Abstract
Background:
Air pollution exposure has been linked with diminished fertility. Identifying the metabolic changes induced by periconception air pollution exposure among women could enhance our understanding of the potential biological pathways underlying air pollution’s reproductive toxicity.
Objective:
To identify serum metabolites associated with periconception air pollution exposure and evaluate the extent to which these metabolites mediate the association between air pollution and live birth.
Methods:
We included 200 women undergoing a fresh assisted reproductive technology (ART) cycle at Massachusetts General Hospital Fertility Center (2005–2015). A serum sample was collected during stimulation, and untargeted metabolic profiling was conducted using liquid chromatography with ultra-high-resolution mass spectrometry. Exposure to nitrogen dioxide (NO2), ozone (O3), fine particulate matter <2.5 µm (PM2.5), and black carbon (BC) was estimated using validated spatiotemporal models. Multivariable linear regression models were used to evaluate the associations between the air pollutants, live birth, and metabolic feature intensities. A meet in the middle approach was used to identify overlapping features and metabolic pathways.
Results:
From the C18 and HILIC chromatography columns, 10,803 and 12,968 metabolic features were extracted. There were 190 metabolic features and 18 pathways that were significantly associated with both air pollution and live birth (P<0.05) across chromatography columns. Eight features were confirmed metabolites implicated in amino acid and nutrient metabolism with downstream effects on oxidative stress and inflammation. Six confirmed metabolites fell into two intuitive clusters – “antioxidants” and “oxidants”- which could potentially mediate some of the association between air pollution and lower odds of live birth. Tryptophan and vitamin B3 metabolism were common pathways linking air pollution exposure to decreased probability of live birth.
Conclusion:
Higher periconception air pollution exposure was associated with metabolites and biologic pathways involved in inflammation and oxidative stress that may mediate the observed associations with lower probability of live birth following ART.
Keywords: air pollution, assisted reproduction, fertility, metabolomics, live birth, in vitro fertilization
Introduction.
Air pollution is a ubiquitous and significant global health threat (Burnett et al. 2018), responsible for many adverse health effects (An et al. 2018; IARC 2013; Lin et al. 2017). While there is a growing consensus that ambient air pollution is related to worse birth outcomes such as preterm birth and low birth weight (Lamichhane et al. 2015; Shah and Balkhair 2011), emerging evidence also suggests that air pollution is related to lower human fertility (Carre et al. 2017a; Checa Vizcaino et al. 2016; Nieuwenhuijsen et al. 2014; Xue and Zhu 2018a, b), with specific adverse effects on spermatogenesis (Deng et al. 2016; Jurewicz et al. 2018; Lafuente et al. 2016), folliculogenesis (Gai et al. 2017; Gaskins et al. 2019c; Ogliari et al. 2013; Veras et al. 2009), and pregnancy maintenance (Gaskins et al. 2019b; Gaskins et al. 2020; Kioumourtzoglou et al. 2019; Leiser et al. 2019). Although several biological mechanisms including oxidative stress, inflammation, endocrine disruption, and DNA damage have been proposed to underlie these associations, very little mechanistic insight has been gained from studies of human pregnancy.
To evaluate the association between air pollution and fertility in further detail, several studies, including our own, have focused on women undergoing assisted reproductive technologies (ART) as it is possible to directly observe many early reproductive outcomes. Across the 12 studies to date, the most consistent finding has been that increased exposure to NO2 (and closer residential distance to major roadways) is related to lower probability of ART success (Boulet et al. 2019; Carre et al. 2017b; Choe et al. 2018; Gaskins et al. 2019a; Gaskins et al. 2018; Legro et al. 2010; Li et al. 2020; Quraishi et al. 2019; Zeng et al. 2020); however, studies have also reported negative relationships with coarse and fine particulate matter (Choe et al. 2018; Gaskins et al. 2019a; Li et al. 2020; Perin et al. 2010a; Perin et al. 2010b; Quraishi et al. 2019; Zeng et al. 2020), ozone (Legro et al. 2010; Qiu et al. 2019; Wang et al. 2019), and sulfur dioxide (Boulet et al. 2019; Li et al. 2020; Wang et al. 2019; Zeng et al. 2020). ART, which requires routine blood collection and externalizes many reproductive processes, enables examination of intermediate outcomes to examine potential biological mediators of environmental associations with fertility. Given the state of the literature, which strongly suggests an adverse effect of air pollutants on fertility, such mechanistic studies are critical as they not only help strengthen the plausibility of the association but also help establish causality.
Untargeted high-resolution metabolomics is an ideal tool to evaluate potential biological pathways underlying the association between ambient air pollution and fertility as it represents a central measure linking exposure to internal dose, biological response, and disease pathobiology (Liang et al. 2018; Oresic et al. 2020; Walker et al. 2019). Many studies, including one in pregnant women (Qi Yan et al. 2019), have previously demonstrated that metabolomics can sensitively reflect internal metabolic perturbations following exposures to ambient air pollution (Liang et al. 2020); however, no studies have investigated the relationships between air pollution, metabolites, and adverse reproductive, pregnancy, or birth outcomes (Inoue et al. 2020). If ambient air pollution perturbs exogenous and endogenous metabolites, then dysregulated metabolic pathways may provide insights into the mechanisms through which air pollution negatively impacts reproduction. Moreover, the metabolites that are significantly altered by air pollution exposure could be investigated as potential intermediate variables, linking air pollution to lower probability of live birth through causal mediation analyses.
Thus, the objective of this analysis was to use untargeted high-resolution metabolomics to identify metabolites and pathways associated with periconception exposure to air pollution and evaluate the extent to which these metabolites mediate the association between air pollution and live birth following ART.
Methods.
Study design and participants.
The women included in our analysis were participants in the Environment and Reproductive Health (EARTH) Study- a prospective cohort aimed at evaluating environmental and nutritional determinants of fertility among couples undergoing fertility evaluation at the Massachusetts General Hospital (MGH) Fertility Center (Messerlian et al. 2018). At enrollment, height and weight were measured by trained research study staff to calculate body mass index (BMI (kg/m2)), and data on demographics, medical and reproductive history, and lifestyle characteristics were collected via questionnaire. Women were then followed prospectively through their ART cycles until failure or live birth. The EARTH study was approved by the Human Studies Institutional Review Boards of the MGH and the Harvard T.H. Chan School of Public Health. All study participants signed an informed consent after the study procedures were explained by research study staff. Among the 345 women with complete air pollution data who underwent a fresh, autologous ART cycle between 2005 and 2015 (Gaskins et al. 2019a), we randomly selected 200 women for our metabolomics sub-study. All demographic and ART cycle characteristics were similar between women who were eligible and included in this sub-study (Supplemental Table 1).
Air pollution assessment.
