Abstract
Chronic diseases, such as chronic kidney disease (CKD), are frequently accompanied by various comorbidities, including anemia, which is considered a surrogate marker of systemic inflammation. Psoriasis is a chronic inflammatory skin disease prevalent in patients with chronic disease. Psoriasis risk in patients with CKD, however, especially in patients with low hemoglobin levels, has never been investigated. In this study, we investigated associations between low hemoglobin levels and psoriasis in patients with CKD using data from the National Health Insurance Service of Korea. During a mean follow-up period of 6.16 ± 1.02 years, psoriasis was recorded in 13,803 patients with CKD (2.39% of CKD patients). The cumulative incidence of psoriasis was significantly higher in CKD patients with anemia (hemoglobin levels < 13 g/dL in men and < 12 g/dL in women) than those without. In multivariate-adjusted Cox proportional hazards regression models, the risk of psoriasis was significantly higher in anemic CKD patients than nonanemic CKD patients (hazard ratio [HR] 1.136, 95% CI 1.089–1.185, p < 0.001). Additionally, we noted that the incidence of psoriasis decreased with increasing hemoglobin levels in CKD patients (HR 0.953, 95% CI 0.942–0.965, p < 0.001). Altogether, our findings indicate that low hemoglobin levels are significantly related to psoriasis risk in patients with CKD. Further study is required to elucidate whether low hemoglobin levels have an impact on the development of psoriasis or are merely a surrogate marker of psoriasis risk in patients with CKD.
Subject terms: Kidney diseases, Skin diseases, Risk factors, Kidney diseases
Introduction
Anemia is a common occurrence in chronic diseases, such as chronic kidney disease (CKD), chronic obstructive pulmonary disease, heart disease, etc., and in healthy older adults1–4. In chronic diseases, anemia has been shown to be closely associated with poor quality of life, higher mortality, and various comorbidities, including cardiovascular disease3,5–8. In patients with CKD in particular, anemia has been found to be associated with symptoms of fatigue, dyspnea, and exercise intolerance, as well as with higher rates of hospitalization and mortality, and treatments for anemia appear to have beneficial effects on cardiovascular comorbidity and hospitalization and mortality rates6,7,9,10.
Psoriasis is chronic inflammatory skin disease in which IL23 and T helper 17 (Th17) cells play a central role11. Cumulative evidence suggests that psoriasis is not merely a cutaneous disease, but a systemic inflammatory disease accompanied by various comorbidities, including psoriatic arthritis, inflammatory bowel disease, and cardiovascular disease12. Recently, population-based cohort studies have indicated that psoriasis is an independent risk factor of CKD13–15. However, to the best of our knowledge, studies have yet to evaluate psoriasis risk in patients with CKD. Since anemia is common in chronic diseases, such as CKD, in this study, we sought to evaluate potential relationships between anemia and psoriasis risk in CKD patients though a population-based cohort study.
Results
Clinical characteristics of the study population
Clinical characteristics of CKD patients with or without anemia are summarized in Table 1. Of the 576,461 CKD patients, anemia was present in 108,304 (18.8%). Mean age (63.37 ± 14.39 vs. 56.13 ± 15.08 years) and the percentages of male patients (28.95 vs. 43.96%), urban residents (47.28 vs. 49.37%), patients with low income (23.1 vs. 19.37%), current smokers (8.21 vs. 17.03%), heavy drinkers (1.67 vs. 4.29%), and patients with regular physical activity (38.52 vs. 49.18%) were significantly different between the CKD patients with and without anemia. Interestingly, the prevalences of hypertension (59.32 vs. 43.3%), dyslipidemia (33.6 vs. 29.52%), stroke (4.27 vs. 2.53%), and heart disease (11 vs. 6.06%) were significantly higher in CKD patients with anemia than those without anemia. In contrast, the prevalence of diabetes (84.7 vs. 73.45%) was significantly higher in CKD patients without anemia. CKD patients with anemia exhibited significantly lower height (156.89 ± 8.55 vs. 160.86 ± 9.29 cm), weight (57.92 ± 9.91 vs. 62.8 ± 10.93 kg), waist circumference (80.61 ± 9.2 vs. 81.93 ± 9.1 cm), body mass index (BMI, 23.48 ± 3.26 vs. 24.2 ± 3.22 kg/m2), diastolic blood pressure (75.95 ± 10.53 vs. 77.37 ± 10.12 mmHg), and total cholesterol (191.38 ± 40.89 vs. 201.05 ± 39.05 mg/dL). Also, systolic blood pressure (126.32 ± 17.33 vs. 125.64 ± 15.92 mmHg), fasting glucose (104.82 ± 32.41 vs. 101.78 ± 27.15 mg/dL), and glomerular filtration rate (45.5 ± 16.11 vs. 42.08 ± 21.21 ml/min/1.73m2) were significantly higher in CKD patients with anemia than in their non-anemic counterparts.
