Abstract
Traditionally, the cerebellum has been linked to motor coordination, but growing evidence points to its involvement in a wide range of non-motor functions. Though the number of studies using transcranial magnetic stimulation (TMS) to investigate cerebellar involvement in cognitive processes is growing exponentially, these findings have not yet been synthesized in a meta-analysis. Here, we used meta-analysis to estimate the effects of cerebellar TMS on performance in cognitive tasks for healthy participants. Outcomes included participants’ accuracy and response times (RTs) of several non-motor tasks performed either during or after the administration of TMS. We included overall 41 studies, of which 44 single experiments reported effects on accuracy and 41 on response times (RTs). The meta-analyses showed medium effect sizes (for accuracy: d = 0.61 [95% CI = 0.48, .073]; for RTs: d = 0.40 [95% CI = 0.30, 0.49]), with leave-one-out analyses indicating that cumulative effects were robust, and with moderate heterogeneity. For both accuracy and RTs, the effect of TMS was moderated by the stimulation paradigm adopted but not by the cognitive function investigated, while the timing of the stimulation moderated only the effects on RTs. Further analyses on lateralization revealed no moderation effects of the TMS site. Taken together, these findings indicate that TMS administered over the cerebellum is able to modulate cognitive performance, affecting accuracy or RTs, and suggest that the various stimulation paradigms play a key role in determining the efficacy of cerebellar TMS.
Subject terms: Cognitive neuroscience, Human behaviour
Introduction
The human cerebellum has been traditionally studied in relation to motor functions. Yet growing evidence supports the involvement of the cerebellum in a wide range of non-motor functions, spanning from the cognitive to the emotional domains (e.g., emotive processing1; perceptual processing2; cognitive processing3; language4; and for an overall overview see5,6). To account for the cerebellar contribution in non-motor processes, Schmahmann7 proposed the so-called dysmetria of thought hypothesis, arguing that the cerebellum performs the same computational processes across all domains in which it is involved in. Evidence supporting this perspective comes from studies showing that the microstructure of the cerebellar cortex is uniform8 and that cerebro-cerebellar connections are segregated9–11. Specifically, structural uniformity would underlie functional uniformity and the segregated cerebro-cerebellar connections would allow specific cerebellar modules to participate in specific cognitive functions12.
Cerebellar modulation of motor and non-motor behavior has been primarily investigated through neurostimulation techniques, such as transcranial direct current stimulation (tDCS) or transcranial magnetic stimulation (TMS). This growing evidence was synthesized in several qualitative reviews13–16 as well as in a recent meta-analysis, demonstrating that anodal and cathodal cerebellar tDCS are effective in modulating participants’ performance (i.e., whether in the form of cognitive impairment or enhancement) and that the effect of cerebellar tDCS on motor functions is higher compared with non-motor functions17. Yet, no systematic review has insofar examined cerebellar modulation by synthesizing the evidence for TMS.
Here, we are therefore interested in quantifying the effect of TMS across non-motor functions. This choice is motivated by the significant increase of TMS studies investigating cerebellar involvement in non-motor functions, as well as by the theoretical difficulty to frame cerebellar involvement in cognitive processing. Furthermore, we note that the inclusion of the studies investigating motor functions could be problematic from a metanalytic point of view as they are highly heterogeneous: that is, the studies that investigated cerebellar involvement in motor processes using TMS highly differ in terms of paradigms adopted18–24, dependent variables used25–27 and population tested28,29, and could not consequently be directly comparable.
TMS is a noninvasive brain stimulation technique that uses electromagnetic induction principles to induce electrical currents in the brain30,31. Albeit the precise mechanisms through which TMS influences brain function are currently not fully understood, this technique is thought to stimulate axons placed in the cortex or in the white matter and to not directly modulate cell bodies activity32. Typically, TMS is used to investigate the link between the activity of a certain brain area and a motor or non-motor function, since a change in behavior induced by TMS (i.e., generally measured using accuracy or response times, RTs) is causally informative of the relationship among the area stimulated and the function investigated33. It should be noted that TMS does not necessarily cause performance disruption; in some specific conditions, TMS can also induce performance enhancement. Performance enhancement has been ascribed to direct modulation of a cortical region involved in one function, but also to indirect modulation (i.e., diaschisis), to non-specific effect of the stimulation (e.g., intersensory facilitation), or to addition-by-subtraction processes, which is a disruption of those processes supposed to compete or distract from task performance34. Within this context is should be noted that, given the assumption that every performance modulation (impairment or enhancement) is thought to be caused by the stimulation, it is impossible to observe “real” negative effects. This point was indeed handled by previous meta-analyses by computing both absolute and signed effect sizes, but focusing mainly on the former17.
TMS has been employed to the study of cerebellar functions, both in the motor and non-motor domains35. Cerebellar TMS aims at investigating temporal features of cerebellar-cortical connectivity36 as well as more basic features of cerebellar involvement in various processes. For instance, for the motor domain, TMS evidence indicates that the cerebellum exerts an inhibitory effect on motor areas18, that it is involved in saccadic adaptation19,20, and in the acquisition and extinction of conditioned responses21–24. Similarly, for the cognitive domain, TMS evidence supports cerebellar involvement in semantic memory37, working memory38, executive functions39, social cognition40 and spatial processing41. However, the cerebellar cortex is significantly different compared with other cortical areas, such as the frontal or temporal regions of the brain, in terms of number of neurons, composition of neurons and glia and their organization8. Because of these key differences, it is not known whether the effects of TMS could be similar to the effects reported for the other areas of the cerebral cortex.
Here, we therefore propose a systematic review and meta-analysis to quantify the effect of TMS in non-motor, cognitive domains. Despite the proliferation of this literature, no such review currently exists. We focus on studies of non-motor function because as a whole they are more homogeneous in terms of paradigms, dependent variables and populations. We also aim to parse variability by looking at potential moderators of effect, such as differences in stimulation paradigms in terms of burst pattern (e.g., double pulse, triple pulse, etc.) or timing (online vs. offline), function investigated, or cerebellar site stimulated. Quantifying the effect of TMS across non-motor functions or possible moderators identified could provide parameters for the use by future TMS studies targeting the cerebellum. As any performance modulation (impairment or enhancement) could be potentially ascribed to TMS influence, in line with previous methodological approaches17 we ran different meta-analyses on absolute and signed effect sizes: the former approach indeed accounts for any cognitive modulation induced by cerebellar TMS (whether in the form of impairment or enhancement), while the latter may be useful to gain insights into the specific direction of the effect (whether cerebellar TMS typically leads to cognitive impairment or enhancement).
Materials and methods
Identification and selection of studies
To identify potential studies for inclusion in the meta-analysis, we systematically searched Pubmed for studies conducted from January 2000 (the first TMS study targeting the cerebellum and investigating non-motor functions has been performed by Rami et al.42) to January 2021. We used the following search string: “((((((transcranial magnetic stimulation) OR tms) OR rtms) OR theta burst) OR tbs)) AND ((cerebellum) OR cerebellar)”. We also manually checked references for narrative reviews investigating cerebellar involvement in cognition using neurostimulation techniques13–16. Study identification and selection was performed by DG.
We included studies with the following characteristics: (i) a sample composed by healthy and adult participants, (ii) the administration of TMS for at least one cerebellar site, (iii) the presence of a cognitive (i.e., non-motor) performance index, (iv) the use of accuracy and/or response times (RTs) as dependent variables, (v) the adoption of cerebellar TMS protocols with the explicit intention to modulate cerebellar function.
From each study, we extracted: the sample size, the dependent variable(s) of interest (accuracy, response times), the cerebellar site stimulated, the stimulation paradigm adopted (e.g., theta-burst stimulation, triple-pulse TMS, single-pulse TMS), the design of the study, the control condition adopted, the timing of the stimulation, the mean and standard deviation of participants’ performance in the various conditions, and the specific cognitive function investigated.
Effect size calculations
Accuracy and RTs were the dependent variables of interest. For each dependent variable, from each study, we included only one effect size (see Supplementary Material for more information regarding which effect was chosen for each study). This procedure is considered as the most straightforward one in case of within-participants dependencies in the same study43.
The effect size used was Cohen’s d44. Cohen’s d for between-participants designs is defined as the mean standardized difference between the two measurements (in our case, cerebellar TMS vs. control area / no TMS / sham stimulation). For within-participants designs, Cohen’s d computation requires taking into account the correlation between the two measurements (cerebellar TMS vs. control area / sham stimulation / no TMS; see Table 1 for more information regarding the control condition used by each study included); that is, the mean difference between the two measurements is divided by:
Table 1.
The studies included in this meta-analysis.
