Abstract
Phenotypic flexibility allows individuals to reversibly modify trait values and theory predicts an individual’s relative degree of flexibility positively correlates with the environmental heterogeneity it experiences. We test this prediction by integrating surveys of population genetic and physiological variation with thermal acclimation experiments and indices of environmental heterogeneity in the Dark-eyed Junco (Junco hyemalis) and its congeners. We combine field measures of thermogenic capacity for 335 individuals, 22,006 single nucleotide polymorphisms genotyped in 181 individuals, and laboratory acclimations replicated on five populations. We show that Junco populations: (1) differ in their thermogenic responses to temperature variation in the field; (2) harbor allelic variation that also correlates with temperature heterogeneity; and (3) exhibit intra-specific variation in thermogenic flexibility in the laboratory that correlates with the heterogeneity of their native thermal environment. These results provide comprehensive support that phenotypic flexibility corresponds with environmental heterogeneity and highlight its importance for coping with environmental change.
Subject terms: Ecophysiology, Evolutionary ecology, Evolutionary genetics, Metabolism
Theory predicts that organisms in varied environments should evolve to be more phenotypically flexible. Evidence combining genetic and physiological variation with thermal acclimation experiments shows that the thermogenic flexibility of wild juncos is greatest in populations where temperatures are most variable.
Introduction
Temporal environmental variation imposes changing selection pressures that can result in multiple fitness optima occurring over time. In an effort to meet these changing fitness optima, an individual may express multiple trait values in the form of phenotypic flexibility (i.e., the ability to reversibly modify a trait value)1. Phenotypic flexibility provides an individual with repeated opportunities to match its phenotype to environmental conditions across its lifetime, and is predicted to evolve in environments characterized by frequent and predictable variation2,3. Accordingly, flexibility can represent an adaptive acclimatization response to environmental variation, especially for long-lived organisms4. Determining the causes and consequences of variation in flexibility among individuals is therefore crucial to our understanding of evolutionary processes and our ability to predict species’ resilience to environmental change.
Because flexibility should increase fitness in variable environments, theory suggests that the magnitude of flexibility that individuals exhibit should positively correlate with the amount of environmental heterogeneity they experience5–7. If flexibility can be locally adapted in this way, empirical evidence for this theory should support three predictions: (1) geographic variation in flexibility corresponds with environmental heterogeneity, (2) genetic variation underlies this phenotypic variation, such that differences in flexibility can be replicated under common garden conditions, and (3) the degree of flexibility should be subjected to natural selection due to its influence on fitness, resulting in a reduction of variability in flexibility for populations occupying more heterogenous environments. Although there is some evidence that geographic variation in the degree of flexibility is associated with spatial patterns of environmental heterogeneity8–10 (i.e., Prediction 1), there are very few empirical demonstrations supporting the other two predictions.
One common axis along which many environments vary is temperature. Whether daily or seasonally, nearly all environments vary in temperature over time. To meet the demands imposed by ambient thermal variation, most organisms exhibit behavioral and physiological responses to maintain homeostasis. For instance, many ectotherms alter their activity patterns and use of microclimates in response to changes in their thermal environment11–13. While endotherms can make similar behavioral alterations, they can also flexibly adjust their physiological ability to endogenously produce heat14,15. The ability to endogenously regulate body temperature can buffer endotherms from ambient thermal variation but it also comes with substantial costs.
Temperate environments, in particular, place a premium on endogenous heat production in small homeothermic endotherms16. Small birds and mammals commonly increase their thermogenic capacity (the ability to generate heat) during high-latitude winters. Failure to achieve adequate thermogenic output can have dire consequences for organismal fitness17–19. Increases in thermogenic capacity are also accompanied by physiological changes occurring at lower levels of biological organization20–23. Thus, a higher thermogenic capacity may be energetically costly to maintain due to the additional metabolic machinery required to achieve elevated thermogenesis24. Phenotypic flexibility in thermogenic capacity could therefore help mediate a balance between thermoregulation and its associated maintenance costs in response to fluctuating selective pressures25. Despite these expectations, it is unknown whether an individual’s capacity for thermogenic flexibility is influenced by the degree of thermal variability it experiences throughout the year.
To test the influence of thermal heterogeneity on variation in thermogenic flexibility, we leveraged the natural environmental variation present across the dark-eyed Junco (Junco hyemalis) distribution. J. hyemalis is a temperate songbird comprised of 14 subspecies that inhabit a broad range of environments and exhibit conspicuous differences in life history, migratory tendency, physiology, size, song, plumage, and behavior26–28. Lab and field studies have demonstrated that J. hyemalis increases its thermogenic capacity in the cold21,29,30 and this heightened thermogenic capacity is associated with a reduced risk of hypothermia30,31. We, therefore, expected that there may be variation in thermogenic flexibility among different Junco populations that correlates with the amount of temperature variation they experience.
In this study, we test this expectation by quantifying associations between environmental heterogeneity, population genetic structure, and flexibility. First, we present a survey of geographic variation in Junco thermogenic capacity (quantified as the maximum O2 consumption under cold exposure, summit metabolism; Msum) from field sites across the United States that host different Junco taxa. We include both J. hyemalis and J. phaeonotus because prior work indicates that the two clades are not reciprocally monophyletic32. We correlate these measures with recent weather data to determine which environmental indices correspond to variation in Msum in the field. If juncos exhibit geographic variation in Msum flexibility (Prediction 1), we expect to find a population × environment interaction in the field. We also expect that the magnitude of this flexibility (modeled as the slope of these population-level reactions norms33) will positively correlate with the annual thermal regime, which we use as a proxy for thermal heterogeneity. Second, we characterize fine-scale, range-wide population genetic variation within the Junco genus. We correlate variation in allele frequencies with environmental heterogeneity using genotype-environment association methods, which can aid in the detection of signatures of natural selection in instances where populations are not clearly distinguishable and environmental gradients are continuous34. We expect that, if there is genetic variation underlying potential differences in Junco flexibility (Prediction 2), genetic variation will be structured by the same environmental index that structures phenotypic variation. Finally, we present an acclimation experiment performed in the lab using five Junco populations to test whether the annual thermal regime (i.e., thermal heterogeneity) predicts the degree of flexibility they exhibit under controlled conditions while accounting for genetic relatedness among populations. We exposed individuals from each population to cold or control temperature treatments and quantified their thermogenic flexibility (defined as their change in Msum with acclimation). We expect that junco populations that experience greater seasonal temperature variation will exhibit higher Msum flexibility in the cold than those from more thermally stable regions (Prediction 2), represented by an interaction between temperature treatment and native temperature range. We also expect to find that populations from the most variable regions will show the least variability in their flexible response, resulting in lower coefficients of variation (CV) in Msum in the cold (Prediction 3). By combining these approaches, our results shed light on the ecological conditions that promote the evolution of increased flexibility and address long-standing hypotheses in the field of evolutionary biology.
Results
Intraspecific variation in thermogenic capacity
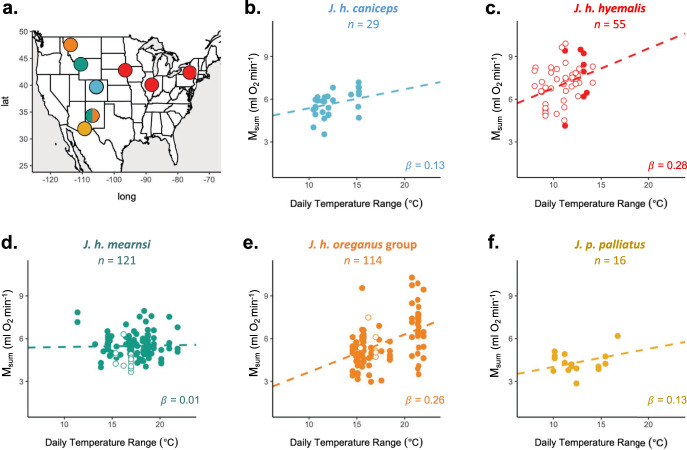
To test whether juncos exhibit geographic variation in Msum flexibility, we assayed Msum for 335 juncos, including five Junco taxa (J. h. caniceps, J. h. hyemalis, J. h. mearnsi, J. h. oreganus group, and J. p. palliatus), at sites across the United States (Fig. 1a). The number of individuals, number of sampling days, seasons (breeding or non-breeding), and years varied across sites with 100 total site-days of environmental variation included in our dataset. Variation in Msum corresponded to recent weather variation, with mean daily temperature range (Td_range) in the 8 days prior to capture explaining more variation than the other environmental variables tested (Table S1). The most well-supported model, explaining 48% of the variation in Msum (AIC = 960.9), included body mass (Mb), Td_range, taxon, and Td_range × taxon. The addition of a term for the season did not improve model fit (∆AIC = 0.14; β = −0.28 ± 0.21, p = 0.18). Mb positively correlated with Msum, such that larger individuals exhibited higher thermogenic capacity (β = 1.17 ± 0.15 ml O2 min−1, p = 1.1 × 10−13). Furthermore, juncos that experienced greater Td_range had elevated Msum, suggesting that they may acclimatize to short-term heterogeneity in their thermal environment (β = 1.87 ± 0.24, p = 6.0 × 10−14). The model also showed persistent differences in Msum among taxa while controlling for differences in Mb and Td_range, with J. p. pallitatus exhibiting the lowest (β = −1.36 ± 0.48, p = 4.1 × 10−3) and J. h. hyemalis the highest Msum values (β = 2.23 ± 0.46, p = 1.8 × 10−6). Moreover, we also found that the inclusion of the Td_range × taxon interaction term substantially improved model fit indicating that some taxa respond to variation in Td_range more strongly than others (model without interaction: ∆AIC = 15.4; Fig. 1b–f). The magnitude of flexibility (i.e., the resulting slopes of the taxon-specific reaction norms from this best model) did not correlate with an index of thermal heterogeneity (measured as the mean annual temperature range across capture sites) among taxa (R2 = 0.10, p = 0.61, n = 5). However, when J. h. mearnsi was removed from the analysis, we found a strong correlation between magnitude of flexibility and annual temperature range (R2 = 0.92, β = 0.02 ± 0.004, p = 0.04, n = 4; Fig. S1), as outlined in our first prediction.