Table 1.
Baseline characteristics of chronic kidney disease patients according to the presence of anemia.
| Anemia* | P value | ||
|---|---|---|---|
| Absent | Present | ||
| n = 468,157 | n = 108,304 | ||
| Age, years | 56.13 ± 15.08 | 63.37 ± 14.39 | < 0.0001 |
| Men, n (%) | 205,798 (43.96) | 31,352 (28.95) | < 0.0001 |
| Urban resident, n (%) | 231,148 (49.37) | 51,201 (47.28) | < 0.0001 |
| Low income, n (%) | 90,677 (19.37) | 25,013 (23.1) | < 0.0001 |
| Current smoker, n (%) | 79,723 (17.03) | 8891 (8.21) | < 0.0001 |
| Heavy drinker, n (%) | 20,080 (4.29) | 1805 (1.67) | < 0.0001 |
| Regular physical activity, n (%) | 230,262 (49.18) | 41,716 (38.52) | < 0.0001 |
| Diabetes, n (%) | 396,514 (84.7) | 79,546 (73.45) | < 0.0001 |
| Hypertension, n (%) | 202,724 (43.3) | 64,244 (59.32) | < 0.0001 |
| Dyslipidemia, n (%) | 138,223 (29.52) | 36,389 (33.6) | < 0.0001 |
| Stroke, n (%) | 8938 (2.53) | 3444 (4.27) | < 0.0001 |
| Heart disease, n (%) | 21,410 (6.06) | 8879 (11) | < 0.0001 |
| Height, cm | 160.86 ± 9.29 | 156.89 ± 8.55 | < 0.0001 |
| Weight, kg | 62.8 ± 10.93 | 57.92 ± 9.91 | < 0.0001 |
| Waist circumference, cm | 81.93 ± 9.1 | 80.61 ± 9.2 | < 0.0001 |
| BMI, kg/m2 | 24.2 ± 3.22 | 23.48 ± 3.26 | < 0.0001 |
| DBP, mmHg | 77.37 ± 10.12 | 75.95 ± 10.53 | < 0.0001 |
| SBP, mmHg | 125.64 ± 15.92 | 126.32 ± 17.33 | < 0.0001 |
| Fasting glucose, mg/dL | 101.78 ± 27.15 | 104.82 ± 32.41 | < 0.0001 |
| Total cholesterol, mg/dL | 201.05 ± 39.05 | 191.38 ± 40.89 | < 0.0001 |
| GFR, ml/min/1.73m2 | 42.08 ± 21.21 | 45.5 ± 16.11 | < 0.0001 |
Data are presented as means ± standard deviations or numbers and percentages.
BMI body mass index; DBP diastolic blood pressure; SBP systolic blood pressure; GFR glomerular filtration rate.
*Anemia was defined as a hemoglobin level of < 13 g/dL in men and < 12 g/dL in women.
