Abstract
Background
The optimal time to deliver adjuvant chemotherapy has not been defined.
Methods
A retrospective study of consecutive patients receiving adjuvant anthracycline and/or taxane 1993–2010. Primary endpoint included 5-year disease-free survival (DFS) in patients commencing chemotherapy <31 versus ≥31 days after surgery. Secondary endpoints included 5-year overall survival (OS) and sub-group analysis by receptor status.
Results
We identified 2003 eligible patients: 1102 commenced chemotherapy <31 days and 901 ≥31 days after surgery. After a median follow-up of 115 months, there was no difference in 5-year DFS rate with chemotherapy <31 compared to ≥31 days after surgery in the overall population (81 versus 82% hazard ratio (HR) 1.15, 95% confidence interval (95% CI) 0.92–1.43, p = 0.230). The 5-year OS rate was similar in patients who received chemotherapy <31 or ≥31 days after surgery (90 versus 91%, (HR 1.21, 95% CI 0.89–1.64, p = 0.228). For 250 patients with triple-negative breast cancer OS was significantly worse in patients who received chemotherapy ≥31 versus <31 days (HR = 2.18, 95% CI 1.11–4.30, p = 0.02).
Discussion
Although adjuvant chemotherapy ≥31 days after surgery did not affect DFS or OS in the whole study population, in TN patients, chemotherapy ≥31 days after surgery significantly reduced 5-year OS; therefore, delays beyond 30 days in this sub-group should be avoided.
Subject terms: Chemotherapy, Breast cancer
Background
Optimal timing for adjuvant chemotherapy is not clearly defined. The first report of a critical time window for commencing adjuvant chemotherapy was suggested by an analysis of three International Breast Cancer Study Group trials, including 1788 pre-menopausal patients treated with cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy, which reported a significant benefit of early adjuvant chemotherapy (defined as the initiation of chemotherapy ≤20 days of surgery), in pre-menopausal women with oestrogen receptor (ER) “absent” cancers. In that sub-group, 10-year disease-free survival (DFS) was increased to 60% compared to 34% with chemotherapy >20 days (hazard ratio (HR) 0.49, 95% confidence interval (CI) 0.33–0.72, p = 0.0003).1 However, a retrospective study from our institution of 1161 patients treated in a similar era was unable to confirm this finding.2 Analysis of three Danish Breast Cancer Cooperative Group trials comprising 7501 patients who received adjuvant CMF or cyclophosphamide, epirubicin and 5-fluorouracil (CEF) chemotherapy within 3 months of surgery was similarly unable to detect any survival detriment from delayed initiation of systemic therapy. However, a British Columbia Cancer Agency study (n = 2594, stages I and II), again including patients mostly treated before the turn of the century, showed inferior survival if chemotherapy was delayed >12 weeks (HR 1.6, 95% CI, 1.2–2.3; p = 0.005), although no impact for delays 4–8 or 8–12 weeks.3 Furthermore, a larger study from the MD Anderson included 6827 stage I–III patients treated from 1997 to 2011 and demonstrated reduced distant relapse-free survival (RFS) in the sub-groups of stage II (HR 1.20, 95% CI 1.02–1.43) or stage III (HR 1.36, 95% CI 1.02–1.80) patients treated ≥61 days after surgery. Overall survival (OS) was also reduced in stage III patients (HR 1.76, 95% CI 1.26–2.36), patients with triple-negative breast cancer (TNBC) (HR 1.54, 95% CI 1.09–2.18) or HER2-positive (HER2+) breast cancers treated with trastuzumab (HR 3.09, 95% CI 1.49–6.39) whose treatment was delayed ≥61 days after surgery.4
The largest study to date, which additionally evaluated contemporary chemotherapy regimens, is a California Cancer Registry study, including 24,843 patients with stage I–III breast cancer treated between 2005 and 2010. An inferior OS was reported in patients treated >90 days after surgery (HR 1.34, 95% CI 1.15–1.57), but there was no apparent detriment in patients treated 30–60 or 60–90 days after surgery. On sub-group analysis, time to chemotherapy >90 days was associated with a significantly reduced survival in the sub-group of patients with TNBC (HR 1.53, 95% CI 1.17–2.00), but not hormone receptor-positive (HR 1.25, 95% CI 0.98–1.59) or HER2+ disease (HR 1.28, 95% CI 0.93–1.75).5 A 2016 meta-analysis of 14 studies, which did not include the Californian Cancer Registry data due to the timing of publication, demonstrated that every 4-week delay in chemotherapy was associated with an increased risk of breast cancer recurrence (relative risk 1.04, 95% CI 1.00–1.08) and death (relative risk 1.04, 95% CI 1.01–1.08).6
In the United Kingdom, NHS cancer targets require the first treatment to be initiated within 62 days of referral with suspected cancer, and subsequent treatments to commence within 31 days; therefore, we designed this study to evaluate whether there was a measurable benefit from initiating adjuvant chemotherapy within 31 days of surgery.
