Abstract
Background:
High-flow nasal oxygen (HFNO) for treatment of adults with acute respiratory failure (ARF) has increased.
Purpose:
Assess HFNO versus noninvasive ventilation (NIV) or conventional oxygen therapy (COT) for ARF in hospitalized adults.
Data Sources:
English language searches of MEDLINE®, Embase, CINAHL, and Cochrane Library from January 2000 to July 2020; systematic review reference lists.
Study Selection:
Twenty-nine randomized controlled trials (RCTs) evaluated HFNO versus NIV (k=11) or COT (k=21).
Data Extraction:
Data extraction by single investigator verified by a second; dual-investigator assessment of risk of bias; consensus determination of evidence certainty.
Data Synthesis:
We reported results separately for HFNO versus NIV and HFNO versus COT and by initial or post-extubation management. Compared to NIV, HFNO may reduce all-cause mortality, intubation, and hospital-acquired pneumonia and improve patient comfort in initial ARF management (low evidence certainty), but not as post-extubation management. Compared to COT, HFNO may reduce reintubation and improve patient comfort in post-extubation ARF management (low evidence certainty).
Limitations:
Trials varied in populations enrolled, ARF etiologies, and treatment protocols. Trial design, sample size, treatment/follow-up duration, and results reporting were often inadequate to adequately assess many outcomes. Protocols, clinician/health system training, cost and resource use were poorly characterized.
Conclusion:
Compared to NIV, HFNO as initial ARF management may improve several clinical outcomes. Compared to COT, HFNO as post-extubation management may reduce reintubations and improve patient comfort. HFNO resulted in fewer harms than NIV or COT. Broad applicability, including required clinician and health system experience and resource use, is not well known.
INTRODUCTION
Acute respiratory failure (ARF) is generally defined as the new onset of clinically important hypoxia, hypercapnia, or both. Noninvasive respiratory treatment options for ARF vary by etiology and severity, and include “conventional oxygenation therapy” (COT)–oxygen delivered through nasal cannula, simple face mask, air-entrainment mask, partial rebreathing mask, or non-rebreather mask, with maximum flow rate of approximately 15 L/min–and more advanced support modalities such as noninvasive ventilation (NIV). NIV encompasses continuous or bilevel positive airway pressure ventilation and requires specialized training and equipment to deliver. High-flow nasal oxygen (HFNO), a newer mode of noninvasive oxygen support, has been increasingly used, in part due to perceived benefits in comparison to COT and NIV. COT, NIV, and HFNO have unique characteristics related to user interface, inspired oxygen concentration and flow rate, heat/humidification, use of positive pressure and ventilatory support(1) (Appendix Table 1).
Compared to COT, HFNO is purported to provide additional support through washout of anatomic dead space(2), higher oxygen flow rates (up to 60 L/min)(3,4), generation of low level positive-end expiratory pressure (PEEP)(5–9), and higher concentrations of heated humidified oxygen (up to 100% FiO2). Compared to NIV, which is typically delivered by full face mask, HFNO is delivered through a small, pliable nasal cannula, potentially improving clearance of secretions, patient comfort, and resource utilization. HFNO is considered to offer a number of physiologic advantages, such as improved oxygenation and ventilation(10,11). However, comparative benefits and harms of HFNO on clinical outcomes including mortality, intubation, hospital length of stay, patient comfort(12–14), clearance of airway secretions(15,16), and reduced work of breathing(13,17,18) are not well known.
The Minnesota Evidence Synthesis and Dissemination Center was commissioned by the American College of Physicians (ACP) to review the evidence regarding the comparative effectiveness and harms of HFNO compared to NIV or COT for ARF in hospitalized adults. This review was used by the ACP-Clinical Guidelines Committee (ACP-CGC) to develop a clinical guideline for the use of HFNO in hospitalized adults with ARF.
METHODS
Our protocol was developed with input from the ACP-CGC as well as an independent technical expert panel and registered in PROSPERO (CRD42019146691). Our protocol underwent additional peer review and was published(19). A summary is presented in Appendix Table 2.
Data Sources and Study Selection
We searched multiple databases (January 2000-July 2020) for peer reviewed, English language, randomized controlled trials (RCTs) (Appendix Table 3). Abstracts and potentially eligible full text articles were independently reviewed by 2 investigators. We included parallel group and crossover studies of hospitalized adults (age ≥18 years) with ARF randomized to receive HFNO or either COT or NIV. We defined HFNO as delivery of humidified oxygen via nasal cannula at a flow rate ≥20 L/min. We excluded studies evaluating HFNO for oxygenation support before and during intubation and studies of pre-hospital HFNO. We included studies if ≥75% of enrollees met at least one ARF criterion: SpO2<90%, PaO2:FIO2 ratio≤300, PaO2≤60 mmHg, or PaCO2≥45 mmHg.
Outcome Measures
Critical outcomes defined by the ACP-CGC were: all-cause mortality (in-hospital and the longest available through 90 days), hospital-acquired pneumonia, intubation/reintubation (days of intubation), intensive care unit (ICU) admission/transfers, patient comfort, and hospital length of stay. Important and intermediate outcomes are described in Appendix Table 2.
Data Extraction and Quality Assessment
Data extraction was completed by one investigator and verified by a second. We assessed risk of bias using a modification of the Cochrane guidance for randomized trials(20). Individual elements were rated low, unclear, or high risk of bias. A study with unclear elements was considered moderate risk of bias.
Data Synthesis and Analysis
We examined clinical and methodological heterogeneity to determine appropriateness of quantitative synthesis. Heterogeneity was assessed using the I2 statistic, Chi-squared test, and visual inspection of the forest plots. An I2 statistic of 75% or greater may indicate substantial heterogeneity. We pooled outcomes from clinically homogeneous studies using Comprehensive Meta Analysis V.3 or R. We calculated risk ratios (RR) or Peto odds ratios (OR) and corresponding 95% confidence intervals for categorical outcomes. The Peto method was applied when events were rare, particularly when trials reported zero events in one of the treatment arms(21). Mean and standardized mean differences (MD, SMD) were calculated for continuous outcomes. If there were at least 5 trials for pooled analysis, the Hartung–Knapp–Sidik–Jonkman method for random-effects models was applied to calculate SMD for continuous outcomes and relative measures of effect for categorical outcomes with corresponding 95% CI(22). If there were fewer than 5 trials and no between-study variance (tau2 at or near 0) data were meta-analyzed with a fixed-effects model(23). When there were no events in a treatment arm, we used the treatment arm continuity correction. Anticipated absolute event rates and corresponding risk differences were generated in GRADEpro software(24,25). In addition, we calculated pooled absolute event rates and 95% CIs for the primary harm outcomes for each study group using the Freeman-Tukey double arcsine transformation(26).
We analyzed results separately for studies of initial ARF management and studies of post-extubation ARF. We conducted subgroup analyses to explore potential causes of heterogeneity by clinical setting, disease indication, treatment duration, and ARF type. If quantitative synthesis was not appropriate, findings were summarized narratively. We used a modification of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology to rate overall certainty of evidence for critical outcomes as high, moderate, low, or insufficient(24,25). At the request of the ACP-CGC, we also assessed certainty of evidence for skin breakdown. The thresholds indicating level of magnitude for our critical outcomes were derived through input by our content experts and technical expert panel (Tables 1 and 2).
Table 1.
