDC
Centers for Disease Control and Prevention
- FFQ
food frequency questionnaire
- I-KIDS
Illinois Kids Development Study
- MBP
mono-n-butyl phthalate
- MBzP
monobenzyl phthalate
- MCNP
monocarboxynonyl phthalate
- MCOCH
cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester
- MCOP
monocarboxyoctyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MECPTP
mono(2-ethyl-5-carboxypentyl) terephthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEHHTP
mono(2-ethyl-5-hydroxyhexyl) terephthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MEP
monoethyl phthalate
- MHBP
mono-hydroxybutyl phthalate
- MHiBP
mono-hydroxy-isobutyl phthalate
- MHiNCH
cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester
- MiBP
mono-isobutyl phthalate
- MiNP
mono-isononyl phthalate
- MONP
monooxononyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- Q1
quartile 1
- Q2
quartile 2
- Q3
quartile 3
- Q4
quartile 4
- SumDBP
sum of di-n-butyl phthalate metabolites
- SumDEHP
sum of di(2-ethylhexyl) phthalate metabolites
- SumDEHTP
sum of di(2-ethylhexyl) terephthalate metabolites
- SumDiBP
sum of di-iso-butyl phthalate metabolites
- SumDiNCH
sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites
- SumDiNP
sum of di-isononyl phthalate metabolites
- SumEstrogens
sum of the eight major urinary estrogen metabolites
- SumPCP
sum of phthalate metabolites of parent compounds found in personal care products
- SumPlastics
sum of phthalate metabolites of parent compounds found in plastics
- SumTestosterones
sum of the two major testosterone metabolites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Statement of ethics: Authors have no conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Centers for Disease Control and Prevention (CDC). National report on human exposure to environmental chemicals, updated tables january 2019. Available at https://www.Cdc.Gov/exposurereport/index.Html.
- Centers for Disease Control and Prevention (CDC). 2015–2016. National health and nutrition examination survey data. National Center for Health Statistics (NCHS). Hyattsville, Maryland:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Banker M, Puttabyatappa M, O’Day P, Goodrich JM, Kelley AS, Domino SE, et al. 2020. Association of maternal-neonatal steroids with early pregnancy endocrine disrupting chemicals and pregnancy outcomes. J Clin Endocrinol Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AL, Waring ME, Leung K, Masiero JV, Stone JM, Scannell EC, et al. 2017. Comparison of self-reported and measured pre-pregnancy weight: Implications for gestational weight gain counseling. Matern Child Health J 21:1469–1478. [DOI] [PubMed] [Google Scholar]
- Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. 2017. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J Hazard Mater 340:360–383. [DOI] [PubMed] [Google Scholar]
- Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. 2006. Validity and reliability of the block98 food-frequency questionnaire in a sample of canadian women. Public Health Nutr 9:84–93. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, McElrath TF, Trabert B, Xu X, Sampson J, Roberts JM, et al. 2019. Estrogen metabolism pathways in preeclampsia and normal pregnancy. Steroids 144:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, Watkins D, Rosario ZY, Velez C, Alshawabkeh AN, Cordero JF, et al. 2019. Associations of phthalates and phthalate replacements with crh and other hormones among pregnant women in puerto rico. J Endocr Soc 3:1127–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn SB, Stanczyk FZ, Falk RT, McGlynn KA, Brinton LA, Sampson J, et al. 2019. Comparability of serum, plasma, and urinary estrogen and estrogen metabolite measurements by sex and menopausal status. Cancer Causes Control 30:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Hopper BR, Siiteri PK. 2017. Third trimester estrogens and maternal breast cancer: Prospective evidence. J Clin Endocrinol Metab 102:3739–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Consumer Product Safety Commission. 2017. Prohibition of children’s toys and child care articles containing specified phthalates: Determinations regarding certain plastics. 2017–18387. [PubMed]
- Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, et al. 2012. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res 72:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Buhrke T, Kasper S, Behr AC, Braeuning A, Jessel S, et al. 2018. The urinary metabolites of dinch((r)) have an impact on the activities of the human nuclear receptors eralpha, erbeta, ar, pparalpha and ppargamma. Toxicol Lett 287:83–91. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. 2014. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortner RT, Hankinson SE, Schairer C, Xu X, Ziegler RG, Eliassen AH. 2012. Association between reproductive factors and urinary estrogens and estrogen metabolites in premenopausal women. Cancer Epidemiol Biomarkers Prev 21:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Caporaso NE, Schairer C, Fortner RT, Xu X, Hankinson SE, et al. 2013. Urinary concentrations of estrogens and estrogen metabolites and smoking in caucasian women. Cancer Epidemiol Biomarkers Prev 22:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Moran V, Leathard HL, Coley J. 2001. Urinary hormone levels during the natural menstrual cycle: The effect of age. J Endocrinol 170:157–164. [DOI] [PubMed] [Google Scholar]
- Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, et al. 2017. Association of prenatal urinary phthalate metabolite concentrations and childhood bmi and obesity. Pediatr Res 82:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, Henttu P, Parker MG, Sumpter JP. 1997. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105:802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E, Moore Simas TA, Doyle Curiale DK, Liao X, Waring ME. 2013. Self-reported pre-pregnancy weight versus weight measured at first prenatal visit: Effects on categorization of pre-pregnancy body mass index. Matern Child Health J 17:1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE Jr. 2007. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: Altered fetal steroid hormones and genes. Toxicol Sci 99:190–202. [DOI] [PubMed] [Google Scholar]
