Abstract
Background
We conducted a systematic review and meta-analysis of prospective studies to clarify the relation of fruit and vegetable consumption with incident breast cancer.
Methods
We searched systematically PubMed and EMBASE databases up to November 2020 to include prospective studies that reported the association of fruit and vegetable consumption with incident breast cancer. The pooled relative risks (RRs) and 95% confidence intervals (CIs) were calculated for the highest versus the lowest category of total fruit and vegetable, total fruit and total vegetable consumption, as well as fruit juice and subgroups of vegetables in relation to breast cancer incidence, using a random-effect model.
Results
Total fruit and vegetable consumption was associated with lower overall (RR = 0.91, 95% CI = 0.87–0.95) and postmenopausal breast cancer risk (RR = 0.88, 95% CI = 0.79–0.99). Total fruit consumption was associated with lower overall (RR = 0.93, 95% CI = 0.88–0.99) and postmenopausal breast cancer risk (RR = 0.93, 95% CI = 0.87–0.99). Total fruit and vegetable intake were associated with 11% and 26% lower risk of oestrogen- and progesterone-receptor-positive (ER+/PR+) and -negative (ER−/PR−) breast cancer, respectively. Total vegetable consumption was associated with 27% lower risk of ER−/PR− breast cancer. Fruit juice consumption was associated with increased overall breast cancer risk (RR = 1.04, 95% CI = 1.01–1.07). We did not find significant associations for subgroups of vegetable intake and breast cancer risk.
Conclusions
These findings suggest that high total fruit and vegetable consumption are associated with reduced risk of overall, postmenopausal, ER+/PR+ and ER−/PR− breast cancer.
Subject terms: Risk factors, Breast cancer
Background
Breast cancer is the most common cancer and the leading cause of cancer-related death among women worldwide.1 That being said, there is a wide variation in breast cancer rates across the globe,1 which may partially reflect vast differences across a range of lifestyle factors. Identifying modifiable lifestyle risk factors could reduce breast cancer risk through large-scale public health initiatives and clinical interventions. Dietary composition is one potential lifestyle factor through which breast cancer incidence may be reduced.2 Fruits and vegetables contain a large number of potentially anti-carcinogenic nutrients and bioactive substances, which may reduce the risk of cancer.3–5 However, epidemiological studies assessing fruit and vegetable intake with risk of breast cancer are inconsistent.6–26
Prospective studies evaluating the associations of fruit and vegetable intake with risk of breast cancer by menopausal status are inconsistent, with most studies reporting associations for postmenopausal breast cancer6,9,12,14–16,18,19,21,23–25 as opposed to premenopausal breast cancer.14,18,19,21,23,25 Moreover, breast cancer is a heterogeneous disease, and evidence suggests that hormone-related factors may be associated with a higher risk of oestrogen-receptor-positive (ER+) tumours as opposed to ER-negative (ER−) tumours;27 on the other hand, intake of fruits and vegetables may be associated with a relatively greater decrease in risk of ER− tumours.20,23 However, the association between fruit and vegetable consumption and tumour hormone-receptor status is unclear.11,14,19,20,23,24 In a meta-analysis published in 2012, based on the data from 15 prospective studies, fruit intake was inversely associated with risk of breast cancer, while there was no significant association between vegetable intake and breast cancer risk.28 Furthermore, in a pooled analysis of 20 prospective studies (mainly from North America) in 2013, fruit or vegetable intake was not associated with a lower risk of overall breast cancer and an inverse association was limited to vegetable consumption and ER– tumours.29 Then, in 2018, the World Cancer Research Fund/American Institute for Cancer Research concluded that data were insufficient to recommend high fruit and vegetable consumption for reducing the risk of breast cancer, except for vegetables and ER− breast cancer.30 Subsequently, several large-cohort studies were published that supported the role of fruits or vegetables in breast cancer prevention.20,23,24 These recent works have not been integrated into a meta-analysis to inform and update recommendations regarding the potential role of fruits and vegetables as part of a healthy diet for the prevention of breast cancer. Also, the role of fruits and vegetables in breast cancer risk needs to be assessed and synthesised for subgroups of women (premenopause and postmenopause) and subtypes of breast cancer (e.g., ER- and progesterone-receptor (PR)-positive (ER+/PR+) and ER- and PR-negative (ER−/PR−). Finally, there is significant public interest in the role of different types of vegetables and fruit juice in breast cancer risk; however, these risk factors were not assessed comprehensively in a previous meta-analysis.28
In this study, we performed a meta-analysis on the consumption of total fruits and vegetables, total fruits and total vegetables, in relation to the overall risk of breast cancer among a geographically and racially/ethnically diverse set of adult women, as well as stratified by menopausal, and ER and PR status, including findings from the more recent prospective longitudinal studies. Meta-analysis was also conducted to determine the associations between intake of fruit juice, yellow/orange vegetables, cruciferous vegetables, green leafy vegetables and tomatoes and risk of breast cancer.
Methods
Study strategy
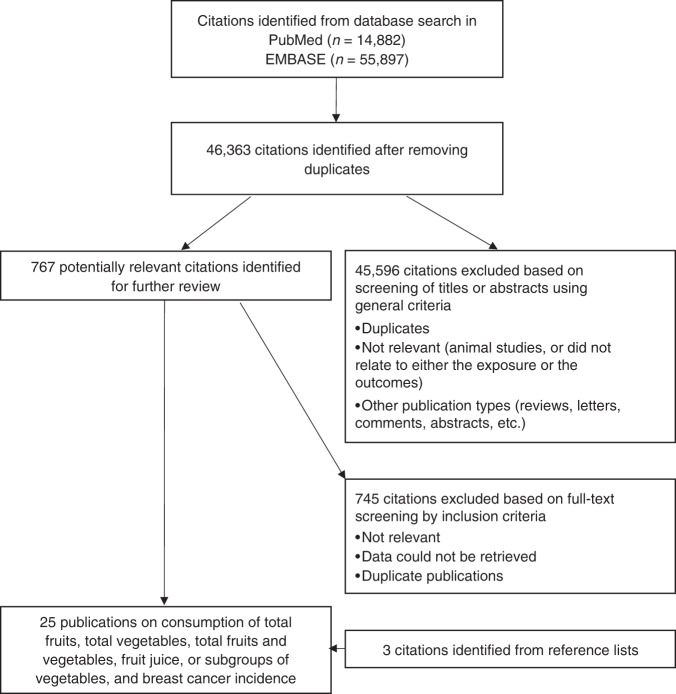
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for title, abstract, introduction, methods, and reporting and interpretation of findings.31 We conducted a systematic literature review of publications using two databases, PubMed and EMBASE, to find prospective studies reporting the associations of fruit, vegetable and fruit juice consumption, with risk of overall, premenopausal, postmenopausal, ER+/PR+ and ER−/PR− breast cancers in adult women, up to November 2020. The search string was developed with assistance from informational specialists at the Harvard Countway Library of Medicine, and it is presented in Supplementary Table S1. We also checked the reference lists in included studies as well as previously published systematic reviews and meta-analyses on the same topic. After identifying 46,363 unique citations, we only selected prospective studies to reduce recall and selection bias. Among prospective studies, we chose studies with risk estimates for the associations of total fruit and vegetable, total fruit, total vegetable, total fruit juice, yellow/orange vegetable, cruciferous vegetable, green leafy vegetable or tomato consumption with breast cancer risk. The following studies were excluded from analysis: retrospective, case–control, cross-sectional or ecological studies. We also excluded non-original research (reviews, editorials and letters) and meeting abstracts. For multiple articles published from the same study population, we included the most up-to-date analyses with the largest number of breast cancer cases and longer follow-up time in this meta-analysis (Fig. 1).