Methods
We conducted a retrospective study of patients treated with adjuvant chemotherapy for early breast cancer at the Royal Marsden Hospital between January 1993 and December 2010. Patients who had received an anthracycline and/or taxane chemotherapy were included, and those who received CMF only were excluded. We additionally excluded patients who had undergone treatment for a previous invasive breast cancer, those who had received neo-adjuvant chemotherapy or primary endocrine therapy for the index cancer and all patients for whom we had inadequate histopathological and treatment details (Fig. 1). Eligible patients from the previously published analysis from our institution2 were included in this study.
Fig. 1.
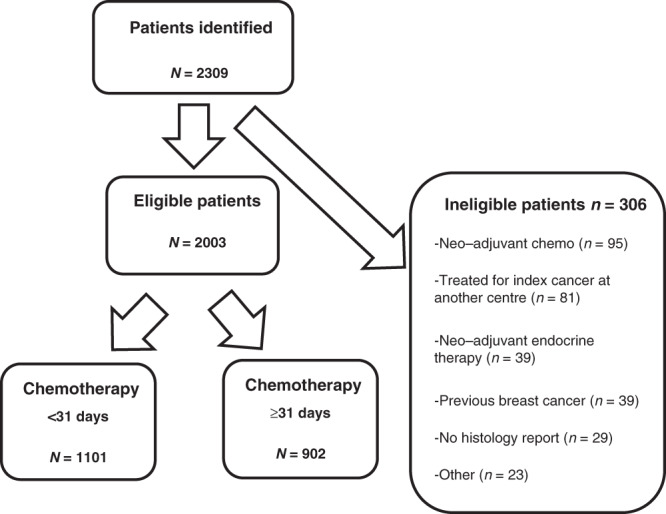
Flow chart of patients assessed for eligibility, those excluded and final study cohort according to timing of initiation of chemotherapy (within 31 days versus 31 days or more) after surgery.
Time to adjuvant chemotherapy was measured from the date of surgery to the date of first chemotherapy. For patients who underwent more than one surgical procedure, the final surgery date before initiation of chemotherapy was used for this analysis.
The primary endpoint of the study was 5-year DFS by chemo <31 days versus ≥31 days. Secondary endpoints of the study were 5-year OS and the effect on DFS of time to chemo as a continuous variable. Groups were compared using Cox’s proportional hazards regression analysis adjusting for age, receptors, grade, size, nodes and the presence or absence of lymphovascular invasion. Pre-specified sub-group analyses included patients with and without nodal involvement, age <40 versus ≥40, patients with tumours that were ER+ (Allred score 3–8/8) versus ER− (Allred score 0–2/8), TNBC, all HER2+ breast cancer patients and HER2+ breast cancer patients who received adjuvant trastuzumab.
Our study was approved as a service evaluation by the Royal Marsden Hospital Clinical Audit Committee, reference SE95.