Certainty of Evidence for HFNO versus NIV
| Outcome: Population № of participants (studies) References | Relative effect or Standardized mean difference (95% CI) | Anticipated absolute event rates* | Absolute risk difference (95% CI) | Certainty | What happens | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFNO | NIV | ||||||||||||
|
Initial management of acute respiratory failure population trials | |||||||||||||
| Intubation: 420 (2 RCTs) (32,34) | RR 0.71 (0.53 to 0.95) | 23.0% | 32.4% | −9.4% (−15.2 to −1.6) | Low†‡ | HFNO may reduce intubations by a moderate amount | |||||||
| All-cause Mortality: 216 (1 RCT) (34) | RR 0.44 (0.24 to 0.79) | 12.4% | 28.2% | −15.8% (−21.4 to −5.9) | Low§ | HFNO may reduce all-cause mortality by a large amount | |||||||
| Hospital-acquired Pneumonia: 216 (1 RCT) (34) |
RR 0.46 (0.15 to 1.45) |
3.8% | 8.2% | −4.4% (−7.0 to 3.7) |
Low‖ | HFNO may reduce hospital-acquired pneumonia by a moderate amount. | |||||||
| ICU Admissions (yes/no): 204 (1 RCT) (32) |
RR 0.98 (0.73 to 1.32) |
46.1% | 47.0% | −0.9% (−12.2 to 15.0) |
Insufficient†‡¶ | It is uncertain if HFNO reduces ICU admissions. | |||||||
| Length of stay, ICU: 420 (2 RCTs) (32,34) |
Mean (days) 7.7 |
Mean (days) 8.3 |
MD −0.64 days (−1.67 to 0.39) |
Insufficient†‡¶ | It is uncertain if HFNO reduces ICU length of stay. | ||||||||
| Length of stay, hospital: 372 (2 RCTs) (29,32) |
Mean (days) 11.6 |
Mean (days) 11.1 |
MD 0.45 days (−0.69 to 1.59) |
Low†‡ | HFNO may make little or no difference in hospital length of stay. | ||||||||
| Patient comfort, including related to dryness, based on VAS or % improved: 644 (7 RCTs) (29,32,34,35,49,50,54) |
One trial (n=216) (34) reported HFNO improved comfort (SMD −0.51 [−0.78 to −0.24]) based on an unmarked 100 mm VAS and 1 (n=168) (29) reported higher percentage of patients feeling comfort with HFNO (88.2% vs. 67.9%; ARD 21.4% [9.4 to 33.4]). One trial (n=204) (32) reported little to no difference in patient comfort based on a 5-point VAS (medians 2 vs. 2 on scale, 5=most discomfort). Among 4 crossover trials (n=56), 3 reported little to no difference (35,50,54) and 1 reported improvement with HFNO in short-term patient comfort based on a 10-point numeric rating scale (49). | Low**†† | HFNO may improve patient comfort. | ||||||||||
| Dyspnea, based on VAS or Borg scale scores or % improved: 464 (7 RCTs) (32,34,35,48–50,54) |
One trial (n=177) (34) reported greater improvement in dyspnea short-term in patients allocated to HFNO compared with NIV (75.6% vs. 58.2%; ARD 17.3% [3.7 to 30.9]). One trial (n=180) (32) reported little to no difference in longer-term (SMD 0.21 [−0.12 to 0.54] dyspnea based on Borg. One trial (n=51) (48) reported little to no difference in short-term dyspnea based on 10-point VAS scale (mean change from baseline −0.1 vs. −0.9). Among 4 crossover trials (n=56), 2 reported little to no difference based on VAS, (50,54) 1 reported worsening based on VAS,(35) and 1 reported improvement in short-term dyspnea based on Borg with HFNO.(49) | Low**†† | HFNO may make little or no difference in dyspnea. | ||||||||||
| Skin breakdown (facial pressure sore or nasal ulceration): | Not reported | ||||||||||||
|
Post-extubation acute respiratory failure population trials | |||||||||||||
| Reintubation: 1476 (3 RCTs) (37,39,53) |
RR 1.13 (0.90 to 1.43) |
17.3% | 15.3% | 2.0% (−1.5 to 6.6) |
Low‖ | HFNO may increase reintubations by a small amount | |||||||
| All-cause Mortality: 1476 (3 RCTs) (37,39,53) |
RR 1.15 (0.88 to 1.51) |
12.9% | 11.2% | 1.7% (−1.3 to 5.7) |
Low‖ | HFNO may increase all-cause mortality by a small amount | |||||||
| Hospital-acquired Pneumonia: 1434 (2 RCTs) (37,53) |
RR 0.90 (0.70 to 1.16) |
13.2% | 14.7% | −1.5% (−4.4 to 2.3) |
Low‖ | HFNO may make little to no difference in hospital-acquired pneumonia | |||||||
| ICU Admissions (yes/no) |
Not applicable | ||||||||||||
| Length of stay, ICU: 1476 (3 RCTs) (37,39,53) |
Pooled mean differences from 2 trials (37,39) (n=646) found HFNO made little or no difference in ICU length of stay (mean days NA; MD −0.98 days [−1.99 to 0.03]. One trial (n=830) reported little to no difference in ICU length of stay (medians 6 vs. 6 days).(53) | Low‖ | HFNO may make little to no difference in ICU length of stay | ||||||||||
| Length of stay, hospital: 1434 (2 RCTs) (37,53) |
One trial (n=604) (37) reported a lower hospital length of stay with HFNO (medians 23 vs. 26 days; MD −3 days [−6.8 to −0.8]). One trial (n=830) (53) reported little to no difference in hospital length of stay (medians 13 vs. 14 days). | Insufficient‖** | It is uncertain if HFNO reduces hospital length of stay. | ||||||||||
| Patient comfort, based on % improved or VAS: 872 (2 RCTs) (39,53) |
One large trial (n=748) (53) reported little to no difference in the percentage of participants reporting good comfort (51.3% vs. 52.9%; ARD −1.6% [−8.7 to 5.6]). One small trial (n=42) (39) found HFNO may improve comfort (SMD −0.75 [−1.38 to −0.12]) based on VAS. | Low**†† | HFNO may make little or no difference in patient comfort. | ||||||||||
| Dyspnea, based on % improved: 752 (1 RCT) (53) |
RR 0.96 (0.86 to 1.08) |
58.0% | 60.4% | −2.4% (−8.5 to 4.8) |
Low‖ | HFNO may make little or no difference in dyspnea. | |||||||
| Skin breakdown (facial pressure sore or nasal ulceration): 1454 (3 RCTs) (37,39,53) |
Peto OR 0.15 (0.02 to 1.13) |
4.6% | 24.3% | −19.7% (−23.7 to 2.3) |
Low‡** | HFNO may reduce skin breakdowns by a large amount | |||||||
Abbreviations
ARD=absolute risk difference; CI=confidence interval; HFNO=high flow nasal oxygen; ICU=intensive care unit; MD=mean difference; NA=not available; NIV=noninvasive ventilation; OR=odds ratio; RCT=randomized controlled trial; RR=risk ratio; SMD=standardized mean difference; VAS=visual analog scale
GRADES of certainty of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate
certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Insufficient certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Thresholds for determining magnitude by outcome
Intubation: Little or no effect: <2%; Small effect: 2–3.9%; Moderate effect: 4–9.9%; Large effect ≥10%
All-cause mortality: Little or no effect: <1%; Small effect: 1–1.9%; Moderate effect: 2–4.9%; Large effect: ≥5%
Pneumonia: Little or no effect: <2%; Small effect: 2–3.9%; Moderate effect: 4–9.9%; Large effect: ≥10%
Length of Stay: Little or no effect: <1 day; Small effect: ≥1 day; Moderate effect: NA; Large effect: ≥3 day
Skin breakdown: Little or no effect: <2%; Small effect: 2–3.9%; Moderate effect: 4–9.9%; Large effect: ≥10%
Pooled event rates calculated with Freeman-Tukey double arcsine variance-stabilizing transformation can be found in Supplementary Table 11
Explanations
Downgraded for study limitations, particularly moderate attrition and/or unclear allocation concealment
Downgraded for imprecision (wide CIs)
Downgraded two levels based on results derived from one trial (n=216) and imprecision, difficult to determine if there is a definitive benefit based on only a single study.
Downgraded two levels for imprecision (very wide CIs) and/or difficult to interpret based on the variability in the reporting of the effects
Downgraded for indirectness, ICU stay possibly protocol driven
Downgraded due to inconsistency
Downgraded due to imprecision, difficult to interpret based on the variability in the reporting of the effects
Table 2.
Certainty of Evidence for HFNO versus COT
| Outcome: Population № of participants (studies) References |
Relative effect or Standardized mean difference (95% CI) |
Anticipated absolute event rates* |
Absolute risk difference (95% CI) |
Certainty | What happens | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFNO | COT | ||||||||||||
|
Initial management of acute respiratory failure population trials | |||||||||||||
| Intubation: 1694 (8 RCTs) (27,28,34,40,41,43,46,52) |
Peto OR 0.98 (0.34 to 2.82) |
26.1% | 26.5% | −0.4% (−15.6 to 23.9) |
Low†‡ | HFNO may make little or no difference in intubation | |||||||
| All-cause Mortality: 1407 (4 RCTs) (27,34,40,43) |
RR 0.97 (0.82 to 1.14) |
26.3% | 27.2% | −0.8% (−4.9 to 3.8) |
Low†‡ | HFNO may make little or no difference in all-cause mortality | |||||||
| Hospital-acquired Pneumonia: 200 (1 RCT) (34) |
RR 0.44 (0.14 to 1.43) |
3.8% | 8.5% | −4.7% (−7.3 to 3.7) |
Low§ | HFNO may result in a reduction in hospital-acquired pneumonia by a moderate amount | |||||||
| ICU Admissions (yes/no): 403 (2 RCTs) (28,40) |
RR 1.11 (0.58 to 2.12) |
8.8% | 7.9% | 0.9% (−3.3 to 8.8) |
Insufficient†‡‖ |
It is uncertain if HFNO reduces ICU admissions. | |||||||
| Length of stay, ICU: 1036 (3 RCTs) (27,34,44) |
Two trials (n=976) (27,34) found little or no difference in ICU length of stay (mean days NA; MD 0.41 days [−1.08 to 1.90]). One trial (n=60) reported little to no difference in ICU length (P=.20, no data reported).(44) | Insufficient†‡‖ | It is uncertain if HFNO reduces ICU length of stay | ||||||||||
| Length of stay, hospital: 1267 (4 RCTs) (27,40,43,44) |
Four trials reported little to no difference in hospital length of stay based on medians (medians ranged from 1 to 24 vs. 1 to 27 days) (27,40,43) and/or p-values.(44) | Low§ | HFNO may make little or no difference in hospital length of stay | ||||||||||
| Patient comfort, including comfort related to dryness, based on VAS or % improved: 1611 (12 RCTs total, some trials reported ≥1 measure of comfort) (27,28,31,34,40,41,43,45,46,49,52,54) |