Fig. 1. Flowchart of study selection.
Search, screening and selection process of prospective studies of fruit, vegetable and fruit juice intake and breast cancer incidence.
Data extraction
The following information was extracted: study characteristics (first author, publication year, study name and country), study design, follow-up period (mean, median or maximum number of follow-up), number of participants, number of cases of overall, premenopausal, postmenopausal, ER+/PR+ and ER−/PR− breast cancers, age at baseline (mean, median or range), dietary assessment method, exposures and covariates controlled in the statistical models. If there was more than one multivariable model, we included the risk estimates of the exposures with the highest number of adjusted variables in this meta-analysis.
Quality assessment
Using the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, the quality of each study was assessed by two authors (M.S.F. and N.D.S.) independently, and any discrepancies were resolved by the third author (J.B.B.). We did not use the quality scores for excluding studies from the meta-analysis.
Data synthesis
We did separate analyses for eight different exposure variables: “total fruits and vegetables”, “total fruits”, “total vegetables”, “fruit juice”, “yellow/orange vegetables”, “cruciferous vegetables”, “green leafy vegetables” and “tomatoes”. Because risk estimates were reported differently across the studies (e.g., tertiles, quartiles or quintiles of intake), the pooled relative risks (RRs) for the highest versus the lowest category of intake were calculated in this meta-analysis. The RRs and the corresponding 95% confidence intervals (CIs) of the studies were illustrated by forest plots. The pooled RRs and 95% CIs were calculated using random-effect models (DerSimonian and Laird method32). For a study that did not use the lowest category as a reference group and the CIs were calculated using the method of floating absolute risks,24 the RRs and 95% CIs for the highest versus lowest category of intake were recalculated.33 For studies that did not report the highest versus lowest category of intake,17–19 we did the following: the RRs and 95% CIs were calculated for comparisons between top versus bottom quintiles using the difference in the medians of the top and bottom quintiles of intake as conversion factors or 2.56 as a conversion factor for a one-SD increase in fruit or vegetable intake. For one study, the RRs and 95% CIs for top versus bottom quartiles have been provided through correspondence with authors.25 For the NHS/NHSII23 that reported RRs and 95% CIs of total fruit and vegetable, total fruit or total vegetable intake and risk of ER+/PR+ and ER−/PR− breast cancer as continuous variables, we reran the programme to get RRs and 95% CIs for the highest versus lowest quintile.
We also estimated the RRs and 95% CIs for each 200 g/day intake of total fruits and vegetables, total fruits and total vegetables in relation to breast cancer. For a study to be included in the dose–response analyses, it had to have risk estimates, standard error or 95% CIs, median intake, number of cases, person-years of follow-up or number of subjects for all exposure categories. For studies that reported the ranges of intake, the midpoint of the lower and upper bound was used as the median of intake for each category. If the highest category was open-ended, the length of open-ended intervals was considered equal to that of adjacent intervals. If studies reported the amount of intake in servings or times, we converted these to grams (g) per day, considering 80 g as one serving size of fruit or vegetable.
We examined the evidence of publication bias using visual inspection of a funnel plot34 and Begg and Mazumdar test.35 We evaluated heterogeneity among studies using the I2 statistic (low, moderate and high heterogeneity was assigned to I2 values of 25%, 50% and 75%, respectively). In addition, we evaluated the potential sources of heterogeneity across studies for total fruits and total vegetables stratified by region (North America/other countries), duration of follow-up (<8 years/≥8 years) and adjustment for energy intake, smoking, benign breast disease, family history of breast cancer, alcohol intake, oral contraceptive use and body mass index (BMI) as well as quality of the study (fair/good). A two-tailed test with P < 0.05 was considered statistically significant. All statistical analyses were conducted using STATA, version 16, software (STATA Corp, College Station, TX).
Results
Study characteristics
For this meta-analysis, we identified 25 publications including 18 prospective cohorts,6,8,10,12,14–25,36–39 four nested case–controls,7,9,13,26 and one clinical trial40 that met inclusion criteria for the analysis (Fig. 1). Findings using the data from Swedish Women’s Lifestyle and Health Study (WLH) were reported in three publications17,18,39, and data from European Prospective Investigation into Cancer and Nutrition (EPIC) were reported in two publications.20,37 Finally, findings using data from combined Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII) were reported in one publication.23 The characteristics of the identified studies are presented in Table 1. The number of participants in each study ranged from 1056 to 691,571. The follow-up time ranged from 4 to 23.7 years. We conducted separate analyses of the association between breast cancer and each of the following exposures: (i) total fruits and vegetables, including 852,425 women with 26,936 cases of breast cancer, (ii) total fruits, including 1,669,858 women with 65,841 cases of breast cancer, (iii) total vegetables, including 1,675,949 women with 66,884 cases of breast cancer, (iv) fruit juice, including 1,274,233 women with 46,112 cases of breast cancer, (v) yellow/orange vegetables, including 292,942 women with 12,850 cases of breast cancer, (vi) cruciferous vegetables, including 640,367 women with 19,379 cases of breast cancer, (vii) green leafy vegetables, including 566,888 women with 16,290 cases of breast cancer and (viii) tomatoes, including 310,545 women with 14,256 cases of breast cancer. Most of the studies used a food-frequency questionnaire (FFQ) to estimate dietary intake, except for two studies (NutriNet-Santé and the SUpplémentation en VItamines et Minéraux AntioXydants [SU.VI.MAX])38,40 that used dietary records. The method of assessing dietary intake was not reported for the Karunagappally Cohort (KC).13 Fruit and vegetable consumption was reported as g/day or g/week, servings/day or g/1000 kcal across studies. Eight publications reported findings using data from populations in North America,6–8,12,14,15,23,36 12 from Europe,9,17,18,20,22,24–26,37–40 and five from Asia.10,13,16,19,21 In 20 publications,7,9,12,14,16–26,36–40 risk estimates were adjusted for confounders (Table 1). Six publications14,18,19,21,23,25 reported findings for the associations of total fruit and vegetable, total fruit or total vegetable intake with premenopausal breast cancer. Twelve publications reported the associations between total fruit and vegetable, total fruit or total vegetable intake and postmenopausal breast cancer.6,9,12,14–16,18,19,21,23–25 Four publications14,19,20,23 reported the association between total fruit and vegetable, total fruit or total vegetable intake and breast cancer by ER/PR status (Table 1).
Table 1.
