Abstract
To develop a predictive model and a nomogram for predicting postoperative hemorrhage in preoperative patients undergoing laparoscopic pancreaticoduodenectomy (LPD). A total of 409 LPD patients that underwent LPD by the same surgical team between January 2014 and December 2020 were included as the training cohort. The preoperative data of patients were statistically compared and analyzed for exploring factors correlated with postoperative hemorrhage. The predictive model was developed by multivariate logistic regression and stepwise (stepAIC) selection. A nomogram based on the predictive model was developed. The discriminatory ability of the predictive model was validated using the receiver operating characteristic (ROC) curve and leave-one-out method. The statistical analysis was performed using R 3.5.1 (www.r-project.org). The predictive model including the risk-associated factors of postoperative hemorrhage was as follows: 2.695843 − 0.63056 × (Jaundice = 1) − 1.08368 × (DM = 1) − 2.10445 × (Hepatitis = 1) + 1.152354 × (Pancreatic tumor = 1) + 1.071354 × (Bile duct tumor = 1) − 0.01185 × CA125 − 0.04929 × TT − 0.08826 × APTT + 26.03383 × INR − 1.9442 × PT + 1.979563 × WBC − 2.26868 × NEU − 2.0789 × LYM − 0.02038 × CREA + 0.00459 × AST. A practical nomogram based on the model was obtained. The internal validation of ROC curve was statistically significant (AUC = 0.7758). The validation by leave-one-out method showed that the accuracy of the model and the F measure was 0.887 and 0.939, respectively. The predictive model and nomogram based on the preoperative data of patients undergoing LPD can be useful for predicting the risk degree of postoperative hemorrhage.
Subject terms: Medical research, Risk factors, Signs and symptoms
Introduction
Laparoscopic surgeries with minimal invasions have become possible because of the new advancements in laparoscopic technology and instruments in the past decade. Laparoscopic pancreaticoduodenectomy (LPD) is widely used for treating periampullary tumors nowadays1,2. Retrospective or prospective comparative studies pertaining to the differences in the clinical outcomes of patients that underwent LPD and that underwent open pancreaticoduodenectomy (OPD) reported that LPD is associated with shorter hospital stay3–5, lesser hospitalization cost5,6 and similar short-term outcomes and long-term survival2,4,7–10 compared with those of OPD. Similar to OPD, the morbidity and mortality of patients that underwent LPD is associated with postoperative complications, including postoperative pancreatic fistula, delayed gastric emptying, and postoperative hemorrhage11,12. Surgeons from various countries try to reduce the incidence of severe complications of LPD by modifying surgical procedures13–16. However, only a few studies have tried to predict the severe complications of LPD. Postoperative hemorrhage is a relatively frequent complication of LPD17, and is mainly induced by the formation of pancreatic fistula. Postoperative hemorrhage often requires reoperation or interventional embolization, which may secondly trigger complications involving perioperative death. There for. in this study, we aimed to develop a predictive model and a nomogram that can predict postoperative hemorrhage in preoperative patients undergoing LPD.
Methods
Patients
A total of 409 patients who underwent successful LPD without unexpected events by the same surgical team in the Department of Hepatobiliary Surgery of the Second Hospital of Hebei Medical University between January 2014 and December 2020 were included in this study as the training cohort. The inclusion criteria for the training cohort were the absence of (1) metastasis in other organs, (2) vascular invasion, (3) coexisting critical diseases, and the presence of comprehensive preoperative data. The preoperative data included general conditions (age, sex and BMI), symptoms before admission (jaundice, abdominal pain and fever), coexisting medical conditions (high blood pressure (HBP), coronary heart disease (CHD), diabetes mellitus (DM), pancreatitis, hepatitis and previous history of operation), preoperative treatment (bile duct drainage), tumor location (duodenum, bile duct, pancreas) and blood tests results (CA125, CA199, TT, fibrinogen (Fib), activated partial thromboplastin time (APTT), INR, PT, WBC, NEU, LYM, red blood cells (RBC), hemoglobin (HGB), platelet (PLT), total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), AST, γ-glutamyl transferase (GGT), CREA.There were 173 females and 236 males with a mean age of 62 years. Among those, 43 patients developed postoperative hemorrhage. In this study, postoperative hemorrhage was defined as gastrointestinal hemorrhage or intra-abdominal hemorrhage and need of immediate reoperation or interventional embolization. The characteristics of the included patients are summarized in Table 1.
Table 1.