All of the women in our study provided their residential address at enrollment for reimbursement purposes. To derive ambient air pollution exposures, we geocoded these addresses using ArcGIS and linked them to several existing spatio-temporal models of air pollution. Daily PM2.5 and NO2 concentrations were both modeled at a 1 km2 resolution using satellite remote sensing data in combination with land use terms (Kloog et al. 2014; Lee and Koutrakis 2014). Daily O3 concentrations were also modelled at a 1 km2 resolution using chemical transport models, O3 vertical profiles, meteorological variables, and other atmospheric compounds (Di et al. 2017). Daily BC exposure was estimated at the home address using support vector machine regression models based on ambient measurements collected from >300 monitors across New England as well as several spatial and temporal predictors (Abu Awad et al. 2017). For each pollutant, we derived daily estimated ambient concentrations starting 3 months prior to the date of blood collection which roughly corresponds to the proposed window of follicular development (Broekmans et al. 2010). Air pollution concentrations were averaged across the 1, 2, and 3 days, 1 and 2 weeks, and 3 months prior to blood draw to evaluate different short- and longer-term exposure periods. Distance to major roadway was defined as the Euclidian distance in meters from the residence to nearest major roadway as defined by the Massachusetts Department of Transportation (MassGIS 2014) and US Census feature class codes (A1, A2, and A3).
Outcome assessment.
Following a pretreatment cycle with oral contraceptives, women began one of three controlled ovarian stimulation protocols on day 3 of induced menses: luteal-phase GnRH agonist, GnRH-antagonist, or a follicular phase GnRH-agonist. During the monitoring phase of controlled ovarian stimulation, women provided a non-fasting blood sample via venipuncture during a routine morning appointment (between 7AM to 10AM). There was some variation in the day of blood collection due to availability of research staff. This variable, however, was not related to air pollution exposures or ART outcomes. Serum was centrifuged, aliquoted, and stored at −20°C initially before being transferred to Harvard for storage at −80°C. After sufficient follicular development, women typically underwent oocyte retrieval on cycle day 14. Embryo transfer occurred 3 to 5 days after oocyte retrieval. Live birth was defined as the delivery of a neonate on or after 24 weeks gestation. For our primary outcome, women were assigned a value of 1 if their fresh, initiated ART cycle resulted in live birth and a value of 0 otherwise.
High-resolution metabolomics.
Metabolomics analyses were conducted on the serum samples using established protocols (Go et al. 2015). Each sample was treated with two volumes of acetonitrile and analyzed in triplicate using liquid chromatography coupled with high-resolution mass spectrometry (LC–HRMS) techniques (Dionex Ultimate 3000 NANO; Thermo Orbitrap Fusion). Two chromatography columns, hydrophilic interaction liquid chromatography (HILIC) with positive electrospray ionization (ESI) and C18 hydrophobic reversed-phase chromatography with negative ESI, were used to enhance the coverage of metabolic feature detection. Two quality control pooled reference plasma samples, including NIST 1950 (Simon-Manso et al. 2013) and pooled human plasma purchased from Equitech Bio were included at the beginning and end of each analytical batch for normalization, batch evaluation, retention time alignment, and post hoc quantification. Following instrument analyses of all samples, raw data files were converted to .mzML files using ProteoWizard. Metabolic features were extracted using apLCMS with modifications by xMSanalyzer, which performed peak detection, noise filtering, m/z and retention time alignment, feature quantification, and data quality filtering (Chambers et al. 2012; Uppal et al. 2013; Yu et al. 2009). Detected signals (referred to as metabolic features) were uniquely defined by their mass-to-charge ratio (m/z), retention time, and ion intensity. To filter out the noise signals and optimize the metabolomics data quality, only metabolic features detected in >10% of the serum samples with median coefficient of variation (CV) among technical replicates <30% and Pearson correlation >0.7 were included in further analyses. Following quality assessment, the median intensity was taken across replicate samples and these intensities were natural log transformed for analysis.
Statistical analysis.
We analyzed the associations between pollutants and metabolic features using multivariable linear regression models adjusted for age, BMI, smoking status, education, and average temperature. Separate models were conducted for each metabolic feature detected in each chromatography chrome (i.e., serum C18 column with negative ESI and HILIC column with positive ESI). Similar multivariable models adjusted for age, BMI, smoking status, education, and protocol were used to examine the association between live birth following ART and metabolic features. Neither of the models were adjusted for intermediate ART outcomes or clinical factors such as number of embryos transferred as these were considered to be intermediate variables, on the casual pathway between exposure and outcome. Multiple comparison correction was conducted using the Benjamini-Hochberg false discovery rate (FDRB-H) procedure, a widely used procedure in MWAS studies, at a 5% false positive threshold.
We conducted pathway enrichment analysis utilizing mummichog (v. 1.0.10), a bioinformatics platform that infers and categorizes functional biological activity directly from mass spectrometry output, without prior metabolite validation (Li et al. 2013). An adjusted p-value for each pathway was calculated from resampling the reference input file in mummichog using a gamma distribution, which penalizes pathways with fewer reference hits, and assigning greater significance to pathways with more reference hits (Li et al. 2013). The samples were analyzed concurrently with pooled reference materials in which hundreds of metabolites were confirmed by Level 1 criteria (Liu et al. 2020), and the mummichog results presented were verified to include at least one metabolite with confirmed identity for each of the pathways discussed. We applied two strategies to select eligible metabolic features for pathway analysis: (i) at raw p-values < 0.05 (less conservative); (ii) at adjusted p-values < 0.05 using Benjamini-Hochberg method for multiple comparison correction (more conservative). For the first approach, to compensate for false discoveries, we excluded pathways identified by mummichog with a p-value higher than 0.05 and those containing less than 3 significant metabolic features that were matched with known compounds by m/z. We conducted pathway analysis separately for each of the air pollutants, during each time frame, and by chromatography column. We also conducted pathway analysis for the outcome of live birth by ionization model. Heat maps were used to display the associations between pollutants and live birth with the top metabolic pathways.
Next, we used a meet-in-the-middle approach to identify the overlapped significant metabolic features and pathways associated with both air pollution and live birth. These overlapped metabolic features were then annotated by matching the m/z value for commonly formed adducts to the METLIN, ChemSpider, Human Metabolome Database (HMDB), and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, using a mass error threshold of 10 ppm (Uppal et al. 2016). To further reduce the possibility of false positive findings, each of the pollutant-driven metabolic features was screened for spectrum peak quality and purity by manual examination of their respective extracted ion chromatographs (EICs). Finally, we used an in-house database of previously confirmed metabolites based on comparison of adduct, m/z, retention time, and liquid chromatography-tandem mass spectrometry (LC-MS/MS) spectra to analytical standards or database spectra to confirm a select number of annotated metabolites with level 1 and level 2 evidence based on the Metabolomics Standards Initiative criteria (Go et al. 2015; Goodacre et al. 2007).
After confirming the identity of the overlapping metabolic features, we estimated the average indirect effect of air pollution on live birth as mediated by the specific metabolite and the direct effect, which is representative of all other causal effects, using the mediation R package (Tingley et al. 2014). P-values for the mediation effects were estimated with 1000 Monte Carlo simulations, using the package default settings.
Results.
The 200 women in our analysis had a mean age of 34.8 y and BMI of 23.9 kg/m2 (Supplemental Table 1). The majority were White (86%), never smokers (74%), with a college degree or higher (92%), who resided in Massachusetts (98%). Serum samples were collected on the day ovarian stimulation began (70%), during the monitoring phase of ovarian stimulation (25%), or on the day of oocyte retrieval (6%). Of the 200 initiated fresh ART cycles, 178 (89%) had at least one embryo transferred and 75 (38%) resulted in live birth.