Characteristics of cohort studies that evaluated fruit and vegetable intake and incidence of breast cancer.
| Author and publication year | Study name and country | Study design | Follow-up period (years) | Total number of participants* | Number of breast cancer events* | Baseline age (years) | Method of dietary assessment and exposures | Adjustment | Quality of study | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Premenopausal | Postmenopausal | ER+/PR + | ER−/PR− | |||||||||
| Shibata et al., 19926 | LWS, US | Cohort | 8 | 11,580 | 219 | – | 219 | – | – | 73.8 ± 7.4 (65–84) |
FFQ Total fruits and vegetables Total fruits Total vegetables Yellow/orange vegetables |
Age, smoking | Good |
| Rohan et al., 19937 | CNBSS, Canada | Nested case–control | 5 | Control: 1182 | 519 | – | – | – | – | 40–59 |
FFQ Total fruits Total vegetables |
Age, age at menarche, surgical menopause, age at first livebirth, education, family history of breast cancer, history of BBD, other contributors of food intake | Good |
| Byrne et al., 19968 | NHEFS, US | Cohort | 4 | 6156 | 53 | – | – | – | – | 32–86 |
FFQ Total fruits and vegetables |
Age | Fair |
| Verhoeven et al., 19979 | NLCS, The Netherlands | Nested case–control | 4.3 | Control: 1598 | 519 | – | 519 | – | – | 55–69 |
FFQ Total fruits Total vegetables |
Age, energy intake, alcohol intake, history of BBD, maternal breast cancer, breast cancer in sister(s), age at menarche, age at menopause, age at first birth, parity | Good |
| Key et al., 199910 | LSS, Japan | Cohort | 14 | 34,759 | 427 | – | – | – | – | – |
FFQ Total fruits |
Age, calendar period, city, age at the time of the bombing, radiation dose | Good |
| van Gils et al., 200537 | EPIC, Europe | Cohort | 5.4 | 285,526 | 3659 | – | – | – | – | 25–70 |
FFQ Fruit juice Cruciferous vegetables Green leafy vegetables |
Age, centre, energy intake, alcohol intake, saturated fat, height, weight, age at menarche, parity, OC use, HRT, menopausal status, smoking, physical activity, education | Good |
| Hirvonen et al., 200640 | SU.VI.MAX, France | Clinical Trial Study | 6.6 | 4396 | 95 | – | – | – | – | 35–60 |
24-hour dietary record Fruit juice |
Age, smoking, number of children, OC use, family history of breast cancer, menopausal status | Good |
| George et al., 200912 | NIH-AARP, US | Cohort | 8 | 195,229 | 5815 | – | 5815 | – | 50–71 |
FFQ Total fruits Total vegetables |
Age, smoking, energy intake, BMI, alcohol intake, physical activity, education, race, marital status, family history, HRT, fruit or vegetable intake | Good | |
| Jayalekshmi, et al., 200913 | KC, India | Nested case–control | 14 | Control: 792 | 264 | – | – | – | 46.4 at time of diagnosis | Total vegetables | - | Fair | |
| Boggs et al., 201014 | BWHS, US | Cohort | 12 | 51,928 | 1268 | 562 | 570 | 366 | 264 | 21–69 |
FFQ Total fruits and vegetables Total fruits Total vegetables Yellow/orange vegetables Cruciferous vegetables Green leafy vegetables Tomatoes |
Age, energy intake, age at menarche, BMI at age 18, family history of breast cancer, education, geographic region, parity, age at first birth, OC use, menopausal status, age at menopause, HRT, vigorous activity, smoking, alcohol intake, multivitamin use | Good |
| Brasky et al., 201015 | VITAL, US | Cohort | 7 | 35,016 | 880 | – | 880 | – | 50–76 |
FFQ Total fruits Total vegetables |
Age | Fair | |
| Butler et al., 201016 | SCHS, Singapore | Cohort | 10.7 | 34,028 | 629 | – | 439 | – | – | 55 (45–74) |
FFQ Total fruits Total vegetables |
Age, dialect group, interview year, education, parity, BMI, first-degree relative with a diagnosis of breast cancer, energy intake | Good |
| Löf et al., 201117 | WLH, Sweden | Cohort | 14 | 44,838 | 1067 | – | – | – | – | 39 ± 6 (30–49) |
FFQ Total fruits and vegetables |
Age, education, BMI, smoking, energy intake, alcohol intake | Good |
| Couto et al., 201318 | WLH, Sweden | Cohort | 16 | 44,840 | 1278 | 736 | 448 | – | – | 30–49 |
FFQ Total vegetables |
Family history of breast cancer, history of BBD, smoking, BMI, height, age at first birth and number of children, education, age at menarche, energy intake, consumption of beverages, potatoes, sweets, eggs, alcohol intake, cereals, dairy products, fish, fruits and nuts, legumes, meat products, the ratio of unsaturated to saturated lipids | Good |
| Suzuki et al., 201319 | JPHC, Japan | Cohort | 10.2 | 47,289 | 452 | 115 | 337 | 105 | 61 | 57 |
FFQ Total fruits and vegetables Total fruits Total vegetables Yellow/orange vegetables Cruciferous vegetables Green leafy vegetables Tomatoes |
Age, area, height, BMI, BMI at age 20, age at menarche, age at first birth, parity, menopausal status, age at menopause, use of exogenous female hormones, smoking, physical activity, alcohol intake, energy-adjusted intake of isoflavones, vitamin C supplement, fruit or vegetable intake | Good |
| Li et al., 201539 | WLH, Sweden | Cohort | 20 | 44,296 | 1464 | – | – | – | – | 29–49 |
FFQ Cruciferous vegetables |
Age, BMI, height, education, smoking, age at menarche, family history of breast cancer, history of BBD, age at first childbirth, number of children, breastfeeding, OC use, alcohol, saturated fat intake, energy intake | Good |
| Emaus et al., 201620 | EPIC, Europe | Cohort | 11.5 | 335,054 | 10,197 | – | – | 3479 | 1021 | 50.8 ± 9.8 |
FFQ Total fruits and vegetables Total fruits Total vegetables |
Age, centre, energy intake, saturated fat intake, age at menarche, OC use, age at first birth, menopausal status, HRT, BMI, BMI×menopausal status, physical activity, smoking, alcohol intake, education, fruits or vegetables | Good |
| Kim et al., 201721 | KNCC, Korea | Cohort | 9.46 | 5046 | 72 | 48 | 23 | – | – | ≥30 |
FFQ Total fruits |
Age, smoking, education, history of BBD, BMI, family history of breast cancer, HRT, menopausal status, age at menopause, alcohol intake, physical activity, age at menarche, parity, OC use | Good |
| Makarem et al., 201836 | FOS, US | Cohort | ~18 | 1688 | 124 | – | – | – | – | 26–84 |
FFQ Fruit juice |
Age, energy intake, smoking, alcohol intake, menopausal status, age at menopause, HRT, number of children | Good |
| Elwood et al., 201822 | UK Biobank, UK | Cohort | 5.1 | 173,435 | 2769 | – | – | – | – | 40–69 |
FFQ Total fruits and vegetables |
Age, Townsend deprivation index, height, smoking, BMI, physical activity, alcohol | Good |
| Bravi et al., 201826 | FRiCaM, Italy | Nested case–control | ~8 | Control: 9,909 | 3303 | – | – | – | – | 41–76 |
FFQ Total fruits Total vegetables |
Age, year of enrollment, education, marital status, BMI, alcohol intake, age at menarche, age at first birth, menopausal status, family history of breast cancer | Good |
| Farvid et al., 201923 | NHS/NHSII, US | Cohort | 23.7 | 182,145 | 10,911 | 2374 | 7578 | 6128 | 1573 | 27–59 |
FFQ Total fruits and vegetables Total fruits Total vegetables Fruit juice Yellow/orange vegetables Cruciferous vegetables Green leafy vegetables Tomatoes |
Age, cohort, family history of breast cancer, history of BBD, height, BMI at age 18, weight change since age 18, smoking, physical activity, OC use, alcohol intake, energy intake, parity and age at first birth, age at menarche, menopausal status, age at menopause, HRT | Good |
| Key et al., 201924 | MWS, UK | Cohort | 12 | 691,571 | 29,005 | – | 29,005 | – | – | 59.9 ± 4.9 |
FFQ Total fruits Total vegetables Fruit juice |
Age, region, socioeconomic status, BMI, height, smoking, HRT, energy intake, alcohol intake, age at menarche, physical activity, parity and age at first birth, education, HRT | Good |
| Chazelas et al., 201938 | NutriNet-Santé, France | Cohort | 5.1 | 79,724 | 693 | – | – | – | – | 40.9 ± 13.9 (18.0–72.7) |
24-hour dietary record Fruit juice |
Age, energy intake, sugar from other dietary sources, alcohol, sodium, lipid, and fruit and vegetable intake, BMI, height, physical activity, smoking, number of 24-h dietary records, family history of breast cancer, education, diabetes, hypertension, CVD, dyslipidemia, number of children, menopausal status, HRT, OC use | Good |
| Dunneram et al., 201925 | UKWCS, UK | Cohort | 18 | 29,183 | 1625 | 291 | 1030 | – | – | 52 ± 9 (35–69) |
FFQ Total fruits Total vegetables Fruit Juice Cruciferous vegetables Tomatoes |
Age, alcohol intake, duration of breastfeeding, physical activity, smoking, social class, menopausal status | Good |
BBD benign breast disease, BMI body mass index, BWHS Black Women’s Health Study, CNBSS Canadian National Breast Screening Study, DCH diet, cancer and health, EPIC European Prospective Investigation into Cancer and Nutrition, FOS Framingham Offspring Study, FRiCaM risk factors for breast cancer, Fattori di Rischio per il Carcinoma della Mammella, HRT hormone-replacement therapy, JPHC Japan Public Health Centre-based Prospective Study, KC Karunagappally Cohort, KNCC Korean National Cancer Centre Cohort, LSS The Radiation Effects Research Foundation’s Life Span Study, LWS Leisure World Study, MWS Million Women Study, NHEFS National Health Epidemiologic Follow-up Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, NIH-AARP National Institute of Health-AARP Diet and Health Study, NLCS The Netherlands Cohort Study, OC oral contraceptive, SCHS the Singapore Chinese Health Study, SU.VI.MAX SUpplémental en VItamines et Minéraux AntioXydants, UKWCS UK Women’s Cohort Study, VITAL VITamins And Lifestyle Cohort, WLH Swedish Women’s Lifestyle and Health Study.