Fig. 1. Relationships between daily temperature range and thermogenic capacity (Msum) differ among five Junco taxa assayed in the field.
a Map of sampling sites for field measurements color-coded by taxon, following color scheme in b–f and plotted in R with package mapdata87. b–f Taxon-specific relationships between Msum and daily temperature range (averaged over the 8 days prior to capture), while controlling for differences in body mass. Each dot represents an individual (n = 335). Sample sizes for each taxon are listed at the top and the slope (β; in ml O2 min−1 °C−1) of each relationship is shown in the lower right corner. Sampling occurred during the breeding season (filled circle) or nonbreeding season (open circle). Lines fit from glm (Msum = Mb + Taxa × Td_range) using mean Mb for each taxon. Source data are provided in the Source Data file.
Genotype-environment associations
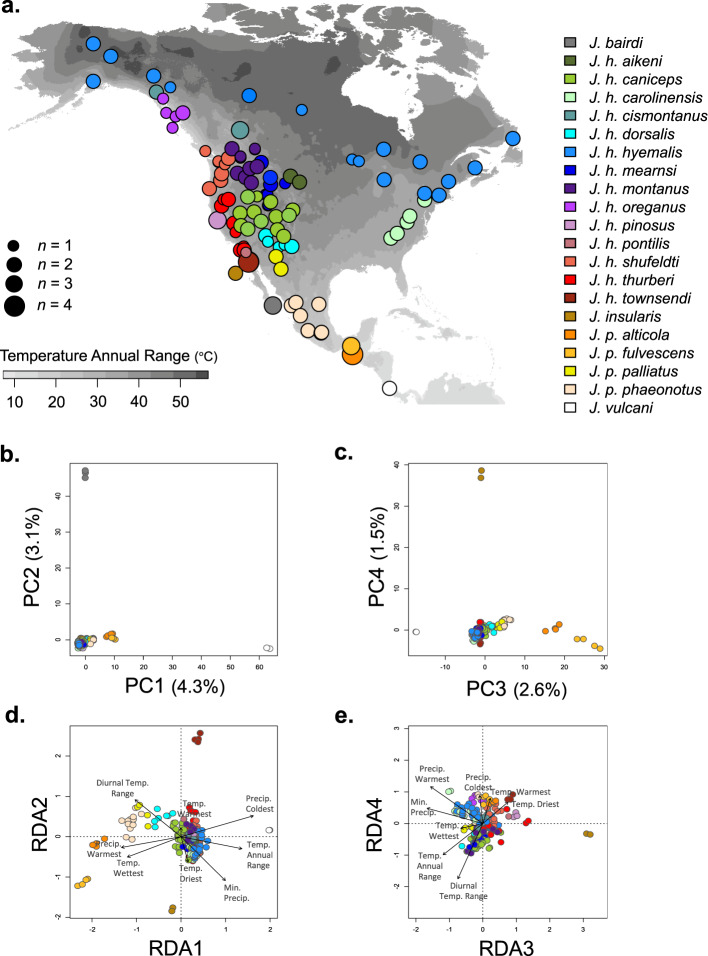
To understand how population genetic variation across the genus might correspond to environmental variation, we genotyped 22,006 biallelic SNPs from 181 individuals representing all recognized Junco species and subspecies (Fig. 2a). The first two axes from a principal component analysis (PCA) explained 7.5% of the total genetic variance. Major clusters identified by the PCA corresponded to the lineages of Costa Rica and Panama (J. vulcani), southern Mexico and Guatemala (J. phaeonotus alticola and J. p. fulvescens), southern Baja (J. bairdi), and Guadalupe Island (J. insularis), with all other taxa (J. p. palliatus, J. p. phaeonotus, and all J. hyemalis) grouping together (Fig. 2b, c). We subtracted the variance explained by PC1 and PC2 in our genotype-environment associations (performed using redundancy analysis; RDA) to control for population history and structure35–37. After controlling for background population structure, the remaining signatures of differentiation can be related to environmental variables and, as such, these relationships may reflect local adaptation to environmental conditions. In our partial RDA, eight axes composed of eight climatic variables explained 2.9% of the genetic variance across Junco (adj. R2). Permutation tests confirmed the significance of the model (F = 1.55, df = 8, p = 0.001) and the first four RDA axes (p ≤ 0.05; Fig. 2d, e). In support of our second prediction, a variance partition analysis of this model found that the annual temperature range explained more allelic variation than any other climatic variable tested (0.6%; Table 1).
Fig. 2. Junco population genetic variation.
a Origin of 181 Junco individuals included in the SNP dataset. Map shading (in grayscale) depicts temperature annual range values from WorldClim66, plotted in R with package raster67. Dot color denotes museum-based taxon assignments; dot size represents the number of individuals from that locale (n = 1 to 4). Population genetic variation from 22,006 genome-wide SNPs structured along four PC axes (b, c) and four RDA axes (d, e) where each dot represents an individual. Colors follow the legend provided in (a). d, e Arrows indicate loadings of eight WorldClim variables (diurnal temperature range, temperature annual range, mean temperature of the warmest quarter, mean temperature of the wettest quarter, mean temperature of the driest quarter, mean minimum precipitation, mean precipitation of the warmest quarter, and mean precipitation of the coldest quarter) in a partial RDA conditioned on PC axes 1 and 2. Source data are provided in the Source Data file.
Table 1.
Genetic variance across 22,006 genome-wide SNPs for 181 Junco individuals partitioned by eight WorldClim variables in a partial RDA (conditioned on background structure using the first two axes of a PCA).
| WorldClim variable | Adj. R2 |
|---|---|
| Diurnal temperature range | 0.0034 |
| Temperature annual range | 0.0057 |
| Temperature wettest quarter | 0.0008 |
| Temperature driest quarter | 0.0016 |
| Temperature warmest quarter | 0.0008 |
| Minimum precipitation | 0.0028 |
| Precipitation warmest quarter | 0.0029 |
| Precipitation coldest quarter | 0.0035 |
| Constraining variable total | 0.0294 |
| Conditional variable total | 0.0642 |
Total variance explained by the constraining (environmental) and conditional (PC1 and PC2) variables shown at the bottom.