The statistical characteristics of the perioperative data in the training cohort.
| Variables | Hemorrhage | No hemorrhage | PComparison | z value | PMultivariate logistic model |
|---|---|---|---|---|---|
| n = 43,10.51% | n = 366,89.49% | ||||
| General conditions | |||||
| Age (years) | 60.42 ± 10.56 | 58.81 ± 11.08 | 0.837 | − 0.010 | 0.664 |
| Sex (M/F) | 26/17 | 210/156 | 0.698 | − 0.411 | 0.681 |
| BMI | 24.09 ± 3.63 | 23.30 ± 3.74 | 0.699 | − 0.992 | 0.321 |
| Sympotoms before admission | |||||
| Jaudice (Y/N) | 25/18 | 193/173 | 0.501 | 0.690 | 0.490 |
| Abdominal pain (Y/N) | 16/27 | 142/224 | 0.840 | 0.210 | 0.833 |
| Fever (Y/N) | 2/41 | 18/348 | 1.000 | 0.379 | 0.705 |
| Coexisting medical conditions | |||||
| HPB (Y/N) | 15/28 | 118/248 | 0.726 | − 0.003 | 0.998 |
| CHD (Y/N) | 4/39 | 22/344 | 0.613 | 0.181 | 0.856 |
| DM (Y/N) | 11/32 | 51/315 | 0.044 | − 2.198 | 0.028 |
| Pancreatitis (Y/N) | 2/41 | 15/351 | 1.000 | − 0.209 | 0.835 |
| Hepatitis (Y/N) | 5/38 | 39/327 | 0.002 | − 3.104 | 0.002 |
| Previous surgical history (Y/N) | 5/38 | 39/327 | 1.000 | 0.457 | 0.647 |
| Preoperative treatment | |||||
| Cholangial drainage (Y/N) | 19/24 | 141/225 | 0.472 | 0.450 | 0.652 |
| Tumor location | |||||
| Duodenum (Y/N) | 22/21 | 120/246 | 0.017 | 0.014 | 0.989 |
| Bile duct (Y/N) | 8/35 | 77/289 | 0.710 | 0.015 | 0.988 |
| Pancreas (Y/N) | 13/30 | 170/196 | 0.043 | 0.015 | 0.988 |
| Blood tests (median) | |||||
| CA125 (U/mL) | 10.82 | 13.00 | 0.119 | − 2.281 | 0.023 |
| CA199 (U/mL) | 66.87 | 63.00 | 0.724 | − 0.261 | 0.794 |
| TT (s) | 13.80 | 13.90 | 0.250 | − 1.503 | 0.133 |
| Fib (g/L) | 4.05 | 3.86 | 0.803 | 0.547 | 0.584 |
| APTT (s) | 31.60 | 30.25 | 0.056 | − 2.007 | 0.038 |
| INR | 0.95 | 0.98 | 0.026 | 1.574 | 0.115 |
| PT (s) | 10.60 | 10.90 | 0.049 | − 1.354 | 0.176 |
| WBC (109/L) | 6.60 | 6.20 | 0.300 | 2.382 | 0.017 |
| NEU (109/L) | 4.40 | 4.02 | 0.209 | − 2.501 | 0.012 |
| LYM (109/L) | 1.50 | 1.45 | 0.816 | − 2.267 | 0.023 |
| RBC (1012/L) | 3.98 | 4.08 | 0.363 | 1.444 | 0.149 |
| HGB (g/L) | 125.00 | 126.00 | 0.910 | − 1.329 | 0.184 |
| PLT (109/L) | 243.00 | 231.00 | 0.989 | 0.221 | 0.825 |
| TBIL (μmol/L) | 54.00 | 42.98 | 0.416 | − 0.708 | 0.479 |
| ALB (g/L) | 37.10 | 38.15 | 0.467 | 0.377 | 0.760 |
| ALT (U/L) | 69.00 | 82.25 | 0.400 | − 1.060 | 0.289 |
| AST (U/L) | 42.70 | 49.70 | 0.188 | 1.435 | 0.151 |
| GGT (U/L) | 279.00 | 273.00 | 0.499 | 0.625 | 0.532 |
| CREA (μmol/L) | 57.00 | 57.80 | 0.350 | − 1.546 | 0.122 |
PComparison: P value of the statistical comparison between the hemorrhage group and no hemorrhage group;
z value: The multivariate logistic regression analysis was performed to develop a full-variable logistic model.
PMultivariate analysis: P value of the multivariate logistic regression analysis of the risk-associated factors of postoperative hemorrhage.