The median (25th, 75th percentile) exposure to air pollution in the three months prior to serum sample was 24.2 (14.1, 33.4) ppb for NO2, 34.0 (26.1, 42.2) ppb for O3, 8.6 (7.6, 10.0) μg/m3 for PM2.5, and 0.5 (0.4, 0.6) μg/m3 for BC (Supplemental Table 2). Exposure distributions were similar for the other time periods (e.g., 1 to 2 weeks and 1–3 days prior to serum sample). The air pollutants were weakly to moderately correlated with one another (ρ=−0.24 for O3 and BC to 0.46 for NO2 and BC) (Supplemental Table 3). All pollutant exposures were positively correlated within a woman, ranging from 0.26 for PM2.5 exposures in the 3 months and 1 day prior to serum sample to 0.94 for NO2 exposures in the 3 months and 2 weeks prior to serum sample.
From the C18 and HILIC chromatography columns, 10,803 and 12,968 metabolic features were extracted, respectively. Hundreds of metabolic features were associated with each air pollution exposure at a raw p-value<0.05 (Table 1); however, after FDR correction, higher exposure to NO2, O3, PM2.5, and BC in the 3 months prior to serum collection was associated with 37, 5, 104, and 1 feature(s), respectively. Distance to A1/A2 roads, A1/A2/A3 roads, and major roads in Massachusetts was associated with 565, 5, and 35 features, respectively, after controlling for FDR. The top metabolic pathways in the C18 chromatography column, ranked by the total number of the significant air pollution associations, were butanoate (21 out of 27), beta-alanine (20 out of 27), and tryptophan (18 out of 27) metabolism (Supplemental Figure 1A). In the HILIC chromatography column, the top pathways were linoleate metabolism (23 out of 27), urea cycle/amino group metabolism (14 out of 27), and vitamin B3 (niacin) metabolism (13 out of 27) (Supplemental Figure 1B).
Table 1.
Significant metabolic features associated with NO2, O3, PM2.5, and black carbon exposure and distance to major roadways among 200 women in the EARTH Study.
| C18 Negative (N=10803) | HILIC Positive (N=12968) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FDR<0.05 | FDR<0.20 | Raw<0.0005 | Raw<0.005 | Raw<0.05 | FDR0.05 | FDR0.20 | Raw0.0005 | Raw0.005 | Raw0.05 | ||
| Black Carbon | 1 day | 0 | 0 | 2 | 20 | 360 | 0 | 2 | 10 | 49 | 518 |
| 2 day | 0 | 0 | 1 | 15 | 351 | 0 | 0 | 0 | 53 | 479 | |
| 3 days | 0 | 0 | 1 | 17 | 375 | 0 | 0 | 5 | 42 | 486 | |
| 1 week | 0 | 0 | 0 | 21 | 385 | 0 | 0 | 5 | 40 | 478 | |
| 2 weeks | 0 | 0 | 1 | 19 | 343 | 0 | 0 | 5 | 43 | 480 | |
| 3 months | 0 | 0 | 0 | 28 | 353 | 1 | 1 | 6 | 54 | 523 | |
| Nitrogen Dioxide | 1 day | 10 | 32 | 31 | 79 | 504 | 14 | 31 | 31 | 108 | 615 |
| 2 day | 21 | 34 | 32 | 84 | 509 | 15 | 35 | 34 | 105 | 595 | |
| 3 days | 9 | 31 | 30 | 78 | 496 | 8 | 33 | 32 | 95 | 565 | |
| 1 week | 9 | 15 | 21 | 80 | 528 | 4 | 19 | 24 | 85 | 623 | |
| 2 weeks | 9 | 33 | 31 | 92 | 573 | 7 | 34 | 33 | 95 | 627 | |
| 3 months | 18 | 33 | 28 | 102 | 568 | 19 | 47 | 42 | 129 | 829 | |
| Ozone | 1 day | 3 | 19 | 22 | 98 | 714 | 1 | 4 | 12 | 87 | 710 |
| 2 day | 3 | 23 | 26 | 105 | 739 | 1 | 7 | 12 | 88 | 710 | |
| 3 days | 3 | 27 | 27 | 113 | 778 | 1 | 7 | 13 | 92 | 728 | |
| 1 week | 3 | 30 | 28 | 125 | 776 | 2 | 7 | 12 | 97 | 783 | |
| 2 weeks | 1 | 32 | 27 | 122 | 785 | 1 | 7 | 11 | 93 | 798 | |
| 3 months | 3 | 30 | 28 | 140 | 858 | 2 | 5 | 12 | 107 | 832 | |
| Fine Particulate Matter | 1 day | 0 | 0 | 6 | 28 | 387 | 0 | 0 | 7 | 68 | 571 |
| 2 day | 0 | 0 | 7 | 56 | 493 | 0 | 2 | 13 | 74 | 594 | |
| 3 days | 0 | 0 | 10 | 74 | 540 | 1 | 2 | 16 | 100 | 695 | |
| 1 week | 0 | 8 | 11 | 82 | 648 | 6 | 26 | 27 | 140 | 846 | |
| 2 weeks | 5 | 14 | 17 | 85 | 628 | 12 | 36 | 34 | 163 | 977 | |
| 3 months | 21 | 37 | 36 | 136 | 721 | 83 | 209 | 102 | 267 | 1092 | |
| Distance to Roadways | A1,A2 | 0 | 2 | 9 | 80 | 625 | 565 | 1235 | 322 | 759 | 1838 |
| A1, A2, A3 | 3 | 5 | 11 | 42 | 348 | 2 | 3 | 9 | 54 | 477 | |
| MassDOT | 3 | 10 | 22 | 74 | 594 | 32 | 62 | 48 | 172 | 1019 | |
There were 503 and 554 metabolic features associated with live birth at a raw p-value<0.05 (Figure 1A & B); however, none of these features remained significant after FDR correction. After using the features with raw p-value<0.05 as inputs into the pathway analysis, 14 and 12 pathways were significantly associated with live birth in the C18 and HILIC chromatography columns, respectively (Supplemental Table 4). Top hits included vitamin B6 (pyridoxine) and B3 metabolism, ascorbate and aldarate metabolism, tryptophan metabolism, bile acid biosynthesis, and urea cycle/amino group metabolism.
Figure 1.

Significant metabolic features associated with live birth following ART on the C18 negative (Panel A) and HILIC positive (Panel B) platforms. No metabolic features were significantly associated with live birth after FDR correction, therefore we focused on features with a raw p-value<0.05. Red dots represent the features that were significantly lower among women with a live birth and blue dots represent the metabolic features that were significantly higher among women with a live birth.