| Study ID | Function | Paradigm | Timing | Design | Dep.var | Exp | N | TMS site | CC | ES.5 | V.5 | ES.75 | V.75 | Agg | ES.5 direction | ES.75 direction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | Semantic Memory | cTBS | Offline | Between | RTs | 1 | 24 | RC | No TMS | 0,83 | 0,18 | 0,83 | 0,18 | N | 0,83 | 0,83 |
| 37 | Semantic Memory | cTBS | Offline | Between | RTs | 1 | 22 | MC | No TMS | 0,24 | 0,18 | 0,24 | 0,18 | N | -0,24 | -0,24 |
| 38 | Working Memory | spTMS | Online | Within | Accuracy | 1 | 17 | RC | No TMS | 0,12 | 0,06 | 0,16 | 0,03 | N | 0,12 | 0,16 |
| 38 | Working Memory | spTMS | Online | Within | RTs | 1 | 17 | RC | No TMS | 0,32 | 0,06 | 0,43 | 0,03 | N | -0,32 | -0,43 |
| 39 | Executive Functions | cTBS | Offline | Between | Accuracy | 1 | 27 | RC vs LC | Cerebellum | 0,79 | 0,16 | 0,79 | 0,16 | N | NA | NA |
| 40 | Social Cognition | tpTMS | Online | Within | Accuracy | 1 | 32 | MC | Vertex | 0,35 | 0,03 | 0,50 | 0,02 | N | -0,35 | -0,50 |
| 40 | Social Cognition | tpTMS | Online | Within | RTs | 1 | 32 | MC | Vertex | 0,10 | 0,03 | 0,14 | 0,02 | N | 0,10 | 0,14 |
| 40 | Social Cognition | tpTMS | Online | Within | Accuracy | 2 | 48 | MC | Vertex | 0,35 | 0,02 | 0,49 | 0,01 | N | -0,35 | -0,49 |
| 40 | Social Cognition | tpTMS | Online | Within | RTs | 2 | 48 | MC | Vertex | 0,03 | 0,02 | 0,04 | 0,01 | N | -0,03 | -0,04 |
| 40 | Social Cognition | tpTMS | Online | Within | Accuracy | 3 | 32 | LC | Vertex | 0,44 | 0,03 | 0,63 | 0,02 | N | -0,44 | -0,63 |
| 40 | Social Cognition | tpTMS | Online | Within | RTs | 3 | 32 | LC | Vertex | 0,09 | 0,03 | 0,13 | 0,02 | N | 0,09 | 0,13 |
| 41 | Spatial cognition | tpTMS | Online | Within | Accuracy | 1 | 12 | MC | Sham | 0,42 | 0,09 | 0,58 | 0,05 | N | -0,42 | -0,58 |
| 41 | Spatial cognition | tpTMS | Online | Within | RTs | 1 | 12 | MC | Sham | 0,04 | 0,08 | 0,05 | 0,04 | N | -0,04 | -0,05 |
| 41 | Spatial cognition | tpTMS | Online | Within | Accuracy | 2 | 12 | LC | Sham | 0,12 | 0,08 | 0,16 | 0,04 | N | 0,12 | 0,16 |
| 41 | Spatial cognition | tpTMS | Online | Within | RTs | 2 | 12 | LC | Sham | 0,06 | 0,08 | 0,09 | 0,04 | N | 0,06 | 0,09 |
| 42 | Memory (various sub-types) | HFrTMS | Online | Within | Accuracy | 1 | 16 | RC | No TMS | 0,40 | 0,04 | 0,56 | 0,02 | N | NA | NA |
| 47 | Spatial cognition | spTMS | Online | Within | Accuracy | 1 | 30 | MC | Vertex | 0,13 | 0,03 | 0,18 | 0,02 | N | NA | NA |
| 47 | Spatial cognition | spTMS | Online | Within | RTs | 1 | 30 | MC | Vertex | 0,02 | 0,03 | 0,03 | 0,02 | N | NA | NA |
| 47 | Spatial cognition | spTMS | Online | Within | Accuracy | 2 | 24 | LC | Vertex | 0,52 | 0,05 | 0,64 | 0,03 | N | NA | NA |
| 47 | Spatial cognition | spTMS | Online | Within | RTs | 2 | 24 | LC | Vertex | 0,53 | 0,05 | 0,71 | 0,02 | N | NA | NA |
| 48 | Semantic Memory | tpTMS | Online | Within | Accuracy | 1 | 24 | RC | Vertex | 0,50 | 0,05 | 0,62 | 0,02 | N | -0,50 | -0,62 |
| 48 | Semantic Memory | tpTMS | Online | Within | RTs | 1 | 24 | RC | Vertex | 0,26 | 0,04 | 0,36 | 0,02 | N | -0,26 | -0,36 |
| 48 | Semantic Memory | tpTMS | Online | Within | Accuracy | 2 | 20 | RC | Visual C | 0,76 | 0,06 | 0,90 | 0,03 | N | -0,76 | -0,90 |
| 48 | Semantic Memory | tpTMS | Online | Within | RTs | 2 | 20 | RC | Visual C | 0,13 | 0,05 | 0,18 | 0,02 | N | -0,13 | -0,18 |
| 49 | Memory (various sub-types) | tpTMS | Online | Within | Accuracy | 1 | 24 | RC | Vertex | 0,11 | 0,05 | 0,15 | 0,02 | N | -0,11 | -0,15 |
| 49 | Memory (various sub-types) | tpTMS | Online | Within | Accuracy | 2 | 32 | RC | Vertex | 0,36 | 0,03 | 0,51 | 0,02 | N | -0,36 | -0,51 |
| 58 | Semantic Memory | cTBS | Offline | Within | RTs | 1 | 19 | LC vs RC | Cerebellum | 0,54 | 0,06 | 0,77 | 0,03 | N | NA | NA |
| 59 | Attention | cTBS | Offline | Between | Accuracy | 1 | 45 | RC vs LC & Sham | Sham & Cerebellum | 0,96 | 0,11 | 0,96 | 0,11 | N | NA | NA |
| 60 | Semantic Memory | cTBS | Offline | Within | Accuracy | 1 | 4 | MC vs RC | Cerebellum | 0,92 | 0,36 | 1,24 | 0,18 | N | NA | NA |
| 60 | Semantic Memory | cTBS | Offline | Within | RTs | 1 | 8 | MC vs RC | Cerebellum | 0,96 | 0,18 | 1,09 | 0,09 | N | NA | NA |
| 61 | Semantic Memory | cTBS | Offline | Between | RTs | 1 | 24 | MC vs RC | Cerebellum | 0,66 | 0,18 | 0,66 | 0,18 | N | NA | NA |
| 62 | Timing | LFrTMS | Offline | Between | Accuracy | 1 | 26 | RC | Sham | 0,51 | 0,16 | 0,51 | 0,16 | N | -0,51 | -0,51 |
| 63 | Working Memory | dpTMS | Online | Within | Accuracy | 1 | 9 | RC | No TMS | 1,51 | 0,24 | 2,12 | 0,12 | N | -1,51 | -2,12 |
| 63 | Working Memory | dpTMS | Online | Within | RTs | 1 | 9 | RC | No TMS | 0,13 | 0,11 | 0,19 | 0,06 | N | -0,13 | -0,19 |
| 64 | Episodic Memory | HFrTMS | Offline | Within | Accuracy | 1 | 24 | RC | Visual C | 0,38 | 0,04 | 0,54 | 0,02 | N | 0,38 | 0,54 |
| 65 | Attention | iTBS | Offline | Within | Accuracy | 1 | 14 | fmri_based | Cerebellum | 0,13 | 0,07 | 0,19 | 0,04 | N | NA | NA |
| 66 | Working Memory | tpTMS | Online | Within | Accuracy | 1 | 18 | RC | Vertex | 0,60 | 0,07 | 0,83 | 0,03 | N | -0,60 | -0,83 |
| 66 | Working Memory | tpTMS | Online | Within | RTs | 1 | 18 | RC | Vertex | 0,15 | 0,06 | 0,21 | 0,03 | N | -0,15 | -0,21 |
| 66 | Working Memory | tpTMS | Online | Within | Accuracy | 2 | 18 | RC | Vertex | 0,42 | 0,06 | 0,55 | 0,03 | N | -0,42 | -0,55 |
| 66 | Working Memory | tpTMS | Online | Within | RTs | 2 | 18 | RC | Vertex | 0,10 | 0,06 | 0,14 | 0,03 | N | -0,10 | -0,14 |
| 67 | Social Cognition | tpTMS | Online | Within | Accuracy | 1 | 36 | LC | Vertex | 0,11 | 0,03 | 0,15 | 0,01 | N | -0,11 | -0,15 |
| 67 | Social Cognition | tpTMS | Online | Within | RTs | 1 | 36 | LC | Vertex | 0,06 | 0,03 | 0,08 | 0,01 | N | -0,06 | -0,08 |
| 67 | Social Cognition | tpTMS | Online | Within | Accuracy | 2 | 20 | LC | Visual C | 0,17 | 0,05 | 0,24 | 0,03 | N | -0,17 | -0,24 |
| 67 | Social Cognition | tpTMS | Online | Within | RTs | 2 | 20 | LC | Visual C | 0,38 | 0,05 | 0,54 | 0,03 | N | 0,38 | 0,54 |
| 67 | Social Cognition | tpTMS | Online | Within | Accuracy | 3 | 20 | LC | Visual C | 0,18 | 0,05 | 0,25 | 0,03 | N | -0,18 | -0,25 |
| 67 | Social Cognition | tpTMS | Online | Within | RTs | 3 | 20 | LC | Visual C | 0,47 | 0,06 | 0,66 | 0,03 | N | -0,47 | -0,66 |
| 68 | Social Cognition | tpTMS | Online | Within | Accuracy | 1 | 20 | LC | Vertex | 0,49 | 0,06 | 0,69 | 0,03 | N | -0,49 | -0,69 |
| 68 | Social Cognition | tpTMS | Online | Within | RTs | 1 | 20 | LC | Vertex | 0,11 | 0,05 | 0,15 | 0,03 | N | 0,11 | 0,15 |
| 68 | Social Cognition | tpTMS | Online | Within | Accuracy | 2 | 20 | LC | Vertex | 0,69 | 0,06 | 0,92 | 0,03 | N | -0,69 | -0,92 |
| 68 | Social Cognition | tpTMS | Online | Within | RTs | 2 | 20 | LC | Vertex | 0,05 | 0,05 | 0,07 | 0,03 | N | 0,05 | 0,07 |
| 69 | Timing | LFrTMS | Offline | Within | Accuracy | 1 | 10 | RC vs LC | Cerebellum | 0,71 | 0,12 | 0,94 | 0,06 | N | NA | NA |
| 70 | Social Cognition | tpTMS | Online | Between | Accuracy | 1 | 40 | RC | No TMS | 0,04 | 0,10 | 0,04 | 0,10 | N | NA | NA |
| 70 | Social Cognition | tpTMS | Online | Between | RTs | 1 | 40 | RC | No TMS | 0,47 | 0,10 | 0,47 | 0,10 | N | NA | NA |
| 71 | Semantic Memory | cTBS | Offline | Within | RTs | 1 | 21 | RC | Vertex | 0,56 | 0,06 | 0,78 | 0,03 | N | 0,56 | 0,78 |
| 71 | Semantic Memory | cTBS | Offline | Within | RTs | 2 | 20 | LC | Vertex | 0,29 | 0,05 | 0,40 | 0,03 | N | -0,29 | -0,40 |
| 72 | Timing | HFrTMS | Online | Within | RTs | 1 | 16 | RC | No TMS | 0,49 | 0,07 | 0,69 | 0,04 | N | 0,49 | 0,69 |
| 73 | Timing | cTBS | Offline | Between | Accuracy | 1 | 24 | MC | Sham | 1,00 | 0,19 | 1,00 | 0,19 | N | -1,00 | -1,00 |
| 74 | Timing | LFrTMS | Offline | Within | Accuracy | 1 | 9 | LC | DLPFC | 0,31 | 0,12 | 0,44 | 0,06 | N | -0,31 | -0,44 |
| 74 | Timing | HFrTMS | Online | Within | Accuracy | 2 | 8 | LC | Vertex | 0,04 | 0,13 | 0,06 | 0,06 | N | 0,04 | 0,06 |
| 75 | Music | LFrTMS | Offline | Within | RTs | 1 | 14 | RC | Sham | 0,56 | 0,08 | 0,78 | 0,04 | N | -0,56 | -0,78 |
| 76 | Semantic Memory | LFrTMS | Offline | Between | RTs | 1 | 43 | RC | Vertex | 0,69 | 0,10 | 0,69 | 0,10 | N | -0,69 | -0,69 |
| 77 | Timing | cTBS | Offline | Within | Accuracy | 1 | 14 | RC | DLPFC | 0,44 | 0,08 | 0,62 | 0,04 | N | -0,44 | -0,62 |
| 78 | Spatial cognition | LFrTMS | Offline | Within | Accuracy | 1 | 8 | LC | Neck | 2,15 | 0,83 | 3,37 | 0,42 | N | NA | NA |
| 79 | Semantic Memory | LFrTMS | Offline | Between | Accuracy | 1 | 24 | RC vs LC | Cerebellum | 3,04 | 0,27 | 3,04 | 0,27 | Y | NA | NA |
| 79 | Semantic Memory | LFrTMS | Offline | Between | RTs | 1 | 24 | RC vs LC | Cerebellum | 0,69 | 0,13 | 0,69 | 0,13 | Y | NA | NA |
| 80 | Executive Functions | cTBS | Offline | Between | Accuracy | 1 | 14 | LC | Sham | 0,30 | 0,29 | 0,30 | 0,29 | N | -0,30 | -0,30 |