BMI body mass index, HPB high blood pressure, CHD coronary heart disease, DM diabetes mellitus.
Procedure and main steps of LPD
The resection of the specimen was performed in the following order. (1) The gastrocolic ligament was dissected and the duodenum was mobilized by performing the Kocher maneuver. (2) The right gastroepiploic and pancreaticoduodenal inferior vessels were dissected, ligated, and transected. (3) The distal stomach 2–3 cm from the pylorus was transected. (4) A tunnel was created between the pancreatic neck and the superior mesenteric vein (SMV) or portal vein at the inferior border of the pancreas. (5) The jejunum was exposed through the Riolan avascular area on the left of the SMV, and transected 15–20 cm distal from the Treitz ligament. (6) Lymphadenectomy of the hepatoduodenal ligament was performed and the gastroduodenal artery was transected. (7) The jejunum and duodenum were completely mobilized from left to right to expose major vasculatures. (8) The pancreas neck was transected. (9) Cholecystectomy was performed and the common hepatic duct was transected. (10). The inferior vena cava and the left renal vein were exposed by performing the Kocher maneuver. (11) The uncinate process was separated from the SMV. (12) Lymphadenectomy was performed, including the lymph node stations of 5, 6, 8a, 13a, 13b, 14a, 14b, 17a, and 17b. (13) The specimen placed in a retrieval bag was extracted through a 5-cm upper abdominal incision.
Reconstruction was performed as follows. (1) Pancreatojejunostomy: a two-layer duct-to-mucosa anastomosis was performed. (2) Choledochojejunostomy: an end-to-side with approximately 10 cm distal to the anastomosis of pancreatojejunostomy was performed. (3) Gastrojejunostomy: ante colic gastrojejunostomy was performed 40–45 cm downstream from the choledochojejunostomy.
All the LPD surgeries were performed by the same surgical team led by Professor Liu Jianhua, who was the operator. Ultrasonic Shears and linear staplers were used in the procedures, and the vessels were ligated using hemolock clips.
Statistical analysis
The preoperative data of 409 patients who underwent LPD were retrospectively analyzed. The data were divided into the following two groups: with postoperative hemorrhage and without postoperative hemorrhage. Normality tests were performed for measurement data of each group. Between the two groups, the Chi-square tests were performed for the variables of sex, jaundice, abdominal pain, fever, HBP, CHD, DM, pancreatitis, hepatitis, previous history of operation, and different tumor locations. The student’s t tests were performed for variables of age and BMI. The Wilcoxon rank-sum tests were performed for the variables of blood test results. All the tests were two tailed, and a P value of less than 0.05 was considered statistically significant.
The predictive model was developed by performing multivariate logistic regression analysis and stepwise (stepAIC) selection. The data were first analyzed by multivariate binary logistic regression analysis to reveal the factors correlated with postoperative hemorrhage and to develop a full-variable predictive model. Stepwise (stepAIC) selection was then performed to obtain the best predictive model. A nomogram based on the predictive model was developed. The discriminatory ability of the predictive model was validated using the receiver operating characteristic (ROC) curve and leave-one-out method. The statistical analyses were performed using R 3.5.1(www.r-project.org).
This study was performed in accordance with the relevant guidelines and regulations and was approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University. Informed consent was obtained from all the study subjects.
Results
Risk-associated factors of postoperative hemorrhage
Among the 409 patients, 43 developed postoperative hemorrhage with an incidence of 10.51%. The statistical comparative tests showed statistically significant differences in the coexisting medical conditions of DM (P = 0.003) and hepatitis (P = 0.004), tumors located in pancreas (P = 0.043) and duodenum (P = 0.017), and the coagulation function blood tests of INR (P = 0.026) and PT (P = 0.049) between the hemorrhage group and the no-hemorrhage group. Risk-associated factors of postoperative hemorrhage identified by multivariate logistic regression analysis in a full-variable predictive model were as follows: the coexisting medical conditions of DM (P = 0.028) and hepatitis (P = 0.002), the tumor marker of CA125 level (P = 0.023), the coagulation function blood tests of APTT (P = 0.038), blood routine examination of WBC (P = 0.017), NEU (P = 0.012), and LYM (P = 0.023). The statistical characteristics of the perioperative data of the patients in the training cohort are summarized in Table 1.