Using a meet-in-the-middle approach, we found 46 and 144 overlapped metabolic features with raw p-value<0.05, associated with both air pollution and live birth from the C18 and HILIC chromatography columns. Moreover, there were 9 metabolic pathways in each chromatography column that were significantly associated with air pollution and live birth (Figure 2A & B). For example, in both chromatography columns, metabolic features in the tryptophan and vitamin B3 pathway were significantly altered with higher exposure to each pollutant and in women who did and did not have a live birth. Of the 190 metabolic features that were significantly association with air pollution and live birth, 8 were confirmed at Level 1 confidence relative to authentic references (Table 2). These included quinoline, 1-methylnicotinamide, N-methyltryptamine, methyl vanillate, 4-pyridoxate, D-pantothenic acid, docosahexaenoic acid, and N6-(delta2-isopentenyl)-adenine. Upon further evaluate, these metabolites tended to fall into one of three common pathways of action (Figure 3). Three metabolites (D-pantothenic acid, methyl vanillate, 4-pyridoxate) appeared to act as antioxidants-being higher among women with lower exposure to air pollution and women with live births-while two metabolites (N-methyltryptamine, N6-(delta2-isopentenyl)-adenine) appeared to act as oxidants/inflammatory agents– being higher among women with high increased exposure to air pollution and women failing prior to live birth (Figure 3). Associations in the third cluster, involving the metabolites quinoline and docosahexaenoic acid, were difficult to interpret. One metabolite, 1-methylnicotinamide, appeared in two clusters (being symbolic of an antioxidant in its association with NO2 and live birth but having a paradoxical relationship with PM2.5 exposure and distance to major road ways).
Figure 2.
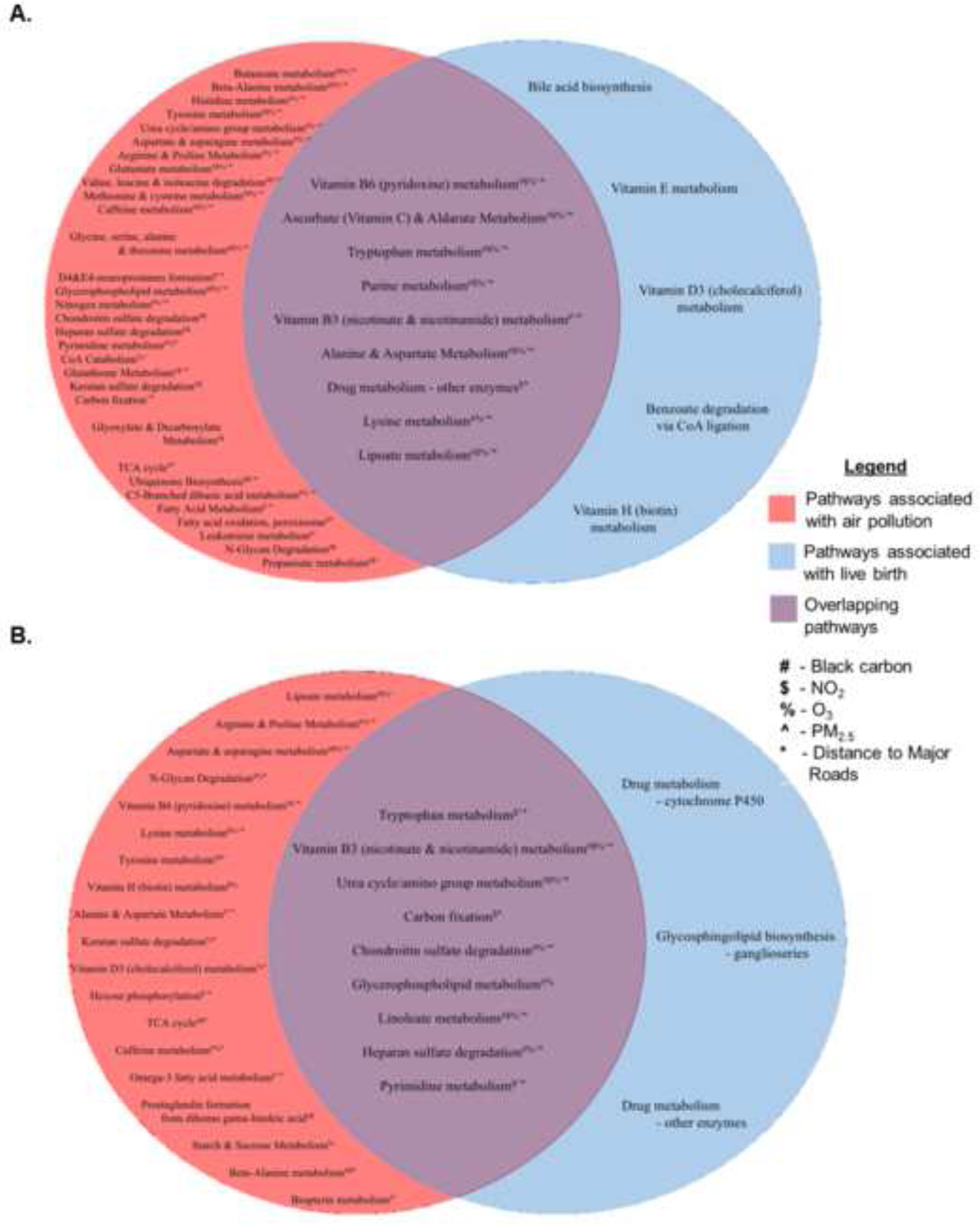
Meet-in-the-middle approach to identify the significant metabolic pathways associated with air pollution exposure and live birth on the C18 negative (Panel A) and HILIC positive (Panel B) platforms.
Table 2.
Chemical identity of the annotated metabolites significantly associated with both air pollution and live birth following ART.
