Summary
We developed a PD-1 B-cell epitope vaccine (PD1-Vaxx) to rival nivolumab therapy which has received ethics approvals for a Phase 1 clinical trial in Australia. The US FDA granted Investigational New Drug approval to Imugene Ltd for clinical testing in NSCLC. We demonstrated synergistic vaccine combinations with an HER-2 targeted vaccine (B-Vaxx).
Subject terms: Peptide vaccines, Cancer immunotherapy
Main
The introduction of checkpoint inhibitors (pembrolizumab/Keytruda, nivolumab/Optivo), and ipilimumab/Yervoy as novel cancer immunotherapeutic approaches has received regulatory approvals in several cancer types culminating in the 2018 Nobel prize awarded for the discovery of immune checkpoint blockade.1 By the end of 2020, a tsunami of immunotherapies has been evaluated in thousands of clinical trials targeting different malignancies with numerous drugs in pharmaceutical companies’ pipeline. Although great clinical success has been noticed by using therapeutic PD-1/PD-L1 signalling inhibitors, only a subset of patients (10–15%) has responded to monotherapy due to the development of primary and secondary resistance. Ongoing studies that exhibit durable responses and efficacy of treatment depends on the rational design of synergistic combinatorial approaches involving different modalities. Our seminal contribution and hypothesis of B-cell epitope vaccine established over decades of research resulting in the development of a polyclonal-induced B-cell antibody response will be more effective, or as effective with improved safety, compared with current monoclonal antibody (mAb) therapies.2 We have advanced a number of combination strategies of HER-2 with VEGF can significantly enhance anti-tumour effects.3 Additionally, we have demonstrated that combination therapies with HER-2 and IGF-1R or HER-2 and HER-3 and HER-1 and IGF-1R exhibit enhanced anti-tumour responses,4,5 and our HER-2 combination vaccines (B-Vaxx) targeting both trastuzumab and pertuzumab (clinical trials.gov: NCT01376505) showed great effects on patients in a Phase 1 clinical trial6 and is presently in a Phase 2 extension trial at the OSU James Cancer Hospital.
We recently introduced a novel PD-1 checkpoint inhibitor vaccine (PD1-Vaxx):7 a chimeric PD-1 B-cell peptide epitope vaccine (amino acid 92–110; PD1-Vaxx) linked to a measles virus fusion “promiscuous” T-cell epitope peptide (MVF) amino acid 288–302 via a four amino acid residue (GPSL). This vaccine when emulsified in Montanide ISA 720VG elicits the production of polyclonal antibodies that block PD-1 signalling and thus trigger anti-cancer effects comparable with the US Food and Drug Administration (FDA)-approved mAb “vivolumab/pembrolizumab” with no evidence of toxicity or autoimmunity.
Our results indicated that MVF-PD-1 (92–110) elicited high levels of anti-peptide antibodies that recognised the human recombinant protein PD-1. In a syngeneic BALB/c model, mice were immunised with PD1-Vaxx three times at three weeks’ intervals followed by challenge with CT26 colon carcinoma cells. A mouse surrogate antagonist antibody anti-mPD-1 mAb 29F.1A12 was used to treat mice twice a week as a positive control or PBS as a negative control. We found that mAb and PD1-Vaxx vaccine treatments had a similar and significant reduction in tumour growth. However, in this study, only the MVF-PD-1(92–110) group observed complete tumour regression after day 9 of tumour challenge, which indicated PD1-Vaxx has a better tumour control ability.
Additionally, we used the CT26/HER-2 tumour model (CT26 colon carcinoma cells engineered to express HER-2) in BALB/c to test for synergistic effects of PD1-Vaxx in combination with B-Vaxx to determine whether this combination could increase immunogenicity, enhance anti-tumour responses, and provide synergistic benefit in inhibiting tumour growth. The mice were immunised with single MVF-PD-1(92–110) [PD1-Vaxx], combo MVF-HER-2 (266–296) + MVF-HER-2(597–626) peptides [B-Vaxx], and triple vaccine combination [PD1-Vaxx +B-Vaxx]. Robust HER-2 and PD-1 antibody responses were elicited in all vaccinated mice. Our results show that the triple vaccination significantly reduced tumour growth in the BALB/c syngeneic model of CT26/HER-2 colon carcinoma versus the single PD1-Vaxx or the combo HER-2 vaccine [B-Vaxx]. The triple vaccination regimen (PD1-Vaxx + B-Vaxx) showed significant inhibition of tumour growth versus the standard mAb 29F.1A12 positive control. Most importantly, after day 16, only triple treatment group mice still observed complete tumour response. The higher rate of complete tumour response in the triple vaccinated group suggests that inhibiting multiple signalling targets may demonstrate synergistic inhibitory properties as compared with individual treatment options. Collectively, our peptide-based vaccine PD1-Vaxx activates both B- and T-cell functions to promote clearance. The treatment is targeted to block signalling pathways that are crucial for tumour growth and maintenance. And by giving this vaccine in combination with an immunotherapy drug, we are essentially super-charging and specifically directing the immune system to target and kill cancer cells.