Predictive model and nomogram for postoperative hemorrhage
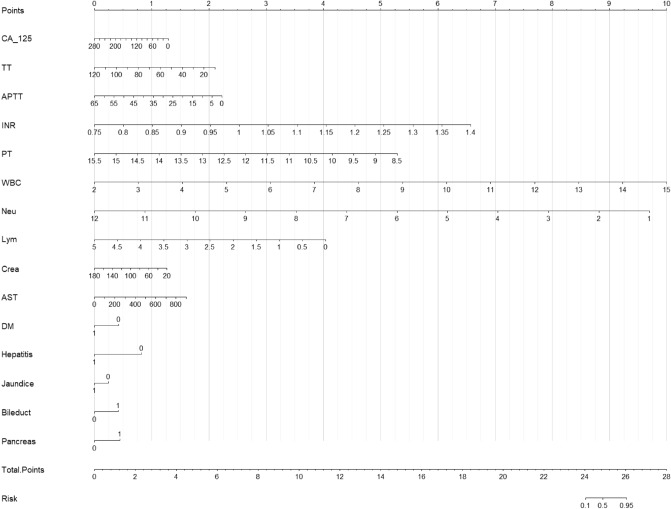
The predictive model was developed by performing the multivariate logistic regression analysis. The best model was obtained by stepwise (stepAIC) selection. The risk-associated variables in the predictive model of postoperative hemorrhage included the symptom of jaundice (P = 0.005, 97.5% CI 0.236–1.165), coexisting medical conditions of DM (P = 0.028, 97.5% CI 0.147–0.807) and hepatitis (P = 0.002,97.5% CI 0.032–0.488), the tumors located in pancreas (P = 0.005, 97.5% CI 1.442–7.278) and bile duct (P = 0.033, 97.5% CI 1.136–8.338), and blood tests of CA125 level (P = 0.039, 97.5% CI 0.978–1.001), TT level (P = 0.147, 97.5% CI NA-0.998), APTT level (P = 0.049, 97.5% CI 0.836–1.004), INR level (P = 0.093, 97.5% CI 0.019–4.43E + 24), PT level (P = 0.154, 97.5% CI 0.010–2.026), WBC level (P = 0.031, 97.5% CI 1.264–41.178), NEU level (P = 0.022, 97.5% CI 0.014–0.679), LYM level (P = 0.046, 97.5% CI 0.015–0.907), CREA level (P = 0.039, 97.5% CI 0.960–0.999) and AST level (P = 0.170, 97.5% CI 0.999–1.012). The statistical characteristics of the model selected by stepwise (stepAIC) selection are summarized in Table 2. The following model was identified as the best predictive model (AIC = 260.86): 2.695843 − 0.63056 × (Jaundice = 1) − 1.08368 × (DM = 1) − 2.10445 × (Hepatitis = 1) + 1.152354 × (Pancreatic tumor = 1) + 1.071354 × (Bile duct tumor = 1) − 0.01185 × CA125 − 0.04929 × TT − 0.08826 × APTT + 26.03383 × INR − 1.9442 × PT + 1.979563 × WBC − 2.26868 × NEU − 2.0789 × LYM − 0.02038 × CREA + 0.00459 × AST. The ROC curve and leave-one-out method were used for the internal validation of the predictive model. The ROC curve was statistically significant (AUC = 0.7758) (Fig. 1). The validation result of the leave-one-out method showed that the accuracy of the model and the F measure were 0.887 and 0.939, respectively, with statistical significances. The nomogram was developed based on the predictive model (Fig. 2).
Table 2.
Statistical characteristics of the stepwise (stepAIC) selected model for postoperative hemorrhage.
| Variables | 97.5% CI | z value | P |
|---|---|---|---|
| Jaundice | 0.236–1.165 | − 1.557 | 0.119 |
| DM | 0.146–0.807 | − 2.513 | 0.012 |
| Hepatitis | 0.032–0.488 | − 3.079 | 0.002 |
| Pancreatic tumor | 1.442–7.278 | 2.810 | 0.005 |
| Bile duct tumor | 1.136–8.338 | 2.127 | 0.033 |
| CA125 | 0.978–1.001 | − 2.006 | 0.039 |
| TT | NA–0.998 | − 1.449 | 0.147 |
| APTT | 0.836–1.004 | − 1.964 | 0.049 |
| INR | 0.018–4.43E+24 | 1.667 | 0.093 |
| PT | 0.010–2.026 | − 1.427 | 0.153 |
| WBC | 1.264–47.178 | 2.147 | 0.032 |
| NEU | 0.014–0.678 | − 2.288 | 0.022 |
| LYM | 0.015–0.907 | − 1.995 | 0.046 |
| AST | 0.999–1.212 | 1.370 | 0.171 |
| CREA | 0.960–0.999 | − 2.058 | 0.039 |
DM diabetes mellitus.