| m/z | RT | Validated Metabolite | Adduct Form | Pollutant | (Lag) | % Change per SD Increase in Exp | % Difference by Live Birth (Y vs. N) | Column |
|---|---|---|---|---|---|---|---|---|
| 130.0657 | 30.8 | quinoline | M+H | Dist to A1/A2 Road | −7.7 (−13.5, −1.5) | 15.5 (0.3, 33.1) | HILIC pos | |
| 137.0709 | 44.0 | 1-methylnicotinamide | M+ | NO2 | (1 day) | −7.0 (−12.8, −0.9) | 18.6 (3.5, 36.0) | HILIC pos |
| NO2 | (2 days) | −6.7 (−12.5, −0.5) | ||||||
| NO2 | (3 days) | −6.4 (−12.2, −0.5) | ||||||
| NO2 | (2 weeks) | −6.2 (−12.1, 0.0) | ||||||
| NO2 | (3 months) | −7.6 (−13.3, −1.5) | ||||||
| PM2.5 | (2 weeks) | 7.5 (0.8, 14.6) | ||||||
| Dist to A1/A2 Road | −7.7 (−13.3, −1.5) | |||||||
| 175.1235 | 29.3 | N-methyltryptamine | M+H | Black Carbon | (1 day) | 38.0 (12.4, 69.5) | −37.8 (−59.0, −5.8) | HILIC pos |
| Black Carbon | (2 days) | 39.1 (13.3, 70.8) | ||||||
| Black Carbon | (3 days) | 31.5 (6.9, 61.7) | ||||||
| 183.0657 | 29.5 | methyl vanillate | M+H | O3 | (1 day) | −6.5 (−11.3, −1.6) | 15.6 (3.3, 29.3) | HILIC pos |
| O3 | (2 days) | −6.6 (−11.3, −1.6) | ||||||
| O3 | (3 days) | −6.6 (−11.3, −1.6) | ||||||
| O3 | (1 week) | −6.3 (−11.0, −1.3) | ||||||
| O3 | (2 weeks) | −6.4 (−11.1, −1.4) | ||||||
| O3 | (3 months) | −6.4 (−11.2, −1.4) | ||||||
| PM2.5 | (2 days) | −6.1 (−10.9, −1.1) | ||||||
| PM2.5 | (3 days) | −6.0 (−10.8, −0.9) | ||||||
| Black Carbon | (2 days) | −5.8 (−10.6, −0.7) | ||||||
| Black Carbon | (3 days) | −6.4 (−11.1, −1.4) | ||||||
| 184.0610 | 40.3 | 4-pyridoxate | M+H | Dist to MassDOT Road | 22.7 (0.8, 49.3 | 73.3 (21.9, 146.5) | HILIC pos | |
| 220.1185 | 35.6 | D-pantothenic acid | M+H | Black Carbon | (1 day) | −18.2 (−26.1, −9.4) | 26.1 (1.1, 57.3) | HILIC pos |
| Black Carbon | (2 days) | −13.5 (−21.9, −4.1) | ||||||
| Black Carbon | (3 days) | −11.8 (−20.4, −2.2) | ||||||
| Black Carbon | (2 weeks) | −12.3 (−20.9, −2.8) | ||||||
| Black Carbon | (3 months) | −14.6 (−22.9, −5.5) | ||||||
| 328.2480 | 27.2 | docosahexaenoic acid | M+H | Black Carbon | (1 day) | 10.3 (1.8, 19.4) | 20.8 (1.9, 43.1) | HILIC pos |
| Black Carbon | (2 days) | 9.0 (0.7, 18.0) | ||||||
| 202.1093 | 37.2 | N6-(delta2-isopentenyl)-adenine | M-H | PM2.5 | (1 day) | 9.5 (0.4, 19.5) | −18.8 (−32.7, −2.0) | C18 neg |
| Black Carbon | (1 day) | 10.2 (1.2, 20.0) | ||||||
Figure 3.

Overview of three common pathways through which the confirmed metabolites mediate the association between air pollution and live birth.
Finally, we evaluated the effect of air pollution on live birth as mediated through the 8 confirmed metabolites. Since there were 31 significant pollutant/exposure periods associated with an annotated metabolite and live birth, we ran a total of 31 models. Five of these models indicated a significant causal mediation effect: exposure to BC in the prior 1, 2, and 3 days and N-methyltryptamine, distance to A1/A2 roads and 1-methylnicotinamide, and exposure to O3 in the prior 3 months and methyl vanillate (Figure 4). In all of these instances, the negative association between the air pollutant and live birth appeared to be completely mediated through the metabolite of interest. In contrast, for the remaining 26 non-significant mediation models, the negative effect of the pollutant on live birth was mediated almost entirely through pathways other than that specific metabolite.
Figure 4.

Significant mediation analyses estimating the indirect (ACME) effect of air pollution on live birth as mediated by annotated metabolites of interest and the average direct effect (ADE). *Not pictured are the mediator models for black carbon exposure in the 1 and 2 days prior to serum sample and N-methyltryptamine as these were identical to the effects shown in Panel A for 3 day average black carbon exposure.
Discussion.
We applied a ‘meet-in-the-middle’ approach to our prospective cohort of 200 women undergoing ART to help improve the biological understanding of the air pollution-fertility associations using untargeted high-resolution metabolomic data. Overall, we identified >20,000 metabolic features in preconception maternal serum samples and demonstrated that air pollution exposures were linked to widespread perturbations in the metabolome. From there, we identified 190 metabolic features and 18 metabolic pathways that were significantly associated with both air pollution and probability of live birth following ART. Eight of these metabolic features were further confirmed, including several metabolites implicated in oxidative stress and inflammation. Following a formal causal mediation analyses, three of these confirmed metabolites (N-methyltryptamine, 1-methylnicotinamide, and methyl vanillate) were found to significantly mediate the association between air pollution and live birth. Our study demonstrates the feasibility of using high-resolution metabolomics in combination with a meet in the middle approach and causal mediation analyses to study human reproduction. Furthermore, our preliminary results provide novel mechanistic insight into the potential biological pathways that may be mediating the association between air pollution and fertility.
To date, over 20 human studies have evaluated the association between air pollution and untargeted high resolution metabolomics (Liang et al. 2020); however, only one previous study has investigated this relationship in women during the prenatal period (Q. Yan et al. 2019). Among 160 pregnant women residing in California, Yan and colleagues assessed exposure to traffic-related air pollution (CO, NOx, PM2.5) during the first trimester in relation to serum metabolites measured in mid-pregnancy (~16 weeks gestation) (Q. Yan et al. 2019). The authors found that higher exposure to air pollution was related to alterations in several oxidative stress and inflammatory pathways including amino acid, fatty acid, phospholipid, linoleate, and eicosanoid metabolism. We too identified the majority of these same pathways. For example, urea cycle/amino group, butanoate, histidine, and linoleate metabolism were some of our top metabolic pathways associated with air pollution, similar to their findings. However, while we observed some perturbations in fatty acid and phospholipid metabolism pathways associated with air pollution exposure, we did not observe the robust associations reported in their study. There are several potential explanations for this discrepancy, including differences in the air pollutants examined (and in composition of PM2.5 between MA and CA), the timing and method of exposure assessment, study population demographics and pre-existing conditions, when the blood sample was drawn (e.g., during pregnancy versus preconception), and stringency of multiple comparison correction.
In the field of assisted reproduction, the majority of studies utilizing metabolomics have focused on the embryonic metabolome by analyzing spent culture media or focused on follicular fluid metabolites to better understand oocyte competence and specific infertility pathologies (McRae et al. 2013). So far, only one small pilot study has used untargeted serum metabolomics to identify biomarkers of response to ovarian stimulation (Borges et al. 2019) and none have focused on clinical outcomes such as live birth. In the study by Borges et al. (n=30), a principal component analysis selected 10 serum metabolites that were predictive of high vs. low response to stimulation; however, none of these ions were chemically identified which precluded comparison with our results. In our study, we found that over 500 metabolic features in each column were associated with probability of live birth at a p-value<0.05 but none of these remained significant after FDR correction. When we used these features as inputs into our pathway analysis, tryptophan metabolism, vitamin B3, B6, C, and D metabolism, and bile acid biosynthesis (in addition to many others) were all found to be dysregulated comparing women who did and did not have a live birth.