Pooled results from 4 trials (n=415) (34,43,46,52) found HFNO improved comfort (SMD −0.61 [−0.81 to −0.41]) based on VAS. Results pertaining to patient comfort based on median or unclear (27) scale scores varied: 1 trial (n=100) (28) reported higher comfort based on a 5 point Likert scale (4 vs. 3 on a 5-point scale, 5=most comfort, P=.04) while 2 trials (n=876) (27,41) reported little to no difference in patient comfort on a 10-point scale (7.9 vs. 6.8, 10=perfect) (27) and medians 3 vs. 3 on a 10-point scale (10=worst).(41) One trial (n=158) (40) reported a lower percentage of participants with discomfort related to dryness (29.8% vs. 45.3%; ARD −15.5% [−30.8 to −0.2]). Four small crossover studies (n=62) (31,45,49,54) reported little to no difference in short-term patient comfort. | Low¶ ** | HFNO may improve patient comfort. | ||||||||||
| Dyspnea, based on VAS and Borg scale scores or % improved: 1799 (13 RCTs) (27,28,31,34,40,41,43,45–47,49,52,54) |
Pooled results from 4 trials (n=258) (43,46,47,52) found HFNO improved dyspnea (SMD −0.56 [−1.35 to 0.24]) based on VAS and Borg scales. Two trials (n=876) (27,41) reported little to no difference in short-term dyspnea based on median scale scores (medians 2.3 to 3 vs. 2.6 to 3 on a 10-point scale, 10=most severe). Three trials (n=417) (28,34,40) reported a greater percentage of participants with improvement in dyspnea or improved breathing (78.0% vs. 55.8%; ARD 22.2% [13.3 to 31.1]). Four small crossover studies (n=62) (31,45,49,54) reported little to no difference in short-term dyspnea. | Low¶ ** | HFNO may improve dyspnea. | ||||||||||
| Skin breakdown (facial pressure sore or nasal ulceration): 431 (2 RCTs) (40,43) |
Both trials reported no incidences of skin breakdown were observed with HFNO. For COT, one trial reported no incidences (40) and the other trial did not report this outcome.(43) | Insufficient†† | It is uncertain if HFNO reduces skin breakdown. | ||||||||||
|
Post-extubation acute respiratory failure population trials | |||||||||||||
| Reintubation: 1065 (7 RCTs) (30,38,42,51,55,56,58) |
Peto OR 0.60 (0.23 to 1.61) |
6.5% | 10.4% | −3.9% (−7.8 to 5.3) |
Low§ | HFNO may reduce reintubations by a small amount | |||||||
| All-cause Mortality: 782 (4 RCTs) (38,42,55,56) |
RR 1.01 (0.60 to 1.72) |
6.3% | 6.2% | 0.1% (−2.5 to 4.5) |
Low§ | HFNO may make little or no difference in all-cause mortality | |||||||
| Hospital-acquired Pneumonia: 527 (1 RCT) (38) |
RR 0.50 (0.13 to 1.97) |
1.1% | 2.3% | −1.1% (−2.0 to 2.2) |
Low§ | HFNO may make little or no difference in hospital-acquired pneumonia | |||||||
| ICU Admissions (yes/no) |
Not applicable | ||||||||||||
| Length of stay, ICU: 1006 (6 RCTs) (30,38,42,55,56,58) | Pooled results from 5 trials (n=479) (30,42,55,56,58) found HFNO makes little or no difference in ICU length of stay (approximately 6 days in each group; MD 0.19 days [−0.19 to 0.57]. One trial not pooled (n=527) (38) reported little to no difference in ICU length of stay (medians 6 vs. 6 days). | Moderate‡ | HFNO probably makes little or no difference in ICU length of stay | ||||||||||
| Length of stay, hospital: 587 (2 RCTs) (38,56) |
Study 1
Median 11 (IQR 6 to 15) Study 2 Mean 37.7 |
Study 1 Median 12 (IQR 6 to 16) Study 2 Mean 25.7 |
Study 1 (38) MD 4 days (−28 to 32) Study 2 (56) MD 12 days (0.15 to 23.85) |
Insufficient†† | It is uncertain if HFNO improves hospital length of stay. | ||||||||
| Patient comfort, including comfort related to dryness and interface, based on VAS or % improved: 324 (4 RCTs total, some trials reported ≥1 measure of comfort) (42,51,55,58) | One trial (n=105) (42) found HFNO improved comfort (SMD −0.70 [−1.10 to −0.31]) based on a 10 point VAS where lower is better. One trial (n=60) (51) reported higher comfort or less dryness with HFNO based on median scale scores. One trial (n=90) (55) reported a lower percentage of participants with discomfort related to dryness with HFNO (38.3% vs. 69.8%; ARD −31.5% [−51.0 to −11.9]). Two trials (n=165) (42,52) reported lower discomfort related to interface with HFNO versus COT (Trial 1 SMD −0.89 [−1.29 to −0.49]) (42) and Trial 2 medians 3 vs. 7 on a 10-point scale, 10=maximal discomfort, P<.001).(51) One trial (n=69) (58) reported little to no difference in any measure of discomfort on a 10-point scale (nasal, oral, or pharynx) based on medians. | Low§ | HFNO may improve patient comfort. | ||||||||||
| Dyspnea, based Borg scale score: 155 (1 RCT) (30) |
One trial reported little to no difference in short-term dyspnea (medians 1 vs. 0 on a 10-point scale, 10=maximal dyspnea). | Insufficient†† | It is uncertain if HFNO improves dyspnea. | ||||||||||
| Skin breakdown (facial pressure sore or nasal ulceration): 527 (1 RCT) (38) |
One trial reported no incidences of skin breakdown were observed with HFNO but this outcome was not reported for the COT arm. | Insufficient†† | It is uncertain if HFNO reduces skin breakdown. | ||||||||||
Abbreviations
ARD=absolute risk difference; CI=confidence interval; COT=conventional oxygen therapy; HFNO=high flow nasal oxygen; ICU=intensive care unit; IQR=interquartile range; MD=mean difference; NA=not available; OR=odds ratio; RCT=randomized controlled trial; RR=risk ratio; SMD=standardized mean difference; VAS=visual analog scale
GRADES of certainty of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Insufficient certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Thresholds for determining magnitude by outcome
Intubation: Little or no effect: <2%; Small effect: 2–3.9%; Moderate effect: 4–9.9%; Large effect ≥10%
All-cause mortality: Little or no effect: <1%; Small effect: 1–1.9%; Moderate effect: 2–4.9%; Large effect: ≥5%
Pneumonia: Little or no effect: <2%; Small effect: 2–3.9%; Moderate effect: 4–9.9%; Large effect: ≥10%
Length of Stay: Little or no effect: <1 day; Small effect: ≥1 day; Moderate effect: NA; Large effect: ≥3 day
Skin breakdown: Little or no effect: <2%; Small effect: 2–3.9%; Moderate effect: 4–9.9%; Large effect: ≥10%
Pooled event rates calculated with Freeman-Tukey double arcsine variance-stabilizing transformation can be found in Supplementary Table 11.
Explanations
Downgraded for imprecision (wide CIs)
Downgraded for study limitations
Downgraded two levels for large imprecision (very wide CIs) and/or sparse data and/or difficult to interpret based on the variability in the reporting of the effects
Downgraded for indirectness, ICU stay possibly protocol driven
Downgraded due to inconsistency
Downgraded due to imprecision, difficult to interpret based on the variability in the reporting of the effect
Downgraded to insufficient based on the enormity of the imprecision or difficult to interpret based on the variability in the reporting of the effects
Role of Funding Source
This review was funded by a contract with the ACP. An ACP representative provided technical support and served as an ACP-CGC and technical expert panel liaison. The ACP-CGC assisted in the development of key questions, study inclusion criteria, and outcome measures of interest but did not participate in data collection, analysis, or manuscript preparation.
RESULTS
Search results are in Appendix Figure 1. We identified 29 eligible RCTs (in 32 articles)(27–58). An overview of included trials is presented in Appendix Table 4 and patient characteristics in Appendix Table 5. Patients typically had at least moderate ARF according to baseline PaO2/FIO2 ratio (<200) or SPO2 (≤88%). In the NIV parallel group studies, the baseline SpO2 weighted mean in the initial management trials was 76% while the baseline PaO2/FIO2 ratio weighted mean in the post-extubation trials was 198. In the COT parallel group studies, the baseline SpO2 weighted mean in the initial management trials was 88% while the baseline PaO2/FIO2 ratio weighted mean in the post-extubation trials was 227. Studies did not require patients to have failed initial oxygen therapy prior to randomization though information was sparse on pre-randomization oxygen treatments. Detailed study and treatment characteristics, individual study risk of bias, and outcomes data are reported in Supplementary Tables 1-10. We report results separately for studies comparing HFNO versus NIV and HFNO versus COT and by whether treatment was for initial or post-intubation ARF management. Treatment protocols varied by study based mostly on physiologic parameters, with most studies targeting SpO2 levels ≥92% (range 88–95%). Information from crossover studies was limited to comfort and dyspnea outcomes in initial management. Pooled absolute event rates within each study arm calculated by the Freeman-Tukey method are provided in Supplementary Table 11. Subgroup analyses for both NIV and COT controls are presented in Supplementary Tables 12-15. The effect of treatments did not differ significantly by clinical setting, disease indication, treatment duration, or type of ARF, although for most outcomes there were few or no studies available for these comparisons. Data on physiologic outcomes were inadequate to derive conclusions due to variable types and timing of physiologic data reported (Supplementary Tables 16-19). The greatest difference in physiologic outcomes was in PaO2/FiO2 ratio, particularly in post-extubation management, where post-treatment values were generally higher in NIV compared to HFNO (Supplementary Table 17) and in HFNO compared to COT (Supplementary Table 19).
HFNO versus NIV
Initial Management of Acute Respiratory Failure
Eight studies (4 parallel design and 4 crossover studies) compared HFNO to NIV for initial management of ARF among patients with multiple diagnoses(32,34,35,49,54), chronic obstructive pulmonary disease (COPD) (29), cystic fibrosis(50), and during bronchoscopy(48) (Appendix Tables 4 and 5). One of these studies reported outcomes on subgroups of acute decompensated heart failure (36) and COPD exacerbation or acute hypercapnic respiratory failure (57). Two were rated low risk of bias while 6 were rated moderate (Supplementary Table 2).