Common garden acclimation experiments
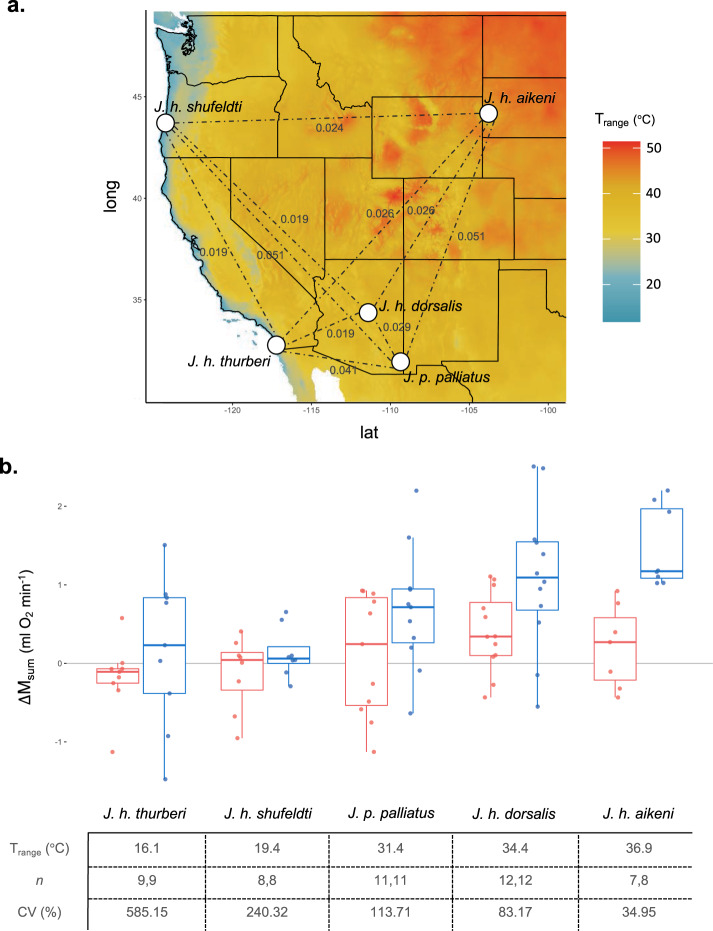
To formally test whether phenotypic flexibility in Msum correlated with thermal heterogeneity, we performed a laboratory acclimation experiment using 95 individuals from five nonmigratory populations across the western United States (J. h. aikeni, J. h. dorsalis, J. h. shufeldti, J. h. thurberi, and J. p. palliatus). The sites at which these populations were captured varied by 21 °C in their annual temperature range (Trange; Fig. 3). Genetic differentiation among populations (FST) ranged from 0.019 to 0.051 (Fig. 3a), but pairwise environmental and genetic distances did not covary (partial Mantel test conditioned on geographic distance: r = −0.40, p = 0.82, n = 5). Prior to acclimation and following an 8-week adjustment period under common conditions at 23 °C, temperature treatment groups did not differ in Msum (posterior μ = 0.18, 95% CI = [−0.06, 0.40], p = 0.14) or Mb (posterior μ = −0.07, 95% CI = [−0.57, 0.41], p = 0.78). However, both Msum and Mb positively correlated with native Trange (Msum: posterior μ = 1.10, 95% CI = [0.26, 1.97], p = 0.02; Mb: posterior μ = 3.71, 95% CI = [3.07, 4.34], p = 2.0 × 10−4).
Fig. 3. Junco thermogenic flexibility correlates with thermal heterogeneity.
a Origin of five Junco populations used in laboratory acclimations (dots) that differ in their native annual temperature range (Trange; values from WorldClim66), shown in shaded colors and plotted in R with package raster67. Pairwise FST indicated by dotted lines and accompanying values in gray. b Thermogenic flexibility, measured as the change in Msum over the 3-week temperature acclimation treatment (post- minus pre-acclimation), for each population. Boxplots show the median values, the 25th and 75th percentiles (lower and upper margins of the box), and the minimum and maximum values ≤1.5 × IQR from the box margin (whiskers). Dots indicate individual measures (n = 95). Control birds (acclimated to 23 °C) in red; cold-acclimated birds (3 °C) in blue. For each population, the native Trange, number of individuals in each treatment (Control, Cold), and coefficient of variation for cold-acclimated individuals (CV) are shown below. Source data are provided in the Source Data file.
After a 3-week acclimation treatment to 3 °C (Cold) or 23 °C (Control), we found that ΔMsum (measured as the difference between post- and pre-acclimation measures) was higher in larger and cold-acclimated birds (Mb: posterior μ = 0.53, 95% CI = [0.03, 1.00], p = 0.03; Cold Treatment: posterior μ = 0.62, 95% CI = [0.35, 0.88], p < 0.001). In support of our second prediction, we also found an interaction between native Trange and temperature treatment, such that populations from more variable thermal climates exhibited the greatest flexibility in Msum in the Cold (posterior μ = 0.60, 95% CI = [0.07, 1.14], p = 0.03), while populations from less variable thermal climates exhibited little or no change in Msum (Fig. 3b). In support of our third prediction, populations from less variable thermal environments also showed larger CVs in their flexibility in Msum in the cold than did those from more variable thermal environments (R2 = 0.80, β = −21.40 ± 6.11, p = 0.04, n = 5). This pattern was not evident in the Control group (β = 0.34 ± 0.18, p = 0.16, n = 5).
Discussion
Our multifaceted approach provides support for three key predictions of theory regarding local adaptation in phenotypic flexibility. We present evidence that Junco populations responded differentially to thermal cues in the field and that temperature variability may be driving these patterns of intraspecific variation in Msum. We replicated this pattern in the laboratory where flexibility in Junco Msum correlated with the heterogeneity of their native thermal environment. Moreover, range-wide variation in allele frequencies also correlated with annual temperature range, providing evidence that thermal heterogeneity may be an important determinant of population structure across the Junco radiation. Together, these results greatly expand our knowledge of endothermic responses to environmental variation and their capacity for thermal acclimatization.
We first predicted that geographic variation in Msum flexibility would correspond with environmental heterogeneity. Several studies have characterized broad-scale interspecific patterns in endothermic Msum38–40, but far less is known about the potential for, or the underlying environmental correlates of, intraspecific variation in Msum (but see refs. 41–43). We found that Junco taxa exhibited constitutive differences in Msum in the field even after controlling for variation in Td_range and Mb among taxa. We also observed variation among individuals in Msum in response to recent changes in ambient thermal conditions—including those occurring within a season. We would not expect this relationship between adult Msum and recent temperature range if the observed variation was attributable to interindividual differences alone. Thus, although we did not perform repeated measures on individuals to quantify flexibility directly, we believe that this pattern is indicative of a thermal acclimatization response. This result is consistent with recent laboratory findings showing that J. h. montanus can substantially increase Msum within 1 week of exposure to low temperatures30. Moreover, we found that these responses differ among Junco taxa, suggesting that Junco populations may differ in their physiological flexibility. Using the reaction norms from this relationship as a measure of the magnitude of flexibility, we found a positive correlation between thermogenic flexibility and annual thermal heterogeneity across four of the five taxa. Yet when the taxa with the lowest flexibility (J. h. mearnsi) is included, this correlation disappears. It is difficult to interpret these absolute differences among taxa because we were not able to control for several factors in the field (e.g., an individual’s diet, reproductive status, use of microclimatic refugia, duration at the site prior to capture, etc.). Additionally, the measure of thermal heterogeneity that we applied is an imperfect approximation of both operative temperature and what these birds likely experience throughout the year due to the migratory tendency of many individuals in this dataset. For example, J. h. mearnsi do not overwinter at the sites where we captured them during the breeding season (at elevations >2000 m near Teton National Park). However, we took this approach because we lacked data regarding the annual movements of each individual. Thus, to provide additional support for our result that temperature variability influenced differences in flexibility across the Junco distribution we also incorporated both genotype-environment association analyses and a laboratory acclimation experiment.
We next predicted that if the phenotypic patterns we observed were indeed a result of local adaptation to temperature heterogeneity, we would find genetic variation that was also structured by temperature range. Indeed, the annual temperature range at a sampling locality explained more genetic variation across the genus than any of the other climatic variables tested. Together, the eight climatic variables explained a small amount of total variation in allele frequencies. However, given that we only surveyed 2% of the genome with our SNP dataset, this amount is well within the range of variation expected from a genotype-environment association analysis (e.g., refs. 36,37,44). Additionally, the climatic data that we employed corresponded to the annual climatic regimes at our collection sites, even though some individuals included in the analysis are likely migratory and therefore not present at these sites year-round. Once again, this approach was necessary because of uncertainty regarding where each individual spent the entire year. In the future, a more refined approach that accurately accounts for the annual environmental conditions experienced by each individual could therefore improve the explanatory power of this analysis.
We also predicted that differences in flexibility would be replicated under common garden conditions. Again, our ability to connect Junco populations to their native climatic regimes is restricted by our limited knowledge of their movements throughout the year; thus we chose to focus our acclimation study on populations that likely remain resident in one narrow geographic area, enabling us to reliably reconstruct their climatic histories. We selected these focal populations to maximize variation in the annual temperature range they experienced based on the results of our field sampling. Importantly, environmental and genetic variation among these populations did not covary, allowing us to simultaneously tease apart the effects of both factors. Furthermore, we accounted for nonindependence among populations by incorporating measures of population genetic differentiation in our acclimation analysis using Markov Chain Monte Carlo generalized linear mixed models45,46. The acclimation experiment, therefore, provided a powerful opportunity to test predictions of flexibility and environmental heterogeneity, and further permitted us to (1) perform repeated measures on individuals to formally quantify flexibility; (2) expose all individuals to the same temperature range stimulus; (3) control for acclimatization history for 2 months prior to temperature treatments; and (4) control for several other factors (e.g., diet, food availability, microclimatic refugia) that may influence observed flexibility. We found that individuals from the two coastal populations in southern California (J. h. thurberi) and Oregon (J. h. shufeldti), which experience the smallest amount of temperature variation, exhibited little to no flexibility in the cold. This inflexibility could result from a failure to sufficiently stress these populations, such that phenotypic change was not necessary to meet the challenge at hand. This does not appear to be the case though, as all individuals were hypothermic at the end of the Msum assay. The only population that was included in both the field and lab studies (J. p. palliatus), meanwhile, exhibited intermediate levels of flexibility in each instance. Finally, birds experiencing the greatest annual temperature variation—those from the Black Hills region (J. h. aikeni) followed by those from the Arizona highlands (J. h. dorsalis)—exhibited the greatest flexibility in their response to the cold. These results, therefore, provide strong support for the prediction that juncos from more thermally variable environments exhibit greater flexibility in Msum.