| 80 | Executive Functions | cTBS | Offline | Between | RTs | 1 | 14 | LC | Sham | 1,62 | 0,38 | 1,62 | 0,38 | N | -1,62 | -1,62 |
| 80 | Executive Functions | cTBS | Offline | Between | Accuracy | 2 | 14 | LC | Sham | 0,00 | 0,29 | 0,00 | 0,29 | N | 0,00 | 0,00 |
| 80 | Executive Functions | cTBS | Offline | Between | RTs | 2 | 14 | LC | Sham | 2,17 | 0,45 | 2,17 | 0,45 | N | -2,17 | -2,17 |
| 81 | Executive Functions | cTBS | Offline | Between | Accuracy | 1 | 28 | LC | Sham | 0,52 | 0,15 | 0,52 | 0,15 | N | 0,52 | 0,52 |
| 81 | Executive Functions | cTBS | Offline | Between | RTs | 1 | 28 | LC | Sham | 0,71 | 0,15 | 0,71 | 0,15 | N | -0,71 | -0,71 |
| 81 | Executive Functions | cTBS | Offline | Between | Accuracy | 2 | 28 | LC | Sham | 0,22 | 0,14 | 0,22 | 0,14 | N | -0,22 | -0,22 |
| 81 | Executive Functions | cTBS | Offline | Between | RTs | 2 | 28 | LC | Sham | 0,19 | 0,14 | 0,19 | 0,14 | N | 0,19 | 0,19 |
| 82 | Executive Functions | cTBS | Offline | Within | RTs | 1 | 12 | LC | Sham | 0,22 | 0,05 | 0,29 | 0,03 | Y | NA | NA |
| 83 | Executive Functions | LFrTMS | Offline | Within | Accuracy | 1 | 16 | RC vs LC | Cerebellum | 0,47 | 0,05 | 0,59 | 0,02 | Y | NA | NA |
| 83 | Executive Functions | LFrTMS | Offline | Within | RTs | 1 | 16 | RC vs LC | Cerebellum | 0,57 | 0,05 | 0,80 | 0,02 | Y | NA | NA |
| 84 | Social Cognition | HFrTMS | Online | Within | RTs | 1 | 15 | MC | Sham | 0,33 | 0,07 | 0,47 | 0,04 | N | -0,33 | -0,47 |
| 85 | Working Memory | dpTMS | Online | Within | Accuracy | 1 | 23 | RC | No TMS | 0,85 | 0,06 | 1,07 | 0,03 | N | -0,85 | -1,07 |
| 85 | Working Memory | dpTMS | Online | Within | RTs | 1 | 23 | RC | No TMS | 0,33 | 0,05 | 0,44 | 0,02 | N | -0,33 | -0,44 |
| 86 | Working Memory | cTBS | Offline | Within | Accuracy | 1 | 10 | RC vs LC | Cerebellum | 0,82 | 0,10 | 1,13 | 0,05 | Y | NA | NA |
| 86 | Working Memory | cTBS | Offline | Within | RTs | 1 | 10 | RC vs LC | Cerebellum | 0,13 | 0,08 | 0,17 | 0,04 | Y | NA | NA |
| 87 | Working Memory | cTBS | Offline | Within | Accuracy | 1 | 10 | RC vs LC | Cerebellum | 0,45 | 0,11 | 0,60 | 0,06 | Y | NA | NA |
| 87 | Working Memory | cTBS | Offline | Within | RTs | 2 | 13 | RC vs LC | Cerebellum | 0,48 | 0,09 | 0,67 | 0,05 | y | NA | NA |
| 88 | Learning | LFrTMS | Offline | Between | RTs | 1 | 36 | RC | No TMS | 0,27 | 0,27 | 0,27 | 0,27 | Y | NA | NA |
| 89 | Learning | LFrTMS | Offline | Between | Accuracy | 1 | 28 | LC | Vertex | 0,88 | 0,21 | 0,88 | 0,21 | N | -0,88 | -0,88 |
TBS theta-burst stimulation, LFrTMS low-frequency repetitive TMS, tpTMS triple-pulse TMS, spTMS single-pulse TMS, HFrTMS high-frequency repetitive TMS, dpTMS double-pulse TMS, LC left cerebellum, RC right cerebellum, MC medial cerebellum, ES.5; V.5 effect size and variance with r = 0.5, ES.75; V.75 effect size and variance with r = 0.75, Exp experiment, N sample numerosity, CC control condition, Agg aggregated data, ES.5 direction; ES.75 direction signed effect size. More information is available in the Supplementary Material.
That is, the mean difference is divided by the square root of the difference between the sum of the two squared standard deviations of the means (SD) and the multiplication among the two SDs and twice the correlation between the means (r).
For between-participants designs the effect size and variance calculation were performed using R45 and its package compute.es46 using the functions mes or pes. The calculation of Cohen’s d for within-participants was performed using a value of r = 0.75. This value was obtained by computing the correlation between measurements (pooling individual participants’ data) in four published papers investigating left cerebellar participation in social cognition41, spatial cognition47 and semantic memory48,49 and from one in preparation from our lab, investigating right cerebellar participation in semantic processing50. The computed correlations ranged from r = 0.64 to r = 0.95 (Mean = 0.78, SD = 0.10). To further control for the possible variability of this measure, we also computed Cohen’s d using a value of r = 0.5 and ran sensitivity analyses. All the Cohens’ d included were either used in their relative sign or transformed in their absolute value, due to the difficulty to estimate “negative” effects when employing brain stimulation techniques: we thus ran separated meta-analyses on signed and absolute effect-sizes.
For the studies employing a task explicitly used as a control task, only the data from the target task was used to calculate the effect size. We expected several studies to employ more than one task: a “target task”, which is thought to measure a specific function, and a “control task”, which is thought to measure a non-relevant (i.e., for the specific purpose of a certain study) function. When performing cerebellar TMS, the adoption of control tasks is particularly important to exclude non-specific effects. The absence of a cerebellar TMS effect in the control task is generally interpreted as evidence of the (possible) main effect of cerebellar TMS in the target task (thus excluding non-specific effects). In such cases, we consequently included only the data from the target task.
The effect size was then computed on the target task, measuring the difference between cerebellar TMS and a control TMS condition using the following rules (in hierarchical order; for more information about the control TMS condition, see Table 1): (i) when available sham, vertex stimulation or no TMS trials within cerebellar TMS session; (ii) if not available, the condition without TMS; (iii) stimulation of a control area; (iv) stimulation of another cerebellar area.
For the studies investigating pre vs. post cerebellar stimulation, if possible, we computed the effect size comparing post cerebellar TMS and post control condition (following the above-mentioned rules). If, within one study, more than one experiment was performed between cerebellar TMS and control conditions (i.e., including different samples of participants), we considered these experiments as independent ones.
For a certain number of studies, it was impossible to identify an effect more relevant than others (e.g., see:42, in which right cerebellar TMS was administered across five different tasks and no significant effects were found). In such cases (see Table 1), the effect sizes were aggregated using the R-package MAd51, with the function agg. This function simultaneously aggregates all the effect sizes implementing Borenstein procedure52 for aggregating dependent effect sizes. Please also note that data from different dependent variables were not aggregated, but kept separated.
When complete data were provided only graphically, effect sizes and variances were computed using the function mes, and the descriptive statistics were extracted from the figures using the WebPlotDigitizer software53.
Meta-analyses
We performed two separate meta-analyses, one for each dependent variable (i.e., accuracy and RTs) on the absolute effect sizes. We also performed four distinct meta-regressions (i.e., two on accuracy and two on RTs as main dependent variables, respectively) to assess if the stimulation timing (online vs. offline) and the stimulation paradigm adopted (e.g., theta-burst stimulation, triple-pulse TMS, single-pulse TMS, etc.) moderated the observed effect. Two additional meta-regressions (i.e., one on each dependent variable) were performed to assess if the specific cognitive function (for a full list of cognitive functions see Table 1) moderated the observed effect.
Next, to investigate if the effect of cerebellar TMS was moderated by the site of stimulation (i.e., left vs. medial vs. right cerebellum), we first performed two meta-analyses (one for each dependent variable) excluding the studies which employed as control condition another cerebellar area, and then we performed two meta-regressions with the site of stimulation as moderator, again using the absolute effect sizes.
All the analyses were performed with restricted maximum-likelihood estimator method. The alpha for the p-values was set at = 0.01 (Bonferroni correction for multiple testing). The meta-analyses and meta-regressions performed, as well as the related plots, were computed using the R-package metafor57.
Heterogeneity was evaluated using the Q-test. In addition, we also report I254, which provides the percentage of the total variability in the effect size estimation that could be attributed to heterogeneity among the true effect (heterogeneity is considered high if I2 > 75%54). To further investigate heterogeneity, we also computed the prediction intervals (PI) of the effect, which quantify the dispersion of effect. That is, 95% PI indicate the range of values that the effect size of a future study similar to those included should probably take.