All mice vaccinated over a period of 8 weeks showed no signs of scruffiness, lesions, or lethargy. No significant lesions were noted in any of the organs (spleen, liver, heart, lung, and kidney) under histological evaluation. In unpublished studies in beagle dogs, we demonstrated high immunogenicity and safety of vaccination with PD1-Vaxx, B-Vaxx and their combinations. A non-human primates (NHPs) investigation to evaluate the potential toxicity of PD1-Vaxx conducted at Charles River laboratories (Ashland, Ohio) in a GLP-compliant toxicology study in cynomolgus monkeys resulted in significant PD-1 antibody induction with a no-observed-adverse-effect-level (NOAEL) of 100 μg dose of PD1-Vaxx. The high immunogenicity and antigenicity of PD1-Vaxx in cynomolgus monkeys with 100% homology indicate that the vaccine was safe and did not develop tolerance and should be equally efficacious in human clinical trials.
Imugene Ltd (ASX:IMU), a clinical stage immune-oncology company, received US FDA Investigational New Drug (IND) approval and is the sponsor to initiate a clinical Phase 1 trial in the US in non-small cell lung cancer (NSCLC) patients. PD1-Vaxx obtained ethics approval for a Phase 1 clinical trial in Sydney and Melbourne and three patients have been successfully dosed in Australia in November/December 2020. Full study details can also be found on clinical trials.gov under study ID: NCT04432207. We believe the field is poised for many more breakthroughs and significant new improvements in the treatment of patients with cancers for maximum clinical benefits.
Future perspectives and challenges
To date, concerted efforts have focused on combinations of approved therapies aiming to improve clinical efficacy and further augment positive outcomes and survivals. Thus, a variety of combinations of checkpoint inhibitors with other modalities targeting different cell types are being pursued based on the need to block multiple pathways of immunosuppression.
In conclusion, despite the proven benefits of passive immunotherapy with mAbs, active immunisation with peptide vaccines that elicit polyclonal antibodies can provide significant advantages for the patient’s quality of life (Fig. 1). Our careful design of suitable antigenic peptides in the HER-2 and PD-1 landscape is a testament to our approach demonstrated to achieve an efficacious safe peptide-based immune therapy. The translation to human clinical trials brings peptide B-cell cancer vaccines to the forefront of cancer treatment without causing potentially serious hypersensitivity reactions, infusion reactions, and immune complex-mediated diseases that inflict substantial mental, biological and financial burdens upon the cancer patients. The potential impact going forward like our three-prong combinations of B-Vaxx and PD1-Vaxx will be fundamental to unlocking the full potential of immunotherapies that mitigates different mechanisms of resistance with synergistic effects that may offer improved efficacy in broader patient populations and in advanced malignancies with high unmet need. It might be possible that blockade of additional immune checkpoint molecules, such as lymphocyte-activation gene 3 (LAG3) and T-cell immunoglobulin mucin-3 (TIM-3) in combinations with RTKs HER-3, IGF-1R and VEGF could improve anti-tumour effects of peptide vaccines.