*Number of participants and breast cancer events were collected relevant to fruit/vegetable/total fruit and vegetable/fruit juice intake.
Publication bias
We did not identify evidence of publication bias for the association of total fruits and vegetables, total fruits, total vegetables, fruit juice or subgroups of vegetables with overall breast cancer by visual inspection of a funnel plot and the Begg and Mazumdar test (Supplemental Fig. S1). Furthermore, we did not identify evidence of publication bias for the association of total fruits and vegetables, total fruits and total vegetables with ER+/PR+ and ER−/PR− breast cancer (data not shown).
Consumption of total fruits and vegetables, total fruits and total vegetables and risk of overall, premenopausal and postmenopausal breast cancers
Using risk estimates extracted from eight publications, the pooled RR comparing the highest versus the lowest category was 0.91 (95% CI = 0.87–0.95, I2 = 0.0%) for total fruit and vegetable consumption and overall breast cancer (Fig. 2a). No single study had a significant impact on the pooled RR for total fruit and vegetable intake and overall breast cancer (Supplementary Fig. S2). Higher intake of total fruits and vegetables was not significantly associated with the risk of premenopausal breast cancer (highest versus lowest category RR = 0.97, 95% CI = 0.76–1.24, I2 = 46.8%) (Fig. 2b), but we observed a significant decreased risk of postmenopausal breast cancer (highest versus lowest category RR = 0.88, 95% CI = 0.79–0.99, I2 = 18.7%) (Fig. 2c).
Fig. 2. Associations of total fruit and vegetable intake and risk of breast cancer.
a Overall, b Premenopausal and c Postmenopausal. Forest plot shows relative risks and 95% confidence intervals comparing highest category versus lowest category, using random-effect models. BWHS Black Women’s Health Study, EPIC European Prospective Investigation into Cancer and Nutrition, JPHC Japan Public Health Center-based Prospective Study, LWS Leisure World Study, NHEFS National Health Epidemiologic Follow-up Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, WLH Swedish Women’s Lifestyle and Health Study.
Using risk estimates extracted from 15 publications, total fruit consumption was associated with a significantly reduced risk of overall breast cancer. The pooled RR comparing the highest versus the lowest category of total fruit intake was 0.93 (95% CI = 0.88–0.99, I2 = 47.6%) (Fig. 3a). In sensitivity analyses excluding one study at a time, the pooled RR of total fruit intake and overall breast cancer ranged from 0.92 (95% CI 0.88–0.96), with the exclusion of UK Women’s Cohort Study (UKWCS),25 to 0.94 (95% CI 0.88–1.00), with the exclusion of the National Institute of Health-AARP Diet and Health Study (NIH-AARP),12 the NHS/NHSII23 or the Million Women Study (MWS)24 (Supplementary Fig. S3). In addition, we found one publication that updated findings from the Netherlands Cohort Study (NLCS) and reported the association of total fruit intake and risk of overall breast cancer, comparing ≥ versus < median of intake41. Similar association was observed by replacing findings from the later publication: highest versus lowest category RR = 0.94, 95% CI = 0.89–0.98, I2 = 45.2%. The pooled RR comparing the highest versus the lowest category was 0.92 (95% CI = 0.74–1.15, I2 = 44.6%) for total fruit intake and premenopausal breast cancer (Fig. 3b) and 0.93 (95% CI = 0.87–0.99, I2 = 38.9%) for total fruit intake and postmenopausal breast cancer (Fig. 3c). A significant positive association was observed between fruit juice consumption and overall breast cancer risk, using risk estimates from seven publications (highest versus lowest category RR = 1.04, 95% CI = 1.01–1.07, I2 = 0.0%) (Fig. 4). In sensitivity analyses excluding one study at a time, the pooled RR of fruit juice intake and overall breast cancer ranged from 1.03 (95% CI 1.00–1.07), with the exclusion of UKWCS,25 to 1.05 (95% CI 1.00–1.11), with the exclusion of the MWS24 (Supplementary Fig. S4).
Fig. 3. Associations of total fruit intake and risk of breast cancer.
a Overall, b Premenopausal and c Postmenopausal. Forest plot shows relative risks and 95% confidence intervals comparing highest category versus lowest category, using random-effect models. BWHS Black Women’s Health Study, CNBSS Canadian National Breast Screening Study, EPIC European Prospective Investigation into Cancer and Nutrition, FRiCaM Risk Factors for Breast Cancer: Fattori di Rischio per il Carcinoma della Mammella, JPHC Japan Public Health Center-based Prospective Study, KNCC Korean National Cancer Center Cohort, LSS The Radiation Effects Research Foundation’s Life Span Study, LWS Leisure World Study, MWS Million Women Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, NIH-AARP National Institute of Health-AARP Diet and Health Study, NLCS The Netherlands Cohort Study, SCHS the Singapore Chinese Health Study, UKWCS UK Women’s Cohort Study, VITAL VITamins And Lifestyle Cohort.
Fig. 4. Associations of fruit juice intake and risk of overall breast cancer.
Forest plot shows relative risks and 95% confidence intervals comparing highest category versus lowest category, using random-effect models. EPIC European Prospective Investigation into Cancer and Nutrition, FOS Framingham Offspring Study, MWS Million Women Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, SU.VI.MAX SUpplémental en VItamines et Minéraux AntioXydants, UKWCS UK Women’s Cohort Study.