Results
Patients
We identified 2003 eligible patients for the analysis, of whom 1101 patients had received chemotherapy <31 days of surgery and 902 had received chemotherapy ≥31 days after surgery. Ninety-six patients (4.8%) commenced chemotherapy within 2 weeks of surgery, 851 (42.5%) in weeks 3 and 4, 699 (34.9%) in weeks 5 and 6, 259 (12.9%) in weeks 7 and 8 and the remaining 98 patients (5.1%) commenced chemotherapy in weeks 10–13 (Supplementary Fig. 1). The clinico-pathological characteristics are summarised in Table 1. Of note, due to the era in which some patients were treated, HER2 status was unknown in approximately one-third of patients, the majority represented in the early chemotherapy group.
Table 1.
Patient characteristics.
| Chemo <31 days, n = 1101 | Chemo ≥31 days, n = 902 | Significance | |
|---|---|---|---|
| N (%) | N (%) | ||
| Gender | |||
| Male | 7 (0.6) | 2 (0.2) | 0.2 |
| Female | 1094 (99.4) | 900 (99.8) | |
| Median age (IQR) | 49 (42–55) | 51 (43–59) | <0.01 |
| Bilateral | |||
| Yes | 18 (1.6) | 28 (3.1) | |
| No | 1083 (98.4) | 874 (96.9) | 0.02 |
| LVI present | |||
| Yes | 539 (49.0) | 386 (42.8) | |
| No | 525 (47.7) | 489 (54.2) | 0.01 |
| Unknown | 37 (3.4) | 27 (3.0) | |
| Grade | |||
| 1 | 49 (4.5) | 49 (5.5) | 0.49 |
| 2 | 409 (37.6) | 344 (38.6) | |
| 3 | 631 (57.9) | 499 (55.9) | |
| Histological sub-type | |||
| IDC | 905 (82.2) | 752 (83.4) | |
| ILC | 117 (10.6) | 95 (10.5) | |
| Mixed IDC/ILC | 35 (3.2) | 24 (2.7) | 0.81 |
| Other | 44 (4.0) | 31 (3.4) | |
| ER | |||
| Negative | 276 (25.1) | 214 (23.7) | |
| Positive | 752 (68.3) | 668 (74.1) | <0.01 |
| Unknown | 73 (6.6) | 20 (2.2) | |
| PgR | |||
| Negative | 272 (24.7) | 252 (27.9) | |
| Positive | 292 (26.5) | 403 (44.7) | <0.01 |
| Unknown | 537 (48.8) | 247 (32) | |
| HER2 | |||
| Positive | 292 (26.5) | 158 (17.5) | |
| Negative | 272 (24.7) | 605 (67.1) | <0.01 |
| Unknown | 537 (48.8) | 139 (15.4) | |
| T stage | |||
| T0 | 11 (1.00) | 9 (1.0) | |
| T1 | 484 (44.0) | 389 (43.1) | |
| T2 | 516 (46.9) | 431 (47.8) | |
| T3 | 76 (6.9) | 66 (7.3) | |
| T4 | 2 (0.2) | 2 (0.2) | |
| Unknown | 11 (1.00) | 5 (0.6) | |
| Median primary tumour size (IQR) | 2.2 (1.6–3) | 2.2 (1.5–3.1) | 0.66 |
| Nodes | |||
| Positive | 669 (60.8) | 475 (52.7) | |
| Micro only | 30 (2.7) | 27 (3.0) | <0.01 |
| Negative | 391 (35.5) | 396 (43.9) | |
| Unknown | 11 (1.0) | 4 (0.4) | |
| Median number of involved nodes (IQR) | 1 (0–3) | 1 (0–2) | <0.01 |
| AJCC stage | |||
| 1A | 221 (20.1) | 185 (20.5) | |
| 2A | 391 (35.5) | 357 (39.6) | |
| 2B | 209 (19.0) | 166 (18.4) | |
| 3A | 184 (16.7) | 128 (14.2) | 0.29 |
| 3B | 2 (0.2) | 1 (0.1) | |
| 3C | 73 (6.6) | 56 (6.2) | |
| Unknown | 21 (1.9) | 9 (1.0) | |
Treatment
Two-thirds of patients underwent breast conservation surgery for a median tumour size of 2.2 cm (interquartile range (IQR) 1.6–3 cm). Although three-quarters of patients underwent axillary node dissection for a median number of 1 involved nodes (IQR 1–3), almost 40% of patients were node negative on histological examination.