Critical Outcomes
Intubation
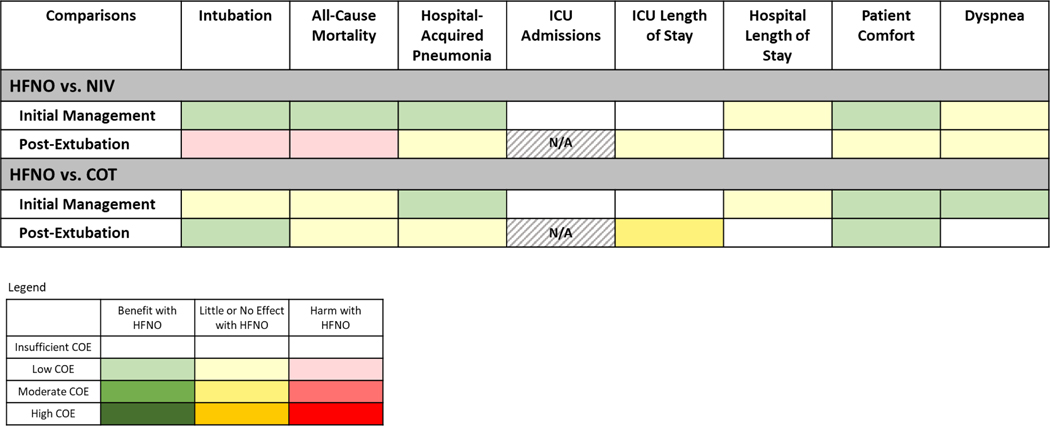
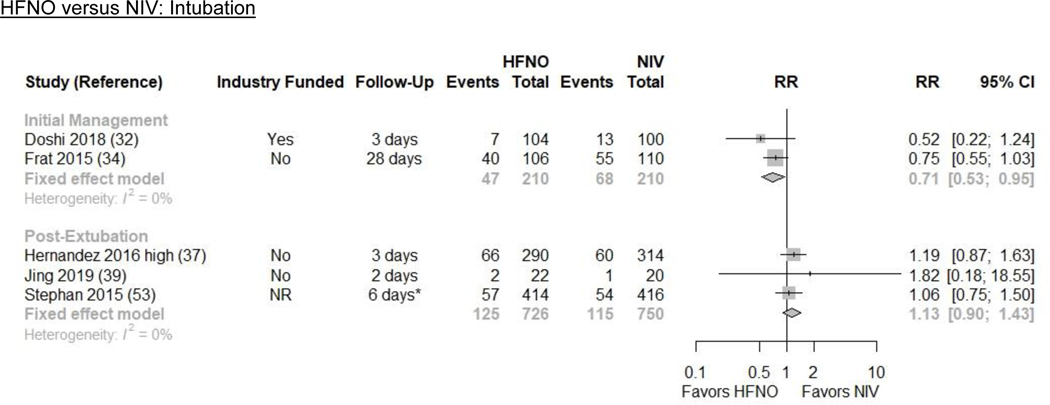
Pooled results from 2 RCTs (n=420) indicate that HFNO may reduce intubations by a moderate amount (23.0% vs. 32.4%; absolute risk difference [ARD] −9.4%, [−15.2, −1.6]) compared with NIV (RR 0.71 [0.53, 0.95]; I2=0%; low evidence certainty) (Figures 1 and 2/Table 1)(32,34).
Figure 1.
Map of Certainty of Evidence
Figure 2. Intubation and Mortality Plots for HFNO versus NIV.
CI=confidence interval; HFNO=high-flow nasal oxygen; ICU=intensive care unit; NIV=non-invasive ventilation; RR=risk ratio
*This is an estimated follow-up time based on the reported median ICU length of stay.
CI=confidence interval; HFNO=high-flow nasal oxygen; ICU=intensive care unit; NIV=non-invasive ventilation; RR=risk ratio*These are estimated follow-up times based on the reported median hospital or ICU length of stay.
All-cause Mortality
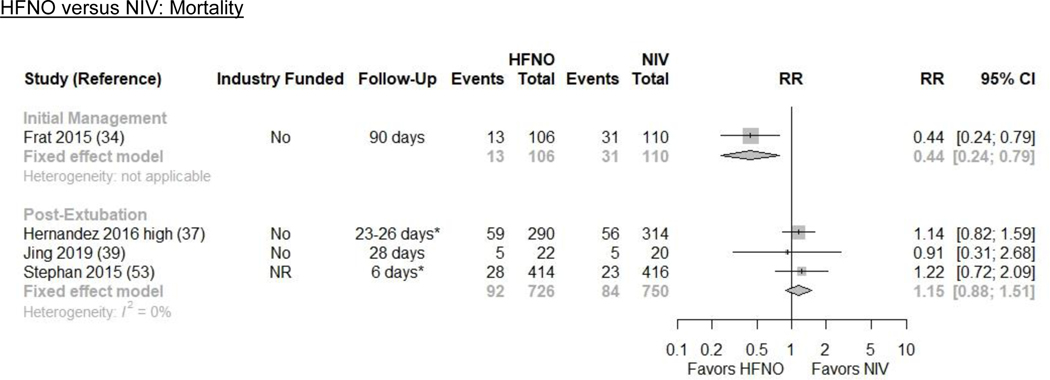
Results from 1 RCT (n=216) indicate that HFNO may reduce all-cause mortality by a large amount (12.4% vs. 28.2%; ARD −15.8% [−21.4, −5.9]) compared with NIV (RR 0.44 [0.24, 0.79]; low evidence certainty) (Figures 1 and 2/Table 1)(34). The trial included patients with hypoxic ARF from multiple etiologies.
Hospital-acquired Pneumonia
One RCT (n=216) among adults with hypoxic ARF due to multiple etiologies evaluated hospital-acquired pneumonia (34). HFNO may reduce hospital-acquired pneumonia by a moderate amount (3.8% vs. 8.2%; ARD −4.4% [−7.0%, 3.7%]) compared to NIV (RR 0.46 [0.15, 1.45]; low evidence certainty) (Figure 1/Table 1).
ICU Admissions and ICU Length of Stay
Few trials reported ICU admissions(32) or length of stay(32,34) (Supplementary Figure 1). Study protocol, rather than clinical outcomes, primarily determined ICU admission and length of stay. It is uncertain whether HFNO reduces ICU admissions or ICU length of stay (insufficient evidence) (Figure 1/Table 1).
Hospital Length of Stay
Two RCTs (n=372) including patients with hypoxic and/or hypercapnic ARF reported hospital length of stay (Supplementary Figure 2)(39,32). HFNO may make little or no difference in hospital length of stay compared to NIV (MD 0.45 days [−0.69, 1.59]; I2=0%; low evidence certainty) (Figure 1/Table 1).
Patient Comfort and Dyspnea
Seven RCTs (n=644) reported comfort measures(28,32,34,35,49,50,54) and 7 RCTs (n=464) provided dyspnea measures(32,34,35,48–50,54); none could be pooled. HFNO may improve patient comfort but may make little or no difference in dyspnea compared to NIV (low evidence certainty) (Figure 1/Table 1).
Important Outcomes
No trials comparing HFNO with NIV reported barotrauma, skin breakdown, discharge disposition, hospital readmissions, compromised nutrition, functional independence, or cost/resource utilization.
Intermediate Outcomes
Treatment escalation, defined as switching from HFNO to NIV or from NIV to HFNO, was rarely reported. One trial(32) suggested higher rates of device switching in HFNO to NIV than from NIV to HFNO. Two trials(48,49) reported higher rates of device intolerance in NIV versus HFNO.
Post-extubation Management of Acute Respiratory Failure
Three RCTs compared HFNO to NIV in post-extubation management of ARF(37,39,53). All were ICU trials in patients with multiple diagnoses, COPD exacerbation, or post-cardiothoracic surgery (Appendix Tables 4 and 5). Two trials were rated low risk of bias; 1 moderate (Supplementary Table 2).
Reintubation
Three RCTs (n=1476) evaluated reintubation(37,39,53). HFNO may increase reintubations by a small amount (17.3% vs. 15.3%; ARD 2.0% [−1.5, 6.6]) compared with NIV (RR 1.13 [0.90, 1.43]; I2=0%; low evidence certainty) (Figures 1 and 2/Table 1).
All-cause Mortality
We pooled 3 RCTs (n=1476) that reported all-cause mortality(37,39,53). HFNO may increase all-cause mortality by a small amount (12.9% vs. 11.2%; ARD 1.7% [−1.3, 5.7]) compared to NIV (RR 1.15 [0.88, 1.51]; I2=0%; low evidence certainty) (Figures 1 and 2/Table 1).
Hospital-acquired Pneumonia
Two RCTs (n=1434) evaluated hospital-acquired pneumonia(37,53). HFNO may make little to no difference in hospital-acquired pneumonia (13.2% vs. 14.7%; ARD −1.5% [−4.4, 2.3%]) compared to NIV (RR 0.90 [0.70, 1.16]; I2=0%; low evidence certainty) (Figure 1/Supplementary Figure 3/Table 1).
ICU Admissions
Not applicable.
ICU Length of Stay
Three RCTs (n=1476) reported ICU length of stay(37,39,53). In pooled results from 2 RCTs in medical patients (n=646), HFNO made little or no difference in ICU mean length of stay compared with NIV (MD −0.98 days [−1.99, 0.03]) (Supplementary Figure 1)(37,39). A third trial of post-cardiothoracic surgery patients(53) (n=830) only reported median length of stay and showed a similar effect. HFNO may make little to no difference in ICU length of stay compared with NIV (low evidence certainty) (Figure 1/Table 1).
Hospital Length of Stay
Two RCTs (n=1434) reporting hospital length of stay(37,63) were not pooled (data reported as means and medians). It is uncertain whether HFNO reduces hospital length of stay compared to NIV (insufficient evidence) (Figure 1/Table 1).
Patient Comfort and Dyspnea
Two RCTs (n=872) provided patient comfort measures (39,53) but could not be pooled, and 1 trial reported dyspnea measures(53) (post-cardiothoracic surgery, n=752). One trial found slight improvement in comfort with HFNO(39) and 1 showed no difference(53). HFNO may make little or no difference in patient comfort compared to NIV (low evidence certainty) (Table 1). HFNO may make little or no difference in dyspnea compared to NIV (58.0% vs. 60.4%; ARD −2.4% [−8.5 to 4.8]; low evidence certainty) (Figure 1/Table 1).