The pooled RR comparing the highest versus the lowest category was 0.96 (95% CI = 0.91–1.00, I2 = 39.2%) for total vegetable consumption and overall breast cancer, using risk estimates extracted from 15 publications (Fig. 5a). In sensitivity analyses excluding one study at a time, the pooled RR of total vegetable intake and overall breast cancer ranged from 0.94 (95% CI 0.90–0.97), with the exclusion of the NIH-AARP,12 to 0.97 (95% CI 0.93–1.02), with the exclusion of EPIC20 (Supplementary Fig. S5). Furthermore, we observed similar result for total vegetable intake and risk of overall breast cancer when the data from the NLCS were replaced with updated findings, comparing ≥ versus < median of intake41: highest versus lowest category RR = 0.95, 95% CI = 0.91–1.00, I2 = 39.5%. With regard to menopausal status, non-significant associations were observed with risk of either premenopausal breast cancer (highest versus lowest category RR = 0.96, 95% CI = 0.87–1.06, I2 = 0.0%), or postmenopausal breast cancer (highest versus lowest category RR = 0.98, 95% CI = 0.93–1.03, I2 = 20.0%) (Fig. 5b and c, respectively). Using meta-regression, region, length of follow-up, adjustment for energy intake, smoking, BBD, family history of breast cancer, alcohol intake or BMI and quality score of the studies were not identified as possible sources of heterogeneity among studies that examined total fruit or total vegetable consumption in relation to overall breast cancer incidence. The only exception was for oral contraceptive use. We observed a significant inverse association between total vegetable consumption and breast cancer risk among studies that adjusted for oral contraceptive use (n = 3), but not among studies that did not adjust for it (n = 12) (P for heterogeneity = 0.02) (Supplementary Table S2). Furthermore, using meta-regression, adjustment for BMI was not identified as a possible source of heterogeneity among studies that examined fruit juice intake in relation to overall breast cancer (P for heterogeneity = 0.36) (data not shown).
Fig. 5. Associations of total vegetable intake and risk of breast cancer.
a Overall, b Premenopausal and c Postmenopausal. Forest plot shows relative risks and 95% confidence intervals comparing highest category versus lowest category, using random-effect models. BWHS Black Women’s Health Study, CNBSS Canadian National Breast Screening Study, EPIC European Prospective Investigation into Cancer and Nutrition, FRiCaM Risk Factors for Breast Cancer: Fattori di Rischio per il Carcinoma della Mammella, JPHC Japan Public Health Center-based Prospective Study, KC Karunagappally Cohort, LWS Leisure World Study, MWS Million Women Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, NIH-AARP National Institute of Health-AARP Diet and Health Study, NLCS the Netherlands Cohort Study, SCHS the Singapore Chinese Health Study, UKWCS UK Women’s Cohort Study, VITAL VITamins And Lifestyle Cohort, WLH Swedish Women’s Lifestyle and Health Study.
In terms of dose–response, each 200 g per day of total fruit and vegetable intake was associated with 3% lower risk of breast cancer (RR = 0.97, 95% CI = 0.94–1.00) (Fig. 6a). Each 200 g per day of total fruit intake was associated with 6% lower risk of breast cancer (RR = 0.94, 95% CI = 0.89–0.99) (Fig. 6b). Similarly, each 200 g per day of total vegetable intake was associated with 6% lower risk of breast cancer (RR = 0.94, 95% CI = 0.92–0.97) (Fig. 6c).
Fig. 6. Associations of total fruit and vegetable, total fruit and total vegetable intake and risk of overall breast cancer.
a Total fruits and vegetables, b Total fruits and c Total vegetables. Forest plot shows relative risks and 95% confidence intervals of each 200 g per day intake of total fruits and vegetables, total fruits and total vegetables, using random-effect models. BWHS Black Women’s Health Study, CNBSS Canadian National Breast Screening Study, EPIC European Prospective Investigation into Cancer and Nutrition, FRiCaM Risk Factors for Breast Cancer, Fattori di Rischio per il Carcinoma della Mammella, JPHC Japan Public Health Center-based Prospective Study, LSS The Radiation Effects Research Foundation’s Life Span Study, MWS Million Women Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, NLCS The Netherlands Cohort Study, UKWCS UK Women’s Cohort Study, VITAL VITamins And Lifestyle Cohort, WLH Swedish Women’s Lifestyle and Health Study.
Fruit and vegetable consumption and risk of ER+/PR+ and ER−/PR− breast cancer
Total fruit and vegetable intake was significantly associated with lower risk of both ER+/PR+ (highest versus lowest category RR = 0.89, 95% CI = 0.83–0.95) and ER−/PR− tumours (highest versus lowest category RR = 0.74, 95% CI = 0.65–0.84) (Fig. 7a1, b1). Total fruit intake was not significantly associated with lower risk of either ER+/PR+ tumours (highest versus lowest category RR = 0.97, 95% CI = 0.91–1.04), or ER−/PR− tumours (highest versus lowest category RR = 0.92, 95% CI = 0.81–1.04) (Fig. 7a2, b2). Although total vegetable intake was not associated with a significantly lower risk of ER+/PR+ tumours (highest versus lowest category RR = 0.92, 95% CI = 0.80–1.07), we noted a significant inverse association with ER−/PR− tumours (highest versus lowest category RR = 0.73, 95% CI = 0.64–0.83) (Fig. 7a3, b3).
Fig. 7. Associations of total fruit and vegetable, total fruit and total vegetable intake and risk of breast cancer based on hormone-receptor status.
a1 Total fruits and vegetables and ER+/PR+, a2 Total fruits and ER+/PR+, a3 Total vegetables and ER+/PR+, b1 Total fruits and vegetables and ER−/PR−, b2 Total fruits and ER−/PR− and b3 Total vegetables and ER−/PR−. Forest plot shows relative risks and 95% CIs comparing highest category versus lowest category, using random-effect models. BWHS Black Women’s Health Study, EPIC European Prospective Investigation into Cancer and Nutrition, JPHC Japan Public Health Center-based Prospective Study, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II.
Subgroups of vegetable consumption and risk of overall breast cancer
We did not find significant associations between consumption of yellow/orange vegetables (Supplementary Fig. S6a) and cruciferous vegetables (Supplementary Fig. S6b), and overall breast cancer risk. In sensitivity analyses excluding one study at a time, the pooled RR of yellow/orange vegetables and overall breast cancer ranged from 0.91 (95% CI 0.84–0.98), with the exclusion of the Japan Public Health Centre-based Prospective Study (JPHC),19 to 0.99 (95% CI 0.81–1.21), with the exclusion of NHS/NHSII23 (Supplementary Fig. S7). The pooled RR of cruciferous vegetables ranged from 0.92 (95% CI 0.87–0.97), with the exclusion of the EPIC,37 to 0.98 (95% CI 0.89–1.07), with the exclusion of Black Women’s Health Study (BWHS)14 (Supplementary Fig. S8). We did not observe a significant association between the intake of green leafy vegetables (Supplementary Fig. S6c) or tomatoes (Supplementary Fig. S6d) and overall breast cancer risk. In sensitivity analyses excluding one study at a time, the pooled RR of green leafy vegetable intake and overall breast cancer ranged from 0.94 (95% CI 0.88–0.99), with the exclusion of the EPIC,37 to 1.05 (95% CI 0.89–1.24), with the exclusion of NHS/NHSII23 (Supplementary Fig. S9). After excluding one study at a time, statistically similar non-significant associations were found between tomato intake and overall breast cancer (Supplementary Fig. S10).