A median of six cycles of adjuvant chemotherapy was delivered (IQR 6–6) and the regimen was most commonly 5-FU, epirubicin and cyclophosphamide (FEC, 69%), or a similar anthracycline-based regimen. Sequential anthracyclines and taxanes were received in just 14.5%, reflecting the standard regimens at the time the study patients were treated. Similarly, few patients (4.3%) received a platinum agent.
Adjuvant endocrine therapy was commenced in 97% of ER+ patients, most commonly tamoxifen, which was delivered in combination with ovarian function suppression with a GNRH analogue in just 3.3%. Adjuvant radiotherapy was delivered to 81% of patients. These data are summarised in Table 2 by early compared to late chemotherapy.
Table 2.
Treatments.
| Column1 | Chemo <31 days, n = 1101 | Chemo ≥31 days, n = 9022 |
|---|---|---|
| N (%) | N (%) | |
| Breast surgery | ||
| Breast conservation | 750 (68.1) | 557 (61.8) |
| Mastectomy | 343 (31.2) | 342 (37.9) |
| None | 8 (0.7) | 3 (0.3) |
| Axillary surgery | ||
| ALND | 838 (76.1) | 672 (74.5) |
| SLNB | 198 (18.0) | 178 (19.3) |
| Axillary sampling | 41 (3.7) | 47 (5.2) |
| None | 22 (2.0) | 5 (0.6) |
| Unknown | 2 (0.2) | 0 |
| Median number of operations before adjuvant chemo (IQR) | 1 (1–2) | 1 (1–2) |
| Chemotherapy regimen | ||
| Anthracycline+/cyclophosphamide or platinum ± 5-FU | 963 (87.5) | 728 (80.9) |
| Anthracycline and taxane | 135 (12.3) | 156 (17.3) |
| Taxane ± cyclophosphamide | 3 (0.3) | 16 (1.8) |
| Median number of chemo cycles (IQR) | 6 (6–6) | 6 (6–6) |
| Adjuvant radiotherapy | ||
| Yes | 921 (83.7) | 703 (77.9) |
| No | 180 (16.4) | 198 (22.0) |
| Unknown | 0 (0.00) | 1 (0.1) |
| Adjuvant endocrine therapy | ||
| AI | 131 (15.4) | 129 (18.4) |
| OFS ± tamoxifen or AI | 22 (2.6) | 36 (5.1) |
| Tamoxifen | 455 (53.5) | 344 (48.9) |
| Tamoxifen and AI switch | 238 (28.0) | 192 (27.3) |
| Unknown | 5 (0.6) | 2 (0.3) |
Disease-free survival
In the overall study population, there was no difference in 5-year DFS between patients receiving chemotherapy <31 compared to ≥31 days after surgery (81% compared to 82%, HR 1.15, 95% confidence interval (CI) 0.92–1.43, p = 0.23) (Fig. 2). Sub-group analysis did not identify a population in whom DFS was significantly affected by the timing of chemotherapy. These results are summarised in Table 2.
Fig. 2.
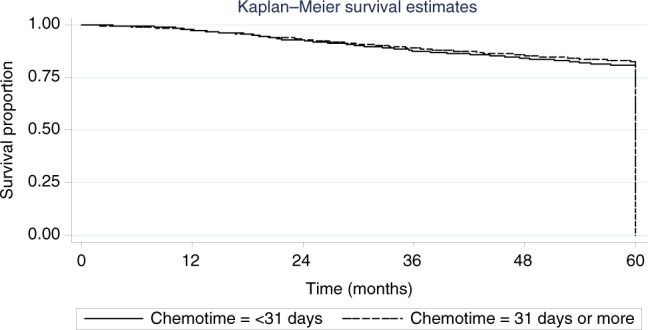
Kaplan-Meier curve representing disease-free survival in all patients by timing of initiation of chemotherapy (within 31 compared to 31 days or more) after surgery.