Publication bias was evaluated using funnel plots, the trim-and-fill method55 and Egger’s test56. The trim-and-fill method provides an estimate of the number of studies missing from the meta-analysis due to the suppression of the most extreme results on one side (generally the left, i.e., non-significant results) of the plot. The Egger’s test examines if the funnel plot is asymmetric performing a regression of the effect size on the standard error weighted by the inverse variance, a significant p value indicates publication bias. To explore the robustness of the results, we performed a leave-one-out analysis: this procedure evaluates the robustness of the effect excluding one study at a time.
Finally, we performed sensitivity analyses performing the two main meta-analyses on accuracy and RTs including the effect sizes of the within-participants designs computed with r = 0.5.
Note that, as discussed before, we also we performed four additional meta-analyses (two on accuracy and two on RTs), this time using signed effect sizes (i.e., not transformed in absolute value; thus disentangling performance impairment from enhancement) and hence recomputing the two possible correlations between measurements. In this case, negative effect sizes index performance impairment, while positive effect sizes index performance enhancement. Yet, the analyses on signed effect sizes were performed only on studies comparing cerebellar vs. control condition different from the stimulation of another cerebellar site (in those comparing two cerebellar sites it is generally not possible to infer performance impairment or enhancement). Similarly, the studies in which there is no default performance impairment or enhancement (e.g., as in the case of pseudoneglect) were excluded from the analyses on signed effect size.
Additional information, including the plots of the meta-analyses performed using signed effect sizes as well as the tables with the number of the studies per condition of the non-significant meta-regressions is reported as Supplementary Material.
Results
Study selection
The literature search identified 590 articles (Fig. 1—PRISMA flowchart). Following the adoption of our selection criteria, a total of 41 studies were included in the present meta-analysis37–42,47–49,58–89). In total, because some studies reported both accuracy and RTs or performed more than one experiment, 85 effects were included, 44 on accuracy and 41 on RTs.
Figure 1.
Flowchart illustrating study selection, review strategy and data extraction, retrieved from: http://prisma-statement.org/documents/PRISMA%202009%20flow%20diagram.pdf.
Study characteristics
The characteristics of the included experiments are reported in Table 1. Studies were conducted between 2003 and 2020.
Of the 44 experiments included using accuracy as dependent variable, 11 were performed with a between-participants design, while 33 with a within-participants design. Of the 41 experiments included using RTs as dependent variable, 11 were performed with a between-participants design and 30 with a within-participants design.
For timing of stimulation, of the 44 experiments included using accuracy as dependent variable, 24 targeted the cerebellum while participants performed the task (i.e., online stimulation), while 20 targeted the cerebellum before the task or between two task sessions (i.e., offline stimulation). Of the 41 experiments included using RTs as dependent variable, 22 adopted online paradigms and 19 offline ones.
Concerning the stimulation paradigm, of the 44 experiments included using accuracy as dependent variable, 2 used double-pulse TMS (dpTMS), 3 high-frequency rTMS (HFrTMS), 7 low-frequency rTMS (LFrTMS), 3 single-pulse TMS (spTMS), 1 intermittent TBS (iTBS), 11 continuous TBS (cTBS), 17 triple-pulse TMS (tpTMS). Of the 41 experiments included using RTs as dependent variable, 2 used dpTMS, 2 HFrTMS, 5 LFrTMS, 3 spTMS, 14 cTBS and 15 tpTMS. Following31, we considered as HFrTMS those paradigms employing a TMS frequency > 1 Hz.
With regards to the specific cognitive function investigated, of the 44 experiments included using accuracy as dependent variable, 2 investigated attention, 1 episodic memory, 6 executive functions, 1 learning, 3 various sub-types of memory, 4 semantic memory, 9 social cognition, 5 spatial cognition, 6 timing and 7 working memory. Of the 41 experiments included using RTs as dependent variable, 6 investigated executive functions, 1 learning, 1 music, 11 semantic memory, 10 social cognition, 4 spatial cognition, 1 timing and 7 working memory. In particular, here the term learning refers to procedural learning, while with various sub-types of memory we target studies that employed tasks thought to measure more than one memory function (e.g., measuring both episodic and semantic memory49) or whose results have been aggregated across several memory functions (comprising episodic, semantic, working and short-term memory42). Finally, with timing we target studies assessing the representation and perception of time, thus not including musical processing.
Accuracy
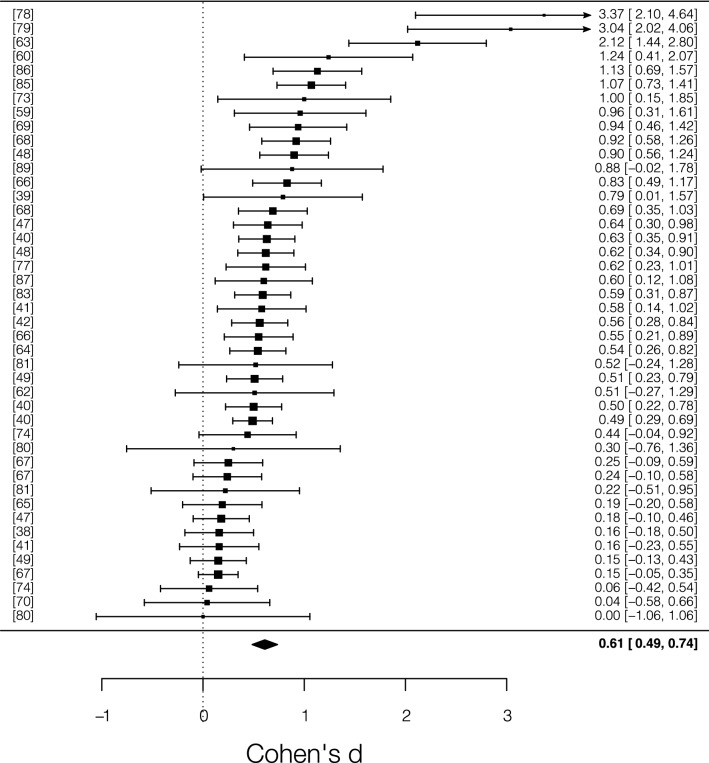
Random effects meta-analysis (N = 44) showed a medium mean effect size, d = 0.61 [95% CI = 0.48, 0.73; 95% PI = − 0.08, 1.30], z = 9.56, p < 0.0001, indicating that cerebellar TMS significantly affects participants’ accuracy compared to control conditions. Total heterogeneity was significant, QT = 157.68, p = < 0001, I2 = 77%, suggesting moderate variance across the experiments included (Fig. 2).
Figure 2.
Forest plot of the studies that used accuracy as main dependent variable included in the meta-analysis. Each row corresponds to one experiment and the lines beside each square represent 95% confidence interval. The size of each square represents the weight of the study. The diamond at the bottom represents the cumulative effect size with 95% confidence interval. Higher positive values indicate higher behavioral modulation in the cerebellar TMS condition.
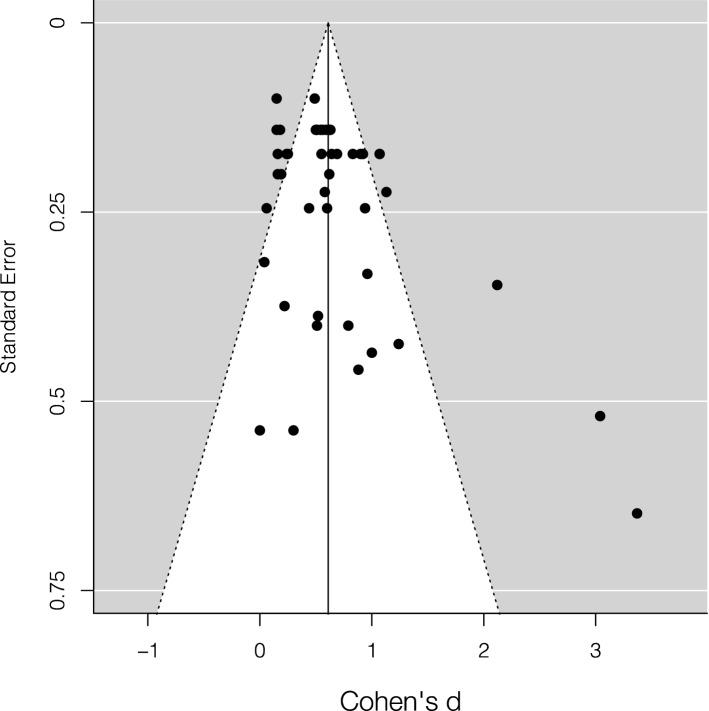
The leave-one-out analysis showed that the effect size was highly robust and ranged between 0.57 and 0.62 (M = 0.61, SD = 0.01). The trim and fill method did not add hypothetical missing studies on the left side of the funnel plot (Fig. 3). The Egger’s test was significant, z = 3.70, p = 0.0002, supporting the possibility of publication bias.
Figure 3.
Funnel plot of the studies that used accuracy as main dependent variable included in the meta-analysis. Black dots represent the studies included. The vertical line represents the corrected effect size.
A first meta-regression did not show any moderation induced by stimulation timing (N = 44), χ2(1) = 3.50, p = 0.06. Heterogeneity remained significant, QT = 149.74, p < 0.0001, I2 = 76%.
A second meta-regression showed that stimulation paradigm moderated effects (N = 44), χ2(6) = 23.23, p = 0.0007. Heterogeneity remained significant, QT = 111.16, p < 0.0001, but I2 decreased, I2 = 67%. The decrease in heterogeneity suggests that the stimulation paradigm plays a role in determining the differences in the effects reported by the various studies (Table 2).
Table 2.
Cohen’s d calculated using the stimulation paradigm as moderator.
| dpTMS | HFrTMS | LFrTMS | spTMS | iTBS | cTBS | tpTMS | |
|---|---|---|---|---|---|---|---|
| Accuracy | 1.43 | 0.42 | 0.98 | 0.32 | 0.19 | 0.71 | 0.48 |
| [0.92, 1.94] | [0.05, 0.79] | [0.66, 1.29] | [−0.03, 0.68] | [−0.47, 0.85] | [0.45, − 0.97] | [0.33, 0.64] | |
| N = 2 | N = 3 | N = 7 | N = 3 | N = 1 | N = 11 | N = 17 |
The effect for the study employing both TBS and HFrTMS (with aggregated effects) are not showed.