Important Outcomes
Three trials (n=1454) comparing HFNO versus NIV reported nasal/facial skin breakdown(37,39,53). All 3 trials consistently showed significantly higher event rates in the NIV group; 2 trials reported no events in the HFNO groups(37,39) but 1 of the trials (n=604) reported that 42.9% of patients, all from the NIV group, had “nasal septum and skin trauma” resulting in discontinuation of NIV(37). The pooled skin breakdown event rate was 24.3% in NIV compared to 4.6% in HFNO (Peto OR 0.15 [0.02, 1.13]; I2=88%) (Supplementary Figure 4). HFNO may reduce nasal/facial skin breakdown by a large amount (low evidence certainty). Reported findings for barotrauma, gastric dysfunction, and cost/resource utilization were inadequate to derive conclusions.
Intermediate Outcomes
Three trials (n=1150) reported “treatment” or “respiratory” failure but did not report specific numbers of patients that were escalated to a different treatment. Results were mixed(37,39,53). As noted above, one trial reported intolerance due to skin trauma(37).
HFNO versus COT
Initial Management of Acute Respiratory Failure
We included 14 trials comparing HFNO to COT for initial ARF management among patients with multiple diseases(28,31,34,40,44,46,49,52,54), cardiogenic pulmonary edema(43), COPD exacerbation(45), those who were immunocompromised(27,41), and in palliative care(47). Nine were parallel design RCTs and 5 were crossover studies. Eight studies enrolled fewer than 100 participants (Appendix Tables 4 and 5). Risk of bias was rated low for 6 studies and moderate for 8 (Supplementary Table 2).
Intubation
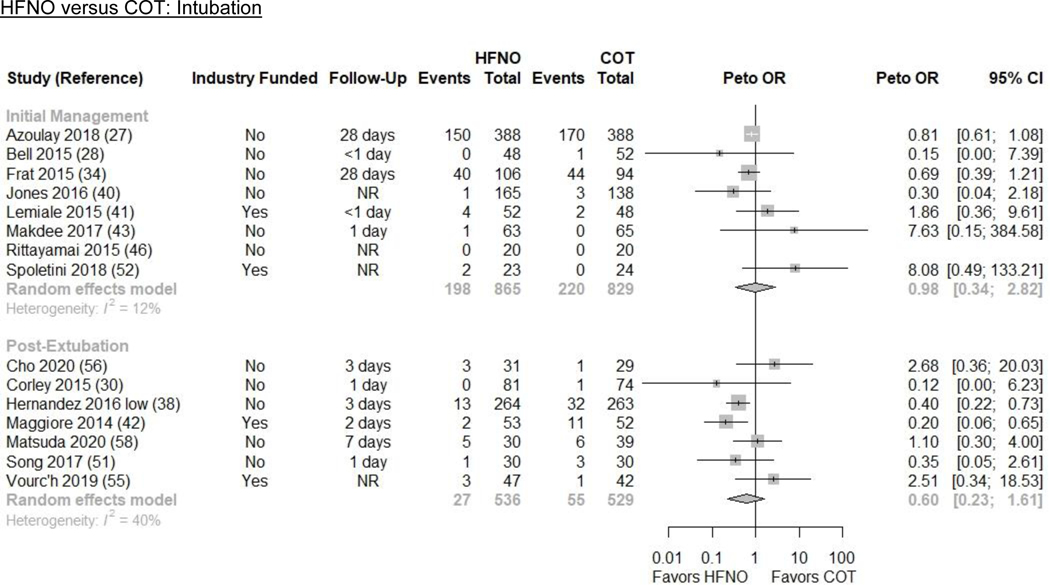
We pooled 8 parallel design RCTs (n=1694) that evaluated intubation(27,28,34,40,41,43,46,52). HFNO may make little or no difference in intubation (26.1% vs. 26.5%; ARD −0.4% [−15.6, 23.9]) compared with COT (Peto OR 0.98 [0.34, 2.82]; I2=12%; low evidence certainty) (Figures 1 and 3/Table 2).
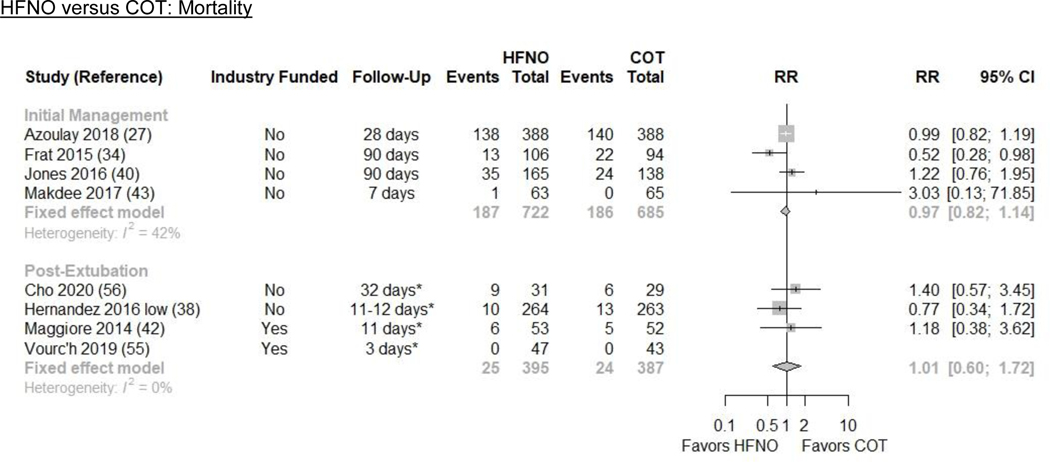
Figure 3. Intubation and Mortality Plots for HFNO versus COT.
CI=confidence interval; COT=conventional oxygen therapy; HFNO=high-flow nasal oxygen; OR=odds ratio; NR=not reported
CI=confidence interval; COT=conventional oxygen therapy; HFNO=high-flow nasal oxygen; ICU=intensive care unit; RR=risk ratio
*These are estimated follow-up times based on the reported mean/median hospital or ICU length of stay.
All-cause Mortality
We pooled 4 RCTs of hypoxic ARF (n=1407) that reported all-cause mortality(27,34,40,43). HFNO may make little or no difference in all-cause mortality (26.3% vs. 27.2%; ARD −0.8% [−4.9, 3.8]) compared with COT (RR 0.97 [0.82, 1.14]; I2=42%; low evidence certainty) (Figures 1 and 3/Table 2).
Hospital-acquired Pneumonia
One RCT (n=200) evaluated hospital-acquired pneumonia in ICU patients with hypoxic ARF from multiple etiologies(34). HFNO may result in a moderate reduction in hospital-acquired pneumonia (3.8% vs. 8.5%; ARD −4.7% [−7.3%, 3.7%]) compared with COT (RR 0.44 [0.14, 1.43]; low evidence certainty) (Figure 1/Table 2).
ICU Admissions
Two RCTs (n=403) reported ICU admissions(28,40). It is uncertain whether HFNO reduces ICU admissions compared to COT (insufficient evidence) (Figure 1/Supplementary Figure 5/Table 2).
ICU Length of Stay
Three RCTs (n=1036) reported ICU length of stay(27,34,44) of which 2 trials of hypoxic ARF (n=976) were pooled(27,34). It is uncertain if HFNO reduces ICU length of stay (insufficient evidence) (Figure 1/Supplementary Figure 6/Table 2).
Hospital Length of Stay
Four RCTs (n=1267) reported hospital length of stay(27,40,43,44) which could not be pooled. HFNO may make little or no difference in hospital length of stay compared to COT (medians ranged from 1 to 24 vs. 1 to 27 days; low evidence certainty) (Figure 1/Table 2).
Patient Comfort and Dyspnea
Twelve RCTs (n=1611) provided patient comfort measures(27,28,31,34,40,41,43,45,46,49,52,54). Four trials (n=415) provided data that permitted pooling. HFNO improved patient comfort based on visual analog scale scores (SMD −0.61 [−0.81, −0.41]; I2=45%)(34,43,46,52) (Supplementary Figure 7). Results from the other 8 RCTs were mixed. Overall, HFNO may improve patient comfort compared with COT (low evidence certainty) (Table 2). Thirteen RCTs (n=1799), including 4 crossover studies, provided dyspnea measures(27,28,31,34,40,41,43,45–47,49,52,54); 4 trials (n=258) could be pooled. HFNO provided moderate improvement in dyspnea compared to COT (SMD −0.56 [−1.35 to 0.24]; I2=67%) (Supplementary Figure 8)(43,46,47,52). HFNO increased the percentage of individuals with improved dyspnea based on results from 3 trials that used different threshold criteria for defining improvement(28,34,40). Based on all 9 studies that included data that could not be pooled results were mixed reported(27,28,31,34,40,41,45,49,54). Overall, HFNO may improve dyspnea compared with COT (low evidence certainty) (Figure 1/Table 2).
Important Outcomes
Two trials (n=431) comparing HFNO versus COT reported skin breakdown (facial pressure sore or nasal ulceration)(40,43). Both trials reported no cases of skin breakdown in the HFNO group. One trial reported no events in the COT group(40) while the other trial did not report skin breakdown in the COT group (insufficient evidence)(43). Other outcomes were rarely or not reported.
Intermediate Outcomes
Seven trials (n=1,503) comparing HFNO versus COT reported treatment escalation from COT to either HFNO or NIV (4 studies) and from HFNO to NIV(27,28,40,41,43,44,46). Studies generally reported higher treatment escalation for COT than for HFNO (Supplementary Figure 9). Six trials reported device intolerance to the assigned treatment(27,40,43,46,47,49). We were unable to derive conclusions due to limited reporting.