Finally, we predicted that we would find a reduction in variability in flexibility for populations occupying more heterogenous environments. In agreement with this prediction, we found a strong negative correlation between the coefficient of variation for Msum flexibility and the annual temperature range. Thus, the patterns observed here may be indicative of selection honing the flexible response in variable climates (i.e., lower CVs) and/or relaxed selection in invariable environments (i.e., higher CVs). Taken together, this work suggests that selection has shaped the junco’s capacity to acclimatize to changes in ambient temperature. The ability to robustly respond to thermal challenges by achieving high Msum may impart survival advantages in the cold, and at the same time, the ability to downregulate metabolic capacity (i.e., Msum) may provide energetic savings under conditions where high thermogenesis is not required.
Nonetheless, a necessary additional step in assessing whether or not flexibility can be locally adapted is to connect differences in flexibility with direct measures of fitness. Unfortunately, we cannot directly address whether differences in flexibility in Junco Msum result in differential fitness outcomes with our dataset. Previous studies have shown that absolute measures of thermogenic capacity do positively correlate with survival rates in other temperate-wintering songbirds17,18. However, this is not analogous to linking thermogenic flexibility and fitness because it does not account for the potential costs of trait alteration (i.e., changing Msum over time). Large-scale demographic studies are therefore also needed to determine the strength of selection on thermogenic flexibility across different environments that vary in thermal heterogeneity.
Quantifying these direct connections between fitness and thermal acclimatization is vital in determining how endotherms can respond to changing environments47. For example, previous work has suggested that many ectotherms are limited in their thermal acclimatization capacity and this has been used as evidence that ectotherms are especially vulnerable to future climatic change48–50. There are several biological differences between ectotherms and endotherms that may contribute to potential disparities in their acclimatization ability, however. In general, many ectotherms rely on behavioral thermoregulatory mechanisms and possess a number of avoidance strategies (e.g., diapause, hibernation, or migration) that may be used to buffer against environmental extremes51. Thus, the amount of thermal heterogeneity that an individual or population experiences may not correspond to broad-scale climatic patterns across the year. Endotherms maintain a relatively constant body temperature in comparison, despite sometimes large temperature differentials with their ambient environment16. While many endotherms also exhibit hibernation and migratory behaviors, small songbirds that reside in temperate regions year-round, like juncos, are particularly exposed to seasonal temperature variation25. These differences could lead to divergent selection pressures on flexibility and thermal performance traits among taxonomic groups.
Ultimately, many of the models that are used to forecast the effects of global change on population dynamics52, make habitat delineations53, and model disease transmission54 rely on accurate characterizations of variation in thermal tolerance. Several recent macrophysiological approaches characterizing potential organismal responses to climatic change employ a single metric of thermal tolerance, for instance, effectively treating tolerance as a trait that is canalized and invariant across a species’ range and an individual’s lifetime (e.g., refs. 50,55,56). Our results instead highlight the capacity for populations to vary geographically in their physiological response to environmental cues. When coupled with datasets like ours, biophysical models that incorporate intraspecific patterns in acclimatization will improve our ability to predict organismal responses to climate change.
Methods
Field data and analysis
Field sampling
We captured adult juncos by mist net or potter trap at sites in Arizona (breeding season), Colorado (breeding), Illinois (non-breeding), Montana (breeding), New Mexico (non-breeding), New York (breeding), South Dakota (non-breeding), and Wyoming (breeding), spanning 16° in latitude and 37° in longitude (Fig. 1). This work was completed with approval from the U.S. Fish and Wildlife Service (MB84376B-1 to M.S.; MB01543B-0 to Z.A.C.; MB758442 to D.L.S.; MB06336A-4 to M.D.C.; MB757670-1 to David Winkler; and MB094297-0 to Christopher Witt,), the State of Arizona Game and Fish Department (SP590760 and SP707897 to D.L.S.), Colorado Parks and Wildlife (10TRb2030A15 to M.D.C.), the Illinois Department of Natural Resources (NH13.5667 to Z.A.C.), the Montana Department of Fish Wildlife and Parks (2016-013 and 2017-067-W to M.S.), the New Mexico Department of Game & Fish (3217 to Christopher Witt), the New York State Division of Fish, Wildlife, & Marine Resources (LCP 1477 to David Winkler), the State of South Dakota Department of Game, Fish, and Parks (06-03, 07-02, 08-03 to D.L.S.), Wyoming Game and Fish (754 to M.D.C.), and the Institutional Animal Care and Use Committees at Cornell University (2001-0051 to David Winkler), the University of Illinois (13385 to Z.A.C.), the University of Montana (010-16ZCDBS-020916 to Z.A.C.), the University of South Dakota (03-08-06-08B to D.L.S), and the University of Wyoming (A-3216-01 to M.D.C.). We classified individuals to taxonomic unit based on plumage (J. h. caniceps, J. h. hyemalis, J. h. mearnsi, J. h. oreganus group, and J. p. palliatus). The J. h. oreganus group encompasses seven subspecies (J. h. montanus, J. h. oreganus, J. h. pinosus, J. h. pontilis, J. h. shufeldti, J. h. thurberi, and J. h. townsendii) with similar plumages and overlapping nonbreeding ranges26,27. For this reason, we were unable to distinguish subspecies of nonbreeding individuals within this group, though all breeding individuals were collected from the J. h. montanus range.
Field metabolic assays
Birds were transported from the site of capture to a nearby laboratory (<50 mi away) for measurement where they were held indoors with ad libitum access to food and water. We assayed the Msum of each individual using open-flow respirometry. Most measurements were completed within 1 day of capture to avoid the effects of captivity on metabolic rates, though some (n = 15) were made within 2 days of capture (mean = 0.85 ± 0.47 day). Mb (in g) was quantified before each measurement began. Msum trials were conducted during daylight hours. A single individual was placed in a metabolic chamber in a dark, temperature-controlled environment. We pumped dry, cooled heliox gas (21% O2 and 79% He) through the animal’s chamber at a constant flow rate. We subsampled the outflow current, dried it (Drierite), scrubbed it of CO2 using ascarite, and dried it again before quantifying the O2 concentration using a FoxBox (Sable Systems). We recorded the output using Expedata software v.1.9.13. Trials were conducted using static cold exposure (−5 °C) for Colorado, Illinois, Montana, New Mexico, New York, and Wyoming birds and sliding cold exposure (starting at 0 °C and descending 3 °C every 20 min) for Arizona and South Dakota birds; however, both methods have been shown to produce similar estimates of Msum57. Trials lasted until O2 consumption plateaued or declined for several minutes. We also sampled a blank chamber before and after trials to account for potential fluctuations in baseline, ambient air.
We used custom R scripts (https://github.com/Mstager/batch_processing_Expedata_files) to correct for drift in baseline O2 content, calculate oxygen consumption (VO2)58, and quantify Msum (calculated as the highest instantaneous VO2 averaged over a 5-min period; ml O2 min−1). We discarded measures characterized by large drift in baseline O2 (owing to ambient temperature fluctuations affecting the FoxBox) or inconsistent flow rates resulting in a total sample size of n = 335 individuals. Following measurements, birds were subject to different fates: either released, exposed to acclimation experiments30, or immediately euthanized and deposited in museums (Source Data). Metabolic data from South Dakota have been previously published59.
Environmental data for field sampling sites
To account for an individual’s recent acclimatization history, we retrieved weather data associated with each collection site (rounded to the nearest hundredth of a degree latitude/longitude) from the DayMet dataset using the R package daymetr v.1.460. This dataset is composed of daily weather parameter estimates derived using interpolation and extrapolation from meteorological observations for a 1 km x 1 km gridded surface across North America61. We downloaded daily estimates of minimum temperature (Tmin; °C), maximum temperature (Tmax; °C), precipitation (prcp; mm/day), water vapor pressure (vp; Pa), shortwave radiation (srad; W/m2), and daylength (dayl; s/day) for the 14 days prior to each individual’s capture date. We additionally calculated daily temperature range (Td_range; °C) as Tmax – Tmin. We selected a conservative potential acclimatization window because, given their migratory nature, we do not know how long juncos were present at a site before sampling occurred, preventing us from incorporating more extensive windows (e.g., months). Prior work in J. hyemalis suggested a window of 7–14 days would be appropriate30,62. We, therefore, calculated averages across acclimatization windows varying from 7 to 14 days preceding capture (i.e., from 1–7 to 1–14) for each weather variable. We also retrieved elevation (elev; m) for each site using the package googleway v.2.7.363.