Overall survival
Similarly, 5-year OS was no different in patients receiving chemotherapy <31 compared to ≥31 days after surgery (89.8% compared to 90.7%, HR 1.21, 95% CI 0.89–1.64, p = 0.23) (Fig. 3). However, on sub-group analysis (Table 3), amongst the 250 patients with TNBC, OS was significantly lower in patients receiving adjuvant chemotherapy ≥31 days compared to <31 days (77% compared to 89%, HR 2.18, 95% CI 1.11–4.30, p = 0.024) (Fig. 4). There were no other sub-groups identified in whom later chemotherapy was detrimental.
Fig. 3.
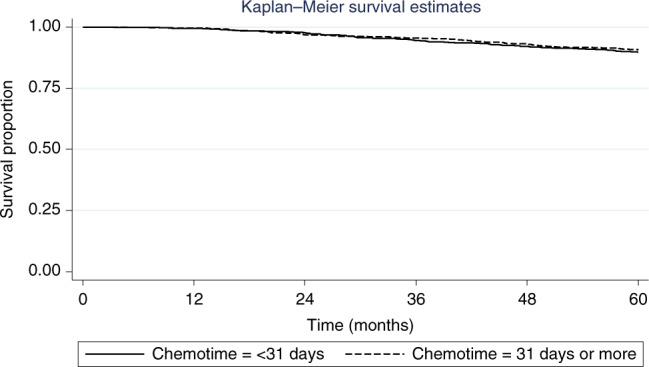
Kaplan-Meier curve representing overall survival in all patients by timing of intiation of chemotherapy (within 31 days compared to 31 days or more) after surgery.
Table 3.
Sub-group analysis for chemotherapy <31 days after surgery versus ≥31 days.
| Sub-group (n) | Unadjusted HR for DFS (95% CI) | Significance | Unadjusted HR for OS (95% CI) | Significance |
|---|---|---|---|---|
| Age | ||||
| <40 (295) | 1.14 (0.72–1.82) | 0.57 | 1.43 (0.61–3.36) | 0.41 |
| ≥40 (1577) | 1.14 (0.88–1.47) | 0.34 | 1.22 (0.87–1.72) | 0.25 |
| AJCC Stage | ||||
| 1 (393) | 1.24 (0.75–2.03) | 0.4 | 1.14 (0.56–2.29) | 0.72 |
| 2 (1096) | 1.00 (0.76–1.34) | 0.96 | 1.24 (0.83–1.83) | 0.29 |
| 3 (430) | 1.02 (0.60–1.73) | 0.94 | 0.65 (0.29–1.47) | 0.3 |
| HER2 | ||||
| Positive (310) | 0.98 (0.58–1.66) | 0.95 | 0.93 (0.43–1.99) | 0.85 |
| Positive and received trastuzumab (185) | 0.91 (0.38–2.15) | 0.83 | 1.12 (0.30–4.21) | 0.87 |
| ER | ||||
| Positive (1394) | 1.14 (0.86–1.53) | 0.36 | 1.14 (0.73–1.77) | 0.56 |
| Negative (478) | 1.15 (0.80–1.65) | 0.44 | 1.28 (0.82–2.00) | 0.28 |
| Triple negative (250) | 1.37 (0.80–2.34) | 0.25 | 2.18 (1.11–4.30) | 0.024 |
Fig. 4.