Figure 1.
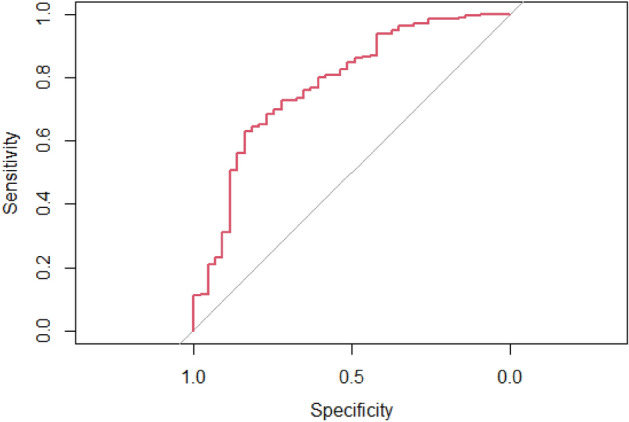
The ROC curve of the predictive model of postoperative hemorrhage of LPD.
Figure 2.
Predictive nomogram for postoperative hemorrhage of LPD.
Discussion
Despite technical complexity and surgical morbidity associated with pancreaticoduodenectomy, LPD has been found superior to OPD in terms of feasibility, safety, and oncological results in several retrospective studies3,18,19. However, some studies have reported that LPD leads to more severe morbidity than OPD20,21. Predictive model based on regression analysis and the deep learning models have been developed for the early detection of clinical issues22,23. Besides modifying the surgical procedures of LPD24–33, the prediction of severe complications before the surgery can be useful for better preoperative preparation and for reducing the incidence of severe complications. The mortality rate of patients due to postoperative hemorrhage after LPD is higher than that due to other complications21,34. To our knowledge, this study is the first to report the risk-associated preoperative factors of LPD and to develop a predictive model and a nomogram of postoperative hemorrhage after LPD, and deep learning models may be potential good tools for improving the prediction performance in the future.
The predictive model required comprehensive preoperative data including admission symptoms, imaging examination, coexisting medical conditions, preoperative treatment, and blood tests (tumor markers, blood routine, liver function, renal function and coagulation function). We found that the preoperative data including coexisting medical conditions of DM and hepatitis were statistically related to the postoperative hemorrhage of LPD. DM and hepatitis are two major public health problems worldwide. The pancreatic fistula was the most common cause of postoperative hemorrhage, which required reoperation or interventional embolization. Some studies have reported that DM is related to pancreatic fistula after pancreatic surgeries35–39. Hepatitis was reported to be related to postoperative hemorrhage after liver transplantation40. Other risk-associated preoperative factors in our studies were tumor-related genomic background, immune microenvironment, tumor locations of pancreas and bile duct, level of tumor marker of CA125, coagulation function of APTT, INR, and PT levels, blood routine of WBC, NEU, and LYM levels41–44. Multivariant binary logistic regression and stepwise (stepAIC) selection were performed to develop the predictive model for postoperative hemorrhage of LPD. We internally validated the model using the ROC curve and leave-one-out method. The results of the internal validation were statistically significant. The nomogram based on the predictive model was developed for the easy use of the model.
This study has some limitations. First, the same LPD procedure was used for all the surgeries, and hence different procedures used at different medical centers were unexplored. Second, the sample size of the training cohort was relatively limited, and the external validation was not performed due to the difficulty to collect additional samples. Further studies are needed to accomplish the validation of the predictive model under a systems-level through integrating genomic and clinical information45–48, and explore the potential causal effects of these risk factors associated with postoperative hemorrhage under a Mendelian randomization framework49–51. As the LPD procedures were recommended by the Study Group of Pancreatic Surgery in the Chinese Society of Surgery of Chinese Medical Association52, our predictive model can be used only in patients who underwent LPD in the same surgical manner. Because of the high mortality caused by severe complications of LPD, studies are required to establish the predictive models for the other severe complications of LPD.
In conclusion, a relationship may exist between the postoperative hemorrhage and the coexisting medical conditions of DM and hepatitis. Postoperative hemorrhage after LPD can be predicted from the preoperative data of the patients. The predictive model with a practical nomogram can be used to estimate the risk degree of postoperative hemorrhage after LPD. Our nomogram will be useful for surgeons to minimize the postoperative complications of LPD by better preoperative preparation and communication with the patients’ families concerned with the intense situation between the doctors and the patients in China.