Relationship between hemoglobin levels and psoriasis in CKD patients
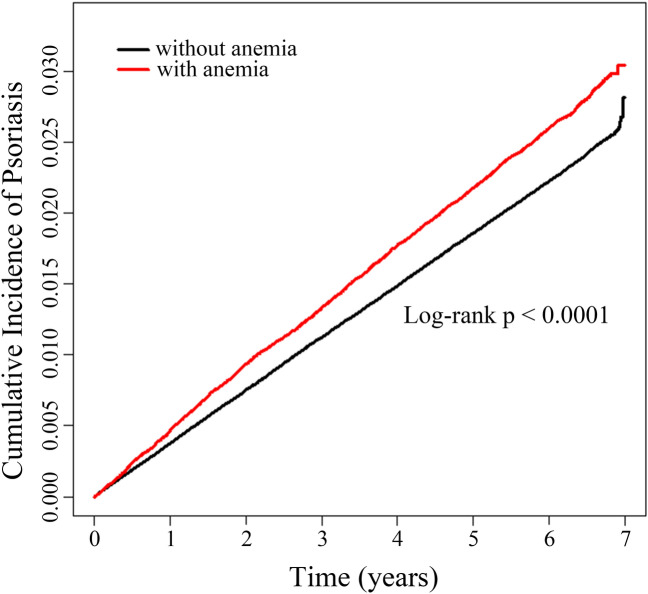
During a mean follow-up period of 6.16 ± 1.02 years, psoriasis developed in 13,803 of the 576,461 CKD patients (2.39%). Interestingly, the CKD patients with anemia showed a higher cumulative incidence of psoriasis than the CKD patients without anemia (log-rank p < 0.0001, Fig. 1). Next, we evaluated the risk of psoriasis in three models adjusting for different confounding variables (see Materials and Methods). As shown in Table 2, CKD patients with anemia exhibited higher risks of psoriasis in all three models (model 1, hazard ratio [HR] 1.168, 95% CI 1.121–1.218; model 2, HR 1.136, 95% CI 1.089–1.185; model 3, HR 1.109, 95% CI 1.062–1.158).
Figure 1.
Cumulative incidences of psoriasis according to the presence of anemia in patients with chronic kidney disease.
Table 2.
Hazard ratios for psoriasis according to the presence and severity of anemia among patients with chronic kidney disease.
| Person years | HR (95% CI) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Anemia* | ||||
| No (n = 10,978) | 2,912,237.2 | 1 (reference) | 1 (reference) | 1 (reference) |
| Yes (n = 2825) | 641,507.4 | 1.168 (1.121–1.218) | 1.136 (1.089–1.185) | 1.109 (1.062–1.158) |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | |
| Hemoglobin quintiles | ||||
| Q1 (n = 3172) | 690,432.9 | 1 (reference) | 1 (reference) | 1 (reference) |
| Q2 (n = 2657) | 676,810.07 | 0.854 (0.811–0.9) | 0.88 (0.836–0.927) | 0.903 (0.858–0.952) |
| Q3 (n = 2786) | 754,565.8 | 0.804 (0.764–0.846) | 0.871 (0.827–0.917) | 0.891 (0.846–0.938) |
| Q4 (n = 2504) | 687,743.26 | 0.793 (0.752–0.835) | 0.873 (0.827–0.921) | 0.889 (0.842–0.939) |
| Q5 (n = 2684) | 744,192.57 | 0.785 (0.746–0.826) | 0.867 (0.823–0.914) | 0.877 (0.831–0.926) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | |
| Hemoglobin of 1 g/dL | 0.995 (0.985–1.006) | 0.953 (0.942–0.965) | 0.957 (0.946–0.969) | |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | |
Model 1 was not adjusted for any variable. Model 2 was adjusted for age and sex. Model 3 was adjusted for age, sex, body mass index, smoking status, alcohol consumption, physical activity, income, glomerular filtration rate, diabetes, hypertension, and dyslipidemia.
*Anemia was defined as a hemoglobin level of < 13 g/dL in men and < 12 g/dL in women.