Analyses for field data
All analyses were conducted in R v.4.0.264. To determine whether junco Msum varied with environmental variation, we constructed eight linear models with Mb, taxon, and a single environmental variable (Tmin, Tmax, prcp, dayl, vp, srad, or Td_range averaged across the acclimatization window or elev) as main effects. We chose to use one variable at a time because several environmental variables were strongly correlated in our dataset (i.e., Tmin and Tmax: r = 0.96). We first standardized continuous predictor variables by centering and dividing by two standard deviations using the package arm v.1.11-265. As an indicator of differences in flexibility, we additionally included an interaction between the environmental variable and taxon to determine if taxa differed in their response to environmental cues. Thus models took the form Msum = Mb + taxon × environmental variable. We present the resulting standardized β to characterize the effect sizes of these relationships. We then used AIC values to evaluate differences in model fit among environmental variables and with that of a null model (including only Mb and taxon as predictors) in order to identify a single “best” environmental variable and acclimatization window explaining variation in Msum. To justify the inclusion of the interaction term, we compared models with and without the term using ∆AIC. We also used ∆AIC to test the inclusion of a variable for the season in which we sampled each individual (breeding or nonbreeding). The J. h. oreganus group served as the reference taxa in reported values.
Lastly, following the results of those analyses, which emphasized the importance of thermal variability, we tested whether junco thermogenic flexibility in the field corresponded with the heterogeneity of their thermal environment. As an index of thermal heterogeneity, for each individual, we downloaded the annual temperature range (˚C) for the site of capture from the WorldClim database66, composed of spatially interpolated climate data aggregated from 1970–2000, at a resolution of 2.5′ using the R package raster v.3.3-1367. Using these values, we calculated the mean annual temperature range for each taxon. We then correlated the reaction norms resulting from our best model (i.e., the taxon-specific slopes; ml O2/min/˚C) with the mean annual temperature range for each taxon using a linear regression.
Population genetic data
Sampling, sequencing, and SNP generation
For phylogeographic reconstruction, we obtained muscle tissue samples (n = 192) from museum specimens collected across the breeding distribution of all 21 Junco taxa identified by Miller26. We tried to maximize the environmental variance in our dataset (per ref. 68) by including 2–30 individuals per taxonomic unit sampled from 94 geographic localities representing the majority of US counties, Canadian provinces, and Mexican states for which tissue samples exist (Fig. 2a). We then employed restriction-site-associated DNA (RAD)-sequencing, which offers a reduced representation of the genome that can be mined for thousands of single nucleotide polymorphisms (SNPs) among individuals69,70.
We extracted whole genomic DNA from each sample using a Qiagen DNeasy Blood and Tissue Extraction Kit and prepared RAD-libraries according to ref. 71 (protocol v.2.3 downloaded from dryad: https://datadryad.org/stash/dataset/doi:10.5061/dryad.m2271pf1). Briefly, we digested whole genomic DNA with two restriction enzymes (EcoRI and Mse1), ligated adapter sequences with unique barcodes (8–10 bp) for each individual (provided in Source Data file), performed PCR amplification (see Table S2 for primers), and then performed automated size selection of 300–400 bp fragments (Sage Science Blue Pippen). We split paired geographic samples between the two libraries such that all taxa were represented in each library of 96, pooled individuals. Samples were additionally randomized on each plate during library preparation. Libraries were sequenced on separate flow-cell lanes of an Illumina HiSeq 4000 at UC Berkeley’s V.C. Genomics Sequencing Lab, yielding over 300 million, 100-nt single-end reads per lane.
We demultiplexed reads, retained reads with intact EcoRI cut sites, removed inline barcodes and adapters, and performed quality filtering (removed Phred score <10) using process_radtags in STACKS v.2.172. This resulted in final reads of μ = 92 bp in length and 2.05 million reads per individual. We removed three individuals in each lane that failed to sequence (<100,000 reads per individual, comprising five J. hyemalis and one J. insularis). We used bwa mem73 to align reads to the J. hyemalis genome37, which we downloaded from NCBI (Accession GCA_003829775.1). An average of 91% of reads mapped and mapping success did not differ among Junco species.
We executed the STACKS pipeline to call SNPs and exported one SNP per locus for loci present in all five Junco species in vcf format (populations: -p 5 --write-random-snp), resulting in 162,856 SNPs. We further filtered the dataset using vcftools v.0.1.1674. We removed sites with mean depth of coverage across all individuals <5 (--min-meanDP) and >50 (--max-meanDP), minimum minor allele count <3 (--mac), more than 50% missing data (--max-missing), and characterized by indels (--remove-indels). We then removed five J. hyemalis individuals with >60% missing data (--remove). Finally, we removed sites with >5% missing data (--max-missing) and filtered three sites that departed from Hardy–Weinberg equilibrium assessed by species (p < 0.001), resulting in 22,006 biallelic SNPs across 181 individuals. We exported the dataset in plink.raw format for downstream analysis.
Genotype-environment association analyses
To determine if environmental variation corresponded to allelic variation across Junco, we employed the SNP dataset in an RDA. RDA is a multivariate ordination technique that has been used to identify multiple candidate loci and several environmental predictors simultaneously35,36. Because RDA requires no missing data, we first imputed data for n = 68,010 missing sites (1.7% of total sites) using the most common genotype for the species at each site. We then quantified population genetic structure using a PCA of the imputed SNP data with the R package ade4 v.1.7-1575. We downloaded 19 spatially interpolated climatic variables aggregated from 1970–2000 corresponding to the geographic origin of each specimen from the WorldClim database66 at a resolution of 2.5′ using the R package raster v.3.3-1367. We centered and standardized climate variables by centering and dividing by two standard deviations65. We excluded highly correlated variables (r ≥ 0.70) resulting in the retention of eight variables: mean diurnal temperature range (BIO2, a measure of monthly temperature variation), temperature annual range (BIO7), mean temperature of the wettest quarter (BIO8), mean temperature of the driest quarter (BIO9), mean temperature of the warmest quarter (BIO10), precipitation of the driest month (BIO14), precipitation of the warmest quarter (BIO18), and precipitation of the coldest quarter (BIO19). We used these eight climatic variables as constraining variables in RDAs executed with the package vegan v.2.5-676. We performed a partial RDA conditioned on background population structure summarized as PC1 and PC2 from the PCA. We tested for, but did not find, multicollinearity among predictor variables (variance inflation factor <5 for all variables). We assessed the significance of the RDA model and of the constrained axes using an ANOVA-like permutation technique77 in vegan at p ≤ 0.05 (n = 999 and n = 299 permutations, respectively). We used variance partitioning78 to quantify the proportion of allelic variation explained by each climatic variable with the function varpart.
Acclimation experiments
Population sampling for acclimation experiments
We combined information gained from a literature search, eBird sightings, and expert opinion to identify five focal populations for phenotypic sampling that (1) were likely to be nonmigratory, (2) represented different morphological subspecies, and (3) maximized variation in annual temperature range within the United States. These populations include J. h. aikeni of the Black Hills, a coastal population of J. h. shufeldti, a highland population of J. h. dorsalis, a sky island population of J. p. palliatus, and a well-studied, urban population of J. h. thurberi79. However, it is possible that some of these populations exhibit seasonal, altitudinal migrations within their geographic area of residence, e.g., J. p. palliatus80.
We captured ≤25 adult individuals from each focal population. Capture periods differed for each population in order to increase the likelihood that targeted individuals were resident year-round, as well as due to time and permitting constraints. For instance, one partially migratory population (J. h. aikeni) with distinct morphological features was caught in the winter to increase the likelihood that the individuals used were nonmigratory. The other four populations, which bred in areas where other, morphologically similar juncos overwinter, were captured in the breeding season when other subspecies were not present. Specifically, J. h. shufeldti (n = 20) were captured 14–15 July 2018 in Coos and Douglas Counties, OR; J. p. palliatus (n = 24) were captured 27 July 2018 in Cochise County, AZ; J. h. dorsalis (n = 25) were captured 30–31 July 2018 in Coconino County, AZ; J. h. aikeni (n = 15) were captured 6–9 March 2019 in Lawrence County, SD; and J. h. thurberi (n = 20) were captured 22–26 July 2019 in San Diego County, CA. In spite of these differences, capture season did not influence acclimation ability (see below). This work was completed with approval from the US Fish and Wildlife Service (MB84376B-1 to M.S. and MB45239B-0 to T.J.G.), the State of Arizona Game and Fish Department (E19253811 to M.S.), the State of California Department of Fish and Wildlife (13971 to M.S.), the Oregon Department of Fish and Wildlife (108-18 to M.S.), the State of South Dakota Department of Game, Fish, and Parks (19-13 to M.S.), and the Institutional Animal Care and Use Committee at the University of Montana (030-18ZCDBS-052918 to Z.A.C.).