Discussion
In this systematic review and meta-analysis study, we found modest significant inverse associations between total fruit and vegetable and total fruit consumption, and overall risk of breast cancer. In dose–response analyses, consumption of total fruits and total vegetables was associated with lower breast cancer risk. With respect to menopausal status, we observed a significant inverse association between total fruit and vegetable intake and total fruit intake and breast cancer risk among postmenopausal women. We also observed a significant inverse association between total fruit and vegetable intake and both ER+/PR+ and ER–/PR– breast cancers, and total vegetable intake and ER–/PR– breast cancer. A higher intake of fruit juice was associated with a greater risk of breast cancer. Yellow/orange and cruciferous vegetables were not significantly associated with the risk of breast cancer. In addition, we did not find significant associations between green leafy vegetables and tomatoes and the risk of breast cancer.
Consistent with findings from a previous meta-analysis,28 in this study, we observed inverse associations of total fruit and vegetable intake and total fruit intake with breast cancer risk after including recently published data. Moreover, in this study, we found an inverse association between vegetable intake and breast cancer risk in dose–response analysis and a positive association between fruit juice consumption and the risk of overall breast cancer.
Whole fruits and vegetables are nutrient- and phytochemically dense with high fibre,5,42 and have various cancer-protective properties.3,4,43–47 Acting in synergy,48,49 via multiple mechanisms, the inclusion of a variety of fruits and vegetables, as part of a healthy daily dietary pattern, has the potential to create and maintain an internal environment that is not conducive to tumour growth and progression. In a pooled analysis of fruit and vegetable consumption and risk of breast cancer using data from 20 prospective cohort studies,29 total vegetable consumption was significantly associated with lower risk of ER−/PR− tumours (highest versus lowest quintile, HR = 0.84, 95% CI = 0.75–0.93, Ptrend = 0.001), but there was no significant association with overall breast cancer. Similarly, the findings of our meta-analysis support the potential protective effect of both total fruit and vegetable intake, and total vegetable consumption in reducing the risk of ER−/PR− breast cancer. There are at least two general mechanisms that may account for the significant inverse association of vegetables with ER–/PR– but not with ER+/PR+ breast cancer. ER+/PR+ breast cancer has been shown to be associated with factors that increase risk of exposure to endogenous and exogenous hormones, including early age at menarche, delayed first birth, later age at menopause and hormone-replacement therapy (HRT) as well as obesity after menopause.27 ER+/PR+ breast cancer is therefore relatively more sensitive to hormonal exposures compared to ER−/PR− breast cancer. In addition, overexpression of epidermal growth factor receptor, which induces nuclear factor B activation,50 and cyclin E, a cell cycle regulator,51 has been observed in ER− compared to ER+ breast cancer. Phytochemicals and bioactive components in vegetables may affect the risk of ER− breast cancer through downregulation of epidermal growth factor receptor52 and cyclin E.53,54 As such, ER−/PR− breast cancer may be relatively more sensitive to the influences of the cancer-protective properties of fruits and vegetables compared to ER+/PR+ breast cancer. To our knowledge, the current meta-analysis is the first to evaluate the risk of breast cancer by ER/PR status in relation to fruit and vegetable consumption and further investigations are needed.
While higher intake of fruit may reduce breast cancer incidence, this meta-analysis showed that high fruit juice consumption was associated with a higher risk. Fruit juice contains a high amount of free sugar that can lead to weight gain and type 2 diabetes,55,56 which may contribute to breast cancer risk.57,58 High amounts of fructose, found in fruit juice, independently, may increase the risk of insulin resistance and may increase inflammatory markers;59–61 these factors may be associated with higher risk of breast cancer.62,63 Also, the increased risk of breast cancer may result from a lack of fibre in these beverages. Fibre has been shown to bind to oestradiol64,65 and increases faecal excretion, leading to decreased circulating sex hormone concentrations.66 However, fruit juice may be good sources of phytochemicals and antioxidants that may provide health benefits.67,68 Future research should investigate this association by type of fruit juice and distinguish pure fruit juice from related fruit beverages with added sugar.
Given the varied nutrient and phytochemical content of vegetables, we examined the associations for yellow/orange vegetables, cruciferous vegetables, green leafy vegetables and tomatoes. It is hypothesised that carotenoids may be associated with reduced cancer risk through several mechanisms, including antioxidant and anti-proliferative activities.69 However, we did not observe a significantly lower risk of breast cancer with high intake of yellow/orange vegetables or tomatoes. In a meta-analysis of prospective cohort studies, although both dietary α-carotene and β-carotene were found to be protective, dietary lycopene, the main carotenoid in tomatoes, was shown not to be significantly associated with reduced incident breast cancer.70
Cruciferous vegetables are rich sources of isothiocyanates and indoles that are hypothesised to reduce cancer risk through antimutagenic and antioxidant activity.71 We found an inverse non-significant association between cruciferous vegetable intake and breast cancer risk. A similar inverse non-significant association between cruciferous vegetables and breast cancer risk was observed in a large pooled analysis.72
With regard to menopausal status, total fruit and vegetable intake and total fruit intake were associated with lower breast cancer risk after menopause; however, findings for premenopausal breast cancer showed non-significant inverse associations. The non-significant associations might be a result of the smaller number of studies on premenopausal women included in the meta-analysis, and hence, lower statistical power.
Our analysis has several strengths. We analysed the data from prospective studies to reduce the effects of recall and selection bias. Although there were wide variations in study populations across the studies, we observed low-to-moderate heterogeneity in the data, indicating the external validity of pooling findings from different studies. Also, major breast cancer risk factors were controlled for in the majority of studies included in the meta-analysis. Furthermore, we were able to examine the association of fruit and vegetable intake and breast cancer events in different populations with large variations in consumption of fruits and vegetables, and to evaluate the associations stratified by menopausal and ER/PR status.
Our analysis has a few limitations. As in any meta-analysis, publication bias is a common issue. In this work, no evidence of publication bias was noted for the exposures examined. While the majority of the studies adjusted for potential breast cancer risk factors, residual confounding is an issue with all observational studies and may potentially explain away some of the associations described in this paper. Because the majority of studies used an FFQ to estimate dietary intake, we cannot exclude measurement error due to under- or over-reporting of the number of food groups. Finally, the pooled RRs were calculated for the highest versus the lowest categories of intake, but levels of intake were not always consistent across studies. Because of the limited data, we were not able to include all the studies in the dose–response analyses; however, the findings are comparable with pooled RRs using the highest versus the lowest categories.
In summary, this systematic review and meta-analysis, including prospective studies of fruit and vegetable consumption, provides evidence that higher consumption of fruits and vegetables is protective of breast cancer risk, including overall, postmenopausal, ER+/PR+ and ER−/PR− breast cancer. High intake of fruit juice may increase breast cancer risk, which is consistent with nutritional guidelines distinguishing between the relative benefits of the intake of whole fruits versus fruit beverages. These findings provide some support for the increased consumption of fruits and vegetables as part of a healthy diet to reduce breast cancer risk and inform public health recommendations and clinical guidelines.
Supplementary information
Acknowledgements
We would like to thank Jacqueline Cellini MLIS, MPH—Research & Instruction Librarian, Harvard T.H. Chan School of Public Health for her valuable contributions in developing the search string.