Acknowledgements
This work was supported by two grants from the Key R&D Program of Hebei Province of China (Grant no. 19277758D) and the Nature Science Foundation of Hebei Province of China (Grant no. H2019206571).
Author contributions
D.R.L. and J.H.L. designed the study, analyzed the data and prepared figures and tables. D.R.L., C.X.D., J.S.Z. and Z.Q.X. collected the data. D.R.L. wrote the main manuscript. All authors read and approved the final version of manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maher H, Jin W, Mou Y, Davies H. The prospective of laparoscopic pancreaticoduodenectomy for cancer management. Chin. Clin. Oncol. 2017;6:8. doi: 10.21037/cco.2017.01.03. [DOI] [PubMed] [Google Scholar]
- 2.Conrad C, et al. Comparable long-term oncologic outcomes of laparoscopic versus open pancreaticoduodenectomy for adenocarcinoma: A propensity score weighting analysis. Surg. Endosc. 2017;10:3970–3978. doi: 10.1007/s00464-017-5430-3. [DOI] [PubMed] [Google Scholar]
- 3.Palanivelu C, Senthilnathan P, Sabnis SC. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br. J. Surg. 2017;104:1443–1450. doi: 10.1002/bjs.10662. [DOI] [PubMed] [Google Scholar]
- 4.Khaled YS, et al. Matched case–control comparative study of laparoscopic versus open pancreaticoduodenectomy for malignant lesions. Surg. Laparosc. Endosc. Percutan. Tech. 2017;1:47–51. doi: 10.1097/SLE.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 5.Tran TB, et al. The first decade of laparoscopic pancreaticoduodenectomy in the united states: Costs and outcomes using the nationwide inpatient sample. Surg. Endosc. 2016;30:1778–1783. doi: 10.1007/s00464-015-4444-y. [DOI] [PubMed] [Google Scholar]
- 6.Gerber MH, et al. Analysis of the cost effectiveness of laparoscopic pancreatoduodenectomy. J. Gastrointest. Surg. 2017;9:1404–1410. doi: 10.1007/s11605-017-3466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stauffer JA, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: Long-term results at a single institution. Surg. Endosc. 2017;31:2233–2241. doi: 10.1007/s00464-016-5222-1. [DOI] [PubMed] [Google Scholar]
- 8.Kantor O, et al. Laparoscopic pancreaticoduodenectomy for adenocarcinoma provides short-term oncologic outcomes and long-term overall survival rates similar to those for open pancreaticoduodenectomy. Am. J. Surg. 2017;213:512–515. doi: 10.1016/j.amjsurg.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Kantor O, et al. Minimally invasive pancreatoduodenectomy: Is the incidence of clinically relevant postoperative pancreatic fistula comparable to that after open pancreatoduodenectomy? Surgery. 2018;163:587–593. doi: 10.1016/j.surg.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YH, Zhang CW, Hu ZM, Hong DF. Pancreatic cancer: Open or minimally invasive surgery? World. J. Gastroenterol. 2016;22:7301–7310. doi: 10.3748/wjg.v22.i32.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, et al. Minimally invasive pancreaticoduodenectomy for periampullary disease: A comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC. Gastroentrol. 2017;17:120. doi: 10.1186/s12876-017-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, et al. Minimally invasive pancreaticoduodenectomy: A comprehensive review. Int. J. Surg. 2016;35:139–146. doi: 10.1016/j.ijsu.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, et al. Imbedding pancreaticojejunostomy used in pure laparoscopic pancreaticoduodenectomy for nondilated pancreatic duct. Surg. Endosc. 2017;31:1986–1992. doi: 10.1007/s00464-016-4805-1. [DOI] [PubMed] [Google Scholar]
- 14.Qin RY. The optimization of surgical approach selection and technical process in total laparoscopic pancreatoduodenectomy. Zhonghua Wai Ke Za Zhi. 2017;55:343–345. doi: 10.3760/cma.j.issn.0529-5815.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Murata Y, et al. Superiority of stapled side-to-side gastrojejunostomy over conventional hand-sewn end-to-side gastrojejunostomy for reducing the risk of primary delayed gastric emptying after subtotal stomach-preserving pancreaticoduodenectomy. Surg. Today. 2017;8:1007–1017. doi: 10.1007/s00595-017-1504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong DF, et al. The role of Hong's single-stitch duct to mucosa pancreaticojejunostomy in laparoscopic pancreaticoduodenectomy. Zhonghua Wai Ke Za Zhi. 2017;55:136–140. doi: 10.3760/cma.j.issn.0529-5815.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Chopinet S, Fuks D, Gregoire E, Hardwigsen J, Gayet B. Authors' reply: Postoperative bleeding after laparoscopic pancreaticoduodenectomy: The Achilles' heel? World. J. Surg. 2018;42:3060–3061. doi: 10.1007/s00268-018-4583-0. [DOI] [PubMed] [Google Scholar]