Acclimation treatments
Within days of capture, we ground-transported all birds to facilities at the University of Montana where birds were housed individually under common conditions (23 °C with 12 h dark: 12 h light) for ≥8 weeks (μ = 63 days, range = 57–71 days) to minimize the effects of prior acclimatization. We had previously determined that a period of 6 weeks in common conditions is sufficient to reduce variation in metabolic traits among J. hyemalis individuals (Fig. S2). Following this adjustment period, we assayed Msum (see below). We allowed birds ~24 h to recover and then randomly assigned individuals from each population into treatment groups and exposed them to either cold (3 °C) or control (23 °C) temperatures. Treatments lasted 21 days in duration. Constant 12 h dark: 12 h light days were maintained for the duration of the experiment, and food and water were supplied ad libitum. The diet consisted of a 2:1 ratio by weight of white millet and black oil sunflower seed, supplemented with ground dog food, live mealworms, and water containing vitamin drops (Wild Harvest D13123 Multi Drops). These experimental conditions were chosen based on previous work in J. h. hyemalis exposed to the same temperatures, which revealed substantial increases in Msum over the same duration21. At the end of treatments, we euthanized individuals using cervical dislocation.
Eight individuals died during the capture-transport and adjustment periods (one J. h. dorsalis, four J. h. shufeldti, one J. h. thurberi, and two J. p. palliatus). Additionally, one J. h. thurberi individual exhibited lethargy upon introduction to the cold treatment, died within the first 24 h of cold acclimation, and was removed from analyses. This resulted in a total sample size of n = 95 individuals.
Metabolic assays for acclimation experiments
We quantified Msum in a temperature-controlled cabinet using open-flow respirometry both before and after acclimation treatments as described above. We measured Mb immediately before each assay. During the post-acclimation Msum trial, we measured body temperature with a pit tag inserted into the cloaca (per ref. 30) to verify that individuals were hypothermic (body temperature ≤37 °C). Msum trials were conducted using static cold exposure at −5 °C for pre-acclimation measures and −17 °C for post-acclimation measures. Although this experimental temperature difference could have elicited higher post-acclimation Msum, we did not find variation in Msum between the two time points in Control birds, suggesting that the two procedures provided similar levels of cold challenge (paired t-test: n = 47, t = −1.24, df = 46, p = 0.22). Because trials occurred at various times throughout the day (08:00–19:00), we tested for, but did not find, a linear effect of the trial start time on Msum either before or after acclimation (before: R2 = 0.0, p = 0.54; after: R2 = 0.0, p = 0.94).
Climate data for acclimation populations
We reconstructed the annual thermal regime experienced by a population using interpolated monthly climate data downloaded from the WorldClim dataset66 with package raster v.3.3-13. We extracted all 19 variables at a resolution of 2.5′ for the site of capture. However, our analysis mainly focuses on BIO7, the temperature annual range variable (Trange).
Pairwise genetic distance of acclimated populations
To quantify genetic differentiation among acclimated populations, we extracted whole genomic DNA from the muscle tissue of acclimated individuals and prepared RAD-libraries as above with an additional PCR step (per protocol v.2.6)71. We pooled all 95 individuals into a single flow-cell lane of an Illumina HiSeq X for sequencing at Novogene Corporation Inc. yielding ~1 billion, 150-nt paired-end reads. We demultiplexed reads, removed inline barcodes and adapters, and performed quality filtering (removed Phred score <10) using process_radtags in STACKS. This resulted in final reads of μ = 147 bp in length and 7 million reads per individual. We removed six individuals that sequenced poorly (<200,000 reads/individual, comprising one J. h. dorsalis, one J. p. palliatus, one J. h. shufeldti, and three J. h. thurberi). We aligned reads to the J. h. carolinensis genome with bwa mem (51% of reads mapped). We then sorted, merged, and removed read duplicates with samtools v.1.381. We used the STACKS pipeline to call SNPs from loci present in all five populations and exported one SNP per locus in vcf format resulting in 282,929 SNPs (populations: -p 5 --write-random-snp).
We filtered the dataset and estimated patterns of genetic differentiation using vcftools. We removed sites with mean depth of coverage across all individuals <20 (--min-meanDP) and >300 (--max-meanDP), minor allele frequency <5% (--min-maf), and >10% missing data (--max-missing), as well as removed indels (--remove-indels), resulting in 1069 biallelic SNPs across 89 individuals. Finally, we estimated pairwise FST among the focal taxa by calculating the weighted Weir’s theta82.
Analyses for acclimation data
We first performed a partial mantel test to ascertain that environmental and genetic distances did not covary among our sampling sites. We calculated pairwise genetic distance as FST/(1 – FST)83. We estimated pairwise environmental differences among the five sites as the Euclidean distance for five WorldClim variables (after removing redundant variables at r ≥ 0.70 from the original 19 WorldClim variables). We simultaneously controlled for geographic distance, estimated as pairwise geodesic distance among sampling sites with the package geosphere v.1.5-1084. We then employed these matrices of pairwise genetic and environmental distances in a partial Mantel test (conditioned on geographic distance) with the package vegan v.2.5-676.
We quantified the effects of our acclimation treatments while simultaneously incorporating population demography using Markov Chain Monte Carlo generalized linear mixed models45,46 with the package MCMCglmm v.2.3285. This allowed us to include pairwise FST as a random effect in all analyses. Because individual measures were used in these models, the pairwise FST among two individuals corresponded to that of their respective subspecies. We standardized all continuous predictor variables by centering and dividing by two standard deviations65. In all cases, we used default priors and ran the model for 1,000,000 iterations with a burn-in of 10,000 and a thinning interval of 100. We examined the resulting trace plots to verify proper convergence.
We first verified that phenotypic differences did not exist among treatment groups before acclimations began. To do this, we constructed a model to explain variation in pre-acclimation Msum with temperature treatment as the main effect and pairwise FST as a random effect. We then repeated this procedure to test for differences among treatments in pre-acclimation Mb. We constructed similar models to test the effect of Trange on pre-acclimation measures of each Msum and Mb. We also tested for (but did not find) an effect of capture season (breeding or nonbreeding) on pre-acclimation Msum while including Mb as a covariate (season: posterior μ = 0.42, 95% CI = [−1.48, 2.30], p = 0.57; Mb: posterior μ = 0.45, 95% CI = [0.01, 0.88], p = 0.04).
To evaluate whether environmental variation corresponded with thermogenic flexibility, we constructed a model explaining variation in thermogenic flexibility (ΔMsum; post- minus pre-acclimation Msum) with pre-acclimation Mb, temperature treatment, Trange, and a treatment × Trange interaction term as main effects and pairwise FST as a random effect. We tested for (but did not find) an effect of capture season on ΔMsum while including Mb as a covariate (season: posterior μ = 0.09, 95% CI = [−0.42, 0.58], p = 0.72; Mb: posterior μ = 0.67, 95% CI = [0.33, 1.03], p = 6.0 × 10−4).
Finally, we calculated CV, (expressed as percentages)86 for ΔMsum across cold-acclimated individuals within each population using the package raster. To test whether populations from more variable environments expressed less variability in their flexible response, we then regressed the five CV values on Trange for each population using a linear regression. We also repeated this analysis for control-acclimated birds.
Supplementary information
Acknowledgements
We are indebted to the natural history museums that contributed to this project, including the Burke Museum, the American Museum of Natural History, the Bell Museum of Natural History, the Cleveland Museum of Natural History, the Cornell University Museum of Vertebrates, the Field Museum of Natural History, the Louisiana State University Museum of Natural Science, the Museum of Southwest Biology, the Museum of Vertebrate Zoology, the New York State Museum, the Royal Alberta Museum, the San Diego Natural History Museum, the Smithsonian National Museum of Natural History, the University of Alaska Museum, the University of Montana Zoological Museum, and the University of Wyoming Museum of Vertebrates. We are also thankful to the help of Brett Addis, Phred Benham, Jeff Brawn, Kevin Burns, Charles Dardia, Eleanor Diamant, Eric Gulson, Andy Johnson, John Klicka, Thom Nelson, Henry Pollock, Trey Sasser, Rena Schweizer, Nick Sly, Link Smith, Gregory Toreev, Phil Unitt, David Winkler, Chris Witt, Blair Wolf, Cole Wolf, Sally Woodin, Pamela Yeh, Point Loma Nazarene University, the UNM Sevilleta Field Station, the UW-NPS Research Station, the DU Mt. Evans Field Station, and the AMNH Southwestern Research Station, as well as the Cheviron lab for feedback on an earlier version of this manuscript. This work was supported by generous funds from the American Museum of Natural History Chapman Fund, the American Philosophical Society Lewis and Clark Fund, the Explorers Club, the Illinois Ornithological Society, the Nuttall-Ornithological Society, Sigma Xi, the Society for Integrative and Comparative Biology, the Society of Systematic Biologists, the University of Illinois Graduate School, the University of Illinois School of Integrative Biology, and the Wilson Ornithological Society (to M.S.); and the University of Montana (startup to Z.A.C). M.S. was supported by the National Science Foundation Graduate Research Fellowship Program, P.E.O. International, and the University of Montana Graduate School Bertha Morton Fellowship.