Using analytical standards, we were able to confirm the identity of 8 metabolites associated with both air pollution and live birth with Level 1 confidence. Most of these metabolites are implicated in amino acid and nutrient metabolism with downstream effects on oxidative stress and inflammation. We were also able to sort these 8 confirmed metabolites into 3 clusters that may help shed insight into similar biologic functions. The first cluster consisted of four metabolites acting as possible antioxidants (e.g., women with higher levels of the metabolite had lower exposure to air pollution had higher probability of live birth). Interestingly, three of these metabolites, 1-methylnicotainamide, D-pantothenic acid, and 4-pyridoxate, are related to essential nutrient metabolism (e.g., niacin, pantothenate (Coenzyme A), pyridoxine) which each play a critical role in human reproduction. Vitamin B3 (nicotinate/nicotinamide) and Vitamin B6 (pyridoxine) metabolism were also two key metabolic pathways identified in the meet in the middle approach linking air pollution to live birth. A confirmed metabolite of specific interest was 1-Methylnicotinamide, the methylation product of nicotinamide under insufficient ATP supply for energy, which was lower among women with higher NO2 exposure and higher among women with live births. A study in skin cells demonstrated that nicotinamide can act as an antioxidant, protecting against PM2.5 induced oxidative stress and cell damage (Zhen et al. 2019). In our study, levels of 1-methylnicotinamide were lower with increasing exposure to NO2 suggesting similar mechanisms could be at play. An experimental study in mice found that dietary administration of nicotinamide reduced embryonic deaths, prolonged pregnancy, and ameliorated fetal growth restriction by inhibiting adenosine diphosphate ribosyl cyclase which relaxes blood vessels and improves the condition of the endothelium (Li et al. 2016). Nicotinamide is also hypothesized to act through the NAD+ salvage pathway, improving the metabolic state of hypoxic embryos by normalizing their production of ATP (Takahashi et al. 2018). Therefore, the higher levels of 1-methylnicotinamide we observed among women with live births is biologically supported by mechanistic research in animals.
The second, intuitive cluster of confirmed metabolites consisted of N-methyltryptamine and N6-(delta2-isopentenyl)-adenine, which were both compounds that increased with higher exposure to air pollution (specifically PM2.5 and black carbon) and were lower among women with live births. Moreover, N-methyltryptamine, a tryptophan metabolite, was found to significantly mediate the negative association between black carbon exposure in the 1–3 days prior to serum sample and lower odds of live birth. N-methyltryptamine levels increased with higher exposure to black carbon, which may be a possible indicator of increased immune system activity (Schrocksnadel et al. 2006). Previous data from both experimental and observational human studies suggests that air pollution alters tryptophan metabolism (Li et al. 2021; Liang et al. 2019; Liang et al. 2020). For example, a randomized cross-over trial of sham and real air purifiers in Chinese college students found that higher exposure to PM2.5 significantly altered several amino acids related to tryptophan metabolism in serum (Li et al. 2017). In addition to its antioxidant properties (Xu et al. 2017), tryptophan (and its metabolites) have also been shown to play an important role in pregnancy due to the increase demand for: 1) proteins to support fetal growth and development (Badawy 2015), 2) serotonin for signaling pathways (O’Mahony et al. 2015), 3) kynurenic acid for neuronal protection (Wirthgen et al. 2017), 4) quinolinic acid for NAD+ synthesis (Sahm et al. 2013), and 5) and other kynurenines for suppressing fetal rejection (Badawy 2015). If tryptophan is depleted in mother during early pregnancy, none of these processes can be optimally achieved. It is also interesting that vitamin B6 metabolism was implicated in the association between air pollution and fertility because several rate-limiting enzymes in tryptophan catabolism use pyridoxal 5′-phosphate (PLP), the active form of vitamin B6, as a cofactor (da Silva et al. 2013). 4-pyridoxate, a downstream metabolite of PLP, was also identified as a key mediator of the association between distance to roadways and live birth in our analysis with further distance from roads being associated with higher levels and higher levels being associated with higher odds of live birth. N6-(delta2isopentenyl)-adenine, the terpene derivative of adenine could indicate a novel and specific mechanism that is worthy of follow-up in future studies.
Finally, the associations identified between confirmed metabolites in the third cluster with air pollution and live birth were non-intuitive and difficult to interpret. For example, exposure to black carbon, which is typically negatively associated with live birth, was positively associated with docosahexaenoic acid, which was positively associated with live birth. There are several common explanations for these paradoxical findings such as residual confounding by some other environmental/behavior factor or chance due to the high number of statistical tests performed; however, in either case, these will require further investigation.
While our study provides many insights into potential biological mechanisms, it is also not without limitations. Given the large number of pollution exposures and time windows we examined in combination with the tens of thousands of metabolites we identified, there is substantial risk of false positives due to multiple comparisons and Type 1 errors. While we attempted to address this using different levels of FDRB-H correction, we often had to apply a cut-off of raw p-value<0.05 for input into the pathway analyses to ensure that there would be sufficient features for a meaningful interpretation. Because our study was relatively small and designed to be exploratory, we ultimately decided on less stringent criteria for statistical significance to decrease the likelihood of false negatives. Therefore, considering the elevated chance of false positive findings, results should be interpreted with caution and requires validation in larger cohorts. Second, although we used validated, spatio-temporal models with high spatial resolution to estimate air pollution exposure at women’s residential address, this is still an imperfect proxy of personal air pollution exposure and likely decreased the precision of our effect estimates. However, because this was a prospective study and women were unaware of their air pollution exposure, there is no reason to believe that the error or uncertainty in our air pollution exposure assessment was related probability of live birth. Therefore, we would only expect bias towards the null. Third, while we were able to account for a wide variety of lifestyle characteristics, since this was an observational study, there still remains the possibility of residual confounding. This is particularly relevant to our causal mediation analyses where we needed to make fairly strong exchangeability assumptions for our inferences to remain valid. Moreover, while we adjusted for environmental factors such as ambient temperature that are often highly correlated with air pollution exposure, it is possible that the metabolites and metabolic pathways we identified as probable mediators are not specific to air pollution. Future work examining the effects of other environmental chemicals on the serum metabolome and human fertility will enable us to further evaluate the specificity of these metabolites and pathways. In addition, the use of non-fasting blood samples may have had an impact on the metabolomics results. To minimize the potential impact of non-fasting state, we applied a comprehensive metabolomics workflow, which we and others have successfully applied to the analysis of non-fasting samples, using pooled standards and internal references. Finally, the majority of our women were White and highly educated, which is typical of studies focusing on infertility clinic populations but may limit the applicability of our findings to other race/ethnicities and socioeconomic status. The women in our study’s exposure to air pollution also tended to be low. Therefore, it is possible we may have underestimated or missed associations that could be present in other, more highly polluted regions.
In summary, we successfully applied a ‘meet-in-the-middle’ approach using untargeted high-resolution metabolomics to produce evidence of common and disease-specific pathway perturbations in the etiological relationship between air pollution exposure and fertility among a prospective cohort of women undergoing assisted reproduction. These results further support high resolution metabolomics as a powerful platform for elucidating biologically relevant pathways associated with exposures to key environmental pollutants and health outcomes, such as fertility. While highly intriguing, our findings warrant replication in future targeted and untargeted studies.
Supplementary Material
Highlights.
We used untargeted high-resolution metabolomics to identify metabolites and pathways associated with periconception exposure to air pollution and probability of live birth following assisted reproductive technology (ART).
In total, we identified 190 metabolic features and 18 metabolic pathways that were significantly associated with both air pollution and probability of live birth following ART.
Eight metabolic features were confirmed at Level 1 confidence, including several metabolites implicated in oxidative stress and inflammation.
Our results provide novel mechanistic insight into the potential biological pathways that may be mediating the association between air pollution and fertility.