A third meta-regression did not show any moderation induced by the specific cognitive function investigated (N = 44), χ2(9) = 10.77, p = 0.29. Heterogeneity remained significant, QT = 127.97, p < 0.0001, I2 = 79%.
Response times
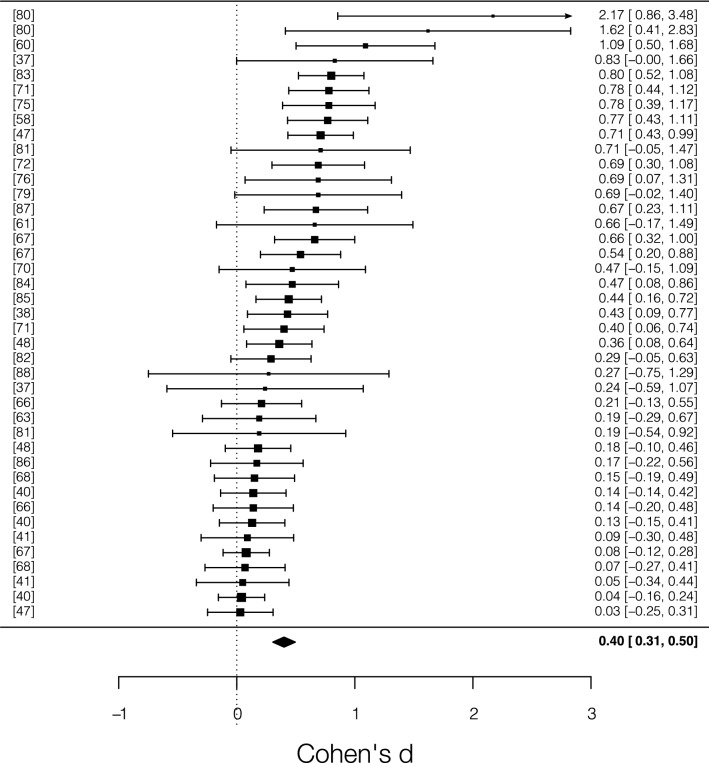
The random effect meta-analysis (N = 41) showed a medium effect size, d = 0.40 [95% CI = 0.30, 0.49; 95% PI = -0.05, 0.85], z = 8.19, p < 0.0001, meaning that cerebellar TMS significantly affects participants’ RTs compared to control conditions. Total heterogeneity was significant and moderate, QT = 105.95, p < 0.0001, I2 = 60% (Fig. 4).
Figure 4.
Forest plot of the studies that used RTs as main dependent variable included in the meta-analysis. Each row corresponds to one experiment and the lines beside each square represent 95% confidence interval. The size of each square represents the weight of the study. The diamond at the bottom represents the cumulative effect size with 95% confidence interval. Higher positive values indicate higher behavioral modulation in the cerebellar TMS condition.
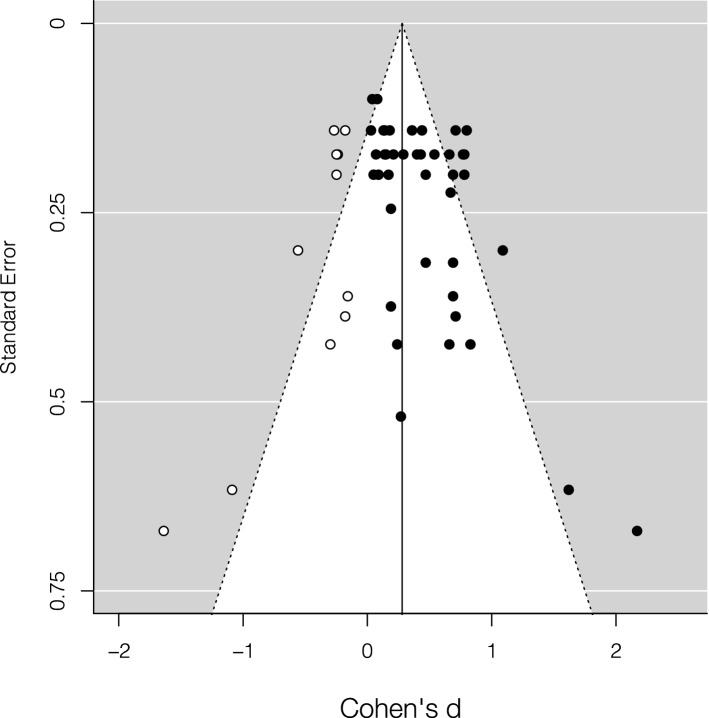
The leave-one-out analysis showed that the effect size ranged between 0.38 and 0.41 (M = 0.40, SD = 0.008). The trim and fill method added 11 hypothetical missing studies on the left side of the funnel plot (Fig. 5). Adding these hypothetical studies, the effect size became smaller but still significant, d = 0.28 [95% CI = 0.17, 0.38], z = 5.11, p < 0.0001, and the heterogeneity remained significant, QT = 186.49, p < 0.0001, I2 = 73%. The Egger’s test was significant, z = 3.28, p = 0.001, supporting the possibility of publication bias.
Figure 5.
Funnel plot of the studies that used RTs as main dependent variable included in the meta-analysis. Black dots represent the studies included, while white dots represent hypothetical missing studies on the left side (estimated using the trim and fill method). The vertical line represents the corrected effect size.
A first meta-regression showed that stimulation timing (N = 41) moderated effects, χ2(1) = 17.25, p < 0.0001. Heterogeneity remained significant, QT = 71.45, p = 0.001, and I2 decreased, I2 = 44%, suggesting that the stimulation timing plays a role in determining the differences in the effects reported by the various studies. In particular, offline paradigms reported significantly higher effect sizes comparted with online ones, with both paradigms reporting cumulative effect sizes significantly different from zero (Table 3).
Table 3.
Cohen’s d calculated using the stimulation timing as moderator.
| Online | Offline | |
|---|---|---|
| RTs | 0.27 | 0.63 |
| [0.17, 0.36] | [0.59, 0.77] | |
| N = 22 | N = 19 |
A second meta-regression showed stimulation paradigm moderated effects (N = 41), χ2(5) = 26.44, p < 0.0001. Heterogeneity remained significant, QT = 57.22, p = 0.01, but I2 decreased, I2 = 37%. The decrease in heterogeneity suggests that the stimulation paradigm plays a role in determining the differences in the effects reported by the various studies (Table 4).
Table 4.
Cohen’s d calculated using the stimulation paradigm as moderator.
| dpTMS | HFrTMS | LFrTMS | spTMS | cTBS | tpTMS | |
|---|---|---|---|---|---|---|
| RTs | 0.35 | 0.58 | 0.73 | 0.38 | 0.59 | 0.20 |
| [0.03, 0.67] | [0.23, 0.92] | [0.48, 0.99] | [0.15, 0.62] | [0.43, 0.75] | [0.09, 0.31] | |
| N = 2 | N = 2 | N = 5 | N = 3 | N = 14 | N = 15 |
A third meta-regression did not show any moderation induced by the specific cognitive function investigated (N = 41), χ2(7) = 15.11, p = 0.03. Heterogeneity decreased slightly but remained significant, QT = 70.59, p = 0.0002, I2 = 53%.
Cerebellar lateralization
Accuracy
The random effects meta-analysis (N = 35) showed a medium mean effect size, d = 0.54 [95% CI = 0.42, 0.66; 95% PI = − 0.03, 1.11], z = 8.79, p < 0.0001, indicating that cerebellar TMS significantly affects participants’ accuracy compared to control conditions. Total heterogeneity was significant, QT = 114.93, p ≤ 0001, I2 = 72%, suggesting moderate variance across the experiments included.
The leave-one-out analysis showed that the effect size was highly robust and ranged between 0.50 and 0.55 (M = 0.54, SD = 0.01). The trim and fill method did not add hypothetical missing studies on the left side of the funnel plot. The Egger’s test was significant, z = 2.17, p = 0.02, supporting the possibility of publication bias.
A meta-regression did not show any moderation induced by the stimulation site (N = 35), χ2(2) = 1.37, p = 0.50. Heterogeneity remained significant, QT = 108.17, p < 0.0001, I2 = 71%.
Response times
The random effects meta-analysis (N = 34) showed a medium mean effect size, d = 0.34 [95% CI = 0.25, 0.44; 95% PI = − 0.04, 0.73], z = 7.12, p < 0.0001, indicating that cerebellar TMS significantly affects participants’ accuracy compared to control conditions. Total heterogeneity was significant, QT = 76.82, p ≤ 0001, I2 = 54%, suggesting moderate variance across the experiments included.
The leave-one-out analysis showed that the effect size was highly robust and ranged between 0.32 and 0.36 (M = 0.34, SD = 0.009). The trim and fill method added 4 hypothetical missing studies on the left side of the funnel plot. Adding these hypothetical studies, the effect size became smaller but still significant, d = 0.30 [95% CI = 0.21, 0.40], z = 6.26, p < 0.0001, and the heterogeneity remained significant, QT = 95.50, p < 0.0001, I2 = 56%. The Egger’s test was significant, z = 3.23, p = 0.001, supporting the possibility of publication bias.
A meta-regression did not show any moderation induced by the stimulation site (N = 34), χ2(2) = 6.19, p = 0.04. Heterogeneity decreased slightly but remained significant, QT = 61.86, p < 0.0001, I2 = 46%.
Sensitivity analyses
The sensitivity analyses performed are reported in Table 5. Besides the two meta-analyses reported above, with r = 0.75 for correlation between measures in within-participants designs, we also performed two meta-analyses with r = 0.5. The meta-analyses performed with r = 0.5 show lower cumulative effect sizes (d = 0.41 for accuracy; d = 0.27 for RTs), but both are still significant. The two meta-analysis performed with r = 0.5 report also substantially different indexes in all the other measures assessed. In particular, heterogeneity is reduced for r = 0.5, but funnel plot asymmetry is still problematic, with the trim and fill method estimating a large number of studies missing (7 for accuracy; 11 for RTs). Egger’s test is also significant.
Table 5.