Source data
Author contributions
M.S. and Z.A.C. conceived of the study; M.D.C. and N.R.S. helped perform field work; D.L.S. performed field measurements in AZ and SD; D.K.E. helped perform field measurements in CO and WY; T.J.G. provided sampling permits; M.S. performed all other data collection and analyses and drafted the manuscript; all authors contributed edits to the manuscript.
Data availability
Source Data are provided with this paper. Additionally, raw sequence reads have been deposited in the NCBI Sequence Read Archive under accession number PRJNA678344. Environmental data were accessed in R from the Daymet [https://daymet.ornl.gov/] and WorldClim [https://www.worldclim.org/data/bioclim.html] datasets. Source data are provided with this paper.
Code availability
The scripts used for processing and analysis are deposited on github (10.5281/zenodo.4968784).
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Trevor Price and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24588-6.
References
- 1.Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evolution. 2003;18:228–233. doi: 10.1016/S0169-5347(03)00036-3. [DOI] [Google Scholar]
- 2.Botero CA, Weissing FJ, Wright J, Rubenstein DR. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA. 2015;112:184–189. doi: 10.1073/pnas.1408589111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lande R. Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. J. Evol. Biol. 2014;27:866–875. doi: 10.1111/jeb.12360. [DOI] [PubMed] [Google Scholar]
- 4.Piersma, T. & van Gils, J. A. The Flexible Phenotype: A Body-centred Integration of Ecology, Physiology, and Behaviour (Oxford Univ. Press, 2011).
- 5.Moran NA. The evolutionary maintenance of alternative phenotypes. Am. Naturalist. 1992;139:971–989. doi: 10.1086/285369. [DOI] [Google Scholar]
- 6.Sultan SE, Spencer HG. Metapopulation structure favors plasticity over local adaptation. Am. Naturalist. 2002;160:271–283. doi: 10.1086/341015. [DOI] [PubMed] [Google Scholar]
- 7.Ernande B, Dieckmann U. The evolution of phenotypic plasticity in spatially structured environments: implications of intraspecific competition, plasticity costs and environmental characteristics. J. Evolut. Biol. 2004;17:613–628. doi: 10.1111/j.1420-9101.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- 8.Cavieres G, Sabat P. Geographic variation in the response to thermal acclimation in rufous-collared sparrows: are physiological flexibility and environmental heterogeneity correlated? Funct. Ecol. 2008;22:509–515. doi: 10.1111/j.1365-2435.2008.01382.x. [DOI] [Google Scholar]
- 9.Riddell EA, Sears MW. Geographic variation of resistance to water loss within two species of lungless salamanders: implications for activity. Ecosphere. 2015;6:art86. doi: 10.1890/ES14-00360.1. [DOI] [Google Scholar]
- 10.Griffith RW. Environment and salinity tolerance in the genus Fundulus. Copeia. 1974;1974:319–331. doi: 10.2307/1442526. [DOI] [Google Scholar]
- 11.Stevenson RD. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Naturalist. 1985;126:362–386. doi: 10.1086/284423. [DOI] [Google Scholar]
- 12.Huey RB, Peterson CR, Arnold SJ, Porter WP. Hot rocks and not-so-hot rocks: retreat-site selection by garter snakes and its thermal consequences. Ecology. 1989;70:931–944. doi: 10.2307/1941360. [DOI] [Google Scholar]
- 13.Huey RB, Pianka ER. Seasonal variation in thermoregulatory behavior and body temperature of diurnal Kalahari lizards. Ecology. 1977;58:1066–1075. doi: 10.2307/1936926. [DOI] [Google Scholar]
- 14.McKechnie AE. Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J. Comp. Physiol. B. 2008;178:235–247. doi: 10.1007/s00360-007-0218-8. [DOI] [PubMed] [Google Scholar]
- 15.Williams JB, Tieleman BI. Physiological flexibility in hoopoe larks. J. Exp. Biol. 2005;203:3153–3159. doi: 10.1242/jeb.203.20.3153. [DOI] [PubMed] [Google Scholar]
- 16.McNab, B. The Physiological Ecology of Vertebrates: A View from Energetics Vol. 2002 (Comstock Publishing Associates, 2002).
- 17.Hayes JP, O’Connor CS. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution. 1999;53:1280–1287. doi: 10.1111/j.1558-5646.1999.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 18.Petit M, Clavijo-Baquet S, Vézina F. Increasing winter maximal metabolic rate improves intrawinter survival in small birds. Physiol. Biochem. Zool. 2017;90:166–177. doi: 10.1086/689274. [DOI] [PubMed] [Google Scholar]
- 19.Latimer CE, Cooper SJ, Karasov WH, Zuckerberg B. Does habitat fragmentation promote climate-resilient phenotypes? Oikos. 2018;127:1069–1080. doi: 10.1111/oik.05111. [DOI] [Google Scholar]
- 20.Swanson DL. Seasonal variation of vascular oxygen transport in the Dark-Eyed Junco. Condor. 1990;92:62–66. doi: 10.2307/1368383. [DOI] [Google Scholar]
- 21.Swanson D, Zhang Y, Liu J-S, Merkord CL, King MO. Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. J. Exp. Biol. 2014;217:866–875. doi: 10.1242/jeb.096677. [DOI] [PubMed] [Google Scholar]
- 22.Stager M, Swanson DL, Cheviron ZA. Regulatory mechanisms of metabolic flexibility in the dark-eyed junco (Junco hyemalis) J. Exp. Biol. 2015;218:767–777. doi: 10.1242/jeb.113472. [DOI] [PubMed] [Google Scholar]
- 23.Stager M, Cheviron ZA. Is there a role for sarcolipin in avian facultative thermogenesis in extreme cold? Biol. Lett. 2020;16:20200078. doi: 10.1098/rsbl.2020.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vezina F, et al. Consequences of being phenotypically mismatched with the environment: rapid muscle ultrastructural changes in cold-shocked black-capped chickadees (Poecile atricapillus) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R274–R283. doi: 10.1152/ajpregu.00203.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson, D. L. In Current Ornithology Vol. 17 (ed. Thompson, C. F.) 75–129 (Springer, 2010).
- 26.Miller, A. H. Speciation in the Avian Genus Junco Vol. 44 (University of California Press, 1941).
- 27.Nolan, V. Jr. et al. Dark-eyed Junco (Junco hyemalis). Birds N. Am. 10.2173/bna.716 (2002).
- 28.Ketterson, E. D. & Atwell, J. W. Snowbird: Integrative Biology and Evolutionary Diversity in the Junco. (University of Chicago Press, 2016).