Acknowledgments:
We would like to thank all members of the EARTH study team, specifically principal investigator Russ Hauser, research nurse Myra G. Keller, senior research staff Ramace Dadd, the physicians and staff at Massachusetts General Hospital Fertility Center, and all the EARTH study participants.
Funding:
Supported by grants ES009718, ES022955, ES000002, and ES026648 from the National Institute of Environmental Health Sciences (NIEHS). The study was also supported by HERCULES through the NIEHS (P30 ES019776). This publication was also made possible by U.S. Environmental Protection Agency (U.S. EPA): RD-834798 and RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests: All authors declare no actual or potential competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References.
- Abu Awad Y, Koutrakis P, Coull BA, Schwartz J. 2017. A spatio-temporal prediction model based on support vector machine regression: Ambient Black Carbon in three New England States. Environ Res 159: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z, Jin Y, Li J, Li W, Wu W. 2018. Impact of Particulate Air Pollution on Cardiovascular Health. Curr Allergy Asthma Rep 18(3): 15. [DOI] [PubMed] [Google Scholar]
- Badawy AA. 2015. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep 35(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges E Jr., Montani DA, Setti AS, Zanetti BF, Figueira RCS, Iaconelli A Jr., et al. 2019. Serum metabolites as predictive molecular markers of ovarian response to controlled stimulation: a pilot study. JBRA Assist Reprod 23(4): 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet SL, Zhou Y, Shriber J, Kissin DM, Strosnider H, Shin M. 2019. Ambient air pollution and in vitro fertilization treatment outcomes. Hum Reprod 34(10): 2036–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. 2010. The antral follicle count: practical recommendations for better standardization. Fertil Steril 94(3): 1044–1051. [DOI] [PubMed] [Google Scholar]
- Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA 3rd, et al. 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed]
- Carre J, Gatimel N, Moreau J, Parinaud J, Leandri R. 2017a. Does air pollution play a role in infertility?: a systematic review. Environ Health 16(1): 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre J, Gatimel N, Moreau J, Parinaud J, Leandri R. 2017b. Influence of air quality on the results of in vitro fertilization attempts: A retrospective study. Eur J Obstet Gynecol Reprod Biol 210: 116–122. [DOI] [PubMed] [Google Scholar]
- Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, et al. 2012. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30(10): 918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa Vizcaino MA, Gonzalez-Comadran M, Jacquemin B. 2016. Outdoor air pollution and human infertility: a systematic review. Fertil Steril 106(4): 897–904 e891. [DOI] [PubMed] [Google Scholar]
- Choe SA, Jun YB, Lee WS, Yoon TK, Kim SY. 2018. Association between ambient air pollution and pregnancy rate in women who underwent IVF. Hum Reprod 33(6): 1071–1078. [DOI] [PubMed] [Google Scholar]
- da Silva VR, Rios-Avila L, Lamers Y, Ralat MA, Midttun O, Quinlivan EP, et al. 2013. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr 143(11): 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Chen F, Zhang M, Lan L, Qiao Z, Cui Y, et al. 2016. Association between air pollution and sperm quality: A systematic review and meta-analysis. Environ Pollut 208(Pt B): 663–669. [DOI] [PubMed] [Google Scholar]
- Di Q, Rowland S, Koutrakis P, Schwartz J. 2017. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc 67(1): 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai HF, An JX, Qian XY, Wei YJ, Williams JP, Gao GL. 2017. Ovarian Damages Produced by Aerosolized Fine Particulate Matter (PM2.5) Pollution in Mice: Possible Protective Medications and Mechanisms. Chin Med J (Engl) 130(12): 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Fong KC, Abu Awad Y, Di Q, Minguez-Alarcon L, Chavarro JE, et al. 2019a. TimeVarying Exposure to Air Pollution and Outcomes of in Vitro Fertilization among Couples from a Fertility Clinic. Environ Health Perspect 127(7): 77002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Hart JE, Chavarro JE, Missmer SA, Rich-Edwards JW, Laden F, et al. 2019b. Air pollution exposure and risk of spontaneous abortion in the Nurses’ Health Study II. Hum Reprod 34(9): 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Hart JE, Minguez-Alarcon L, Chavarro JE, Laden F, Coull BA, et al. 2018. Residential proximity to major roadways and traffic in relation to outcomes of in vitro fertilization. Environ Int 115: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Minguez-Alarcon L, Fong KC, Abdelmessih S, Coull BA, Chavarro JE, et al. 2019c. Exposure to Fine Particulate Matter and Ovarian Reserve Among Women from a Fertility Clinic. Epidemiology 30(4): 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Minguez-Alarcon L, Williams PL, Chavarro JE, Schwartz JD, Kloog I, et al. 2020. Ambient air pollution and risk of pregnancy loss among women undergoing assisted reproduction. Environ Res 191: 110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, et al. 2015. Reference Standardization for Mass Spectrometry and High-resolution Metabolomics Applications to Exposome Research. Toxicol Sci 148(2): 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre R, Broadhurst D, Smilde AK, Kristal BS, Baker JD, Beger R, et al. 2007. Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics 3(3): 231–241. [Google Scholar]
- I International Agency for Research on Cancer (IARC). 2013. Air Pollution and Cancer. France: World Health Organization. [Google Scholar]
- Inoue K, Yan Q, Arah OA, Paul K, Walker DI, Jones DP, et al. 2020. Air Pollution and Adverse Pregnancy and Birth Outcomes: Mediation Analysis Using Metabolomic Profiles. Curr Environ Health Rep 7(3): 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Dziewirska E, Radwan M, Hanke W. 2018. Air pollution from natural and anthropic sources and male fertility. Reprod Biol Endocrinol 16(1): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Raz R, Wilson A, Fluss R, Nirel R, Broday DM, et al. 2019. Traffic-related Air Pollution and Pregnancy Loss. Epidemiology 30(1): 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. 2014. A New Hybrid Spatio-Temporal Model For Estimating Daily Multi-Year PM2.5 Concentrations Across Northeastern USA Using High Resolution Aerosol Optical Depth Data. Atmos Environ (1994) 95: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente R, Garcia-Blaquez N, Jacquemin B, Checa MA. 2016. Outdoor air pollution and sperm quality. Fertil Steril 106(4): 880–896. [DOI] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, Kim HC. 2015. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol 30: e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Koutrakis P. 2014. Daily ambient NO2 concentration predictions using satellite ozone monitoring instrument NO2 data and land use regression. Environ Sci Technol 48(4): 2305–2311. [DOI] [PubMed] [Google Scholar]
- Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. 2010. Effect of air quality on assisted human reproduction. Hum Reprod 25(5): 1317–1324. [DOI] [PubMed] [Google Scholar]
- Leiser CL, Hanson HA, Sawyer K, Steenblik J, Al-Dulaimi R, Madsen T, et al. 2019. Acute effects of air pollutants on spontaneous pregnancy loss: a case-crossover study. Fertil Steril 111(2): 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Fushima T, Oyanagi G, Townley-Tilson HW, Sato E, Nakada H, et al. 2016. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc Natl Acad Sci U S A 113(47): 13450–13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. 2017. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation 136(7): 618–627. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou L, Feng T, Hao G, Yang S, Wang N, et al. 2020. Ambient air pollution exposed during preantral-antral follicle transition stage was sensitive to associate with clinical pregnancy for women receiving IVF. Environ Pollut 265(Pt B): 114973. [DOI] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, et al. 2013. Predicting network activity from high throughput metabolomics. PLoS Comput Biol 9(7): e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liang D, Ye D, Chang HH, Ziegler TR, Jones DP, et al. 2021. Application of high-resolution metabolomics to identify biological pathways perturbed by traffic-related air pollution. Environ Res 193: 110506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Ladva CN, Golan R, Yu T, Walker DI, Sarnat SE, et al. 2019. Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environment international 127: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Li Z, Vlaanderen J, Tang Z, Vermeulen R, Sarnat JA. 2020. Systematic Review on Untargeted Metabolomics Application for Air Pollution Health Research: Current Progress, Analytical Challenges, and Future Direction. Presented at the Annual Meeting of the International Society for Exposure Science. [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, et al. 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ Int 120: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Guo Y, Di Q, Zheng Y, Kowal P, Xiao J, et al. 2017. Ambient PM2.5 and Stroke: Effect Modifiers and Population Attributable Risk in Six Low- and Middle-Income Countries. Stroke 48(5): 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Nellis M, Uppal K, Ma C, Tran V, Liang Y, et al. 2020. Reference Standardization for Quantification and Harmonization of Large-Scale Metabolomics. Anal Chem 92(13): 8836–8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MassGIS Data - Massachusetts Department of Transportation (MassDOT) Roads. The Massachusetts Department of Transportation - Office of Transportation Planning; 2014. Available: http://www.mass.gov/anf/research-and-tech/it-serv-and-support/application-serv/office-of-geographic-information-massgis/datalayers/eotroads.html [accessed June 2 2017].