Results of the meta-analyses performed with the effect sizes of the within-participants studies included computed using r = 0.75 or r = 0.5.
| Dependent variable | Corr | Effect size | Q-test | Higgins’I2 | Trim and fill | Egger’s test |
|---|---|---|---|---|---|---|
| Accuracy | r = 0.75 |
0.60 [CI = .47, .73; PI = -.09, 1.30] |
p < .0001 | 77% | 0 | p = .0002 |
| r = 0.5 |
0.41 [CI = 0.33, 0.49; PI = 0.22, 0.60] |
p = .005 | 11% | 7 | p < .0001 | |
| RTs | r = 0.75 |
0.40 [CI = 0.30, 0.49; PI = −0.05, 0.85] |
p < .0001 | 60% | 11 | p = .001 |
| r = 0.5 |
0.27 [CI = 0.19, 0.35; PI = 0.16, 0.38] |
p = .27 | 3% | 11 | p < .0001 |
Signed effect sizes
The results of the meta-analyses on signed effect sizes are reported in Table 6. As above, we performed on accuracy and RTs two meta-analyses with r = 0.75 and r = 0.5 for correlation between measures in within-participants designs. In this case we used the signed effect sizes, with negative values indicating performance impairment and positive values indicating performance enhancement. For accuracy, both meta-analyses reported a negative cumulative effect size, indicating that generally the studies included reported performance impairment. Conversely, for RTs, both meta-analyses reported non-significant cumulative effect sizes, likely indicating that positive and negative effects countered each other. As above, with higher correlation between measures, heterogeneity was higher and publication bias followed the same pattern (but was less evident for accuracy).
Table 6.
Results of the meta-analyses performed on signed effect sizes with the effect sizes of the within-participants studies included computed using r = 0.75 or r = 0.5.
| Dependent variable | Corr | Signed effect size | Q-test | Higgins’ I2 | Trim and fill | Egger’s test |
|---|---|---|---|---|---|---|
| Accuracy | r = 0.75 |
−0.45 [CI = −0.62, −0.27; PI = −1.29, 0.39] |
p < .0001 | 84% | 0 | p = .67 |
| r = 0.5 |
−0.32 [CI = −0.45, −0.20; PI = −0.76, 0.10] |
p = .007 | 42% | 3 | p = .34 | |
| RTs | r = 0.75 |
−0.13 [CI = −0.30, 0.02; PI = −0.92, 0.65] |
p < .0001 | 83% | 3 | p = .02 |
| r = 0.5 |
−0.09 [CI = −0.21, 0.02; PI = −0.51, 0.31] |
p = .0009 | 41% | 4 | p = .006 |
Discussion
Because of the main propriety of magnetic stimulation, which allows to infer causal relationships between a targeted brain area and a specific cognitive function, as well as the growing interest around cerebellar involvement in non-motor functions, the number of studies targeting the cerebellum using TMS has largely increased in the past few years. In particular, while several studies showed that the cerebellum is clearly involved in motor coordination and adaptation19–24, the findings about cerebellar involvement in non-motor functions were qualitatively more variable. In the present meta-analysis, we thus aimed at quantifying the effects of TMS applied over the cerebellum on non-motor functions for both accuracy and RTs. Our results showed that TMS is a reliable technique for investigating cerebellar participation in cognitive processes. TMS administered over the cerebellum was indeed found to successfully modulate cognitive performance (either in terms of cognitive impairment or enhancement), affecting accuracy and RTs. The cumulative effects calculated were robust and heterogeneity was partly accounted by the moderators added in meta-regressions for both accuracy and RTs data. Critically, the effects of TMS were significant not only when considering a strong correlation among measurements (r = 0.75) for within-participants designs, but also for a moderate correlational value (r = 0.5).
In this study, we further investigated whether other potentially crucial variables, namely stimulation timing, stimulation paradigm or the specific cognitive function investigated, could moderate the observed effects. We found that stimulation timing moderated the observed effects on RTs only (and not on accuracy), with cumulative effect sizes being significantly higher for offline compared with online paradigms. For both dependent variables, the stimulation paradigm moderated the observed effects, suggesting that the various stimulation paradigms do play a role in determining the effect of cerebellar TMS. These results indicate that certain TMS paradigms can be more reliable than others when investigating cerebellar functions. Conversely, for both dependent variables, the specific cognitive function investigated did not moderate the observed effects, indicating similar effects across the various functions at hand.
Finally, we also investigated the possible effect of lateralization (i.e., whether cerebellar TMS effects depend on the stimulation site being left vs. medial vs. right), including this variable as a moderator in a meta-regression. Across both accuracy and RTs, we found that TMS site did not moderate the observed effects, suggesting similar effects across the three cerebellar sites tested (left vs. medial vs. right cerebellum). Unfortunately, a deeper relationship between TMS site and the specific cognitive function investigated could not be handled here due to the low numerosity within each group, but would likely modulate the observed effects, since certain cognitive functions appear to be lateralized in the cerebellum90,91. Indeed, a large number of studies focusing on social cognition specifically targeted the left cerebellum only67,68, in line with neuroimaging evidence showing left cerebellar activations during social tasks92,93. Similarly, studies focusing on semantic and linguistic processing, as well as on verbal working memory, mainly targeted the right cerebellum49,60. The laterality of these cognitive functions reflects the fact that cerebro-cerebellar interactions and cerebro-cerebellar connections are crossed94. Therefore, because many studies targeted only the left or the right cerebellum as a function of the specific cognitive process tested, we could not address the interaction between laterality and cognitive function directly in our meta-analysis. Indeed, only a small number of available studies directly focused on cerebellar asymmetries (e.g.,71,79), with this topic being particularly promising for future research in order to distinguish between left vs. medial vs. right cerebellar involvement in cognitive processing.
Another critical point is related to the effect size differences between the two dependent variables considered (i.e., RTs and accuracy), which may seem surprising, as from an experimental point of view both these measures quantify participants’ performance and are generally highly related (e.g., as in the case of speed-accuracy tradeoff). Moreover, because of cerebellar involvement in event timing95,96, one may have expected higher effect sizes for RTs than for accuracy. However, the specific stimulation paradigm adopted, the specific stimulation timing (i.e., offline vs. online stimulation) and the specific task adopted can all play a critical role on the observed behavior. That is, the pattern found may be affected by the interaction among these variables as well as by the involvement of other cerebral areas in the specific function tested. This interpretation is consistent with previous evidence showing that TMS effects depend on various factors such as stimulation intensity, brain state and timing97, and that TMS does not simply cause a generalized “virtual lesion”98.
We believe that our findings contribute to the debate on the role of the cerebellum in cognitive functions from both methodological and theoretical points of view. Firstly, our findings provide cumulative information quantifying the effect of the various TMS paradigms on cerebellar functions, as well as the effect of the various TMS timing and sites on cerebellar functions. Secondly, our meta-analysis supports cerebellar involvement in non-motor processing, indicating that the cerebellum does participate in cognitive processing and that this involvement is not moderated by the specific function investigated. This evidence further supports theories regarding cerebellar involvement in cognitive processing7,99. In addition to this, the cumulative effect sizes computed may have a direct application, allowing researchers to use them when estimating the minimum sample size needed to observe the hypothesized effect in future studies.
Regarding the effects of TMS on cerebellar cortex, it has been suggested that TMS directly modulates inhibitory activity of Purkinje cells placed in cerebellar cortex, thus affecting the activity of the cerebral cortex via the thalamus100. Purkinje cells activity can modulate size, speed, and timing of movements101 and it has been shown that when posterior cerebellar areas are targeted with TMS, the activity of other brain areas (e.g., deep nuclei, prefrontal areas, thalamus, etc.36,65,102,103) is modulated. Critically, the effects of cerebellar TMS on the activity of other brain areas as measured by motor-evoked potential (MEP) are frequency dependent. It has indeed been shown that LFrTMS and iTBS enhance MEPs104,105, while cTBS exerts the opposite effect106. Investigating such effects in future studies may be critical from a clinical point of view, particularly for the use of TMS in rehabilitation protocols for neuropsychiatric disorders107,108.
In interpreting our findings, three main limitations should be considered; namely, the potential publication bias, the fact that we mainly focused on absolute effect sizes and the level of heterogeneity. First, evidence for publication bias was particularly strong in sensitivity analyses considering a smaller correlation for within-participant designs. Various authors pointed to the need of interpreting null TMS results and making such findings available to the scientific community109, possibly providing more detailed evidence for the involvement of certain brain areas in certain functions. Second, concerning the issue of heterogeneity, our findings were strictly dependent on the correlation used for effect sizes estimation. This pattern of results must be interpreted by considering that almost none of the within-participants study included reported the correlation between measurements, leading to imprecise effect size computation. Nevertheless, for both dependent variables we found that prediction intervals were large and their lower bound negative, indicating that although cerebellar TMS is effective on average in modulating human cognitive performance, heterogeneity is also high. Given cerebellar anatomo-physiological characteristics, we believe that the high heterogeneity is likely related to differences in timing, intensity and TMS procedures adopted by the studies included, some of which could not be analyzed here. In addition, using a smaller correlation coefficient between the two measurements, heterogeneity was more contained, emphasizing the importance of reporting the correlation in within-participant designs. Finally, in our main meta-analyses we focused on absolute values of effect sizes, as we were interested in probing whether TMS generally modulates cognitive performance, regardless of the specific direction of the considered effect (i.e., whether TMS induces cognitive impairment or enhancement). In addition to this, defying a priori whether a certain TMS paradigm would result in impairment or enhancement may be problematic and rather unrealistic. Our approach may have therefore increased the likelihood of observing significant effects, affecting in turn the publication bias and inducing consequently an asymmetric funnel plot. Yet, we also note that when we computed signed effect sizes, thus accounting for the specific direction of modulation (i.e., impairment or enhancement), we still found that TMS administered over the cerebellum was able to successfully modulate cognitive performance, showing that cerebellar TMS typically results in cognitive impairment in terms of accuracy.
In conclusion, the present meta-analysis indicates that TMS is effective in modulating cerebellar activity. These results therefore substantiate the well-established dysmetria of thought hypothesis7, corroborating the idea that the cerebellum is involved in non-motor, cognitive functions.
Supplementary Information
Author contributions
D.G. and L.R. wrote the manuscript and analyzed the data. D.G., L.R., I.C., and T.V. designed the study and contributed to the revision process. All authors approved the final version of the manuscript.
Funding
This work was supported by funding from the Italian Ministry of University and Research (PRIN 2017 no. 201755TKFE) and from Italian Ministry of Health (Ricerca Corrente 2021) to TV and LR.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94051-5.