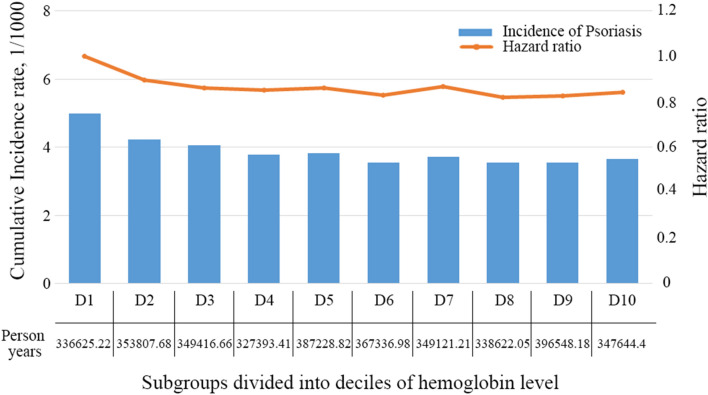
Also, we assessed psoriasis risk according to quintiles of hemoglobin levels (Q1-Q5). The mean hemoglobin levels were 11.4 ± 1.1 g/dL in Q1, 12.9 ± 0.9 g/dL in Q2, 13.5 ± 0.9 g/dL in Q3, 14.2 ± 1.0 g/dL in Q4, and 15.1 ± 1.2 g/dL in Q5. Compared to lowest hemoglobin quintile (Q1), the risk of psoriasis decreased with increases in hemoglobin and followed a linear trend across hemoglobin quintiles. This finding was consistent across models 1, 2, and 3 (Table 2). Moreover, in model 3, the cumulative incidences and HRs of psoriasis showed similar trends across subgroups divided into deciles of hemoglobin levels (Fig. 2). As expected, psoriasis risk decreased significantly with an increase in hemoglobin, and this finding was consistent in models 1, 2, and 3 (Table 2).
Figure 2.
Cumulative incidences and hazard ratios of psoriasis according to deciles of hemoglobin levels in chronic kidney disease patients.
Discussion
Psoriasis is a chronic inflammatory skin disease accompanied by systemic inflammation, therefore, several comorbidities, such as cardiovascular disease, inflammatory bowel disease and psoriatic arthritis, are associated with psoriasis12. In addition, there have been several reports regarding the disease with a higher risk of psoriasis. In addition to diseases shared immunopathologic connection with psoriasis, such as Crohn’s disease, several diseases, which are not mainly caused by activation of Th17 cell-mediated pathway, such as rheumatoid arthritis, celiac disease, and chronic periodontitis, also revealed a higher risk of psoriasis16–19. This finding might be partially explained by inflammatory microenviroment in these disease might provide favorable conditions for the development of psoriasis.
Chronic diseases, such as CKD, are frequently accompanied by inflammation20, and chronic inflammatory status in CKD has been shown to be associated with several underlying factors, increased infection rates, elevated cytokine levels, and other cardiovascular comorbidities21. Due to impaired renal function, patients with CKD typically present with increased serum levels of inflammatory molecules, including interleukin-1 (IL-1), IL-6, and C-reactive protein22,23. Based on various clinical and experimental findings, researchers have suggested that inflammation in patients with CKD is not only a predisposing factor for cardiovascular comorbidities, but also an aggravating factor for CKD24,25.
The clinical significance of chronic inflammation in CKD is further emphasized by its causative effect on anemia. In patients with chronic disease, such as CKD, chronic heart disease, and chronic pulmonary disease, persistent systemic inflammation restricts erythropoiesis and hinders erythrocyte survival through inflammatory cytokines26,27. In this study, we found that anemia and hemoglobin levels were closely associated with psoriasis risk in CKD patients. The association between hemoglobin levels and psoriasis was independent of age, sex, BMI, current smoking status, alcohol consumption, physical activity, income level, glomerular filtration rate, diabetes, hypertension, and dyslipidemia. Since the development of anemia and resistance to treatment for anemia are induced by systemic inflammation in CKD, anemia may be a surrogate marker of psoriasis risk. However, further investigation of the effect of anti-inflammation treatment on the development of psoriasis in CKD patients would be required to address this hypothesis.
Hypoxia-inducible factors (HIFs), which are transcription factors required for cellular adaptation of hypoxia, play a crucial role in pathogenesis of CKD, as well as CKD-associated comorbidities, through multiple mechanisms28. Interestingly, HIFs have also been found to be upregulated in the lesional skin of psoriasis patients29. Meanwhile, several studies have indicated that HIF-1α plays a role in the development of psoriasis: HIF-1α induces the expression of vascular endothelial growth factor (VEGF), which is also upregulated in psoriatic lesions, and the activation of VEGF induces psoriasis like lesions, possibly through induction of angiogenesis29,30. In addition, HIF-1α is involved in the differentiation of T helper cell 17 (Th17), a key cellular component in the immunopathogenesis of psoriasis, and in the production of IL-1731. Interestingly, research suggests that a Th17 immune response could induce anemia through suppression of the hematopoietic system32. Moreover, studies have shown that Th17 cells and IL17 are important in renal injury induced by high salt intake or ischemia/reperfusion injury33,34. Accordingly, we suspect that the development of both CKD and anemia could be accompanied by activation of a Th17 immune response associated with psoriasis development, making psoriasis a comorbidity of CKD, especially in anemic individuals.