- 29.Swanson DL. Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the dark-eyed Junco (Junco hyemalis) Auk. 1990;107:6. [Google Scholar]
- 30.Stager M, Senner NR, Tobalske BW, Cheviron ZA. Body temperature maintenance acclimates in a winter-tenacious songbird. J. Exp. Biol. 2020;223:jeb221853. doi: 10.1242/jeb.221853. [DOI] [PubMed] [Google Scholar]
- 31.Swanson D. Are summit metabolism and thermogenic endurance correlated in winter-acclimatized passerine birds? J. Comp. Physiol. B. 2001;171:475–481. doi: 10.1007/s003600100197. [DOI] [PubMed] [Google Scholar]
- 32.Friis G, Aleixandre P, Rodríguez‐Estrella R, Navarro‐Sigüenza AG, Milá B. Rapid postglacial diversification and long‐term stasis within the songbird genus Junco: phylogeographic and phylogenomic evidence. Mol. Ecol. 2016;25:6175–6195. doi: 10.1111/mec.13911. [DOI] [PubMed] [Google Scholar]
- 33.Via S, Lande R. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution. 1985;39:505. doi: 10.1111/j.1558-5646.1985.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones MR, et al. Integrating landscape genomics and spatially explicit approaches to detect loci under selection in clinal populations. Evolution. 2013;67:3455–3468. doi: 10.1111/evo.12237. [DOI] [PubMed] [Google Scholar]
- 35.Forester BR, Jones MR, Joost S, Landguth EL, Lasky JR. Detecting spatial genetic signatures of local adaptation in heterogeneous landscapes. Mol. Ecol. 2016;25:104–120. doi: 10.1111/mec.13476. [DOI] [PubMed] [Google Scholar]
- 36.Forester BR, Lasky JR, Wagner HH, Urban DL. Comparing methods for detecting multilocus adaptation with multivariate genotype–environment associations. Mol. Ecol. 2018;27:2215–2233. doi: 10.1111/mec.14584. [DOI] [PubMed] [Google Scholar]
- 37.Friis G, et al. Genome-wide signals of drift and local adaptation during rapid lineage divergence in a songbird. Mol. Ecol. 2018;27:5137–5153. doi: 10.1111/mec.14946. [DOI] [PubMed] [Google Scholar]
- 38.Stager M, et al. Disentangling environmental drivers of metabolic flexibility in birds: the importance of temperature extremes versus temperature variability. Ecography. 2016;39:787–795. doi: 10.1111/ecog.01465. [DOI] [Google Scholar]
- 39.Wiersma P, Chappell MA, Williams JB. Cold- and exercise-induced peak metabolic rates in tropical birds. Proc. Natl Acad. Sci. USA. 2007;104:20866–20871. doi: 10.1073/pnas.0707683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson DL, Garland T., Jr. The evolution of high summit metabolism and cold tolerance in birds and its impact on present-day distributions. Evolution. 2009;63:184–194. doi: 10.1111/j.1558-5646.2008.00522.x. [DOI] [PubMed] [Google Scholar]
- 41.Dawson WR, Marsh RL, Buttemer WA, Carey C. Seasonal and geographic variation of cold resistance in house finches Carpodacus mexicanus. Physiol. Zool. 1983;56:353–369. doi: 10.1086/physzool.56.3.30152600. [DOI] [Google Scholar]
- 42.O’Connor TP. Geographic variation in metabolic seasonal acclimatization in house finches. Condor. 1996;98:371–381. doi: 10.2307/1369155. [DOI] [Google Scholar]
- 43.Olson JR, Cooper SJ, Swanson DL, Braun MJ, Williams JB. The relationship of metabolic performance and distribution in black‐capped and Carolina chickadees. Physiol. Biochem. Zool. 2010;83:263–275. doi: 10.1086/648395. [DOI] [PubMed] [Google Scholar]
- 44.Meirmans PG. Seven common mistakes in population genetics and how to avoid them. Mol. Ecol. 2015;24:3223–3231. doi: 10.1111/mec.13243. [DOI] [PubMed] [Google Scholar]
- 45.Hadfield JD, Nakagawa S. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evolut. Biol. 2010;23:494–508. doi: 10.1111/j.1420-9101.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 46.Stone GN, Nee S, Felsenstein J. Controlling for non-independence in comparative analysis of patterns across populations within species. Philos. Trans. R. Soc. B. 2011;366:1410–1424. doi: 10.1098/rstb.2010.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyles JG, Seebacher F, Smit B, McKechnie AE. Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 2011;51:676–690. doi: 10.1093/icb/icr053. [DOI] [PubMed] [Google Scholar]
- 48.Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- 49.Huey RB, et al. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B. 2012;367:1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunderson AR, Stillman JH. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B. 2015;282:20150401. doi: 10.1098/rspb.2015.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearney M, Shine R, Porter WP. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl Acad. Sci. USA. 2009;106:3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFarlane SE, Ålund M, Sirkiä PM, Qvarnström A. Difference in plasticity of resting metabolic rate – the proximate explanation to different niche breadth in sympatric Ficedula flycatchers. Ecol. Evol. 2018;8:4575–4586. doi: 10.1002/ece3.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du, W. G. D. G., Shou, L. S. & Shen, J. Y. S. Y. Habitat selection in two sympatric Chinese skinks, Eumeces elegans and Sphenomorphus indicus: do thermal preferences matter? Can. J. Zool.10.1139/z06-116 (2006).
- 54.Miazgowicz KL, et al. Age influences the thermal suitability of Plasmodium falciparum transmission in the Asian malaria vector Anopheles stephensi. Proc. R. Soc. B: Biol. Sci. 2020;287:20201093. doi: 10.1098/rspb.2020.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunday JM, et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA. 2014;111:5610–5615. doi: 10.1073/pnas.1316145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riddell EA, Iknayan KJ, Wolf BO, Sinervo B, Beissinger SR. Cooling requirements fueled the collapse of a desert bird community from climate change. Proc. Natl Acad. Sci. USA. 2019;116:21609–21615. doi: 10.1073/pnas.1908791116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson DL, Drymalski MW, Brown JR. Sliding vs static cold exposure and the measurement of summit metabolism in birds. J. Therm. Biol. 1996;21:221–226. doi: 10.1016/0306-4565(96)00005-8. [DOI] [Google Scholar]
- 58.Lighton, J. Measuring Metabolic Rates: A Manual for Scientists. (Oxford Univ. Press, 2008).
- 59.Swanson DL, Thomas NE, Liknes ET, Cooper SJ. Intraspecific correlations of basal and maximal metabolic rates in birds and the aerobic capacity model for the evolution of endothermy. PLoS ONE. 2012;7:e34271. doi: 10.1371/journal.pone.0034271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hufkens K, Basler D, Milliman T, Melaas E, Richardson A. An integrated phenology modelling framework in R. Methods Ecol. Evol. 2018;9:1276–1285. doi: 10.1111/2041-210X.12970. [DOI] [Google Scholar]
- 61.Thornton, P. E. et al. Daymet: daily surface weather data on a 1-km grid for North America, Version 3. ORNL DAAC10.3334/ORNLDAAC/1328. (2016).
- 62.Swanson DL, Olmstead KL. Evidence for a proximate influence of winter temperature on metabolism in passerine biirds. Physiol. Biochem. Zool. 1999;72:566–575. doi: 10.1086/316696. [DOI] [PubMed] [Google Scholar]
- 63.Cooley, D. & Barcelos, P. (Author of c++ & pacakge), R. (Functions written for the L. googleway: accesses google maps APIs to retrieve data and plot maps. (2018).
- 64.R Core Team. R: the R project for statistical computing. R Foundation for Statistical Computinghttps://www.r-project.org/ (2018).
- 65.Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008;27:2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- 66.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 67.Hijmans, R. J. et al. raster: geographic data analysis and modeling. (2020).
- 68.Lotterhos KE, Whitlock MC. The relative power of genome scans to detect local adaptation depends on sampling design and statistical method. Mol. Ecol. 2015;24:1031–1046. doi: 10.1111/mec.13100. [DOI] [PubMed] [Google Scholar]
- 69.Davey JW, Blaxter ML. RADSeq: next-generation population genetics. Brief. Funct. Genomics. 2010;9:416–423. doi: 10.1093/bfgp/elq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baird NA, et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE. 2008;3:e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parchman TL, et al. Genome-wide association genetics of an adaptive trait in lodgepole pine. Mol. Ecol. 2012;21:2991–3005. doi: 10.1111/j.1365-294X.2012.05513.x. [DOI] [PubMed] [Google Scholar]
- 72.Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: building and genotyping loci de novo from short-read sequences. G3. 2011;1:171–182. doi: 10.1534/g3.111.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at ArXiv1303 (2013).
- 74.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dray S, Dufour A-B. The ade4 package: implementing the duality diagram for ecologists. J. Stat. Softw. 2007;22:1–20. doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 76.Oksanen, J. et al. vegan: Community Ecology Package. (2019).
- 77.Legendre P, Oksanen J, ter Braak CJF. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2011;2:269–277. doi: 10.1111/j.2041-210X.2010.00078.x. [DOI] [Google Scholar]
- 78.Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. doi: 10.2307/1940179. [DOI] [Google Scholar]
- 79.Yeh PJ, Price TD. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Naturalist. 2004;164:531–542. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]
- 80.Lundblad CG, Conway CJ. Variation in selective regimes drives intraspecific variation in life-history traits and migratory behaviour along an elevational gradient. J. Anim. Ecol. 2020;89:397–411. doi: 10.1111/1365-2656.13134. [DOI] [PubMed] [Google Scholar]
- 81.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 83.Rousset F. Genetic differentiation and estimation of gene flow from F statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hijmans, R. J., Williams, E. & Vennes, C. geosphere: Spherical Trigonometry. (2019).
- 85.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i02. [DOI] [Google Scholar]
- 86.Pélabon C, Hilde CH, Einum S, Gamelon M. On the use of the coefficient of variation to quantify and compare trait variation. Evol. Lett. 2020;4:180–188. doi: 10.1002/evl3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brownrigg, O. S. code by R. A. B. and A. R. W. R. version by R. mapdata: Extra Map Databases. (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source Data are provided with this paper. Additionally, raw sequence reads have been deposited in the NCBI Sequence Read Archive under accession number PRJNA678344. Environmental data were accessed in R from the Daymet [https://daymet.ornl.gov/] and WorldClim [https://www.worldclim.org/data/bioclim.html] datasets. Source data are provided with this paper.
The scripts used for processing and analysis are deposited on github (10.5281/zenodo.4968784).