Post-extubation Management of Acute Respiratory Failure
Seven parallel group RCTs (n=1,065) compared HFNO with COT for post-extubation ARF. All were ICU trials in medical (mixed diagnoses)(38,42,51,56,58) and post-cardiothoracic surgery patients(30,55) (Appendix Tables 4 and 5). Three studies were rated low risk of bias and 4 moderate (Supplementary Table 2).
Reintubation
Based on pooled results from 7 RCTs (n=1065), HFNO may reduce reintubations by a small amount (6.5% vs. 10.4%; ARD −3.9% [−7.8%, 5.3%]) compared to COT (Peto OR 0.60 [0.23, 1.61]; I2=40%; low evidence certainty) (Figures 1 and 3/Table 2)(30,38,42,51,55,56,58).
All-cause Mortality
We pooled 4 RCTs of ICU patients with hypoxic ARF (n=782) that reported all-cause mortality(38,42,55,56). HFNO may make little or no difference in all-cause mortality (6.3% vs. 6.2%; ARD 0.1% [−2.5%, 4.5%]) compared with COT (RR 1.01 [0.60, 1.72]; I2=0%; low evidence certainty) (Figures 1 and 3/Table 2)
Hospital-acquired Pneumonia
One RCT (n=527) evaluated hospital-acquired pneumonia in the ICU in medical patients with post-extubation hypoxic (non-hypercapnic) ARF from multiple etiologies(38). HFNO may make little or no difference (1.1% vs. 2.3%; ARD −1.1% [−2.0%, 2.2%]) in hospital-acquired pneumonia compared with COT (RR 0.50 [0.13, 1.97]; low evidence certainty) (Figure 1/Table 2).
ICU Length of Stay
Six RCTs (n=1006) reported ICU length of stay(30,38,42,55,56,58) of which 5 (n=479) were pooled. Compared to COT, HFNO probably makes little or no difference in ICU length of stay (approximately 6 days in each group; MD 0.19 [−0.19, 0.57]; moderate evidence certainty) (Figure 1/Supplementary Figure 6/Table 2)(30,42,55,56,58).
Hospital Length of Stay
Two RCTs reported hospital length of stay; results could not be pooled as one reported medians and one reported means(38,56). It is uncertain whether HFNO reduces hospital length of stay compared to COT (insufficient evidence) (Figure 1/Table 2).
Patient Comfort and Dyspnea
Four parallel design RCTs (n=324) provided patient comfort measures(42,51,55,58) which could not be pooled due to variation in measures reported. Three trials showed that HFNO resulted in improved patient comfort compared to COT and one reported little or no difference(58). HFNO may improve patient comfort compared with COT (low evidence certainty) (Table 2). Only 1 parallel design RCT (n=155) reported dyspnea with little or no difference in median values(30). It is uncertain whether HFNO improves dyspnea compared to COT (insufficient evidence) (Figure 1/Table 2).
Important outcomes
One trial reported no incidences of skin breakdown were observed with HFNO but this outcome was not reported for the COT arm (insufficient evidence)(38). No trials reported gastric dysfunction, hospital readmissions, compromised nutrition, or functional independence. Only 1 trial reported a measure of cost/resource utilization(42).
Intermediate outcomes
Five RCTs (n=479) reported treatment escalation from COT to either HFNO or NIV and HFNO to NIV(30,42,51,55,58). All trials reported lower treatment escalation in the HFNO versus COT groups [8.1% vs. 18.9%; RR 0.43 [0.27, 0.70]) (Supplementary Figure 9). Two additional trials reported a higher rate of “treatment” or “respiratory” failure in the COT versus HFNO group but ensuing treatment was not clearly defined(38,51). No RCTs comparing HFNO with COT reported device intolerance outcomes.
DISCUSSION
Our review of HFNO versus NIV or COT found that compared to NIV, HFNO may reduce intubation, all-cause mortality, and hospital-acquired pneumonia, and improve patient comfort in initial ARF management. However, compared to NIV, HFNO may increase reintubations and mortality in post-extubation ARF management. Compared to COT, HFNO may reduce reintubation and improve patient comfort in post-extubation ARF management. Benefits of HFNO were less clear compared to COT in initial ARF management. HFNO may reduce facial skin breakdown compared to NIV and decrease treatment escalation. We analyzed results separately for initial or post-extubation ARF management. Such patients are clinically distinct and may have different ARF etiologies and severities. For example, post-extubation ARF frequently results in reintubation, resulting in prolonged intubation duration and higher ICU mortality(59). Our results are generally consistent with past systematic reviews(1,60–76). However, we limited our inclusion criteria to hospitalized adults meeting ARF criteria, included a broader scope of clinical conditions and settings, assessed HFNO against both NIV and COT, evaluated a more comprehensive list of key clinical outcomes, and updated our search through July 2020. We prioritized patient-centered outcomes such as intubation, mortality, pneumonia, length of hospitalization or length of ICU stay, rather than physiologic outcomes.
As respiratory treatment options vary by ARF etiology and severity, we analyzed results separately for NIV and COT. The baseline physiologic parameters of patients enrolled in NIV trials were worse than those enrolled in COT trials. For example, the baseline mean SpO2 of patients in initial management NIV parallel group trials was 76% compared to 88% in COT trials. Additionally, 5 of 21 (24%) COT trials versus 4 of 11 (36%) NIV trials included patients with hypercapnic ARF. Intubation rates in NIV trials were higher compared to COT trials in both initial and post-extubation ARF management, likely reflecting the higher ARF severity in the NIV versus COT trials. European Respiratory Society and American Thoracic Society Guidelines(77) identify specific indications for NIV, such as hypercapnia with COPD exacerbation and cardiogenic pulmonary edema. However, many patients are treated with NIV for indications beyond these recommendations(78,79) and in trials included in this review. Because populations were combined, it is not possible to determine if HFNO was equally beneficial over NIV in cases where NIV is recommended versus areas where it is not(77).
Subanalyses were conducted to assess the effect of different study designs and recently published studies. Removing a study that used a broader escalation strategy than only the initial management strategy by allowing crossover(32) did not change the strength of findings. An updated bridge search through February 2021 identified 10 additional eligible RCTs that provided critical or important outcomes(80–89). Three were considered large (n≥100) and reported on mortality and intubation(80–82). Of these three, one was in individuals with hematologic malignancies(80) and not further assessed. The second was a moderate risk of bias study that evaluated HFNO vs. NIV in post-extubation patients with ARF (n=140)(81). When adding this trial (81) the absolute risk difference of HFNO vs. NIV on reintubation decreased from 2.0% to 1.8%, but did not change the overall certainty of evidence. The third was a low risk of bias study that evaluated HFNO vs. COT as initial management of COPD exacerbation and acute hypoxic respiratory failure with compensated hypercapnea (n=320)(82). Adding this trial(82) did not alter the effect magnitude estimates for either outcome. Findings from these studies were consistent with our overall findings and inclusion of results made little to no difference in effect estimates.
We identified gaps in the existing literature that limited our conclusions and for which future research is needed. Trials varied in populations enrolled, ARF etiology, and protocols used. When numerous causes of ARF were included in a single trial, results were often not stratified or sample sizes were too small to adequately evaluate outcomes across disease states or clinical settings. We were unable to distinguish relative effectiveness of therapies in specific populations. Studies often excluded patients with life-threatening comorbidities or at imminent risk of mechanical ventilation. No studies reported outcomes in patients with SARS CoV-2 infection. Many studies used surrogate endpoints, such as physiologic outcomes, rather than patient-centered outcomes such as mortality. Trial design, sample size, treatment/follow-up duration, and results reporting were often inadequate to accurately assess our pre-specified outcomes. No RCTs evaluated delirium, compromised nutrition, functional independence at discharge, or discharge disposition. Finally, treatment protocols, clinician/health system training, and cost and resource use were poorly characterized. These represent a key part of HFNO utility for a health system.
In conclusion, compared to NIV, HFNO used as initial ARF management may improve several clinical outcomes. Compared to COT, HFNO used as post-extubation management may reduce reintubations and improve patient comfort. HFNO resulted in fewer harms than either NIV or COT. Broad applicability, including required clinician and health system experience and resource use, remain unknown.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge input and feedback on review scope, treatment categorization, critical outcomes, and draft findings from the members of the Technical Expert Panel: Charles Carpati, MD; Matthew G. Drake, MD; Andrew Dunn, MD, MPH, SFHM, MACP; and Robert C. Hyzy, MD.
Funding
The evidence review was commissioned by the American College of Physicians. The material is the result of work supported by and conducted at the Minneapolis VA Health Care System.
Dr. Baldomero was also supported, in part, by National Institutes of Health National Center for Advancing Translational Sciences grants KL2TR002492 and UL1TR002494.
Primary Funding Source: American College of Physicians
PROSPERO registration: CRD42019146691
Footnotes
Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the Annals of Internal Medicine © 2021 American College of Physicians Document Publication Date: 04/27/21 PAGE 18 article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Reproducible Research Statement
Study Protocol: Baldomero AK, Melzer A, Greer N, et al. Effectiveness and harms of high-flow nasal oxygen (HFNO) for acute respiratory failure: A systematic review protocol. BMJ Open. 2020;10(2). [PMID: 32051320] doi: 10.1136/bmjopen-2019–034956
Statistical Code: Not available
Data Set: Supplementary Tables
DISCLOSURE
The materials presented here solely represent the views of the authors and do not represent the view of the U.S. Department of Veterans Affairs, the United States Government or the National Institutes of Health’s National Center for Advancing Translational Sciences.