Psoriasis is well known for its association with poor quality of life35. Moreover, in CKD patients with anemia, who likely have severely impaired renal function, systemic treatment of psoriasis, such as that with cyclosporine or methotrexate, is limited. Therefore, in CDK patients with anemia, we propose that controlling the risk of psoriasis by effectively managing anemia and systemic inflammation could help with limiting psoriasis and its adverse effects on quality of life. Since inflammatory cytokines underlie the development of anemia and resistance to erythropoietin treatment, anti-inflammatory treatment could be beneficial in the treatment of anemia and the prevention of psoriasis in CKD patients36. Indeed, research has shown that pentoxifylline, which inhibits the production of T-helper cell-derived cytokines, improves anemia in CKD patients37,38.
In conclusion, we found that low hemoglobin levels were significantly related to an increased risk of psoriasis in CKD patients. Although it is unclear whether the observed increased risk in psoriasis is directly affected by an anemic condition or is merely a surrogate marker of systemic inflammation underlying the development of psoriasis, proactive treatment for inflammation might play crucial role in managing both anemia and psoriasis in CKD patients.
Materials and methods
Data source and study population
The dataset used in this retrospective cohort study was provided by the National Health Insurance Service (NHIS) of Korea and included information on age, sex, socioeconomic variables, disease diagnoses based on the ICD-10 Clinical Modification (ICD-10-CM), medical treatments and procedures, and health examination results recorded in inpatient and outpatient claims for the majority of the South Korean population39. The study population was recommended to undergo standardized medical examinations every 2 years by the National Health Insurance Corporation.
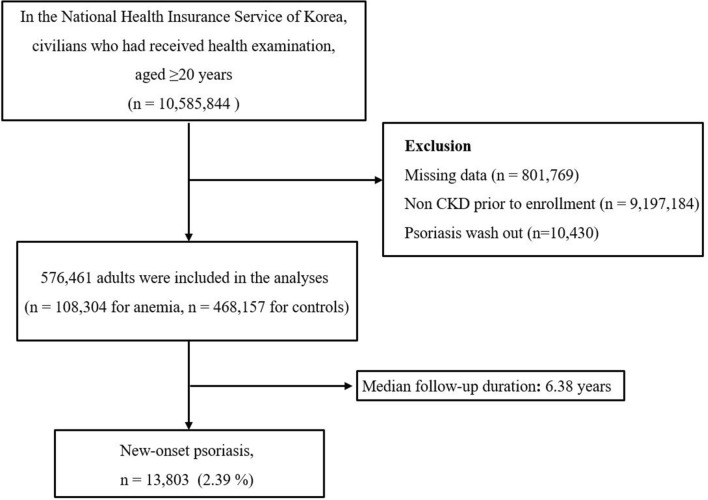
In the NHIS database, individuals with chronic kidney disease (CKD) were identified using the International Classification of Disease, 10th revision code N18. CKD patients with a confirmed ICD-10-CM diagnostic code given during health screening from January 2009 to December 2009 were enrolled in this study. CKD patients diagnosed with psoriasis before this study period were excluded (Fig. 3). This study was approved by the Institutional Review Board of the Korean National Institute for Bioethics Policy (NHIS-2019–01-076) and the Ethics Committee of Seoul St. Mary’s Hospital, the Catholic University of Korea (KC19ZESI 0236). All data were anonymized and de-identified, and informed consent was waived by the Institutional Review Board of the Korean National Institute for Bioethics Policy. This study was performed in accordance with the principles of the Declaration of Helsinki.
Figure 3.
Flow chart of enrolled population.
Measurement and definition of covariates
Medical records included measurements of height, weight, waist circumference, and blood pressure; laboratory test results for fasting glucose, total cholesterol, and glomerular filtration rate; and past medical history. Information on health-related behaviors, such as smoking, drinking, and physical activity, were obtained using a standardized self-reporting questionnaire. Smoking status was categorized as nonsmoker, former smoker, and current smoker. Heavy drinkers were defined as individuals who consumed ≥ 30 g of alcohol per day40. Regular physical activity was categorized as strenuous physical activity for ≥ 20 min at least three times per week or moderate physical activity for ≥ 30 min at least five times per week. Household income level in the lower 25% was defined as low income.