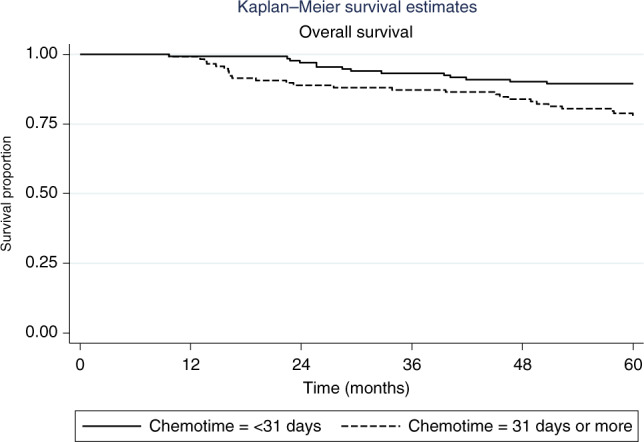
Kaplan-Meier curve representing overall survival in teh sub-group of patients with triple negative breast cancer by timing of initiation of chemotherapy (within 31 days versus 31 days or more) after surgery.
Discussion
Adjuvant chemotherapy is delivered to eradicate micrometastases and reduce the risk of breast cancer recurrence. Immediately following surgery, micrometastases undergo accelerated growth as a result of up-regulation of genes involved in wound healing7 and angiogenesis.8 Although chemotherapy confers a relative risk reduction in patients with all breast cancer sub-types,9 the absolute benefit is greatest for women at highest risk of recurrence, so chemotherapy is routinely delivered to high-risk patients, such as those with TNBC or HER2+ breast cancer, and those with ER+ breast cancer with adverse features such as lymph node involvement and/or a high genomic risk score.10 Intuitively, especially for patients with more aggressive disease, adjuvant chemotherapy should be commenced soon after recovery from surgery to minimise the time in which micro-metastatic residual disease can proliferate.
Our study demonstrates no overall difference in DFS or OS in patients with early breast cancer treated at our institution when chemotherapy was delivered <31 or ≥31 days of surgery This is consistent with two large published studies evaluating the timing of contemporary adjuvant chemotherapy regimens, which demonstrated detriment only if chemotherapy was delayed beyond 604 or 905 days. We did not evaluate the impact of delays beyond these time points, and in our cohort, only 73/2003 patients (3.6%) received treatment ≥61 days after surgery, and only 12 patients (0.6%) were treated ≥91 days after surgery; therefore, it is unlikely that any difference would be detectable. Of note, the MD Anderson study of 6827 patients demonstrated no significant differences in DFS or OS by the timing of chemotherapy in their overall study population of stage I–III patients, but sub-group analysis revealed reduced distant RFS in stage II and stage III patients and reduced OS in stage III, triple-negative or HER2+ patients treated ≥61 days after surgery.4 In contrast, the larger study of 24,843 Californian Cancer Registry stage I–III patients detected inferior OS (HR 1.34, 95% CI 1.15–1.57) in the overall study population when chemotherapy was delayed beyond 90 days, but not 23–60 or 61–90 days after surgery. DFS was not evaluated in this study. Of interest, delayed chemotherapy was particularly detrimental to the OS of patients with TNBC treated ≥91 days after surgery (HR 1.53, 95% CI 1.17–2.00), with no impact in women with ER+ or HER2+ breast cancer. This detriment was again not detectable in women treated 31–60 or 61–90 days after surgery.5 In contrast, our results demonstrated a significant worsening of OS (although not DFS) in women with TNBC in whom chemotherapy was delivered ≥31 days after surgery. The absence of a difference in DFS is difficult to explain and may simply be a consequence of our sample size (250 women with TNBC) being inadequate to detect a smaller difference: With our sample size, we were only able to detect a difference of 9.2% in 5-year DFS (HR 1.952, with 80% power and two-sided α 0.05).