Current mailing address for AKB, ACM:
1 Veterans Drive, Pulmonary 111-N
Minneapolis, MN, USA
Current mailing address for all other authors:
One Veterans Drive (111–0)
Minneapolis, MN 55417
References
- 1.Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145–155. [PMID: 29144160] doi: 10.1513/AnnalsATS.201707-548FR [DOI] [PubMed] [Google Scholar]
- 2.Möller W, Feng S, Domanski U, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122(1):191–197. [PMID:27856714] doi: 10.1152/japplphysiol.00584.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie JE, Williams AB, Gerard C, et al. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011;39(6):1103–10. [PMID:22165366] doi: [DOI] [PubMed] [Google Scholar]
- 4.Sim MA, Dean P, Kinsella J, et al. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63(9):938–40. [PMID:18540928] doi: 10.1111/j.1365-2044.2008.05536.x [DOI] [PubMed] [Google Scholar]
- 5.Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58(10):1621–4. [PMID:23513246] doi: 10.4187/respcare.02358 [DOI] [PubMed] [Google Scholar]
- 6.Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56(8):1151–5 doi: 10.4187/respcare.01106. [DOI] [PubMed] [Google Scholar]
- 7.Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4):126–31. [PMID:17931878] doi: 10.1016/j.aucc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 8.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103(6):886–90. [PMID:19846404] doi: 10.1093/bja/aep280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107(6):998–1004. [PMID:21908497] doi: 10.1093/bja/aer265 [DOI] [PubMed] [Google Scholar]
- 10.Tiruvoipati R, Lewis D, Haji K, et al. High-flow nasal oxygen vs high-flow face mask: A randomized crossover trial in extubated patients. J Crit Care. 2010;25(3):463–8. [PMID:19781896]. doi: 10.1016/j.jcrc.2009.06.050 [DOI] [PubMed] [Google Scholar]
- 11.Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: A prospective observational study. J Crit Care. 2012;27(3):324.e9–13. [PMID:21958974] doi: 10.1016/j.jcrc.2011.07.075 [DOI] [PubMed] [Google Scholar]
- 12.Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–13. [PMID:20406507] [PubMed] [Google Scholar]
- 13.Rittayamai N, Tscheikuna J, Rujiwit P . High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: A randomized crossover physiologic study. Respir Care. 2014;59(4):485–90. [PMID:24046462] doi: 10.4187/respcare.02397 [DOI] [PubMed] [Google Scholar]
- 14.Cortegiani A, Crimi C, Noto A, et al. Effect of high-flow nasal therapy on dyspnea, comfort, and respiratory rate. Crit Care. 2019;23(1):201. [PMID: 31167660] doi: 10.1186/s13054-019-2473-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams R, Rankin N, Smith T, et al. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med. 1996;24(11):1920–9. [PMID:8917046] doi: 10.1097/00003246-199611000-00025 [DOI] [PubMed] [Google Scholar]
- 16.Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81–6. [PMID: 18539721]. doi: 10.1177/1479972307087190 [DOI] [PubMed] [Google Scholar]
- 17.Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011;37(11):1780–6. [PMID: 21946925] doi: 10.1007/s00134-011-2354-6 [DOI] [PubMed] [Google Scholar]
- 18.Lenglet H, Sztrymf B, Leroy C, et al. Humidified high flow nasal oxygen during respiratory failure in the emergency department: Feasibility and efficacy. Respir Care. 2012;57(11):1873–8. [PMID: 22417844] doi: 10.4187/respcare.01575 [DOI] [PubMed] [Google Scholar]
- 19.Baldomero AK, Melzer A, Greer N, et al. Effectiveness and harms of high-flow nasal oxygen (HFNO) for acute respiratory failure: A systematic review protocol. BMJ Open. 2020;10(2). [PMID: 32051320] doi: 10.1136/bmjopen-2019-034956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 22.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard Dersimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. [PMID: 24548571] doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veroniki AA, Jackson D, Bender R, et al. Methods to calculate uncertainty in the estimated overall effect size from a random‐effects meta‐analysis. Res Synth Methods. 2019;10(1):23–43. [PMID: 30129707] doi: 10.1002/jrsm.1319 [DOI] [PubMed] [Google Scholar]
- 24.. University McMaster and Prime Evidence, Inc. GRADEpro GDT: GRADEpro Guideline development tool. Ontario, Canada, 2015 [Google Scholar]
- 25.. Schünemann H, Brożek J, Guyatt G, et al. GRADE Handbook, 2019. Available: https://gdt.gradepro.org/app/handbook/handbook.html [Accessed 9 Oct 2019]
- 26.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann. Math. Statist. 1950:607–11. [Google Scholar]
- 27.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: The HIGH randomized clinical trial. JAMA. 2018;320(20):2099–10. [PMID: 30357270] doi: 10.1001/jama.2018.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell N, Hutchinson CL, Green TC, et al. Randomised control trial of humidified high flow nasal cannulae versus standard oxygen in the emergency department. Emerg Med Australas. 2015;27(6):537–41. [PMID: 26419650] doi: 10.1111/1742-6723.12490 [DOI] [PubMed] [Google Scholar]
- 29.Cong L, Zhou L, Liu H, et al. Outcomes of high-flow nasal cannula versus non-invasive positive pressure ventilation for patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Clin Exp Med. 2019;12(8):10863–7 [Google Scholar]
- 30.Corley A, Bull T, Spooner AJ, et al. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI >/=30: A randomised controlled trial. Intensive Care Med. 2015;41(5):887–94. [PMID:25851385] doi: 10.1007/s00134-015-3765-6 [DOI] [PubMed] [Google Scholar]
- 31.Delorme M, Bouchard PA, Simon M, et al. Effects of high-flow nasal cannula on the work of breathing in patients recovering from acute respiratory failure. Crit Care Med. 2017;45(12):1981–8. [PMID: 28857852] doi: 10.1097/CCM.0000000000002693 [DOI] [PubMed] [Google Scholar]
- 32.Doshi P, Whittle JS, Bublewicz M, et al. High-velocity nasal insufflation in the treatment of respiratory failure: A randomized clinical trial. Ann Emerg Med. 2018;72(1):73–83 e5. [PMID: 29310868] doi: 10.1016/j.annemergmed.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 33.Frat JP, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: A post-hoc analysis of a randomised trial. Lancet Respir Med. 2016;4(8):646–52. [PMID: 27245914] doi: 10.1016/S2213-2600(16)30093-5 [DOI] [PubMed] [Google Scholar]
- 34.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–96 [PMID:2598190] doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 35.Grieco DL, Menga LS, Raggi V, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201(3):303–12. [PMID:31687831] doi: 10.1164/rccm.201904-0841OC [DOI] [PubMed] [Google Scholar]
- 36.Haywood ST, Whittle JS, Volakis LI, et al. HVNI vs NIPPV in the treatment of acute decompensated heart failure: Subgroup analysis of a multi-center trial in the ED. Am J Emerg Med. 2019;37(11):2084–90. [PMID: 30880040] doi: 10.1016/j.ajem.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 37.Hernandez G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: A randomized clinical trial. JAMA. 2016;316(15):1565–74. [PMID: 27706464] doi: 10.1001/jama.2016.14194 [DOI] [PubMed] [Google Scholar]
- 38.Hernandez G, Vaquero C, Gonzalez P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: A randomized clinical trial. JAMA. 2016;315(13):1354–61. [PMID: 26975498] doi: 10.1001/jama.2016.271132 [DOI] [PubMed] [Google Scholar]
- 39.Jing G, Li J, Hao D, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: A pilot randomized controlled trial. Res Nurs Health. 2019;42(3):217–25. [PMID: 30887549] doi: 10.1002/nur.21942 [DOI] [PubMed] [Google Scholar]
- 40.Jones PG, Kamona S, Doran O, et al. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: The HOT-ER study. Respir Care. 2016;61(3):291–9. [PMID: 26577199] doi: 10.4187/respcare.04252 [DOI] [PubMed] [Google Scholar]
- 41.Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: A multicenter randomized trial. Crit Care. 2015;19:380. [PMID: 26521922] doi: 10.1186/s13054-015-1097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282–8. [PMID: 25003980] doi: 10.1164/rccm.201402-0364OC [DOI] [PubMed] [Google Scholar]
- 43.Makdee O, Monsomboon A, Surabenjawong U, et al. High-flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: A randomized controlled trial. Ann Emerg Med. 2017;70(4):465–72 e2 [PMID: 28601264] doi: 10.1016/j.annemergmed.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 44.Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56(3):265–70. [PMID: 21255498] doi: 10.4187/respcare.00801 [DOI] [PubMed] [Google Scholar]
- 45.Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: A randomized controlled cross-over trial. Respirology. 2017;22(6):1149–55. [PMID: 28470831] doi: 10.1111/resp.13050 [DOI] [PubMed] [Google Scholar]
- 46.Rittayamai N, Tscheikuna J, Praphruetkit N, et al. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. 2015;60(10):1377–82. [PMID: 26060321] doi: 10.4187/respcare.03837 [DOI] [PubMed] [Google Scholar]