References
- 1.Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Orsi L. Consensus paper: Cerebellum and emotion. Cerebellum. 2017;16:552–576. doi: 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- 2.Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Paulin MG. Consensus paper: The role of the cerebellum in perceptual processes. Cerebellum. 2015;14(2):197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, Pezzulo G. Consensus paper: The cerebellum's role in movement and cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariën P, Ackermann H, Adamaszek M, Barwood CH, Beaton A, Desmond J, Leggio M. Consensus paper: language and the cerebellum: An ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Angelo E. The cerebellum gets social. Science. 2019;363(6424):229–229. doi: 10.1126/science.aaw2571. [DOI] [PubMed] [Google Scholar]
- 6.D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural Circuits. 2013;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmahmann JD. An emerging concept: The cerebellar contribution to higher function. Arch. Neurol. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 8.Ramnani N. The primate cortico-cerebellar system: Anatomy and function. Nat. Rev. Neurosci. 2006;7(7):511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex. 2009;19(10):2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolov AA, Erb M, Grodd W, Pavlova MA. Structural loop between the cerebellum and the superior temporal sulcus: Evidence from diffusion tensor imaging. Cereb. Cortex. 2014;24(3):626–632. doi: 10.1093/cercor/bhs346. [DOI] [PubMed] [Google Scholar]
- 12.Schmahmann JD. The cerebellum and cognition. Neurosci. Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Lesage E. Non-invasive cerebellar stimulation—A consensus paper. Cerebellum. 2014;13:121–138. doi: 10.1007/s12311-013-0514-7. [DOI] [PubMed] [Google Scholar]
- 14.Pleger B, Timmann D. The role of the human cerebellum in linguistic prediction, word generation and verbal working memory: Evidence from brain imaging, non-invasive cerebellar stimulation and lesion studies. Neuropsychologia. 2018;115:204–210. doi: 10.1016/j.neuropsychologia.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson SP, Davis NJ, Bracewell RM. Brain stimulation studies of non-motor cerebellar function: A systematic review. Neurosci. Biobehav. Rev. 2013;37(5):766–789. doi: 10.1016/j.neubiorev.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 16.van Dun K, Bodranghien F, Manto M, Marien P. Targeting the cerebellum by noninvasive neurostimulation: A review. Cerebellum. 2017;16:695–741. doi: 10.1007/s12311-016-0840-7. [DOI] [PubMed] [Google Scholar]
- 17.Oldrati V, Schutter DJ. Targeting the human cerebellum with transcranial direct current stimulation to modulate behavior: A meta-analysis. Cerebellum. 2018;17:228–236. doi: 10.1007/s12311-017-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson N, Miall RC. Disruption of saccadic adaptation with repetitive transcranial magnetic stimulation of the posterior cerebellum in humans. Cerebellum. 2010;9:548–555. doi: 10.1007/s12311-010-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panouilleres M, Neggers SF, Gutteling TP, Salemme R, Stigchel SVD, van der Geest JN, Pelisson D. Transcranial magnetic stimulation and motor plasticity in human lateral cerebellum: Dual effect on saccadic adaptation. Hum. Brain Mapp. 2012;33:1512–1525. doi: 10.1002/hbm.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonietti A, Monaco J, D’Angelo E, Pedrocchi A, Casellato C. Dynamic redistribution of plasticity in a cerebellar spiking neural network reproducing an associative learning task perturbed by TMS. Int. J. Neural Syst. 2018;28(09):1850020. doi: 10.1142/S012906571850020X. [DOI] [PubMed] [Google Scholar]
- 22.Hoffland BS, Bologna M, Kassavetis P, Teo JT, Rothwell JC, Yeo CH, Edwards MJ. Cerebellar theta burst stimulation impairs eyeblink classical conditioning. J. Physiol. 2012;590:887–897. doi: 10.1113/jphysiol.2011.218537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco J, Casellato C, Koch G, D'Angelo E. Cerebellar theta burst stimulation dissociates memory components in eyeblink classical conditioning. Eur. J. Neurosci. 2014;40:3363–3370. doi: 10.1111/ejn.12700. [DOI] [PubMed] [Google Scholar]
- 24.Monaco, J., Rocchi, L., Ginatempo, F., D’Angelo, E., & Rothwell, J.C. Cerebellar theta-burst stimulation impairs memory consolidation in eyeblink classical conditioning. Neural Plast. (2018). [DOI] [PMC free article] [PubMed]
- 25.Ferrari, C., Fiori, F., Suchan, B., Plow, E. B., & Cattaneo, Z. TMS over the posterior cerebellum modulates motor cortical excitability in response to facial emotional expressions. Eur. J. Neurosci. (2020). [DOI] [PubMed]
- 26.Harrington, A., & Hammond-Tooke, G. D. Theta burst stimulation of the cerebellum modifies the TMS-evoked N100 potential, a marker of GABA inhibition. PloS One10(11), e0141284 (2015). [DOI] [PMC free article] [PubMed]
- 27.Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3(3):161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Hoffland BS, Kassavetis P, Bologna M, Teo JTH, Bhatia KP, Rothwell JC, Van De Warrenburg BP. Cerebellum-dependent associative learning deficits in primary dystonia are normalized by r TMS and practice. Eur. J. Neurosci. 2013;38(1):2166–2171. doi: 10.1111/ejn.12186. [DOI] [PubMed] [Google Scholar]
- 29.Oechsner M, Zangemeister WH. Prolonged postexcitatory inhibition after transcranial magnetic stimulation of the motor cortex in patients with cerebellar ataxia. J. Neurol. Sci. 1999;168(2):107–111. doi: 10.1016/S0022-510X(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 30.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of the human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 31.Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neurosci. Biobehav. Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat. Rev. Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 33.Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: The problems solved and created by transcranial magnetic stimulation. J. Cogn. Neurosci. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- 34.Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuroimage. 2014;85:961–970. doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveri M, Torriero S, Koch G, Salerno S, Petrosini L, Caltagirone C. The role of transcranial magnetic stimulation in the study of cerebellar cognitive function. Cerebellum. 2007;6(1):95. doi: 10.1080/14734220701213421. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez, L., Rogasch, N.C., Do, M., Clark, G., Major, B.P., Teo, W.P., & Enticott, P. G. Cerebral cortical activity following non-invasive cerebellar stimulation—A systematic review of combined TMS and EEG studies. Cerebellum 1–27 (2020). [DOI] [PubMed]
- 37.Argyropoulos GP, Muggleton NG. Effects of cerebellar stimulation on processing semantic associations. Cerebellum. 2013;12(1):83–96. doi: 10.1007/s12311-012-0398-y. [DOI] [PubMed] [Google Scholar]
- 38.Desmond JE, Chen SA, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann. Neurol. 2005;58:553–560. doi: 10.1002/ana.20604. [DOI] [PubMed] [Google Scholar]
- 39.Arasanz CP, Staines WR, Roy EA, Schweizer TA. The cerebellum and its role in word generation: A cTBS study. Cortex. 2012;48:718–724. doi: 10.1016/j.cortex.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari, C., Ciricugno, A., Battelli, L., Grossman, E.D., & Cattaneo, Z. Distinct cerebellar regions for body motion discrimination. Soc. Cognit. Affect. Neurosci. (2019). [DOI] [PMC free article] [PubMed]
- 41.Cattaneo Z, Renzi C, Casali S, Silvanto J, Vecchi T, Papagno C, D’Angelo E. Cerebellar vermis plays a causal role in visual motion discrimination. Cortex. 2014;58:272–280. doi: 10.1016/j.cortex.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Rami L, Gironell A, Kulisevsky J, Garcıa-Sánchez C, Berthier M, Estevez-Gonzalez A. Effects of repetitive transcranial magnetic stimulation on memory subtypes: A controlled study. Neuropsychologia. 2003;41:1877–1883. doi: 10.1016/S0028-3932(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 43.Quintana DS. From pre-registration to publication: A non-technical primer for conducting a meta-analysis to synthesize correlational data. Front. Psychol. 2015;6:1549. doi: 10.3389/fpsyg.2015.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen, J. Statistical Power Analysis for the Behavioral Sciences. (Routledge Academic, 1988).
- 45.R Core Team. R: A Language and Environment for Statistical Computing (2020).
- 46.Del Re, A.C. compute.es: Compute Effect Sizes. R Package Version 0.2-2. http://cran.r-project.org/web/packages/compute.es (2013).
- 47.Ciricugno, A., Ferrari, C., Rusconi, M.L., & Cattaneo, Z. The left posterior cerebellum is involved in orienting attention along the mental number line: An online-TMS study. Neurospychologia (2020). [DOI] [PubMed]
- 48.Gatti D, VanVugt F, Vecchi T. A causal role for the cerebellum in semantic integration: A transcranial magnetic stimulation study. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gatti D, Vecchi T, Mazzoni G. Cerebellum and semantic memory: A TMS study using the DRM paradigm. Cortex. 2021;135:78–91. doi: 10.1016/j.cortex.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Gatti, D., Rinaldi, L., Marelli, M., & Vecchi, T. Processing of semantic associations in the cerebellum: A TMS study (in preparation).
- 51.Del Re, A.C. & Hoyt W.T. MAd: Meta-Analysis with Mean Differences. R Package Version 0.8-2. https://cran.r-project.org/package=MAd (2014).
- 52.Borenstein. Effect sizes for continuous data. in The Handbook of Research Synthesis and Meta Analysis (Cooper, H., Hedges, L.V., & Valentine, J.C., eds.) 279–293. (Russell Sage Foundation, 2009).
- 53.Rohatgi, A. WebPlotDigitizer (Version 4.4) [Computer Software]. http://arohatgi.info/WebPlotDigitizer (2015).
- 54.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duval, S.J. The trim and fill method. in Publication Bias in Meta-analysis: Prevention, Assessment, and Adjustments (Rothstein, H.R., Sutton, A.J., Borenstein, M. eds.) 127–144 (Wiley, 2005).
- 56.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw.36(3), 1–48. http://www.jstatsoft.org/v36/i03/ (2010).