- Ipapo KN, Factor-Litvak P, Whyatt RM, Calafat AM, Diaz D, Perera F, et al. 2017. Maternal prenatal urinary phthalate metabolite concentrations and visual recognition memory among infants at 27 weeks. Environ Res 155:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. 1995. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect 103:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV, et al. 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: A longitudinal analysis. Reprod Biol Endocrinol 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragie L 2002. Aromatase in primate pregnancy: A review. Endocr Res 28:121–128. [DOI] [PubMed] [Google Scholar]
- MacPherson S, Arbuckle TE, Fisher M. 2018. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 28:481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makieva S, Saunders PT, Norman JE. 2014. Androgens in pregnancy: Roles in parturition. Hum Reprod Update 20:542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. 2006. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect 114:805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. 2002. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am J Clin Nutr 76:1261–1271. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. 2009. Urinary phthalate metabolites in relation to preterm birth in mexico city. Environ Health Perspect 117:1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry HD, Eisele N, Escher G, Dick B, Surbek D, Delles C, et al. 2015. Gestation-specific reference intervals for comprehensive spot urinary steroid hormone metabolite analysis in normal singleton pregnancy and 6 weeks postpartum. Reprod Biol Endocrinol 13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset AS, Dube MC, Drolet R, Pelletier M, Labrie F, Luu-The V, et al. 2013. Androgens in the maternal and fetal circulation: Association with insulin resistance. J Matern Fetal Neonatal Med 26:513–519. [DOI] [PubMed] [Google Scholar]
- Natamba BK, Sanchez SE, Gelaye B, Williams MA. 2016. Concordance between self-reported pre-pregnancy body mass index (bmi) and bmi measured at the first prenatal study contact. BMC Pregnancy Childbirth 16:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyola-Martinez N, Halhali A, Barrera D. 2019. Steroid hormones and pregnancy. Gynecol Endocrinol 35:376–384. [DOI] [PubMed] [Google Scholar]
- O’Leary P, Boyne P, Flett P, Beilby J, James I. 1991. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem 37:667–672. [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58:339–349. [DOI] [PubMed] [Google Scholar]
- Perez-Albaladejo E, Fernandes D, Lacorte S, Porte C. 2017. Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in jeg-3 human placental cells. Toxicol In Vitro 38:41–48. [DOI] [PubMed] [Google Scholar]
- Qian Y, Shao H, Ying X, Huang W, Hua Y. 2020. The endocrine disruption of prenatal phthalate exposure in mother and offspring. Front Public Health 8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. 1990. No adjustments are needed for multiple comparisons. Epidemiology 1:43–46. [PubMed] [Google Scholar]
- Saadeldin IM, Hussein MA, Suleiman AH, Abohassan MG, Ahmed MM, Moustafa AA, et al. 2018. Ameliorative effect of ginseng extract on phthalate and bisphenol a reprotoxicity during pregnancy in rats. Environ Sci Pollut Res Int 25:21205–21215. [DOI] [PubMed] [Google Scholar]
- Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. 2006. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol 126:16–19. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. 2014. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction 147:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, et al. 2017. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab 102:1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso HA, Idahl A, et al. 2016. Hormone concentrations throughout uncomplicated pregnancies: A longitudinal study. BMC Pregnancy Childbirth 16:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, et al. 2019. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int 122:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860:106–112. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Schantz SL. 2018. Using experimental models to assess effects of bisphenol a (bpa) and phthalates on the placenta: Challenges and perspectives. Toxicol Sci 166:250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succop PA, Clark S, Chen M, Galke W. 2004. Imputation of data values that are less than a detection limit. J Occup Environ Hyg 1:436–441. [DOI] [PubMed] [Google Scholar]
- Tal R, Taylor HS, Burney RO, Mooney SB, Giudice LC. 2000. Endocrinology of pregnancy. In: Endotext, (Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., eds). South Dartmouth (MA). [Google Scholar]
- Toft G, Jonsson BA, Lindh CH, Jensen TK, Hjollund NH, Vested A, et al. 2012. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect 120:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, et al. 2019. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology 30:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel C, Salinas A, Lopez P, Kohen P, Rencoret G, Devoto L, et al. 2017. Pregestational type 2 diabetes and gestational diabetes exhibit different sexual steroid profiles during pregnancy. Gynecol Endocrinol 33:212–217. [DOI] [PubMed] [Google Scholar]
- Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. 2012. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol 258:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Arbuckle TE, Fisher M, Ramsay T, Mallick R, Hauser R, et al. 2015. Temporal variability and sources of triclosan exposure in pregnancy. Int J Hyg Environ Health 218:507–513. [DOI] [PubMed] [Google Scholar]
- Wesselink AK, Fruh V, Hauser R, Weuve J, Taylor KW, Orta OR, et al. 2020. Correlates of urinary concentrations of phthalate and phthalate alternative metabolites among reproductive-aged black women from detroit, michigan. J Expo Sci Environ Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. 2005. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem 77:6646–6654. [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Faupel-Badger JM, Sue LY, Fuhrman BJ, Falk RT, Boyd-Morin J, et al. 2010. A new approach to measuring estrogen exposure and metabolism in epidemiologic studies. J Steroid Biochem Mol Biol 121:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environ Health Perspect 122:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