Study design
To examine the relationship between anemia and psoriasis risk in CKD patients, the patients were subdivided into subgroups according to the presence of anemia and the severity thereof. In this study, anemia was defined as a hemoglobin level of < 13 g/dL in men and < 12 g/dL in women. In addition, CKD patients were subdivided into five and 10 groups according to quintiles and deciles of hemoglobin levels, respectively. Among these subgroups, we identified patients newly diagnosed with psoriasis (ICD-10-CM code, L40) over a mean follow-up period of 6.16 years through claims data.
Statistical analysis
The baseline characteristics of the CKD patients are presented as means with standard deviations or numbers and percentages. The cumulative incidences of psoriasis according to the presence of anemia in CKD patients were depicted using Kaplan–Meier curves, and the log-rank test was used to analyze differences therein between groups. To analyze the presence or severity of anemia in relation to psoriasis risk, multivariate-adjusted Cox proportional hazards regression models were used in three different ways: Model 1 was not adjusted for any variable. Model 2 was performed adjusting for age and sex. BMI, smoking status, alcohol consumption, physical activity, income, glomerular filtration rate, diabetes, hypertension, and dyslipidemia were further adjusted in model 3. Variables included in these models were chosen according to it’s clinical in the development of psoriasis and statistical significance in our study. Compared to controls (CKD patients without anemia and in the lowest hemoglobin quintile), hazard ratios (HRs) and 95% CIs were calculated. Statistical significance was defined as a two-sided P value less than 0.05. All statistical analyses were performed using SAS software (ver. 9.4; SAS Institute, Cary, NC, USA) and R programming (version 3.1.0; The R Foundation for Statistical Computing, Vienna, Austria).
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2018R1D1A1B07044100).
Author contributions
S.H.L., M.K. and J.H.L. wrote the main manuscipt and K.H. prepared figures and tables. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanudel MR, et al. Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am. J. Physiol. Renal Physiol. 2016;311:F1369–F1377. doi: 10.1152/ajprenal.00281.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opasich C, et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur. Heart. J. 2005;26:2232–2237. doi: 10.1093/eurheartj/ehi388. [DOI] [PubMed] [Google Scholar]
- 3.Boutou AK, Hopkinson NS, Polkey MI. Anaemia in chronic obstructive pulmonary disease: An insight into its prevalence and pathophysiology. Clin. Sci. (Lond) 2015;128:283–295. doi: 10.1042/CS20140344. [DOI] [PubMed] [Google Scholar]
- 4.Peters R, et al. Haemoglobin, anaemia, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:18. doi: 10.1186/1471-2318-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters H, et al. Association of anemia with health-related quality of life and survival: A large population-based cohort study. Haematologica. 2019;104:468–476. doi: 10.3324/haematol.2018.195552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J. Am. Soc. Nephrol. 1999;10:610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 7.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63:1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 8.Kdoqi & National Kidney, F KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am. J. Kidney Dis. 2006;47:S11–145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Locatelli F, et al. Anemia management for hemodialysis patients: Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines and Dialysis Outcomes and Practice Patterns Study (DOPPS) findings. Am. J. Kidney Dis. 2004;44:27–33. doi: 10.1053/j.ajkd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Parfrey PS, et al. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J. Am. Soc. Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 11.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Invest. Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: Classical and emerging comorbidities. Ann. Bras. Dermatol. 2015;90:9–20. doi: 10.1590/abd1806-4841.20153038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi CC, et al. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J. Dermatol. Sci. 2015;78:232–238. doi: 10.1016/j.jdermsci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Wan J, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: Population based cohort study. BMJ. 2013;347:f5961. doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, et al. Risk of end-stage renal disease in psoriatic patients: Real-world data from a nationwide population-based cohort study. Sci. Rep. 2019;9:16581. doi: 10.1038/s41598-019-53017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn's disease. J. Am. Acad. Dermatol. 2003;48:805–821. doi: 10.1067/mjd.2003.540. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Lindelof B, Zingone F, Ciacci C. Psoriasis in a nationwide cohort study of patients with celiac disease. J. Invest. Dermatol. 2011;131:2010–2016. doi: 10.1038/jid.2011.162. [DOI] [PubMed] [Google Scholar]