It is perhaps surprising that few studies4 have demonstrated that delayed time to chemotherapy affects the outcome for women with HER2+ early breast cancer, given that it is, like TNBC, a highly proliferative and aggressive sub-type. A possible explanation is the transformation of the prognosis of this cancer following the introduction of adjuvant trastuzumab.11, 12 This is supported by a Chinese single-institution study, which demonstrated impaired DFS in women with ER−/HER2+ breast cancer who did not receive adjuvant trastuzumab (HR 2.41, 95% CI 1.36–4.26).13 This detrimental effect was also seen in patients with triple-negative (HR 2.55, 95% CI 1.25–5.18) and those with ER-positive cancers with luminal B-like features (HER2+ and/or high Ki67; HR 1.93, 95% CI, 1.10–3.34), but not those with luminal A-like features (HER2− and Ki67 <14%; HR 1.15, 95% CI, 0.54–2.43). It is possible that intrinsic sub-type would better predict the impact of delaying adjuvant chemotherapy. TNBC comprises 414–615 gene expression sub-types, and, although basal sub-types dominate, characterised by a higher expression of DNA repair genes and growth factors,14 the luminal androgen receptor sub-type are mostly luminal A or B and are associated with a more favourable prognosis,15 and therefore a delay might be less impactful. Similarly, whilst the majority of ER+ breast cancers are luminal (~60% luminal A and 30% luminal B), at least 1% will be basal and 7% HER2 enriched. Amongst HER2+ breast cancers, when divided by hormone receptor status, a higher proportion of ER+HER2+ cancers are luminal (33% luminal A, 46% luminal B), whereas ER−HER2+ cancers are dominated by the HER2 enriched sub-type (66%) and 11% are basal-like.16
Due to the retrospective nature of our study, although all patients received either an anthracycline or taxane, only 14.5% received both; a strategy that is now known to confer a superior survival.17 Similarly, our study included very few patients who received a platinum agent, which has recently been integrated into standard treatment for TNBC at many centres: The BrighTNess trial demonstrated a significant increase in pathological complete response (pCR) for women with TNBC who received carboplatin as part of their neo-adjuvant chemotherapy,18 and this has been extrapolated also to the adjuvant setting for this patient group, although long-term outcome data are awaited. However, it seems unlikely that these more intensive regimens would have significantly altered our results in the triple-negative cohort.
The change in practice towards neo-adjuvant rather than adjuvant chemotherapy for both TNBCs and HER2+ breast cancers has been driven by pre-operative studies demonstrating a correlation between achievement of pCR and breast cancer outcomes. Furthermore, for HER2+ breast cancer, studies demonstrating high rates of pCR from the addition of pertuzumab to standard regimens19–21 has made this approach the treatment of choice for all but small, node-negative cancers, for whom outcomes are excellent with abrogated chemotherapy with weekly paclitaxel and trastuzumab only.22 More recently, the availability of effective salvage therapies for women who do not achieve pCR with neo-adjuvant therapy for triple-negative23 or HER2+24 early breast cancer has confirmed the neo-adjuvant strategy as the optimal approach. Neo-adjuvant chemotherapy also has the advantage of avoiding delays to adjuvant chemotherapy, which may occur in women undergoing mastectomy and autologous reconstruction. However, for women who do undergo surgery as their first treatment, our data support previously published studies to suggest that for patients with TNBC in particular, adjuvant chemotherapy should be initiated within 31 days and that delays beyond that should be avoided if possible. Whether this also applies to women who received neo-adjuvant chemotherapy and are scheduled to receive an adjuvant salvage chemotherapy with capecitabine or T-DM1, is currently not known.
Limitations of our study include its retrospective nature, the small sample size of women with TNBC, in part due to the historical nature of our study including some patients whose full receptor status was not known, plus the lack of information on the duration of and compliance with endocrine therapy in the women with ER+ cancers. Furthermore, we did not collect data on whether radiotherapy was delivered before or after chemotherapy, although at our institution, routine practice is to sequence chemotherapy first. The optimal sequencing of adjuvant chemotherapy and radiotherapy, balancing the risks of distant and loco-regional relapse has been the subject of extensive debate. A 2013 Cochrane meta-analysis was unable to resolve this uncertainty,25 which arguably may be influenced by breast cancer phenotype. Given it would be difficult to deliver even hypo-fractionated radiotherapy and commence chemotherapy within 31 days of surgery, our data would argue in favour of sequencing chemotherapy first in patients with TNBC.