- 58.Allen-Walker LS, Bracewell RM, Thierry G, Mari-Beffa P. Facilitation of fast backward priming after left cerebellar continuous theta-burst stimulation. Cerebellum. 2018;17(2):132–142. doi: 10.1007/s12311-017-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arasanz CP, Staines WR, Schweizer TA. Isolating a cerebellar contribution to rapid visual attention using transcranial magnetic stimulation. Front. Behav. Neurosci. 2012;6:55. doi: 10.3389/fnbeh.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Argyropoulos GP. Cerebellar theta-burst stimulation selectively enhances lexical associative priming. Cerebellum. 2011;10:540–550. doi: 10.1007/s12311-011-0269-y. [DOI] [PubMed] [Google Scholar]
- 61.Argyropoulos GP, Kimiskidis VK, Papagiannopoulos S. Theta-burst stimulation of the right neocerebellar vermis selectively disrupts the practice-induced acceleration of lexical decisions. Behav. Neurosci. 2011;125(5):724. doi: 10.1037/a0025134. [DOI] [PubMed] [Google Scholar]
- 62.Avanzino, L., Bove, M., Pelosin, E., Ogliastro, C., Lagravinese, G., & Martino, D. The cerebellum predicts the temporal consequences of observed motor acts. PLoS One10 (2015). [DOI] [PMC free article] [PubMed]
- 63.Chen SA, Heng GJ, Ng THB, Eng GK, Kwok FY, Lim JYY, Chew E. Involvement of the inferior cerebellum in working memory: An fMRI-guided TMS study. Brain Stimul. 2015;8:375–376. doi: 10.1016/j.brs.2015.01.204. [DOI] [Google Scholar]
- 64.Dave, S., VanHaerents, S., & Voss, J.L. Cerebellar theta and beta noninvasive stimulation rhythms differentially influence episodic memory versus semantic prediction. J. Neurosci. (2020). [DOI] [PMC free article] [PubMed]
- 65.Esterman M, Thai M, Okabe H, DeGutis J, Saad E, Laganiere SE, Halko MA. Network-targeted cerebellar transcranial magnetic stimulation improves attentional control. Neuroimage. 2017;156:190–198. doi: 10.1016/j.neuroimage.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrari C, Cattaneo Z, Oldrati V, Casiraghi L, Castelli F, D’Angelo E, Vecchi T. TMS over the cerebellum interferes with short-term memory of visual sequences. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-018-25151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrari C, Oldrati V, Gallucci M, Vecchi T, Cattaneo Z. The role of the cerebellum in explicit and incidental processing of facial emotional expressions: A study with transcranial magnetic stimulation. Neuroimage. 2018;169:256–264. doi: 10.1016/j.neuroimage.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 68.Ferrari, C., Ciricugno, A., Urgesi, C., & Cattaneo, Z. Cerebellar contribution to emotional body language perception: A TMS study. Soc. Cognit. Affect. Neurosci. (2019). [DOI] [PMC free article] [PubMed]
- 69.Fierro B, Palermo A, Puma A, Francolini M, Panetta ML, Daniele O, Brighina F. Role of the cerebellum in time perception: A TMS study in normal subjects. J. Neurol. Sci. 2007;263:107–112. doi: 10.1016/j.jns.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 70.Gamond L, Ferrari C, La Rocca S, Cattaneo Z. Dorsomedial prefrontal cortex and cerebellar contribution to in-group attitudes: A transcranial magnetic stimulation study. Eur. J. Neurosci. 2017;45(7):932–939. doi: 10.1111/ejn.13529. [DOI] [PubMed] [Google Scholar]
- 71.Gilligan TM, Rafal RD. An opponent process cerebellar asymmetry for regulating word association priming. Cerebellum. 2019;18:47–55. doi: 10.1007/s12311-018-0949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gironell A, Rami L, Kulisevsky J, García-Sánchez C. Lack of prefrontal repetitive transcranial magnetic stimulation effects in time production processing. Eur. J. Neurol. 2005;12:891–896. doi: 10.1111/j.1468-1331.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- 73.Grube M, Lee KH, Griffiths TD, Barker AT, Woodruff PW. Transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Front. Psychol. 2010;1:171. doi: 10.3389/fpsyg.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koch G, Oliveri M, Torriero S, Salerno S, Gerfo EL, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp. Brain Res. 2007;179:291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- 75.Lega C, Vecchi T, D’Angelo E, Cattaneo Z. A TMS investigation on the role of the cerebellum in pitch and timbre discrimination. Cerebellum Ataxias. 2016;3:6. doi: 10.1186/s40673-016-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lesage E, Morgan BE, Olson AC, Meyer AS, Miall RC. Cerebellar rTMS disrupts predictive language processing. Curr. Biol. 2012;22:R794–R795. doi: 10.1016/j.cub.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Méndez JC, Rocchi L, Jahanshahi M, Rothwell J, Merchant H. Probing the timing network: A continuous theta burst stimulation study of temporal categorization. Neuroscience. 2017;356:167–175. doi: 10.1016/j.neuroscience.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 78.Oliver R, Opavsky R, Vyslouzil M, Greenwood R, Rothwell JC. The role of the cerebellum in ‘real’ and ‘imaginary’ line bisection explored with 1-Hz repetitive transcranial magnetic stimulation. Eur. J. Neurosci. 2011;33(9):1724–1732. doi: 10.1111/j.1460-9568.2011.07664.x. [DOI] [PubMed] [Google Scholar]
- 79.Oliveri, M., Bonnì, S., Turriziani, P., Koch, G., Gerfo, E.L., Torriero, S., & Caltagirone, C. Motor and linguistic linking of space and time in the cerebellum. PloS One4 (2009). [DOI] [PMC free article] [PubMed]
- 80.Picazio S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Cerebellar contribution to mental rotation: A cTBS study. Cerebellum. 2013;12:856–861. doi: 10.1007/s12311-013-0494-7. [DOI] [PubMed] [Google Scholar]
- 81.Picazio, S., Oliveri, M., Koch, G., Caltagirone, C., & Petrosini, L. Continuous theta burst stimulation (cTBS) on left cerebellar hemisphere affects mental rotation tasks during music listening. PloS One8(5) (2013). [DOI] [PMC free article] [PubMed]
- 82.Picazio, S., Foti, F., Oliveri, M., Koch, G., Petrosini, L., Ferlazzo, F., & Sdoia, S. Out with the old and in with the new: The contribution of prefrontal and cerebellar areas to backward inhibition. Cerebellum 1–11 (2020). [DOI] [PubMed]
- 83.Runnqvist E, Bonnard M, Gauvin HS, Attarian S, Trébuchon A, Hartsuiker RJ, Alario FX. Internal modeling of upcoming speech: A causal role of the right posterior cerebellum in non-motor aspects of language production. Cortex. 2016;81:203–214. doi: 10.1016/j.cortex.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 84.Schutter DJ, Enter D, Hoppenbrouwers SS. High-frequency repetitive transcranial magnetic stimulation to the cerebellum and implicit processing of happy facial expressions. J. Psychiatry Neurosci. 2009;34:60. [PMC free article] [PubMed] [Google Scholar]
- 85.Sheu YS, Liang Y, Desmond JE. Disruption of cerebellar prediction in verbal working memory. Front. Hum. Neurosci. 2019;13:61. doi: 10.3389/fnhum.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tomlinson SP, Davis NJ, Morgan HM, Bracewell RM. Cerebellar contributions to verbal working memory. Cerebellum. 2014;13(3):354–361. doi: 10.1007/s12311-013-0542-3. [DOI] [PubMed] [Google Scholar]
- 87.Tomlinson SP, Davis NJ, Morgan HM, Bracewell RM. Cerebellar contributions to spatial memory. Neurosci. Lett. 2014;578:182–186. doi: 10.1016/j.neulet.2014.06.057. [DOI] [PubMed] [Google Scholar]
- 88.Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rTMS with procedural learning. J. Cogn. Neurosci. 2004;16:1605–1611. doi: 10.1162/0898929042568488. [DOI] [PubMed] [Google Scholar]
- 89.Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. The what and how of observational learning. J. Cogn. Neurosci. 2007;19(10):1656–1663. doi: 10.1162/jocn.2007.19.10.1656. [DOI] [PubMed] [Google Scholar]
- 90.King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019;22:1371–1378. doi: 10.1038/s41593-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 92.Sokolov AA, Erb M, Gharabaghi A, Grodd W, Tatagiba MS, Pavlova MA. Biological motion processing: The left cerebellum communicates with the right superior temporal sulcus. Neuroimage. 2012;59(3):2824–2830. doi: 10.1016/j.neuroimage.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 93.Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JD, Leggio M. Consensus paper: Cerebellum and social cognition. Cerebellum. 2020;19(6):833–868. doi: 10.1007/s12311-020-01155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 95.Ivry RB, Keele SW. Timing functions of the cerebellum. J. Cogn. Neurosci. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 96.Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann. N. Y. Acad. Sci. 2002;978(1):302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- 97.Silvanto J, Bona S, Cattaneo Z. Initial activation state, stimulation intensity and timing of stimulation interact in producing behavioral effects of TMS. Neuroscience. 2017;363:134–141. doi: 10.1016/j.neuroscience.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silvanto J, Cattaneo Z. Common framework for “virtual lesion” and state-dependent TMS: The facilitatory/suppressive range model of online TMS effects on behavior. Brain Cogn. 2017;119:32–38. doi: 10.1016/j.bandc.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vecchi T, Gatti D. Memory as Prediction: From Looking Back to Looking Forward. MIT Press; 2020. [Google Scholar]
- 100.Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heiney SA, Kim J, Augustine GJ, Medina JF. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J. Neurosci. 2014;34:2321–2330. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farzan F, Pascual-Leone A, Schmahmann JD, Halko M. Enhancing the temporal complexity of distributed brain networks with patterned cerebellar stimulation. Sci. Rep. 2016;6:23599. doi: 10.1038/srep23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp. Brain Res. 2001;140:505–510. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- 104.Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci. Lett. 2005;376(3):188–193. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 105.Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp. Brain Res. 2007;176(3):440–447. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- 106.Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, Caltagirone C. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin. Neurophysiol. 2008;119(11):2559–2569. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 107.Koch G, Brusa L, Carrillo F, Gerfo EL, Torriero S, Oliveri M, Stanzione P. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73(2):113–119. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- 108.Koch G. Repetitive transcranial magnetic stimulation: A tool for human cerebellar plasticity. Funct. Neurol. 2010;25(3):159. [PubMed] [Google Scholar]
- 109.De Graaf TA, Sack AT. Null results in TMS: From absence of evidence to evidence of absence. Neurosci. Biobehav. Rev. 2011;35(3):871–877. doi: 10.1016/j.neubiorev.2010.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.