- 47.Ruangsomboon O, Dorongthom T, Chakorn T, et al. High-flow nasal cannula versus conventional oxygen therapy in relieving dyspnea in emergency palliative patients with do-not-intubate status: A randomized crossover study. Ann Emerg Med. 2019;18:18. [PMID: 31864728] doi: 10.1016/j.annemergmed.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 48.Saksitthichok B, Petnak T, So-Ngern A, et al. A prospective randomized comparative study of high-flow nasal cannula oxygen and non-invasive ventilation in hypoxemic patients undergoing diagnostic flexible bronchoscopy. J Thorac Dis. 2019;11(5):1929–39. [PMID: 31285886] doi: 10.21037/jtd.2019.05.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwabbauer N, Berg B, Blumenstock G, et al. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: Effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol. 2014;14:66. [PMID: 25110463] doi: 10.1186/1471-2253-14-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sklar MC, Dres M, Rittayamai N, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: A randomized crossover physiological study. Ann Intensive Care. 2018;8(1):85. [PMID: 30187270] doi: 10.1186/s13613-018-0432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song HZ, Gu JX, Xiu HQ, et al. The value of high-flow nasal cannula oxygen therapy after extubation in patients with acute respiratory failure. Clinics (Sao Paulo). 2017;72(9):562–7. [PMID: 29069260] doi: 10.6061/clinics/2017(09)07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spoletini G, Mega C, Pisani L, et al. High-flow nasal therapy vs standard oxygen during breaks off noninvasive ventilation for acute respiratory failure: A pilot randomized controlled trial. J Crit Care. 2018;48:418–25. [PMID: 30321833] doi: 10.1016/j.jcrc.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 53.Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: A randomized clinical trial. JAMA. 2015;313(23):2331–9. [PMID: 25980660] doi: 10.1001/jama.2015.5213 [DOI] [PubMed] [Google Scholar]
- 54.Vargas F, Saint-Leger M, Boyer A, et al. Physiologic effects of high-flow nasal cannula oxygen in critical care subjects. Respir Care. 2015; 60(10):1369–76. [PMID: 25944940] doi: 10.4187/respcare.03814 [DOI] [PubMed] [Google Scholar]
- 55.Vourc’h M, Nicolet J, Volteau C, et al. High-flow therapy by nasal cannulae versus high-flow face mask in severe hypoxemia after cardiac surgery: A single-center randomized controlled study-the heart flow study. J Cardiothorac Vasc Anesth. 2020;34(1):157–65. [PMID: 31230964] doi: 10.1053/j.jvca.2019.05.039 [DOI] [PubMed] [Google Scholar]
- 56.Cho JY, Kim H-S, Kang H, et al. Comparison of postextubation outcomes associated with high-flow nasal cannula vs. conventional oxygen therapy in patients at high risk of reintubation: a randomized clinical trial. J Korean Med Sci. 2020;35(25):e194. ]PMID: 32597041] doi: 10.3346/jkms.2020.35.e194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doshi PB, Whittle JS, Dungan II G, et al. The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive pressure ventilation in the treatment of hypercapneic respiratory failure: a subgroup analysis. Heart Lung. 2020. [PMID: 32273085] doi: 10.1016/j.hrtlng.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 58.Matsuda W Hagiwara A, Uemura T, et al. High-flow nasal cannula may not reduce the re-intubation rate compared with large-volume nebulization-based humidifier. Respir Care. 2020;65(5):610–617. [PMID: 31992669] doi: 10.4187/respcare.07095 [DOI] [PubMed] [Google Scholar]
- 59.Thille A, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–8. [PMID: 21765357] doi: 10.1097/CCM.0b013e3182282a5a [DOI] [PubMed] [Google Scholar]
- 60.Cheng LC, Chang SP, Wang JJ, et al. The impact of high-flow nasal cannula on the outcome of immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. Medicina (Kaunas). 2019;55(10):16. [PMID: 31623276] doi: 10.3390/medicina55100693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corley A, Rickard CM, Aitken LM, et al. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev. 2017;5. [PMID: 28555461] doi: 10.1002/14651858.CD010172.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortegiani A, Crimi C, Sanfilippo F, et al. High flow nasal therapy in immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. J Crit Care. 2019;50:250–6. [PMID: 30622042] doi: 10.1016/j.jcrc.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 63.Helviz Y, Einav S. A systematic review of the high-flow nasal cannula for adult patients. Crit Care. 2018;22(1):71. [PMID: 29558988] doi: 10.1186/s13054-018-1990-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang HB, Peng JM, Weng L, et al. High-flow oxygen therapy in immunocompromised patients with acute respiratory failure: A review and meta-analysis. J Crit Care. 2018;43:300–5. [PMID: 28968525] doi: 10.1016/j.jcrc.2017.09.176 [DOI] [PubMed] [Google Scholar]
- 65.Huang CC, Lan HM, Li CJ, et al. Use high-flow nasal cannula for acute respiratory failure patients in the emergency department: a meta-analysis study. Emerg Med Int. 2019. [PMID: 31737365] doi: 10.1155/2019/2130935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang H, Zhao Z, Tong Z. Effect of high-flow nasal cannula oxygen therapy in immunocompromised subjects with acute respiratory failure. Respir Care. 2020;65(3):369–376. [PMID: 31744865] doi: 10.4187/respcare.07205 [DOI] [PubMed] [Google Scholar]
- 67.Leeies M, Flynn E, Turgeon AF, et al. High-flow oxygen via nasal cannulae in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Syst Rev. 2017;6(1):202. [PMID: 29037221] doi: 10.1186/s13643-017-0593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liesching TN, Lei Y. Efficacy of high-flow nasal cannula therapy in intensive care units. J Intensive Care Med. 2017. [PMID: 28110612] doi: 10.1177/0885066616689043 [DOI] [PubMed] [Google Scholar]
- 69.Lin SM, Liu KX, Lin ZH, et al. Does high-flow nasal cannula oxygen improve outcome in acute hypoxemic respiratory failure? A systematic review and meta-analysis. Respir Med. 2017;131:58–64. [PMID: 28947043] doi: 10.1016/j.rmed.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 70.Monro-Somerville T, Sim M, Ruddy J, et al.The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):e449–e56. [PMID: 27611978] doi: 10.1097/CCM.0000000000002091 [DOI] [PubMed] [Google Scholar]
- 71.Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest. 2017;151(4):764–75. [PMID: 28089816] doi: 10.1016/j.chest.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 72.Ou X, Hua Y, Liu J, et al. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189(7):E260–E7. [PMID: 28246239] doi: 10.1503/cmaj.160570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–72. [PMID: 30888444] doi: 10.1007/s00134-019-05590-5 [DOI] [PubMed] [Google Scholar]
- 74.Tinelli V, Cabrini L, Fominskiy E, et al. High flow nasal cannula oxygen vs. conventional oxygen therapy and noninvasive ventilation in emergency department patients: a systematic review and meta-analysis. J Emerg Med. 2019;57(3):322–8. [PMID: 31421952] doi: 10.1016/j.jemermed.2019.06.033 [DOI] [PubMed] [Google Scholar]
- 75.Xu Z, Li Y, Zhou J, et al. High-flow nasal cannula in adults with acute respiratory failure and after extubation: a systematic review and meta-analysis. Respir Res. 2018;19(1):202. [PMID: 30326893] doi: 10.1186/s12931-018-0908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Yin H, Zhang R, et al. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Crit Care. 2019;23(1):180. [PMID: 31101127] doi: 10.1186/s13054-019-2465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50:1602426. [PMID: 28860265] doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 78.Mehta AB, Douglas IS, Walkey AJ. Evidence-based utilization of noninvasive ventilation and patient outcomes. Ann Am Thorac Soc. 2017;14(11):1667–1673. [PMID: 28541747] doi: 10.1513/AnnalsATS.201703-208OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000–2009: a population-based study. Ann Am Thorac Soc. 2013;10(1):10–17. [PMID: 23509327[ doi: 10.1513/AnnalsATS.201206-034OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.AlptekİnoĞlu Mendİl NÖ, Temel Ş, YÜksel RC, et al. The use of high-flow nasal oxygen vs. standard oxygen therapy in hematological malignancy patients with acute respiratory failure in hematology wards. Turk J Med Sci. 2021. [PMID:33517607] doi: 10.3906/sag-2007-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Theerawit P, Natpobsuk N, Petnak T, et al. The efficacy of the WhisperFlow CPAP system versus high flow nasal cannula in patients at risk for postextubation failure: a Randomized controlled trial. J Crit Care. 2020. [PMID:33012589] doi: 10.1016/j.jcrc.2020.09.031 [DOI] [PubMed] [Google Scholar]
- 82.Li XY, Tang X, Wang R, et al. High-flow nasal cannula for chronic obstructive pulmonary disease with acute compensated hypercapnic respiratory failure: A randomized, controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:3051–61. [PMID:33262584] doi: 10.2147/COPD.S283020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andino R, Vega G, Pacheco SK, et al. High-flow nasal oxygen reduces endotracheal intubation: a randomized clinical trial. Ther Adv Respir Dis. 2020;14:1753466620956459. [PMID:32976085] doi: 10.1177/1753466620956459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho JY, Kim HS, Kang H, et al. Comparison of Postextubation Outcomes Associated with High-Flow Nasal Cannula vs. Conventional Oxygen Therapy in Patients at High Risk of Reintubation: a Randomized Clinical Trial. J Korean Med Sci. 2020;35(25):e194. [PMID:32597041] doi: 10.3346/jkms.2020.35.e194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cortegiani A, Longhini F, Madotto F, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1):692. [PMID:33317579] doi: 10.1186/s13054-020-03409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geng W, Batu W, You S, et al. High-Flow Nasal Cannula: a Promising Oxygen Therapy for Patients with Severe Bronchial Asthma Complicated with Respiratory Failure. Can Respir J. 2020;2301712. [PMID:32211084] doi: 10.1155/2020/2301712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papachatzakis Y, Nikolaidis PT, Kontogiannis S, et al. High-Flow Oxygen through Nasal Cannula vs. Non-Invasive Ventilation in Hypercapnic Respiratory Failure: A Randomized Clinical Trial. Int J Environ Res Public Health. 2020;17(16):5994. [PMID:32824771] doi: 10.3390/ijerph17165994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rezaei A, Fakharian A, Ghorbani F, et al. Comparison of high-flow oxygenation with noninvasive ventilation in COPD exacerbation: a crossover clinical trial. Clin Respir J. 2020. [PMID:33269553] doi: 10.1111/crj.13315 [DOI] [PubMed] [Google Scholar]
- 89.Tan D, Walline JH, Ling B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. 2020;24(1):489. [PMID:32762701] doi: 10.1186/s13054-020-03214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.