In conclusion, receiving adjuvant chemotherapy ≥31 days after surgery did not affect outcomes for all patients with early breast cancer in our study, consistent with most previous reports. However, in patients with TNBC, 5-year OS was significantly reduced if chemotherapy was received ≥31 days after surgery, suggesting that delivering chemotherapy within 31 days of surgery should be prioritised in this sub-group.
Supplementary information
Acknowledgements
These data were presented at the ESMO Breast Cancer Symposium 2019.26
Author contributions
A.F.C.O. collected data and wrote the manuscript. E.K., T.I., M.C. and V.A. collected data and edited/approved the manuscript. B.A. and K.M. performed the statistical analyses and approved the manuscript. G.W. collected data and approved the manuscript. A.R., S.R.D.J., M.P. and N.C.T. provided data and edited/approved the manuscript. I.E.S. designed the study and edited/approved the manuscript.
Ethics approval and consent to participate
The study was approved by the RM audit committee and ethics approval and consent were not required. The study was performed in accordance with the Declaration of Helsinki
Data availability
The data are available on request to the corresponding author.
Competing interests
The authors declare no competing interests.
Funding information
We wish to acknowledge the support of the Royal Marsden NIHR Biomedical Research Centre for Cancer.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01428-4.
References
- 1.Colleoni M, Bonetti M, Coates AS, Castiglione-Gertsch M, Gelber RD, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J. Clin. Oncol. 2000;18:584–590. doi: 10.1200/JCO.2000.18.3.584. [DOI] [PubMed] [Google Scholar]
- 2.Shannon C, Ashley S, Smith IE. Does timing of adjuvant chemotherapy for early breast cancer influence survival? J. Clin. Oncol. 2003;21:3792–3797. doi: 10.1200/JCO.2003.01.073. [DOI] [PubMed] [Google Scholar]
- 3.Lohrisch C, Paltiel C, Gelmon K, Speers K, Taylor S, Barnett J, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J. Clin. Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 4.Gagliato D, de M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J. Clin. Oncol. 2014;32:735–744. doi: 10.1200/JCO.2013.49.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322–329. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. Treat. 2016;160:17–28. doi: 10.1007/s10549-016-3960-3. [DOI] [PubMed] [Google Scholar]
- 7.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retsky M, Bonadonna G, Demicheli R, Folkman J, Hrushesky W, Valagussa P. Hypothesis: Induced angiogenesis after surgery in premenopausal node-positive breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients. Breast Cancer Res. 2004;6:R372–R374. doi: 10.1186/bcr804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 10.Sparano JA, Gray RJ, Ravdin PM, Makower DF, Pritchard KI, Albain KS, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N. Engl. J. Med. 2019;380:2395–2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Yu KD, Fan L, Qiu LX, Ling H, Jiang YZ, Shao ZM. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549–46556. doi: 10.18632/oncotarget.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group (EBCTC) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 19.Gianni L, Pienkowski T, Im YH, Roman L, Tseng L-M, Liu M-C, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 20.Gianni L, Pienkowski T, Im YH, Tseng L-M, Liu M-C, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 21.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann. Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 22.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yolota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 24.von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 25.Hickey BE, Francis DP, Lehman M. Sequencing of chemotherapy and radiotherapy for early breast cancer. Cochrane Database Syst. Rev. 2013;30:CD005212. doi: 10.1002/14651858.CD005212.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Okines AFC, Kipps E, Irfan T, Coakley M, Aggelis V, Asare B, et al. Impact of delayed adjuvant chemotherapy: the Royal Marsden Hospital (RMH) experience. Ann. Oncol. 2019;30:iii28. doi: 10.1093/annonc/mdz096.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available on request to the corresponding author.