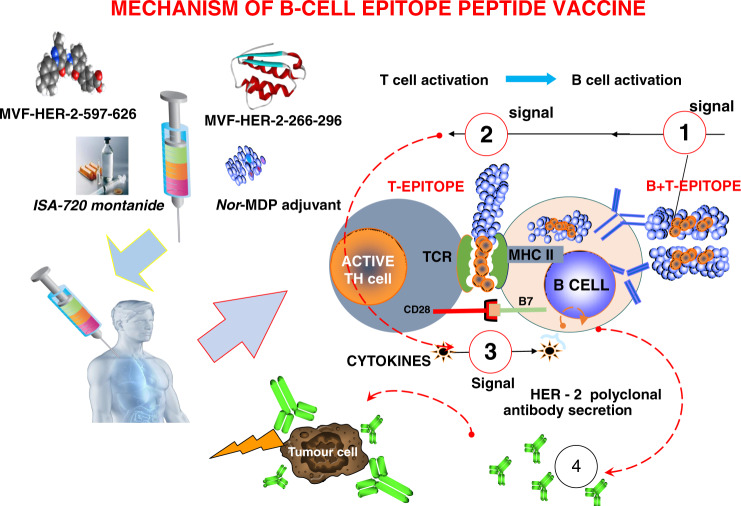
Fig. 1. Mechanism of B-cell epitope vaccine.
(1) The chimeric B-cell and MVF-T-cell epitope are presented intact (without processing) to antigen presenting cells or B cells; (2) The MVF-T-cell epitope binds MHC class II and activates the T cells, whereas the B-cell epitope engages the B-cell receptor; (3) Cytokines are liberated to help B cells make anti-peptide polyclonal antibodies; (4) Both HER-2 B-cell and PD1-Vaxx elicited polyclonal antibodies bind tumour cells and prevent metastasis or block PD-1:PD-L1 binding respectively.
Acknowledgements
Not applicable.
Author contributions
L.G. wrote the first draft. P.K. edited the manuscript, wrote the revised version and drafted the figure. Both authors approved this manuscript.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
All the data relevant to this manuscript are included in the article or in our previous publications (see refs. 6,7). Other data information’s available on reasonable request from the corresponding author.
Competing interests
P.T.P.K. is co-inventor on patents regarding PD1-Vaxx which has licensed such patents from OSU to Imugene Ltd. P.T.P.K. has partial inventor rights to PD1-Vaxx which has been licensed for commercial development. P.T.P.K. is consultant to Imugene Ltd. L.G. declares no competing interests. No writing assistance was utilised in the production of this manuscript.
Funding information
Funded by National Institutes of Health NIH CA84356, NIH R21CA13508 and Imugene Ltd to P.T.P. Kaumaya.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ledford H, Else H, Warren M. Cancer immunologists scoop medicine Nobel prize. Nature. 2018;562:20–21. doi: 10.1038/d41586-018-06751-0. [DOI] [PubMed] [Google Scholar]
- 2.Kaumaya PT. B-cell epitope peptide cancer vaccines: a new paradigm for combination immunotherapies with novel checkpoint peptide vaccine. Future Oncol. 2020;16:1767–1791. doi: 10.2217/fon-2020-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J. Biol. Chem. 2011;286:13626–13637. doi: 10.1074/jbc.M110.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MJ, Foy KC, Overholser JP, Nahta R, Kaumaya PTP. HER-3 peptide vaccines/mimics: combined therapy with IGF-1R, HER-2, and HER-1 peptides induces synergistic antitumor effects against breast and pancreatic cancer cells. Oncoimmunology. 2014;3:e956012. doi: 10.4161/21624011.2014.956012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foy KC, Miller MJ, Overholser J, Donnelly SM, Nahta R, Kaumaya PTP. IGF-1R peptide vaccines/mimics inhibit the growth of BxPC3 and JIMT-1 cancer cells and exhibit synergistic antitumor effects with HER-1 and HER-2 peptides. Oncoimmunology. 2014;3:e956005. doi: 10.4161/21624011.2014.956005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekaii-Saab T, Wesolowski R, Ahn DH, Wu C, Mortazavi A, Lustberg M, et al. Phase I immunotherapy trial with two chimeric HER-2 B-cell peptide vaccines emulsified in montanide ISA 720VG and nor-MDP adjuvant in patients with advanced solid tumors. Clin. Cancer Res. 2019;25:3495–3507. doi: 10.1158/1078-0432.CCR-18-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaumaya PTP, Guo L, Overholser J, Penichet ML, Bekaii-Saab T. Immunogenicity and antitumor efficacy of a novel human PD-1 B-cell vaccine (PD1-Vaxx) and combination immunotherapy with dual trastuzumab/pertuzumab-like HER-2 B-cell epitope vaccines (B-Vaxx) in a syngeneic mouse model. Oncoimmunology. 2020;9:1818437. doi: 10.1080/2162402X.2020.1818437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data relevant to this manuscript are included in the article or in our previous publications (see refs. 6,7). Other data information’s available on reasonable request from the corresponding author.