Author contributions
Study concept and design: M.F.S., data collection and collation: M.S.F., statistical analysis: M.S.F., writing—original draft: M.S.F., writing—review and editing: M.S.F., J.B.B. and N.D.S, study supervision: M.S.F. Interpretation of the data, critical revision of the paper for important intellectual content and approval of the final paper for submission: M.S.F., J.B.B. and N.D.S. The corresponding authors prove that all listed authors meet the authorship criteria, and that no other eligible authors have been omitted.
Ethics approval and consent to participate
All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
Consent to publish
Not applicable.
Data availability
The datasets used and analysed during this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01373-2.
References
- 1.Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A. & Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin.10.3322/caac.21660 (2021). [DOI] [PubMed]
- 2.Rock, C. L., Thomson, C., Gansler, T., Gapstur, S. M., McCullough, M. L., Patel, A. V. et al. American cancer society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J. Clin. 70, 245–271 (2020). [DOI] [PubMed]
- 3.Farvid, M. S., Spence, N. D., Holmes, M. D. & Barnett, J. B. Fiber consumption and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Cancer126, 3061–3075 (2020). [DOI] [PubMed]
- 4.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J. Natl Cancer Inst. 2012;104:1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br. J. Cancer. 1992;66:673–679. doi: 10.1038/bjc.1992.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohan TE, Howe GR, Friedenreich CM, Jain M, Miller AB. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993;4:29–37. doi: 10.1007/BF00051711. [DOI] [PubMed] [Google Scholar]
- 8.Byrne C, Ursin G, Ziegler RG. A comparison of food habit and food frequency data as predictors of breast cancer in the NHANES I/NHEFS cohort. J. Nutr. 1996;126:2757–2764. doi: 10.1093/jn/126.11.2757. [DOI] [PubMed] [Google Scholar]
- 9.Verhoeven DT, Assen N, Goldbohm RA, Dorant E, van ‘t Veer P, Sturmans F, et al. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br. J. Cancer. 1997;75:149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br. J. Cancer. 1999;81:1248–1256. doi: 10.1038/sj.bjc.6690837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonestedt E, Borgquist S, Ericson U, Gullberg B, Landberg G, Olsson H, et al. Plant foods and oestrogen receptor alpha- and beta-defined breast cancer: observations from the Malmo Diet and Cancer cohort. Carcinogenesis. 2008;29:2203–2209. doi: 10.1093/carcin/bgn196. [DOI] [PubMed] [Google Scholar]
- 12.George SM, Park Y, Leitzmann MF, Freedman ND, Dowling EC, Reedy J, et al. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am. J. Clin. Nutr. 2009;89:347–353. doi: 10.3945/ajcn.2008.26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayalekshmi P, Varughese SC, Kalavathi, Nair MK, Jayaprakash V, Gangadharan P, et al. A nested case-control study of female breast cancer in Karunagappally cohort in Kerala, India. Asian Pac. J. Cancer Prev. 2009;10:241–246. [PubMed] [Google Scholar]
- 14.Boggs DA, Palmer JR, Wise LA, Spiegelman D, Stampfer MJ, Adams-Campbell LL, et al. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. Am. J. Epidemiol. 2010;172:1268–1279. doi: 10.1093/aje/kwq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brasky TM, Lampe JW, Potter JD, Patterson RE, White E. Specialty supplements and breast cancer risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer Epidemiol. Biomark. Prev. 2010;19:1696–1708. doi: 10.1158/1055-9965.EPI-10-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler LM, Wu AH, Wang R, Koh WP, Yuan JM, Yu MC. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am. J. Clin. Nutr. 2010;91:1013–1019. doi: 10.3945/ajcn.2009.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Löf M, Sandin S, Lagiou P, Trichopoulos D, Adami HO, Weiderpass E. Fruit and vegetable intake and risk of cancer in the Swedish women’s lifestyle and health cohort. Cancer Causes Control. 2011;22:283–289. doi: 10.1007/s10552-010-9696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couto E, Sandin S, Löf M, Ursin G, Adami HO, Weiderpass E. Mediterranean dietary pattern and risk of breast cancer. PLoS ONE. 2013;8:e55374. doi: 10.1371/journal.pone.0055374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki R, Iwasaki M, Hara A, Inoue M, Sasazuki S, Sawada N, et al. Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: the Japan Public Health Center-based Prospective Study. Cancer Causes Control. 2013;24:2117–2128. doi: 10.1007/s10552-013-0289-7. [DOI] [PubMed] [Google Scholar]
- 20.Emaus MJ, Peeters PH, Bakker MF, Overvad K, Tjønneland A, Olsen A, et al. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am. J. Clin. Nutr. 2016;103:168–177. doi: 10.3945/ajcn.114.101436. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. H., Lee, J., Jung, S. Y. & Kim, J. Dietary factors and female breast cancer risk: a prospective cohort study. Nutrients9, 1331 (2017). [DOI] [PMC free article] [PubMed]
- 22.Elwood PC, Whitmarsh A, Gallacher J, Bayer A, Adams R, Heslop L, et al. Healthy living and cancer: evidence from UK Biobank. Ecancermedicalscience. 2018;12:792. doi: 10.3332/ecancer.2018.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farvid MS, Chen WY, Rosner BA, Tamimi RM, Willett WC, Eliassen AH. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up. Int. J. Cancer. 2019;144:1496–1510. doi: 10.1002/ijc.31653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key TJ, Balkwill A, Bradbury KE, Reeves GK, Kuan AS, Simpson RF, et al. Foods, macronutrients and breast cancer risk in postmenopausal women: a large UK cohort. Int. J. Epidemiol. 2019;48:489–500. doi: 10.1093/ije/dyy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunneram Y, Greenwood DC, Cade JE. Diet and risk of breast, endometrial and ovarian cancer: UK Women’s Cohort Study. Br. J. Nutr. 2019;122:564–574. doi: 10.1017/S0007114518003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravi F, Decarli A, Russo AG. Risk factors for breast cancer in a cohort of mammographic screening program: a nested case–control study within the FRiCaM study. Cancer Med. 2018;7:2145–2152. doi: 10.1002/cam4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol. Biomark. Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 28.Aune D, Chan DS, Vieira AR, Rosenblatt DA, Vieira R, Greenwood DC, et al. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012;134:479–493. doi: 10.1007/s10549-012-2118-1. [DOI] [PubMed] [Google Scholar]
- 29.Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J. Natl Cancer Inst. 2013;105:219–236. doi: 10.1093/jnci/djs635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physcal activity, and breast cancer. Revised 2018. https://www.wcrf.org/sites/default/files/Breast-cancer-report.pdf.