- 18.Torphy RJ, et al. Comparing short-term and oncologic outcomes of minimally invasive versus open pancreaticoduodenectomy across low and high volume centers. Ann. Surg. 2018;6:1147–1155. doi: 10.1097/SLA.0000000000002810. [DOI] [PubMed] [Google Scholar]
- 19.Pedziwiatr M, et al. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks. Arch. Surg. 2017;402:841–851. doi: 10.1007/s00423-017-1583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dokmak S, et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J. Am. Coll. Surg. 2015;220:831–838. doi: 10.1016/j.jamcollsurg.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Chopinet S, et al. Postoperative bleeding after laparoscopic pancreaticoduodenectomy: The Achilles' Heel? World. J. Surg. 2018;42:1138–1146. doi: 10.1007/s00268-017-4269-z. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, et al. LEPR hypomethylation is significantly associated with gastric cancer in males. Exp. Mol. Pathol. 2020;116:104493. doi: 10.1016/j.yexmp.2020.104493. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, et al. A multi-model deep convolutional neural network for automatic hippocampus segmentation and classification in Alzheimer's disease. Neuroimage. 2020;208:116459. doi: 10.1016/j.neuroimage.2019.116459. [DOI] [PubMed] [Google Scholar]
- 24.Kuroki T, Tajima Y, Kitasato A, Adachi T, Kanematsu T. Pancreas-hanging maneuver in laparoscopic pancreaticoduodenectomy: A new technique for the safe resection of the pancreas head. Surg. Endosc. 2010;24:1781–1783. doi: 10.1007/s00464-009-0814-7. [DOI] [PubMed] [Google Scholar]
- 25.Addeo P, Marzano E, Rosso E, Pessaux P. Hanging maneuver during pancreaticoduodenectomy: A technique to improve R0 resection. Surg. Endosc. 2011;25:1697–1698. doi: 10.1007/s00464-010-1421-3. [DOI] [PubMed] [Google Scholar]
- 26.Ogiso S, Conrad C, Araki K, Basso V, Gayet B. Posterior approach for laparoscopic pancreaticoduodenectomy to prevent replaced hepatic artery injury. Ann. Surg. Oncol. 2013;20:3120. doi: 10.1245/s10434-013-3058-7. [DOI] [PubMed] [Google Scholar]
- 27.Pointer DT, Jr, Slakey LM, Slakey DP. Safety and effectiveness of vessel sealing for dissection during pancreaticoduodenectomy. Am. Surg. 2013;79:290–295. doi: 10.1177/000313481307900329. [DOI] [PubMed] [Google Scholar]
- 28.Gall TM, et al. Surgical techniques for improving outcomes in pancreatic ductal adenocarcinoma. Expert. Rev. Gastroenterol. Hepatol. 2014;8:241–246. doi: 10.1586/17474124.2014.881251. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, et al. Laparoscopic pancreaticoduodenectomy via a reverse-"V" approach with four ports: Initial experience and perioperative outcomes. World. J. Gastroenterol. 2015;21:1588–1594. doi: 10.3748/wjg.v21.i5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagakawa Y, et al. A novel, "artery first" approach allowing safe resection in laparoscopic pancreaticoduodenectomy: The uncinate process first approach. Hepatogastroenterology. 2015;62:1037–1040. [PubMed] [Google Scholar]
- 31.Pittau G, Sanchez-Cabus S, Laurenzi A, Gelli M, Cunha AS. Laparoscopic pancreaticoduodenectomy: Right posterior superior mesenteric artery "first" approach. Ann. Surg. Oncol. 2015;22(Suppl 3):S345–348. doi: 10.1245/s10434-015-4913-5. [DOI] [PubMed] [Google Scholar]
- 32.Zimmitti G, et al. Laparoscopic pancreatoduodenectomy with superior mesenteric artery-first approach and pancreatogastrostomy assisted by mini-laparotomy. Surg. Endosc. 2016;30:1670–1671. doi: 10.1007/s00464-015-4359-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen YJ, Lau WY, Zhen ZJ, He YT. Long-sleeve-working-port assisted laparoscopic pancreaticoduodenectomy—a new technique in laparoscopic surgery. Int. J. Surg. Case. Rep. 2017;30:190–193. doi: 10.1016/j.ijscr.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu M, Xia Q. Letter to the Editor: Postoperative bleeding after laparoscopic pancreaticoduodenectomy: The Achilles' heel? World. J. Surg. 2018;42:3058–3059. doi: 10.1007/s00268-018-4506-0. [DOI] [PubMed] [Google Scholar]