- McRae C, Sharma V, Fisher J. 2013. Metabolite Profiling in the Pursuit of Biomarkers for IVF Outcome: The Case for Metabolomics Studies. International Journal of Reproductive Medicine 2013: 603167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Minguez-Alarcon L, Dadd R, et al. 2018. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open 2018(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Basagana X, Dadvand P, Martinez D, Cirach M, Beelen R, et al. 2014. Air pollution and human fertility rates. Environ Int 70: 9–14. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. 2015. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277: 32–48. [DOI] [PubMed] [Google Scholar]
- Ogliari KS, Lichtenfels AJ, de Marchi MR, Ferreira AT, Dolhnikoff M, Saldiva PH. 2013. Intrauterine exposure to diesel exhaust diminishes adult ovarian reserve. Fertil Steril 99(6): 1681–1688. [DOI] [PubMed] [Google Scholar]
- Oresic M, McGlinchey A, Wheelock CE, Hyotylainen T. 2020. Metabolic Signatures of the Exposome-Quantifying the Impact of Exposure to Environmental Chemicals on Human Health. Metabolites 10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin PM, Maluf M, Czeresnia CE, Januario DA, Saldiva PH. 2010a. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET). J Assist Reprod Genet 27(7): 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januario DA, Nascimento Saldiva PH. 2010b. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril 93(1): 301–303. [DOI] [PubMed] [Google Scholar]
- Qiu J, Dong M, Zhou F, Li P, Kong L, Tan J. 2019. Associations between ambient air pollution and pregnancy rate in women who underwent in vitro fertilization in Shenyang, China. Reprod Toxicol 89: 130–135. [DOI] [PubMed] [Google Scholar]
- Quraishi SM, Lin PC, Richter KS, Hinckley MD, Yee B, Neal-Perry G, et al. 2019. Ambient Air Pollution Exposure and Fecundability in Women Undergoing In Vitro Fertilization. Environ Epidemiol 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm F, Oezen I, Opitz CA, Radlwimmer B, von Deimling A, Ahrendt T, et al. 2013. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res 73(11): 3225–3234. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. 2006. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 364(1–2): 82–90. [DOI] [PubMed] [Google Scholar]
- Shah PS, Balkhair T. 2011. Air pollution and birth outcomes: a systematic review. Environ Int 37(2): 498–516. [DOI] [PubMed] [Google Scholar]
- Simon-Manso Y, Lowenthal MS, Kilpatrick LE, Sampson ML, Telu KH, Rudnick PA, et al. 2013. Metabolite profiling of a NIST Standard Reference Material for human plasma (SRM 1950): GC-MS, LC-MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Anal Chem 85(24): 11725–11731. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Li F, Fushima T, Oyanagi G, Sato E, Oe Y, et al. 2018. Vitamin B3 Nicotinamide: A Promising Candidate for Treating Preeclampsia and Improving Fetal Growth. Tohoku J Exp Med 244(3): 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. 2014. Mediation: R package for causal mediation analysis. Journal of Statistical Software 59(5): 1–38.26917999 [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, et al. 2013. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP. 2016. Computational Metabolomics: A Framework for the Million Metabolome. Chem Res Toxicol 29(12): 1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Guimaraes Silva RM, Scoriza JN, Saldiva PH, Caldini EG, et al. 2009. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res 109(5): 536–543. [DOI] [PubMed] [Google Scholar]
- Walker DI, Valvi D, Rothman N, Lan Q, Miller GW, Jones DP. 2019. The metabolome: A key measure for exposome research in epidemiology. Curr Epidemiol Rep 6: 93–103. [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cai J, Liu L, Jiang X, Li P, Sha A, et al. 2019. Association between outdoor air pollution during in vitro culture and the outcomes of frozen-thawed embryo transfer. Hum Reprod 34(3): 441–451. [DOI] [PubMed] [Google Scholar]
- Wirthgen E, Hoeflich A, Rebl A, Gunther J. 2017. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front Immunol 8: 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Liu H, Bai M, Gao J, Wu X, Yin Y. 2017. Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy. Int J Mol Sci 18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T, Zhu T. 2018a. Association between fertility rate reduction and pre-gestational exposure to ambient fine particles in the United States, 2003–2011. Environ Int 121(Pt 1): 955–962. [DOI] [PubMed] [Google Scholar]
- Xue T, Zhu T. 2018b. Increment of ambient exposure to fine particles and the reduced human fertility rate in China, 2000–2010. Sci Total Environ 642: 497–504. [DOI] [PubMed] [Google Scholar]
- Yan Q, Liew Z, Uppal K, Cui X, Ling C, Heck JE, et al. 2019. Maternal serum metabolome and traffic-related air pollution exposure in pregnancy. Environment International 130: 104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Liew Z, Uppal K, Cui X, Ling C, Heck JE, et al. 2019. Maternal serum metabolome and traffic-related air pollution exposure in pregnancy. Environ Int 130: 104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Johnson JM, Jones DP. 2009. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics 25(15): 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Jin S, Chen X, Qiu Y. 2020. Association between Ambient Air Pollution and Pregnancy Outcomes in Patients Undergoing In Vitro Fertilization in Chengdu, China: A retrospective study. Environ Res 184: 109304. [DOI] [PubMed] [Google Scholar]
- Zhen AX, Piao MJ, Kang KA, Fernando P, Kang HK, Koh YS, et al. 2019. Niacinamide Protects Skin Cells from Oxidative Stress Induced by Particulate Matter. Biomol Ther (Seoul): 562–569. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