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials7, 177–188 (1986). [DOI] [PubMed]
- 33.Orsini N. From floated to conventional confidence intervals for the relative risks based on published dose-response data. Comput Methods Prog. Biomed. 2010;98:90–93. doi: 10.1016/j.cmpb.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 36.Makarem N, Bandera EV, Lin Y, Jacques PF, Hayes RB, Parekh N. Consumption of sugars, sugary foods, and sugary beverages in relation to adiposity-related cancer risk in the Framingham Offspring Cohort (1991-2013) Cancer Prev. Res (Philos.). 2018;11:347–358. doi: 10.1158/1940-6207.CAPR-17-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Gils CH, Peeters PH, Bueno-de-Mesquita HB, Boshuizen HC, Lahmann PH, Clavel-Chapelon F, et al. Consumption of vegetables and fruits and risk of breast cancer. J. Am. Med. Assoc. 2005;293:183–193. doi: 10.1001/jama.293.2.183. [DOI] [PubMed] [Google Scholar]
- 38.Chazelas E, Srour B, Desmetz E, Kesse-Guyot E, Julia C, Deschamps V, et al. Sugary drink consumption and risk of cancer: results from NutriNet-Santé prospective cohort. BMJ. 2019;366:l2408. doi: 10.1136/bmj.l2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Roswall N, Sandin S, Strom P, Adami HO, Weiderpass E. Adherence to a healthy Nordic food index and breast cancer risk: results from a Swedish cohort study. Cancer Causes Control. 2015;26:893–902. doi: 10.1007/s10552-015-0564-x. [DOI] [PubMed] [Google Scholar]
- 40.Hirvonen T, Mennen LI, de Bree A, Castetbon K, Galan P, Bertrais S, et al. Consumption of antioxidant-rich beverages and risk for breast cancer in French women. Ann. Epidemiol. 2006;16:503–508. doi: 10.1016/j.annepidem.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 41.van den Brandt, P. A. & Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int. J. Cancer 140, 2220–2231 (2017). [DOI] [PubMed]
- 42.Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J. Am. Diet. Assoc. 2008;108:1716–1731. doi: 10.1016/j.jada.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J. Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 44.Reuben SC, Gopalan A, Petit DM, Bishayee A. Modulation of angiogenesis by dietary phytoconstituents in the prevention and intervention of breast cancer. Mol. Nutr. Food Res. 2012;56:14–29. doi: 10.1002/mnfr.201100619. [DOI] [PubMed] [Google Scholar]
- 45.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J. Agric Food Chem. 2009;57:4467–4477. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 46.Collins AR, Harrington V, Drew J, Melvin R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis. 2003;24:511–515. doi: 10.1093/carcin/24.3.511. [DOI] [PubMed] [Google Scholar]
- 47.Riso P, Martini D, Moller P, Loft S, Bonacina G, Moro M, et al. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis. 2010;25:595–602. doi: 10.1093/mutage/geq045. [DOI] [PubMed] [Google Scholar]
- 48.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr. 2004;134(12 Suppl):3479S–3485SS. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 49.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–843. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 50.Biswas DK, Cruz AP, Gansberger E, Pardee AB. Epidermal growth factor-induced nuclear factor kappa B activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl Acad. Sci. USA. 2000;97:8542–8547. doi: 10.1073/pnas.97.15.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landberg G. Multiparameter analyses of cell cycle regulatory proteins in human breast cancer: a key to definition of separate pathways in tumorigenesis. Adv. Cancer Res. 2002;84:35–56. doi: 10.1016/S0065-230X(02)84002-7. [DOI] [PubMed] [Google Scholar]
- 52.Moiseeva EP, Heukers R, Manson MM. EGFR and Src are involved in indole-3-carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis. 2007;28:435–445. doi: 10.1093/carcin/bgl171. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen HH, Aronchik I, Brar GA, Nguyen DH, Bjeldanes LF, Firestone GL. The dietary phytochemical indole-3-carbinol is a natural elastase enzymatic inhibitor that disrupts cyclin E protein processing. Proc. Natl Acad. Sci. USA. 2008;105:19750–19755. doi: 10.1073/pnas.0806581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh V, Singh R, Kujur PK, Singh RP. Combination of resveratrol and quercetin causes cell growth inhibition, DNA damage, cell cycle arrest, and apoptosis in oral cancer cells. Assay. Drug Dev. Technol. 2020;18:226–238. doi: 10.1089/adt.2020.972. [DOI] [PubMed] [Google Scholar]
- 55.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. J. Am. Med. Assoc. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 56.Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Engin A. Obesity-associated breast cancer: analysis of risk factors. Adv. Exp. Med. Biol. 2017;960:571–606. doi: 10.1007/978-3-319-48382-5_25. [DOI] [PubMed] [Google Scholar]
- 58.Vrachnis N, Iavazzo C, Iliodromiti Z, Sifakis S, Alexandrou A, Siristatidis C, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch. Gynecol. Obstet. 2016;293:239–246. doi: 10.1007/s00404-015-3858-z. [DOI] [PubMed] [Google Scholar]
- 59.Taskinen, M. R., Packard, C. J. & Boren, J. Dietary fructose and the metabolic syndrome. Nutrients11, 1987 (2019). [DOI] [PMC free article] [PubMed]
- 60.Gambaro SE, Zubiria MG, Portales AE, Rey MA, Rumbo M, Giovambattista A. M1 macrophage subtypes activation and adipocyte dysfunction worsen during prolonged consumption of a fructose-rich diet. J. Nutr. Biochem. 2018;61:173–182. doi: 10.1016/j.jnutbio.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Pektas MB, Koca HB, Sadi G, Akar F. Dietary fructose activates insulin signaling and inflammation in adipose tissue: modulatory role of resveratrol. Biomed. Res. Int. 2016;2016:8014252. doi: 10.1155/2016/8014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan K, Chlebowski RT, Mortimer JE, Gunter MJ, Rohan T, Vitolins MZ, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126:3638–3647. doi: 10.1002/cncr.33002. [DOI] [PubMed] [Google Scholar]
- 63.Michels N, van Aart C, Morisse J, Mullee A, Huybrechts I. Chronic inflammation towards cancer incidence: a systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol. Hematol. 2021;157:103177. doi: 10.1016/j.critrevonc.2020.103177. [DOI] [PubMed] [Google Scholar]
- 64.Arts CJ, Govers CA, van den Berg H, Wolters MG, van Leeuwen P, Thijssen JH. In vitro binding of estrogens by dietary fiber and the in vivo apparent digestibility tested in pigs. J. Steroid Biochem. Mol. Biol. 1991;38:621–628. doi: 10.1016/0960-0760(91)90321-U. [DOI] [PubMed] [Google Scholar]
- 65.Shultz TD, Howie BJ. In vitro binding of steroid hormones by natural and purified fibers. Nutr. Cancer. 1986;8:141–147. doi: 10.1080/01635588609513887. [DOI] [PubMed] [Google Scholar]
- 66.Schaefer EJ, Lamon-Fava S, Spiegelman D, Dwyer JT, Lichtenstein AH, McNamara JR, et al. Changes in plasma lipoprotein concentrations and composition in response to a low-fat, high-fiber diet are associated with changes in serum estrogen concentrations in premenopausal women. Metabolism. 1995;44:749–756. doi: 10.1016/0026-0495(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 67.Tonin FS, Steimbach LM, Wiens A, Perlin CM, Pontarolo R. Impact of natural juice consumption on plasma antioxidant status: a systematic review and meta-analysis. Molecules. 2015;20:22146–22156. doi: 10.3390/molecules201219834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyson DA. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutr. 2015;6:37–51. doi: 10.3945/an.114.005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertram JS. Dietary carotenoids, connexins and cancer: what is the connection? Biochem Soc. Trans. 2004;32:985–989. doi: 10.1042/BST0320985. [DOI] [PubMed] [Google Scholar]
- 70.Hu F, Wang YiB, Zhang W, Liang J, Lin C, Li D, et al. Carotenoids and breast cancer risk: a meta-analysis and meta-regression. Breast Cancer Res. Treat. 2012;131:239–253. doi: 10.1007/s10549-011-1723-8. [DOI] [PubMed] [Google Scholar]
- 71.Fuentes F, Paredes-Gonzalez X, Kong AN. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3’-diindolylmethane: anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharm. Rep. 2015;1:179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. J. Am. Med. Assoc. 2001;285:769–776. doi: 10.1001/jama.285.6.769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during this study are available from the corresponding author on reasonable request.