- 35.Chu CK, et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J. Am. Coll. Surg. 2010;210:463–473. doi: 10.1016/j.jamcollsurg.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Addeo P, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: A multicentre study of the French Surgical Association. HPB (Oxford) 2014;16:46–55. doi: 10.1111/hpb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghavan SR, Ballehaninna UK, Chamberlain RS. The impact of perioperative blood glucose levels on pancreatic cancer prognosis and surgical outcomes: An evidence-based review. Pancreas. 2013;42:1210–1217. doi: 10.1097/MPA.0b013e3182a6db8e. [DOI] [PubMed] [Google Scholar]
- 38.Subhedar PD, et al. Risk factors for pancreatic fistula after stapled gland transection. Am. Surg. 2011;77:965–970. doi: 10.1177/000313481107700811. [DOI] [PubMed] [Google Scholar]
- 39.Xia X, Huang C, Cen G, Qiu ZJ. Preoperative diabetes as a protective factor for pancreatic fistula after pancreaticoduodenectomy: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2015;14:132–138. doi: 10.1016/S1499-3872(15)60330-7. [DOI] [PubMed] [Google Scholar]
- 40.Schrem H, et al. Post-operative hemorrhage after liver transplantation: Risk factors and long-term outcome. Ann. Transplant. 2016;21:46–55. doi: 10.12659/AOT.895605. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, et al. Associations between maternal vitamin D status during three trimesters and cord blood 25(OH)D concentrations in newborns: A prospective Shanghai birth cohort study. Eur. J. Nutr. 2021 doi: 10.1007/s00394-021-02528-w. [DOI] [PubMed] [Google Scholar]
- 42.Jin G, Xu M, Zou M, Duan S. The processing, gene regulation, biological functions, and clinical relevance of N4-acetylcytidine on RNA: A systematic review. Mol. Ther. Nucleic Acids. 2020;20:13–24. doi: 10.1016/j.omtn.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng S, et al. Immunodeficiency promotes adaptive alterations of host gut microbiome: An observational metagenomic study in mice. Front. Microbiol. 2019;10:2415. doi: 10.3389/fmicb.2019.02415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan X, Zhao X, Li J, He L, Xu M. Effects of early-life malnutrition on neurodevelopment and neuropsychiatric disorders and the potential mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;83:64–75. doi: 10.1016/j.pnpbp.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Li H, et al. Co-expression network analysis identified hub genes critical to triglyceride and free fatty acid metabolism as key regulators of age-related vascular dysfunction in mice. Aging (Albany NY) 2019;11:7620–7638. doi: 10.18632/aging.102275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, et al. Genetic regulatory subnetworks and key regulating genes in rat hippocampus perturbed by prenatal malnutrition: Implications for major brain disorders. Aging (Albany NY) 2020;12:8434–8458. doi: 10.18632/aging.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, et al. Multi-trait analysis for genome-wide association study of five psychiatric disorders. Transl. Psychiatry. 2020;10:209. doi: 10.1038/s41398-020-00902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang L, et al. Sex-specific association of circulating ferritin level and risk of type 2 diabetes: A dose-response meta-analysis of prospective studies. J. Clin. Endocrinol. Metab. 2019;104:4539–4551. doi: 10.1210/jc.2019-00495. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F, et al. Causal influences of neuroticism on mental health and cardiovascular disease. Hum. Genet. 2021 doi: 10.1007/s00439-021-02288-x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, et al. Genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder. J. Clin. Invest. 2021 doi: 10.1172/JCI145942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, et al. Genetic support of a causal relationship between iron status and type 2 diabetes: A Mendelian randomization study. J. Clin. Endocrinol. Metab. 2021 doi: 10.1210/clinem/dgab454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Expert consensus of laparoscopic pancreaticoduodenectomy (postscript of operation process and main steps). Zhonghua Wai Ke Za Zhi55, 335–339 (2017). [DOI] [PubMed]