- 18.Keller JJ, Lin HC. The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. Br. J. Dermatol. 2012;167:1338–1344. doi: 10.1111/j.1365-2133.2012.11126.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen ML, Ku YH, Yip HT, Wei JC. Tonsillectomy and the subsequent risk of psoriasis: A nationwide population-based cohort study. J. Am. Acad. Dermatol. 2021 doi: 10.1016/j.jaad.2021.01.094. [DOI] [PubMed] [Google Scholar]
- 20.Stenvinkel P. Inflammation in end-stage renal disease: A fire that burns within. Contrib. Nephrol. 2005;149:185–199. doi: 10.1159/000085525. [DOI] [PubMed] [Google Scholar]
- 21.Malyszko J, Mysliwiec M. Hepcidin in anemia and inflammation in chronic kidney disease. Kidney Blood Press Res. 2007;30:15–30. doi: 10.1159/000098522. [DOI] [PubMed] [Google Scholar]
- 22.Poole S, et al. Fate of injected interleukin 1 in rats: Sequestration and degradation in the kidney. Cytokine. 1990;2:416–422. doi: 10.1016/1043-4666(90)90050-4. [DOI] [PubMed] [Google Scholar]
- 23.Panichi V, et al. C reactive protein in patients with chronic renal diseases. Renal Fail. 2001;23:551–562. doi: 10.1081/jdi-100104737. [DOI] [PubMed] [Google Scholar]
- 24.Tonelli M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 25.Zebrack JS, et al. Do associations with C-reactive protein and extent of coronary artery disease account for the increased cardiovascular risk of renal insufficiency? J. Am. Coll. Cardiol. 2003;42:57–63. doi: 10.1016/s0735-1097(03)00564-3. [DOI] [PubMed] [Google Scholar]
- 26.Ganz T. Anemia of Inflammation. N. Engl. J. Med. 2019;381:1148–1157. doi: 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
- 27.Cartwright GE. The anemia of chronic disorders. Semin. Hematol.. 1966;3:351–375. [PubMed] [Google Scholar]
- 28.Liu J, et al. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18050950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberger C, et al. Upregulation of hypoxia-inducible factors in normal and psoriatic skin. J Invest Dermatol. 2007;127:2445–2452. doi: 10.1038/sj.jid.5700874. [DOI] [PubMed] [Google Scholar]
- 30.Xia YP, et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 31.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Latour RP, et al. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116:4175–4184. doi: 10.1182/blood-2010-01-266098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo L, et al. Lymphocyte-specific deletion of IKK2 or NEMO mediates an increase in intrarenal Th17 cells and accelerates renal damage in an ischemia-reperfusion injury mouse model. Am. J. Physiol. Renal Physiol. 2016;311:F1005–F1014. doi: 10.1152/ajprenal.00242.2016. [DOI] [PubMed] [Google Scholar]
- 34.Mehrotra P, et al. IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: Compensatory role of natural killer cells in athymic rats. Am. J. Physiol. Renal Physiol. 2017;312:F385–F397. doi: 10.1152/ajprenal.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: A systematic literature review. J. Investig. Dermatol. Symp. Proc. 2004;9:140–147. doi: 10.1046/j.1087-0024.2003.09110.x. [DOI] [PubMed] [Google Scholar]
- 36.Gluba-Brzozka A, Franczyk B, Olszewski R, Rysz J. The influence of inflammation on anemia in CKD patients. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolignano D, D'Arrigo G, Pisano A, Coppolino G. Pentoxifylline for anemia in chronic kidney disease: A systematic review and meta-analysis. PLoS ONE. 2015;10:e0134104. doi: 10.1371/journal.pone.0134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benbernou N, Esnault S, Potron G, Guenounou M. Regulatory effects of pentoxifylline on T-helper cell-derived cytokine production in human blood cells. J. Cardiovasc. Pharmacol. 1995;25(Suppl 2):S75–79. doi: 10.1097/00005344-199500252-00016. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: A review of putative mechanisms. Alcohol Alcohol. 2002;37:409–415. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]